Regímenes de tratamiento acortados versus régimen estándar para la tuberculosis pulmonar sensible a los fármacos
Resumen
Antecedentes
La tuberculosis causa más muertes que cualquier otra enfermedad infecciosa en el mundo, siendo la tuberculosis pulmonar la forma más común. El tratamiento estándar de primera línea para la tuberculosis pulmonar sensible a los fármacos durante seis meses incluye isoniazida, rifampicina, pirazinamida y etambutol (HRZE) durante dos meses, seguido de HRE (en áreas de alta resistencia a los fármacos contra la tuberculosis) o HR, durante una fase de continuación de cuatro meses. Muchos pacientes no completan este curso completo. Los regímenes de tratamiento acortados que presentan la misma efectividad y seguridad podrían mejorar el éxito del tratamiento.
Objetivos
Evaluar la eficacia y la seguridad de los regímenes de tratamiento acortados versus régimen de tratamiento estándar de seis meses para los individuos con tuberculosis pulmonar sensible a los fármacos.
Métodos de búsqueda
Se realizaron búsquedas en las siguientes bases de datos hasta el 10 de julio 2019: el Registro Especializado del Grupo Cochrane de Enfermedades Infecciosas (Cochrane Infectious Diseases Group Specialized Register); el Registro Cochrane Central de Ensayos Controlados (Central Register of Controlled Trials, CENTRAL), la Cochrane Library; MEDLINE (PubMed); Embase; la Latin American Caribbean Health Sciences Literature (LILACS); Science Citation Index‐Expanded; Indian Medlars Center; y la South Asian Database of Controlled Clinical Trials. También se realizaron búsquedas de ensayos en curso en la International Clinical Trials Registry Platform de la Organización Mundial de la Salud (OMS), ClinicalTrials.gov, la Clinical Trials Unit of the International Union Against Tuberculosis and Lung Disease, la UK Medical Research Council Clinical Trials Unit, y el Clinical Trials Registry India. Se verificaron las listas de referencias de los artículos identificados para encontrar estudios adicionales pertinentes.
Criterios de selección
Se buscaron ensayos controlados aleatorizados (ECA) o cuasialeatorizados que compararan regímenes de menor duración (menos de seis meses) versus el régimen estándar de seis meses para pacientes de todas las edades, independientemente de su estado serológico respecto al VIH, que habían sido diagnosticados recientemente con tuberculosis pulmonar por medio de un cultivo de esputo o una GeneXpert positiva, y con tuberculosis sensible a los fármacos presunta o comprobada. El resultado primario de interés fue la recaída dentro de los dos años posteriores a la finalización del tratamiento antituberculoso (TAT).
Obtención y análisis de los datos
Dos autores de la revisión, de forma independiente, seleccionaron los ensayos, extrajeron los datos y evaluaron el riesgo de sesgo de los ensayos incluidos. Para los resultados dicotómicos, se utilizaron los riesgos relativos (RR) con intervalos de confianza (IC) del 95%. Cuando fue apropiado, se agruparon los datos de los ensayos incluidos en los metanálisis. La certeza de la evidencia se evaluó con los criterios GRADE.
Resultados principales
Se incluyeron cinco ensayos aleatorizados que comparaban regímenes de TAT de cuatro meses que contenían fluoroquinolona versus regímenes estándar de TAT de seis meses y que habían reclutado a 5825 adultos con tuberculosis pulmonar sensible a los fármacos recién diagnosticada de 14 países con alta transmisión de tuberculosis de Asia, África y América Latina. Tres eran ensayos multinacionales que incluían a un total de 572 pacientes con pruebas positivas para el VIH. Estos ensayos excluyeron a los niños, a las mujeres embarazadas o que amamantaban, a los pacientes con afecciones comórbidas graves y a los pacientes con diabetes mellitus. Cuatro ensayos tenían brazos de tratamiento múltiples.
La moxifloxacina reemplazó el etambutol en los regímenes estándar de TAT de cuatro meses, administrados una vez al día o tres veces por semana en dos ensayos; la moxifloxacina reemplazó la isoniazida en los regímenes de TAT de cuatro meses en dos ensayos, se administró una vez al día en un ensayo y se administró con rifapentina en lugar de rifampicina una vez al día durante dos meses y dos veces por semana durante dos meses en un ensayo. La moxifloxacina se agregó a los fármacos del TAT estándar durante tres a cuatro meses en un ensayo en curso que informó resultados provisionales. La gatifloxacina reemplazó el etambutol en los regímenes estándar de TAT administrados diariamente o tres veces por semana durante cuatro meses en dos ensayos. El seguimiento varió de 12 a 24 meses después de la finalización del tratamiento para la mayoría de los participantes.
Regímenes de TAT de cuatro meses que contienen moxifloxacina
Los regímenes de TAT de cuatro meses que contienen moxifloxacina que reemplazaron el etambutol o la isoniazida probablemente aumentaron las proporciones que experimentaron la recaída después del tratamiento exitoso en comparación con los regímenes de TAT estándar (RR 3,56; IC del 95%: 2,37 a 5,37; 2265 participantes, tres ensayos; evidencia de certeza moderada). Para la muerte por cualquier causa, probablemente hubo poca o ninguna diferencia entre los dos regímenes (2760 participantes, tres ensayos; evidencia de certeza moderada). El fracaso del tratamiento fue poco frecuente, y probablemente hubo poca o ninguna diferencia en las proporciones que presentaron el fracaso del tratamiento entre los regímenes de TAT (2282 participantes, tres ensayos; evidencia de certeza moderada). Ninguno de los participantes que recibieron regímenes que contenían moxifloxacina desarrolló resistencia a la rifampicina, y es posible que estos regímenes no aumenten el riesgo de resistencia adquirida (2282 participantes, tres ensayos; evidencia de certeza baja). Los eventos adversos graves probablemente presentaron poco o ninguna diferencia con los regímenes de cuatro meses que contenían moxifloxacina que reemplazaron el etambutol o la isoniazida, y con los regímenes de tres a cuatro meses que aumentaron el TAT estándar con moxifloxacina, en comparación con los regímenes estándar de TAT de seis meses (3548 participantes, cuatro ensayos; evidencia de certeza moderada).
Regímenes de TAT de cuatro meses que contienen gatifloxacina
Los regímenes de TAT de cuatro meses con gatifloxacina que reemplazaron el etambutol probablemente aumentaron la recaída en comparación con los regímenes estándar de TAT de seis meses en adultos con tuberculosis pulmonar sensible a los fármacos (RR 2,11; IC del 95%: 1,56 a 2,84; 1633 participantes, dos ensayos; evidencia de certeza moderada). El régimen de cuatro meses probablemente logró poca o ninguna diferencia en la muerte en comparación con el régimen de seis meses (1886 participantes, dos ensayos; evidencia de certeza moderada). El fracaso del tratamiento fue poco frecuente y probablemente presentó poca o ninguna diferencia entre los regímenes de cuatro y seis meses (1657 participantes, dos ensayos; evidencia de certeza moderada). No se detectó resistencia adquirida a la isoniazida ni a la rifampicina en los que recibieron el régimen de TAT acortado que contiene gatifloxacina, pero no se sabe si la resistencia adquirida al fármaco difiere con relación a los regímenes de cuatro y seis meses (429 participantes, un ensayo; evidencia de certeza muy baja). Los eventos adversos graves probablemente no fueron diferentes con ninguno de los dos regímenes (1993 participantes, dos ensayos; evidencia de certeza moderada).
Conclusiones de los autores
La evidencia hasta la fecha no apoya el uso de regímenes acortados de TAT en adultos con tuberculosis pulmonar sensible a los fármacos recién diagnosticada. Los regímenes de TAT de cuatro meses que reemplazan el etambutol con moxifloxacina o gatifloxacina, o la isoniazida con moxifloxacina, aumentan de forma considerable la recaída en comparación con los regímenes estándar de TAT de seis meses, aunque el éxito del tratamiento y los eventos adversos graves presentan poca o ninguna diferencia. Los resultados de seis ensayos grandes en curso ayudarán a informar las decisiones sobre si los regímenes de TAT acortados pueden reemplazar los regímenes estándar de TAT de seis meses.
PICO
Resumen en términos sencillos
Regímenes de tratamiento más cortos para los pacientes con tuberculosis pulmonar
¿Cuál era el objetivo de esta revisión?
El objetivo de esta revisión Cochrane fue averiguar si la duración del tratamiento antituberculoso (TAT) para las personas con tuberculosis pulmonar sensible a los fármacos diagnosticada recientemente puede acortarse a menos de seis meses. Los autores de la revisión Cochrane recopilaron y analizaron todos los estudios relevantes para responder a esta pregunta y encontraron cinco estudios relevantes.
Mensajes clave
Los regímenes de TAT acortados probablemente logran poca o ninguna diferencia en la muerte, el fracaso del tratamiento o los eventos adversos graves en comparación con los regímenes de TAT de seis meses, aunque probablemente aumentan la recaída de la tuberculosis. Hay seis ensayos grandes en curso que están estudiando esta cuestión.
¿Qué se estudió en la revisión?
La tuberculosis es una enfermedad infecciosa, y la tuberculosis que afecta los pulmones (tuberculosis pulmonar) es la presentación más frecuente de la tuberculosis en adultos. La tuberculosis es un problema importante de salud pública en todo el mundo y, entre las enfermedades infecciosas, es la causa principal de muerte.
Los pacientes con tuberculosis pulmonar actualmente reciben tratamiento durante seis meses con una combinación de fármacos que incluyen isoniazida, rifampicina, etambutol y pirazinamida durante dos meses, seguido de isoniazida y rifampicina (con o sin etambutol) durante cuatro meses. Muchos pacientes no terminan el tratamiento o toman los fármacos de manera irregular debido a la duración larga del tratamiento o a los efectos secundarios de los fármacos. El tratamiento incompleto o irregular puede dar lugar al fracaso del tratamiento y puede aumentar la recaída de la enfermedad. Este tratamiento también puede dar lugar a resistencia a los fármacos. Si las combinaciones de medicamentos más nuevas administradas durante menos de seis meses presentan las misma efectividad y seguridad que los regímenes de TAT de seis meses recomendados actualmente, es posible que más pacientes se adhieran y completen el tratamiento. Este hecho podría ayudar a reducir la resistencia a los fármacos y podría ayudar a detener la infección por tuberculosis a nivel mundial.
¿Cuáles son los principales resultados de la revisión?
Los cinco ensayos incluidos estudiaron a 5825 adultos con tuberculosis pulmonar sensible a los fármacos recién diagnosticada de 14 países con alta transmisión de tuberculosis de Asia, África y América Latina. Tres ensayos incluyeron a 572 pacientes con pruebas positivas, pero todos excluyeron a los pacientes con otras afecciones comórbidas graves y a los pacientes con diabetes mellitus. Este hecho redujo la aplicabilidad de los resultados de los estudios. Todos fueron financiados por el gobierno o por agencias internacionales.
Cuatro estudios reemplazaron la isoniazida o el etambutol con moxifloxacina o gatifloxacina en regímenes de TAT de cuatro meses. El seguimiento se realizó durante 12 a 24 meses después de la finalización del tratamiento. En un estudio en curso, la moxifloxacina se agregó al TAT de cuatro meses, pero los autores del estudio solo proporcionaron resultados provisionales.
Cuando se comparan los regímenes de TAT de cuatro meses con los regímenes estándar de TAT de seis meses, esta revisión muestra lo siguiente:
‐ La recaída probablemente aumenta después de un tratamiento exitoso (evidencia de certeza moderada).
‐ La muerte por cualquier causa, el fracaso del tratamiento y los eventos adversos graves probablemente presentan poca o ninguna diferencia (evidencia de certeza moderada).
‐ La resistencia a los fármacos puede no aumentar con los regímenes de cuatro meses que contienen moxifloxacina (evidencia de certeza baja), aunque no se conoce si lo anterior se aplica a los regímenes que contienen gatifloxacina (evidencia de certeza muy baja).
¿Cuál es el grado de actualización de esta revisión?
Los autores de la revisión buscaron los estudios disponibles hasta el 10 de julio 2019.
Conclusiones de los autores
Summary of findings
| Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimen for drug‐sensitive pulmonary tuberculosis | ||||||
| Patient or population: adults with drug‐sensitive pulmonary tuberculosis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with 6‐month standard ATT | Risk with 4‐month moxifloxacin‐containing ATT | |||||
| Relapse | 32 per 1000 | 82 more relapses per 1000 | RR 3.56 | 2265 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably increases relapse compared to the 6‐month regimen |
| Death from any cause Follow‐up: range 18 to 24 months | 21 per 1000 | 2 more deaths per 1000 | RR 1.06 | 2760 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably makes little or no difference in death from any cause compared to the 6‐month regimen |
| Treatment failure | 16 per 1000 | 5 fewer treatment failures per 1000 | RR 0.71 | 2282 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably results in little or no difference in treatment failure compared to the 6‐month regimen |
| Acquired drug resistance | 7 per 1000 | 5 fewer with acquired drug resistance per 1000 (6 fewer to 2 more) | RR 0.33 | 2282 (3 RCTs)e | ⊕⊕⊝⊝ Due to indirectness and imprecision | The 4‐month regimen may be little or no different than the 6‐month regimen in the incidence of acquired drug resistance |
| Serious adverse events Follow‐up: range 18 to 24 months | 62 per 1000 | 2 fewer with serious adverse events per 1000 | RR 0.97 | 3548 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably results in little or no difference in serious adverse events compared to the 6‐month regimen |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aNo serious risk of bias: although Jawahar 2013 was at high risk of allocation bias, exclusion of this trial from the sensitivity analysis did not change the direction of effect. Not downgraded. | ||||||
| Gatifloxacin‐containing 4‐month ATT regimens compared to standard 6‐month ATT regimens for drug‐sensitive pulmonary tuberculosis | ||||||
| Patient or population: adults with drug‐sensitive pulmonary tuberculosis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with 6‐month standard ATT | Risk with gatifloxacin‐containing | |||||
| Relapse | 70 per 1000 | 77 more relapses per 1000 | RR 2.11 | 1633 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably increases relapse compared to the 6‐month regimen |
| Death from any cause | 29 per 1000 | 3 fewer deaths per 1000 | RR 0.90 | 1886 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably makes little or no difference in death compared to the 6‐month regimen |
| Treatment failure | 25 per 1000 | 1 less treatment failure per 1000 | RR 0.93 | 1657 | ⊕⊕⊝⊝ Due to indirectness | The 4‐month regimen probably makes little or no difference in treatment failure compared to the 6‐month regimen |
| Acquired drug resistance | 12 per 1000 | 9 fewer with acquired drug resistance per 1000 (12 fewer to 49 more) | RR 0.24 (0.01 to 5.01) | 301 (1 RCT)d | ⊕⊝⊝⊝ Due to indirectness, risk of bias, and imprecision | We do not know if acquired drug resistance is any different in the 4‐month and the 6‐month regimens |
| Serious adverse events | 24 per 1000 | 0 fewer serious adverse events per 1000 | RR 1.02 | 1993 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably results in little or no difference in serious adverse events compared to the 6‐month regimen |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. | ||||||
| aNo serious risk of bias: although Jawahar 2013 was assigned high risk of bias for allocation concealment, removal of this trial from the sensitivity analysis did not significantly alter the direction, magnitude, or precision of the effect estimate. Not downgraded. | ||||||
Antecedentes
Descripción de la afección
La tuberculosis (TB), una enfermedad infecciosa crónica causada por la transmisión aérea de pequeñas gotas aerosolizadas de Mycobacteriumtuberculosis, es un problema importante de salud pública a nivel mundial (OMS 2018). Se estima que en 2017 se produjeron 10 000 000 de casos nuevos de tuberculosis y 1 600 000 de muertes relacionadas con la tuberculosis, lo que convierte a la tuberculosis en una de las 10 causas principales de muerte en todo el mundo (OMS 2018). Entre los casos nuevos identificados, el 90% eran adultos, el 58% hombres, el 10% niños y el 9% presentaban coinfección por el VIH (OMS 2018). Entre las enfermedades transmisibles, la tuberculosis es una causa principal de mortalidad en el grupo etario económicamente productivo (15 a 49 años) (OMS 2017). Los ocho países afectados con la carga más alta de tuberculosis en el mundo son la India, China, Filipinas, Indonesia, Pakistán, Nigeria, Bangladesh y Sudáfrica (OMS 2018), y el 87% de la tuberculosis ocurre en 30 países de carga alta (OMS 2018). Como agregado a la carga existente, en 2017 se diagnosticaron 558 000 casos nuevos de tuberculosis resistente a la rifampicina, y de estos pacientes, el 82% tenía tuberculosis resistente a múltiples fármacos (TB‐RMF) (OMS 2018). Aunque la mortalidad relacionada con la tuberculosis se redujo en un 23% entre 2000 y 2017 en todo el mundo, aún hay brechas en el diagnóstico y el tratamiento (OMS 2018).
En mayo 2014 la World Health Assemby aprobó la "End TB Strategy (Estrategia para poner fin a la tuberculosis)" de la Organización Mundial de la Salud (OMS), cuyo objetivo es lograr una reducción del 95% de la mortalidad causada por la tuberculosis y una reducción del 90% de la incidencia de casos nuevos para el año 2035 en comparación con las estimaciones de 2015 (OMS 2015). Lo anterior puede ser el resultado de una disminución considerable del número de casos de tuberculosis y de muertes en los años venideros. Sin embargo, la tasa de disminución de la incidencia de la tuberculosis fue del 1,9% entre 2015 y 2016; para alcanzar los objetivos de la "End TB Strategy", esta tasa de disminución debe aumentar al 4% o al 5% anual para 2020. Al administrar el régimen de tratamiento estándar actual aprobado por la OMS, la tasa de éxito del tratamiento para los individuos con casos nuevos y con recidivas de la tuberculosis sensible a los fármacos, según se informó para la cohorte de 2015; fue del 83% (OMS 2017a). Aunque esta tasa de éxito es alta en comparación con la de los pacientes con TB‐RMF (tasa de éxito del 54%), los resultados deficientes como la falta de respuesta, la muerte y las pérdidas durante el seguimiento son motivo de gran preocupación, debido a que uno de los objetivos de desarrollo sostenible de la OMS para 2030 es poner fin a la epidemia mundial de tuberculosis (OMS 2015; OMS 2018).
El régimen estándar actual aprobado por la OMS consiste en isoniazida, rifampicina, pirazinamida y etambutol (HRZE) durante dos meses (fase intensiva), seguido de isoniazida y rifampicina con etambutol (HRE) en áreas de alta resistencia, o sin etambutol (HR) durante cuatro meses (fase de continuación) (OMS 2010). Esta duración del tratamiento de seis meses puede tener un impacto adverso en la adherencia al tratamiento por parte del paciente (Zumla 2014). La adherencia deficiente da lugar al desarrollo de resistencia a los fármacos y aumenta la posibilidad de recaída en estos individuos (Ginsberg 2010; Ma 2010). Por lo tanto, se necesitan nuevas combinaciones de fármacos para acortar el curso del tratamiento mientras se mantienen tasas altas de éxito y tasas bajas de recaída. La posibilidad de acortar la duración del tratamiento para los pacientes con tuberculosis sensible a los fármacos o resistente a los fármacos es una prioridad de investigación mundial y, sin duda, será muy beneficiosa tanto para los pacientes como para los profesionales sanitarios. Han comenzado a surgir nuevos fármacos para la tuberculosis a partir del flujo de desarrollo clínico, y los regímenes de corta duración que contienen compuestos nuevos podrían mejorar la adherencia al tratamiento y al mismo tiempo promover el control de la infección y dar lugar a un mejor manejo de la enfermedad (Ma 2010).
Descripción de la intervención
La necesidad de una terapia de combinación para la tuberculosis es el resultado de la estructura celular distintiva de la Mtuberculosis (un conjunto complejo de lípidos, proteínas y glicolípidos) y la tendencia de los bacilos a desarrollar resistencia a la monoterapia (Kerantzas 2017). Se necesitan combinaciones de fármacos para tratar la Mtuberculosis: la combinación de fármacos con actividad bactericida y actividad esterilizante puede ayudar a dirigir los objetivos a las diversas subpoblaciones bacterianas (bacilos de división activa, crecimiento lento y latentes) presentes (Mitchison 1985). La actividad bactericida de un fármaco se refiere a su capacidad de matar bacilos metabólicamente activos. Un fármaco bactericida efectivo previene la transmisión de los bacilos y el desarrollo de resistencia a otros fármacos administrados como parte del régimen. La actividad esterilizante de un fármaco se refiere a su capacidad para matar todos los bacilos viables, incluidos los microorganismos tolerantes al tratamiento con fármacos. Los fármacos con buena capacidad de esterilización tienen el potencial de acortar la duración del tratamiento contra la tuberculosis (Ma 2010). En los últimos años, se han probado varios fármacos en diferentes combinaciones para acortar el régimen de tratamiento estándar de seis meses, los cuales han mostrado resultados preliminares prometedores (Conde 2011).
Algunas de las características deseadas de los nuevos compuestos de fármacos antituberculosos son las siguientes (Ma 2010).
-
Efectividad contra los bacilos de la tuberculosis tanto replicadores como latentes.
-
Nuevo mecanismo de acción.
-
Perfil de seguridad mejorado (versus el régimen de tratamiento estándar).
-
Buena biodisponibilidad oral.
-
Barrera de desarrollo de baja resistencia.
-
Mínima interacción con las enzimas del citocromo p450.
-
Bajo coste.
En la actualidad se están desarrollando clínicamente 10 compuestos para el tratamiento de la tuberculosis, se han desarrollado seis específicamente y se han reorientado cuatro fármacos existentes. Los fármacos que están a la vanguardia de esta búsqueda incluyen fluoroquinolonas (moxifloxacina, levofloxacina y gatifloxacina), rifamicinas (rifabutina y rifapentina), nitroimidazoles, diarilquinolinas, oxazolidinedionas y etilendiaminas. Estos fármacos han sido investigados en ensayos clínicos en combinación con, o como sustitutos de, uno de los fármacos antituberculosos estándar de primera línea, con el objetivo de acortar la duración del tratamiento (Lienhardt 2010). Los fármacos de segunda línea contra la tuberculosis, que incluyen clavulanato de amoxicilina, linezolid, carbapenems y clofazimina, también son candidatos potenciales para los regímenes antituberculosos de menor duración (D'Ambrosio 2015).
De qué manera podría funcionar la intervención
Fluoroquinolonas
Las fluoroquinolonas poseen una buena actividad bactericida in vivo e in vitro contra la M tuberculosis (Moadebi 2007). Esta clase de fármacos actúa sobre la enzima ADN‐girasa, y de esa forma previene la síntesis bacteriana de ADN (Lienhardt 2010). Este mecanismo de acción es distinto al de otros fármacos antituberculosos, lo que aumenta la posibilidad de actividad sinérgica. En general, las quinolonas son bien toleradas y presentan efectos secundarios mínimos en la administración a largo plazo (Schluger 2013). Las fluoroquinolonas, cuando se agregan a un régimen de tratamiento antituberculoso, pueden mejorar los efectos esterilizantes y bactericidas del tratamiento combinado, al mismo tiempo que aumentan la penetración del fármaco en las lesiones crónicas de la tuberculosis. Las fluoroquinolonas presentan una mejor tolerabilidad que los fármacos estándar de primera línea y pueden acortar la duración del tratamiento, mejorando así la adherencia al tratamiento por parte del paciente (Ginsburg 2003).
La principal preocupación con las quinolonas es que pueden prolongar el intervalo QT, lo cual puede causar arritmias ventriculares y paro cardíaco repentino (Schluger 2013). La frecuencia de las torsades de pointes ‐el tipo de arritmia inducida por las fluoroquinolonas‐ ha sido informada en 1 por millón con ciprofloxacina o levofloxacina, 3,8 por millón con grepafloxacina, y 14,5 por millón con esparfloxacina. La probabilidad de arritmia es mayor para los pacientes que presentan trastornos metabólicos asociados, como hipopotasemia o enfermedad cardíaca, o que están tomando otros fármacos que pueden prolongar el intervalo QT (Rubinstein 2002). Sin embargo, un análisis agrupado de los datos de ensayos clínicos de fase 2; 3 y 4 que compararon la moxifloxacina con otros antibióticos no mostró diferencias clínicamente relevantes en los efectos adversos cardíacos entre la moxifloxacina y los comparadores (Haverkamp 2012).
Rifamicinas
La rifapentina es una rifamicina de nueva generación que actúa inhibiendo la polimerasa de ARN dependiente del ADN de la Mtuberculosis. Al igual que otras rifamicinas, la rifapentina puede (con poca frecuencia) causar hepatitis inducida por fármacos y trombocitopenia (Munsiff 2006). Lo que hace que la rifapentina sea un buen candidato para el acortamiento del tratamiento de la tuberculosis y la simplificación de la dosis es su vida media larga (10 a 15 horas para la rifapentina frente a 2 a 3 horas para la rifampicina) y su potencia contra la Mtuberculosis (Temple 1999). Sin embargo, en comparación con la rifampicina, la rifapentina tiene una penetración deficiente en las lesiones de la cavidad pulmonar, en particular en el material caseoso licuado que contiene altas concentraciones de bacterias (Rifat 2018). En consecuencia, la rifapentina requiere dosis considerablemente más altas que las que se recomiendan con frecuencia para mejorar los resultados clínicos en la tuberculosis pulmonar; algunos pacientes con lesiones cavitarias pulmonares grandes presentan una respuesta menor al tratamiento incluso con dosis altas de rifapentina (Savic 2017). Debido a que las dosis actualmente recomendadas de rifampicina son menos efectivas que las dosis más altas de rifampicina para lograr una conversión temprana del cultivo, si se puede demostrar que las dosis más altas de rifampicina reducen las tasas de recaída, este hecho podría mejorar la eficacia de las combinaciones de TAT acortadas (Boeree 2017).
