Neuroprótesis motoras para promover la recuperación de la funcionalidad después de un accidente cerebrovascular
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: RCT Instruments used: MMSE, BDI, 10MWT, SIS, device‐related SAE rate, 6MWT, GaitRite FAP, mEFAP, BBS, TUG, SSQoL Study design as described in the article: Quote: "This study was an unblinded, parallel‐group RCT" Study duration: 24 months Year of study: trial ran between April 2010 and April 2012 | |
| Participants | Inclusion criteria: ≥ 6 months poststroke; inadequate dorsiflexion with inadequate limb clearance during swing phase of gait; positive response to peroneal nerve stimulation testing; adequate cognitive function (MMSE score > 17); not currently using FES for the treatment of foot drop; ≥ 30 days post‐inpatient or outpatient stroke, cardiac, pulmonary, or any other lower extremity physical rehabilitation; able to walk at least 10 meters with or without an assist device; initial gait speed of > 0.0 m/s and < 0.8 m/s; eligible for Medicare or Medicare Choice/Advantage benefits at time of consent; ≥ 90 days post‐MI; ≥ 90 days post‐stenting procedure (i.e. peripheral, cardiac, carotid, and/or renal); ≥ 90 days post‐major orthopedic surgery (i.e. hip, knee, and/or ankle joint replacement); ≥ 6 months post‐CABG or cardiac valve procedure; able and willing to give written consent and comply with study procedures, including follow‐up visits Exclusion criteria: ankle joint instability other than foot drop; needs AFO for stance control of the foot, ankle, and/or knee; unable to safely clear toes in swing phase on the involved lower extremity, defined as > −5 degrees plantar flexion with the WalkAide device (determined at fitting); diagnosed with peripheral neuropathy, and symptoms obstruct or limit ambulation or participation in study; diagnosed with significant peripheral vascular disease accompanied by lower extremity ulceration and/or disabling claudication; underlying condition(s) that would limit study participation; severe hypertonicity resulting in the need for more involved orthotic strategies; excessive dysesthetic pain secondary to neurological involvement; moderate to very severe chronic obstructive pulmonary disease, as defined by the Global Initiative for Chronic Obstructive Lung Disease (GOLD); New York Heart Association (NYHA) Class III‐IV; malignant skin lesion below the knee on the affected lower extremity; history of seizure disorder and is currently on seizure control medication for this disorder; aphasia, defined as inability to verbalize commands; BDI score of > 29 indicating severe depression; life expectancy less than 12 months; received botulinum toxin injections in the lower extremity within the past 6 months; baclofen pump with unstable dosing in the last 3 months; participating in another clinical trial that, according to the principal investigator, is likely to affect study outcome or confound results; patient has existing electrical stimulation devices (implantable cardioverter defibrillator, pacemaker, spinal stimulation, TENS) Age: MN group mean age (± SD): 63.87 years (± 11.33); control group mean age (± SD): 64.30 years (± 12.01) Country: USA Sample size: 495 participants Sex: MN group: 147 (60.74%) men, 95 (39.26%) women; control group: 157 (62.06%) men, 96 (37.94%) women Time poststroke: ≥ 6 months poststroke. MN group mean time poststroke (± SD): 6.90 years (± 6.43); control group mean time poststroke (± SD): 6.86 years (± 6.64) Type of stroke: not stated | |
| Interventions | Motor neuroprosthesis
Another assistive technology device
| |
| Outcomes | Activities involving limbs: walking speed measured with the 10MWT (m/s)
Activities involving limbs: mEFAP (s)
Activities involving limbs: TUG (s)
Balance: BBS
Exercise capacity: 6MWT (m)
Participation scale of HRQoL: SSQoL
Participation scale of HRQoL: SIS Social participation domain
Adverse events: dropouts during the intervention period
Adverse events: serious adverse events related to the intervention
Adverse events: falls
| |
| Identification | Author's name: Francois Bethoux Institution: The Cleveland Clinic Foundation Email: [email protected] Address: The Cleveland Clinic Foundation, Desk U10, 9500 Euclid Avenue, Cleveland, OH 44195, USA | |
| Funding source | Innovative Neurotronics | |
| Notes | This study consisted of 2 articles (Bethoux 2014; Bethoux 2015). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a centralized computer‐generated randomization scheme built into the electronic data capture system for this study" |
| Allocation concealment (selection bias) | Low risk | Quote: "Centralized computer‐generated randomization scheme" |
| Blinding of participants and personnel (performance bias) | High risk | There was no blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | High risk | Unblinded outcome assessment |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "We conducted an ITT analysis using multiple imputations to account for missing data" |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "We conducted an ITT analysis using multiple imputations to account for missing data" |
| Selective reporting (reporting bias) | Unclear risk | Although all of the study’s prespecified primary outcomes were reported, a secondary variable was included in the study that was not prespecified in the protocol. |
| Other bias | High risk | This study was sponsored by Innovative Neurotronics. |
| Methods | Study design: RCT Instruments used: 10MWT, lower extremity Fugl‐Meyer, TUG, 6MWT, BBS, FRT, SIS Study design as described in the article: "single‐blinded randomized controlled trial" Study duration: 32 months Year of study: trial ran between May 2010 and December 2012 | |
