Probióticos para el tratamiento del eccema
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias de los estudios en curso
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Three month parallel group randomised controlled trial. | |
| Participants | Fifty infants under five months age with mild/moderate eczema diagnosed using Hanifin and Rajka criteria, and a clinical history suggestive of cow's milk allergy. All participants were exclusively formula fed, and received an extensively hydrolysed formula for three to five weeks prior to receiving the study intervention. Infants receiving antihistamines, oral corticosteroids or any probiotic/antibiotic/antimycotic in the preceding four weeks were excluded, as were those with a congenital gastrointestinal malformation. Setting primary care in the Netherlands. One participant lost to follow‐up. | |
| Interventions | Extensively hydrolysed whey‐based formula given alone, with Lactobacillus rhamnosus at 5x109 cfu/100mls or with Lactobacillus GG at 5x109 cfu/100mls. The study formula was offered at all feeds during the intervention period. | |
| Outcomes | SCORAD assessed at baseline, one, two and three months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Eight week parallel group randomised controlled trial. | |
| Participants | Fifty‐three children aged 1 to 55 months with eczema diagnosed using Hanifin and Rajka criteria. Setting German Dermatology Centre. Six participants lost to follow‐up. | |
| Interventions | Lactobacillus GG at 1010 cfu/day as a twice daily dose, or microcrystalline cellulose placebo. Interventions given as capsules, which were mixed with milk if bottle fed, or mixed with water if not bottle fed. | |
| Outcomes | 1. Parent global assessment of disease severity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Twelve week parallel group randomised controlled trial. The last observation carried forward approach was used for missing continuous data. | |
| Participants | 106 children aged 3 to12 months with mild/moderate eczema and SCORAD 15 to 40, not receiving antiinflammatory treatment. Four participants excluded from analysis after randomisation due to protocol breaches. | |
| Interventions | Lactobacillus GG at 1010 cfu/day as a twice daily dose, or placebo. | |
| Outcomes | 1. SCORAD | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Parallel group three arm randomised controlled trial. Duration of treatment unclear. | |
| Participants | Twenty‐seven infants ‐ ages not stated ‐ with eczema diagnosed using the Hanifin and Rajka criteria. All infants were exclusively breast fed, and were tolerant of the study formula without added probiotic. Setting paediatric service in Finland. Unclear how many participants lost to follow‐up. | |
| Interventions | Extensively hydrolysed whey‐dominant cow's milk formula with no probiotic added, with Lactobacillus GG added at 3x108 cfu/g or with Bifidobacterium lactis Bb‐12 added at 1x109 cfu/g. | |
| Outcomes | SCORAD ‐ interval of assessment unclear. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Parallel group randomised controlled trial. Intended duration of treatment not clear. | |
| Participants | Twenty‐seven infants (mean age 5.5 months) with eczema and suspected cow's milk allergy. Method for diagnosing eczema not described. Setting Hospital Paediatric department in Finland. Unclear how many participants lost to follow‐up. | |
| Interventions | Lactobacillus GG at 3x1010 cfu/kg/day, mixed with extensively hydrolysed whey formula, or the same formula without probiotic. A third treatment arm (excluded from this review) used heat‐inactivated LGG at 3x1010 cfu/kg/day, mixed with extensively hydrolysed whey formula. | |
| Outcomes | SCORAD | |
| Notes | Study terminated early due to adverse effects in a third treatment arm. The third treatment arm was not included in this systematic review since it involved the use of killed bacteria. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | One month parallel group randomised controlled trial. | |
| Participants | Thirty‐one children aged 2 to 16 months with eczema diagnosed using the Hanifin and Rajka criteria, and a history suggestive of cow's milk allergy. Children currently receiving systemic corticosteroid treatment were excluded. Setting of a paediatric clinic in Finland. Unclear how many participants lost to follow‐up. | |
