Momento adecuado para la inhalación de solución salina hipertónica en la fibrosis quística
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised, cross‐over trial with concealed allocation, intention‐to‐treat analysis and blinded assessors; investigating the impact of timing of hypertonic saline inhalation in relation to airway clearance techniques. The inhalation blocks were for one day. The three treatments of the allocated timing regimen were performed on each of the three trial days, with no washout day. | |
| Participants | 50 adults (mean age 31 years, SD 10, range 18 ‐ 64 years) with a confirmed diagnosis of cystic fibrosis who were clinically stable with an FEV₁ within 10% of the best recorded value in the last 6 months. This trial excluded people who were hypertonic saline naïve or previously intolerant, a lung transplant recipient, colonised with Burkholderia cepacia complex, not clinically stable, haemoptysis greater than 60 mL within the last month, thrombocytopenia or pregnancy. | |
| Interventions | 4 mL of 6% hypertonic saline was nebulised via an LC Star nebuliser 3 times per day with the allocated timing regimen for that day. Hypertonic saline was nebulised immediately before or after airway clearance or during (with blocks of inhalation and pauses for airway clearance). The airway clearance technique was optimised for each participant on recruitment to the trial and was standardised for all 3 trial days. | |
| Outcomes | The primary outcome was the change in FEV₁ and FVC (in litres and percentage of the predicted value) recorded prior to and two hours following the middle treatment session of each trial day. Symptom scores at the end of each intervention arm were also recorded: perceived effectiveness, tolerability and satisfaction rated on a 100 mm visual analogue scale. Adverse events and adherence were also recorded. | |
| Notes | PEDro Score: 8/10 [Eligibility criteria: yes; random allocation: yes; concealed allocation: yes; baseline comparability: yes; blind participants: no; blind therapists: no; blind assessors: yes; adequate follow up: yes; intention‐to‐treat analysis: yes; Between‐group comparisons: yes; point estimates and variability: yes. Note: eligibility criteria item does not contribute to total score]. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random allocation list. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes. |
| Incomplete outcome data (attrition bias) | Low risk | Stated "no withdrawals" and intention‐to‐treat analysis used. |
| Selective reporting (reporting bias) | Low risk | Consistent with the prospectively registered trial protocol. |
| Other bias | Low risk | |
| Blinding of participants and personnel (performance bias) | High risk | Participants were unblinded to the timing regimen. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were blinded. |
| Methods | Randomised, cross‐over trial with concealed allocation, intention to treat analysis and blinded assessors; investigating the impact of timing of hypertonic saline inhalation in relation to airway clearance techniques. The inhalation blocks were for one day. The two treatment days that contributed data to this review involved single treatment of 30 minutes with differing timing of hypertonic saline in relation to physiotherapy airway clearance techniques (i.e. hypertonic saline before or during autogenic drainage). The other day was solely autogenic drainage and did not include hypertonic saline inhalation, so it is not discussed further in this review. | |
| Participants | 13 hospitalised participants who were over 14 years (mean age 27 years, range 18 ‐ 37 years). All were productive daily and performed autogenic drainage for their airway clearance. The lung function of participants was not stated but some were noted to be on oxygen therapy. One participant withdrew and outcome data was not included in analysis. | |
| Interventions | 4 mL of 6% hypertonic saline before or during 30 minutes of autogenic drainage. The type of nebuliser and use of co‐interventions were not reported. | |
| Outcomes | Outcomes included change in dyspnoea scores at the conclusion of each intervention arm, wet weight of sputum in grams produced during the treatment session, and adverse events and adherence. | |
| Notes | PEDro Score: 8/10 [Eligibility criteria: yes; random allocation: yes; concealed allocation: yes; baseline comparability: yes; blind participants: no; blind therapists: no; blind assessors: yes; adequate follow up: yes; intention‐to‐treat analysis: yes; between‐group comparisons: yes; point estimates and variability: yes. Note: eligibility criteria item does not contribute to total score]. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocations were drawn from a box after the box had been shaken. |
| Allocation concealment (selection bias) | Low risk | Allocations were sealed in opaque envelopes. |
| Incomplete outcome data (attrition bias) | Low risk | One participant was lost to follow up but intention to treat analysis was conducted. |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol. No protocol available from the author. |
| Other bias | Low risk | |
| Blinding of participants and personnel (performance bias) | High risk | Participants were unblinded to the timing regimen. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were blinded. |