| Participants | Inclusion criteria: at least 1 stroke ≥ 3 months before study enrollment, resulting in drop foot; ankle dorsiflexion response with test stimulation in sitting and standing, and adequate ankle and knee stability during gait with test stimulation; medically stable; score ≥ 24 on the MMSE, or have a competent caregiver if < 24; age ≥ 18 year or older; able to walk ≥ 10 meters with a maximum of 1 person assist; self‐selected gait speed ≤ 0.80 m/s without orthotic effect Exclusion criteria: fixed ankle contracture at ≥ 5 degrees of plantar flexion in the hemiplegic leg with the knee extended; pain in the affected leg, rated ≥ 4 on a 10‐point visual analogue scale; participating in physical therapy, occupational therapy, new exercise program, or any other interventional clinical research studies without the sponsor's approval; botulinum toxin to the hemiplegic leg or arm within the past 6 weeks or planned during the course of the study; expectation of a significant change in oral medications for spasticity; complete lower extremity hemisensory loss; use of any FDS device for foot drop for an accumulative > 3 hours within the last 6 months before study enrollment; any electric or metallic implant; significant swelling/edema in the lower leg; chronic skin problems or cancerous lesion in close proximity to the site of FDS stimulation; pregnant or planning on becoming pregnant; unstable seizure disorder; orthopedic conditions that would affect ambulation; major untreated depression Sample size: 197 participants Country: USA Age: mean age (± SD): 61.14 years (± 11.61) Sex: 79 women and 118 men. MN group: 51 (51.5%) men; control group: 67 (68.4%) men Time poststroke: this study considered 2 subgroups: participants 3 to 6 months after stroke and participants > 6 months after stroke. Mean time poststroke (± SD): 4.55 years (± 4.72) Type of stroke: 145 ischemic, 46 hemorrhagic, 6 data not available | |
| Interventions | Motor neuroprosthesis
Another assistive technology device
| |
| Outcomes | Activities involving limbs: walking speed measured with the 10MWT (m/s)
Activities involving limbs: fast walking speed measured with the 10MWT (m/s)
Activities involving limbs: TUG (s)
Exercise capacity: 6MWT (m)
Balance: BBS
Balance: functional reach (cm)
Participation scale of HRQoL: SIS ‐ Social participation
Adverse events: dropouts during the intervention period
Adverse events: serious adverse events related to intervention
Adverse events: falls
| |
| Identification | Author's name: Patricia M Kluding Institution: Department of Physical Therapy and Rehabilitation Sciences, University of Kansas Medical Center Email: [email protected] Address: University of Kansas Medical Center, 3901 Rainbow Blvd, Mail Stop 3051, Kansas City, KS 66160, USA | |
| Funding source | Bioness Inc | |
| Notes | Associated reference: Dunning 2013 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Once study eligibility was confirmed, random group assignment was performed by the sponsor using a web‐based application prepared by the study statistician." |
| Allocation concealment (selection bias) | Unclear risk | Although the study protocol mentioned that the process is concealed by the site, the method of concealment is not described in sufficient detail to permit a definitive judgement. |
| Blinding of participants and personnel (performance bias) | High risk | There was no blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "To maintain blinding, a nonblinded research team member coordinates outcome testing and all subjects wear loose pants, a lower leg and shoe cover ('gaiter') on the involved lower extremity (to conceal the AFO or FDS cuff and pressure sensor), and an FDS control unit" |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis was performed. |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis was performed. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available, and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way. |
| Other bias | High risk | This trial was funded by Bioness Inc. |
| Methods | Study design: RCT Instruments used: 6MWT, Vicon system, activPAL Professional, surface electromyographic (sEMG) activity, SF‐36, DIP, EQ‐5D Study design as described in the article: "Randomized controlled trial" Study duration: not stated Year of study: not stated | |
| Participants | Inclusion criteria: drop foot identified by an inability to achieve a normal heel strike during walking; first hemiplegia of at least 6 months in duration as a result of a cerebrovascular accident with a stable neurology; individual is an outdoor walker; able to give an informed consent Exclusion criteria: under age 18 years; passive dorsiflexion of the ankle 5 degrees with knee in extension; medical conditions other than cerebrovascular accident, i.e. neurologic, rheumatic, cardiovascular, or systemic disorders (including diabetes mellitus) limiting the function of walking; injury to deep and superficial peroneal nerve and sciatic nerve; any medical condition that would exclude the use of a surgical procedure or anesthetic; not able to don and doff the equipment; pregnancy Age: MN group mean age (± SD): 55.2 years (± 11.36); control group mean age (± SD): 52.87 years (± 9.87) Country: the Netherlands Sample size: 29 Sex: MN group: 10 men and 4 women; control group: 10 men and 5 women Time poststroke: ≥ 6 months poststroke. MN group mean time poststroke (± SD): 9.07 years (± 9.29); control group mean time poststroke (± SD): 5.67 years (± 4.64) Type of stroke: not stated | |
| Interventions | Motor neuroprosthesis
Another assistive technology device
| |
| Outcomes | Activities involving limbs: walking speed (m/s)
Participation scale of HRQoL: SF‐36 ‐ Social functioning
Adverse events: dropouts during the intervention period
| |
| Identification | Author's name: Anke I Kottink Institution: Roessingh Research and Development Email: [email protected] Address: Roessingh Research and Development, PO Box 310, 7500 AH, Enschede, the Netherlands | |
| Funding source | Not reported | |
| Notes | This study consisted of 4 articles that were part of a PhD thesis (Kottink 2007; Kottink 2008; Kottink 2010; Kottink 2012). We did not include outcomes of 6MWT and walking speed assessed with and without devices because these data were presented only as figures (Kottink 2007; Kottink 2008). We contacted the principal investigator, but the author did not respond to our request for data. References associated with this study: Kottink 2008; Kottink 2010; Kottink 2012 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study authors stated that blocked randomization was used, but it is not clear if the method used for selecting the blocks describes a random component in the sequence generation process. |
