肺癌と診断された患者の禁煙のための介入
アブストラクト
背景
肺癌は世界中で癌で死亡する最もよく見られる原因の一つである。喫煙は、血管疾患、呼吸器疾患および癌などの多くの健康上の問題を誘発し悪化させる。喫煙によって肺癌の最も重要な危険因子が生じる。大部分の肺癌患者は、診断時に依然として能動喫煙者であるか、あるいは禁煙後にまた喫煙者に逆戻りしている場合が多い。喫煙者にとって、早期死亡および障害のリスクを減らすためには禁煙は最も効果的な方法である。肺癌患者は禁煙によって利益を得るであろう。肺癌患者にとって禁煙介入が有効であるかどうか、そして禁煙の一つの方法が他のいかなる方法に比較してより有効であるかどうかということは系統的に再検討されていない。
目的
肺癌患者に対する禁煙プログラムの有効性を確認すること。
検索戦略
2015年6月22日までCochrane Central Register of Controlled Trials (CENTRAL), MEDLINE ( PubMedからアクセス) および EMBASEを検索した。2015年7月1日までAmerican Society of Clinical Oncology (ASCO) Annual Meeting proceedings, ESMO Congress議事録の肺癌セクション, European Conference of Clinical Oncology (ECCO) Congress議事録の肺癌セクション, World Conference on Lung Cancerの議事録, Society for Research on Nicotine and Tobacco Annual Meeting (2013年から), Food and Drug Administrationのウェブサイト, European Medicine Agency for drug registrationのウェブサイト, World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP)のサーチポータル,ClinicalTrials.gov, および metaRegister of Controlled Trials (mRCT)を検索した。発表の言語に関して制限を設けなかった。
選択基準
肺癌患者において、以下のあらゆるランダム化比較試験(RCT)を組み入れる予定であった。介入なし、それぞれ異なる心理社会的または薬理学的介入(または両方)もしくは薬理学的介入のプラセボと比較した、あらゆる心理社会的あるいは薬理学的禁煙介入あるいはそれらの組み合わせ。
データ収集と分析
2名のレビュー著者らはそれぞれ組み入れのために可能性のある試験の初期検索から得られた試験のスクリーニングを行った。コクランが予想した標準的方法を用いる予定であった。選択基準に合致した試験は確認されなかった。
主な結果
選択基準に合致したRCTは確認されなかった。我々の検索法を用いて検索された1052件の記録の中で、さらに検討するために13件の試験を検索した。10件の試験を除外した:5件の試験は肺癌患者を他の参加者と区別できなかったため、もしくは参加者が肺癌でなかったため、4件はランダム化試験あるいはRCTでなかったため。2004年に終了したが結果が入手できないため1件の試験を除外した。データが入手可能になった場合、組み入れのために3件の進行中の試験を評価した。
著者の結論
肺癌患者用のあらゆる種類の禁煙プログラムの有効性を確認したRCTはなかった。禁煙介入が肺癌患者に有効かどうか、そして1つのプログラムが他のいずれのプログラムと比較してもより有効であるかどうかを確認するためのエビデンスが不十分であった。肺癌患者には禁煙を奨励し、禁煙介入を提案する必要がある。しかしながら、RCTの不足のため肺癌患者のための禁煙介入の効力が評価できず、結論も下すこともできなかった。この系統的レビューはこれらを調査するためにRCTの必要性を確認した。
PICO
一般語訳
肺癌患者のための禁煙介入肺癌患者のための禁煙介入
背景
肺癌は世界中で癌による死亡の最もよく見られる原因である。喫煙によって肺癌の最も重要な危険因子が生じる。肺癌患者の大部分は診断時に依然として能動喫煙者であるか、あるいは禁煙後にまた喫煙者に逆戻りしている場合が多い。喫煙者にとって、早期死亡および障害のリスクを減らすためには禁煙は最も効果的な方法である。禁煙介入は、薬剤を使用しないアドバイスやカウンセリングなどの心理社会的介入と、ニコチンパッチなどの薬剤を用いる薬理学的介入とに類別することができる。患者の行動を変える行動療法および薬理的治療は、禁煙を補完する方法であり、自立して長期の禁煙状態を維持する可能性を向上させると考えられている。肺癌診断後の禁煙は、死亡率または死亡数、術後合併症、二次原発肺癌の再発率および発症率の低下、ならびに治療の有効性の増大およびQOLと関連があるかもしれない。禁煙介入は癌患者の管理に重要な役割を果たす。現喫煙者および元喫煙者、とりわけ肺癌患者は禁煙を奨励される必要がある。しかしながら、肺癌患者に対するあらゆる種類の禁煙プログラムの効果は不明である。
レビューの論点
肺癌患者に対するあらゆる種類の禁煙プログラムの有効性を確認することを目指している。
主な結果
2015年6月22日までの医学データベースおよび2015年7月1日までの他のウェブサイトを検索した。組み入れのために、複数の治療法のうちの1つに参加者を無作為に割り付けるランダム化比較試験はなかった。データが入手可能な場合、組み入れのために3件の進行中の試験を確認した。今日現在、禁煙介入が肺癌患者にとって有効であるかどうか、そして1つの方法が他のあらゆる方法に比較してより有効であるかどうか結論づけることはできない。肺癌患者は禁煙を奨励され、禁煙に関した助けを提供される必要があるが、肺癌患者に対していかなる種類の禁煙介入も推奨することができない。高品質のランダム化比較試験がこの質問に答える必要がある。
