Motor neuroprosthesis for promoting recovery of function after stroke
Abstract
Background
Motor neuroprosthesis (MN) involves electrical stimulation of neural structures by miniaturized devices to allow the performance of tasks in the natural environment in which people live (home and community context), as an orthosis. In this way, daily use of these devices could act as an environmental facilitator for increasing the activities and participation of people with stroke.
Objectives
To assess the effects of MN for improving independence in activities of daily living (ADL), activities involving limbs, participation scales of health‐related quality of life (HRQoL), exercise capacity, balance, and adverse events in people after stroke.
Search methods
We searched the Cochrane Stroke Group Trials Register (searched 19 August 2019), the Cochrane Central Register of Controlled Trials (CENTRAL) (August 2019), MEDLINE (1946 to 16 August 2019), Embase (1980 to 19 August 2019), and five additional databases. We also searched trial registries, databases, and websites to identify additional relevant published, unpublished, and ongoing trials.
Selection criteria
Randomized controlled trials (RCTs) and randomized controlled cross‐over trials comparing MN for improving activities and participation versus other assistive technology device or MN without electrical stimulus (stimulator is turned off), or no treatment, for people after stroke.
Data collection and analysis
Two review authors independently selected trials, extracted data, and assessed risk of bias of the included studies. Any disagreements were resolved through discussion with a third review author. We contacted trialists for additional information when necessary and performed all analyses using Review Manager 5. We used GRADE to assess the certainty of the evidence.
Main results
We included four RCTs involving a total of 831 participants who were more than three months poststroke. All RCTs were of MN that applied electrical stimuli to the peroneal nerve. All studies included conditioning protocols to adapt participants to MN use, after which participants used MN from up to eight hours per day to all‐day use for ambulation in daily activities performed in the home or community context. All studies compared the use of MN versus another assistive device (ankle‐foot orthosis [AFO]). There was a high risk of bias for at least one assessed domain in three of the four included studies.
No studies reported outcomes related to independence in ADL. There was low‐certainty evidence that AFO was more beneficial than MN on activities involving limbs such as walking speed until six months of device use (mean difference (MD) −0.05 m/s, 95% confidence interval (CI) −0.10 to −0.00; P = 0.03; 605 participants; 2 studies; I2 = 0%; low‐certainty evidence); however, this difference was no longer present in our sensitivity analysis (MD −0.07 m/s, 95% CI −0.16 to 0.02; P = 0.13; 110 participants; 1 study; I2 = 0%). There was low to moderate certainty that MN was no more beneficial than AFO on activities involving limbs such as walking speed between 6 and 12 months of device use (MD 0.00 m/s, 95% CI −0.05 to 0.05; P = 0.93; 713 participants; 3 studies; I2 = 17%; low‐certainty evidence), Timed Up and Go (MD 0.51 s, 95% CI −4.41 to 5.43; P = 0.84; 692 participants; 2 studies; I2 = 0%; moderate‐certainty evidence), and modified Emory Functional Ambulation Profile (MD 14.77 s, 95% CI −12.52 to 42.06; P = 0.29; 605 participants; 2 studies; I2 = 0%; low‐certainty evidence). There was no significant difference in walking speed when MN was delivered with surface or implantable electrodes (test for subgroup differences P = 0.09; I2 = 65.1%).
For our secondary outcomes, there was very low to moderate certainty that MN was no more beneficial than another assistive device for participation scales of HRQoL (standardized mean difference 0.26, 95% CI −0.22 to 0.74; P = 0.28; 632 participants; 3 studies; I2 = 77%; very low‐certainty evidence), exercise capacity (MD −9.03 m, 95% CI −26.87 to 8.81; P = 0.32; 692 participants; 2 studies; I2 = 0%; low‐certainty evidence), and balance (MD −0.34, 95% CI −1.96 to 1.28; P = 0.68; 692 participants; 2 studies; I2 = 0%; moderate‐certainty evidence). Although there was low‐ to moderate‐certainty evidence that the use of MN did not increase the number of serious adverse events related to intervention (risk ratio (RR) 0.35, 95% CI 0.04 to 3.33; P = 0.36; 692 participants; 2 studies; I2 = 0%; low‐certainty evidence) or number of falls (RR 1.20, 95% CI 0.92 to 1.55; P = 0.08; 802 participants; 3 studies; I2 = 33%; moderate‐certainty evidence), there was low‐certainty evidence that the use of MN in people after stroke may increase the risk of participants dropping out during the intervention (RR 1.48, 95% CI 1.11 to 1.97; P = 0.007; 829 participants; 4 studies; I2 = 0%).
Authors' conclusions
Current evidence indicates that MN is no more beneficial than another assistive technology device for improving activities involving limbs measured by Timed Up and Go, balance (moderate‐certainty evidence), activities involving limbs measured by walking speed and modified Emory Functional Ambulation Profile, exercise capacity (low‐certainty evidence), and participation scale of HRQoL (very low‐certainty evidence). Evidence was insufficient to estimate the effect of MN on independence in ADL. In comparison to other assistive devices, MN does not appear to increase the number of falls (moderate‐certainty evidence) or serious adverse events (low‐certainty evidence), but may result in a higher number of dropouts during intervention period (low‐certainty evidence).
PICO
Plain language summary
Motor neuroprosthesis for improving activities and participation of people in their natural environment after stroke
Review question
Is motor neuroprosthesis (MN) effective for improving activities and participation of people in their natural environment after stroke?
Background
Stroke survivors usually face long‐term impairment, activity limitation, and reduced participation. MN consists of electronic devices that electrically stimulate a nervous system structure to help the performance of daily activities in the natural environment in which people live, as an orthosis (a device applied to a body segment to optimize position, or to limit or assist movement). However, the role of MN for improving activities and participation after stroke is unclear.
Study characteristics
We found four studies of MN involving a total of 831 participants who more than three months poststroke, with mean ages from 53 to 64 years. All participants were able to walk from less than 0.5 m/s to more than 0.7 or even 0.9 m/s. The included studies were published between 2007 and 2015 in the USA and the Netherlands. All included studies applied MN directed to a nerve in the leg (peroneal nerve) to promote the contraction of a muscle at the front of the leg, thus preventing the foot 'dropping' as the leg was swung forward while the participant walked. MN was used from up to eight hours per day to all‐day use for walking about in the natural environment in which people live. Three studies used an MN device that interfaces with the nervous system through electrodes positioned over the skin in the projection of the peroneal nerve in the leg. Only one study used a implantable device whose electrical stimulus is released directly on the nerve by electrodes placed under the layer that surrounds the nerve. All studies compared MN versus ankle‐foot orthosis (AFO), that is an assistive device usually made of a rigid material and placed externally on the lower leg to hold the foot and ankle to prevent the foot dropping.
Key results
There is limited evidence that people after stroke who receive MN as an orthosis for walking in the home or community context may not improve activities involving limbs such as walking speed between 6 and 12 months of device use (low‐certainty evidence), Timed Up and Go (moderate‐certainty evidence), and modified Emory Functional Ambulation Profile (low‐certainty evidence); as well as participation scale of health‐related quality of life (very low‐certainty evidence), exercise capacity (low‐certainty evidence), and balance (moderate‐certainty evidence), compared with people after stroke who receive AFO. There was evidence of an effect that the control intervention (AFO) attained a higher walking speed after six months of device use (low‐certainty evidence), but this evidence showed that the improvements were too small to indicate a meaningful change to patients, and when we excluded the study in which the people that assessed the outcomes were aware of the intervention details, this effect was no longer found. There was no difference in effects on walking speed between MN with surface versus MN with implantable electrodes. No study reported outcomes related to independence in activities of daily living.
The majority of studies reported adverse events such as falls and serious adverse events related to device use, which were found to be similar for MN and AFO use (moderate‐ and low‐certainty evidence, respectively). One study considered serious adverse events related to device use as serious falls. More people who received MN withdrew from the studies than did people who received AFO (low‐certainty evidence). The results of this review indicate that little is known about the effects of MN and that further information is required.
It is unknown if people less than three months poststroke could benefit from MN use as an assistive device to perform activities in daily life. The impact of MN applied to the upper limb or MN that uses brain or muscle signals to trigger the stimulation is unknown in people with stroke. We found no evidence evaluating the costs of delivering MN.
Certainty of the evidence
The certainty of the evidence ranged from moderate to very low.
Authors' conclusions
Summary of findings
| Motor neuroprosthesis compared to another assistive technology device for promoting recovery of function after stroke | |||||||
| Patient or population: promoting recovery of function after stroke | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | ||
| Risk with another assistive technology device | Risk with motor neuroprosthesis | ||||||
| Independence in activities of daily living | (No data) | ‐ | ‐ | No studies | Insufficient evidence | No trials measured this outcome. | |
| Activities involving limbs | Walking speed until 6 months of device use (m/s) timed measures at the end of treatment | The mean walking speed in the control group was on average 0.58 m/s. | 0.05 mean difference lower | ‐ | 605 (2 RCTs) | ⊕⊕⊕⊝ | Minimal important difference for comfortable walking speed in chronic stroke participant is 0.2 m/s (Hiengkaew 2012). |
| Walking speed between 6 and 12 months of device use (m/s) timed measures at the end of treatment | The mean walking speed in the control group was on average 0.69 m/s. | 0 mean difference (0.05 lower to 0.05 higher) | ‐ | 713 (3 RCTs) | ⊕⊕⊝⊝ | Minimal important difference for comfortable walking speed in chronic stroke participant is 0.2 m/s (Hiengkaew 2012). | |
| TUG (s) timed measures at the end of treatment | The mean TUG in the control group was on average 27.57 s. | 0.51 mean difference higher (4.41 lower to 5.43 higher) on intervention group | ‐ | 692 | ⊕⊕⊕⊝ | ||
| mEFAP (s) timed measures at the end of treatment | The mean mEFAP in the control group was on average 286.43 s. | 14.77 mean difference higher (12.52 lower to 42.06 higher) on intervention group | ‐ | 605 | ⊕⊕⊝⊝ | ||
| Participation scales of HRQoL timed measures at the end of treatment | The mean participation scales of HRQoL in the control groups was NA.d | 0.26 standardized mean difference (0.22 lower to 0.74 higher) | ‐ | 632 (3 RCTs) | ⊕⊝⊝⊝ | Using Cohen's rules of thumb, 0.26 represents a small effect. | |
| Exercise capacity: 6MWT (m) timed measures at the end of treatment | The mean 6MWT in the control group was on average 208.12 m. | 9.03 mean difference lower (26.87 lower to 8.81 higher) on intervention group | ‐ | 692 | ⊕⊕⊝⊝ | There are no accurate indices of minimal important difference for 6MWT in people poststroke whose gait speed was ≥ 0.40 m/s (Fulk 2018). | |
| Balance: BBS timed measures at the end of treatment | The mean BBS in the control group was on average 44.15. | 0.34 mean difference lower (1.96 lower to 1.28 higher) on intervention group | ‐ | 692 | ⊕⊕⊕⊝ | Minimal detectable change for BBS in chronic stroke participant is 5 points (Hiengkaew 2012). | |
| Adverse events | Number of dropouts during the intervention period | Study population | RR 1.48 | 829 | ⊕⊕⊝⊝ | ||
| 96 per 1000** | 142 per 1000** | ||||||
| Falls | Study population | RR 1.20 | 802 | ⊕⊕⊕⊝ | |||
| 296 per 1000** | 355 per 1000** | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). **We used the median control group risk across studies. 6MWT: 6‐minute walk test; BBS: Berg Balance Scale; CI: confidence interval; HRQoL: health‐related quality of life; mEFAP: modified Emory Functional Ambulation Profile; NA: not applicable; RCT: randomized controlled trial; RR: risk ratio; TUG: Timed Up and Go test. | |||||||
| GRADE Working Group grades of evidence | |||||||
| aThe outcome assessors were not blinded in the larger study. | |||||||
Background
Description of the condition
Among the cardiovascular diseases, hemorrhagic and ischemic strokes were considered to be the second and third most common causes of disability‐adjusted life‐years, respectively, in 2015 (Roth 2017). They present a higher prevalence among individuals aged 74 to 79 years (Roth 2017). Projections indicate that by 2030 there will be 70 million stroke survivors worldwide (Feigin 2014). The stroke survivors will face long‐term impairment, activity limitation, and reduced participation that will impact not only on their own lives, but also on the lives of their families (Langhorne 2009). Among them, approximately one‐third will have functional dependence during the first year after stroke (de Campos 2017). One of the important factors that contributes to being unable to live independently is motor impairment by hemiparesis, because it leads to difficulties in performing functional activities (Schiemanck 2006). Lower limb impairment typically affects the performance of gait, and it is common to observe foot drop when the individual tries to take a step with the paretic limb (Stein 2008). Upper limb impairment affects the interaction with objects in the environment, involving movements such as grasp, grip, pinch, and others (Lang 2013). In this scenario, the use of contextual factors, such as assistive technology devices (e.g. orthosis), work as a resource to facilitate the performance of daily activities and the recovery of motor function after stroke (Eng 2007).
