Neuroprótesis motoras para promover la recuperación de la funcionalidad después de un accidente cerebrovascular
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012991.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 14 enero 2020see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Accidentes cerebrovasculares
- Copyright:
-
- Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Luciana Mendes: conceived the review question; developed, completed, and edited the first draft of the protocol; drafted the final protocol; and made an intellectual contribution to the protocol. She searched some electronic databases with the help of the Information Specialist, screened titles and abstracts of publications identified by the search, selected and assessed trials, extracted trial and outcome data, contacted trialists about unpublished data, assessed the methodological quality of selected trials, carried out statistical analysis and interpretation of the data, drafted the review, and approved the final manuscript of the review.
Íllia Lima: developed and completed part of the first draft of the protocol and made an intellectual contribution to the protocol. Together with Luciana Mendes she screened titles and abstracts of publications identified by the search and selected and assessed trials; she also checked the outcome data extracted by Luciana Mendes.
Túlio Souza: contributed with clinical expertise, advised on and developed the protocol, and made an intellectual contribution to the protocol. He advised in case of disagreement on the selection of studies, data extraction, and assessment of risk of bias; contributed to the interpretation of the data; and approved the final manuscript of the review.
George Nascimento: contributed with clinical expertise on devices, advised on and developed the protocol, made an intellectual contribution to the protocol, and approved the final version prior to submission. He contributed to the interpretation of the data and approved the final manuscript of the review.
Vanessa Resqueti: advised on and developed the protocol, participated as an arbiter, and made an intellectual contribution to the protocol. She advised in case of disagreement on the selection of studies, data extraction, and assessment of risk of bias; contributed to the interpretation of the data; and approved the final manuscript of the review.
Guilherme Fregonezi: developed and co‐ordinated the protocol, secured funding, advised on and made an intellectual contribution to the protocol, and approved the final version prior to submission. He interpreted the data and the analysis, and corrected and approved the final manuscript of the review.
Sources of support
Internal sources
-
Federal University of Rio Grande do Norte, Brazil.
External sources
-
No sources of support supplied
Declarations of interest
Luciana Mendes: none known
Íllia Lima: none known
Túlio Souza: none known
George Nascimento: none known
Vanessa Resqueti: none known
Guilherme Fregonezi: none known
Acknowledgements
We thank Hazel Fraser, Cochrane Stroke Group Managing Editor; Joshua Cheyne, Cochrane Stroke Group Information Specialist; Peter Langhorne, Cochrane Stroke Group Co‐ordinating Editor; Jan Mehrholz and Alex Pollock, Cochrane Stroke Group Associate Editors; Aryelly Rodriguez, Cochrane Stroke Group Statistical Editor; Bernhard Elsner, Cochrane author, Department of Public Health, Technical University Dresden; and Dee Shneiderman and Sunita Gudwani, consumer reviewers, for their valuable advice on writing the protocol and the review.
Version history
| Published | Title | Stage | Authors | Version |
| 2020 Jan 14 | Motor neuroprosthesis for promoting recovery of function after stroke | Review | Luciana A Mendes, Illia NDF Lima, Tulio Souza, George C do Nascimento, Vanessa R Resqueti, Guilherme AF Fregonezi | |
| 2018 Mar 29 | Motor neuroprosthesis for promoting recovery of function after stroke | Protocol | Luciana A Mendes, Illia NDF Lima, Tulio Souza, George C do Nascimento, Vanessa R Resqueti, Guilherme A F Fregonezi | |
Differences between protocol and review
We used Covidence software for the selection of studies, data extraction, and assessment of risk of bias (Covidence). We included another review author (TS) to help the third review author (VR) in the evaluation of discrepancies and providing advice in case of disagreement on the selection of studies, data extraction, and assessment of risk of bias.
We conducted an extensive search, and are therefore confident that we have identified all relevant studies in the field. However, we did not use Science Citation Index Cited Reference Search for forward tracking of important articles. Due to technical problems with OpenDOAR repository, we used CORE for this repository content search.
We only identified individually randomized trials for this review, so we did not need to analyze for unit of analysis issues as planned in our protocol (Mendes 2018).