エビデンスの質
コクランの選択基準に合致した試験は存在しなかったため、質の良いエビデンスは確認されなかった。
Authors' conclusions
Background
Description of the condition
Lung cancer is one of the most common causes of death from cancer worldwide. There were 43,463 new cases of lung cancer diagnosed in the UK in 2011 (Cancer Research UK), and 1.82 million people diagnosed all over the world in 2012, accounting for 13% of all cancers (GLOBOCAN 2012). The World Health Organization (WHO) divides lung cancer into non‐small‐cell lung carcinoma (NSCLC) and small‐cell lung carcinoma (SCLC). NSCLC accounts for more than 85% of all lung cancer cases and includes two major types: non‐squamous carcinoma (including adenocarcinoma, large‐cell carcinoma and other cell types) and squamous cell carcinoma (Brambilla 2001). Because obvious clinical manifestations, such as the haemoptysis, cough, dyspnoea, chest pain, hoarseness, weight loss and finger clubbing are unusual in the early stages of lung cancer, over three‐quarters of people are only diagnosed when the disease has progressed (National Cancer Institute). This difficulty in diagnosing lung cancer at an early stage results in a higher death rate compared to other types of cancers where most people can be diagnosed early and treated curatively (Ettinger 2013). The five‐year survival rate varies markedly depending on the stage at diagnosis, from 58% to 73% in stage I, from 36% to 46% in stage II, 24% in stage IIIA and from 9% to 13% in stage IIIB and Ⅳ (Goldstraw 2007).
Tobacco smoking, which contributes to approximately 85% to 90% of lung cancer cases, constitutes the most important risk factor for lung cancer (Ettinger 2012). Lung cancer risk increases with the number of packs of cigarettes smoked per day and with the number of years spent smoking (i.e. pack‐years of smoking history) (Ettinger 2013). Compared with lifetime non‐smokers, lifetime smokers have a mean 20‐fold increase in the risk of developing lung cancer (Alberg 2013). More than 90% of people with lung cancer report positive smoking histories, among whom up to 64% people have been smoking for one year before diagnosis and 40% are still active smokers at diagnosis (Eng 2014). Up to 50% of people who smoke and have lung cancer do not stop smoking after their diagnosis or frequently relapse after smoking cessation (Park 2012; Walker 2006).