Description of the intervention
The first application of electric current to nervous tissue in order to promote movement dates back to the experiment of Galvani in the 1790s (Cambridge 1977). Since then, there have been advances in the use of electrical stimulation of motor neurons to activate paralyzed or paretic muscles, and it is widely used in clinical rehabilitation (Sheffler 2007). This electrical stimulus applied to excite peripheral sensory and motor nerves is known as neuromuscular electrical stimulation (NMES) (Alon 2003b); when the aim is to employ this stimulus to achieve functional tasks, the term used is functional electrical stimulation (FES) (Sheffler 2007). FES is a routine therapeutic approach that physiotherapists use during stroke rehabilitation in a clinical setting to improve strength, upper extremity function, and gait, and to prevent hemiplegic shoulder subluxation (Auchstaetter 2016).
Due to technological advances, especially in electronics, electrical stimulation devices have become increasingly miniaturized and lightweight, and with more refined control and sensor configurations, they can be worn as an orthosis beyond the clinical setting (Melo 2015; Popović 2014). By integrating the electric stimulator with control algorithms and sensors, it is possible to determine the time of delivery of the electrical current in response to the sensor signals (Melo 2015). This integration was implemented for the first time in 1961 when Liberson applied electrical stimuli to the common peroneal nerve to activate the tibialis anterior muscle during the swing phase of gait. He used a heel switch as a sensor to control the timing of the stimulation. The train of stimuli was only released when the heel came off the ground at the end of the stance phase and ended when the heel contacted the ground again at the beginning of the stance phase (Liberson 1961). Since then, much progress has been made, with devices becoming portable, battery powered, and wireless, allowing them to be worn and implemented as an assistive technology device (e.g. an orthosis) that acts as an environmental facilitator for expanded capacity and performance in walking and moving and also carrying and handling objects (Bosch 2014; Cowan 2012). In addition to this direct effect on performance, the orthotic use of the electric current enables people with stroke to experience a greater amount of practice in their current environment. This orthotic use is often referred to as motor neuroprosthesis (MN), which is considered to be an electronic device that interfaces with the nervous system and attempts to restore functions, generally by electrical stimulation (Naik 2014; Ziat 2015).
The activation of neural structures to promote movement through electrical stimulation is used in both MN and FES, meaning there may be overlap between FES and MN concepts (Popović 2014). Although both MN and FES use electrical stimulation, MN has a system technology configuration that allows its use in the actual context in which people live (real‐world setting). In this way MN allows the electrical stimulus to be used as an environmental facilitator (e.g. an orthosis) to achieve a greater level of practice, producing effects during the performance of functional abilities in the individual's current environment (Laufer 2009). Several studies and guidelines already consider comparisons of MN with other orthotic devices for decision‐making purposes (Bethoux 2015; Bosch 2014; Kluding 2014; NICE 2009). We focused on this perspective within the scope of this review, that is that MN consists of a category that uses stimuli to allow the performance of tasks in the actual context in which a person lives, and is being used daily for increasing the activities and participation of people with stroke, while FES comprises the use of electrical stimulation to enhance function (Martin 2012; Sheffler 2007), and is especially used in the context of the clinical setting. Several Cochrane Reviews have already shown evidence of therapies for improving activities of daily living (ADL) such as virtual reality (Laver 2017), action observation (Borges 2018), and mirror therapy (Thieme 2018). Within the context of rehabilitation, these therapies may be additional and further enhanced by MN daily use.
In order to operate autonomously during the performance of functional activities, MN has a typical architecture composed of a network of sensors, control unit, and a stimulation unit (Melo 2015). The stimulation unit is responsible for generating the electric current that is delivered to the nervous system via electrodes placed in different locations, ranging from the skin surface to directly implanted into different areas of the nervous system (Collinger 2013). Regardless of the location of this interface in the nervous system, all devices that stimulate it electrically for the previously described purposes are considered to be MN. It is possible to use biological signals, such as electromyography, electroencephalography, and electroneurography signals, eye tracking, and voice control, or non‐biological signals such as force/pressure and inertial sensors as an input to trigger the electrical stimulus to the desired motor function (Ambrosini 2014). Consequently, there is a need to translate and to adjust the command signal provided by sensors as an input to the stimulation unit, a function of the control unit (Horch 2004; Naik 2014). Besides the described requirements, the device needs to be portable, lightweight, autonomously controlled, and battery powered to be an assistive technology device (Melo 2015).
How the intervention might work
MN allows people with stroke to enhance the performance of functional activities in the home and community, including the manipulation of objects with the paretic upper limb or gait activities with the paretic lower limb (Cowan 2012; Moss 2011; Sheffler 2009). The use of these assistive devices can lead people with stroke to benefit from their orthotic effect, reflecting the direct improvement in tasks while using the MN (Dunning 2015; Kottink 2004; Prenton 2016). Furthermore, the daily use of MN allows people with stroke to perform repetitive activities that lead to a longer‐lasting improvement (as an effect of relearning) after the stimulation is turned off (Ambrosini 2011; Dunning 2015; Prenton 2016). This may be explained by plasticity mechanisms from peripheral effects in muscles and central effects from the central nervous system reorganization. It is hypothesized that these devices activate the motor‐related areas of the cortex and their residual corticospinal pathways induce neural plasticity in people with stroke (Everaert 2010). Thus far, direct signs of brain injury repair after one year of using the MN in people with chronic stroke were seen by cortical metabolism improvement over the damaged motor areas, leading to recovery of near‐to‐normal brain metabolism (Thibaut 2017).
Why it is important to do this review
Some systematic reviews have been conducted on the topic of FES that considered devices with the architecture configuration of MN to promote recovery of function after stroke (Bolton 2004; Dunning 2015; Kottink 2004; Meilink 2008). Only one of these reviews considered daily use of MN devices in the home or community context as an assistive device; however, this review only analyzed surface MN directed to a specific part of the lower limb, without performing a meta‐analysis (Dunning 2015). In order to determine the level of evidence of the effects of the daily use of the whole category of upper limb and lower limb MN for improving activities and participation in the natural environment in which people with stroke live, it was essential to conduct this high‐quality systematic review.
Due to the wide variety of MN, there is a need to clarify which device has the best evidence for improving activity and participation after stroke, the best phase in which to use the device, the optimal frequency of use, and which target shows the best results. Moreover, to support clinical practice, healthcare managers, policymakers, and consumers, and the acceptability of using MN, costs, and benefits, must be considered. This review aimed to synthesize the evidence for the use of MN for improving activities and participation after stroke and hence to assist clinical decision‐making.
Objectives
To assess the effects of motor neuroprosthesis (MN) for improving independence in activities of daily living (ADL), activities involving limbs, participation scales of health‐related quality of life (HRQoL), exercise capacity, balance, and adverse events in people after stroke.
Methods
Criteria for considering studies for this review
Types of studies
We planned to review published and unpublished randomized controlled trials (RCTs) and randomized controlled cross‐over trials. For randomized controlled cross‐over trials, we only analyzed the first period as a parallel‐group trial. Cross‐over trials were only eligible if comparison groups included placebo; the evaluation of outcomes was blinded to allocation; and a minimum period of follow‐up was clearly described. Trials reported in abstract form were eligible for inclusion only when adequate information was provided in the abstract or was available from the trial authors. We excluded quasi‐RCTs, that is trials in which the method of allocating participants to a treatment is not strictly random (e.g. by date of birth, hospital record number, or alternation). If we included a study that was described as randomized, but while assessing risk of bias we learned that it was a quasi‐RCT, we excluded the data from this study from the analysis.
Types of participants
We included studies whose participants were clinically diagnosed with stroke, were over 18 years of age, of both sexes, at any stage of the disease. A diagnosis of stroke fulfills the clinical criteria of the World Health Organization (WHO); stroke is defined as a "neurological deficit of cerebrovascular cause that lasts more than 24 hours or leads to death within 24 hours" (WHO 1989). A diagnosis of stroke encompasses ischemic and hemorrhagic stroke (including subarachnoid, intraventricular, or intracerebral hemorrhage).
Types of interventions
This review included studies that used motor neuroprosthesis (MN) devices for improving activities and participation after stroke. Considering that this approach focuses on the use of MN as an orthosis, we only included studies that used MN in the home or community context and that fulfilled some device requirements, such as working autonomously, being battery powered to ensure its autonomy, and have stimulus triggered by a sensor. We also included studies that used implanted or superficial electrodes whose application is directed to upper or lower limbs, and studies that addressed hybrid MN, which combines an exoskeleton or a mechanical orthosis with an electrical stimulation device. We excluded studies that used sensory stimulation as transcutaneous electrical nerve stimulation (TENS).
We selected studies that included the following comparisons.
-
MN with electrical stimulus versus no treatment.
-
MN with electrical stimulus versus MN without electrical stimulus, where both groups used the device, but in one group the stimulator was turned off.
-
MN versus another assistive technology device (e.g. foot drop stimulator versus ankle foot orthosis, electromyographic (EMG)‐triggered stimulation versus hand orthosis, etc).
Types of outcome measures
We included outcome measures falling into the International Classification of Functioning, Disability and Health (ICF) categories for activity and participation (Brehm 2011; Mudge 2007; Sullivan 2013). According to the ICF, 'activity' corresponds to the execution of a task or action by an individual, while 'participation' means the involvement in a life situation (WHO 2001).
Primary outcomes
-
Independence in ADL, e.g. Functional Independence Measure (FIM) (Hamilton 1994), Barthel Index (BI) (Quinn 2011), Motor Assessment Scale (MAS) (Dean 1992).
-
Activities involving limbs, e.g. Jebsen Taylor Hand Function Test (Stern 1992), Wolf Motor Function Test (WMFT) (Wolf 2001), 9‐Hole Peg Test (9HPT) (Heller 1987), Box & Blocks Test (BBT) (Mathiowetz 1985), Motor Activity Log (MAL) (Uswatte 2005), Timed Up and Go test (TUG) (Podsiadlo 1991), Rivermead Mobility Index (Collen 1991), Functional Ambulation Categories (FAC) (Holden 1984), Dynamic Gait Index (Jonsdottir 2007), modified Emory Functional Ambulation Profile (mEFAP) (Baer 2001; Wolf 1979), walking speed.
Secondary outcomes
-
Participation scales of HRQoL, e.g. 36‐Item Short Form Health Survey (SF‐36) (Anderson 1996), Stroke Impact Scale (SIS) (Duncan 1999).