Nitroimidazoles
Los nitroimidazoles actúan tanto contra los bacilos multiplicadores como contra los bacilos latentes, por lo que pueden ser adecuados para acortar potencialmente la duración del tratamiento contra la tuberculosis (Ma 2010). En la actualidad se están investigando dos nitroimidazoles en ensayos clínicos para el tratamiento de los individuos con tuberculosis: pretomanid y delamanid. Estos agentes son igualmente activos contra la tuberculosis sensible a los fármacos y resistente a los fármacos. Actúan sobre los bacilos a través de la biorreducción del farmacóforo nitroimidazol, la generación de especies de oxígeno reactivo y la inhibición de la síntesis de ácido micólico (Matsumoto 2006). En los ensayos de fase 2 la prolongación del QT se observó con frecuencia en pacientes con TB‐RMF que recibieron delamanid (Gler 2012). La actividad bactericida de una nueva combinación de pirazinamida, moxifloxacina, clofazimina y pretomanida se ha comparado con la del régimen de tratamiento estándar en individuos con tuberculosis sensible a los fármacos y resistente a los fármacos. Este nuevo régimen fue bien tolerado y mostró una mayor actividad bactericida que el régimen estándar (Dawson 2015).
Diarilquinolinas
Un miembro de esta clase de fármacos, la bedaquilina, ha sido aprobado como fármaco contra la tuberculosis por la European Medicines Agency (EMA) y la Food and Drug Administration (FDA) de los Estados Unidos (Lessem 2015). La bedaquilina interrumpe el metabolismo bacteriano al afectar la síntesis de trifosfato de adenosina (ATP) (Andries 2005). El fármaco se utiliza actualmente para el tratamiento de la TB‐RMF, luego de los hallazgos de un ensayo de fase 2 que demostró una rápida conversión del cultivo de esputo y tasas bajas de resistencia adquirida a los fármacos coadministrados (Diacon 2014). Al igual que las quinolonas, la bedaquilina puede causar prolongación del QT (Diaconía 2012). La bedaquillina tiene propiedades bactericidas tardías potentes que superan las de la rifampicina, en especial durante el segundo mes de tratamiento, y puede tener una actividad esterilizante superior, en particular cuando se combina con pirazinamida, con el potencial de acortar la duración del tratamiento para los pacientes con tuberculosis sensible a los fármacos (Andries 2005).
Oxazolidinedionas
El linezolid y el sutezolid inhiben el inicio de la síntesis de proteínas bacterianas al actuar sobre la subunidad ribosomal 50S. El linezolid, un fármaco con un nuevo propósito, es efectivo en el tratamiento de la tuberculosis resistente a los fármacos, aunque los efectos adversos como la mielosupresión y la neuropatía periférica restringen su uso a largo plazo (Sotgiu 2012). Un nuevo agregado a esta clase ‐ sutezolid ‐ está ganando atención, ya que ha demostrado una mayor potencia como fármaco antituberculoso que el linezolid en modelos murinos (Williams 2009). Los estudios de fase 1 en humanos han encontrado que el sutezolid es seguro y presenta buena tolerabilidad (Wallis 2010).
Etilendiaminas
La etilendiamina, SQ109; inhibe la síntesis de proteínas al dirigirse al transportador de la membrana, MmpL3; en la Mtuberculosis, y es efectiva contra la tuberculosis sensible a los fármacos y resistente a los fármacos. Los estudios in vitro mostraron efectos sinérgicos con la administración de bedaquilina e interacciones favorables con sutezolid (D'Ambrosio 2015; Sacksteder 2012). Sin embargo, la SQ109 no acortó el tiempo hasta la conversión del cultivo en los estudios clínicos cuando se utilizó en lugar del etambutol en los regímenes antituberculosos (Boeree 2017; Svensson 2018). Se necesita investigación adicional para determinar la dosis óptima e identificar las combinaciones de fármacos que podrían optimizar la utilidad de la SQ109; si se considera para la inclusión en los regímenes de acortamiento del tratamiento.
Por qué es importante realizar esta revisión
Se necesitan nuevos regímenes farmacológicos para considerar los desafíos asociados con la adherencia por parte de los pacientes al régimen de tratamiento estándar actual de seis meses para la tuberculosis (Ma 2010). Los ensayos clínicos recientes han investigado la eficacia de los regímenes más nuevos administrados durante menos de seis meses para el tratamiento de los pacientes con tuberculosis sensible a los fármacos. Una revisión sistemática de estos ensayos ayudará a guiar la comprensión de la eficacia y la seguridad de estos regímenes más cortos entre los individuos con tuberculosis pulmonar sensible a los fármacos. Una revisión Cochrane anterior ‐ Gelband 1999 ‐ estableció la conclusión de que los períodos más largos de tratamiento (al menos seis meses) resultaron en tasas de éxito más altas entre los individuos con tuberculosis activa, aunque la mejoría fue pequeña en comparación con los regímenes administrados durante menos de seis meses. Otra revisión Cochrane sobre el uso de fluoroquinolonas para el tratamiento de la tuberculosis, publicada en 2013; estableció la conclusión de que la evidencia era insuficiente para apoyar las conclusiones, aunque señaló que se estaban realizando ensayos más amplios que investigaban los regímenes basados en fluoroquinolonas de corta duración (Ziganshina 2013). El tratamiento de primera línea con combinaciones de fármacos nuevos administrados durante un período de tiempo más corto que el régimen de tratamiento estándar actual de seis meses podría mejorar los resultados del tratamiento, y reducir así las probabilidades de transmisión de la enfermedad y la carga en esta población.
Objetivos
Evaluar la eficacia y la seguridad de los regímenes de tratamiento acortados versus régimen de tratamiento estándar de seis meses para los individuos con tuberculosis pulmonar sensible a los fármacos.
Métodos
Criterios de inclusión de estudios para esta revisión
Tipos de estudios
Ensayos controlados aleatorizados (ECA) y cuasialeatorizados.
Tipos de participantes
Pacientes con un diagnóstico reciente de tuberculosis pulmonar, según lo definido por un cultivo de esputo positivo o de una GeneXpert MTB/RIF positiva, con tuberculosis sensible a los fármacos presunta o comprobada, de todas las edades, independientemente del estado serológico respecto al VIH. Los ensayos que incluían a pacientes con tuberculosis extrapulmonar fueron elegibles cuando dichos participantes constituían menos del 10% de los participantes, o cuando se disponía de datos desglosados.
Tipos de intervenciones
Intervención
Regímenes de tratamiento de menos de seis meses de duración, incluidos todos los fármacos antituberculosos o sus combinaciones (fármacos nuevos o fármacos antituberculosos estándar en dosis superiores a las recomendadas).
Control
Tratamiento estándar de primera línea para la tuberculosis pulmonar, definido como un régimen que comprende dos meses de HRZE y cuatro meses de HR o HRE.
Tipos de medida de resultado
Resultados primarios
-
Recaída de la tuberculosis, definida como recurrencia clínica o bacteriológica dentro de los dos años siguientes a la finalización del tratamiento antituberculoso
Resultados secundarios
-
Muerte por cualquier causa durante el tratamiento antituberculoso o dentro de los dos años siguientes a la finalización del tratamiento
-
Interrupción del tratamiento: tasas de interrupción del tratamiento en cualquier momento durante el tratamiento
-
Cultivo/frotis de esputo positivo a las ocho semanas: proporción de participantes que siguen presentando pruebas positivas al final de las ocho semanas de tratamiento
-
Fracaso del tratamiento: cultivos de esputo positivos persistentes o recurrentes en el momento de la finalización del tratamiento
-
Resistencia adquirida a los fármacos: desarrollo de resistencia secundaria a los fármacos antituberculosos, identificada mediante pruebas de susceptibilidad a los fármacos
Eventos adversos.
-
Eventos adversos graves: eventos adversos mortales o potencialmente mortales, o que dieron lugar a un cambio en el régimen de tratamiento
-
Otros eventos adversos: otros eventos adversos informados por los autores del ensayo, como hepatitis, prolongación del intervalo QT, reacciones de hipersensibilidad, trombocitopenia, neuropatía periférica, toxicidad ocular y artralgia
Métodos de búsqueda para la identificación de los estudios
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press, or in progress).
Búsquedas electrónicas
We searched the following databases up to 10 July 2019 using the search terms and strategy we have described in Appendix 1: the Cochrane Infectious Diseases Group Specialized Register; the Central Register of Controlled Trials (CENTRAL), in the Cochrane Library; MEDLINE (PubMed, from 1966); Embase (OVID, from 1947); the Latin American and Caribbean Health Science Information database (LILACS, from 1982); and Science Citation Index‐Expanded (Web of Science, from 1900). We also searched the website of the Indian Medlars Center (indmed.nic.in/, 10 July 2019) and the South Asian Database of Controlled Clinical Trials (cochrane‐sadcct.org/, 10 July 2019). We searched the WHO International Clinical Trials Registry Platform (who.int/ictrp/en/), ClinicalTrials.gov (clinicaltrials.gov/ct2/home), the Clinical Trials Unit of the International Union Against Tuberculosis and Lung Disease (theunion.org/what‐we‐do/research/clinical‐trials), the UK Medical Research Council Clinical Trials Unit (ctu.mrc.ac.uk/), and Clinical Trials Registry India (ctri.nic.in/) for trials in progress (all accessed on 10 July 2019).
Búsqueda de otros recursos
We searched the following conference proceedings for abstracts of relevant trials: World Congress on TB, World Lung Conferences of the International Union Against Tuberculosis Lung Disease (2004‐2018), American Thoracic Society Meeting Proceedings (2009 to 2019), and the British Society for Antimicrobial Therapy (2010‐2019). We contacted relevant organizations, including the Global Partnership to Stop TB and the WHO, for ongoing or completed but unpublished trials. We contacted researchers and experts in the field of clinical trials to identify any additional eligible studies. We checked the references of all included studies to identify additional relevant trials.
Obtención y análisis de los datos
Selección de los estudios
Two review authors (AG and AM) independently screened all citations and abstracts identified by the search strategy for inclusion. After eliminating duplicates, we scrutinized each report to ensure that multiple publications from the same trial were linked. If eligibility was not clear, or if we noted discrepancies, we resolved them through discussion or through consultation with another review author (SJ or JT). AG and AM obtained and scrutinized full texts of potentially eligible studies for inclusion and exclusion. Another review author (PT) independently screened the selected trials and the potentially eligible trials. We listed the excluded studies and tabulated reasons for their exclusion. We presented the study selection process in a PRISMA flow diagram.
Extracción y manejo de los datos
Two review authors (AG and AM) independently extracted data using a pre‐tested data extraction form. We resolved discrepancies in the extracted data through discussion and by referring to the original articles.
We extracted the following data from the included studies.
-
Trial details: publication year, country where the trial was undertaken, study authors, year in which the study was done, study design, number of participants recruited, inclusion criteria, exclusion criteria, recruitment sites.
-
Baseline characteristics of participants: age, gender, nutritional status, comorbid illnesses including HIV, sputum smear grading, disease severity, chest X‐ray findings.
-
Intervention and control arms: numbers allocated to each arm, numbers completing the trial, description of the drugs used in the trial, drug dosage, route and frequency of administration, duration of treatment in the intensive and continuation phases.
-
Outcomes: we extracted data for the primary and secondary outcomes as defined above.
For each outcome, we extracted information on the number of participants randomized. For dichotomous outcomes, we extracted the number of participants who experienced the event and the number of people assessed for the event.
Two other review authors (PT and RK) independently verified all extracted data.
Evaluación del riesgo de sesgo de los estudios incluidos
Two review authors (AG and AM) independently assessed risk of bias in the trials included in this review using Cochrane’s ‘Risk of bias’ tool in Review Manager 5 (RevMan 5) (Review Manager 2014). We assessed each of the included trials for risk of bias in seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment (assessed at end of treatment and at end of follow‐up), incomplete outcome data, selective outcome reporting, and other potential biases. We resolved disagreements through discussion with a third review author (JT or SS). For each domain in the 'Risk of bias' assessment, we judged the risk of bias as low, high, or unclear. Another review author (PT) independently verified all assessments. We recorded our judgements and support for these judgements in 'Risk of bias' tables accompanying the characteristics of each included study, and we summarized our findings in a 'Risk of bias' summary and graph.
Medidas del efecto del tratamiento
All outcomes were dichotomous, and we compared them using risk ratios and presented these with their 95% confidence intervals.
Cuestiones relativas a la unidad de análisis
The included studies were parallel‐group RCTs. For trials with multiple intervention arms, we undertook pair‐wise comparisons of only relevant interventions and when possible combined the results of trial arms with similar ATT regimens. When adverse events were reported as the numbers of events (counts) as well as the numbers of participants experiencing adverse events (rates), we recorded both but used the latter for data synthesis.
Manejo de los datos faltantes
When data for outcomes were missing from the primary trial report, we sought these in supplementary data provided in appendices or related publications. When trials reported intention‐to‐treat (ITT) or modified intention‐to‐treat (m‐ITT) or per‐protocol analyses (available case analyses), we recorded the numbers excluded from analyses from among those randomized and allocated to each arm before and during treatment and during follow‐up. We also noted the reasons for their exclusion. Post‐randomization exclusions are not uncommon in trials comparing newer ATT regimens. One type occurs when sputum smear positive participants are randomized, but when sputum culture and drug susceptibility results become available, they may not confirm tuberculosis or may indicate infection with other mycobacteria, or the presence of drug resistance. These ineligible participants are excluded from the trials (late screening failures). Modified‐ITT analysis in such situations excludes late screening failures from ITT analyses, and all other participants are analysed in their allocated arms. In this deviation from the standard ITT analysis, post‐randomization exclusions are unrelated to compliance, withdrawals, or losses to follow‐up, or to the likelihood of getting the intervention; when ineligible participants do not represent populations to which trial results are likely to be applied, the risk of bias may not differ from traditional ITT analysis (Fergusson 2002). However, if m‐ITT analyses exclude participants post‐randomization for reasons other than late screen failure, this can lead to overestimation of treatment effects compared to standard ITT analyses (Abraha 2015). For this review, we used the data provided in ITT or m‐ITT analysis of the included trials for our main analysis, because this analysis included more eligible participants than were included in the reported per‐protocol analyses and it did not require us to make assumptions about missing data. When ITT or m‐ITT analyses reported in the trials differed from standard interpretations, we assessed the impact of missing data by performing sensitivity analysis for the review's primary outcome of relapse. In imputing missing data, we had intended to perform the commonly used 'best‐worst case' analysis, in which the ‘best‐case’ scenario is that all participants with missing outcomes in the experimental intervention group had good outcomes (no relapse), and all those with missing outcomes in the control intervention group had poor outcomes (relapse); the ‘worst‐case’ scenario is the converse. However, these are extreme assumptions, especially with rare outcomes such as relapse. Instead, we used relapse proportions in the treatment and control arms from per‐protocol analysis in these trials to impute relapse rates for the missing population.
Evaluación de la heterogeneidad
We assessed clinical heterogeneity by looking at variability among trial participants, interventions, outcomes, and trial methods, including risk of bias. We assessed statistical heterogeneity by inspecting forest plots for non‐overlapping confidence intervals, and we used the Chi² test with a 10% level of statistical significance to denote that the inconsistency is not due to random error. We used the I² statistic, with a value of 50% or greater to generally denote moderate heterogeneity (the proportion of intertrial inconsistency that exceeds random error). However, we acknowledge that absolute thresholds for interpretation of I² can be misleading. Therefore we interpreted I² between 0% and 40% as possibly unimportant; from 30% to 60% as possibly representing moderate heterogeneity; from 50% to 90% as representing substantial heterogeneity; and from 75% to 100% as showing considerable heterogeneity, depending on the magnitude and direction of effects and the strength of evidence for heterogeneity (e.g. P value from the Chi² test) (Deeks 2011).
Evaluación de los sesgos de notificación
We intended to evaluate the possibility of publication bias by evaluating funnel plots for asymmetry, but because we included fewer than 10 trials, this was not possible.
Síntesis de los datos
We used risk ratios (RRs) with 95% confidence intervals (CIs) as summary effect estimates for dichotomous outcomes, and we synthesized data using RevMan 5 (Review Manager 2014). We conducted meta‐analyses using a fixed‐effect model when heterogeneity was low and a random‐effects model when heterogeneity was moderate (see Assessment of heterogeneity section). However, if heterogeneity was moderate and inconsistency was due to trials with large and small effects favouring an intervention, this need not necessarily denote imprecision of clinical importance (Guyatt 2011c). In such instances, if using a random‐effects model in sensitivity analyses also resulted in 95% CIs indicating appreciable effects of the intervention (see Sensitivity analysis), we used the fixed‐effect model in meta‐analysis but also reported random‐effects estimates in the results. If random‐effects meta‐analysis had resulted in imprecision (as indicated by the 95% CI including non‐appreciable benefits) or had changed the direction of effect, we would have retained the random‐effects model in meta‐analysis. If heterogeneity was substantial but could be explained in subgroup analyses (see below), we provided effect estimates for the subgroups without an overall pooled effect estimate.
Certainty of the evidence
We assessed the certainty of evidence by using the GRADE approach for the primary outcome of relapse and for the secondary outcomes important for clinical decision‐making, that is, death due to any cause, treatment failure, development of drug resistance, and serious adverse events (Guyatt 2011a). For each of these outcomes, we assessed how certain we were that pooled effect estimates were true (Balshem 2011), and that their 95% CIs represented the range of effects that were plausible and likely to be useful (Hultcrantz 2017). Certainty of evidence for each outcome is influenced by risk of bias in the studies contributing to pooled effect estimates for each outcome, as well as other factors such as unexplained inconsistency, indirectness, imprecision, and publication bias (Balshem 2011). Pooled effect estimates from RCTs are generally considered to provide high‐certainty evidence, but if there were serious or very serious concerns that any of the above‐mentioned factors may have compromised the certainty of effect estimates, we rated down the certainty for that outcome by one or two levels. In making these assessments, we used the overall guidance provided in Schünemann 2011 and Schünemann 2013. We also used guidance provided in Guyatt 2011b to assess the impact of imprecision on the certainty of evidence for each outcome. According to this guidance, precision is considered adequate if the 95% CI excludes an RR of 1.0, and the total number of events or patients in the total sample size is large enough to satisfy or exceed that required for an adequately powered individual trial (optimal information size, or OIS). However, when event rates are very low, as is likely with trials comparing shortened versus standard ATT regimens that were designed to assess equivalence or non‐inferiority within prespecified non‐inferiority margins, CIs around relative effects may be wide but CIs around absolute effects will be narrow. In such instances, rating down for imprecision may be inappropriate (Guyatt 2011b). For rating inconsistency, we used guidance provided in Guyatt 2011c, particularly when heterogeneity was moderate in fixed‐effect meta‐analysis but inconsistency in results was due to trials with large and small effects favouring an intervention. In such instances, if using a random‐effects model did not result in 95% CIs that now included non‐appreciable effects or no benefit associated with the intervention, we did not rate down for imprecision. We incorporated the ratings on certainty of evidence for effect estimates for each outcome along with relative and absolute measures of effect in 'Summary of findings' tables for each comparison in this review, using the GRADEpro Guideline Development Tool (GRADEpro GDT).
Análisis de subgrupos e investigación de la heterogeneidad
When we considered heterogeneity to be moderate or substantial, we explored potential causes in subgroup analyses based on categories of shortened treatment regimens. We subgrouped four‐month regimens according to whether they replaced components of standard ATT drugs or augmented them in comparison with standard six‐month ATT regimens.
Análisis de sensibilidad
We re‐analysed data using a random‐effects model in sensitivity analysis if fixed‐effect meta‐analysis revealed moderate heterogeneity but inconsistency in results of the trials was due to differences in the magnitude of effect favouring an intervention, rather than to differences in the direction of effects. Moderate inconsistency need not necessarily reduce our confidence in the pooled estimate if inconsistency is largely due to differences between large and small effects favouring an intervention (Guyatt 2011c). Thus, when we judged heterogeneity to be moderate but inconsistency in results was due to large and small effects favouring an intervention, we assessed the robustness of the results by changing from a fixed‐effect to a random‐effects meta‐analysis. If pooled effect estimates in random‐effects meta‐analysis continued to favour the intervention, and if both limits of the 95% CI continued to indicate appreciable benefit, we used the fixed‐effect model in the analysis but reported both fixed‐effect and random‐effects meta‐analysis in the results. We retained the fixed‐effect model in meta‐analysis in such instances to avoid compromising grading of imprecision in evaluating certainty of the evidence while summarizing the findings. Random‐effects meta‐analyses provide pooled estimates of the range of possible effects, with point estimates representing the mean of their distribution; this inherently denotes imprecision. Using the random‐effects model under such circumstances would warrant rating down for imprecision while assessing the certainty of evidence when this is not warranted.
We also assessed the impact of risk of bias on effect estimates of the primary outcome in sensitivity analysis by excluding studies judged to be at high risk of bias. We explored the effects of missing outcome data for the primary outcome of relapse in sensitivity analysis comparing results of the main analysis with results of the per‐protocol analysis, and including all randomized participants (excluding late screening failures, treatment failures, and deaths), and we imputed missing data using relapse rates from available data.
Results
Description of studies
Results of the search
We identified 1489 articles through database screening and two articles by searching other sources. After screening the 1491 titles and abstracts, we excluded 1457 records that were not relevant. We retrieved 34 full‐text records of potentially eligible studies (Figure 1). We excluded 11 records of RCTs that did not fulfil the inclusion criteria for the review (see Characteristics of excluded studies). We identified 23 relevant records for inclusion that reported on 11 RCTs. Eight of these records pertained to six ongoing studies that are detailed in Characteristics of ongoing studies. The remaining 15 records related to five RCTs that met criteria for selection to this review. No studies await assessment.