Tobacco smoke is an aerosol containing more than 4000 substances, more than 50 of which are known to be carcinogenic, such as polonium 210, benzene, formaldehyde, lead and cadmium (Andreas 2007). Smoking induces and aggravates many health problems, including vascular diseases, respiratory illnesses and cancers (Rigotti 2012). There are, for understandable ethical reasons, no randomised controlled trials (RCTs) establishing the effectiveness of smoking cessation in people diagnosed with lung cancer. There are many studies, mostly retrospective, and thus based on incomplete reporting of smoking history (Land 2012), which suggest that quitting smoking after a diagnosis of lung cancer may be associated with significantly decreased mortality (Parsons 2010; Videtic 2003), postoperative complications, recurrence and incidence of second primary lung cancer (Andreas 2013; Cataldo 2010). In addition, there might be greater treatment effectiveness and quality of life (QoL) (Balduyck 2011; Baser 2006; Chen 2012; Garces 2004). Tobacco smoking cessation interventions play an important role in the management of people with cancer (Nayan 2013). Current and former smokers, especially people with lung cancer, should be encouraged to quit smoking (De Groot 2012; Hurt 2011). The guidelines of the European Society for Medical Oncology (ESMO) recommends that people with all stages of NSCLC should be offered smoking cessation interventions, as this leads to superior treatment outcomes (ESMO Guidelines Working Group 2014). The guidelines of the American College of Chest Physicians (ACCP) includes a similar recommendation (ACCP 2013).
Description of the intervention
Smoking cessation interventions can be divided into psychosocial interventions and pharmacological interventions. Psychosocial interventions refer to intervention strategies that are designed to increase tobacco abstinence rates through psychological or social support mechanisms. These interventions include treatment strategies such as counselling, self help materials and behavioural support from individuals or groups. Behavioural treatment includes any psychotherapeutic approach aimed at identifying and modifying smoking‐related behaviours (Tobacco Use and Dependence Guideline Panel 2008). Pharmacological therapies include all forms of nicotine replacement therapy (NRT), the nicotine receptor partial agonists (NRPAs) varenicline and cytisine, and the antidepressants, bupropion and nortriptyline. Counselling and pharmacotherapy may also be combined (Cahill 2012; Hughes 2014; Rigotti 2012; Vaidya 2014). NRT, bupropion and varenicline are commonly prescribed. NRTs are available as non‐prescription medications. They are authorised for use as first‐line smoking cessation treatment, mainly used in the USA and in the European Union, and are widely recommended in many national guidelines (Cahill 2013). NRT is available as skin patches that deliver nicotine slowly, chewing gum, nasal and oral sprays, inhalers, lozenges and sublingual tablets. All forms of NRT can help people who try to quit smoking and increase their chances of successfully stopping smoking with a rate of quitting of 50% to 70% (Stead 2012). Bupropion was developed as a non‐tricyclic antidepressant, and is sometimes the best choice for smokers who do not want to use a nicotine‐based treatment, or who have already failed to quit using NRT (Hughes 2014). It has been licensed as a prescription aid to smoking cessation in many countries.
How the intervention might work
Quitting smoking is the most effective way for smokers to reduce the risk of premature death and disability. Behavioural and pharmacological treatments are believed to have complementary modes of action, and to improve the chances of maintaining long‐term abstinence independently (Stead 2012). One systematic review and meta‐analysis on smoking cessation interventions concluded that integrated pharmacological and behavioural counselling approaches were more effective than pharmacological approaches alone (De Groot 2012). There is evidence that standard, print‐based self help, individually tailored self help materials, or more intensive advice or counselling increase the success of quit attempts (Hartmann‐Boyce 2014; Lancaster 2005). One systematic review found that advice, counselling, or both, given by nurses improved abstinence rates compared with no intervention (Rice 2013). Another systematic review showed that telephone counselling was more effective than providing self help materials or brief advice or pharmacotherapy alone (Stead 2013a). Advice from doctors to quit can increase the rate of quitting compared with no intervention (Stead 2013b). Group therapy doubles success compared with self help interventions and is more effective than other less‐intensive interventions (Stead 2005).
The aim of NRT is to temporarily replace much of the nicotine from cigarettes, reduce motivation to smoke, and reduce the physiological and psychomotor withdrawal symptoms often experienced during an attempt to stop smoking, and therefore increase the likelihood of remaining abstinent (Stead 2012). In addition, when nicotine is provided by NRT, the user avoids about 4000 other toxins that are inhaled in tobacco smoke (Coleman 2012). Bupropion, which has both dopaminergic and adrenergic actions, seems to block nicotine effects and withdrawal symptoms by inhibiting the nicotinic acetylcholinergic receptor (Hughes 2014). Varenicline is a selective NRPA that plays the same role nicotine plays in the brain. It can decrease nicotine craving and nicotine withdrawal symptoms (Cahill 2013). Varenicline is more effective than placebo, doubling the rate of smoking cessation (Cahill 2013), and more effective than bupropion (Hughes 2014). Anxiety can contribute to increase smoking, and may be a symptom of nicotine withdrawal (Hughes 2007; Morissette 2007). Therefore, anxiolytics may help in smoking cessation by abating a withdrawal symptom or by replacing the reinforcing effects of nicotine (Hughes 2000).