-
Exercise capacity, e.g. 6‐minute walk test (6MWT) (Seale 2006).
-
Balance, e.g. Berg Balance Scale (BBS) and Functional Reach Test (FRT) (Berg 1992; Martins 2012).
-
Adverse events, i.e. pain, skin irritation, dropouts, acceptance, number of falls.
Adverse events
To measure the acceptance of MN we considered the number of withdrawals or dropouts from the study due to any reason during the study period. We used the incidence of serious adverse events related to intervention and number of falls to investigate the safety of MN. We considered the number of falls due to the nature of the use of MN in the home or community context for walking activities.
Search methods for identification of studies
See the 'Specialized register' information at the Cochrane Stroke Group's website. We searched for trials in all languages and arranged for the translation of relevant articles when necessary.
Electronic searches
We searched the Cochrane Stroke Group Trials Register (last searched 19 August 2019) and the following electronic bibliographic databases.
-
The Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 8 of 12, August 2019) in the Cochrane Library (searched 19 August 2019; Appendix 1)
-
MEDLINE Ovid (1946 to 16 August 2019; Appendix 2)
-
Embase Ovid (1980 to 2019 Week 33; searched 19 August 2019; Appendix 3)
-
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1982 to 19 August 2019; Appendix 4)
-
AMED Ovid (Allied and Complementary Medicine Database; 1985 to 19 August 2019; Appendix 5)
-
PEDro (Physiotherapy Evidence Database; www.pedro.org.au/; searched 19 August 2019; Appendix 6)
-
REHABDATA (www.naric.com/?q=en/REHABDATA; searched 19 August 2019; Appendix 7);
-
IEEE (Institute of Electrical and Electronics Engineers; www.ieee.org/; searched 19 August 2019; Appendix 8)
We developed the MEDLINE search strategy with the help of the Cochrane Stroke Group Information Specialist and modified it for the other databases. The search strategy included Cochrane's highly sensitive search strategies for identification of RCTs, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011), and the Cochrane Stroke Group's search strategies for the identification of stroke studies in respective databases and other resources.
We also searched the following electronic registries, databases, and websites to identify additional relevant published, unpublished, and ongoing trials.
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 19 August 2019; Appendix 9)
-
World Health Organization (WHO) International Clinical Trials Registry Platform (www.who.int/ictrp/en/; searched 19 August 2019; Appendix 10)
-
Stroke Trials Registry (www.strokecenter.org/trials/; searched 21 August 2019; Appendix 11)
-
ISRCTN registry (www.isrctn.com/; searched 20 August 2019; Appendix 12)
-
Australian New Zealand Clinical Trials Registry (www.anzctr.org.au/; searched 20 August 2019; Appendix 13)
-
Health Technology Assessment (HTA) database ‐ Centre for Reviews and Dissemination, University of York (www.crd.york.ac.uk/PanHTA; searched 19 August 2019; Appendix 14)
-
OAIster (oaister.worldcat.org/; searched 19 August 2019; Appendix 15)
-
The Directory of Open Access Repositories – OpenDOAR (searched using CORE; searched 19 August 2019; Appendix 16)
-
British Library Ethos (ethos.bl.uk/; searched 19 August 2019; Appendix 17)
-
ProQuest Dissertations & Theses Global (www.proquest.com/products‐services/pqdtglobal.html; 19 August 2019; Appendix 18)
Searching other resources
We contacted equipment manufacturers to ask for information about any relevant trials that address MN (Appendix 19). We contacted some original study authors for clarification and further data if trial reports were unclear. Additionally, we used Grey Matters: a practical tool for searching health‐related grey literature checklist, published by the Canadian Agency for Drugs and Technologies in Health (CADTH) to conduct additional searches of grey literature (Grey Matters; www.cadth.ca/resources/finding‐evidence/grey‐matters; searched 19 August 2019; Appendix 20).
Data collection and analysis
Selection of studies
Two review authors (LM and IN) independently screened the titles and abstracts of the studies identified by the search and removed obviously irrelevant reports. We obtained the full‐text of the remaining studies, and the same two review authors selected studies for inclusion according to the predefined inclusion criteria. In the case of any questions on methodology, we contacted the study authors for clarification to determine study eligibility. Two other review authors (VR or TS) evaluated any discrepancies as required and advised in case of disagreement. We recorded the reasons for exclusion and completed a PRISMA flow chart (Liberati 2009).
Data extraction and management
Two review authors (LM and IN) independently extracted data from the included studies and summarized trial details on a data extraction template in Covidence that was developed specifically for this review (Covidence). In the case of incomplete or unclear data, we contacted the study authors for clarification. Any disagreements were resolved by discussion or by involving a third review author (VR or TS). We extracted the following information according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
-
General information: title of the review, study ID, and contact details.
-
Methods: study design, instruments used, study duration, 'Risk of bias' criteria (sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting), year of study.
-
Participants: total number of participants, setting, age, sex, country, motor impairment, type of stroke, phase (acute, subacute, or chronic).
-
Intervention: intervention details regarding time (number and duration of exposure, weeks of use, and in the case of follow‐up, describe the duration), devices (type of electrode and sensor), and place of application (upper or lower limb); methods used in the control group.
-
Outcomes: definition of primary and secondary outcome(s).
-
Results: number of participants allocated to each group, number of withdrawals (by group, with reasons), adverse events, overall sample size and methods used to estimate statistical power (regarding the target number of participants to be recruited, the clinical difference to be detected, and the ability of the trial to detect this difference).
-
Notes: contact with authors (information obtained or not), article in a language other than English, funding source and noteworthy conflicts of interest of study authors.
Assessment of risk of bias in included studies
Two review authors (LM and IN) independently assessed the risk of bias for each included study using the Cochrane 'Risk of bias' tool (Higgins 2011b). Any disagreements were resolved by discussion or by involving a third review author (VR or TS). We assessed risk of bias according to the following domains.
-
Random sequence generation
-
Allocation concealment
-
Blinding of participants and personnel
-
Blinding of outcome assessment
-
Incomplete outcome data
-
Selective outcome reporting
-
Other bias
We graded the risk of bias for each domain as low, high, or unclear and entered this information along with the reasons for each decision into the 'Risk of bias' table produced for each study. We used Table 8.5.d in the Cochrane Handbook for Systematic Reviews of Interventions, which provides criteria for making judgements regarding risk of bias in each of the seven domains of the tool (Higgins 2011b). We considered the risk of bias of the studies and its contribution to the treatment effect. We then entered the data into Review Manager 5 (Review Manager 2014).
Measures of treatment effect
We performed the data analysis according to Cochrane guidelines. One review author (LM) entered the quantitative data into Review Manager 5 (Review Manager 2014), which was checked by another review author (IN), and analyzed. We presented the results for each outcome with 95% confidence intervals (CIs). We measured treatment effects using the risk ratio (RR) for dichotomous outcomes, mean difference (MD) or standardized mean difference (SMD) (if different methods of measurement were used in the studies) and overall effect size (with 95% CI calculated) for continuous outcomes.
Unit of analysis issues
We considered the number of individual participants as the unit of analysis in this review. As we only identified individually randomized trials, we did not need to analyze for unit of analysis issues as planned in our protocol (Mendes 2018).
Dealing with missing data
When data were missing, we contacted the original researchers to request these data whenever possible. When this was not possible, and we considered that the missing data might introduce serious bias, we performed a sensitivity analysis to explore the impact of including such studies on the overall assessment of results. We assessed and reported dropout rates for each study, and used intention‐to‐treat (ITT) analyses (analysis of all participant data according to group allocation). We considered the amount of missing data when determining the risk of bias within included studies.
Assessment of heterogeneity
We assessed heterogeneity visually by observing the non‐overlapping of CIs in the forest plots. Once identified, we quantified statistical heterogeneity using the I2 statistic. The I2 statistic estimates the percentage of total variation across trials due to heterogeneity rather than to chance. We categorized heterogeneity as I2 values of 40% or less as indicating heterogeneity might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; and 75% or above indicating considerable heterogeneity (Higgins 2011c).
Assessment of reporting biases
We intended to perform a funnel plot analysis to assess reporting bias if a sufficient number of studies was identified (i.e. 10 or more). Had asymmetry been present, we would have explored possible causes, including publication bias, poor methodological quality, and true heterogeneity.
Data synthesis
We planned to perform a random‐effects meta‐analysis and use the fixed‐effect method as a sensitivity analysis.
GRADE and 'Summary of findings' table
We assessed the certainty of the evidence by creating a 'Summary of findings' table using the following outcomes: independence in ADL, activities involving limbs, participation, exercise capacity, balance, and adverse events. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of a body of evidence as it relates to the studies contributing data to the review for the outcomes (Atkins 2004). We used GRADEpro GDT to prepare the 'Summary of findings' table for the main comparison and to report the certainty of the evidence (GRADEpro GDT).
Subgroup analysis and investigation of heterogeneity
We planned to examine the following subgroup analyses if data were available.
-
Type of effect (first subgroup defined as immediate effect or orthotic effect, i.e. the effect seen while using MN; second subgroup defined as relearn effect, i.e. the effect seen after the stimulation is turned off).
-
Effect of MN when device was used for varying durations (4 weeks of use, between 5 and 24 weeks of use, 25 weeks of use).
-
Effect of MN when used by participants at different phases of disease (< 3 months, 3 months).
-
Effect of MN with surface or implantable electrodes.
-
Effect of MN when applied on lower limb or upper limb.
We planned to use random‐effects methods to produce this subgroup analysis for primary outcomes only.
Sensitivity analysis
We used Cochrane's tool for assessing risk of bias to judge the study methods (Higgins 2011b). We performed sensitivity analyses to assess the robustness of the findings by excluding studies from the analysis that were at high risk of bias in one or more of these three domains: random sequence generation, allocation concealment, and blinding of outcome assessors.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
Our electronic searches and searches of other resources identified a total of 18,579 references. After removal of 4881 duplicates, a total of 13,698 references remained for title and abstract screening. Of these, we excluded 13,616 references as irrelevant. We obtained the full text of 82 reports, and from these identified and included four studies (9 reports) in the review. The results of the search are summarized in the study flow diagram (Figure 1).

Study flow diagram.
Included studies
See Characteristics of included studies.
Sample size and study location
The four included RCTs involved a total of 831 participants of both sexes, and were published between 2007 and 2015 in the USA, Bethoux 2014; Kluding 2013; Sheffler 2013a, and the Netherlands, Kottink 2007. Sample sizes ranged from 29 (14 and 15 in each group; Kottink 2007) to 497 (242 and 253 in each group; Bethoux 2014).
Participant characteristics
The mean age of participants ranged from 53, in Sheffler 2013a, to 64 years, in Bethoux 2014. Time poststroke varied between studies: Bethoux 2014 and Kottink 2007 recruited participants with a poststroke period of six months or more, whereas Kluding 2013 and Sheffler 2013a included participants with a poststroke period of three months or more. All participants were able to walk: in Sheffler 2013a they were able to walk at least 9.1 meters without an ankle‐foot orthosis; in Bethoux 2014 and Kluding 2013 they were able to walk at least 10 meters with or without an assistive device or with a maximum of one person assisting, respectively; and in Kottink 2007 participants needed to walk independently on level and non‐level surfaces, stairs, and inclines. The mean walking speed varied among studies, from less than 0.5 m/s, Bethoux 2014; Kluding 2013; Sheffler 2013a, to more than 0.7 and 0.9 m/s, Kottink 2007.