| Interventions | Cow's milk elimination diet, topical eczema treatment and extensively hydrolysed cow's milk formula with or without addition of probiotic Lactobacillus GG. Probiotic given at 5x108 cfu/g formula. | |
| Outcomes | SCORAD assessed at one and two months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Three month parallel group randomised controlled trial. | |
| Participants | Forty‐eight children aged 2 to 12 years with moderate/severe eczema diagnosed by UK Working Party Criteria and total SCORAD over 14. Exclusion criteria were current flare of eczema, exposure to systemic corticosteroids or immunosuppressants in the previous three months or other known immune deficiency. Setting hospital dermatology clinic in France. Nine participants lost to follow‐up. | |
| Interventions | Skim milk powder, potato starch and lactose containing prebiotic, with or without Lactobacillus rhamnosus Lcr35 at 3.6x109 cfu/day given as a three times daily dose mixed with cold water or other liquid. | |
| Outcomes | 1. Parent or participant global assessment of eczema severity | |
| Notes | Three episodes of mild abdominal pain reported ‐ Two in probiotic group, one in placebo (prebiotic alone) group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Six week randomised controlled cross‐over trial. | |
| Participants | Fifty‐eight children aged 1 to 13 years with eczema diagnosed using the UK Working Party Criteria. Children who had received systemic corticosteroids at any time were excluded. Setting: hospital paediatric and dermatology departments in Denmark. 15 participants lost to follow‐up. | |
| Interventions | Skimmed milk powder with dextrose anhydrate 2 g/day or a mix of Lactobacillus rhamnosus 19070‐2 and Lactobacillus reuteri DSM12246 at 2x1010 cfu/day of each strain. Both placebo and probiotic preparations administered twice daily with 2.5 to 5ml water. | |
| Outcomes | 1. Global self assessment by participant or parent | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Twelve week parallel group randomised controlled trial. | |
| Participants | Sixty children aged 1to 10 years with eczema diagnosed by UK Working Party criteria, SCORAD of at least ten at recruitment and a positive skin prick or RAST test to at least one common evironmental or food allergen. Exclusion criteria were oral corticosteroid, immunosuppressant or antibiotic in the previous month, previous immune deficiency or malignancy and greater than ten point improvement in SCORAD during two weeks prior to commencing study treatment. Setting hospital clinic in New Zealand. One participant lost to follow‐up. | |
| Interventions | Microcrystalline cellulose placebo or Lactobacillus rhamnosus and Bifidobacterium lactis given together once daily at a combined total dose of 2x1010 cfu/day. Treatment capsules administered as either a powder mixed with food or drink, or taken in capsule form. | |
| Outcomes | SCORAD assessed at two weeks before treatment, on commencing treatment, then 2, 12 and 16 weeks later. | |
| Notes | One participant noted to be taking other non‐investigational probiotic. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Three month parallel group randomised controlled trial. | |
| Participants | Seventeen children aged 3 to 18 months with eczema diagnosed by Hanifin and Rajka criteria, and cows milk hypersensitivity diagnosed by suggestive history plus evidence of cow milk specific IgE. All participants had reduced levels of Bifidobacteria in their faeces (under 30% of total bacteria) and were receiving extensively hydrolysed cow's milk formula for at least two weeks prior to randomisation. Setting unclear. Unclear how many participants lost to follow‐up. | |
| Interventions | Raffinose prebiotic containing extensively hydrolysed cow's milk formula with or without Bifidobacterium breve M‐16V at 5‐15x109cfu/day. | |
| Outcomes | Investigator‐rated eczema scoring system | |
| Notes | No numerical outcome data available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Four week parallel group randomised controlled trial. | |