Why it is important to do this review
Although people with lung cancer may benefit from stopping smoking, it is not clear whether smoking cessation interventions are effective for people with lung cancer and whether one programme is more effective than any other. We systematically reviewed the literature and planned to summarise the effectiveness of tobacco cessation interventions for people with lung cancer, reporting smoking cessation rates.
Objectives
To determine the effectiveness of smoking cessation programmes for people with lung cancer.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include RCTs only in this review.
Types of participants
We included people diagnosed with lung cancer at any stage and who were smokers at the time of intervention.
Types of interventions
We planned to include any RCT of any psychosocial or pharmacological smoking cessation intervention or combinations of both, compared with no intervention, a different psychosocial or pharmacological (or both) intervention, or placebo for pharmacological interventions, in people with lung cancer.
We planned to investigate the following comparisons:
-
psychosocial intervention versus no intervention;
-
any psychosocial intervention versus another psychosocial intervention;
-
pharmacological interventions versus no intervention or placebo;
-
any pharmacological intervention versus another pharmacological intervention;
-
psychosocial plus pharmacological interventions versus no intervention;
-
any combination of psychosocial plus pharmacological interventions with another combination.
Types of outcome measures
Primary outcomes
-
Percentage of participants with continuous or prolonged abstinence over a period of six months or longer.
Secondary outcomes
-
Percentage of participants with point prevalence abstinence over a period of six months or longer.
-
Overall survival (OS).
-
Progression‐free survival (PFS).
-
Quality of life (QoL).
-
Any adverse events.
Search methods for identification of studies
Electronic searches
The search strategy was constructed in the following databases:
-
the Cochrane Central Register of Controlled Trials (CENTRAL) (to 1 July 2015) (Appendix 1);
-
MEDLINE (from January 1950 to 1 July 2015), accessed via PubMed (Appendix 2);
-
EMBASE (from January 1980 to 22 June 2015) (Appendix 3).
We applied no restriction on language of publication. We performed the search in collaboration with the Trials Search Co‐ordinator of the Cochrane Lung Cancer Group.
We searched MEDLINE and EMBASE using both controlled vocabulary (namely, MeSH in MEDLINE and EMTREE in EMBASE) and a wide range of free‐text terms. We performed the search on MEDLINE using the Cochrane Highly Sensitive Search Strategy, sensitivity‐maximising version (2008 version), as referenced in Chapter 6.4.11.1 and detailed in Box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Searching other resources
We sought to identify abstracts of the following conferences on lung cancer and smoking cessation:
-
lung cancer sections of the proceedings of the American Society of Clinical Oncology (ASCO) Annual Meeting, from 2013 onwards;
-
lung cancer sections of the proceedings of the ESMO Congress, from 2013 onwards;
-
lung cancer sections of the proceedings of the European Conference of Clinical Oncology (ECCO) Congress, 2013 and 2014;
-
the World Conference on Lung Cancer, 2013 and 2014;
-
the Society for Research on Nicotine and Tobacco Annual Meeting from 2013 (www.srnt.org/index.cfm).
We planned to include RCTs published only as abstracts and to contact study authors to request further information.
We checked reference lists of the included articles and any relevant reviews for any studies that the primary search of electronic resources did not identify.
We also searched:
-
studies submitted to the Food and Drug Administration and to the European Medicine Agency for drug registration (www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm; (www.ema.europa.eu/ema/);
-
the WHO International Clinical Trials Registry Platform (ICTRP) search portal (apps.who.int/trialsearch/; www.controlled‐trials.com), ClinicalTrials.gov (clinicaltrials.gov/), and the metaRegister of Controlled Trials (mRCT) (www.isrctn.com/page/mrctcontrolledtrials.com/mrct/).