Intervention approaches
The included studies applied MN on a lower limb to facilitate walking in daily activities performed in the home or community context. All studies applied electrical stimuli to the peroneal nerve during the swing phase of gait using commercially available battery‐powered devices. Three studies used a single‐channel surface peroneal nerve stimulator composed of a stimulator, control unit, sensor, and two surface electrodes (Bethoux 2014; Kluding 2013; Sheffler 2013a), whereas one study used a two‐channel implantable device composed of implantable components such as a stimulator, two leads, and two intraneural electrodes, and non‐implantable components such as an external transmitter with a built‐in antenna and sensor (Kottink 2007). Only one study used a tilt sensor and an accelerometer placed on the participant's leg to trigger the stimulation (Bethoux 2014); the other three studies used a heel switch placed inside the shoe to control the timing of the stimulation. Participants used MN from up to eight hours per day (Sheffler 2013a), to all‐day use for ambulation (Bethoux 2014; Kluding 2013; Kottink 2007). During the first weeks of the intervention, all studies used conditioning protocols to adapt participants to MN use; after that, participants used MN for a long time during the day. All conditioning protocols included fitting the device to the participant’s leg and giving the participant instructions on the use of MN (Table 1). The duration of the conditioning protocols ranged from two weeks, in Bethoux 2014, to six weeks, in Kluding 2013. These protocols were comprised of different activities, such as a progressive home‐wear schedule to gradually increase time of device use, Bethoux 2014; Kluding 2013; Kottink 2007, and physical therapy sessions, which included gait training with the device, performed once or twice a week, Kluding 2013, and additional activities such as passive and active range‐of‐motion exercises, lower extremity strengthening, standing balance, and weight‐shifting activities to the affected limb (Sheffler 2013a). In Kottink 2007, participants underwent a surgical procedure for the implantation of the MN components, and stimulation during walking started in the third week after surgery. The total duration of exposure to interventions varied from 12 weeks, in Sheffler 2013a, to 12 months, in Bethoux 2014. All studies compared the use of MN versus another assistive device (ankle‐foot orthosis [AFO]).
| Study ID (report) | MN device | Duration of exposure to MN intervention | Conditioning protocol used to adapt participants to MN use | MN use/daily use for increasing the activities and participation in the home or community context |
| The MN used was the WalkAide device (Innovative Neurotronics, Austin, TX, USA). It is a commercially available, battery‐operated, single‐channel surface peroneal nerve stimulator that consists of a cuff worn around the proximal part of the lower leg, which holds the control module and surface electrodes. This device uses a tilt sensor and an accelerometer to trigger ankle dorsiflexion and control the timing and duration of peroneal nerve stimulation during the swing phase of gait to alleviate foot drop. | The duration of MN intervention was 12 months. The conditioning protocol occurred in the first 2 weeks, after which participants started daily use of MN device. | The first part consisted of fitting and programming the MN device as well as patient education performed by WalkAide‐certified orthotist or licensed physical therapist. The conditioning protocol included a 2‐week progressive wearing schedule of MN device. | Participants were instructed to wear MN device on a full‐time basis (quote: "ie, for all walking activities throughout the day"). | |
| The MN used was the NESS L300 device (Bioness Inc, Valencia, CA, USA). It is a commercially available, battery‐operated, single‐channel surface peroneal nerve stimulator that consists of a cuff with integrated stimulation unit and electrodes, a control unit, and an in‐shoe pressure sensor. The pressure sensor detects heel off and initial contact events during gait. It transmits wireless signals to the stimulation cuff, which initiates or pauses the stimulation of deep and superficial branches of the peroneal nerve via 2 surface electrodes that activate dorsiflexors and evertors muscles to ensure foot clearance during the swing phase of gait and prevent excessive ankle inversion during early stance. | The duration of MN intervention was 30 weeks. The conditioning protocol occurred in the first 6 weeks. Participants used the MN device all day between week 4 and week 30. | The first part consisted of initial fitting of the device, gait training, wearing schedule, home exercise program, and participant education based on manufacturer standardized protocols. For the first 3 weeks, participants followed the standard conditioning protocol (gradually increasing walking with the MN from 15 minutes each day to all‐day use). During the same period, participants also used the MN for cyclic stimulation while not walking in order to gradually strengthen and condition the muscles to avoid fatigue when using the device (Dunning 2013).* During the first 6 weeks of the study, participants also received 8 dose‐matched sessions of physical therapy. The first 2 to 4 therapy visits focused on education on device use, initial gait training, and an individualized home exercise program. The remaining physical therapy sessions focused on gait training (Kluding 2013). | Participants used the MN all day for ambulation (Dunning 2013).* | |
| The MN used was the STIMuSTEP device (FineTech Medical Ltd, Hertfordshire, UK). It is a commercially available, battery‐operated, 2‐channel implantable device composed of implantable components such as a stimulator, 2 leads, and bipolar intraneural electrodes, and non‐implantable components such as an external transmitter with a built‐in antenna and a pressure sensor. 1 electrode is surgically positioned under the epineurium of the superficial peroneal nerve and the other under the epineurium of the deep peroneal nerve. This device promotes the ankle dorsiflexion/eversion during gait to correct foot drop, and a pressure sensor placed inside the shoe determines the on and off switching of the stimulation. | The duration of MN intervention was 26 weeks. The intervention began with the surgical procedure for placement of the implant. After 2 weeks of the surgery, the wound was checked and first test stimulation took place. The conditioning protocol began at the third week, and all‐day MN use began at the sixth week. | Quote: "Two weeks after the surgery the wound was checked and a first test stimulation took place. In the third week, stimulation during walking was tested and the stimulator was taken home by the patient. The use of the stimulator was gradually increased over 2 weeks to prevent severe muscle pain and fatigue. After this period patients were allowed to use the system all day." | Participants were allowed to use the system all day between week 6 and week 26. | |
| The MN used to correct foot drop was the Odstock Dropped‐Foot Stimulator (ODFS) device (Odstock Medical Ltd, Salisbury Wiltshire, UK). The ODFS is a commercially available, battery‐operated, single‐channel surface peroneal nerve stimulator consisting of an electrical stimulator, a control module, pressure sensors, and surface electrodes. The stimulation is triggered by an insole pressure‐sensing foot switch that detects heel rise at pre‐swing. | The duration of MN intervention was 12 weeks. The conditioning protocol occurred over the 12 weeks. Daily MN use began once device safety was demonstrated by participants. | In the first 5 weeks the Functional Training phase (2 x 1‐hour sessions per week) took place, in which participants were trained to use MN device for home and community mobility with an assistive device, if needed. Activities included passive and active range‐of‐motion exercises, lower extremity strengthening, standing balance and weight‐shifting activities to the affected limb with transition to least‐restrictive assistive device, and refinement of a reciprocal gait pattern. Exercises were done with multiple repetitions with an increase in difficulty and a decrease in cues, with and without the MN device, as appropriate. In the last 7 weeks the Post‐Functional Training Phase (3 x 1‐hour sessions) took place, in which device function, application, and usage guidelines were reviewed with each participant to maximize MN compliance. | The article did not explicitly mention when participants started all‐day MN use, but reported that as soon as participants demonstrated safe use of the device, it was used up to 8 hours per day. |
MN: motor neuroprosthesis
*Dunning 2013 corresponds to the published protocol of the study Kluding 2013.
Outcomes
Outcome measures for each of the predefined outcome categories are shown in Table 2. No study included primary outcomes related to independence in ADL. As we listed walking speed as a primary outcome related to activities involving limbs, we considered measures that assess speed for a distance of 10 meters (the 10‐meter walk test (10MWT)) and kinematic assessment with a motion analysis system (Watson 2002). All studies included comfortable walking speed as a primary outcome measured with the 10MWT, Bethoux 2014; Kluding 2013, or with a motion analysis system, Kottink 2007; Sheffler 2013a. We pooled comfortable walking speed data because both measures assessed speed with the same unit (m/s) and the same distance. Only one study assessed fast walking speed, also using the 10MWT (Kluding 2013). Other outcomes related to activities involving limbs were assessed with TUG, Bethoux 2014; Kluding 2013, and mEFAP, Bethoux 2014; Sheffler 2013a. We included the data for outcomes related to participation in the Stroke‐Specific Quality of Life (SSQoL) (Williams 1999), reported in two studies (Bethoux 2014; Sheffler 2013a). It can therefore be said that all studies included outcomes related to participation, but the scale used varied among studies: SF‐36 (Kottink 2007), SIS (Bethoux 2014; Kluding 2013), and the SSQoL previously mentioned. The total value of the quality of life scale was only presented in studies that reported SSQoL. One study reported the values of each domain of the scale SF‐36 separately as well as the Physical Component Summary (PCS‐36) and Mental Component Summary (MCS‐36) (Kottink 2007). Kluding 2013 reported the values of some domains of the SIS. Two studies assessed the exercise capacity‐related outcome using the 6MWT (Bethoux 2014; Kluding 2013), and the balance‐related outcomes using the BBS, Bethoux 2014; Kluding 2013, and FRT, Kluding 2013. Considering that the only study that described FRT also evaluated BBS, and that functional reach is an outcome assessed in the BBS as well, we decided to present only data for BBS in outcomes related to balance.
| Study ID (report) | Independence in ADL | Activities involving limbs | Participation scales of HRQoL | Exercise capacity | Balance |
| (Bethoux 2014; 6‐month assessment) | ‐ | Comfortable walking speed measured by 10MWT, TUG, mEFAP | SSQoL (total value); SIS (all domains) | 6MWT | BBS |
| (Bethoux 2015; 12‐month assessment) | ‐ | Comfortable walking speed measured by 10MWT, mEFAP | ‐ | 6MWT | ‐ |
| ‐ | Comfortable and fast walking speed measured by 10MWT, TUG | SIS (ADL/iADL, Mobility, Participation domains) | 6MWT | BBS; FRT | |
| Kottink 2007 (Kottink 2007; Kottink 2008; Kottink 2010; Kottink 2012) | ‐ | Comfortable walking speed motion analysis system | SF‐36 (all domains) | ‐ | ‐ |
| ‐ | Comfortable walking speed measured by motion analysis system, mEFAP | SSQoL (total value) | ‐ | ‐ |
6MWT: 6‐minute walk test
10MWT: 10‐meter walk test
ADL: activities of daily living
BBS: Berg Balance Scale
FRT: Functional Reach Test
HRQoL: health‐related quality of life
iADL: instrumental activities of daily living
mEFAP: modified Emory Functional Ambulation Profile
SF‐36: 36‐item Short Form Health Survey
SIS: Stroke Impact Scale
SSQoL: Stroke‐Specific Quality of Life
TUG: Timed Up and Go test
All studies reported outcomes at baseline and at intervention end (endpoint values), except for Kluding 2013, which presented outcomes as the change from baseline values. However, we were able to extract all outcomes from Kluding 2013 because they were presented in an reference associated with the study (Dunning 2015). Only one study had repeated observations of participants, with an assessment of outcomes in the middle part of the intervention period (Bethoux 2014), and only one study assessed follow‐up 12 and 24 weeks post‐treatment (Sheffler 2013a).
All of these outcomes except those related to participation/quality of life were assessed either while the participants were using MN or while they were not using MN. Two studies performed final assessments while participants were using MN: one study investigated the training effect of the intervention, so the baseline assessment was performed while the participants used MN (Bethoux 2014), while the other study investigated the total effect of intervention, so the baseline assessment was performed while the participants were not using MN (Kottink 2007). One study assessed the training effect of MN, thus all assessments were performed without the use of MN (Sheffler 2013a). One study evaluated both the training and therapeutic effect, so assessment of each outcome was conducted while participants were using MN and while they were not using MN (Kluding 2013).