FEV₁: forced expiratory volume at one second
FVC: forced vital capacity
SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Did not involve administration of hypertonic saline. | |
| Did not involve administration of hypertonic saline. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. | |
| Intervention not related to the timing of hypertonic saline inhalation. |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised cross‐over trial. The physiotherapist collecting the outcome measures was blinded. |
| Participants | 14 adults with CF recruited, 13 completed the trial (mean (SD) age 33 years (12), FEV₁% predicted 51 (22), LCI (no. turnovers) 14 (4)). |
| Interventions | ACTs after HTS inhalation or ACTs during HTS inhalation on alternate days. Between days 10 – 14 of intravenous antibiotic course during a pulmonary exacerbation. ACT treatment consisted of 10 cycles of active cycle of breathing technique using an Acapella®. |
| Outcomes | Participants completed a multiple breath washout (MBW) test to obtain LCI and spirometry (FEV₁) at baseline and 90 min post treatment. Sputum collection during 90 min, ease of clearance and satisfaction with treatment was also recorded. Wilcoxon test was used and P < 0.05 was considered significant. |
| Notes | Abstract only. |
ACTs: airways clearance techniques
CF: cystic fibrosis
FEV₁: forced expiratory volume in one second
HTS: hypertonic saline
LCI: lung clearance index
SD: standard deviation
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 FEV1 (L) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 1 FEV1 (L). | ||||
| 2 FEV1 (% pred) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 2 FEV1 (% pred). | ||||
| 3 FVC (L) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 3 FVC (L). | ||||
| 4 FVC (% pred) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 4 FVC (% pred). | ||||
| 5 Perceived efficacy (mm) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 1.5  Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 5 Perceived efficacy (mm). | ||||
| 6 Tolerability (mm) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 1.6  Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 6 Tolerability (mm). | ||||
| 7 Satisfaction (mm) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 1.7  Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 7 Satisfaction (mm). | ||||
| 8 Dyspnoea Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 1.8  Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 8 Dyspnoea. | ||||
| 9 Sputum wet weight Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 1.9  Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 9 Sputum wet weight. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 FEV1 (L) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 Inhalation before versus after airway clearance techniques, Outcome 1 FEV1 (L). | ||||
| 2 FEV1 (% pred) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Inhalation before versus after airway clearance techniques, Outcome 2 FEV1 (% pred). | ||||
| 3 FVC (L) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 Inhalation before versus after airway clearance techniques, Outcome 3 FVC (L). | ||||
| 4 FVC (% pred) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 2.4  Comparison 2 Inhalation before versus after airway clearance techniques, Outcome 4 FVC (% pred). | ||||
| 5 Perceived efficacy (mm) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 2.5  Comparison 2 Inhalation before versus after airway clearance techniques, Outcome 5 Perceived efficacy (mm). | ||||
| 6 Tolerability (mm) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 2.6  Comparison 2 Inhalation before versus after airway clearance techniques, Outcome 6 Tolerability (mm). | ||||
| 7 Satisfaction (mm) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 2.7  Comparison 2 Inhalation before versus after airway clearance techniques, Outcome 7 Satisfaction (mm). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 FEV1 (L) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 3.1  Comparison 3 Inhalation during versus after airway clearance techniques, Outcome 1 FEV1 (L). | ||||
| 2 FEV1 (% pred) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 3.2 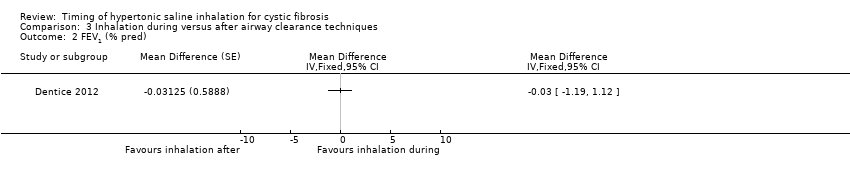 Comparison 3 Inhalation during versus after airway clearance techniques, Outcome 2 FEV1 (% pred). | ||||
| 3 FVC (L) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 3.3  Comparison 3 Inhalation during versus after airway clearance techniques, Outcome 3 FVC (L). | ||||
| 4 FVC (% pred) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 3.4  Comparison 3 Inhalation during versus after airway clearance techniques, Outcome 4 FVC (% pred). | ||||
| 5 Perceived efficacy (mm) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 3.5 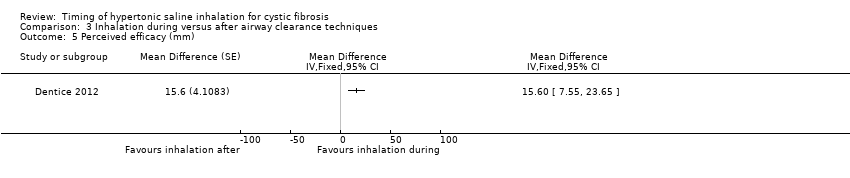 Comparison 3 Inhalation during versus after airway clearance techniques, Outcome 5 Perceived efficacy (mm). | ||||
| 6 Tolerability (mm) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 3.6  Comparison 3 Inhalation during versus after airway clearance techniques, Outcome 6 Tolerability (mm). | ||||
| 7 Satisfaction (mm) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 3.7  Comparison 3 Inhalation during versus after airway clearance techniques, Outcome 7 Satisfaction (mm). | ||||