Data collection and analysis
Selection of studies
Two review authors (XY and TY) independently checked the abstracts of retrieved studies for relevance, and acquired full trial reports of potential candidates for inclusion. The review authors resolved any disagreements by mutual consent, or by recourse to a third review author (YH). We classified studies for which full reports were obtained but which did not meet the inclusion criteria as excluded. We recorded the selection process in sufficient detail to complete a Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) (Liberati 2009) flow diagram and a 'Characteristics of excluded studies' table, giving reasons for the decisions to exclude.
Data extraction and management
Two review authors (XY and TY) planned to extract study data independently into a data extraction form and to compare their findings. A third review author (YH) would have resolved any disagreement by discussion. Two review authors (XY and TY) planned to double‐enter the data into Review Manager 5 (RevMan 2014). We planned to record the following information in a 'Characteristics of included studies' table:
-
methods: study design, study name (if applicable), study recruitment period, country, number of study centres, study recruitment procedures;
-
participants: number (intervention/control), definition of smoker used, specific demographic characteristics (e.g. age, gender, ethnicity, socio‐economic status), cigarettes smoked per day, mean score on Fagerström Test for Nicotine Dependence (FTND), lung cancer stage, histology, any other relevant inclusion and exclusion criteria;
-
intervention and control description: description of intervention(s) (treatment, dosage, regimen, behavioural support);
-
outcomes: primary and secondary outcomes, including reported time points, definitions of abstinence, biochemical validation of abstinence;
-
sources of funding and potential conflicts of interest of trial authors.
Assessment of risk of bias in included studies
Two review authors (XY and TY) planned to undertake assessment of the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We planned to resolve any disagreements by discussion or by involving a third review author (YH).
We planned to rate each trial as being at high, low or unclear risk of bias for sequence generation, allocation concealment, blinding of participants and personnel, incomplete outcome data, selective outcome reporting and other potential sources of bias. This would have provided a direct quote or details from the study report, together with a justification for our judgement in the 'Risk of bias' table. We planned to address the domains of sequence generation, allocation concealment and selective outcome reporting in the tool by a single entry for each study. For blinding and incomplete outcome data, we intended to use two or more entries separately for different outcomes (or for the same outcome at different time points). We planned to note in the 'Risk of bias' table the proportion of participants for whom the outcome was imputed, and whether there was either high or differential loss to follow‐up between the groups. We planned to assess 'other sources of bias' after single entry for studies as a whole. Where information on risk of bias related to unpublished data or correspondence with a trialist, we planned to note this in the table. We planned to summarise the risk of bias judgements across different studies for each of the domains listed, and planned to display the summary results in a 'Risk of bias' figure ('traffic lights' or bar chart, depending on the number of included studies) (Higgins 2011).
Measures of treatment effect
We planned to present the treatment effect of dichotomous outcomes (such as continuous or prolonged abstinence and point prevalence) as risk ratio (RR) and 95% confidence interval (CI). We planned to present the results for time‐to‐event outcomes (such as PFS and OS) as hazard ratio (HR) and 95% CI. For continuous data outcomes (such as QoL), we planned to present data as standardised mean difference (SMD) or mean difference (MD).
Unit of analysis issues
We planned to extract data on smoking outcomes from RCTs. In the case of cluster‐randomised controlled trials, we would have attempted to extract, where available, a direct estimate of the required effect from an analysis that properly accounts for the cluster design. When such data would have been unavailable, we intended to perform an approximately correct analysis if the required information could be extracted (Higgins 2011).
In the case of trials with multiple intervention groups, we intended to combine all relevant experimental intervention groups of the study into a single group, and to combine all relevant control intervention groups into a single control group.
Dealing with missing data
We intended to attempt to contact investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data when possible (e.g. when a study was identified as abstract only).
Furthermore, regarding smoking cessation, we planned to consider participants with missing outcome data as smokers.
Assessment of heterogeneity
We planned to evaluate levels of clinical heterogeneity (study characteristics, methods, outcomes) between included studies, to decide whether or not it would have been appropriate to pool study data.