All studies reported withdrawal or dropouts for several reasons during the intervention period (Table 3). Only two studies reported data for serious adverse events related to the intervention (Bethoux 2014; Kluding 2013). Although Sheffler 2013a included data for serious adverse events in its trial registry, it was not clear which data were related to the intervention, therefore we did not extract these data. Three studies presented data for falls (Bethoux 2014; Kluding 2013; Sheffler 2013a). Kottink 2007 did not mention any adverse event data in its reports.
| Study ID (report) | Motor neuroprosthesis | Another assistive technology device |
| (Bethoux 2014; 6‐month assessment)* | 2 deceased; 25 non‐compliance with protocol; 15 participant request; 7 medical reasons; 4 lost to follow‐up; 2 investigator withdrew | 2 deceased; 13 non‐compliance with protocol; 18 participant request; 4 medical reasons; 3 lost to follow‐up; 1 investigator withdrew |
| (Bethoux 2015; 12‐month assessment)** | 2 deceased; 25 non‐compliance with protocol; 16 participant request; 7 medical reasons; 6 lost to follow‐up; 6 investigator withdrew | 3 deceased; 15 non‐compliance with protocol; 19 participant request; 4 medical reasons; 6 lost to follow‐up; 2 investigators withdrew |
| 2 lost to follow‐up; 23 discontinued intervention | 1 lost to follow‐up; 9 discontinued intervention | |
| Kottink 2007 (Kottink 2007; Kottink 2008; Kottink 2010; Kottink 2012) | 1 technical defect in the epineural electrode | 1 psychological issues not related to the study |
| 6 non‐medical reasons; 1 medical reason
| 2 non‐medical reasons; 3 medical reasons
|
*Bethoux 2014 (six‐month assessment) corresponds to the first report of Bethoux 2014 study whose assessment was made after six months of motor neuroprosthesis use.
**Bethoux 2014 (12‐month assessment) corresponds to the second report of Bethoux 2014 study whose assessment was made after 12 months of motor neuroprosthesis use.
Excluded studies
We excluded 68 studies (see Characteristics of excluded studies and Figure 1 for further information).
Studies awaiting assessment
Four studies are still awaiting assessment. We were unable to contact the principal investigator of one study because our email was undelivered (Wright 2004). We contacted the principal investigators of ISRCTN91639560 and NCT03574623 to learn if the electrical stimulation was used as an orthosis in the home or community context, but we have not received a response to date. We also contacted the principal investigator of UMIN000018648, who stated that the electrical stimulation protocol was applied at home. To date, we have received no response clarifying whether the study has already been published. See Characteristics of studies awaiting classification.
Ongoing studies
We identified one ongoing study that appeared to be eligible for inclusion (Ghedira 2014). See Characteristics of ongoing studies.
Risk of bias in included studies
'Risk of bias' assessments are presented for individual studies in Characteristics of included studies. See Figure 2 and Figure 3 for summaries of the results.
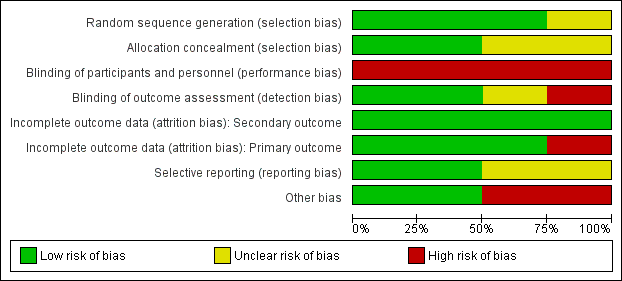
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
Generation of randomization sequence was conducted correctly in three studies (Bethoux 2014; Kluding 2013; Sheffler 2013a), which we deemed to be at low risk of bias. One study did not clearly report if the method used for selecting the blocks described a random component in the sequence generation process, therefore we classified it as at unclear risk of bias (Kottink 2007).
Allocation concealment
We judged two trials to be at low risk of bias for allocation concealment (Bethoux 2014; Kottink 2007). We considered the other two included studies to be at unclear risk of bias: Kluding 2013 because the method of concealment was not described in sufficient detail to permit a definitive judgement, and Sheffler 2013a because the investigators did not report whether the envelopes used were sealed or not.
Blinding
Blinding of participants and personnel
None of the studies utilized blinded participants or personnel because of the nature of the intervention. We judged all studies as having a high risk of detection bias.
Blinding of outcome assessment
We assessed two studies where the outcome assessors were blinded to treatment allocation as at low risk of detection bias (Kluding 2013; Sheffler 2013a). One study had a high risk of detection bias because outcome assessments were unblinded (Bethoux 2014), whereas another study provided insufficient information to permit an assessment of level of bias and was therefore classified as at unclear risk of bias (Kottink 2007).
Incomplete outcome data
All studies reported withdrawals or dropouts, but we classified them as having a low risk of bias considering that ITT analyses were performed. Only Kottink 2007 did not perform ITT analysis for the primary outcome, hence we classified it as having a high risk of detection bias.
Selective reporting
We classified two studies as having a low risk of selective reporting because they had protocols available, and all of the prespecified outcomes were reported in the prespecified way (Kluding 2013; Sheffler 2013a). We considered two studies as having an unclear risk of selective reporting: Bethoux 2014 included a secondary variable in the study that was not prespecified in the protocol, and Kottink 2007 provided insufficient information to permit a judgement.
Other potential sources of bias
We assessed two studies that were sponsored by manufacturers of MN as having a high risk of bias (Bethoux 2014; Kluding 2013). No other bias was detected in Kottink 2007 and Sheffler 2013a.
Effects of interventions
See summary of findings Table for the main comparison.
We included all four studies in the quantitative analysis (Bethoux 2014; Kluding 2013; Kottink 2007; Sheffler 2013a). All studies compared MN versus another assistive technology device. The outcomes used in these studies were: activities involving limbs (Bethoux 2014; Kluding 2013; Kottink 2007; Sheffler 2013a); participation scales of HRQoL (Bethoux 2014; Kluding 2013; Kottink 2007; Sheffler 2013a); exercise capacity (Bethoux 2014; Kluding 2013); balance (Bethoux 2014; Kluding 2013); number of dropouts (Bethoux 2014; Kluding 2013; Kottink 2007; Sheffler 2013a); serious adverse events related to intervention (Bethoux 2014; Kluding 2013); and falls (Bethoux 2014; Kluding 2013; Sheffler 2013a).
We contacted the principal investigator of Kottink 2007 to request data for outcomes of the 6MWT and walking speed assessed with ITT analysis with and without devices, which were presented in Kottink 2007 and Kottink 2008 only as figures. However, we could not obtain these data, so we excluded the 6MWT results of this study from the quantitative analysis and extracted the walking numerical speed data analyzed without ITT presented in Kottink 2012.
We considered for meta‐analysis only Kluding 2013 assessments performed with the participants using a device (MN or another assistive device). The study of Bethoux 2014 had two publications that performed an assessment at different time intervals during the intervention period (repeated observations of the participants). In order to gain a better understanding of the effect of MN on different time periods, we decided to include data from both Bethoux 2014 publications in the meta‐analysis.
Kluding 2013 used two measures to assess walking speed: comfortable and fast walking speed. As all studies assessed comfortable walking speed, and no study assessed fast walking speed, we decided to include only the Kluding 2013 data for comfortable walking speed in the meta‐analysis.
Comparison: motor neuroprosthesis versus another assistive technology device
Independence in ADL
None of the four included studies reported outcomes related to independence in ADL.
Activities involving limbs
Walking speed until six months of device use
Two studies (605 participants) measured walking speed until six months of device use. One study performed a final assessment with participants using MN; the other study did not perform a final assessment with participants using MN. We found low‐certainty evidence that the control intervention (another assistive technology device) had a greater effect than MN on walking speed in six months of device use: the mean difference (MD) (random‐effects model) was ‐0.05 m/s (95% confidence interval (CI) −0.10 to −0.00; P = 0.03; I2 = 0%; Analysis 1.1). But considering that the minimal important difference for comfortable walking speed in chronic stroke participant is 0.2 m/s, this effect was not enough to be clinically meaningful.
We conducted sensitivity analysis by excluding Bethoux 2014 since this study presented a high risk of bias in the blinding of outcome assessment. The sensitivity analysis showed that there is low certainty that the effect of the control intervention on improving walking speed is no longer present (MD −0.07 m/s, 95% CI −0.16 to 0.02; P = 0.13; I2 = 0%; 110 participants; Table 4).
| Outcome | Study ID (report) | Analysis results |
| Activities involving limbs: walking speed until 6 months of device use | MD −0.07, 95% CI −0.16 to 0.02; P = 0.13; participants = 110; I2 = 0% | |
| Activities involving limbs: walking speed between 6 and 12 months of device use | MD 0.04, 95% CI −0.09 to 0.16; P = 0.57; participants = 218; I2 = 52% | |
| Activities involving limbs: TUG | MD 0.88, 95% CI −6.36 to 8.12; P = 0.81; participants = 197; I2 = 0% | |
| Activities involving limbs: mEFAP | MD 14.45, 95% CI −13.97 to 42.87; P = 0.32; participants = 110; I2 = 0% | |
| Participation scale of HRQoL | SMD 0.60, 95% CI −0.39 to 1.59; P = 0.24; participants = 137; I2 = 79% | |
| Exercise capacity: 6MWT | MD −8.39, 95% CI −38.01 to 21.23; P = 0.58; participants = 197; I2 = 0% | |
| Balance: BBS | MD −1.50, 95% CI −4.38 to 1.38; P = 0.31; participants = 197; I2 = 0% |
6MWT: 6‐minute walk test
BBS: Berg Balance Scale
CI: confidence interval
HRQoL: health‐related quality of life
MD: mean difference
mEFAP: modified Emory Functional Ambulation Profile
SMD: standardized mean difference
TUG: Timed Up and Go test
Walking speed between six and 12 months of device use
Three studies (713 participants) measured the walking speed of participants between six and 12 months of device use. All three studies performed final assessments while the participants used MN. There is low‐certainty evidence that MN is no more beneficial than another assistive device on walking speed between six and 12 months of device use (MD 0.00 m/s, 95% CI −0.05 to 0.05; P = 0.93; I2 = 17%; Analysis 1.2).
We conducted a sensitivity analysis excluding the study that was at high risk of bias for blinding of outcome assessment, which highlighted that we are very uncertain whether MN is more beneficial than another assistive device on walking speed between six and 12 months of device use (Table 4). Kottink 2007 had a high risk of bias for incomplete outcome data relating to this outcome, but as we did not consider this 'Risk of bias' domain on sensitivity analysis, we decided to maintain these data. However, caution should be used in interpreting these data as their results were visually different from data for other studies.
Walking speed: subgroup analysis for type of MN
We analyzed subgroups considering the type of MN used (823 participants). We compared studies in which the MN used consisted of a superficial device with those in which MN consisted of an implantable device. For this subgroup analysis, we considered the walking speed assessment performed in Bethoux 2014 at 12 months. The test for subgroup differences (between surface MN and implantable MN) revealed no significant difference (P = 0.09; I2 = 65.1%; Analysis 1.3).
TUG at the end of the intervention phase
Two studies (692 participants) assessed TUG. In both studies, the final assessments of participants were performed while they were using MN. As Bethoux 2014 presented TUG assessment only for six months, these data were included for TUG analysis. There is moderate‐certainty evidence that MN is no more beneficial than another assistive device on TUG (MD 0.51 s, 95% CI −4.41 to 5.43; P = 0.84; I2 = 0%; Analysis 1.4).