| Participants | 252 infants aged under 12 months with a clinical diagnosis of eczema and a clinical history suggestive of cow's milk allergy. Infants who had received a probiotic preparation for over a week in the preceding six weeks were excluded. Participants were selected from primary care referrals to a hospital clinic in Finland. Twenty‐two participants lost to follow‐up. | |
| Interventions | Cow's milk elimination diet, extensively hydrolysed formula and capsules of either microcrystalline cellulose placebo, Lactobacillus GG (1010 cfu/day) or probiotic mix (Lactobacillus GG 1010 cfu/day, Bifidobacterium breve Bbi 99 at 4x108 cfu/day, Lactobacillus rhamnosus LC705 at 1010 cfu/day and Propionibacterium JS 4x109 cfu/day). Capsules were mixed with food twice daily. | |
| Outcomes | SCORAD assessed at the end of treatment and four weeks later. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Eight week parallel group block randomised controlled trial. | |
| Participants | Fifty‐six children aged 6 to 18 months with moderate/severe eczema diagnosed by Hanifin and Rajka criteria and modified SCORAD score of at least 25 at enrolment. Those previously exposed to probiotics, currently receiving antibiotics or with other major medical problems were excluded. Community and hospital outpatient clinic setting in Australia. Three participants lost to follow‐up. | |
| Interventions | Lactobacillus fermentum VR1‐003PCC 2x109cfu/day as a sachet reconstituted by parents with 5 to 10 ml water twice daily, or maltodextrin placebo. | |
| Outcomes | 1. Global self assessment by parent | |
| Notes | One probiotic treated participant withdrew due to gastrointestinal illness (vomiting). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
SCORAD ‐ Scoring Atopic Dermatitis
UK ‐ United Kingdom
cfu ‐ Colony Forming Units
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Participants didn't have eczema. | |
| Intervention was not a probiotic. | |
| Participants didn't have eczema. | |
| Study did not report changes in eczema symptoms or severity | |
| Intervention was not a probiotic | |
| Study did not report changes in eczema symptoms or severity | |
| Study did not report changes in eczema symptoms or severity | |
| Study did not report changes in eczema symptoms or severity | |
| Study did not report changes in eczema symptoms or severity | |
| Study did not report changes in eczema symptoms or severity | |
| Study did not report changes in eczema symptoms or severity |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Cow's milk allergy mediated by elimination and probiotics |
| Methods | |
| Participants | 118 infants under 6 months with proven cow's milk allergy |
| Interventions | Casein hydrolysate formula with or without probiotics |
| Outcomes | |
| Starting date | |
| Contact information | Dr. Jeroen Hol, [email protected], Tel +31 104636946 |
| Notes | ISRCTN 04799749; Trial due to complete 2008 |
| Trial name or title | Effects of synbiotics in infants with atopic dermatitis |
| Methods | Multicenter randomised double blind placebo controlled parallel group intervention study |
| Participants | Infants aged 0 to7 months with eczema |
| Interventions | Infant formula with or without synbiotics |
| Outcomes | SCORAD change after 12 weeks treatment |
| Starting date | 1st September 2005 |
| Contact information | Dr DAM Goossens, Numico Research B.V., PO Box 7005, 6700 CA, Alkmaar, Netherlands |
| Notes | ISRCTN 69085979 |
| Trial name or title | The effects of probiotics in atopic dermatitis |
| Methods | Randomised double blind placebo controlled parallel group intervention study |
| Participants | Children aged six months to three years with moderate to severe eczema |
| Interventions | An oral probiotic |
| Outcomes | SCORAD one month after commencing treatment with probiotic or placebo |
| Starting date | July 2007 |
| Contact information | Michael H Land, UCLA Medical Center, Los Angeles, California, 90095 |
| Notes |
| Trial name or title | Probiotics in Atopic Dermatitis in Infancy |
| Methods | Randomised double blind placebo controlled parallel group intervention study |
| Participants | Infants aged three to six months with eczema |
| Interventions | Lactobacillus paracasei and Bifidobacterium lactis |
| Outcomes | SCORAD at the end of treatment |
| Starting date | 1st March 2002 |
| Contact information | Dr. Clare Murray, North West Lung Research Centre, Wythenshawe Hospital, Southmoor Road, Manchester M23 9LT [email protected] |