Study flow diagram.
Included studies
We included five RCTs that randomized a total of 5825 participants (Gillespie 2014; Jawahar 2013; Jindani 2014; Merle 2014; Velayutham 2014). Refer to Characteristics of included studies for a summary of included trial characteristics. Table 1 provides additional descriptive details.
| Study ID (Acronym) | (REMoxTB) | (RIFAQUIN) | (OFLOTUB) | |||||||
| Setting | Multiple sites in Africa (Kenya, South Africa, Tanzania, Zambia), Asia (China, India, Malaysia Thailand), Latin America (Mexico) | 6 sites in 2 cities in India | 6 cities in 4 countries in Africa (Botswana, South Africa, Zambia, Zimbabwe) | 5 countries in Africa (Benin, Guinea, Kenya, Senegal, South Africa) | 2 cities in India | |||||
| Participants | ||||||||||
| Number randomized | 1931 | 429 | 827 | 1836 | 801 | |||||
| Age | Adults (> 18 years) | Adults (> 18 years) | Adults (> 18 years) | Adults (18 to 65 years) | Adults (> 18 years) | |||||
| HIV infection | Included (if CD4 count > 250 cells/μL and not on ART); 110 (7%) | Excluded | Included (if CD4 count > 150/mm³ and not on ART; 158 (27%) | Included if not stage 3 or 4 disease and not on ART; 304 (17%) | Excluded | |||||
| Diagnosis of TB | Positive sputum smears on 2 occasions Culture‐confirmed susceptibility to rifampicin, isoniazid, pyrazinamide, and moxifloxacin | Newly diagnosed pulmonary TB with at least 2 positive sputum cultures. Confirmed by culture and MDR‐TB excluded, susceptibility to ofloxacin (as proxy for moxifloxacin) | 2 sputum samples that were positive for tubercle bacilli on direct smear microscopy No resistance to isoniazid, rifampicin, or moxifloxacin | Acid‐fast bacilli in 2 consecutive sputum smears; confirmed by culture (solid medium) and drug sensitivity tests to rifampicin, isoniazid, ethambutol, streptomycin, and gatifloxacin | 2 positive sputum smear smears for tuberculosis. Culture‐confirmed and MDR‐TB ruled out; susceptible to ofloxacin (as proxy for moxifloxacin) | |||||
| Intervention(s) and comparator | ||||||||||
| Duration of ATT | 4 monthsa | 6 months | 4 monthsb | 6 months | 4 months | 6 monthsc | 4 months | 6 months | 4 monthsa | 6 months |
| Regimens | 2HRZM/2HRM + 2MRZE/2MR | 2HRZE/4HR | 2(HRZG)₃/ 2 (HRG)₃ 2(HRZM)₃/2(HRM)₃ | 2(HRZE)₃ /4(HR)₃ | 2MRZE/ 2P₂M₂ | 2HRZE/ 4HR | 2HRZG/ 2HRG | 2HRZE/4HR | 3HRZM + 2HRZM/ 2RHM + 2HRZM/ 2(RHM)₃ + 2HRZM/ 2(RHEM)₃ | 2(HRZE)₃/ 4(HR)₃ |
| Number allocated | 655 + 636 = 1291 | 640 | 141 118 | 170 | 275 | 275 | 917 | 919 | 629 | 172 |
| Late screening failures excluded after allocation | 38 + 32 = 70 | 40 | 5 3 | 5 | 36 | 35 | 62 | 51 | 13 | 8 |
| Number eligible | 1231 | 600 | 136 115 | 165 | 239 | 240 | 852 | 868 | 616 | 164 |
| Number analysed in m‐ITT analysis (% of those allocated) | 568 + 551 = 1119 (87) | 555 (87) | 136 (97) 115 (98) | 165 (97) | 193 (70) | 188 (68) | 791 (86) | 794 (86) | 590 (94) | 151 (88) |
| Number analysed in per‐protocol analysis (% of those allocated) | 514 + 524 = 1038 (80) | 510 (80) | 131 (93) 113 (96) | 159 (94) | 165 (60) | 163 (59) | 651 (71) | 601 (65) | As above | |
| Number analysed in ancillary analysis (ITT) (% of those allocated) | 617 + 604 = 1221 (94) | 600 (94) | Not done | 239 (87) | 240 (87) | Not reported | Not reported | |||
| Outcomes reported | ||||||||||
| Relapse | Relapse within 18 months after randomization in those with negative culture with treatment. Relapse strains were those shown to be identical on 24‐locus MIRU analysis LJ solid media and MGIT liquid media used for culture | Recurrence of TB over 24 months after treatment in those with a favourable response with treatment: either bacteriologic recurrence (LJ solid media) or clinical/radiologic recurrence | Relapse within 12 to 18 months after treatment. Two positive cultures within a period of 4 months without an intervening negative culture). Re‐infections differentiated from relapse through genotyping (MIRU‐VNTRs) LJ solid media used for culture in some centres, MGIT liquid media in others, and both in some centres | Recurrence of TB over 24 months after treatment proven bacteriologically (2 consecutive positive sputum samples a day apart) or clinically Genotyping (MIRU‐VNTRs) results available for only 70/140 (55%) of those with culture confirmed recurrence. Most were relapses | Not reported | |||||
| Deaths | All deaths TB deaths | Reported (only non‐TB deaths occurred) | All deaths TB deaths | Death during treatment Death after treatment | Not reported | |||||
| Treatment discontinuation | Includes those who did not complete treatment, relocated, or withdrew consent | Includes those who did not complete treatment and those lost to follow‐up | Includes change in treatment due to adverse events, loss to follow‐up, and other treatment changes | Includes those who withdrew consent during treatment and dropouts | Reported but disaggregated data for each group not available | |||||
| Positive smear/ sputum culture at 2 months | Reported using LJ solid media (used in this review) and MGIT liquid media for all randomized participants excluding late screening failures | Reported using LJ solid media for all randomized participants excluding late screening failures | Reported but disaggregated data for moxifloxacin 4‐month and 6‐month treatment groups not available Data also not available for all participants from LJ media | Reported for 752 in the 4‐month and 759 in the 6‐month regimens (88% and 87% of those eligible, respectively) Culture using LJ solid media | Reported for 590 (94%) in the 4‐month and 151 (88%) in the 6‐month regimens | |||||
| Acquired drug resistance | Reported | Reported | Reported | Not reported | Not reported | |||||
| Treatment failure | Includes culture confirmed and not confirmed | Includes culture confirmed and unconfirmed | Culture confirmed | Includes culture confirmed failure | Not reported | |||||
| Serious adverse events | Reported for all randomized participants excluding late screening failures. Grade 3 and 4 severity (DAIDS 2009) | Deduced from adverse events reported for all randomized participants excluding late screening failures. Not graded | Reported for all participants randomized who took 1 dose and assessed as severe or life‐threatening during and 2 weeks after treatment. grade 3 and 4 severity (DAIDS 2009) | Reported for 1692 (92%) of all randomized participants. grade 3 and 4 severity (DAIDS 2009) | Deduced from adverse events reported. Not graded | |||||
| Other adverse events | Not reported | Reported | Not reported | QT prolongation Hyperglycaemic episodes | Reported | |||||
Abbreviations: ART: anti‐retroviral treatment; ATT: anti‐tuberculosis treatment; E: ethambutol; G: gatifloxacin; H: isoniazid; ITT: intention‐to‐treat; LJ: Löwenstein‐Jensen; M: moxifloxacin; MGIT: mycobacterial growth indicator tube; MIRU‐VNTRs: mycobacterial interspersed repetitive unit–variable number tandem repeats; m‐ITT: modified intention‐to‐treat; P: rifapentine; R: rifampicin; Z: pyrazinamide.
Leading numbers in regimens indicate duration in months. Drugs were administered daily, except when given thrice weekly as indicated by subscripts.
aData from moxifloxacin‐containing shortened regimens combined for data synthesis.
bData from the 2 shortened regimens compared separately with standard 6‐month regimens.
cData from an additional arm evaluating moxifloxacin‐containing 6‐month regimen not included.
Setting
Three of the included trials were multi‐country trials. Gillespie 2014 (REMoxTB study) included participants from multiple sites in nine countries: four in Africa (Kenya, South Africa, Tanzania, Zambia), four in Asia (China, India, Malaysia, Thailand), and one in Latin America (Mexico). Jindani 2014 (RIFAQUIN trial) recruited participants from six cities in four countries in Africa (Botswana, South Africa, Zambia, Zimbabwe). Merle 2014 (OFLOTUB/Gatifloxacin) included participants from five cities in five countries in Africa (Benin, Guinea, Kenya, Senegal, South Africa). The other two trials were conducted in two cities in south India (Jawahar 2013; Velayutham 2014).
Study participants
The five trials recruited only adults (> 18 years of age). Most participants were male, ranging from 64% to 74% across the five trials. Two trials excluded HIV‐positive participants (Jawahar 2013; Velayutham 2014). Gillespie 2014 included 110 HIV‐positive participants (7% in each arm) whose CD4 counts were > 250 cells/μL, and who were not receiving antiretroviral treatment (ART). Merle 2014 included 304 (18.1%) individuals with HIV who were not in stage 3 or 4 disease and were not receiving ART (17.4% in the shortened regimen, 18.7% in the standard regimen). Jindani 2014 included the largest proportion of HIV‐positive participants (158; 27%) after excluding those with CD4 count < 150/mm³ and those on ART; 28% were allocated to the shortened regimen and 29% to the six‐month regimen.
All five trials included patients with lung cavitation. In Gillespie 2014, this accounted for 71% overall (69% and 70% in the intervention groups, 72% in the control group). In Jindani 2014, 67% given the control regimen and 65% receiving the shortened regimen had cavitation. Merle 2014 included 50% in the control regimen and 52% in the shortened regimen with cavitation. Velayutham 2014 reported that cavitation was present in 36% of those allocated to the shortened regimen and in 41% of those given the control regimen. Jawahar 2013 did not provide numerical data about proportions with lung cavitation.
Gillespie 2014 and Jindani 2014 excluded those with body weight less than 35 kg; in Gillespie 2014 and in Jindani 2014, 9% to 11% and 4% to 5% of included participants, respectively, had body weight < 40 kg. Jawahar 2013 and Velayutham 2014 excluded participants who weighed < 30 kg. In Jawahar 2013, mean body weight ranged from 43.7 kg to 44.2 kg in the shortened treatment arms and was 43 kg in the control arm. In Velayutham 2014, 53% in the shortened‐treatment arms and 54% in the standard treatment arm weighed > 42 kg. Merle 2014 required participants to weigh between 38 kg and 80 kg; mean weight was 53.8 kg in the intervention arm and 54.2 kg in the control arm.
The diagnosis was made by using two positive sputum samples and was confirmed by culture in all trials. Gillespie 2014 required culture‐confirmed susceptibility to rifampicin, isoniazid, pyrazinamide, and moxifloxacin; Jindani 2014 additionally required susceptibility to isoniazid; and Merle 2014 required susceptibility to ethambutol and gatifloxacin. All trials excluded people with MDR‐TB (Table 1).
Shorter ATT regimens
The five included studies evaluated shorter regimens involving two fluoroquinolones (moxifloxacin and gatifloxacin) given to 3512 participants compared to 2176 participants given standard six‐month ATT regimens. We did not find trials evaluating other fluoroquinolones, nitroimidazoles, diarylquinolines, oxazolidinediones, or ethylenediamines in shortened ATT regimens compared to standard ATT regimens. We also did not find eligible trials that included other candidate drugs for shorter regimens, such as amoxicillin clavulanate, linezolid, carbapenems, or clofazimine.
Comparision 1. Moxifloxacin‐containing four‐month ATT regimens
Four trials compared moxifloxacin‐containing shortened ATT regimens (three to four months) versus standard six‐month ATT regimens.They differed in whether moxifloxacin was used to replace one of the standard ATT drugs in the four‐month ATT arm (Gillespie 2014; Jawahar 2013; Jindani 2014), or to augment them (Velayutham 2014). Treatments were supervised in all trials.
Moxifloxacin replacing standard ATT drugs
Gillespie 2014 (REMoxTB study) randomized 1931 participants to three arms. Two arms compared moxifloxacin‐containing daily regimens for four months (17 weeks) versus a control intervention for six months (26 weeks) of a daily ATT regimen. One arm (isoniazid group, where moxifloxacin (M) (400 mg) replaced ethambutol (E); N = 655) received eight weeks of M with isoniazid, rifampicin, and pyrazinamide (HRZ) plus E placebo administered daily, followed by nine weeks of MHR, followed by nine weeks of H and R placebo. The second intervention arm (ethambutol group, where moxifloxacin (400 mg) replaced isoniazid; N = 636) received eight weeks of MRZE plus H placebo administered daily, followed by nine weeks of MR plus H placebo daily, followed by nine weeks of H and R placebo. The control arm (N = 640) received eight weeks of HRZE and M placebo given daily, followed by nine weeks of HR and M placebo given daily, followed by nine weeks of HR. Results of the two moxifloxacin arms did not differ significantly. We combined the data for these two intervention arms compared to the six‐month regimen in data synthesis for our primary analysis.
Jawahar 2013 randomized 429 participants to three arms. In the two intervention arms, gatifloxacin (G) or moxifloxacin (M) replaced ethambutol in the shortened regimen. The moxifloxacin arm (N = 118) received two months of moxifloxacin (400 mg) and HRZ thrice weekly, followed by two months of MHR thrice weekly. The control arm (N = 170) received two months of HRZE thrice weekly, followed by four months of HR thrice weekly. This trial was stopped early by the data safety monitoring board at a planned interim analysis, after it had recruited only a third of the 1200 estimated sample, due to higher relapse rates in the intervention arms.
Jindani 2014 (RIFAQUIN trial) also had three arms randomizing 827 participants (of the estimated sample size of 1095). In two intervention arms, moxifloxacin (400 mg) replaced isoniazid throughout, and high‐dose (900 mg) rifapentine (P) replaced rifampicin in the continuation phase. We did not include one of these arms in data synthesis because the four‐month continuation phase resulted in a six‐month ATT regimen. In the other arm, 275 participants were given eight weeks of MRZE administered daily, followed by nine weeks of MP administered twice weekly. In the control arm, 275 participants were given eight weeks of HRZE administered daily, followed by 18 weeks of HR daily.
Moxifloxacin augmenting standard ATT drugs
Velayutham 2014 is the interim report of an ongoing trial ‐ CTRI/2008/091/000024 ‐ that compared four different regimens in which moxifloxacin (400 mg) was added to HRZE in shortened courses. The four arms randomized 629 participants to receive HRZEM daily for three months, or daily for two months followed by RHM daily for two months, or daily for two months followed by RHM thrice weekly for two months, or daily for two months followed by RHEM thrice weekly for two months. The standard six‐month (2HRZE/4HR) regimen was given thrice weekly to 172 participants. The report presented planned interim outcomes and final results are awaited.
Comparison 2. Gatifloxacin‐based four‐month ATT regimens
Gatifloxacin replacing standard ATT drugs
Merle 2014 (OFLOTUB/gatifloxacin) randomized 1836 participants, of whom 917 were given two months of gatifloxacin (400 mg; replacing ethambutol) and HRZ daily, followed by two months of daily HRG. In the control arm, 919 participants were given the standard daily six‐month (2HRZE/4HR) regimen.
In Jawahar 2013, the gatifloxacin arm replaced ethambutol in 141 participants who received two months of HRZG thrice weekly, followed by two months of HRG thrice weekly. The 170 participants in the control arm received 2HRZE/2HR given thrice weekly.
Follow‐up
Participants in three of the included trials were followed for a period of 24 months after end of treatment (Jawahar 2013; Merle 2014; Velayutham 2014). Gillespie 2014 and Jindani 2014 followed‐up participants for a period of 18 months after randomization (12 months after treatment). However, 14% of participants in Jindani 2014 who were randomized in the last six months of enrolment received follow‐up for 12 or 15 months after randomization. All trials reported regular scheduled assessments for efficacy and safety outcomes for participants in the intervention and control arms (see Characteristics of included studies).
Outcomes
Four trials provided data on relapse ‐ the primary outcome of this review (Gillespie 2014; Jawahar 2013; Jindani 2014; Merle 2014). In Gillespie 2014 and Jindani 2014, relapse was differentiated from re‐infection through genotyping of patients with culture‐confirmed recurrence. In Merle 2014, genotyping results were available for only 77 of 140 (55%) of those with culture‐confirmed recurrence. However, 79% of the 77 with genotyping results were confirmed as relapses. In Jawahar 2013, relapse was not differentiated from re‐infection but most recurrences occurred within six months after treatment, suggesting that these were instances of relapse.