Our protocol prespecified a number of subgroup analyses including type of effect and duration of use of device. However, as we analyzed the outcome data only as endpoint values (and not changes from baseline), we decided not to perform a subgroup analysis for type of effect. Regarding the subgroup analysis duration of use of device, we decided to define some primary outcomes based on different periods (such as walking speed up to six months of device use, walking speed between six and 12 months of device use) instead of carrying out the proposed subgroup analysis. This change was based on the fact that we could gain a better understanding of the effect of MN on different periods of use without unit of analysis error (Higgins 2011c), considering that studies could have repeated observations on participants for the same study. We did not perform subgroup analysis for the effect of MN when applied to lower limb or upper limb or for the effect of MN when used by participants in different phases of stroke because there were no data available for MN applied to upper limb, and there were no data for participants less than three months since stroke onset.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO
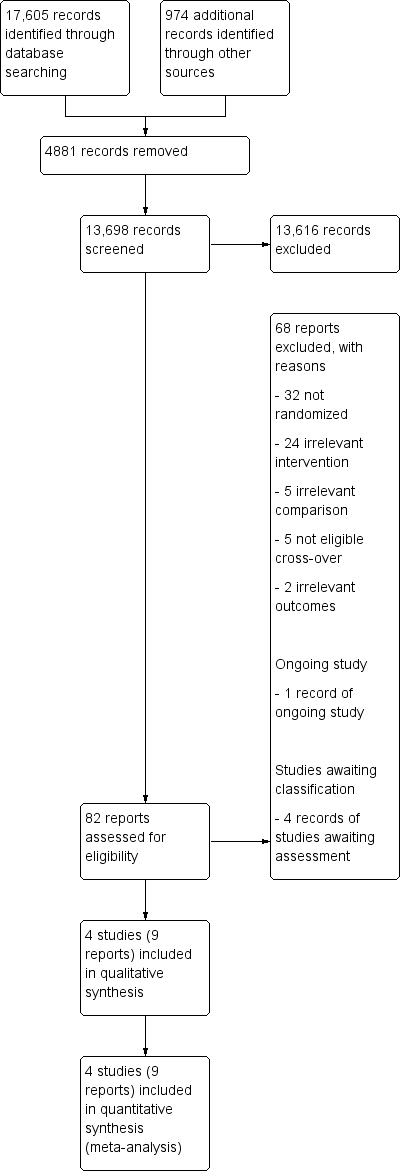
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
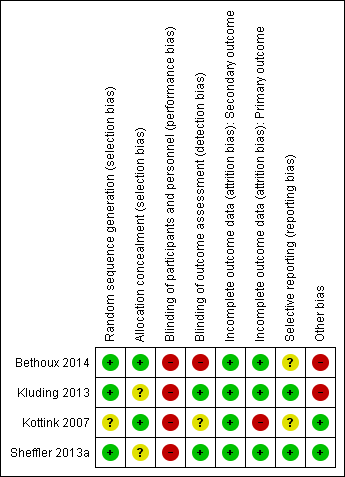
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
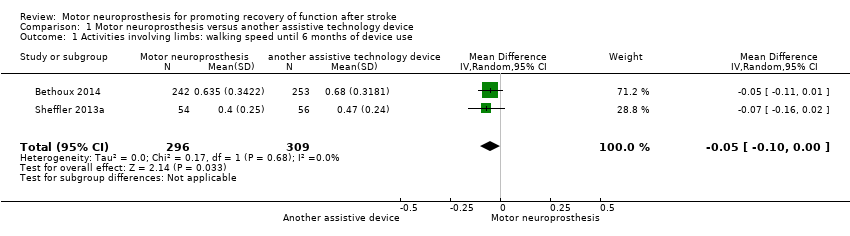
Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 1 Activities involving limbs: walking speed until 6 months of device use.

Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 2 Activities involving limbs: walking speed between 6 and 12 months of device use.

Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 3 Activities involving limbs: walking speed.

Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 4 Activities involving limbs: TUG.

Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 5 Activities involving limbs: mEFAP.

Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 6 Participation scale of HRQoL.