| Notes | ISRCTN 41490500 Trial completed |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participant or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment Show forest plot | 5 | Mean difference (Random, 95% CI) | ‐0.90 [‐2.84, 1.04] | |
| Analysis 1.1  Comparison 1 Probiotic vs Placebo, Outcome 1 Participant or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment. | ||||
| 1.1 Parallel group trials | 4 | Mean difference (Random, 95% CI) | ‐0.68 [‐3.07, 1.71] | |
| 1.2 Cross‐over trials | 1 | Mean difference (Random, 95% CI) | ‐1.80 [‐3.59, ‐0.01] | |
| 2 Participant or parent‐rated global change in eczema symptoms during treatment Show forest plot | 3 | Odds ratio (Random, 95% CI) | 0.40 [0.14, 1.15] | |
| Analysis 1.2  Comparison 1 Probiotic vs Placebo, Outcome 2 Participant or parent‐rated global change in eczema symptoms during treatment. | ||||
| 2.1 Parallel group trials | 2 | Odds ratio (Random, 95% CI) | 0.70 [0.27, 1.77] | |
| 2.2 Cross‐over trials | 1 | Odds ratio (Random, 95% CI) | 0.18 [0.05, 0.60] | |
| 3 Parent or participant‐rated eczema severity (SCORAD part C) (Long term) Show forest plot | 2 | 102 | Mean Difference (IV, Random, 95% CI) | ‐2.27 [‐3.97, ‐0.58] |
| Analysis 1.3  Comparison 1 Probiotic vs Placebo, Outcome 3 Parent or participant‐rated eczema severity (SCORAD part C) (Long term). | ||||
| 4 Global eczema severity score (Total SCORAD) (Short term) Show forest plot | 7 | Mean difference (Random, 95% CI) | ‐2.46 [‐7.45, 2.53] | |
| Analysis 1.4  Comparison 1 Probiotic vs Placebo, Outcome 4 Global eczema severity score (Total SCORAD) (Short term). | ||||
| 4.1 Parallel group studies | 6 | Mean difference (Random, 95% CI) | ‐1.68 [‐7.15, 3.80] | |
| 4.2 Cross‐over studies | 1 | Mean difference (Random, 95% CI) | ‐6.27 [‐11.21, ‐1.32] | |
| 5 Global eczema severity score (Total SCORAD) ‐ Sensitivity analysis ‐ Change score Show forest plot | 5 | Mean difference (Random, 95% CI) | ‐2.47 [‐4.72, ‐0.21] | |
| Analysis 1.5  Comparison 1 Probiotic vs Placebo, Outcome 5 Global eczema severity score (Total SCORAD) ‐ Sensitivity analysis ‐ Change score. | ||||
| 5.1 Parallel group trial | 4 | Mean difference (Random, 95% CI) | ‐2.15 [‐4.64, 0.34] | |
| 5.2 Cross‐over trial | 1 | Mean difference (Random, 95% CI) | ‐3.93 [‐9.25, 1.40] | |
| 6 Investigator‐rated eczema severity (SCORAD parts A/B) (Long term) Show forest plot | 2 | 102 | Mean Difference (IV, Fixed, 95% CI) | ‐8.11 [‐13.14, ‐3.09] |
| Analysis 1.6  Comparison 1 Probiotic vs Placebo, Outcome 6 Investigator‐rated eczema severity (SCORAD parts A/B) (Long term). | ||||
| 7 Participant/parent‐rated symptoms of eczema (SCORAD part C)(Short term)‐Stratified by Age group Show forest plot | 5 | Mean difference (Random, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Probiotic vs Placebo, Outcome 7 Participant/parent‐rated symptoms of eczema (SCORAD part C)(Short term)‐Stratified by Age group. | ||||
| 7.1 Age under 2 years | 2 | Mean difference (Random, 95% CI) | ‐0.13 [‐4.18, 3.91] | |
| 7.2 Age 2‐12 years | 1 | Mean difference (Random, 95% CI) | 0.33 [‐1.94, 2.60] | |
| 7.3 Age not categorised | 2 | Mean difference (Random, 95% CI) | ‐2.23 [‐3.71, ‐0.74] | |
| 8 Participant/parent‐rated global change in symptoms of eczema (Short term) ‐ Stratified by Age Show forest plot | 3 | Odds ratio (Random, 95% CI) | Totals not selected | |
| Analysis 1.8  Comparison 1 Probiotic vs Placebo, Outcome 8 Participant/parent‐rated global change in symptoms of eczema (Short term) ‐ Stratified by Age. | ||||
| 8.1 Age under 2 years | 1 | Odds ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Age 2‐ 12 years | 1 | Odds ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Age not categorised | 1 | Odds ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Global eczema severity score (Total SCORAD) (Short term) ‐ Stratified by Age group Show forest plot | 7 | Mean difference (Random, 95% CI) | Subtotals only | |
| Analysis 1.9  Comparison 1 Probiotic vs Placebo, Outcome 9 Global eczema severity score (Total SCORAD) (Short term) ‐ Stratified by Age group. | ||||
| 9.1 Age under 2 years | 3 | Mean difference (Random, 95% CI) | 0.74 [‐5.06, 6.54] | |