We intended to use the I2 statistic to assess the statistical heterogeneity among studies considering an I2 value greater than 50% as substantial heterogeneity (Higgins 2011). In the case of heterogeneity among studies, we intended to explore clinical heterogeneity and methodological heterogeneity as potential causes, and it would have been noted and discussed. We also planned to explore heterogeneity in the subgroup analysis.
Assessment of reporting biases
We intended to address any suspected selective reporting of outcomes, as assessed by either of the review authors, by contacting the study authors for more information about the reported and unreported outcomes. When this would have not been not possible, and the missing data would have been thought to introduce serious bias, we would have explored the impact of including such studies in the overall assessment of results using a sensitivity analysis.
We planned to create and examine funnel plots if we included more than 10 trials in the review, to explore possible publication biases.
Data synthesis
We planned to use Review Manager 5 to summarise the data of interest and to produce forest plot graphics, using a random‐effects model (RevMan 2014). If we had judged data aggregation unfeasible, we planned to discuss and present the results in the form of tables or graphics.
We intended to present three 'Summary of findings' tables, one for each major comparison (psychosocial versus no intervention, pharmacological versus no intervention or placebo, psychosocial plus pharmacological versus no intervention) according to the Cochrane Handbook of Systematic Reviews of Intervention (Higgins 2011). We planned to include data regarding the following outcomes: percentage of participants with prolonged or continuous (or both) abstinence, percentage of participants with point prevalence abstinence, PFS, OS, QoL and adverse events.
We planned to use the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it was related to the studies that contribute data to the meta‐analyses for the pre‐specified outcomes. We planned to use methods and recommendations as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and as used in GRADEpro software. We planned to justify all decisions to downgrade or upgrade the quality of studies by using footnotes and making comments to aid the reader's understanding of the review when necessary. We planned to provide a narrative summary of the included studies and, where appropriate, would have pooled these data in meta‐analyses. For dichotomous data, we planned to use a random‐effect Mantel‐Haenszel model to estimate the pooled RR with 95% CI. For continuous data, we planned to use a random‐effect inverse variance model to estimate the pooled SMD or MD with 95% CI. For time‐to‐event data, we planned to use a random‐effect inverse variance model to estimate the pooled HR with 95% CI.
Subgroup analysis and investigation of heterogeneity
Whenever possible, we planned to perform the following subgroup analyses:
-
cancer stage at diagnosis;
-
curative versus non‐curative treatment;
-
demographic characteristics: age, gender, ethnicity, socio‐economic status.
Sensitivity analysis
We planned to perform sensitivity analysis using smoking cessation outcomes, by limiting analysis only to trials with biochemical validation of smoking status.
Results
Description of studies
See Characteristics of excluded studies table.
Results of the search
Searches returned 1052 references. We retrieved 984 references after duplicates were removed. We discarded 971 clearly irrelevant records at first‐level screening by reviewing titles and abstracts. After second‐level screening we were left with 13 articles for further assessment (Bastian 2011; Bastian 2013; Browning 2000; Clavero 2014; Schnoll 2003; Schnoll 2004a; Schnoll 2004b; Wewers 1997; NCT00032084; NCT01192256; NCT01434342; NCT01457469; NCT02048917). Following assessment of the full text of these studies, we excluded 10 articles (Bastian 2011; Bastian 2013; Browning 2000; Clavero 2014; Schnoll 2003; Schnoll 2004a; Schnoll 2004b; Wewers 1997; NCT00032084; NCT01457469). We identified three ongoing study (NCT01192256; NCT01434342; NCT02048917) (see Characteristics of ongoing studies table). No studies met our inclusion criteria. The study selection flow chart is shown in Figure 1.