Again, four trials provided data on death from any cause, including tuberculosis, that occurred on treatment and during follow‐up (Gillespie 2014; Jawahar 2013; Jindani 2014; Merle 2014). No deaths were reported in the interim analysis provided in Velayutham 2014. Rates of treatment discontinuation and treatment failure were reported in four trials (Gillespie 2014; Jawahar 2013; Jindani 2014; Merle 2014), with different definitions used to compute these outcomes (Table 1).
Four trials reported the outcome of sputum culture positivity at eight weeks (Gillespie 2014; Jawahar 2013; Merle 2014; Velayutham 2014). In Velayutham 2014, data for this outcome were presented for all participants allocated to four groups combined, but because participants in the four groups had received identical regimens for the first two months, we used these data in the meta‐analysis. In the fifth trial (Jindani 2014), these results were presented as combined data for the four‐month and six‐month moxifloxacin arms, and disaggregated data for sputum positivity at two months were not available. Gillespie 2014, Jawahar 2013, and Jindani 2014 provided data on acquired drug resistance. Merle 2014 and Velayutham 2014 did not report on this.
Acquired drug resistance was assessed and reported in three trials (Gillespie 2014; Jawahar 2013; Jindani 2014), which assessed drug susceptibility at baseline as well as in those who were culture positive at end of treatment, or who experienced relapse/recurrence. Resistance results were missing for isoniazid in 24 patients and for pyrazinamide in 27 patients at baseline in Gillespie 2014, and the cases of acquired drug resistance reported were only probable and were not unequivocal in the absence of whole genome sequencing. Jawahar 2013 did not directly assess susceptibility to moxifloxacin and gatifloxacin but used susceptibility to ofloxacin as a proxy indicator. Merle 2014 assessed drug susceptibility at baseline and performed indirect drug susceptibility tests during follow‐up but did not report acquired drug resistance.
Serious adverse events experienced by trial participants were reported in all trials or could be deduced from the adverse events reported. Gillespie 2014 and Jindani 2014 did not report adverse events other than serious adverse events. Merle 2014 also reported the proportions of participants with QT prolongation and with hyperglycaemic episodes.
Excluded studies
We excluded 11 studies for reasons detailed under Characteristics of excluded studies. One trial, Alavi 2009, studied the effects of rifampicin, isoniazid, and ofloxacin in people with smear negative pulmonary tuberculosis, diagnosed solely on the basis of clinical criteria. Five were phase 2b trials with no six‐month standard ATT comparator arm (Burman 2006; Conde 2009; Conde 2016; Dorman 2009; Rustomjee 2008). These trials, along with El‐Sadr 1998 which we excluded because it compared levofloxacin added for the first two months of the standard six‐month ATT regimen versus six to nine months of standard ATT regimens, are included in an earlier Cochrane Review (Ziganshina 2013). We excluded three other trials because they lacked comparisons with a standard six‐month ATT arm (Kohno 1992; Tuberculosis Research Centre 1986; Tuberculosis Research Centre 2002). Johnson 2009 evaluated the effects of four months of standard ATT drugs versus six months of standard ATT but randomized only those who were sputum negative after four months of treatment to receive no further treatment or two more months of ATT.
Risk of bias in included studies
Please refer to Figure 2 for the summary of 'Risk of bias' assessments for each included study, and to Figure 3 for a risk of bias graph regarding each item presented as percentages across all included trials. Please also see 'Risk of bias' tables for individual trials under Characteristics of included studies for supporting evidence on the judgement of risk of bias for the included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
All included studies were judged to be at low risk of bias for generating the random sequence. All but one ‐ Jawahar 2013 ‐ were judged as having low risk of bias for allocation concealment. Jawahar 2013 was judged to be at high risk of bias because recruitment ratios were altered during the course of the trial, thus likely compromising concealment of allocation. In conjunction with premature termination of the trial following a planned interim analysis, this led to baseline imbalance in some prognostic indicators.
Blinding
In Jawahar 2013, participants and care providers were not blinded to the interventions, and allocation concealment was likely to have been compromised. Jindani 2014 was an open‐label trial and treating physicians were aware of the treatment allocated. However, we believe this did not increase the risk of performance bias in these trials because we found no evidence that this influenced the administration of interventions or co‐interventions differentially between four‐month and six‐month regimens. We judged the other three trials to have low risk of performance bias, and we judged the five included trials as having low risk for detection bias.
Incomplete outcome data
We judged the five trials to be at low risk of attrition bias for outcomes assessed at the end of ATT and at the end of follow‐up. These trials had low attrition (Jawahar 2013; Velayutham 2014), or, if attrition exceeded 10% (Gillespie 2014; Jindani 2014; Merle 2014), differential attrition was not substantial and the results of per‐protocol analysis, modified intention‐to‐treat analyses, and other sensitivity analyses reported in the trials were consistent. In Jawahar 2013, although the power of the trial to prove equivalence was reduced due to early termination, we judged this study as having low risk of attrition bias, as attrition was low with similar reasons for exclusion, and this was unlikely to have altered the relative estimates of effects.
Selective reporting
The five studies reported all outcomes stated in the methods sections of their trial publications, or their protocols, or their clinical trial registry documents, and we judged them to be at low risk of reporting bias.
Other potential sources of bias
In three trials (Gillespie 2014; Jindani 2014; Merle 2014), study drugs were provided by their manufacturers, but we judged these studies to be at low risk of bias because the trial publications provided explicit statements that the manufacturers had no role in the study nor in the publication of results. We did not detect any other sources of bias.
Effects of interventions
See: Summary of findings for the main comparison Moxifloxacin‐containing 4‐month ATT regimens versus standard 6‐month ATT regimen for drug‐sensitive pulmonary tuberculosis; Summary of findings 2 Gatifloxacin‐containing 4‐month ATT regimens compared to standard 6‐month ATT regimens for drug‐sensitive pulmonary tuberculosis
Comparison 1. Moxifloxacin‐containing four‐month ATT regimens versus standard six‐month ATT regimens
Primary outcome
Relapse
Three trials provided data on relapse over 12 to 24 months following treatment in people with drug‐sensitive pulmonary tuberculosis (Gillespie 2014; Jawahar 2013; Jindani 2014). Two trials differentiated relapse from re‐infection using molecular methods (Gillespie 2014; Jindani 2014). Of 2769 participants randomized to the three regimens, 2265 participants (82%) were culture negative at the end of treatment and were evaluated for relapse or recurrence. Relapse proportions for the two regimens compared in the three trials are shown in Table 2.
| Primary outcome: relapse | ||||||
| Trial ID | ||||||
| Regimens | 4 months | 6 months | 4 months | 6 months | 4 months | 6 months |
| aModified‐ITT analysis (primary analysis) | 110/1119 (9.8%) | 13/555 (2.3%) | 11/108 (10.1%) | 10/155 (6.5%) | 27/165 (16.4%) | 6/163 (3.7%) |
| aPer‐protocol analysis | 110/1038 (10.6%) | 12/510 (2.4%) | 11/107 (10.1%) | 10/152 (6.6%) | 26/165 (15.8%) | 5/163 (3.1%) |
| bSensitivity analysis imputing missing data | 126/1184 (10.7%) | 14/577 (2.4%) | 11/114 (9.7%) | 10/159 (6.3%) | 36/225 (16.0%) | 71/232 (2.6%%) |
Abbreviations: ATT: anti‐tuberculosis treatment; ITT: intention‐to‐treat.
aAs reported in trial reports.
bIncludes in the denominators for each trial arm all randomized participants minus those excluded post randomization due to ineligibility (not confirmed TB, or drug resistant), those who died, and those who experienced treatment failure. The difference in this denominator and the denominator in per‐protocol analyses are missing data. Relapse rates for missing people were imputed from rates in the per‐protocol analysis for each trial arm.
Overall, 177 (5.2%) in the two groups included in the primary modified ITT analysis experienced a recurrence; most cases (156/178; 88%) were confirmed as relapse through genotyping; and 17 of 21 (81%) tuberculosis recurrences in Jawahar 2013 occurred in the first six months after treatment, suggesting that they were relapses rather than re‐infections. Relapse in the six‐month ATT arm varied from 2.3% of 555 participants in Gillespie 2014, to 6.5% of 155 participants in Jawahar 2013 and 3.7% of 163 participants in Jindani 2014. The corresponding incidence of relapse in the moxifloxacin‐containing shorter ATT regimens was 9.8% of 1119 in Gillespie 2014, 10.1% of 108 in Jawahar 2013, and 16.4% of 165 in Jindani 2014. Meta‐analysis showed that risk of relapse was thrice more common with moxifloxacin‐containing four‐month ATT regimens than with the standard six‐month regimen (RR 3.56, 95% CI 2.37 to 5.37; 2265 participants, 3 trials; Analysis 1.1). Results showed some heterogeneity (I² = 54%), but inconsistency between large and small effects favoured the six‐month ATT regimen, with considerable overlap in the 95% CI of the effect estimates. Re‐analysing data in sensitivity analysis using a random‐effects model did not introduce imprecision into estimates of appreciable benefit with the six‐month regimen (RR 3.18, 95% CI 1.69 to 5.97).
In the main analysis, we combined modified‐ITT data from the two moxifloxacin‐containing intervention arms in Gillespie 2014. We subgrouped data according to whether moxifloxacin was used in the four‐month regimen to replace ethambutol in the intensive phase (one of the intervention arms in Gillespie 2014 and the moxifloxacin arm in Jawahar 2013), or to replace isoniazid in the four‐month regimen (the other intervention arm in Gillespie 2014, and the moxifloxacin with high‐dose rifapentine arm in Jindani 2014). Results again favoured the six‐month ATT regimen (Analysis 1.2), irrespective of whether moxifloxacin replaced isoniazid in four‐month ATT regimens (RR 2.74, 95% CI 1.69 to 4.43; 747 participants, 3 trials; Analysis 1.2: subgroup 1), or whether moxifloxacin replaced ethambutol (RR 4.89, 95% CI 3.02 to 7.92; 1424 participants, 2 trials; Analysis 1.2: subgroup 2). We did not undertake subgroup analysis based on HIV status as there were only three trials, and one excluded HIV‐positive people (Jawahar 2013). However, Gillespie 2014 and Jindani 2014 reported no significant interaction effects between HIV status and unfavourable outcomes in subgroup analyses.
Jawahar 2013 was at high risk of bias for allocation concealment, and we explored the impact of this in sensitivity analysis by removing this study's data. Pooled estimates from the two studies without high risk of bias also show that the four‐month regimen increases relapse compared to the standard six‐month regimen (RR 4.26, 95% CI 2.65 to 6.84; 2002 participants, 2 trials; I² = 0%).
We used data for relapse from m‐ITT analyses reported in the three trials for the main meta‐analysis in this review. However results did not differ substantially when we performed sensitivity analyses using data from the per‐protocol analyses in the three trials in meta‐analysis (Table 2; Analysis 1.3: subgroup 2). When we explored the impact of missing data for all randomized participants (excluding late screening failures, treatment failures, and deaths) and imputed relapse rates for missing participants using relapse proportions reported in per‐protocol analyses of individual trials, results were consistent with the main meta‐analysis (Table 2; Analysis 1.3: subgroup 3; Figure 4).
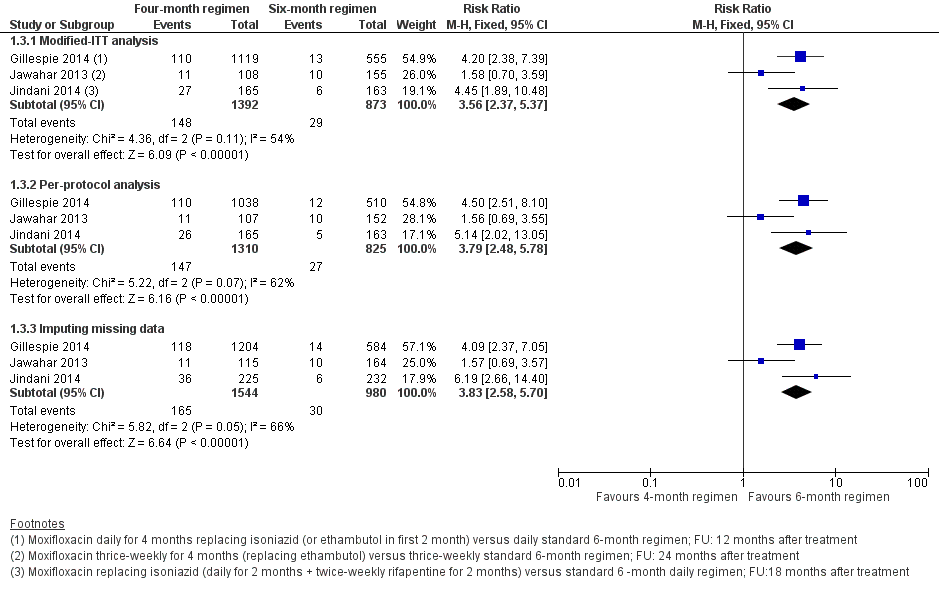
Forest plot of comparison: 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, outcome: 1.3 Relapse: sensitivity analysis accounting for missing data.
Secondary outcomes
Death from any cause
Three trials reported 62 deaths (Gillespie 2014; Jawahar 2013; Jindani 2014). Gillespie 2014 reported 27 deaths with four‐month ATT; 19 (70%) were tuberculosis‐related deaths, and 11 of 16 (69%) deaths with six‐month ATT were tuberculosis‐related. The one death (non‐tuberculosis) in Jawahar 2013 occurred with six‐month ATT. Jindani 2014 reported 2 of 12 (16%) deaths as tuberculosis‐related with the four‐month regimen, and 1 of 6 (16%) as tuberculosis‐related deaths with six‐month ATT. Pooled estimates of the risk of death due to any cause did not significantly differ between four‐month and six‐month ATT regimens (2760 participants, 3 trials; Analysis 1.4).
Treatment discontinuation
Of 2335 evaluable participants in three trials (Gillespie 2014; Jawahar 2013; Jindani 2014), 121 (5.2%) discontinued treatment for different reasons (Table 1). In meta‐analysis, treatment discontinuation showed little or no difference between the two groups (2335 participants, 3 studies; Analysis 1.5).
Sputum culture/smear positivity at eight weeks
Data for sputum culture conversion at the end of the intensive phase of ATT treatment were reported by all trials in the review; however, Jindani 2014 reported only combined sputum conversion data for the four‐month and six‐month moxifloxacin‐containing ATT arms of the trial. Disaggregated data for the four‐month moxifloxacin arm were not available for inclusion in meta‐analysis.
The pooled point estimate from the three trials with usable data for sputum culture/smear positivity at eight weeks favoured the four‐month moxifloxacin‐containing ATT regimen (Gillespie 2014; Jawahar 2013; Velayutham 2014), but the 95% CI did not rule out a small benefit for the standard six‐month ATT regimen, and heterogeneity was substantial (I² = 91%; 2828 participants, 3 trials; Analysis 1.6). We explored heterogeneity by subgrouping the data according to whether moxifloxacin replaced isoniazid or ethambutol in four‐month ATT regimens (Gillespie 2014; Jawahar 2013), or augmented standard ATT drugs in four‐month ATT regimens in random‐effects meta‐analysis(Velayutham 2014). Four‐month moxifloxacin‐containing regimens that replaced isoniazid or ethambutol were not unequivocally better than standard six‐month ATT regimens in achieving sputum culture conversion at eight weeks (2087 participants, 2 trials; Analysis 1.6: subgroup 1). However, moxifloxacin augmentation of standard ATT drugs in four‐month regimens was more effective than standard six‐month ATT in sterilizing sputum (sputum positivity at eight weeks 4.6% versus 19.2%; RR 0.24, 95% CI 0.15 to 0.39, 741 participants; Analysis 1.6: subgroup 2) (Velayutham 2014). The test for subgroup differences confirmed that moxifloxacin augmentation rather than substitution of standard ATT drugs achieves better sputum conversion at eight weeks compared to standard six‐month ATT regimens (P = 0.001; I² = 90.2%; Analysis 1.6).
Treatment failure
In the three trials that reported this outcome (Gillespie 2014; Jawahar 2013; Jindani 2014), treatment failures were equally rare, with only 14 failures reported among 1399 participants evaluated in the four‐month arm and 14 among 883 participants evaluated in the six‐month arm (2282 participants, 3 trials; Analysis 1.7). Most of these were culture confirmed treatment failures.
Acquired drug resistance
Acquired drug resistance was evaluated in three of the four included trials among those who had treatment failure, or who suffered a relapse with the four‐month regimen and the six‐month regimen (Gillespie 2014; Jawahar 2013; Jindani 2014). Due to the greater proportion of relapses in the four‐month ATT arm, proportions assessed for acquired drug resistance differed between the four‐month regimen (162/1392; 11.7%) and the six‐month regimen (43/873; 4.9%). Overall, eight people were judged to have developed acquired drug resistance. Two persons in the four‐month moxifloxacin‐containing ATT regimens in the three trials were detected with acquired drug resistance ‐ one to moxifloxacin and one to isoniazid. The incidence of acquired drug resistance ranged from 0.83% (1/120) in Gillespie 2014 to 7.7% (1/13) in Jawahar 2013 to 0% (of 29 assessed) in Jindani 2014. Six people developed acquired drug resistance in the six‐month standard ATT arms ‐ three to isoniazid and three to rifampicin. The incidence ranged from 15% (3/20) in Gillespie 2014 to 13% (2/15) in Jawahar 2013 to 12.5% (1/8) in Jindani 2014. Results for the four people with acquired drug resistance in Gillespie 2014 were not unequivocal but were judged probable. We pooled the data for acquired drug resistance from these trials using numbers evaluated for treatment failure in each trial as a more appropriate denominator for assessing acquired drug resistance than only those who experienced treatment failure or relapse. The pooled effect estimate suggests that acquired drug resistance was less frequent with the four‐month moxifloxacin‐containing ATT regimen than with the standard six‐month ATT regimen, but events were rare and 95% CIs were imprecise (2282 participants, 3 trials; Analysis 1.8).