| 9.2 Age 2 ‐ 12 years | 1 | Mean difference (Random, 95% CI) | ‐3.28 [‐13.78, 7.22] | |
| 9.3 Age not categorised | 3 | Mean difference (Random, 95% CI) | ‐5.66 [‐14.82, 3.50] | |
| 10 Global eczema severity score (Total SCORAD) (Short term) ‐ Stratified by Presence of Atopy Show forest plot | 7 | Mean difference (Random, 95% CI) | Subtotals only | |
| Analysis 1.10  Comparison 1 Probiotic vs Placebo, Outcome 10 Global eczema severity score (Total SCORAD) (Short term) ‐ Stratified by Presence of Atopy. | ||||
| 10.1 Participants with atopy | 2 | Mean difference (Random, 95% CI) | ‐5.50 [‐23.87, 12.87] | |
| 10.2 Participants with unknown atopic status | 5 | Mean difference (Random, 95% CI) | ‐1.73 [‐7.29, 3.83] | |
| 11 Global eczema severity score (Total SCORAD) (Short term) ‐ Stratified by Challenge‐Proven Food Allergy Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.11  Comparison 1 Probiotic vs Placebo, Outcome 11 Global eczema severity score (Total SCORAD) (Short term) ‐ Stratified by Challenge‐Proven Food Allergy. | ||||
| 11.1 Food allergy present | 1 | 120 | Mean Difference (IV, Random, 95% CI) | 1.15 [‐3.20, 5.50] |
| 11.2 Unknown food allergic status | 5 | 300 | Mean Difference (IV, Random, 95% CI) | ‐3.19 [‐10.59, 4.20] |
| 12 Global eczema severity score (Total SCORAD) (Short term) ‐ Stratified by Eczema Severity Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.12  Comparison 1 Probiotic vs Placebo, Outcome 12 Global eczema severity score (Total SCORAD) (Short term) ‐ Stratified by Eczema Severity. | ||||
| 12.1 Severe eczema (SCORAD over 40) | 3 | 70 | Mean Difference (IV, Random, 95% CI) | ‐7.00 [‐15.34, 1.34] |
| 12.2 Moderate eczema (SCORAD 15‐40) | 4 | 175 | Mean Difference (IV, Random, 95% CI) | ‐3.45 [‐10.34, 3.45] |
| 12.3 Mild eczema (SCORAD under 15) | 1 | 8 | Mean Difference (IV, Random, 95% CI) | ‐5.53 [‐15.29, 4.23] |
| 13 Global eczema severity (Total SCORAD) (Short term)‐Stratified by Probiotic Show forest plot | 7 | Mean difference (Random, 95% CI) | Subtotals only | |
| Analysis 1.13  Comparison 1 Probiotic vs Placebo, Outcome 13 Global eczema severity (Total SCORAD) (Short term)‐Stratified by Probiotic. | ||||
| 13.1 Lactobacillus rhamnosus GG, either alone or in combination with different probiotic bacteria | 3 | Mean difference (Random, 95% CI) | 3.37 [0.55, 6.20] | |
| 13.2 Other Lactobacillus strains, either alone or in combination with different probiotic bacteria | 4 | Mean difference (Random, 95% CI) | ‐7.64 [‐11.65, ‐3.62] | |
| 14 Adverse events (Short term) Show forest plot | 5 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.14  Comparison 1 Probiotic vs Placebo, Outcome 14 Adverse events (Short term). | ||||
| 14.1 Gastrointestinal symptoms | 5 | 304 | Odds Ratio (M‐H, Random, 95% CI) | 1.57 [0.78, 3.15] |
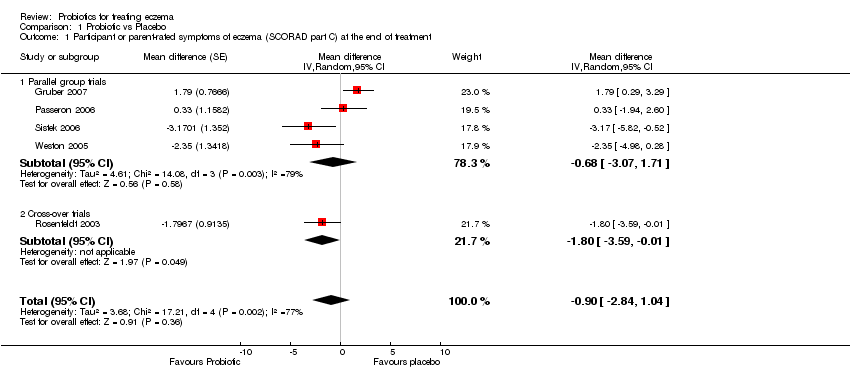
Comparison 1 Probiotic vs Placebo, Outcome 1 Participant or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment.

Comparison 1 Probiotic vs Placebo, Outcome 2 Participant or parent‐rated global change in eczema symptoms during treatment.

Comparison 1 Probiotic vs Placebo, Outcome 3 Parent or participant‐rated eczema severity (SCORAD part C) (Long term).

Comparison 1 Probiotic vs Placebo, Outcome 4 Global eczema severity score (Total SCORAD) (Short term).

Comparison 1 Probiotic vs Placebo, Outcome 5 Global eczema severity score (Total SCORAD) ‐ Sensitivity analysis ‐ Change score.

Comparison 1 Probiotic vs Placebo, Outcome 6 Investigator‐rated eczema severity (SCORAD parts A/B) (Long term).

Comparison 1 Probiotic vs Placebo, Outcome 7 Participant/parent‐rated symptoms of eczema (SCORAD part C)(Short term)‐Stratified by Age group.

Comparison 1 Probiotic vs Placebo, Outcome 8 Participant/parent‐rated global change in symptoms of eczema (Short term) ‐ Stratified by Age.
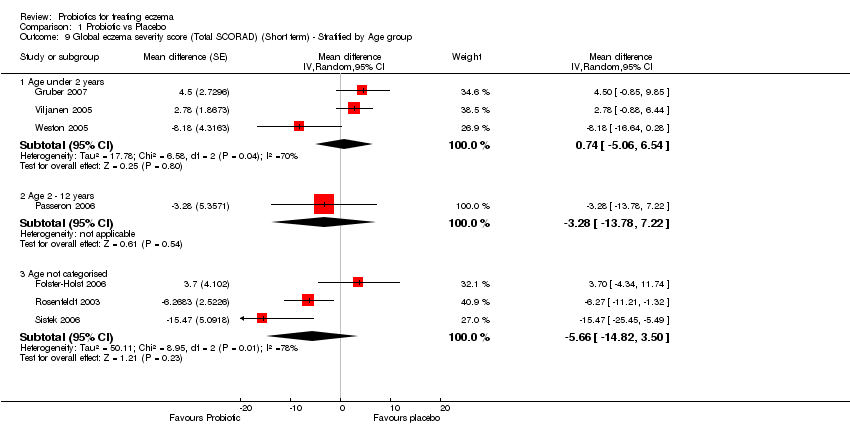
Comparison 1 Probiotic vs Placebo, Outcome 9 Global eczema severity score (Total SCORAD) (Short term) ‐ Stratified by Age group.

Comparison 1 Probiotic vs Placebo, Outcome 10 Global eczema severity score (Total SCORAD) (Short term) ‐ Stratified by Presence of Atopy.
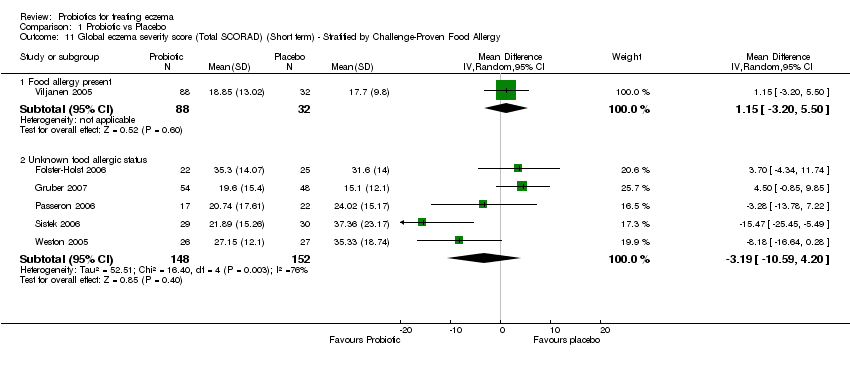
Comparison 1 Probiotic vs Placebo, Outcome 11 Global eczema severity score (Total SCORAD) (Short term) ‐ Stratified by Challenge‐Proven Food Allergy.