| Allocation concealment (selection bias) | Low risk | Random procedure was carried out by an independent person. |
| Blinding of participants and personnel (performance bias) | High risk | There was no blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis was performed. |
| Incomplete outcome data (attrition bias) | High risk | The study had withdrawals, and no ITT was performed for the primary outcome of 10MWT (Kottink 2012). |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Other bias | Low risk | No other bias detected. |
| Methods | Study design: RCT Instruments used: lower limb portion of the Fugl‐Meyer Assessment, mEFAP, SSQoL, gait analysis with Vicon system Study design as described in the article: "Single‐blinded randomized controlled trial" Study duration: not stated Year of study: not stated | |
| Participants | Inclusion criteria: age ≥ 18 years, ≥ 12 weeks poststroke with unilateral hemiparesis and ankle dorsiflexion strength of ≤ 4/5 on the Medical Research Council (MRC) scale. Participants were required to ambulate ≥ 30 feet without an AFO, score ≥ 24 on the BBS, and demonstrate correction of foot drop using a PNS without evidence of knee hyperextension during stance. Exclusion criteria: lower extremity edema, skin breakdown, or absent sensation; serious cardiac arrhythmias, pacemakers or other implanted electronic systems; pregnancy; uncontrolled seizure disorder; concomitant lower motor neuron dysfunction and non‐stroke upper motor neuron dysfunction; uncompensated hemineglect; sensory or motor peripheral neuropathy; fixed ankle plantarflex or contracture; or lower extremity botulinum toxin injection within the 3 months prior to study enrollment Age: MN group mean age (± SD): 52.8 years (± 12.2); control group mean age (± SD): 53.2 years (± 10.1) Country: USA Sample size: 110 participants Sex: MN group: 30 men and 24 women; control group: 37 men and 19 women Time poststroke: > 12 weeks poststroke. MN group mean time poststroke (± SD): 44.7 months (± 97.5); control group mean time poststroke (± SD): 44.9 months (± 79.2) Type of stroke: MN group: 13 embolic, 17 thrombotic, 9 lacunar, and 15 hemorrhagic; control group: 12 embolic, 23 thrombotic, 6 lacunar, and 15 hemorrhagic | |
| Interventions | Motor neuroprosthesis
Another assistive technology device
| |
| Outcomes | Activities involving limbs: mEFAP (s)
Activities involving limbs: walking speed (m/s)
Participation scale of HRQoL: SSQoL
Adverse events: dropouts during the intervention period
Adverse events: falls
| |
| Identification | Author's name: Lynne R Sheffler Institution: Department of Physical Medicine and Rehabilitation, Case Western Reserve University, Cleveland, OH; Cleveland FES Center; Dept of PM&R, MetroHealth Rehabilitation Institute of Ohio, USA Email: [email protected] Address: MetroHealth Medical Center, 4229 Pearl Road, N5‐524, Cleveland, OH 44109, USA | |
| Funding source | MetroHealth Medical Center | |
| Notes | This study consisted of 2 articles (Sheffler 2013a; Sheffler 2015). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators described that envelopes were used as a random component in the sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Although the investigators stated that the randomization sequence was concealed, there is no mention as to whether the envelopes were sealed or not. |
| Blinding of participants and personnel (performance bias) | High risk | There was no blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "blinded outcomes assessor". |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis was performed. |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis was performed. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available, and all of the study's prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way. |
| Other bias | Low risk | No other bias detected. |
6MWT: 6‐minute walk test
10MWT: 10‐meter walk test
AFO: ankle‐foot orthosis
BDI: Beck Depression Inventory
BBS: Berg Balance Scale
CABG: coronary artery bypass grafting
DIP: Disability Impact Profile
FAP: Functional Ambulation Profile
FDS: foot drop stimulator
FES: functional electrical stimulation
FRT: Functional Reach Test
HRQoL: health‐related quality of life
ITT: intention‐to‐treat
MMSE: Mini Mental State Exam
mEFAP: modified Emory Functional Ambulation Profile
MI: myocardial infarction
MN: motor neuroprosthesis
PNS: peroneal nerve stimulation
pps: pulses per second
RCT: randomized controlled trial
SAE: serious adverse event
SD: standard deviation
SF‐36: 36‐item Short Form Health Survey
SIS: Stroke Impact Scale
SSQoL: Stroke‐Specific Quality of Life
TENS: transcutaneous electrical nerve stimulation
TUG: Timed Up and Go
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not randomized | |
| Not randomized | |
| Irrelevant intervention | |
| Irrelevant intervention | |
| Irrelevant comparison | |
| Not randomized | |
| Not randomized | |
| Not randomized | |
| Irrelevant intervention | |
| Irrelevant outcomes | |
| Not randomized | |
| Not randomized | |
| Not randomized | |
| Not randomized | |
| Irrelevant intervention | |
| Irrelevant intervention | |
| Irrelevant comparison | |
| Irrelevant intervention | |
| Irrelevant intervention | |
| Not eligible cross‐over trial | |
| Not randomized | |
| Not randomized | |
| Not eligible cross‐over trial | |
| Not randomized | |
| Not eligible cross‐over trial | |
| Irrelevant comparison | |
| Not randomized | |
| Irrelevant intervention | |
| Not randomized | |
| Irrelevant intervention | |
| Not randomized (quasi‐randomized controlled trial) | |
| Irrelevant comparison | |
| Irrelevant intervention | |
| Irrelevant intervention | |
| Irrelevant intervention | |
| Not randomized | |
| Not randomized | |
| Not randomized | |
| Irrelevant intervention | |
| Irrelevant intervention | |
| Irrelevant intervention | |
| Not eligible cross‐over trial | |
| Irrelevant intervention (electrical stimulation performed in clinical setting) | |
| Irrelevant intervention (electrical stimulation performed in clinical setting) | |
| Irrelevant intervention | |
| Not eligible cross‐over trial | |
| Not randomized | |
| Irrelevant intervention | |
