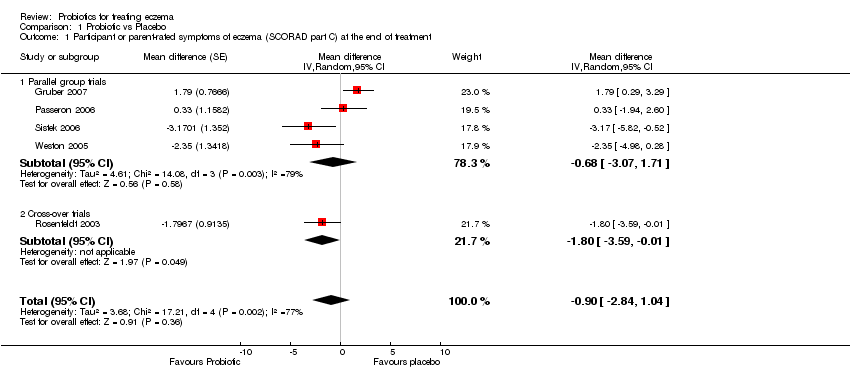
| 1 Participant or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment Show forest plot | 5 | | Mean difference (Random, 95% CI) | ‐0.90 [‐2.84, 1.04] |
|
| 1.1 Parallel group trials | 4 | | Mean difference (Random, 95% CI) | ‐0.68 [‐3.07, 1.71] |
| 1.2 Cross‐over trials | 1 | | Mean difference (Random, 95% CI) | ‐1.80 [‐3.59, ‐0.01] |
| 2 Participant or parent‐rated global change in eczema symptoms during treatment Show forest plot | 3 | | Odds ratio (Random, 95% CI) | 0.40 [0.14, 1.15] |
|
| 2.1 Parallel group trials | 2 | | Odds ratio (Random, 95% CI) | 0.70 [0.27, 1.77] |
| 2.2 Cross‐over trials | 1 | | Odds ratio (Random, 95% CI) | 0.18 [0.05, 0.60] |
| 3 Parent or participant‐rated eczema severity (SCORAD part C) (Long term) Show forest plot | 2 | 102 | Mean Difference (IV, Random, 95% CI) | ‐2.27 [‐3.97, ‐0.58] |
|
| 4 Global eczema severity score (Total SCORAD) (Short term) Show forest plot | 7 | | Mean difference (Random, 95% CI) | ‐2.46 [‐7.45, 2.53] |
|
| 4.1 Parallel group studies | 6 | | Mean difference (Random, 95% CI) | ‐1.68 [‐7.15, 3.80] |
| 4.2 Cross‐over studies | 1 | | Mean difference (Random, 95% CI) | ‐6.27 [‐11.21, ‐1.32] |
| 5 Global eczema severity score (Total SCORAD) ‐ Sensitivity analysis ‐ Change score Show forest plot | 5 | | Mean difference (Random, 95% CI) | ‐2.47 [‐4.72, ‐0.21] |
|
| 5.1 Parallel group trial | 4 | | Mean difference (Random, 95% CI) | ‐2.15 [‐4.64, 0.34] |
| 5.2 Cross‐over trial | 1 | | Mean difference (Random, 95% CI) | ‐3.93 [‐9.25, 1.40] |
| 6 Investigator‐rated eczema severity (SCORAD parts A/B) (Long term) Show forest plot | 2 | 102 | Mean Difference (IV, Fixed, 95% CI) | ‐8.11 [‐13.14, ‐3.09] |
|
| 7 Participant/parent‐rated symptoms of eczema (SCORAD part C)(Short term)‐Stratified by Age group Show forest plot | 5 | | Mean difference (Random, 95% CI) | Subtotals only |
|
| 7.1 Age under 2 years | 2 | | Mean difference (Random, 95% CI) | ‐0.13 [‐4.18, 3.91] |
| 7.2 Age 2‐12 years | 1 | | Mean difference (Random, 95% CI) | 0.33 [‐1.94, 2.60] |
| 7.3 Age not categorised | 2 | | Mean difference (Random, 95% CI) | ‐2.23 [‐3.71, ‐0.74] |
| 8 Participant/parent‐rated global change in symptoms of eczema (Short term) ‐ Stratified by Age Show forest plot | 3 | | Odds ratio (Random, 95% CI) | Totals not selected |
|
| 8.1 Age under 2 years | 1 | | Odds ratio (Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Age 2‐ 12 years | 1 | | Odds ratio (Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Age not categorised | 1 | | Odds ratio (Random, 95% CI) | 0.0 [0.0, 0.0] |