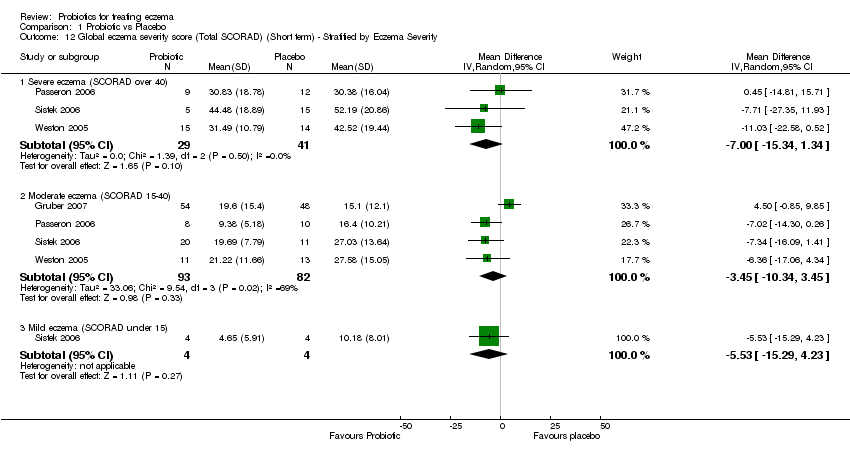
Comparison 1 Probiotic vs Placebo, Outcome 12 Global eczema severity score (Total SCORAD) (Short term) ‐ Stratified by Eczema Severity.

Comparison 1 Probiotic vs Placebo, Outcome 13 Global eczema severity (Total SCORAD) (Short term)‐Stratified by Probiotic.

Comparison 1 Probiotic vs Placebo, Outcome 14 Adverse events (Short term).
| Forms of eczema included | Forms of eczema excluded |
| Atopic eczema | Seborrheic eczema |
| Atopic dermatitis | Contact eczema |
| Besnier's prurigo | Allergic contact eczema |
| Neurodermatitis atopica (German) | Irritant contact eczema |
| Flexural eczema/ dermatitis | Discoid/ nummular eczema |
| Periorbital eczema | Asteatotic eczema |
| Childhood eczema | Varicose/ stasis eczema |
| Infantile eczema | Photo‐/ light‐sensitive eczema |
| 'Eczema' unspecified | Chronic actinic dermatitis |
| Constitutional eczema | Dishydrotic eczema |
| Endogenous eczema | Pompholyx eczema |
| Chronic eczema | Hand eczema |
| Neurodermatitis | Frictional lichenoid dermatitis |
| Neurodermatitis (German) | Lichen simplex |
| Occupational dermatitis | |
| Prurigo |
| Study | Treatment allocation | Blinding | Loss to follow‐up | Clarity of methods | Compliance | Dietary management |
| Brouwer 2006 | Method not described | Unclear | One1 participant lost to follow‐up after randomisation. Available case analysis used, with no exclusions after randomisation and no imputation of data. | Clear | No compliance measures described | Adequate exclusion of other probiotics during study |
| Gruber 2007 | Method not described | Described as 'double blind' but no details given | No loss to follow‐up. Four participants excluded from analysis after randomisation. | Unclear what the placebo was; otherwise clear | 92.5% of doses taken by probiotic group; 94.4% by placebo group | Not stated, other than an encouragement to avoid allergens |
| Isolauri 2000 | Method not described | Described as 'double blind' but no details given | Loss to follow‐up not stated. Not clear whether available case analysis was used. | Unclear ‐ dose and duration of probiotic treatment received not clearly described. Severity of participant eczema at baseline not described. | No compliance measures reported | Not stated |
| Majamaa 1997 | Method not described | Described as 'double blind' but no details given | Loss to follow‐up data not given. Not clear whether available case analysis was used. Four participants excluded from analysis after randomisation, based on later negative food challenge. | Unclear ‐ precise dose of probiotic received by participants not stated | No compliance measures described | Not stated |
| Passeron 2006 | Treatment allocated by hospital pharmacy according to a computer generated randomisation sequence. | Participants, clinicians and outcome assessers were all blinded | Nine participants lost to follow‐up. Available case analysis used, with no exclusions after randomisation. A secondary analysis was performed by the authors using imputation of missing data, but was not included in this meta‐analysis. | Clear | No compliance measures described | Not stated |
| Rosenfeldt 2003 | Method not described | Described as 'double blind' but no details given | Fifteen participants excluded from analysis after randomisaton, for reasons including poor compliance, exacerbation of eczema and loss to follow‐up. No available case analysis performed. | Clear | No compliance measures described | Adequate exclusion of other probiotics during study |
| Sistek 2006 | Treatment allocated by a third party according to a computer generated randomisation sequence. Third party not otherwise involved in the study. | Participants, clinician and outcome assessor blind | One study withdrawal. Available case analysis was used, with no exclusions after randomisation and no imputation of data. | Clear | Assessed by two telephone calls | One participant noted to have taken non‐study probiotic |