| Irrelevant intervention | |
| Not randomized | |
| Not randomized | |
| Not randomized | |
| Irrelevant intervention | |
| Not randomized | |
| Not randomized | |
| Irrelevant intervention | |
| Irrelevant comparison | |
| Not randomized | |
| Not randomized | |
| Irrelevant intervention | |
| Not randomized | |
| Irrelevant outcomes | |
| Not randomized | |
| Not randomized | |
| Not randomized | |
| Not randomized | |
| Irrelevant intervention | |
| Not randomized |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Study design: the study author only stated at trial registry that this is a randomized controlled pilot study Instruments used: walking speed; Physiological Cost Index; visual gait analysis from video using Rivermead Visual Gait Assessment; 6MWT; Canadian Occupational Performance Measure; Hospital Anxiety and Depression Scale; Rivermead Mobility Index Study duration: not stated Year of study: registered in 2007 |
| Participants | Inclusion criteria: participants will be over 18 years; participants will be medically fit enough to undertake physiotherapy (consultant and GP approval will be sought prior to starting the trial); current inpatient stay will be for rehabilitation following first stroke; during the inpatient period participants will have demonstrated they have sufficient motivation, memory, and cognitive ability to participate in treatment within physiotherapy and practice outside of treatment sessions; participants will be able to understand spoken instructions; participants' goals must include improving gait; suitable participants will be returning home after hospital discharge with a Rivermead Mobility Index of between 6 and 10; participants will be able to attend the hospital for twice‐weekly physiotherapy, i.e. will have suitable transport and live within 25 miles of the hospital Exclusion criteria: unable to tolerate sensation of stimulation (assessed prior to acceptance into the trial); poor skin condition making stimulation unsuitable; previous neurological conditions likely to influence response to treatment; orthopedic/other health problems limiting ability to participate or use stimulation/physiotherapy; score of 25 or under on Mini Mental Test; pacemaker and other active implant users; poorly controlled epileptics; pregnancy Age: stated only that participants were adults Sample size: 30 participants Sex: men and women Time poststroke: less than 6 months of stroke Type of stroke: not stated |
| Interventions | Motor neuroprosthesis
Another assistive technology device
|
| Outcomes |
|
| Notes | We contacted the principal investigator to request more detailed information about the intervention to determine if the intervention was used as an orthosis in the home or community context, but as of yet have not received a response. |
| Methods | Study design: the study author only stated at trial registry that this is a randomized parallel‐assignment trial Instruments used: Stroke Upper Limb Capacity Scale (SULCS); Box & Blocks Test Study duration: not stated Year of study: registered in 2018 |
| Participants | Inclusion criteria: 6 to 24 months since a first clinical cortical or subcortical, hemorrhagic or non‐hemorrhagic stroke; unilateral upper limb hemiparesis with finger extensor strength of grade no more than 4 out of 5 on the Medical Research Council (MRC) scale; score of at least 1 and no more than 11 out of 14 on the hand section of the upper extremity Fugl‐Meyer Assessment; adequate active movement of the shoulder and elbow to position the hand in the workspace for table‐top task practice (necessary for the lab task practice sessions); able to follow 3‐stage commands; able to recall at least 2 of a list of 3 items after 30 minutes; skin intact on the hemiparetic arm; surface stimulation of the paretic finger and thumb extensors produces functional hand opening without pain (this will exclude those who have too much flexor spasticity); able to hear and respond to cues from stimulator; not receiving occupational therapy (no concomitant occupational therapy); full voluntary opening/closing of the contralateral (less affected) hand; demonstrates ability to follow instructions for operating the stimulator or have a caregiver who will assist them Exclusion criteria: co‐existing neurologic diagnosis of peripheral nerve injury, Parkinson's disease, spinal cord injury, traumatic brain injury, or multiple sclerosis; uncontrolled seizure disorder; brainstem stroke; uncompensated hemineglect; severe shoulder or hand pain; insensate forearm or hand; history of potentially fatal cardiac arrhythmias with hemodynamic instability; implanted electronic systems (e.g. pacemaker); botulinum toxin injections to any upper extremity muscle within 3 months of enrolling; pregnant women due to unknown risks of surface NMES during pregnancy; lack of functional passive range of motion of the wrist or fingers of affected side; diagnosis (apart from stroke) that substantially affects paretic arm and hand function; deficits in communication that interfere with reasonable study participation; lacking sufficient visual acuity to see the stimulator's display; concurrent enrollment in another investigational study Age: 21 to 90 years Sample size: 129 participants Sex: men and women Time poststroke: not stated Type of stroke: not stated |
| Interventions | Motor neuroprosthesis
Another assistive technology device
|
| Outcomes |
|
| Notes | We contacted the principal investigator to request more detailed information about the intervention to determine if the intervention was used as an orthosis in the home or community context, but as of yet have not received a response. |
| Methods | Study design: the study author only stated at trial registry that this is a randomized cross‐over trial Instruments used: Fugl‐Meyer Assessment, Mortor Activity Log, Box & Blocks Test, Motor Assessment Scale Study duration: not stated Year of study: not stated |
| Participants | Inclusion criteria: time from stroke onset > 5 months; no cognitive deficit; no severe proprioceptive deficit; no severe contracture in paretic hand; independent for locomotion Exclusion criteria: severe heart failure; severe pulmonary dysfunction; severe hypertension; uncontrolled seizure; pacemaker and other implants; other serious medical condition Age: 15 to 80 years old Sample size: 40 participants Sex: men and women Time poststroke: not stated Type of stroke: not stated |