The sensitivity analysis performed by excluding Bethoux 2014, which was at high risk of bias for blinding of outcome assessment, highlighted that more information is required to be certain as to whether MN is no more beneficial than another assistive device on TUG (Table 4).
mEFAP at the end of the intervention phase
Two studies (605 participants) assessed mEFAP. One study performed a final assessment of participants while using MN, whereas the other study did not perform a final assessment of the participants while using MN. As Bethoux 2014 presented mEFAP assessment only for six months, these data were included for mEFAP analysis. There is low‐certainty evidence that MN is no more beneficial than another assistive device on mEFAP (MD 14.77 s, 95% CI −12.52 to 42.06; P = 0.29; I2 = 0%; Analysis 1.5).
The sensitivity analysis performed by excluding Bethoux 2014 data highlighted that more information is required to be certain as to whether MN is no more beneficial than another assistive device on mEFAP (Table 4).
Participation scales of HRQoL
All studies assessed at least one participation scale of HRQoL. There was heterogeneity between the selection of scales of HRQoL as well as their presentation (some scales presented the total value of a full version, while others presented the value of some domains separately). In light of this, we decided to include only scales or scale components that represented the whole domain of a scale and to combine data from these different scales using standardised mean difference (SMD) as stated in our protocol. Bethoux 2014 presented two participation scales of HRQoL at six months' assessment; we decided to use the SSQoL for analysis, as the total value was available. We did not include measures that represented only some domains of a scale of HRQoL. We included data from three studies (632 participants) that assessed HRQoL with a participation scale. There is very low‐certainty evidence that MN is no more beneficial than another assistive device on participation scale of HRQoL. The random‐effects pooled estimate for all trials was SMD 0.26 (95% CI −0.22 to 0.74; P = 0.28; I2 = 77%; Analysis 1.6).
The sensitivity analysis performed by excluding Bethoux 2014 data highlighted that we are very uncertain as to whether MN is any more beneficial than another assistive device on participation scale of HRQoL, although the magnitude of the effect changed from a small effect (Analysis 1.6) to a moderate effect based on Cohen's rules of thumb (Table 4).
Exercise capacity
Two studies (692 participants) assessed exercise capacity using the 6MWT. Both studies performed final assessments on participants using MN. As Bethoux 2014 presented 6MWT assessment only for six months, these data were included for 6MWT analysis. There is low‐certainty evidence that MN is no more beneficial than another assistive device on exercise capacity (MD −9.03 m, 95% CI −26.87 to 8.81; P = 0.32; I2 = 0%; Analysis 1.7).
The sensitivity analysis performed by excluding Bethoux 2014, which was at high risk of bias for blinding of outcome assessment, highlighted that more information is required to be certain as to whether MN is no more beneficial than another assistive device on exercise capacity (Table 4).
Balance
Two studies (692 participants) assessed balance using the BBS. Both studies performed final assessments on participants using MN. There is moderate‐certainty evidence that MN is no more beneficial than another assistive device on balance (MD −0.34, 95% CI −1.96 to 1.28; P = 0.68; I2 = 0%; Analysis 1.8).
The sensitivity analysis excluding Bethoux 2014 data highlighted that we are very uncertain as to whether MN is more beneficial than another assistive device on balance (Table 4).
Number of dropouts
All studies (829 participants) reported dropouts during the intervention period; the reasons for dropouts are described in detail for each trial in Table 3. For this outcome, we considered the number of dropouts for Bethoux 2014 at 12 months. There is low‐certainty evidence that the risk of participants dropping out of the study was increased by 51% with MN when compared with control. The risk ratio (RR) (random‐effects model) for dropouts was 1.48 (95% CI 1.11 to 1.97; P = 0.007; I2 = 0%; Analysis 1.9). The highest dropout rate occurred in Bethoux 2014 (12 months of intervention): 26% in the MN group (62 dropouts out of 242 participants) and 19% in the control group (49 dropouts out of 253 participants). The lowest dropout rate occurred in Kottink 2007: 7% in the MN group (1 dropout out of 14 participants) and 8% in the control group (1 dropout out of 13 participants).
Adverse events
Only one study reported deaths during the intervention period (Bethoux 2014). The death rate was less than 1% for both groups in the six‐month intervention report of Bethoux 2014. In the MN group, deaths were due to a nervous system disorder or renal and urinary disorders (2 deaths of 242 participants), whereas deaths in the control group were due to nervous system disorders (2 deaths of 253 participants). In the 12‐month intervention report of the Bethoux 2014 study (Bethoux 2015), the death rate was maintained in the MN group (less than 1%; 2 deaths of 242 participants), but was increased by 1% in the control group (3 deaths of 253 participants).
Serious adverse events related to intervention
Two studies (692 participants) provided data on serious adverse events related to the intervention during the treatment period. Bethoux 2014 considered serious adverse events related to device use as serious falls; as this study presented this outcome in both six‐ and 12‐month reports, we decided to include the longer assessment (12 months). Overall, there is low‐certainty evidence that the use of MN in people after stroke does not have an effect on risk of adverse events during the treatment period when compared to other assistive devices: RR (random‐effects model) of 0.35 (95% CI 0.04 to 3.33; P = 0.36; I2 = 0%; Analysis 1.10).
Falls
Three studies (802 participants) provided data on falls. There is moderate‐certainty evidence that the use of MN in people after stroke does not have an effect on risk of falls during the whole treatment period when compared to other assistive devices: RR (random‐effects model) of 1.20 (95% CI 0.92 to 1.55; P = 0.08; I2 = 33%; Analysis 1.11).
Discussion
Summary of main results
The aim of this review was to assess the effects of MN for improving independence in ADL, activities involving limbs, participation scales of HRQoL, exercise capacity, balance, and adverse events in people after stroke. We included four studies (9 articles) involving a total of 831 participants that compared MN with another assistive technology device. All studies addressed MN application directed to the lower limbs, specifically in the peroneal nerve, to correct foot drop during walking activities in the home or community context, such as an orthosis. In all studies, another assistive technology device (control intervention) used was ankle‐foot orthosis (AFO). No studies compared MN with no treatment or with MN without electrical stimulus. Overall, the certainty of the evidence for outcomes ranged from moderate to very low. The main results are presented in the summary of findings Table for the main comparison.
No studies reported outcomes related to independence in ADL, so we could not assess the evidence of the effects of MN on ADL. Although we found low‐certainty evidence that AFO had an effect on improving walking speed until six months of device use (MD −0.05 m/s, 95% CI −0.10 to −0.00), this effect did not appear to be clinically relevant, given that the minimally significant difference for comfortable walking speed in chronic stroke participants is 0.2 m/s (Hiengkaew 2012). Furthermore, when we excluded one study at high risk of bias related to unblinded outcome assessment from the meta‐analysis, this effect was absent, that is both strategies (MN and AFO) proved to have similar effects on walking speed until six months of device use. We found low‐certainty evidence that there is no beneficial effect of MN, when compared to another assistive device, for walking speed between six and 12 months of device use. We also observed no difference in effect on walking speed between the surface and implantable MN. We have very little confidence in our estimate of the effect of implantable MN because only one small study with a high risk of bias for incomplete outcome data and unclear risk for random sequence generation, blinding of outcome assessment, and selective reporting investigated its effect. For this reason, the exact effect of implantable MN is likely to be substantially different from the estimate of its effect.
We found moderate‐certainty evidence that MN has no effect on balance measured with BBS and activities involving limbs such as TUG. We found low‐certainty evidence that MN has no effect on exercise capacity measured with 6MWT and activities involving limbs such as mEFAP. Although there were some limited moderate‐ or low‐certainty evidence, this apparent lack of effect (MN is not any different to AFO) should be interpreted with caution due to the high risk of bias (outcome assessors were unblinded) in the largest study and the broad confidence intervals these outcomes presented. Regarding the secondary outcome measure participation scale of HRQoL, we found very low‐certainty evidence that MN does not differ in effect compared to AFO. However, due to the quality of evidence, any potential benefits of the interventions remain uncertain.
We found low‐certainty evidence that the use of MN in people after stroke increases the risk of participants dropping out of the study. However, when considering safety, we found that the number of falls (moderate‐certainty evidence) and the number of serious adverse events (low‐certainty evidence) were not increased related to the use of MN. We considered serious adverse events related to device use as serious falls. Limited data contributed to the results for this outcome, and due to the wide confidence intervals, further information is needed.
Overall completeness and applicability of evidence
Our search results identified a considerable number of studies that applied MN devices in a clinical context for ADL training or even as a home‐based program to increase the dose therapy usually done with cyclic stimulation. As this was not the focus of this review, we excluded these studies. We found only four studies (nine articles) that focused on the effects of MN for improving activities and participation in people after stroke, considering its use as an environmental facilitator to enhance the performance of functional abilities in the home or community context. While the number of such studies was small, the number of participants was not (831 participants). We also found trials that used the brain‐machine interface (BMI) to control the signals of electrical stimulation devices; however, no study met the inclusion criteria of this review, especially with regard to study design.
The results of this review indicate that little is known about the effect of MN and that further information is needed. There is currently insufficient high‐certainty evidence to make conclusions about the benefits or harms of MN. Overall, there is no substantial evidence that MN has an effect on improving activities involving limbs, participation scales of HRQoL, exercise capacity, balance, and adverse events. The only possible effect found was that the use of MN in people after stroke increased the risk of participants dropping out the study for several reasons, which included participant request to discontinue the intervention, lost to follow‐up, medical reasons, non‐compliance with protocol, and others as mentioned in Table 3.
Considering that we only included studies with MN applied on the lower limb, these results cannot be generalized to include improvement of activities related to the upper limb. Even considering MN directed to the lower limb, the following factors produce uncertainty.
-
The majority of interventions were focused on single‐channel surface MN for stimulation of the peroneal nerve.
-
All interventions consisted of MN applied on the peroneal nerve to facilitate walking activities.
-
The activities performed and their duration throughout the conditioning protocols varied between studies. These conditioning protocols prepared participants for MN use for a long period during the day, and all studies included them in the first few weeks of the intervention period.
As all the included studies used non‐biological signals such as force/pressure and inertial sensors as an input to trigger the electrical stimulus, the results of this review cannot be generalized for MN triggered with electromyography or electroencephalography signals. Since we considered the use of MN as an orthosis, the only comparison we found was MN versus another assistive technology device. In this way, this review provides incipient data that can help in decision making on the use of MN or AFO, not considering other assistive devices or MN versus no device.
Since none of the studies included participants within three months of stroke, we did not assess if the effects of MN can be generalized for individuals in the early stages of stroke. In addition, the mean walking speed at baseline assessment varied widely between studies that assessed superficial and implantable MN devices.
The most common outcome was walking speed, which was part of activities involving limbs, but no study assessed other important outcomes for people with stroke, such as independence in ADL. Consequently, there is a need to monitor this outcome in future updates to determine the effect of MN on independence in ADL.
Additionally, MN using an environmental facilitator to enhance the performance of functional abilities in the home or community context could possibly create additional costs of rehabilitation after stroke due to the nature of the device and because the structure requires experts to fit, adapt, and train users to use the device; researchers did not quantify the costs of its application. In this context, the results of this review seem to be quite generalizable for industrialized countries that have services and facilities available for the application and adaptation of MN to users.
Quality of the evidence
According to the GRADE criteria, the certainty of evidence ranged from moderate to very low due to the small number of studies included in the review, which led to wide confidence intervals for seven out of the nine included outcomes, and the presence of some judgements of high risk and unclear risk of detection bias in the included studies (see summary of findings Table for the main comparison). Three out of four trials showed a high risk of bias for one or two of these 'Risk of bias' domains: blinding of outcome assessment, incomplete outcome data for primary outcome, or other potential sources of bias. The outcome assessors in the largest study were not blinded. The two most significant studies, which represented 83% of included participants, were multicenter and sponsored by manufacturers of MN; because of this, we classified them as being at high risk of other potential sources of bias. However, these studies adopted some precautions that may have minimized the presence of other biases, such as clearly described adverse events related and not related to MN and AFO groups on trial registries, sufficient methodological detail presented with prior protocol publication, or even inclusion of an independent Clinical Events Committee to adjudicate serious adverse events and their connection to the device. All studies had a high risk of bias for blinding participants or personnel because of the nature of the intervention. Poor reporting and lack of clarification from the authors led us to assess studies as being at unclear risk of bias for important criteria such as random sequence generation, allocation concealment, blinding of outcome assessment, and selective reporting. Nevertheless, most of the results were consistent (low heterogeneity).