Adverse events
Serious adverse events
All five included studies reported serious adverse events (SAEs) that were fatal or life‐threatening, or required hospitalization or a change in treatment regimen. Gillespie 2014 reported that a total of 349 SAEs occurred in 173 participants, with 246 events occurring during the treatment period and 103 during follow‐up. Serious adverse events occurred in 62 of 655 (9%) in the isoniazid group and in 52 of 636 (8%) in the ethambutol group, compared with 59 of 639 (9%) in the control group. The incidence of adverse events, including seizures, clinically significant cardiac toxicity, hypoglycaemia or hyperglycaemia, and peripheral neuropathy, did not significantly differ. Jawahar 2013 noted only two SAEs ‐ a case of jaundice in a person on the six‐month regimen and QTc prolongation in a person on the moxifloxacin‐ATT regimen. Jindani 2014 reported 12 SAEs among 11 participants on the four‐month ATT regimen, four of which were considered possibly or probably related to study medicines. In the control arm, 16 events were reported among 12 participants, with six possibly or probably related to treatment. Velayutham 2014 reported QTc prolongation in five participants in the moxifloxacin group and in one on standard ATT, but all cases were reversible. Other SAEs included hepatitis (12 in the moxifloxacin arm and 2 in the control arm) and seizures (four in the moxifloxacin arm and two in the control arm).
The meta‐analysis did not show significant differences between treatment regimens in the incidence of SAEs among 3548 participants in the four trials (Analysis 1.9).
Other adverse events
In Jawahar 2013, the most common adverse events were gastrointestinal symptoms (nausea, vomiting, abdominal discomfort), which occurred in 25 of 115 (22%) in the moxifloxacin group and in 15 of 165 (9%) in the control group. Giddiness or dizziness was also more frequent with moxifloxacin‐containing regimens (17/115; 15%) than with standard ATT (5/165; 3%). Arthralgia attributable to pyrazinamide was seen in 3 of 115 (3%) in the four‐month regimen and in 4 of 165 (2%) in the six‐month regimen.
Velayutham 2014 also reported that arthralgia was significantly greater in the moxifloxacin group (25% of 616 participants) than in the control group (4% of 164 participants). Skin rash with or without pruritis occurred in 5% of 616 participants in the moxifloxacin arms and in 4% of 164 participants in the six‐month ATT arm. The other three trials did not report adverse events other than SAEs.
Comparison 2. Gatifloxacin‐based four‐month ATT regimens versus standard six‐month ATT regimens
Two trials provided data for this intervention. Jawahar 2013 was a three‐armed, open‐label, equivalence trial, one arm of which randomized 141 adults with drug‐sensitive pulmonary tuberculosis to two months of supervised gatifloxacin 400 mg (replacing ethambutol), isoniazid, rifampicin, and pyrazinamide thrice weekly, followed by two months of gatifloxacin, isoniazid, and rifampicin thrice weekly. The 170 participants in the control arm were administered thrice‐weekly supervised standard six‐month ATT. Merle 2014 was an open‐label, two‐arm, non‐inferiority trial that randomized 917 participants to a similar gatifloxacin‐containing regimen (also replacing ethambutol) but given daily and compared the effects with 919 participants given a daily, supervised, standard six‐month ATT regimen.
We did not find trials that used gatifloxacin to replace isoniazid or to augment standard ATT regimens.
Primary outcome
Relapse
Both trials reported on relapse after confirming culture conversion by Löwenstein–Jensen (LJ) solid media to confirm tuberculosis recurrence over 24 months after treatment in people who had become culture negative with treatment. Jawahar 2013 did not differentiate relapse from recurrence but reported that all 19 recurrences in the gatifloxacin‐containing ATT arm and 8 of 10 recurrences in the six‐month ATT arm occurred within six months after treatment (suggestive of relapse rather than re‐infection). In Merle 2014, of 140 participants with culture‐positive recurrence, 77 (55%) had strains genotyped by means of a 15‐locus mycobacterial interspersed repetitive unit–variable‐number tandem‐repeat analysis. Of these 77 patients, 15 of 20 (75%) in the gatifloxacin arm and 46 of 57 (81%) in the standard ATT arm had a verified relapse. Relapse was diagnosed in 6.5% of 155 participants given six months of ATT in Jawahar 2013 and in 7.1% of 662 people given six months of ATT in Merle 2014. Relapse was more common with the gatifloxacin‐containing regimens: 15.6% of 122 in Jawahar 2013, and 14.6% of 694 in Merle 2014. Meta‐analysis of the two trials showed that relapse was twice as common with the gatifloxacin‐containing four‐month regimen than with the six‐month ATT regimen (RR 2.11, 95% CI 1.56 to 2.84; 1633 participants, 2 trials; Analysis 2.1).
Jawahar 2013 was at high risk of bias for allocation concealment and excluded HIV‐positive individuals. However, meta‐analysis results did not reveal any inconsistency in the results. Merle 2014 included HIV‐positive participants and undertook subgroup analysis based on HIV status. No significant interaction effects were detected between HIV status and unfavourable outcomes.
As in the previous comparison, we used m‐ITT analysis data from both trials for meta‐analysis in this review. Sensitivity analyses comparing m‐ITT data and per‐protocol data showed similar results, as did meta‐analysis using all randomized participants (minus late screening failures, treatment failures, and deaths) with imputed relapse rates for missing participants from relapse proportions in the per‐protocol analyses reported in the two trials (Table 3; Analysis 2.2; Figure 5).
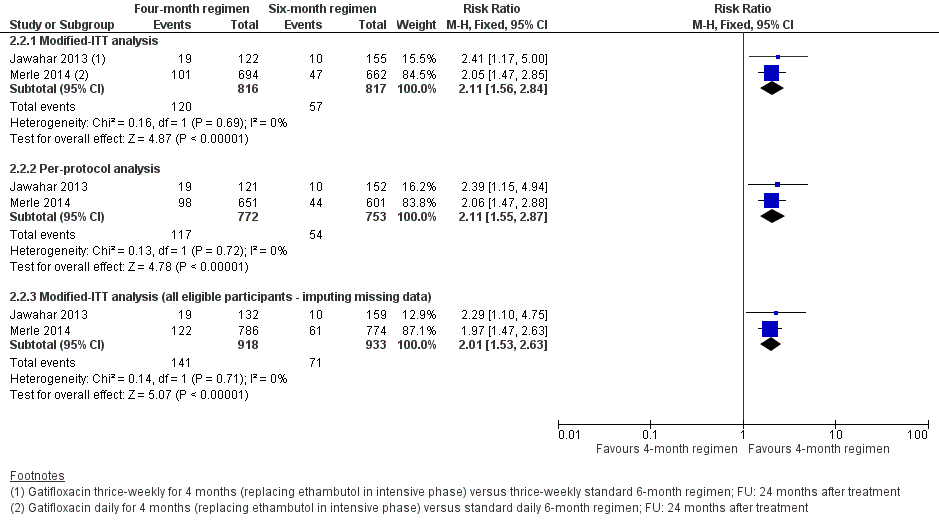
Forest plot of comparison: 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, outcome: 2.2 Relapse: sensitivity analysis accounting for missing data.
| Primary outcome: relapse | ||||
| Trial ID | ||||
| Regimen | 4 months | 6 months | 4 months | 6 months |
| aModified‐ITT analysis (primary analysis) | 19/122 (15.6%) | 10/155 (6.5%) | 101/694 (14.6%) | 47/662 (7.1%) |
| aPer‐protocol analysis | 19/121 (15.7%) | 10/152 (6.6%) | 98/651 (15.1%) | 44/601 (7,3%) |
| bSensitivity analysis imputing missing data | 19/132 (14.4%) | 10/159 (6.3%) | 122/786 (15.5%) | 61/774 (7,9%) |
Abbreviations: ATT: anti‐tuberculosis treatment; ITT: intention‐to‐treat.
aAs reported in trial reports.
bIncludes in the denominators for each trial arm all randomized participants minus those excluded post randomization due to ineligibility (not confirmed TB, or drug resistant), those who died, and those who experienced treatment failure. The difference in this denominator and the denominator in per‐protocol analyses are missing data. Relapse rates for missing people were imputed from rates in the per‐protocol analysis for each trial arm.
Secondary outcomes
Death from any cause
One non‐tuberculosis‐related death was reported in each of the four‐month and six‐month arms in Jawahar 2013, In Merle 2014, five deaths in the gatifloxacin arm and nine deaths in the six‐month ATT arm occurred during treatment. Two deaths in the gatifloxacin arm and three in the control arm were defined as SAEs. An additional 19 deaths in the gatifloxacin arm and 18 deaths in the standard ATT arm were reported after treatment. Meta‐analysis did not reveal significant differences in risk of death due to any cause between ATT regimens (1886 participants, 2 trials; Analysis 2.3).
Treatment discontinuation
In Jawahar 2013, seven people in each arm discontinued treatment (5,2% of 136 with gatifloxacin‐containing ATT, and 4.2% of 165 with standard ATT); in Merle 2014, 27 of 694 (3.9%) and 41 of 662 (6.2%) discontinued treatment. Although the control arm included more people who discontinued treatment in Merle 2014, and the reverse was seen in Jawahar 2013, risk of treatment discontinuation between the two ATT regimens was not appreciably different (1657 participants, 2 trials; Analysis 2.4).
Positive sputum culture at eight weeks
Gatifloxacin replacing ethambutol in ATT regimens did not offer any advantage over standard ATT in sterilizing sputum at the end of the intensive phase of anti‐tuberculosis treatment. At eight weeks 16% of 1818 participants on the two ATT regimens were sputum positive. Pooled data did not reveal that either intervention was better in sputum conversion at eight weeks (1818 participants, 2 trials; Analysis 2.5).
Treatment failure
Treatment failure was rare in both trials. In the gatifloxacin‐containing ATT arms, 19 of 830 in the two trials (2.1%) had positive sputum cultures at end of treatment. In the control arms, 21 of 827 (2.5%) participants experienced treatment failure. Pooled data did not show significant differences in treatment failure between the two ATT regimens (1657 participants, 2 trials; Analysis 2.6).
Acquired drug resistance
Of the two included trials evaluating gatifloxacin‐containing four‐month ATT regimens versus standard six‐month ATT regimens, only Jawahar 2013 reported on acquired drug resistance among 41 participants who experienced culture confirmed treatment failure, or who suffered a recurrence in the six‐month ATT arm. Rifampicin resistance developed in one participant and isoniazid resistance in another. None of the participants given the gatifloxacin‐containing four‐month ATT regimen was detected to have acquired drug resistance. Acquired drug resistance did not differ significantly between the two ATT regimens when the number of participants in each ATT regimen assessed for treatment failure was used as the denominator rather than only the number with treatment failure (301 participants; Analysis 2.7). However, susceptibility to gatifloxacin was not directly evaluated in this trial.
Serious adverse events
Five people in Jawahar 2013 had SAEs; with gatifloxacin‐containing ATT, three had seizures and one had QTc prolongation requiring termination of treatment; and with control ATT, one person had jaundice. In Merle 2014, 20 people in the gatifloxacin arm had 20 SAEs, of which 14 were considered unrelated to treatment; two of three SAEs considered treatment related were deaths. With control ATT, 23 people had 23 SAEs, of which 20 were considered unrelated to treatment; two of three considered treatment related were deaths. Pooled effect estimates were similar for both regimens (1993 participants, 2 trials; Analysis 2.8).
Other adverse events
In Jawahar 2013, nausea, vomiting, and abdominal discomfort (23%) and giddiness (18%) were more frequent among 136 participants given the four‐month regimen than among 165 participants on standard ATT (9% and 3%, respectively). Merle 2014 systematically assessed participants for QTc and blood sugar abnormalities and reported no differences in abnormal peak values of the QTc interval between ATT regimens, nor in episodes of high or low blood sugar, between ATT regimens.
Discusión
Se incluyeron cinco ensayos que compararon regímenes de tratamiento antituberculoso (TAT) de cuatro meses con fluoroquinolona versus regímenes de TAT estándar de seis meses, y que reclutaron a 5825 adultos con tuberculosis pulmonar sensible a los fármacos de 14 países con alta transmisión de tuberculosis en Asia, África y América Latina. Tres eran ensayos multinacionales que incluían a un total de 572 pacientes con pruebas positivas para el VIH que no estaban recibiendo tratamiento antirretroviral (TAR).
Resumen de los resultados principales
Los regímenes de TAT de cuatro meses con moxifloxacina que sustituyen el etambutol o a la isoniazida probablemente aumentan la recaída después del tratamiento en adultos con tuberculosis pulmonar sensible a los fármacos en comparación con los regímenes estándar de TAT de seis meses (evidencia de certeza moderada; Resumen de resultados, tabla 1). En comparación con el TAT estándar de seis meses, los regímenes de TAT de cuatro meses que sustituyen la gatifloxacina por etambutol probablemente aumentan la recaída después del tratamiento en adultos con tuberculosis sensible a los fármacos (evidencia de certeza moderada; Resumen de resultados, tabla 2).
En comparación con el TAT de seis meses, los regímenes de TAT de cuatro meses que contienen moxifloxacina o gatifloxacina probablemente logran poca o ninguna diferencia en el fracaso del tratamiento, la muerte o los eventos adversos graves (evidencia de certeza moderada). Los regímenes de cuatro meses que contienen moxifloxacina pueden no aumentar la incidencia de resistencia adquirida a los fármacos (evidencia de certeza baja). No se conoce si los regímenes de TAT de cuatro meses que contienen gatifloxacina aumentan la incidencia de resistencia adquirida a los fármacos (evidencia de certeza muy baja). Véase la tabla 1 de Resumen de resultados para observar los regímenes de cuatro meses que contienen moxifloxacina, y la tabla 2 de Resumen de resultados para observar los regímenes de cuatro meses que contienen gatifloxacina.
Compleción y aplicabilidad general de las pruebas
Los ensayos que cumplieron con los criterios de inclusión evaluaron solo dos de las fluoroquinolonas de tercera generación que se utilizan (moxifloxacina y gatifloxacina). Cuatro de los cinco ensayos evaluaron sus efectos en cuanto al reemplazo del etambutol o la isoniazida en regímenes de TAT acortados. Solo un ensayo en curso evaluó los efectos del agregado de una fluoroquinolona (moxifloxacina) a los fármacos del TAT estándar en regímenes acortados, y se esperan los hallazgos de los resultados clínicamente relevantes del fracaso del tratamiento y la recaída. La evidencia disponible de los estudios de esta revisión indica que los regímenes acortados que reemplazan el etambutol o la isoniazida con moxifloxacina pueden no aumentar la resistencia adquirida a los fármacos. No se sabe si los regímenes que contienen gatifloxacina aumentarán la resistencia adquirida a los fármacos, debido a que este hecho solo se evaluó en un ensayo que utilizó la susceptibilidad a la ofloxacina como un indicador sustituto. La resistencia a las fluoroquinolonas en pacientes con tuberculosis pulmonar recién diagnosticada, y en los que son sometidos a un nuevo tratamiento, se reconoce cada vez más como un problema, en particular en partes del mundo donde el uso de fluoroquinolonas es muy generalizado y no está regulado (Agarwal 2009; Devasia 2009; Selvakumar 2015). Los efectos perjudiciales relacionados con la fluoroquinolona se evaluaron de forma sistemática en los cinco ensayos, en particular en los tres ensayos multinacionales. Los ensayos en curso están comparando otros regímenes de cuatro meses versus TAT estándar de seis meses (Características de los estudios en curso). Los resultados de estos estudios se agregarán a la evidencia disponible para informar las decisiones sobre si el tratamiento de primera línea para la tuberculosis pulmonar sensible a los fármacos puede acortarse de forma efectiva sin comprometer la seguridad o aumentar la recaída o la resistencia adquirida a los fármacos.
Dos de los tres ensayos de los regímenes que contenían moxifloxacina diferenciaron la recaída de la nueva infección mediante el genotipo. En los otros dos ensayos, la mayoría de las recurrencias se consideraron recaídas en lugar de nuevas infecciones, aunque en una proporción menor, la nueva infección puede haber causado recurrencia. El TAT afecta principalmente las tasas de recaída, no las tasas de nueva infección; estas últimas dependerían de otros factores como la infección por VIH comórbida y la intensidad de la transmisión de tuberculosis (Wood 2011). Los ensayos de esta revisión se realizaron en países con carga alta de tuberculosis, donde la presión de la transmisión de tuberculosis es alta. Estos países de carga alta representan el 84% de la carga de tuberculosis en todo el mundo (OMS 2018). La recaída y la reinfección no suelen estar diferenciadas en los programas de control de la tuberculosis en estos países. Es tranquilizador observar que, con respecto a este tema, uno de los análisis de sensibilidad realizados en Gillespie 2014 y Jindani 2014 incluyó todas las nuevas infecciones en los resultados desfavorables, y las estimaciones de los efectos no difirieron de forma considerable del metanálisis que excluyó la nueva infección.