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
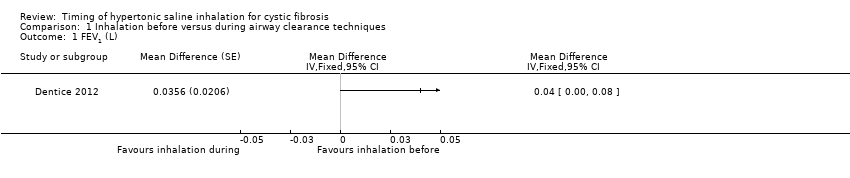
Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 1 FEV1 (L).

Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 2 FEV1 (% pred).

Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 3 FVC (L).

Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 4 FVC (% pred).

Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 5 Perceived efficacy (mm).

Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 6 Tolerability (mm).

Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 7 Satisfaction (mm).
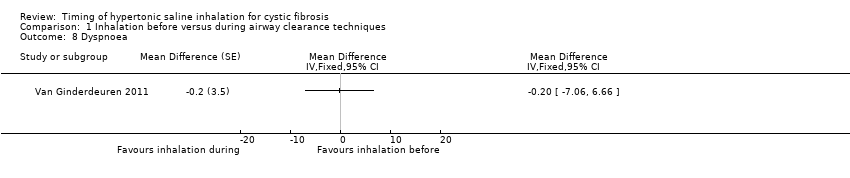
Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 8 Dyspnoea.

Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 9 Sputum wet weight.

Comparison 2 Inhalation before versus after airway clearance techniques, Outcome 1 FEV1 (L).

Comparison 2 Inhalation before versus after airway clearance techniques, Outcome 2 FEV1 (% pred).

Comparison 2 Inhalation before versus after airway clearance techniques, Outcome 3 FVC (L).

Comparison 2 Inhalation before versus after airway clearance techniques, Outcome 4 FVC (% pred).

Comparison 2 Inhalation before versus after airway clearance techniques, Outcome 5 Perceived efficacy (mm).
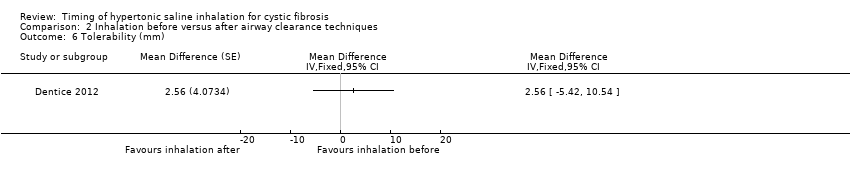
Comparison 2 Inhalation before versus after airway clearance techniques, Outcome 6 Tolerability (mm).

Comparison 2 Inhalation before versus after airway clearance techniques, Outcome 7 Satisfaction (mm).

Comparison 3 Inhalation during versus after airway clearance techniques, Outcome 1 FEV1 (L).

Comparison 3 Inhalation during versus after airway clearance techniques, Outcome 2 FEV1 (% pred).
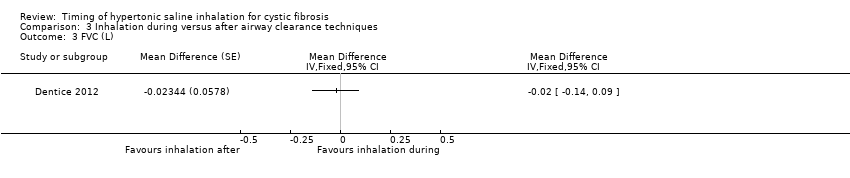
Comparison 3 Inhalation during versus after airway clearance techniques, Outcome 3 FVC (L).

Comparison 3 Inhalation during versus after airway clearance techniques, Outcome 4 FVC (% pred).
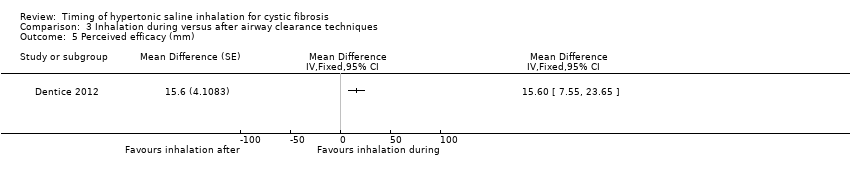
Comparison 3 Inhalation during versus after airway clearance techniques, Outcome 5 Perceived efficacy (mm).

Comparison 3 Inhalation during versus after airway clearance techniques, Outcome 6 Tolerability (mm).

Comparison 3 Inhalation during versus after airway clearance techniques, Outcome 7 Satisfaction (mm).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 FEV1 (L) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 2 FEV1 (% pred) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 3 FVC (L) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 4 FVC (% pred) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 5 Perceived efficacy (mm) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 6 Tolerability (mm) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 7 Satisfaction (mm) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 8 Dyspnoea Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 9 Sputum wet weight Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 FEV1 (L) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 2 FEV1 (% pred) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 3 FVC (L) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 4 FVC (% pred) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 5 Perceived efficacy (mm) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 6 Tolerability (mm) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 7 Satisfaction (mm) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 FEV1 (L) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 2 FEV1 (% pred) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 3 FVC (L) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 4 FVC (% pred) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 5 Perceived efficacy (mm) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 6 Tolerability (mm) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 7 Satisfaction (mm) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |