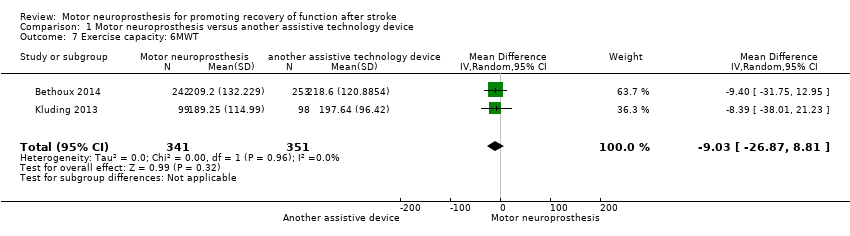
Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 7 Exercise capacity: 6MWT.
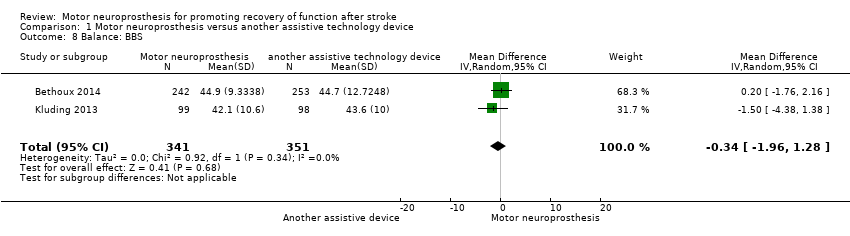
Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 8 Balance: BBS.
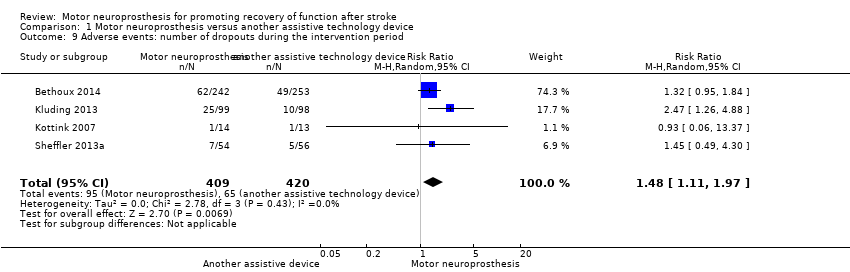
Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 9 Adverse events: number of dropouts during the intervention period.
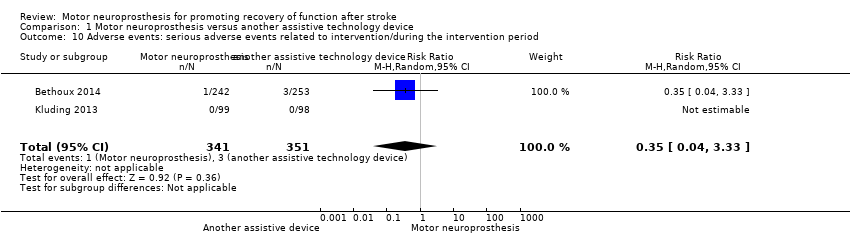
Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 10 Adverse events: serious adverse events related to intervention/during the intervention period.

Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 11 Adverse events: falls.
| Motor neuroprosthesis compared to another assistive technology device for promoting recovery of function after stroke | |||||||
| Patient or population: promoting recovery of function after stroke | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | ||
| Risk with another assistive technology device | Risk with motor neuroprosthesis | ||||||
| Independence in activities of daily living | (No data) | ‐ | ‐ | No studies | Insufficient evidence | No trials measured this outcome. | |
| Activities involving limbs | Walking speed until 6 months of device use (m/s) timed measures at the end of treatment | The mean walking speed in the control group was on average 0.58 m/s. | 0.05 mean difference lower | ‐ | 605 (2 RCTs) | ⊕⊕⊕⊝ | Minimal important difference for comfortable walking speed in chronic stroke participant is 0.2 m/s (Hiengkaew 2012). |
| Walking speed between 6 and 12 months of device use (m/s) timed measures at the end of treatment | The mean walking speed in the control group was on average 0.69 m/s. | 0 mean difference (0.05 lower to 0.05 higher) | ‐ | 713 (3 RCTs) | ⊕⊕⊝⊝ | Minimal important difference for comfortable walking speed in chronic stroke participant is 0.2 m/s (Hiengkaew 2012). | |
| TUG (s) timed measures at the end of treatment | The mean TUG in the control group was on average 27.57 s. | 0.51 mean difference higher (4.41 lower to 5.43 higher) on intervention group | ‐ | 692 | ⊕⊕⊕⊝ | ||
| mEFAP (s) timed measures at the end of treatment | The mean mEFAP in the control group was on average 286.43 s. | 14.77 mean difference higher (12.52 lower to 42.06 higher) on intervention group | ‐ | 605 | ⊕⊕⊝⊝ | ||
| Participation scales of HRQoL timed measures at the end of treatment | The mean participation scales of HRQoL in the control groups was NA.d | 0.26 standardized mean difference (0.22 lower to 0.74 higher) | ‐ | 632 (3 RCTs) | ⊕⊝⊝⊝ | Using Cohen's rules of thumb, 0.26 represents a small effect. | |
| Exercise capacity: 6MWT (m) timed measures at the end of treatment | The mean 6MWT in the control group was on average 208.12 m. | 9.03 mean difference lower (26.87 lower to 8.81 higher) on intervention group | ‐ | 692 | ⊕⊕⊝⊝ | There are no accurate indices of minimal important difference for 6MWT in people poststroke whose gait speed was ≥ 0.40 m/s (Fulk 2018). | |
| Balance: BBS timed measures at the end of treatment | The mean BBS in the control group was on average 44.15. | 0.34 mean difference lower (1.96 lower to 1.28 higher) on intervention group | ‐ | 692 | ⊕⊕⊕⊝ | Minimal detectable change for BBS in chronic stroke participant is 5 points (Hiengkaew 2012). | |
| Adverse events | Number of dropouts during the intervention period | Study population | RR 1.48 | 829 | ⊕⊕⊝⊝ | ||
| 96 per 1000** | 142 per 1000** | ||||||
| Falls | Study population | RR 1.20 | 802 | ⊕⊕⊕⊝ | |||
| 296 per 1000** | 355 per 1000** | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). **We used the median control group risk across studies. 6MWT: 6‐minute walk test; BBS: Berg Balance Scale; CI: confidence interval; HRQoL: health‐related quality of life; mEFAP: modified Emory Functional Ambulation Profile; NA: not applicable; RCT: randomized controlled trial; RR: risk ratio; TUG: Timed Up and Go test. | |||||||
| GRADE Working Group grades of evidence | |||||||
| aThe outcome assessors were not blinded in the larger study. | |||||||
| Study ID (report) | MN device | Duration of exposure to MN intervention | Conditioning protocol used to adapt participants to MN use | MN use/daily use for increasing the activities and participation in the home or community context |
| The MN used was the WalkAide device (Innovative Neurotronics, Austin, TX, USA). It is a commercially available, battery‐operated, single‐channel surface peroneal nerve stimulator that consists of a cuff worn around the proximal part of the lower leg, which holds the control module and surface electrodes. This device uses a tilt sensor and an accelerometer to trigger ankle dorsiflexion and control the timing and duration of peroneal nerve stimulation during the swing phase of gait to alleviate foot drop. | The duration of MN intervention was 12 months. The conditioning protocol occurred in the first 2 weeks, after which participants started daily use of MN device. | The first part consisted of fitting and programming the MN device as well as patient education performed by WalkAide‐certified orthotist or licensed physical therapist. The conditioning protocol included a 2‐week progressive wearing schedule of MN device. | Participants were instructed to wear MN device on a full‐time basis (quote: "ie, for all walking activities throughout the day"). | |
| The MN used was the NESS L300 device (Bioness Inc, Valencia, CA, USA). It is a commercially available, battery‐operated, single‐channel surface peroneal nerve stimulator that consists of a cuff with integrated stimulation unit and electrodes, a control unit, and an in‐shoe pressure sensor. The pressure sensor detects heel off and initial contact events during gait. It transmits wireless signals to the stimulation cuff, which initiates or pauses the stimulation of deep and superficial branches of the peroneal nerve via 2 surface electrodes that activate dorsiflexors and evertors muscles to ensure foot clearance during the swing phase of gait and prevent excessive ankle inversion during early stance. | The duration of MN intervention was 30 weeks. The conditioning protocol occurred in the first 6 weeks. Participants used the MN device all day between week 4 and week 30. | The first part consisted of initial fitting of the device, gait training, wearing schedule, home exercise program, and participant education based on manufacturer standardized protocols. For the first 3 weeks, participants followed the standard conditioning protocol (gradually increasing walking with the MN from 15 minutes each day to all‐day use). During the same period, participants also used the MN for cyclic stimulation while not walking in order to gradually strengthen and condition the muscles to avoid fatigue when using the device (Dunning 2013).* During the first 6 weeks of the study, participants also received 8 dose‐matched sessions of physical therapy. The first 2 to 4 therapy visits focused on education on device use, initial gait training, and an individualized home exercise program. The remaining physical therapy sessions focused on gait training (Kluding 2013). | Participants used the MN all day for ambulation (Dunning 2013).* | |
| The MN used was the STIMuSTEP device (FineTech Medical Ltd, Hertfordshire, UK). It is a commercially available, battery‐operated, 2‐channel implantable device composed of implantable components such as a stimulator, 2 leads, and bipolar intraneural electrodes, and non‐implantable components such as an external transmitter with a built‐in antenna and a pressure sensor. 1 electrode is surgically positioned under the epineurium of the superficial peroneal nerve and the other under the epineurium of the deep peroneal nerve. This device promotes the ankle dorsiflexion/eversion during gait to correct foot drop, and a pressure sensor placed inside the shoe determines the on and off switching of the stimulation. | The duration of MN intervention was 26 weeks. The intervention began with the surgical procedure for placement of the implant. After 2 weeks of the surgery, the wound was checked and first test stimulation took place. The conditioning protocol began at the third week, and all‐day MN use began at the sixth week. | Quote: "Two weeks after the surgery the wound was checked and a first test stimulation took place. In the third week, stimulation during walking was tested and the stimulator was taken home by the patient. The use of the stimulator was gradually increased over 2 weeks to prevent severe muscle pain and fatigue. After this period patients were allowed to use the system all day." | Participants were allowed to use the system all day between week 6 and week 26. | |
| The MN used to correct foot drop was the Odstock Dropped‐Foot Stimulator (ODFS) device (Odstock Medical Ltd, Salisbury Wiltshire, UK). The ODFS is a commercially available, battery‐operated, single‐channel surface peroneal nerve stimulator consisting of an electrical stimulator, a control module, pressure sensors, and surface electrodes. The stimulation is triggered by an insole pressure‐sensing foot switch that detects heel rise at pre‐swing. | The duration of MN intervention was 12 weeks. The conditioning protocol occurred over the 12 weeks. Daily MN use began once device safety was demonstrated by participants. | In the first 5 weeks the Functional Training phase (2 x 1‐hour sessions per week) took place, in which participants were trained to use MN device for home and community mobility with an assistive device, if needed. Activities included passive and active range‐of‐motion exercises, lower extremity strengthening, standing balance and weight‐shifting activities to the affected limb with transition to least‐restrictive assistive device, and refinement of a reciprocal gait pattern. Exercises were done with multiple repetitions with an increase in difficulty and a decrease in cues, with and without the MN device, as appropriate. In the last 7 weeks the Post‐Functional Training Phase (3 x 1‐hour sessions) took place, in which device function, application, and usage guidelines were reviewed with each participant to maximize MN compliance. | The article did not explicitly mention when participants started all‐day MN use, but reported that as soon as participants demonstrated safe use of the device, it was used up to 8 hours per day. | |
| MN: motor neuroprosthesis *Dunning 2013 corresponds to the published protocol of the study Kluding 2013. | ||||
| Study ID (report) | Independence in ADL | Activities involving limbs | Participation scales of HRQoL | Exercise capacity | Balance |
| (Bethoux 2014; 6‐month assessment) | ‐ | Comfortable walking speed measured by 10MWT, TUG, mEFAP | SSQoL (total value); SIS (all domains) | 6MWT | BBS |
| (Bethoux 2015; 12‐month assessment) | ‐ | Comfortable walking speed measured by 10MWT, mEFAP | ‐ | 6MWT | ‐ |
| ‐ | Comfortable and fast walking speed measured by 10MWT, TUG | SIS (ADL/iADL, Mobility, Participation domains) | 6MWT | BBS; FRT | |
| Kottink 2007 (Kottink 2007; Kottink 2008; Kottink 2010; Kottink 2012) | ‐ | Comfortable walking speed motion analysis system | SF‐36 (all domains) | ‐ | ‐ |
| ‐ | Comfortable walking speed measured by motion analysis system, mEFAP | SSQoL (total value) | ‐ | ‐ | |
| 6MWT: 6‐minute walk test | |||||
| Study ID (report) | Motor neuroprosthesis | Another assistive technology device |
| (Bethoux 2014; 6‐month assessment)* | 2 deceased; 25 non‐compliance with protocol; 15 participant request; 7 medical reasons; 4 lost to follow‐up; 2 investigator withdrew | 2 deceased; 13 non‐compliance with protocol; 18 participant request; 4 medical reasons; 3 lost to follow‐up; 1 investigator withdrew |
| (Bethoux 2015; 12‐month assessment)** | 2 deceased; 25 non‐compliance with protocol; 16 participant request; 7 medical reasons; 6 lost to follow‐up; 6 investigator withdrew | 3 deceased; 15 non‐compliance with protocol; 19 participant request; 4 medical reasons; 6 lost to follow‐up; 2 investigators withdrew |
| 2 lost to follow‐up; 23 discontinued intervention | 1 lost to follow‐up; 9 discontinued intervention | |
| Kottink 2007 (Kottink 2007; Kottink 2008; Kottink 2010; Kottink 2012) | 1 technical defect in the epineural electrode | 1 psychological issues not related to the study |
| 6 non‐medical reasons; 1 medical reason
| 2 non‐medical reasons; 3 medical reasons
| |
| *Bethoux 2014 (six‐month assessment) corresponds to the first report of Bethoux 2014 study whose assessment was made after six months of motor neuroprosthesis use. | ||
| Outcome | Study ID (report) | Analysis results |
| Activities involving limbs: walking speed until 6 months of device use | MD −0.07, 95% CI −0.16 to 0.02; P = 0.13; participants = 110; I2 = 0% | |
| Activities involving limbs: walking speed between 6 and 12 months of device use | MD 0.04, 95% CI −0.09 to 0.16; P = 0.57; participants = 218; I2 = 52% | |
| Activities involving limbs: TUG | MD 0.88, 95% CI −6.36 to 8.12; P = 0.81; participants = 197; I2 = 0% | |
| Activities involving limbs: mEFAP | MD 14.45, 95% CI −13.97 to 42.87; P = 0.32; participants = 110; I2 = 0% | |
| Participation scale of HRQoL | SMD 0.60, 95% CI −0.39 to 1.59; P = 0.24; participants = 137; I2 = 79% | |
| Exercise capacity: 6MWT | MD −8.39, 95% CI −38.01 to 21.23; P = 0.58; participants = 197; I2 = 0% | |
| Balance: BBS | MD −1.50, 95% CI −4.38 to 1.38; P = 0.31; participants = 197; I2 = 0% | |
| 6MWT: 6‐minute walk test | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Activities involving limbs: walking speed until 6 months of device use Show forest plot | 2 | 605 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.10, ‐0.00] |
| 2 Activities involving limbs: walking speed between 6 and 12 months of device use Show forest plot | 3 | 713 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.05, 0.05] |
| 3 Activities involving limbs: walking speed Show forest plot | 4 | 823 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.06, 0.04] |
| 3.1 Surface MN | 3 | 802 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.06, 0.02] |
| 3.2 Implantable MN | 1 | 21 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.04, 0.28] |
| 4 Activities involving limbs: TUG Show forest plot | 2 | 692 | Mean Difference (IV, Random, 95% CI) | 0.51 [‐4.41, 5.43] |
| 5 Activities involving limbs: mEFAP Show forest plot | 2 | 605 | Mean Difference (IV, Random, 95% CI) | 14.77 [‐12.52, 42.06] |
| 6 Participation scale of HRQoL Show forest plot | 3 | 632 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.22, 0.74] |
| 7 Exercise capacity: 6MWT Show forest plot | 2 | 692 | Mean Difference (IV, Random, 95% CI) | ‐9.03 [‐26.87, 8.81] |
| 8 Balance: BBS Show forest plot | 2 | 692 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐1.96, 1.28] |
| 9 Adverse events: number of dropouts during the intervention period Show forest plot | 4 | 829 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [1.11, 1.97] |
| 10 Adverse events: serious adverse events related to intervention/during the intervention period Show forest plot | 2 | 692 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.04, 3.33] |
| 11 Adverse events: falls Show forest plot | 3 | 802 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.92, 1.55] |