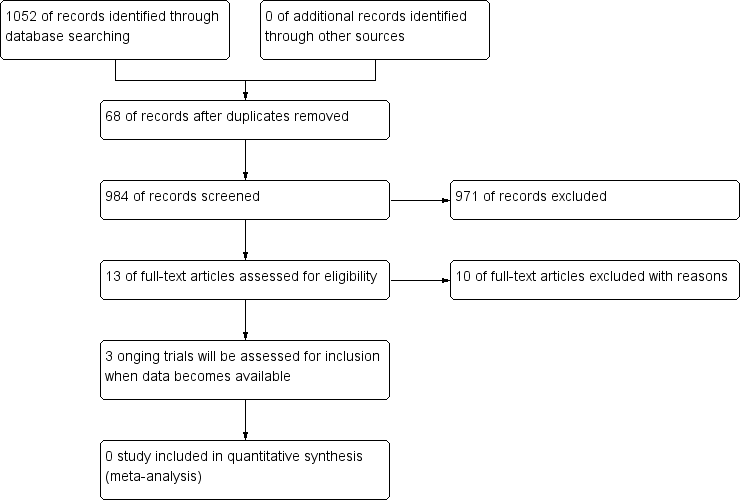
Study flow diagram.
Included studies
We included no studies in this review.
Excluded studies
We excluded 10 studies from the review for the following reasons (Bastian 2011; Bastian 2013; Browning 2000; Clavero 2014; Schnoll 2003; Schnoll 2004a; Schnoll 2004b; Wewers 1997; NCT00032084; NCT01457469) (see Characteristics of excluded studies table):
-
in Bastian 2011 and Bastian 2013, we were unable to identify people with lung cancer;
-
in Schnoll 2003, Schnoll 2004a, and Schnoll 2004b, we were unable to distinguish people with lung cancer from other participants;
-
Clavero 2014 and Wewers 1997 had no control group, and were not randomised;
-
Browning 2000 was not an RCT;
-
in NCT01457469, it reports that it was "randomized" but it was also described as "Phase 1" (which is not an RCT). There was no clear description of the experimental intervention or the comparator on the website. Thus, it probably is not an RCT;
-
NCT00032084 was completed in 2004, but no results were available.
Risk of bias in included studies
The searches retrieved no trials relevant to this review and thus we conducted no assessment of methodological quality.
Effects of interventions
We included no studies in this review, so the effects could not be evaluated.
Discussion
Although people with lung cancer may benefit from stopping smoking, we found no data from adequately powered RCTs to determine whether smoking cessation interventions are effective for people with lung cancer and whether one intervention is more effective than any other.
Summary of main results
No studies met the inclusion criteria for this review. Three ongoing RCTs included people with lung cancer participating in a smoking cessation programme (NCT01192256; NCT01434342; NCT02048917). These RCTs will be eligible for inclusion in future updates of this review.
Overall completeness and applicability of evidence
We found no high‐quality RCTs fulfilling the study eligibility criteria.
Quality of the evidence
We found no RCTs for analysis.
Potential biases in the review process
The Trials Search Co‐ordinator of the Cochrane Lung Cancer Group designed our search strategy. A strength of this systemic review is the comprehensive literature search, including several databases and clinical trials registers. We applied no language restrictions in our search strategy.
Agreements and disagreements with other studies or reviews
Our results identified no studies for analysis. Neither did we identify any high‐quality observational studies that addressed this issue.
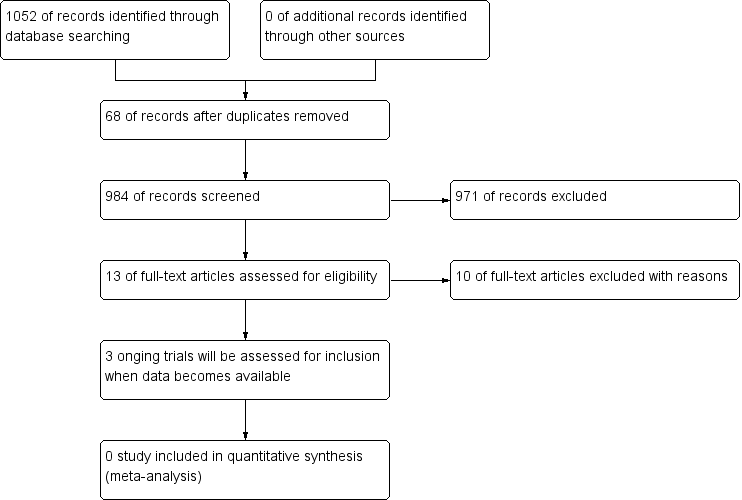
Study flow diagram.