| Interventions | Motor neuroprosthesis
Another assistive technology device
|
| Outcomes |
|
| Notes | The principal investigator stated that the HANDS protocol was applied at home. We wrote to the principal investigator to ask if this study is already published but as of yet have not received a response. |
| Methods | Study design: not stated. The study author reported that participants were randomly assigned to groups. Instruments used: 10MWT, Physiological Cost Index, endurance (3‐minute test), modified Ashworth Scale, Rivermead Mobility Index Study duration: not stated Year of study: not stated |
| Participants | Inclusion criteria: single stroke of vascular origin with hemiplegia (< 6 months); assessed by a clinical specialist physiotherapist to confirm that both a stimulator and an AFO would be suitable for the participant; affected by a drop‐foot, identified by failure to achieve a heel strike, and corrected by FES; inability to achieve an effective push‐off at terminal stance, identified by clinical observation Exclusion criteria: use of a dropped‐foot stimulator or AFO in the 4 weeks prior to start of the intervention; required an AFO other than that selected for the trial Age: not stated Sample size: 22 participants Sex: not stated Time poststroke: not stated Type of stroke: not stated |
| Interventions | Motor neuroprosthesis
Another assistive technology device
|
| Outcomes |
|
| Notes | We were unable to contact the principal investigator (email returned undeliverable). |
6MWT: 6‐minute walk test
10MWT: 10‐meter walk test
AFO: ankle‐foot orthosis
FES: functional electrical stimulation
GP: general practitioner
HANDS: Hybrid Assistive Neuromuscular Dynamic Stimulation
EMG‐controlled NMES: electromyography‐controlled neuromuscular electrical stimulation
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Randomized controlled trial comparing implanted peroneal nerve stimulation and ankle foot orthosis in spastic paresis |
| Methods | Not stated Random allocation |
| Participants | 24 participants with chronic paresis |
| Interventions | Motor neuroprosthesis
Another assistive technology device
|
| Outcomes | Activities involving limbs: walking speed (m/s) |
| Starting date | Not stated |
| Contact information | Mouna Ghédira, PhD Laboratoire ARM ‐ Analyse et Restauration du Mouvement email: [email protected] |
| Notes | This study was published only as an abstract. We contacted the principal investigator, who reported that the full text has not yet been published. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
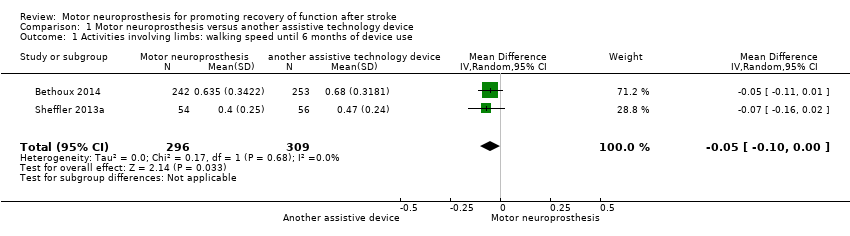
| 1 Activities involving limbs: walking speed until 6 months of device use Show forest plot | 2 | 605 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.10, ‐0.00] |
| Analysis 1.1  Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 1 Activities involving limbs: walking speed until 6 months of device use. | ||||
| 2 Activities involving limbs: walking speed between 6 and 12 months of device use Show forest plot | 3 | 713 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.05, 0.05] |
| Analysis 1.2  Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 2 Activities involving limbs: walking speed between 6 and 12 months of device use. | ||||
| 3 Activities involving limbs: walking speed Show forest plot | 4 | 823 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.06, 0.04] |
| Analysis 1.3  Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 3 Activities involving limbs: walking speed. | ||||
| 3.1 Surface MN | 3 | 802 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.06, 0.02] |
| 3.2 Implantable MN | 1 | 21 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.04, 0.28] |
| 4 Activities involving limbs: TUG Show forest plot | 2 | 692 | Mean Difference (IV, Random, 95% CI) | 0.51 [‐4.41, 5.43] |
| Analysis 1.4  Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 4 Activities involving limbs: TUG. | ||||
| 5 Activities involving limbs: mEFAP Show forest plot | 2 | 605 | Mean Difference (IV, Random, 95% CI) | 14.77 [‐12.52, 42.06] |
| Analysis 1.5  Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 5 Activities involving limbs: mEFAP. | ||||
| 6 Participation scale of HRQoL Show forest plot | 3 | 632 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.22, 0.74] |
| Analysis 1.6  Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 6 Participation scale of HRQoL. | ||||
| 7 Exercise capacity: 6MWT Show forest plot | 2 | 692 | Mean Difference (IV, Random, 95% CI) | ‐9.03 [‐26.87, 8.81] |
| Analysis 1.7  Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 7 Exercise capacity: 6MWT. | ||||
| 8 Balance: BBS Show forest plot | 2 | 692 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐1.96, 1.28] |
| Analysis 1.8  Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 8 Balance: BBS. | ||||
| 9 Adverse events: number of dropouts during the intervention period Show forest plot | 4 | 829 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [1.11, 1.97] |
| Analysis 1.9  Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 9 Adverse events: number of dropouts during the intervention period. | ||||
| 10 Adverse events: serious adverse events related to intervention/during the intervention period Show forest plot | 2 | 692 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.04, 3.33] |
| Analysis 1.10  Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 10 Adverse events: serious adverse events related to intervention/during the intervention period. | ||||
| 11 Adverse events: falls Show forest plot | 3 | 802 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.92, 1.55] |
| Analysis 1.11  Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 11 Adverse events: falls. | ||||
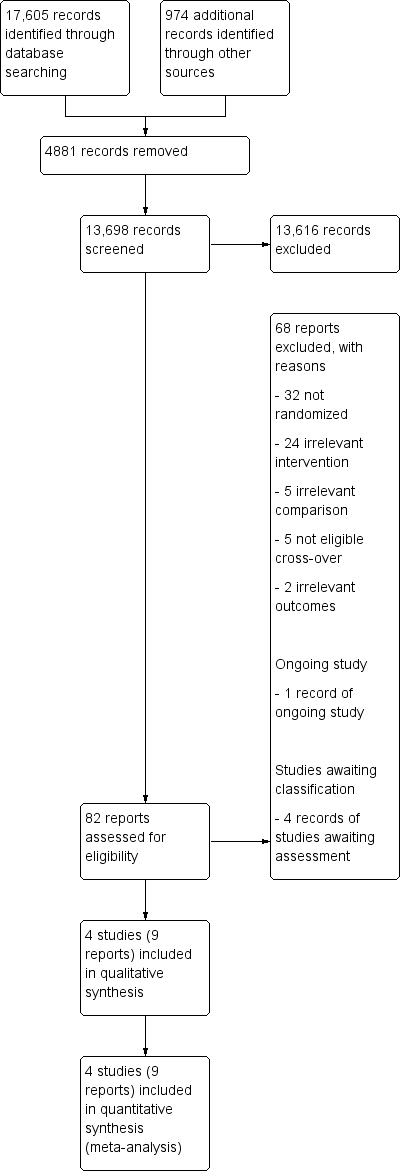
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
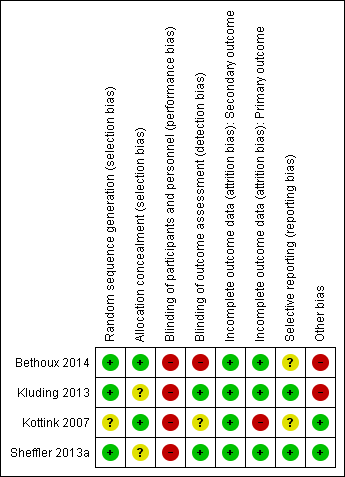
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 1 Activities involving limbs: walking speed until 6 months of device use.

Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 2 Activities involving limbs: walking speed between 6 and 12 months of device use.

Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 3 Activities involving limbs: walking speed.

Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 4 Activities involving limbs: TUG.

Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 5 Activities involving limbs: mEFAP.

Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 6 Participation scale of HRQoL.
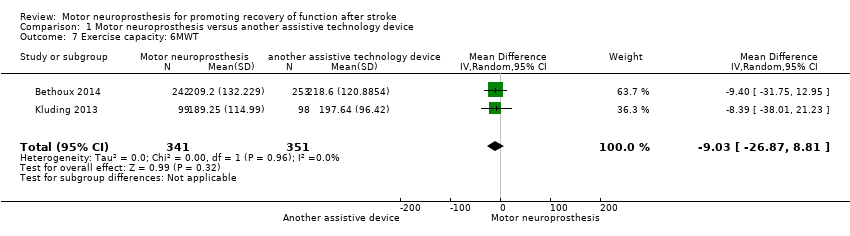
Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 7 Exercise capacity: 6MWT.
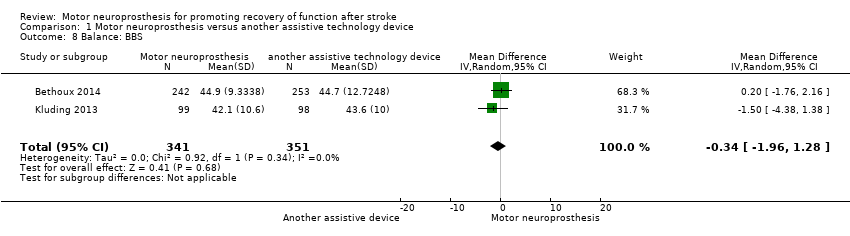
Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 8 Balance: BBS.
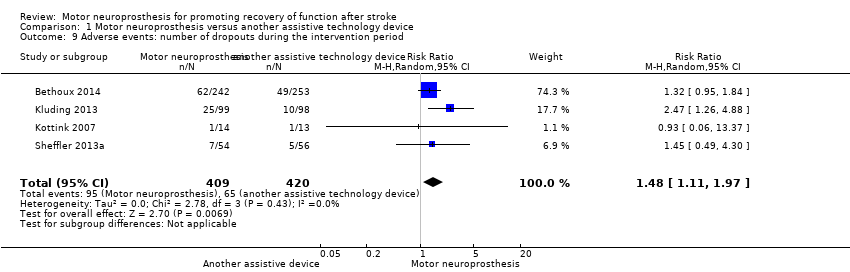
Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 9 Adverse events: number of dropouts during the intervention period.
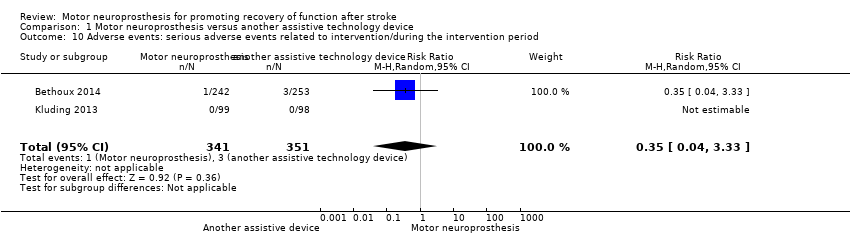
Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 10 Adverse events: serious adverse events related to intervention/during the intervention period.

Comparison 1 Motor neuroprosthesis versus another assistive technology device, Outcome 11 Adverse events: falls.
| Motor neuroprosthesis compared to another assistive technology device for promoting recovery of function after stroke | |||||||
| Patient or population: promoting recovery of function after stroke | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | ||
| Risk with another assistive technology device | Risk with motor neuroprosthesis | ||||||
| Independence in activities of daily living | (No data) | ‐ | ‐ | No studies | Insufficient evidence | No trials measured this outcome. | |
| Activities involving limbs | Walking speed until 6 months of device use (m/s) timed measures at the end of treatment | The mean walking speed in the control group was on average 0.58 m/s. | 0.05 mean difference lower | ‐ | 605 (2 RCTs) | ⊕⊕⊕⊝ | Minimal important difference for comfortable walking speed in chronic stroke participant is 0.2 m/s (Hiengkaew 2012). |
| Walking speed between 6 and 12 months of device use (m/s) timed measures at the end of treatment | The mean walking speed in the control group was on average 0.69 m/s. | 0 mean difference (0.05 lower to 0.05 higher) | ‐ | 713 (3 RCTs) | ⊕⊕⊝⊝ | Minimal important difference for comfortable walking speed in chronic stroke participant is 0.2 m/s (Hiengkaew 2012). | |
| TUG (s) timed measures at the end of treatment | The mean TUG in the control group was on average 27.57 s. | 0.51 mean difference higher (4.41 lower to 5.43 higher) on intervention group | ‐ | 692 | ⊕⊕⊕⊝ | ||
| mEFAP (s) timed measures at the end of treatment | The mean mEFAP in the control group was on average 286.43 s. | 14.77 mean difference higher (12.52 lower to 42.06 higher) on intervention group | ‐ | 605 | ⊕⊕⊝⊝ | ||
| Participation scales of HRQoL timed measures at the end of treatment | The mean participation scales of HRQoL in the control groups was NA.d | 0.26 standardized mean difference (0.22 lower to 0.74 higher) | ‐ | 632 (3 RCTs) | ⊕⊝⊝⊝ | Using Cohen's rules of thumb, 0.26 represents a small effect. | |
| Exercise capacity: 6MWT (m) timed measures at the end of treatment | The mean 6MWT in the control group was on average 208.12 m. | 9.03 mean difference lower (26.87 lower to 8.81 higher) on intervention group | ‐ | 692 | ⊕⊕⊝⊝ | There are no accurate indices of minimal important difference for 6MWT in people poststroke whose gait speed was ≥ 0.40 m/s (Fulk 2018). | |
| Balance: BBS timed measures at the end of treatment | The mean BBS in the control group was on average 44.15. | 0.34 mean difference lower (1.96 lower to 1.28 higher) on intervention group | ‐ | 692 | ⊕⊕⊕⊝ | Minimal detectable change for BBS in chronic stroke participant is 5 points (Hiengkaew 2012). | |
| Adverse events | Number of dropouts during the intervention period | Study population | RR 1.48 | 829 | ⊕⊕⊝⊝ | ||
| 96 per 1000** | 142 per 1000** | ||||||
| Falls | Study population | RR 1.20 | 802 | ⊕⊕⊕⊝ | |||
| 296 per 1000** | 355 per 1000** | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). **We used the median control group risk across studies. 6MWT: 6‐minute walk test; BBS: Berg Balance Scale; CI: confidence interval; HRQoL: health‐related quality of life; mEFAP: modified Emory Functional Ambulation Profile; NA: not applicable; RCT: randomized controlled trial; RR: risk ratio; TUG: Timed Up and Go test. | |||||||
| GRADE Working Group grades of evidence | |||||||
| aThe outcome assessors were not blinded in the larger study. | |||||||
| Study ID (report) | MN device | Duration of exposure to MN intervention | Conditioning protocol used to adapt participants to MN use | MN use/daily use for increasing the activities and participation in the home or community context |