Los ensayos incluidos en esta revisión excluyeron a los niños y a las mujeres embarazadas o que amamantaban. También excluyeron a los pacientes con muchas afecciones comórbidas, como la tuberculosis anterior, los pacientes con VIH que recibían TAR o con recuentos bajos de células CD4 y los pacientes con diabetes. Por lo tanto, los resultados de esta revisión se pueden aplicar principalmente a los adultos con tuberculosis pulmonar sensible a los fármacos sin afecciones comórbidas graves. Esta revisión aporta evidencia de certeza moderada de que los regímenes de TAT de cuatro meses que sustituyen la moxifloxacina o la gatifloxacina por isoniazida o etambutol probablemente son inferiores a los regímenes estándar de TAT de seis meses para prevenir la recaída (aunque probablemente haya poca o ninguna diferencia en la resolución). Se puede argumentar que la recaída con los regímenes de cuatro meses probablemente no sería menor en las poblaciones con afecciones comórbidas graves de lo que se informó en los ensayos de esta revisión.
Sin embargo, la extrapolación de los resultados de esta revisión a los pacientes con diabetes (muchos de los cuales pueden presentar otras enfermedades comórbidas graves) puede ser más problemática. En primer lugar, la diabetes mellitus (DM) y la tuberculosis comórbidas son cada vez más frecuentes, debido a que los pacientes con DM tienen un mayor riesgo de desarrollar tuberculosis activa; la mayor parte de esta doble carga se encuentra en países de ingresos bajos y medios y con una alta carga de tuberculosis (Al‐Rifai 2017; Jeon 2008; Tegegegne 2018). Los pacientes con DM y tuberculosis tienen más probabilidades de presentar peores resultados del tratamiento que los pacientes para los que la DM no es comórbida con la tuberculosis (Baker 2011). La diabetes aumenta el riesgo de fracaso del tratamiento, muerte, recaída y recurrencia debido a una nueva infección entre los pacientes con tuberculosis (Baker 2011). La diabetes también aumenta las probabilidades de desarrollar tuberculosis resistente a múltiples fármacos (TB‐RMF) (Liu 2017; Tegegne 2018). El tratamiento de la tuberculosis y la DM también plantea problemas debido a las interacciones entre los fármacos antituberculosos, en particular la rifampicina, y los fármacos antidiabéticos, y los eventos adversos de los fármacos son más frecuentes entre los que presentan tuberculosis y DM que entre los que solo presentan tuberculosis (Riza 2014).
Certeza de la evidencia
Se utilizaron los criterios GRADE para juzgar la certeza de la evidencia para los resultados preseleccionados para cada comparación en esta revisión (Guyatt 2011a). Uno de los ensayos que contribuyó con datos a ambas comparaciones en esta revisión fue juzgado como en riesgo alto de sesgo de selección debido a la ocultación comprometida de la asignación (Jawahar 2013). Sin embargo, la eliminación de los datos de este ensayo en los análisis de sensibilidad no alteró la dirección de las estimaciones del efecto, por lo que no se redujo la calificación del riesgo de sesgo en la comparación de los regímenes de TAT de cuatro meses con moxifloxacina versus los regímenes estándar de seis meses. Se redujeron todos los resultados en un nivel a causa de la falta de direccionalidad debido a los criterios de inclusión restringidos en todos los ensayos, en particular la exclusión de los pacientes con DM y tuberculosis. Los pacientes con tuberculosis y DM tienen cuatro veces más probabilidades de presentar la recaída que los que no presentan DM (Baker 2011). Sin embargo, también son más propensos a morir que los pacientes sin DM, lo cual puede afectar los cálculos de la recaída de manera variable. Estas diferencias en la vulnerabilidad entre los pacientes con DM y tuberculosis comórbidas reducen la certeza de las estimaciones del efecto para la recaída con regímenes de TAT acortados versus estándar, que se evaluaron con mayor frecuencia en pacientes sin DM comórbida reclutados para los ensayos en esta revisión. Los hallazgos para este resultado en la comparación del TAT de cuatro meses con moxifloxacina versus el TAT estándar muestran inconsistencia entre los efectos grandes y pequeños a favor del régimen de seis meses. La certeza de la evidencia para el resultado primario de la recaída se calificó como moderada en ambas comparaciones (Resumen de resultados, tabla 1; Resumen de resultados, tabla 2).
La certeza de la evidencia para la muerte por cualquier causa, la interrupción del tratamiento y los eventos adversos graves en ambas comparaciones también se calificó como moderada, y se disminuyó en un nivel a causa de la falta de direccionalidad debido a los criterios de inclusión restringidos, en particular para los pacientes con DM. Para estos resultados, los intervalos de confianza (IC) del 95% para los riesgos relativos (RR) fueron amplios, aunque los eventos fueron pocos y el tamaño de las muestras fue suficientemente grande. El RR y el IC del 95% alrededor del RR fueron precisos e indicaron poca o ninguna diferencia en los efectos clínicamente apreciables con cualquier régimen de tratamiento. Además, los estudios primarios se diseñaron como ensayos de no inferioridad, el margen de no inferioridad se estableció en 6%, y los IC del 95% para las estimaciones absolutas agrupadas del riesgo para los resultados de la muerte, el fracaso del tratamiento y los eventos adversos graves en ambos grupos de comparaciones se encontraban bien incluidos dentro de este margen. Por lo tanto, no se disminuyó la certeza de estos resultados debido a la imprecisión. La certeza de la evidencia para el resultado de la resistencia adquirida a los fármacos se calificó como baja para la comparación de los regímenes de combinación basados en moxifloxacina y como muy baja para la comparación de los regímenes basados en gatifloxacina, debido a que, además de la falta de direccionalidad, estos resultados se disminuyeron por la imprecisión y, además, por el riesgo alto de sesgo para la comparación con el régimen que contenía gatifloxacina, debido a que el único ensayo informó un desequilibrio inicial entre las proporciones con resistencia a los fármacos (Jawahar 2013).
Sesgos potenciales en el proceso de revisión
Se utilizaron los métodos estándar descritos en el Manual Cochrane para Revisiones Sistemáticas de Intervenciones (Higgins 2011). La búsqueda bibliográfica abarcó múltiples bases de datos; además, se evaluaron las listas de referencias de los estudios incluidos y de las revisiones sistemáticas pertinentes para obtener ensayos potencialmente elegibles. No fue posible evaluar formalmente el sesgo de publicación mediante el uso de gráficos en embudo debido a que solo se identificaron cinco ensayos relevantes con resultados pertinentes a esta revisión. Se conocen seis estudios en curso que informarán las actualizaciones de esta revisión. Al menos dos autores de la revisión seleccionaron de forma independiente los estudios para su inclusión, lo cual fue verificado de forma independiente por un autor principal de la revisión. La extracción de datos fue realizada de forma independiente por dos autores de la revisión y fue verificada de forma independiente por otros dos autores de la revisión.
Se intentó representar la pérdida durante el seguimiento para el resultado primario de la recaída mediante el uso de los datos de los análisis principales proporcionados en el análisis de intención de tratar (ITT‐m) modificado del informe de cada ensayo, debido a que los ensayos incluyeron a más participantes aleatorizados que los que se incluyeron en sus análisis por protocolo. Los tres ensayos multinacionales habían demostrado que los resultados de los análisis de sensibilidad que comparaban los análisis por protocolo y los análisis ITT‐m eran consistentes. Sin embargo, cuando los datos de los ensayos se incluyen en un metanálisis, las estimaciones agrupadas pueden variar dependiendo de la cantidad de información faltante para los resultados del ensayo, así como de la magnitud y de la dirección de las estimaciones del efecto en los ensayos individuales. La serie de análisis de sensibilidad que se realizó no indicó que los datos faltantes para la recaída influyeran en los resultados generales.
Se excluyeron muchos ensayos que comparaban regímenes de TAT que contenían fluoroquinolonas versus regímenes de TAT estándar (ver Características de los estudios excluidos) y se informaron los datos para la positividad del cultivo de esputo a las ocho semanas, un resultado secundario de esta revisión. Los criterios de inclusión de esta revisión requirieron la comparación de regímenes de tratamiento de la tuberculosis acortados versus regímenes de tratamiento de la tuberculosis estándar de seis meses, y debido a que estos ensayos de fase 2b fueron diseñados principalmente para evaluar e informar la conversión del esputo solo a los dos meses, no cumplieron con los criterios de inclusión de la revisión. Por otro lado, se incluyó Velayutham 2014; que informó los resultados del cultivo de esputo a los dos meses, pero no proporcionó datos sobre el éxito del tratamiento o la recaída. Sin embargo, a diferencia de los ensayos de fase 2b que se excluyeron, este estudio se diseñó como un ensayo de fase 3 que cumplió con los criterios de selección de esta revisión. Los datos de la conversión del esputo a las ocho semanas fueron un resultado secundario preestablecido, y el informe provisional incluyó los eventos adversos durante el tratamiento, lo que justifica aún más su inclusión. También se excluyeron los datos para la conversión del esputo de uno de los brazos del ensayo en Jindani 2014; que utilizó moxifloxacina en lugar de isoniazida, pero durante seis meses. Se combinaron e informaron los datos para la conversión del cultivo del esputo a los dos meses a partir de los brazos de moxifloxacina de cuatro y seis meses, y no fue posible utilizar estos datos. Sin embargo, en la siguiente sección se revisarán los datos para la conversión del cultivo de esputo de estos ensayos para otros trabajos publicados, a fin de tener en cuenta la totalidad de la evidencia de los ensayos para este resultado.
Acuerdos y desacuerdos con otros estudios o revisiones
Una revisión Cochrane anterior sobre las fluoroquinolonas para el tratamiento de la tuberculosis pulmonar encontró solo ensayos en curso de los regímenes acortados que contienen fluoroquinolona en comparación con los regímenes estándar de TAT de seis meses que ahora se han incluido en esta revisión (Ziganshina 2013). Ziganshina 2013 incluyó los ensayos de fase 2b que se excluyeron de la presente revisión. Otras revisiones sistemáticas sobre las fluoroquinolonas para el tratamiento de los pacientes con tuberculosis pulmonar sensible a los fármacos también incluyeron estos ensayos de fase 2b (Lee 2016; Li 2016; Ruan 2016), así como los ensayos de fase 3 incluidos en esta revisión. Los metanálisis de los datos para la positividad del esputo a las ocho semanas de los ensayos de fase 2b y fase 3 (incluidos los datos combinados de los brazos de cuatro y seis meses que contienen moxifloxacina en Jindani 2014) incluidos en estas revisiones mostraron resultados similares a los de esta revisión para la conversión del esputo a las ocho semanas. Estas revisiones sistemáticas también informaron resultados similares para los otros resultados informados en esta revisión.
El efecto de los regímenes de tratamiento acortados para los pacientes con enfermedad no cavitaria no fue un objetivo de esta revisión. Sin embargo, otra revisión sistemática intentó evaluar si los pacientes con tuberculosis no cavitaria pueden tener mejores resultados que los pacientes con enfermedad cavitaria con los regímenes más cortos, debido a que la carga bacteriana es menor con la tuberculosis pulmonar no cavitaria. Solo los tres ensayos multinacionales incluidos en esta revisión cumplieron con sus criterios de inclusión y proporcionaron datos para los participantes con enfermedad no cavitaria (Gillespie 2014; Jindani 2014; Merle 2014). Utilizaron datos en el metanálisis de 1066 participantes a partir de los tres ensayos que incluían la tuberculosis pulmonar no cavitaria. Su intención era estudiar los efectos de los regímenes que contienen fluoroquinolona sobre la recaída y la resolución, aunque no pudieron encontrar datos desglosados para estos resultados para los pacientes con tuberculosis no cavitaria en los tres ensayos. Utilizaron el "resultado desfavorable" compuesto en estos ensayos y utilizaron un margen del 6% en los riesgos relativos (RR) para las estimaciones agrupadas con el fin de indicar la no inferioridad. El intervalo de confianza (IC) del 95% para el RR agrupado para los resultados desfavorables al utilizar los datos de los tres ensayos superó este margen, y los resultados fueron heterogéneos. En los análisis de subgrupos de los datos agrupados de los ensayos que utilizaron el tratamiento diario (Gillespie 2014; Merle 2014), los resultados fueron homogéneos y el IC del 95% para el RR estuvo dentro del margen de no inferioridad (RR 1%, IC del 95%: ‐3% a 5%; 965 participantes, dos ensayos). Además, en el análisis de subgrupos que utilizó datos agrupados de los brazos de estos dos ensayos cuando las fluoroquinolonas fueron sustituidas por etambutol y se compararon con el TAT de seis meses, el IC del 95% para el RR agrupado para un resultado desfavorable estuvo dentro del margen de no inferioridad (RR ‐1%, IC del 95%: ‐5% a 4%; 857 participantes, dos ensayos). Los datos agrupados de los tres ensayos para los eventos adversos graves entre los participantes con enfermedad no cavitaria tampoco mostraron diferencias para los regímenes que contenían flouroquinolona versus los regímenes de seis meses, con el IC del 95% para el RR claramente dentro del margen de no inferioridad (RR 0%, IC del 95%: ‐2% a 1%; 4881 participantes, tres ensayos). Alipanah 2016 estableció la conclusión de que los regímenes diarios de cuatro meses que sustituyen el etambutol por gatifloxacina o moxifloxacina pueden no ser inferiores al tratamiento estándar para pacientes con tuberculosis pulmonar confirmada por cultivo, no cavitaria y sensible a los fármacos. Estos autores de la revisión reconocieron que estas estimaciones pueden ser propensas a errores debido a que tuvieron que utilizar datos de una combinación de datos del análisis por protocolo y de intención de tratar de los ensayos en su análisis.
Debe verificarse la sugerencia a partir de los resultados de Alipanah 2016 de que las proporciones mayores de recaídas observadas con los regímenes que contienen moxifloxacina y gatifloxacina en comparación con el régimen estándar de seis meses de la presente revisión pueden deberse a la inclusión de pacientes con enfermedad pulmonar cavitaria debido a la tuberculosis. El apoyo a esta observación proviene de un análisis agrupado de conjuntos de datos de pacientes individuales de 3411 participantes de Gillespie 2014; Jindani 2014 y Merle 2014 (Imperial 2018). Este análisis identificó dos subgrupos de participantes que difirieron en su respuesta a los regímenes de cuatro meses. Un subgrupo de pacientes con tuberculosis sensible a los fármacos y grados bajos de positividad del esputo o ausencia de cavitación en las evaluaciones iniciales tuvo un menor riesgo de resultados desfavorables, y esta población (con cualquiera de estas características de bajo riesgo) constituyó el 47% de los 3405 participantes en los tres ensayos. Los regímenes de fluoroquinolona de cuatro meses en estos ensayos fueron efectivos para reducir el riesgo de resultados desfavorables en esta población con "enfermedad mínima". Otro subgrupo de participantes con un grado de frotis de 3+ y la presencia de cavitación en las radiografías de tórax al inicio (34% de la muestra total) tuvo resultados desfavorables. Los datos de este análisis agrupado sugieren que esta población "difícil de tratar" puede necesitar tratamiento durante más tiempo que los que reciben el régimen estándar de seis meses para lograr resultados óptimos (Imperial 2018). Estas observaciones de Alipanah 2016 e Imperial 2018 tienen implicaciones de valor heurístico para el diseño y la interpretación de los ensayos futuros sobre este tema.
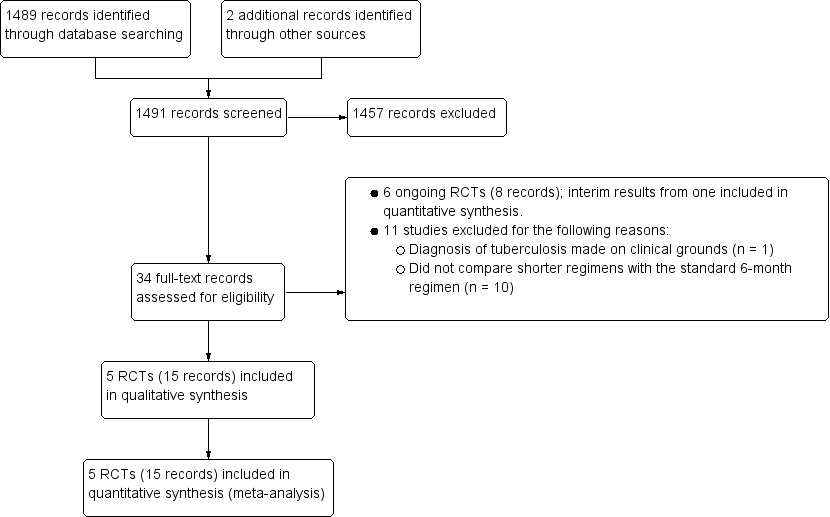
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
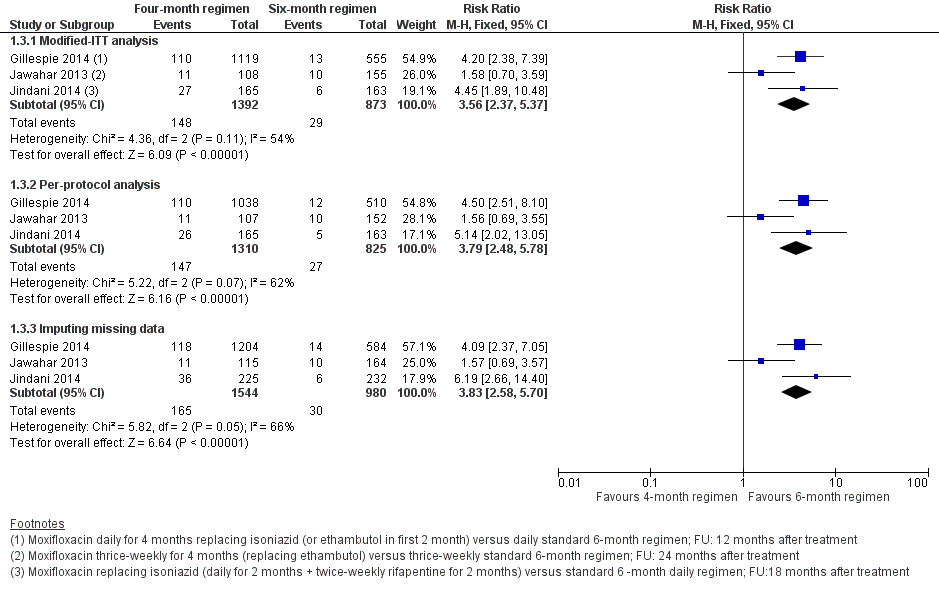
Forest plot of comparison: 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, outcome: 1.3 Relapse: sensitivity analysis accounting for missing data.

Forest plot of comparison: 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, outcome: 2.2 Relapse: sensitivity analysis accounting for missing data.
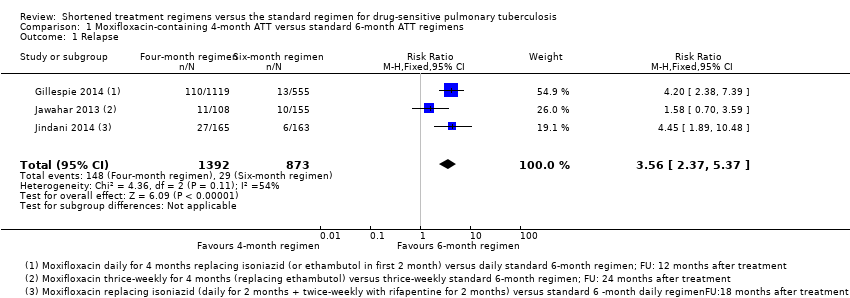
Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 1 Relapse.

Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 2 Relapse: subgroup analysis.
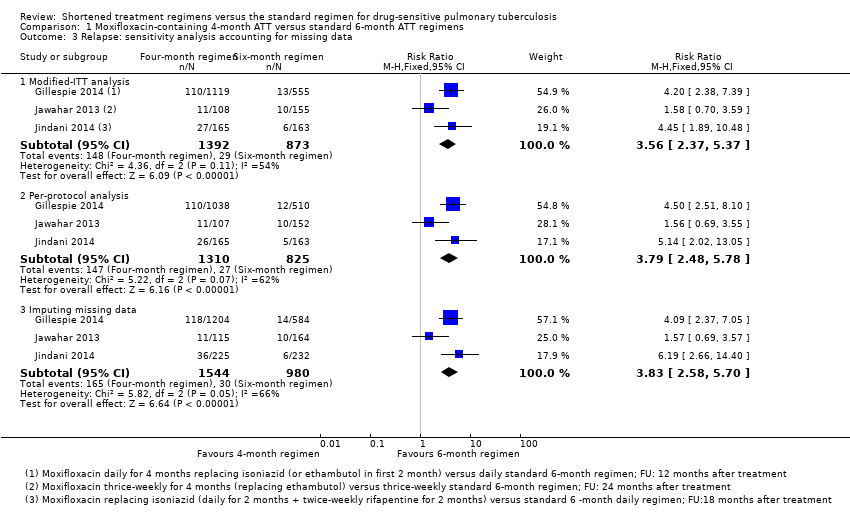
Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 3 Relapse: sensitivity analysis accounting for missing data.

Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 4 Death from any cause.

Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 5 Treatment discontinuation.

Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 6 Positive sputum culture/smear at 8 weeks.
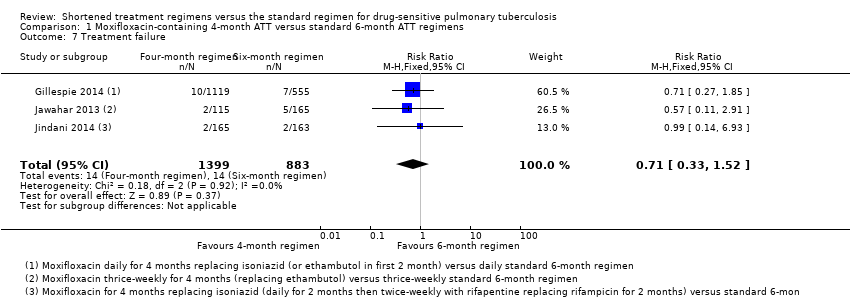
Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 7 Treatment failure.

Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 8 Acquired drug resistance.

Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 9 Serious adverse events.

Comparison 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 1 Relapse.

Comparison 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 2 Relapse: sensitivity analysis accounting for missing data.

Comparison 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 3 Death from any cause.

Comparison 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 4 Treatment discontinuation.

Comparison 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 5 Positive sputum culture at 8 weeks.

Comparison 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 6 Treatment failure.

Comparison 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 7 Acquired drug resistance.
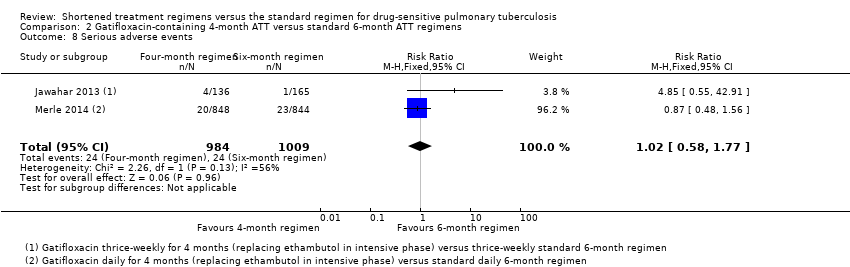
Comparison 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 8 Serious adverse events.
| Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimen for drug‐sensitive pulmonary tuberculosis | ||||||
| Patient or population: adults with drug‐sensitive pulmonary tuberculosis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with 6‐month standard ATT | Risk with 4‐month moxifloxacin‐containing ATT | |||||
| Relapse | 32 per 1000 | 82 more relapses per 1000 | RR 3.56 | 2265 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably increases relapse compared to the 6‐month regimen |
| Death from any cause Follow‐up: range 18 to 24 months | 21 per 1000 | 2 more deaths per 1000 | RR 1.06 | 2760 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably makes little or no difference in death from any cause compared to the 6‐month regimen |
| Treatment failure | 16 per 1000 | 5 fewer treatment failures per 1000 | RR 0.71 | 2282 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably results in little or no difference in treatment failure compared to the 6‐month regimen |
| Acquired drug resistance | 7 per 1000 | 5 fewer with acquired drug resistance per 1000 (6 fewer to 2 more) | RR 0.33 | 2282 (3 RCTs)e | ⊕⊕⊝⊝ Due to indirectness and imprecision | The 4‐month regimen may be little or no different than the 6‐month regimen in the incidence of acquired drug resistance |
| Serious adverse events Follow‐up: range 18 to 24 months | 62 per 1000 | 2 fewer with serious adverse events per 1000 | RR 0.97 | 3548 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably results in little or no difference in serious adverse events compared to the 6‐month regimen |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aNo serious risk of bias: although Jawahar 2013 was at high risk of allocation bias, exclusion of this trial from the sensitivity analysis did not change the direction of effect. Not downgraded. | ||||||
| Gatifloxacin‐containing 4‐month ATT regimens compared to standard 6‐month ATT regimens for drug‐sensitive pulmonary tuberculosis | ||||||
| Patient or population: adults with drug‐sensitive pulmonary tuberculosis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with 6‐month standard ATT | Risk with gatifloxacin‐containing | |||||
| Relapse | 70 per 1000 | 77 more relapses per 1000 | RR 2.11 | 1633 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably increases relapse compared to the 6‐month regimen |
| Death from any cause | 29 per 1000 | 3 fewer deaths per 1000 | RR 0.90 | 1886 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably makes little or no difference in death compared to the 6‐month regimen |
| Treatment failure | 25 per 1000 | 1 less treatment failure per 1000 | RR 0.93 | 1657 | ⊕⊕⊝⊝ Due to indirectness | The 4‐month regimen probably makes little or no difference in treatment failure compared to the 6‐month regimen |
| Acquired drug resistance | 12 per 1000 | 9 fewer with acquired drug resistance per 1000 (12 fewer to 49 more) | RR 0.24 (0.01 to 5.01) | 301 (1 RCT)d | ⊕⊝⊝⊝ Due to indirectness, risk of bias, and imprecision | We do not know if acquired drug resistance is any different in the 4‐month and the 6‐month regimens |
| Serious adverse events | 24 per 1000 | 0 fewer serious adverse events per 1000 | RR 1.02 | 1993 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably results in little or no difference in serious adverse events compared to the 6‐month regimen |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. | ||||||
| aNo serious risk of bias: although Jawahar 2013 was assigned high risk of bias for allocation concealment, removal of this trial from the sensitivity analysis did not significantly alter the direction, magnitude, or precision of the effect estimate. Not downgraded. | ||||||
| Study ID (Acronym) | (REMoxTB) | (RIFAQUIN) | (OFLOTUB) | |||||||
| Setting | Multiple sites in Africa (Kenya, South Africa, Tanzania, Zambia), Asia (China, India, Malaysia Thailand), Latin America (Mexico) | 6 sites in 2 cities in India | 6 cities in 4 countries in Africa (Botswana, South Africa, Zambia, Zimbabwe) | 5 countries in Africa (Benin, Guinea, Kenya, Senegal, South Africa) | 2 cities in India | |||||
| Participants | ||||||||||
| Number randomized | 1931 | 429 | 827 | 1836 | 801 | |||||
| Age | Adults (> 18 years) | Adults (> 18 years) | Adults (> 18 years) | Adults (18 to 65 years) | Adults (> 18 years) | |||||
| HIV infection | Included (if CD4 count > 250 cells/μL and not on ART); 110 (7%) | Excluded | Included (if CD4 count > 150/mm³ and not on ART; 158 (27%) | Included if not stage 3 or 4 disease and not on ART; 304 (17%) | Excluded | |||||
| Diagnosis of TB | Positive sputum smears on 2 occasions Culture‐confirmed susceptibility to rifampicin, isoniazid, pyrazinamide, and moxifloxacin | Newly diagnosed pulmonary TB with at least 2 positive sputum cultures. Confirmed by culture and MDR‐TB excluded, susceptibility to ofloxacin (as proxy for moxifloxacin) | 2 sputum samples that were positive for tubercle bacilli on direct smear microscopy No resistance to isoniazid, rifampicin, or moxifloxacin | Acid‐fast bacilli in 2 consecutive sputum smears; confirmed by culture (solid medium) and drug sensitivity tests to rifampicin, isoniazid, ethambutol, streptomycin, and gatifloxacin | 2 positive sputum smear smears for tuberculosis. Culture‐confirmed and MDR‐TB ruled out; susceptible to ofloxacin (as proxy for moxifloxacin) | |||||
| Intervention(s) and comparator | ||||||||||
| Duration of ATT | 4 monthsa | 6 months | 4 monthsb | 6 months | 4 months | 6 monthsc | 4 months | 6 months | 4 monthsa | 6 months |
| Regimens | 2HRZM/2HRM + 2MRZE/2MR | 2HRZE/4HR | 2(HRZG)₃/ 2 (HRG)₃ 2(HRZM)₃/2(HRM)₃ | 2(HRZE)₃ /4(HR)₃ | 2MRZE/ 2P₂M₂ | 2HRZE/ 4HR | 2HRZG/ 2HRG | 2HRZE/4HR | 3HRZM + 2HRZM/ 2RHM + 2HRZM/ 2(RHM)₃ + 2HRZM/ 2(RHEM)₃ | 2(HRZE)₃/ 4(HR)₃ |
| Number allocated | 655 + 636 = 1291 | 640 | 141 118 | 170 | 275 | 275 | 917 | 919 | 629 | 172 |
| Late screening failures excluded after allocation | 38 + 32 = 70 | 40 | 5 3 | 5 | 36 | 35 | 62 | 51 | 13 | 8 |
| Number eligible | 1231 | 600 | 136 115 | 165 | 239 | 240 | 852 | 868 | 616 | 164 |
| Number analysed in m‐ITT analysis (% of those allocated) | 568 + 551 = 1119 (87) | 555 (87) | 136 (97) 115 (98) | 165 (97) | 193 (70) | 188 (68) | 791 (86) | 794 (86) | 590 (94) | 151 (88) |
| Number analysed in per‐protocol analysis (% of those allocated) | 514 + 524 = 1038 (80) | 510 (80) | 131 (93) 113 (96) | 159 (94) | 165 (60) | 163 (59) | 651 (71) | 601 (65) | As above | |
| Number analysed in ancillary analysis (ITT) (% of those allocated) | 617 + 604 = 1221 (94) | 600 (94) | Not done | 239 (87) | 240 (87) | Not reported | Not reported | |||
| Outcomes reported | ||||||||||
| Relapse | Relapse within 18 months after randomization in those with negative culture with treatment. Relapse strains were those shown to be identical on 24‐locus MIRU analysis LJ solid media and MGIT liquid media used for culture | Recurrence of TB over 24 months after treatment in those with a favourable response with treatment: either bacteriologic recurrence (LJ solid media) or clinical/radiologic recurrence | Relapse within 12 to 18 months after treatment. Two positive cultures within a period of 4 months without an intervening negative culture). Re‐infections differentiated from relapse through genotyping (MIRU‐VNTRs) LJ solid media used for culture in some centres, MGIT liquid media in others, and both in some centres | Recurrence of TB over 24 months after treatment proven bacteriologically (2 consecutive positive sputum samples a day apart) or clinically Genotyping (MIRU‐VNTRs) results available for only 70/140 (55%) of those with culture confirmed recurrence. Most were relapses | Not reported | |||||
| Deaths | All deaths TB deaths | Reported (only non‐TB deaths occurred) | All deaths TB deaths | Death during treatment Death after treatment | Not reported | |||||
| Treatment discontinuation | Includes those who did not complete treatment, relocated, or withdrew consent | Includes those who did not complete treatment and those lost to follow‐up | Includes change in treatment due to adverse events, loss to follow‐up, and other treatment changes | Includes those who withdrew consent during treatment and dropouts | Reported but disaggregated data for each group not available | |||||
| Positive smear/ sputum culture at 2 months | Reported using LJ solid media (used in this review) and MGIT liquid media for all randomized participants excluding late screening failures | Reported using LJ solid media for all randomized participants excluding late screening failures | Reported but disaggregated data for moxifloxacin 4‐month and 6‐month treatment groups not available Data also not available for all participants from LJ media | Reported for 752 in the 4‐month and 759 in the 6‐month regimens (88% and 87% of those eligible, respectively) Culture using LJ solid media | Reported for 590 (94%) in the 4‐month and 151 (88%) in the 6‐month regimens | |||||
| Acquired drug resistance | Reported | Reported | Reported | Not reported | Not reported | |||||
| Treatment failure | Includes culture confirmed and not confirmed | Includes culture confirmed and unconfirmed | Culture confirmed | Includes culture confirmed failure | Not reported | |||||
| Serious adverse events | Reported for all randomized participants excluding late screening failures. Grade 3 and 4 severity (DAIDS 2009) | Deduced from adverse events reported for all randomized participants excluding late screening failures. Not graded | Reported for all participants randomized who took 1 dose and assessed as severe or life‐threatening during and 2 weeks after treatment. grade 3 and 4 severity (DAIDS 2009) | Reported for 1692 (92%) of all randomized participants. grade 3 and 4 severity (DAIDS 2009) | Deduced from adverse events reported. Not graded | |||||
| Other adverse events | Not reported | Reported | Not reported | QT prolongation Hyperglycaemic episodes | Reported | |||||
| Abbreviations: ART: anti‐retroviral treatment; ATT: anti‐tuberculosis treatment; E: ethambutol; G: gatifloxacin; H: isoniazid; ITT: intention‐to‐treat; LJ: Löwenstein‐Jensen; M: moxifloxacin; MGIT: mycobacterial growth indicator tube; MIRU‐VNTRs: mycobacterial interspersed repetitive unit–variable number tandem repeats; m‐ITT: modified intention‐to‐treat; P: rifapentine; R: rifampicin; Z: pyrazinamide. Leading numbers in regimens indicate duration in months. Drugs were administered daily, except when given thrice weekly as indicated by subscripts. aData from moxifloxacin‐containing shortened regimens combined for data synthesis. | ||||||||||
| Primary outcome: relapse | ||||||
| Trial ID | ||||||
| Regimens | 4 months | 6 months | 4 months | 6 months | 4 months | 6 months |
| aModified‐ITT analysis (primary analysis) | 110/1119 (9.8%) | 13/555 (2.3%) | 11/108 (10.1%) | 10/155 (6.5%) | 27/165 (16.4%) | 6/163 (3.7%) |
| aPer‐protocol analysis | 110/1038 (10.6%) | 12/510 (2.4%) | 11/107 (10.1%) | 10/152 (6.6%) | 26/165 (15.8%) | 5/163 (3.1%) |
| bSensitivity analysis imputing missing data | 126/1184 (10.7%) | 14/577 (2.4%) | 11/114 (9.7%) | 10/159 (6.3%) | 36/225 (16.0%) | 71/232 (2.6%%) |
| Abbreviations: ATT: anti‐tuberculosis treatment; ITT: intention‐to‐treat. aAs reported in trial reports. | ||||||
| Primary outcome: relapse | ||||
| Trial ID | ||||
| Regimen | 4 months | 6 months | 4 months | 6 months |
| aModified‐ITT analysis (primary analysis) | 19/122 (15.6%) | 10/155 (6.5%) | 101/694 (14.6%) | 47/662 (7.1%) |
| aPer‐protocol analysis | 19/121 (15.7%) | 10/152 (6.6%) | 98/651 (15.1%) | 44/601 (7,3%) |
| bSensitivity analysis imputing missing data | 19/132 (14.4%) | 10/159 (6.3%) | 122/786 (15.5%) | 61/774 (7,9%) |
| Abbreviations: ATT: anti‐tuberculosis treatment; ITT: intention‐to‐treat. aAs reported in trial reports. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse Show forest plot | 3 | 2265 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.56 [2.37, 5.37] |
| 2 Relapse: subgroup analysis Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Moxifloxacin replacing ethambutol | 2 | 1386 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.74 [1.69, 4.43] |
| 2.2 Moxifloxacin replacing isoniazid | 2 | 1424 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.89 [3.02, 7.92] |
| 3 Relapse: sensitivity analysis accounting for missing data Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Modified‐ITT analysis | 3 | 2265 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.56 [2.37, 5.37] |
| 3.2 Per‐protocol analysis | 3 | 2135 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.79 [2.48, 5.78] |
| 3.3 Imputing missing data | 3 | 2524 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.83 [2.58, 5.70] |
| 4 Death from any cause Show forest plot | 3 | 2760 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.65, 1.75] |
| 5 Treatment discontinuation Show forest plot | 3 | 2335 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.78, 1.61] |
| 6 Positive sputum culture/smear at 8 weeks Show forest plot | 3 | 2828 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.22, 1.13] |
| 6.1 Moxifloxacin replacing isoniazid or ethambutol in 4‐month ATT regimen | 2 | 2087 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.45, 1.20] |
| 6.2 Moxifloxacin augmenting standard 6‐month ATT regimen | 1 | 741 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.15, 0.39] |
| 7 Treatment failure Show forest plot | 3 | 2282 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.33, 1.52] |
| 8 Acquired drug resistance Show forest plot | 3 | 2282 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.08, 1.31] |
| 9 Serious adverse events Show forest plot | 4 | 3548 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.74, 1.27] |
| 9.1 Moxifloxacin replacing standard drugs in 4‐month ATT regimens | 3 | 2760 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.72, 1.26] |
| 9.2 Moxifloxacin augmenting standard 6‐month ATT regimens | 1 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.45, 3.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse Show forest plot | 2 | 1633 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [1.56, 2.84] |
| 2 Relapse: sensitivity analysis accounting for missing data Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Modified‐ITT analysis | 2 | 1633 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [1.56, 2.84] |
| 2.2 Per‐protocol analysis | 2 | 1525 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [1.55, 2.87] |
| 2.3 Modified‐ITT analysis (all eligible participants ‐ imputing missing data) | 2 | 1851 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [1.53, 2.63] |
| 3 Death from any cause Show forest plot | 2 | 1886 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.53, 1.53] |
| 4 Treatment discontinuation Show forest plot | 2 | 1657 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.46, 1.08] |
| 5 Positive sputum culture at 8 weeks Show forest plot | 2 | 1818 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.80, 1.23] |
| 6 Treatment failure Show forest plot | 2 | 1657 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.51, 1.70] |
| 7 Acquired drug resistance Show forest plot | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.01, 5.01] |
| 8 Serious adverse events Show forest plot | 2 | 1993 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.58, 1.77] |