| Taniuchi 2005 | Method not described | Unclear | Loss to follow‐up not reported. Not clear whether available case analysis was used. | Clear | No compliance measure described | Not stated |
| Viljanen 2005 | Treatment allocated by a remote third party according to a computer generated randomisation sequence. | Participants, clinicians and outcome assessor blinded | Twenty‐two participants were lost to follow‐up. Analysis was by 'treatment received' because four participants who did not tolerate the study formula were excluded from analysis. | Method for diagnosing eczema not described | No compliance measures described | Not stated |
| Weston 2005 | Treatment allocated by hospital pharmacy according to a computer generated randomisation sequence. | Outcome assessor blind, and also stated 'double blind' | Three participants lost to follow‐up. Available case analysis was used, with no exclusions after randomisation and no imputation of data. | Clear | Sachet counts and parent‐completed sachet administration chart. Good compliance. | Adequate exclusion of other probiotics during study |
| Kirjavainen 2003 | Method not described | Described as 'double blind' but no details given | No loss to follow‐up data given. Analysis was by 'treatment received' because five participants who did not tolerate the study formula were excluded from analysis after randomisation. | Unclear ‐ intended duration of study treatment not stated | No compliance measures reported | Not stated |
| Folster‐Holst 2006 | Method not described | Described as 'double blind' but no details given | Six participants lost to follow‐up. Available case analysis was used. | Clear | No compliance measures reported | Not stated |
| Probiotic 8 weeks | Placebo 8 weeks | Probiotic 16 weeks | Placebo 16 weeks | |
| N | 26 | 27 | 26 | 27 |
| Median | ‐2 | ‐2 | ‐2.5 | ‐3 |
| IQR | ‐5 to ‐0.7 | ‐6 to +2 | ‐5 to ‐1 | ‐7.2 to +2 |
| Majamaa 1month LGG | Majamaa 1month Place | Majamaa 2mo LGG | Majamaa 2mo Placebo | Isolauri 2mo LGG | Isolauri 2mo Bb12 | Isolauri 2mo Placebo | |
| N | 13 | 14 | 13 | 14 | 9 | 9 | 9 |
| Median | 15 | 19 | 16 | 14 | 1 | 0 | 13.4 |
| IQR | 7‐28 | 13‐31 | 6‐25 | 2‐38 | 0.1‐8.7 | 0‐3.8 | 4.5‐18.2 |
| IQR = Interquartile range | |||||||
| Rosenfeldt Probiotic | Rosenfeldt Placebo | Gruber Probiotic | Gruber Placebo | Weston Probiotic | Weston Placebo | Folster‐H Probiotic | Folster‐H Placebo | |
| N | 39 | 39 | ||||||
| Median grams hydrocortisone butyrate applied | 7.8 | 6.0 | ||||||
| Range | 0 to 67 | 0 to 59 | ||||||
| Mean grams 1% hydrocortisone applied | 0.8 | 3.5 | ||||||
| Standard deviation | 45.0 | 29.8 | ||||||
| Median change in topical corticosteroid use score | 0.25 | ‐1.0 | ||||||
| IQR for change in corticosteroid score | ‐6.7 to +7.0 | ‐8.0 to +0.7 | ||||||
| Mean applications per week | 3.0 | 3.2 | ||||||
| Standard deviation | 0.6 | 0.9 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participant or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment Show forest plot | 5 | Mean difference (Random, 95% CI) | ‐0.90 [‐2.84, 1.04] | |
| 1.1 Parallel group trials | 4 | Mean difference (Random, 95% CI) | ‐0.68 [‐3.07, 1.71] | |
| 1.2 Cross‐over trials | 1 | Mean difference (Random, 95% CI) | ‐1.80 [‐3.59, ‐0.01] | |
| 2 Participant or parent‐rated global change in eczema symptoms during treatment Show forest plot | 3 | Odds ratio (Random, 95% CI) | 0.40 [0.14, 1.15] | |
| 2.1 Parallel group trials | 2 | Odds ratio (Random, 95% CI) | 0.70 [0.27, 1.77] | |
| 2.2 Cross‐over trials | 1 | Odds ratio (Random, 95% CI) | 0.18 [0.05, 0.60] | |
| 3 Parent or participant‐rated eczema severity (SCORAD part C) (Long term) Show forest plot | 2 | 102 | Mean Difference (IV, Random, 95% CI) | ‐2.27 [‐3.97, ‐0.58] |
| 4 Global eczema severity score (Total SCORAD) (Short term) Show forest plot | 7 | Mean difference (Random, 95% CI) | ‐2.46 [‐7.45, 2.53] | |