| The MN used was the WalkAide device (Innovative Neurotronics, Austin, TX, USA). It is a commercially available, battery‐operated, single‐channel surface peroneal nerve stimulator that consists of a cuff worn around the proximal part of the lower leg, which holds the control module and surface electrodes. This device uses a tilt sensor and an accelerometer to trigger ankle dorsiflexion and control the timing and duration of peroneal nerve stimulation during the swing phase of gait to alleviate foot drop. | The duration of MN intervention was 12 months. The conditioning protocol occurred in the first 2 weeks, after which participants started daily use of MN device. | The first part consisted of fitting and programming the MN device as well as patient education performed by WalkAide‐certified orthotist or licensed physical therapist. The conditioning protocol included a 2‐week progressive wearing schedule of MN device. | Participants were instructed to wear MN device on a full‐time basis (quote: "ie, for all walking activities throughout the day"). | |
| The MN used was the NESS L300 device (Bioness Inc, Valencia, CA, USA). It is a commercially available, battery‐operated, single‐channel surface peroneal nerve stimulator that consists of a cuff with integrated stimulation unit and electrodes, a control unit, and an in‐shoe pressure sensor. The pressure sensor detects heel off and initial contact events during gait. It transmits wireless signals to the stimulation cuff, which initiates or pauses the stimulation of deep and superficial branches of the peroneal nerve via 2 surface electrodes that activate dorsiflexors and evertors muscles to ensure foot clearance during the swing phase of gait and prevent excessive ankle inversion during early stance. | The duration of MN intervention was 30 weeks. The conditioning protocol occurred in the first 6 weeks. Participants used the MN device all day between week 4 and week 30. | The first part consisted of initial fitting of the device, gait training, wearing schedule, home exercise program, and participant education based on manufacturer standardized protocols. For the first 3 weeks, participants followed the standard conditioning protocol (gradually increasing walking with the MN from 15 minutes each day to all‐day use). During the same period, participants also used the MN for cyclic stimulation while not walking in order to gradually strengthen and condition the muscles to avoid fatigue when using the device (Dunning 2013).* During the first 6 weeks of the study, participants also received 8 dose‐matched sessions of physical therapy. The first 2 to 4 therapy visits focused on education on device use, initial gait training, and an individualized home exercise program. The remaining physical therapy sessions focused on gait training (Kluding 2013). | Participants used the MN all day for ambulation (Dunning 2013).* | |
| The MN used was the STIMuSTEP device (FineTech Medical Ltd, Hertfordshire, UK). It is a commercially available, battery‐operated, 2‐channel implantable device composed of implantable components such as a stimulator, 2 leads, and bipolar intraneural electrodes, and non‐implantable components such as an external transmitter with a built‐in antenna and a pressure sensor. 1 electrode is surgically positioned under the epineurium of the superficial peroneal nerve and the other under the epineurium of the deep peroneal nerve. This device promotes the ankle dorsiflexion/eversion during gait to correct foot drop, and a pressure sensor placed inside the shoe determines the on and off switching of the stimulation. | The duration of MN intervention was 26 weeks. The intervention began with the surgical procedure for placement of the implant. After 2 weeks of the surgery, the wound was checked and first test stimulation took place. The conditioning protocol began at the third week, and all‐day MN use began at the sixth week. | Quote: "Two weeks after the surgery the wound was checked and a first test stimulation took place. In the third week, stimulation during walking was tested and the stimulator was taken home by the patient. The use of the stimulator was gradually increased over 2 weeks to prevent severe muscle pain and fatigue. After this period patients were allowed to use the system all day." | Participants were allowed to use the system all day between week 6 and week 26. | |
| The MN used to correct foot drop was the Odstock Dropped‐Foot Stimulator (ODFS) device (Odstock Medical Ltd, Salisbury Wiltshire, UK). The ODFS is a commercially available, battery‐operated, single‐channel surface peroneal nerve stimulator consisting of an electrical stimulator, a control module, pressure sensors, and surface electrodes. The stimulation is triggered by an insole pressure‐sensing foot switch that detects heel rise at pre‐swing. | The duration of MN intervention was 12 weeks. The conditioning protocol occurred over the 12 weeks. Daily MN use began once device safety was demonstrated by participants. | In the first 5 weeks the Functional Training phase (2 x 1‐hour sessions per week) took place, in which participants were trained to use MN device for home and community mobility with an assistive device, if needed. Activities included passive and active range‐of‐motion exercises, lower extremity strengthening, standing balance and weight‐shifting activities to the affected limb with transition to least‐restrictive assistive device, and refinement of a reciprocal gait pattern. Exercises were done with multiple repetitions with an increase in difficulty and a decrease in cues, with and without the MN device, as appropriate. In the last 7 weeks the Post‐Functional Training Phase (3 x 1‐hour sessions) took place, in which device function, application, and usage guidelines were reviewed with each participant to maximize MN compliance. | The article did not explicitly mention when participants started all‐day MN use, but reported that as soon as participants demonstrated safe use of the device, it was used up to 8 hours per day. | |