Potential biases in the review process
Given our extensive searching process, we are confident that our search strategy was comprehensive and detailed; this strategy included searching in databases, electronic registries, websites, and a careful search of grey literature. We thus expect that we have identified all relevant studies; however, there is a small possibility that we failed to identify additional (published or unpublished) papers. Two review authors independently assessed the studies and obtained and extracted data, with a third or fourth review author available to resolve disagreements as needed, thereby minimizing bias; several subjective judgements were required during the review process.
We decided to downgrade our assessment of blinding of participants and personnel, even while knowing that such blinding does not seem feasible for the type of intervention. Another limitation of this review is that some of the studies had methodological shortcomings, such as random sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data for the primary outcome, and selective reporting. According to the Cochrane Handbook for Systematic Reviews of Interventions, these biases can lead to overestimation of the intervention effect (Higgins 2011b).
Agreements and disagreements with other studies or reviews
We found only one systematic review with meta‐analysis of randomized controlled trials on MN for improving activities and participation in people after the stroke that considered MN use as an environmental facilitator to enhance the performance of functional abilities (Prenton 2016). Although that review focused on MN directed to peroneal nerve stimulation as well, it differed from the current review by including studies that used MN in the ward environment, which did not represent a real‐life context. The authors referred to the MN device with the use of FES nomenclature. As the comparison included in Prenton 2016 was MN versus AFO on the date of the final assessment of 10‐meter walking speed and 6MWT, its results were similar to the results of the current review, which indicated that AFO had positive orthotic effects on walking that are equivalent to FES for foot drop on stroke participants. Similarly, researchers also found little difference in favor of AFO for evaluations performed between 12 and 13 weeks of device use, but they did not perform sensitivity analysis. Prenton 2016 additionally performed a meta‐analysis for the mobility domain of the Stroke Impact Scale and found no difference between the two interventions, but did not assess the dropout rate between studies.
As far as we know, no systematic review has evaluated the number of dropouts related of MN use with meta‐analysis. There are only separated reports that assess compliance and preference for MN. Among the studies included in this review, only Kluding 2013 evaluated satisfaction related to the use of the devices. The authors of this study used a satisfaction survey that evaluates 12 items with a total range of scores from 0 to 24, with a higher number indicating greater satisfaction with the device. Although there was a higher dropout rate in the MN group (25 of 99 participants) than in the AFO group (10 of 98 participants), the satisfaction was higher in the MN group than in the AFO group. Everaert 2013 recorded the users' preference and asked participants to indicate the reasons for their preference at the end of each arm of a cross‐over study. The majority of participants preferred the MN, the reasons most frequently mentioned being function, confidence, comfort, convenience, easy donning and doffing, and safety.
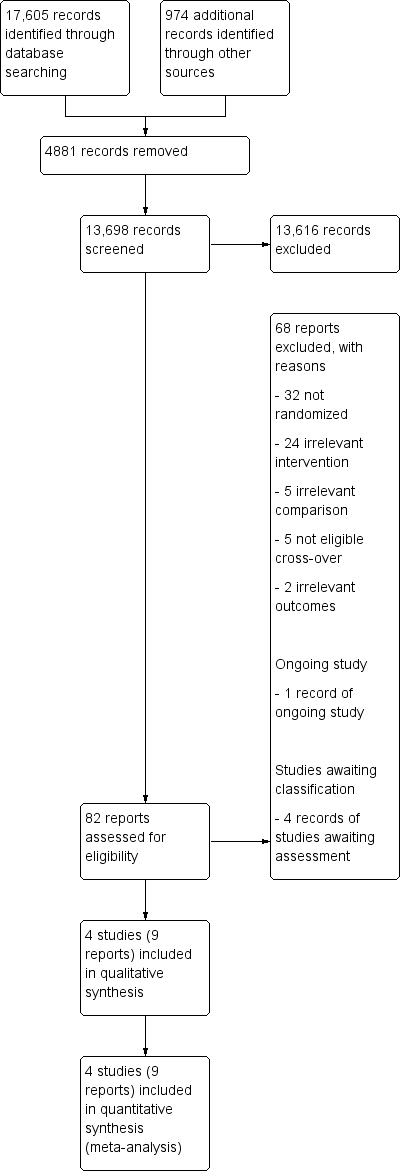
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
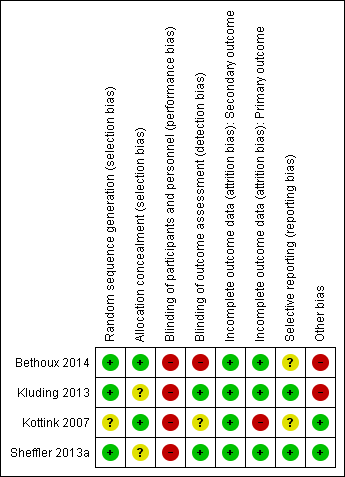
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
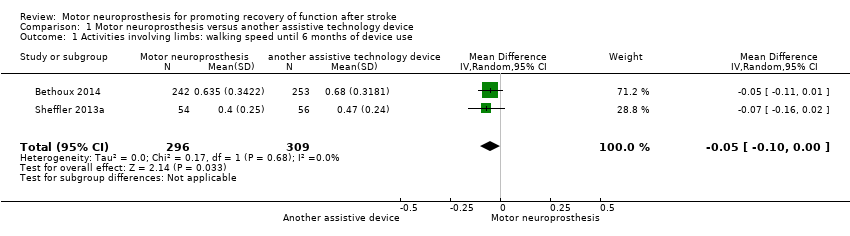
Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 1 Activities involving limbs: walking speed until 6 months of device use.

Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 2 Activities involving limbs: walking speed between 6 and 12 months of device use.

Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 3 Activities involving limbs: walking speed.

Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 4 Activities involving limbs: TUG.

Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 5 Activities involving limbs: mEFAP.

Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 6 Participation scale of HRQoL.
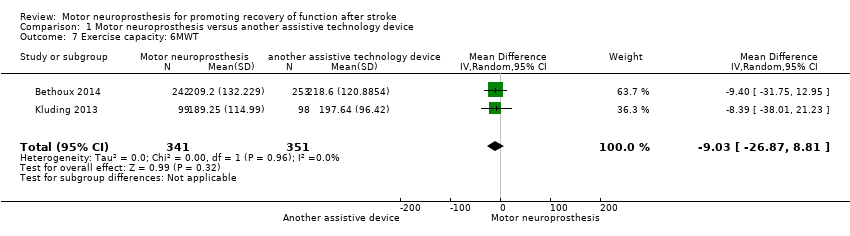
Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 7 Exercise capacity: 6MWT.
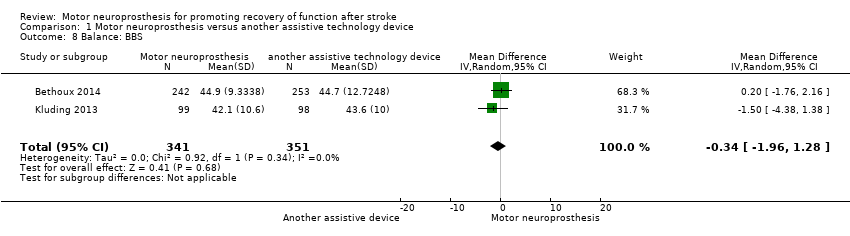
Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 8 Balance: BBS.
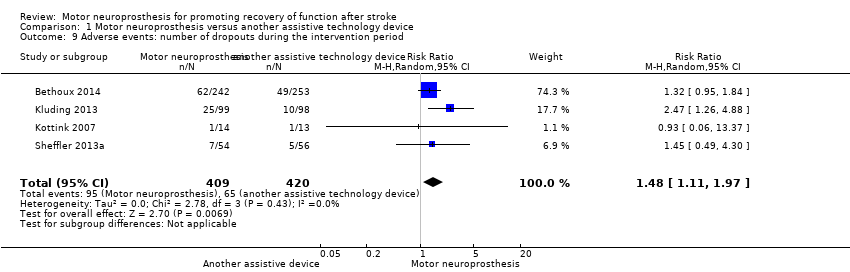
Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 9 Adverse events: number of dropouts during the intervention period.
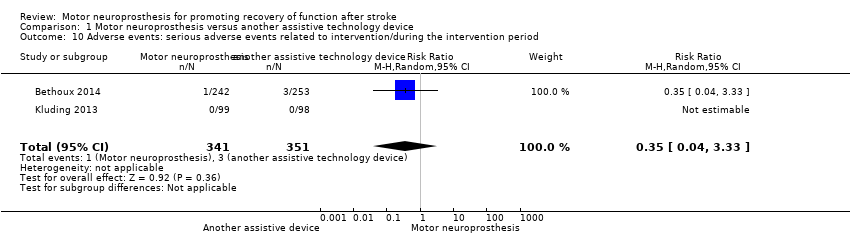
Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 10 Adverse events: serious adverse events related to intervention/during the intervention period.

Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 11 Adverse events: falls.