| 4.1 Parallel group studies | 6 | Mean difference (Random, 95% CI) | ‐1.68 [‐7.15, 3.80] | |
| 4.2 Cross‐over studies | 1 | Mean difference (Random, 95% CI) | ‐6.27 [‐11.21, ‐1.32] | |
| 5 Global eczema severity score (Total SCORAD) ‐ Sensitivity analysis ‐ Change score Show forest plot | 5 | Mean difference (Random, 95% CI) | ‐2.47 [‐4.72, ‐0.21] | |
| 5.1 Parallel group trial | 4 | Mean difference (Random, 95% CI) | ‐2.15 [‐4.64, 0.34] | |
| 5.2 Cross‐over trial | 1 | Mean difference (Random, 95% CI) | ‐3.93 [‐9.25, 1.40] | |
| 6 Investigator‐rated eczema severity (SCORAD parts A/B) (Long term) Show forest plot | 2 | 102 | Mean Difference (IV, Fixed, 95% CI) | ‐8.11 [‐13.14, ‐3.09] |
| 7 Participant/parent‐rated symptoms of eczema (SCORAD part C)(Short term)‐Stratified by Age group Show forest plot | 5 | Mean difference (Random, 95% CI) | Subtotals only | |
| 7.1 Age under 2 years | 2 | Mean difference (Random, 95% CI) | ‐0.13 [‐4.18, 3.91] | |
| 7.2 Age 2‐12 years | 1 | Mean difference (Random, 95% CI) | 0.33 [‐1.94, 2.60] | |
| 7.3 Age not categorised | 2 | Mean difference (Random, 95% CI) | ‐2.23 [‐3.71, ‐0.74] | |
| 8 Participant/parent‐rated global change in symptoms of eczema (Short term) ‐ Stratified by Age Show forest plot | 3 | Odds ratio (Random, 95% CI) | Totals not selected | |
| 8.1 Age under 2 years | 1 | Odds ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Age 2‐ 12 years | 1 | Odds ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Age not categorised | 1 | Odds ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Global eczema severity score (Total SCORAD) (Short term) ‐ Stratified by Age group Show forest plot | 7 | Mean difference (Random, 95% CI) | Subtotals only | |
| 9.1 Age under 2 years | 3 | Mean difference (Random, 95% CI) | 0.74 [‐5.06, 6.54] | |
| 9.2 Age 2 ‐ 12 years | 1 | Mean difference (Random, 95% CI) | ‐3.28 [‐13.78, 7.22] | |
| 9.3 Age not categorised | 3 | Mean difference (Random, 95% CI) | ‐5.66 [‐14.82, 3.50] | |
| 10 Global eczema severity score (Total SCORAD) (Short term) ‐ Stratified by Presence of Atopy Show forest plot | 7 | Mean difference (Random, 95% CI) | Subtotals only | |
| 10.1 Participants with atopy | 2 | Mean difference (Random, 95% CI) | ‐5.50 [‐23.87, 12.87] | |
| 10.2 Participants with unknown atopic status | 5 | Mean difference (Random, 95% CI) | ‐1.73 [‐7.29, 3.83] | |
| 11 Global eczema severity score (Total SCORAD) (Short term) ‐ Stratified by Challenge‐Proven Food Allergy Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 Food allergy present | 1 | 120 | Mean Difference (IV, Random, 95% CI) | 1.15 [‐3.20, 5.50] |
| 11.2 Unknown food allergic status | 5 | 300 | Mean Difference (IV, Random, 95% CI) | ‐3.19 [‐10.59, 4.20] |
| 12 Global eczema severity score (Total SCORAD) (Short term) ‐ Stratified by Eczema Severity Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 Severe eczema (SCORAD over 40) | 3 | 70 | Mean Difference (IV, Random, 95% CI) | ‐7.00 [‐15.34, 1.34] |
| 12.2 Moderate eczema (SCORAD 15‐40) | 4 | 175 | Mean Difference (IV, Random, 95% CI) | ‐3.45 [‐10.34, 3.45] |
| 12.3 Mild eczema (SCORAD under 15) | 1 | 8 | Mean Difference (IV, Random, 95% CI) | ‐5.53 [‐15.29, 4.23] |
| 13 Global eczema severity (Total SCORAD) (Short term)‐Stratified by Probiotic Show forest plot | 7 | Mean difference (Random, 95% CI) | Subtotals only | |
| 13.1 Lactobacillus rhamnosus GG, either alone or in combination with different probiotic bacteria | 3 | Mean difference (Random, 95% CI) | 3.37 [0.55, 6.20] | |
| 13.2 Other Lactobacillus strains, either alone or in combination with different probiotic bacteria | 4 | Mean difference (Random, 95% CI) | ‐7.64 [‐11.65, ‐3.62] | |
| 14 Adverse events (Short term) Show forest plot | 5 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 Gastrointestinal symptoms | 5 | 304 | Odds Ratio (M‐H, Random, 95% CI) | 1.57 [0.78, 3.15] |