| MN: motor neuroprosthesis *Dunning 2013 corresponds to the published protocol of the study Kluding 2013. | ||||
| Study ID (report) | Independence in ADL | Activities involving limbs | Participation scales of HRQoL | Exercise capacity | Balance |
| (Bethoux 2014; 6‐month assessment) | ‐ | Comfortable walking speed measured by 10MWT, TUG, mEFAP | SSQoL (total value); SIS (all domains) | 6MWT | BBS |
| (Bethoux 2015; 12‐month assessment) | ‐ | Comfortable walking speed measured by 10MWT, mEFAP | ‐ | 6MWT | ‐ |
| ‐ | Comfortable and fast walking speed measured by 10MWT, TUG | SIS (ADL/iADL, Mobility, Participation domains) | 6MWT | BBS; FRT | |
| Kottink 2007 (Kottink 2007; Kottink 2008; Kottink 2010; Kottink 2012) | ‐ | Comfortable walking speed motion analysis system | SF‐36 (all domains) | ‐ | ‐ |
| ‐ | Comfortable walking speed measured by motion analysis system, mEFAP | SSQoL (total value) | ‐ | ‐ | |
| 6MWT: 6‐minute walk test | |||||
| Study ID (report) | Motor neuroprosthesis | Another assistive technology device |
| (Bethoux 2014; 6‐month assessment)* | 2 deceased; 25 non‐compliance with protocol; 15 participant request; 7 medical reasons; 4 lost to follow‐up; 2 investigator withdrew | 2 deceased; 13 non‐compliance with protocol; 18 participant request; 4 medical reasons; 3 lost to follow‐up; 1 investigator withdrew |
| (Bethoux 2015; 12‐month assessment)** | 2 deceased; 25 non‐compliance with protocol; 16 participant request; 7 medical reasons; 6 lost to follow‐up; 6 investigator withdrew | 3 deceased; 15 non‐compliance with protocol; 19 participant request; 4 medical reasons; 6 lost to follow‐up; 2 investigators withdrew |
| 2 lost to follow‐up; 23 discontinued intervention | 1 lost to follow‐up; 9 discontinued intervention | |
| Kottink 2007 (Kottink 2007; Kottink 2008; Kottink 2010; Kottink 2012) | 1 technical defect in the epineural electrode | 1 psychological issues not related to the study |
| 6 non‐medical reasons; 1 medical reason
| 2 non‐medical reasons; 3 medical reasons
| |
| *Bethoux 2014 (six‐month assessment) corresponds to the first report of Bethoux 2014 study whose assessment was made after six months of motor neuroprosthesis use. | ||
| Outcome | Study ID (report) | Analysis results |
| Activities involving limbs: walking speed until 6 months of device use | MD −0.07, 95% CI −0.16 to 0.02; P = 0.13; participants = 110; I2 = 0% | |
| Activities involving limbs: walking speed between 6 and 12 months of device use | MD 0.04, 95% CI −0.09 to 0.16; P = 0.57; participants = 218; I2 = 52% | |
| Activities involving limbs: TUG | MD 0.88, 95% CI −6.36 to 8.12; P = 0.81; participants = 197; I2 = 0% | |
| Activities involving limbs: mEFAP | MD 14.45, 95% CI −13.97 to 42.87; P = 0.32; participants = 110; I2 = 0% | |
| Participation scale of HRQoL | SMD 0.60, 95% CI −0.39 to 1.59; P = 0.24; participants = 137; I2 = 79% | |
| Exercise capacity: 6MWT | MD −8.39, 95% CI −38.01 to 21.23; P = 0.58; participants = 197; I2 = 0% | |
| Balance: BBS | MD −1.50, 95% CI −4.38 to 1.38; P = 0.31; participants = 197; I2 = 0% | |
| 6MWT: 6‐minute walk test | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Activities involving limbs: walking speed until 6 months of device use Show forest plot | 2 | 605 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.10, ‐0.00] |
| 2 Activities involving limbs: walking speed between 6 and 12 months of device use Show forest plot | 3 | 713 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.05, 0.05] |
| 3 Activities involving limbs: walking speed Show forest plot | 4 | 823 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.06, 0.04] |
| 3.1 Surface MN | 3 | 802 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.06, 0.02] |
| 3.2 Implantable MN | 1 | 21 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.04, 0.28] |
| 4 Activities involving limbs: TUG Show forest plot | 2 | 692 | Mean Difference (IV, Random, 95% CI) | 0.51 [‐4.41, 5.43] |
| 5 Activities involving limbs: mEFAP Show forest plot | 2 | 605 | Mean Difference (IV, Random, 95% CI) | 14.77 [‐12.52, 42.06] |
| 6 Participation scale of HRQoL Show forest plot | 3 | 632 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.22, 0.74] |
| 7 Exercise capacity: 6MWT Show forest plot | 2 | 692 | Mean Difference (IV, Random, 95% CI) | ‐9.03 [‐26.87, 8.81] |
| 8 Balance: BBS Show forest plot | 2 | 692 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐1.96, 1.28] |
| 9 Adverse events: number of dropouts during the intervention period Show forest plot | 4 | 829 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [1.11, 1.97] |
| 10 Adverse events: serious adverse events related to intervention/during the intervention period Show forest plot | 2 | 692 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.04, 3.33] |
| 11 Adverse events: falls Show forest plot | 3 | 802 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.92, 1.55] |