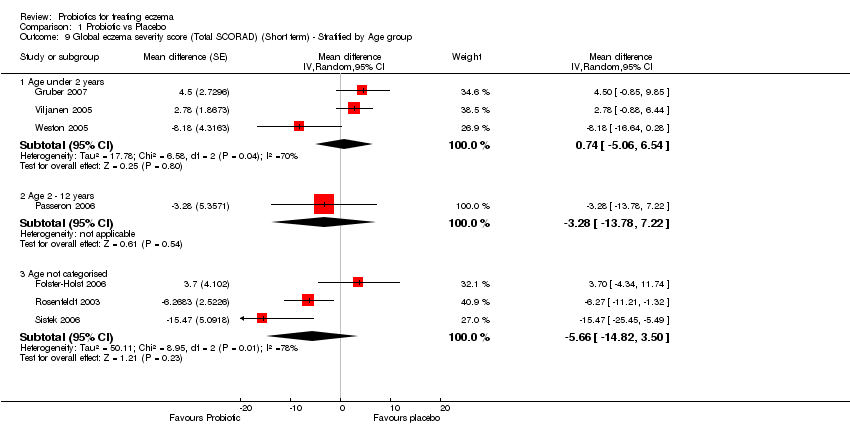
| 9 Global eczema severity score (Total SCORAD) (Short term) ‐ Stratified by Age group Show forest plot | 7 | | Mean difference (Random, 95% CI) | Subtotals only |
|
| 9.1 Age under 2 years | 3 | | Mean difference (Random, 95% CI) | 0.74 [‐5.06, 6.54] |
| 9.2 Age 2 ‐ 12 years | 1 | | Mean difference (Random, 95% CI) | ‐3.28 [‐13.78, 7.22] |
| 9.3 Age not categorised | 3 | | Mean difference (Random, 95% CI) | ‐5.66 [‐14.82, 3.50] |
| 10 Global eczema severity score (Total SCORAD) (Short term) ‐ Stratified by Presence of Atopy Show forest plot | 7 | | Mean difference (Random, 95% CI) | Subtotals only |
|
| 10.1 Participants with atopy | 2 | | Mean difference (Random, 95% CI) | ‐5.50 [‐23.87, 12.87] |
| 10.2 Participants with unknown atopic status | 5 | | Mean difference (Random, 95% CI) | ‐1.73 [‐7.29, 3.83] |
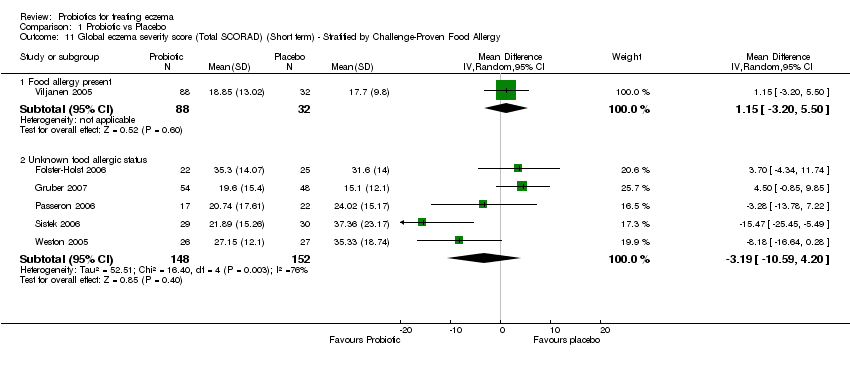
| 11 Global eczema severity score (Total SCORAD) (Short term) ‐ Stratified by Challenge‐Proven Food Allergy Show forest plot | 6 | | Mean Difference (IV, Random, 95% CI) | Subtotals only |
|
| 11.1 Food allergy present | 1 | 120 | Mean Difference (IV, Random, 95% CI) | 1.15 [‐3.20, 5.50] |
| 11.2 Unknown food allergic status | 5 | 300 | Mean Difference (IV, Random, 95% CI) | ‐3.19 [‐10.59, 4.20] |
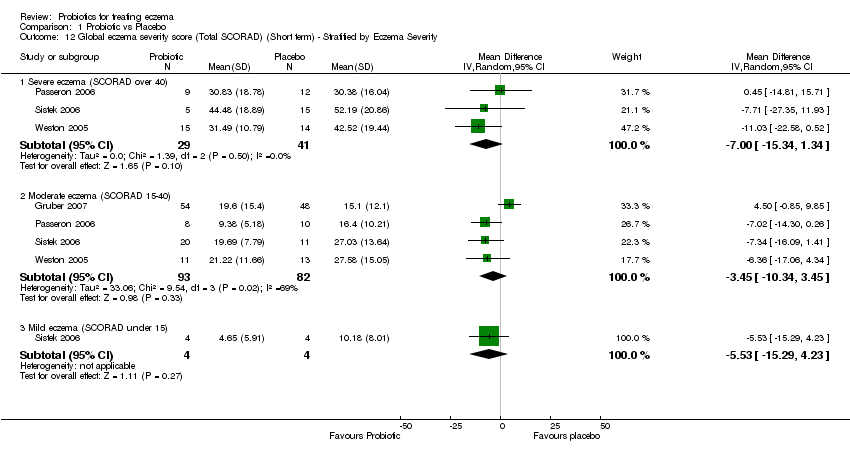
| 12 Global eczema severity score (Total SCORAD) (Short term) ‐ Stratified by Eczema Severity Show forest plot | 4 | | Mean Difference (IV, Random, 95% CI) | Subtotals only |
|
| 12.1 Severe eczema (SCORAD over 40) | 3 | 70 | Mean Difference (IV, Random, 95% CI) | ‐7.00 [‐15.34, 1.34] |
| 12.2 Moderate eczema (SCORAD 15‐40) | 4 | 175 | Mean Difference (IV, Random, 95% CI) | ‐3.45 [‐10.34, 3.45] |
| 12.3 Mild eczema (SCORAD under 15) | 1 | 8 | Mean Difference (IV, Random, 95% CI) | ‐5.53 [‐15.29, 4.23] |
| 13 Global eczema severity (Total SCORAD) (Short term)‐Stratified by Probiotic Show forest plot | 7 | | Mean difference (Random, 95% CI) | Subtotals only |
|
| 13.1 Lactobacillus rhamnosus GG, either alone or in combination with different probiotic bacteria | 3 | | Mean difference (Random, 95% CI) | 3.37 [0.55, 6.20] |
| 13.2 Other Lactobacillus strains, either alone or in combination with different probiotic bacteria | 4 | | Mean difference (Random, 95% CI) | ‐7.64 [‐11.65, ‐3.62] |
| 14 Adverse events (Short term) Show forest plot | 5 | | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 14.1 Gastrointestinal symptoms | 5 | 304 | Odds Ratio (M‐H, Random, 95% CI) | 1.57 [0.78, 3.15] |