| Motor neuroprosthesis compared to another assistive technology device for promoting recovery of function after stroke | |||||||
| Patient or population: promoting recovery of function after stroke | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | ||
| Risk with another assistive technology device | Risk with motor neuroprosthesis | ||||||
| Independence in activities of daily living | (No data) | ‐ | ‐ | No studies | Insufficient evidence | No trials measured this outcome. | |
| Activities involving limbs | Walking speed until 6 months of device use (m/s) timed measures at the end of treatment | The mean walking speed in the control group was on average 0.58 m/s. | 0.05 mean difference lower | ‐ | 605 (2 RCTs) | ⊕⊕⊕⊝ | Minimal important difference for comfortable walking speed in chronic stroke participant is 0.2 m/s (Hiengkaew 2012). |
| Walking speed between 6 and 12 months of device use (m/s) timed measures at the end of treatment | The mean walking speed in the control group was on average 0.69 m/s. | 0 mean difference (0.05 lower to 0.05 higher) | ‐ | 713 (3 RCTs) | ⊕⊕⊝⊝ | Minimal important difference for comfortable walking speed in chronic stroke participant is 0.2 m/s (Hiengkaew 2012). | |
| TUG (s) timed measures at the end of treatment | The mean TUG in the control group was on average 27.57 s. | 0.51 mean difference higher (4.41 lower to 5.43 higher) on intervention group | ‐ | 692 | ⊕⊕⊕⊝ | ||
| mEFAP (s) timed measures at the end of treatment | The mean mEFAP in the control group was on average 286.43 s. | 14.77 mean difference higher (12.52 lower to 42.06 higher) on intervention group | ‐ | 605 | ⊕⊕⊝⊝ | ||
| Participation scales of HRQoL timed measures at the end of treatment | The mean participation scales of HRQoL in the control groups was NA.d | 0.26 standardized mean difference (0.22 lower to 0.74 higher) | ‐ | 632 (3 RCTs) | ⊕⊝⊝⊝ | Using Cohen's rules of thumb, 0.26 represents a small effect. | |
| Exercise capacity: 6MWT (m) timed measures at the end of treatment | The mean 6MWT in the control group was on average 208.12 m. | 9.03 mean difference lower (26.87 lower to 8.81 higher) on intervention group | ‐ | 692 | ⊕⊕⊝⊝ | There are no accurate indices of minimal important difference for 6MWT in people poststroke whose gait speed was ≥ 0.40 m/s (Fulk 2018). | |
| Balance: BBS timed measures at the end of treatment | The mean BBS in the control group was on average 44.15. | 0.34 mean difference lower (1.96 lower to 1.28 higher) on intervention group | ‐ | 692 | ⊕⊕⊕⊝ | Minimal detectable change for BBS in chronic stroke participant is 5 points (Hiengkaew 2012). | |
| Adverse events | Number of dropouts during the intervention period | Study population | RR 1.48 | 829 | ⊕⊕⊝⊝ | ||
| 96 per 1000** | 142 per 1000** | ||||||
| Falls | Study population | RR 1.20 | 802 | ⊕⊕⊕⊝ | |||
| 296 per 1000** | 355 per 1000** | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). **We used the median control group risk across studies. 6MWT: 6‐minute walk test; BBS: Berg Balance Scale; CI: confidence interval; HRQoL: health‐related quality of life; mEFAP: modified Emory Functional Ambulation Profile; NA: not applicable; RCT: randomized controlled trial; RR: risk ratio; TUG: Timed Up and Go test. | |||||||
| GRADE Working Group grades of evidence | |||||||
| aThe outcome assessors were not blinded in the larger study. | |||||||
| Study ID (report) | MN device | Duration of exposure to MN intervention | Conditioning protocol used to adapt participants to MN use | MN use/daily use for increasing the activities and participation in the home or community context |
| The MN used was the WalkAide device (Innovative Neurotronics, Austin, TX, USA). It is a commercially available, battery‐operated, single‐channel surface peroneal nerve stimulator that consists of a cuff worn around the proximal part of the lower leg, which holds the control module and surface electrodes. This device uses a tilt sensor and an accelerometer to trigger ankle dorsiflexion and control the timing and duration of peroneal nerve stimulation during the swing phase of gait to alleviate foot drop. | The duration of MN intervention was 12 months. The conditioning protocol occurred in the first 2 weeks, after which participants started daily use of MN device. | The first part consisted of fitting and programming the MN device as well as patient education performed by WalkAide‐certified orthotist or licensed physical therapist. The conditioning protocol included a 2‐week progressive wearing schedule of MN device. | Participants were instructed to wear MN device on a full‐time basis (quote: "ie, for all walking activities throughout the day"). | |
| The MN used was the NESS L300 device (Bioness Inc, Valencia, CA, USA). It is a commercially available, battery‐operated, single‐channel surface peroneal nerve stimulator that consists of a cuff with integrated stimulation unit and electrodes, a control unit, and an in‐shoe pressure sensor. The pressure sensor detects heel off and initial contact events during gait. It transmits wireless signals to the stimulation cuff, which initiates or pauses the stimulation of deep and superficial branches of the peroneal nerve via 2 surface electrodes that activate dorsiflexors and evertors muscles to ensure foot clearance during the swing phase of gait and prevent excessive ankle inversion during early stance. | The duration of MN intervention was 30 weeks. The conditioning protocol occurred in the first 6 weeks. Participants used the MN device all day between week 4 and week 30. | The first part consisted of initial fitting of the device, gait training, wearing schedule, home exercise program, and participant education based on manufacturer standardized protocols. For the first 3 weeks, participants followed the standard conditioning protocol (gradually increasing walking with the MN from 15 minutes each day to all‐day use). During the same period, participants also used the MN for cyclic stimulation while not walking in order to gradually strengthen and condition the muscles to avoid fatigue when using the device (Dunning 2013).* During the first 6 weeks of the study, participants also received 8 dose‐matched sessions of physical therapy. The first 2 to 4 therapy visits focused on education on device use, initial gait training, and an individualized home exercise program. The remaining physical therapy sessions focused on gait training (Kluding 2013). | Participants used the MN all day for ambulation (Dunning 2013).* | |
| The MN used was the STIMuSTEP device (FineTech Medical Ltd, Hertfordshire, UK). It is a commercially available, battery‐operated, 2‐channel implantable device composed of implantable components such as a stimulator, 2 leads, and bipolar intraneural electrodes, and non‐implantable components such as an external transmitter with a built‐in antenna and a pressure sensor. 1 electrode is surgically positioned under the epineurium of the superficial peroneal nerve and the other under the epineurium of the deep peroneal nerve. This device promotes the ankle dorsiflexion/eversion during gait to correct foot drop, and a pressure sensor placed inside the shoe determines the on and off switching of the stimulation. | The duration of MN intervention was 26 weeks. The intervention began with the surgical procedure for placement of the implant. After 2 weeks of the surgery, the wound was checked and first test stimulation took place. The conditioning protocol began at the third week, and all‐day MN use began at the sixth week. | Quote: "Two weeks after the surgery the wound was checked and a first test stimulation took place. In the third week, stimulation during walking was tested and the stimulator was taken home by the patient. The use of the stimulator was gradually increased over 2 weeks to prevent severe muscle pain and fatigue. After this period patients were allowed to use the system all day." | Participants were allowed to use the system all day between week 6 and week 26. | |
| The MN used to correct foot drop was the Odstock Dropped‐Foot Stimulator (ODFS) device (Odstock Medical Ltd, Salisbury Wiltshire, UK). The ODFS is a commercially available, battery‐operated, single‐channel surface peroneal nerve stimulator consisting of an electrical stimulator, a control module, pressure sensors, and surface electrodes. The stimulation is triggered by an insole pressure‐sensing foot switch that detects heel rise at pre‐swing. | The duration of MN intervention was 12 weeks. The conditioning protocol occurred over the 12 weeks. Daily MN use began once device safety was demonstrated by participants. | In the first 5 weeks the Functional Training phase (2 x 1‐hour sessions per week) took place, in which participants were trained to use MN device for home and community mobility with an assistive device, if needed. Activities included passive and active range‐of‐motion exercises, lower extremity strengthening, standing balance and weight‐shifting activities to the affected limb with transition to least‐restrictive assistive device, and refinement of a reciprocal gait pattern. Exercises were done with multiple repetitions with an increase in difficulty and a decrease in cues, with and without the MN device, as appropriate. In the last 7 weeks the Post‐Functional Training Phase (3 x 1‐hour sessions) took place, in which device function, application, and usage guidelines were reviewed with each participant to maximize MN compliance. | The article did not explicitly mention when participants started all‐day MN use, but reported that as soon as participants demonstrated safe use of the device, it was used up to 8 hours per day. | |
| MN: motor neuroprosthesis *Dunning 2013 corresponds to the published protocol of the study Kluding 2013. | ||||
| Study ID (report) | Independence in ADL | Activities involving limbs | Participation scales of HRQoL | Exercise capacity | Balance |
| (Bethoux 2014; 6‐month assessment) | ‐ | Comfortable walking speed measured by 10MWT, TUG, mEFAP | SSQoL (total value); SIS (all domains) | 6MWT | BBS |
| (Bethoux 2015; 12‐month assessment) | ‐ | Comfortable walking speed measured by 10MWT, mEFAP | ‐ | 6MWT | ‐ |
| ‐ | Comfortable and fast walking speed measured by 10MWT, TUG | SIS (ADL/iADL, Mobility, Participation domains) | 6MWT | BBS; FRT | |
| Kottink 2007 (Kottink 2007; Kottink 2008; Kottink 2010; Kottink 2012) | ‐ | Comfortable walking speed motion analysis system | SF‐36 (all domains) | ‐ | ‐ |
| ‐ | Comfortable walking speed measured by motion analysis system, mEFAP | SSQoL (total value) | ‐ | ‐ | |
| 6MWT: 6‐minute walk test | |||||
| Study ID (report) | Motor neuroprosthesis | Another assistive technology device |
| (Bethoux 2014; 6‐month assessment)* | 2 deceased; 25 non‐compliance with protocol; 15 participant request; 7 medical reasons; 4 lost to follow‐up; 2 investigator withdrew | 2 deceased; 13 non‐compliance with protocol; 18 participant request; 4 medical reasons; 3 lost to follow‐up; 1 investigator withdrew |
| (Bethoux 2015; 12‐month assessment)** | 2 deceased; 25 non‐compliance with protocol; 16 participant request; 7 medical reasons; 6 lost to follow‐up; 6 investigator withdrew | 3 deceased; 15 non‐compliance with protocol; 19 participant request; 4 medical reasons; 6 lost to follow‐up; 2 investigators withdrew |
| 2 lost to follow‐up; 23 discontinued intervention | 1 lost to follow‐up; 9 discontinued intervention | |
| Kottink 2007 (Kottink 2007; Kottink 2008; Kottink 2010; Kottink 2012) | 1 technical defect in the epineural electrode | 1 psychological issues not related to the study |
| 6 non‐medical reasons; 1 medical reason
| 2 non‐medical reasons; 3 medical reasons
| |
| *Bethoux 2014 (six‐month assessment) corresponds to the first report of Bethoux 2014 study whose assessment was made after six months of motor neuroprosthesis use. | ||
| Outcome | Study ID (report) | Analysis results |
| Activities involving limbs: walking speed until 6 months of device use | MD −0.07, 95% CI −0.16 to 0.02; P = 0.13; participants = 110; I2 = 0% | |
| Activities involving limbs: walking speed between 6 and 12 months of device use | MD 0.04, 95% CI −0.09 to 0.16; P = 0.57; participants = 218; I2 = 52% | |
| Activities involving limbs: TUG | MD 0.88, 95% CI −6.36 to 8.12; P = 0.81; participants = 197; I2 = 0% | |
| Activities involving limbs: mEFAP | MD 14.45, 95% CI −13.97 to 42.87; P = 0.32; participants = 110; I2 = 0% | |
| Participation scale of HRQoL | SMD 0.60, 95% CI −0.39 to 1.59; P = 0.24; participants = 137; I2 = 79% | |
| Exercise capacity: 6MWT | MD −8.39, 95% CI −38.01 to 21.23; P = 0.58; participants = 197; I2 = 0% | |
| Balance: BBS | MD −1.50, 95% CI −4.38 to 1.38; P = 0.31; participants = 197; I2 = 0% | |
| 6MWT: 6‐minute walk test | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Activities involving limbs: walking speed until 6 months of device use Show forest plot | 2 | 605 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.10, ‐0.00] |
| 2 Activities involving limbs: walking speed between 6 and 12 months of device use Show forest plot | 3 | 713 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.05, 0.05] |
| 3 Activities involving limbs: walking speed Show forest plot | 4 | 823 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.06, 0.04] |
| 3.1 Surface MN | 3 | 802 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.06, 0.02] |
| 3.2 Implantable MN | 1 | 21 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.04, 0.28] |
| 4 Activities involving limbs: TUG Show forest plot | 2 | 692 | Mean Difference (IV, Random, 95% CI) | 0.51 [‐4.41, 5.43] |
| 5 Activities involving limbs: mEFAP Show forest plot | 2 | 605 | Mean Difference (IV, Random, 95% CI) | 14.77 [‐12.52, 42.06] |
| 6 Participation scale of HRQoL Show forest plot | 3 | 632 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.22, 0.74] |
| 7 Exercise capacity: 6MWT Show forest plot | 2 | 692 | Mean Difference (IV, Random, 95% CI) | ‐9.03 [‐26.87, 8.81] |
| 8 Balance: BBS Show forest plot | 2 | 692 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐1.96, 1.28] |
| 9 Adverse events: number of dropouts during the intervention period Show forest plot | 4 | 829 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [1.11, 1.97] |
| 10 Adverse events: serious adverse events related to intervention/during the intervention period Show forest plot | 2 | 692 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.04, 3.33] |
| 11 Adverse events: falls Show forest plot | 3 | 802 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.92, 1.55] |

