Schémas thérapeutiques raccourcis par rapport au schéma standard pour la tuberculose pulmonaire sensible aux médicaments
Résumé scientifique
Contexte
La tuberculose est la maladie infectieuse qui cause le plus de décès dans le monde, la tuberculose pulmonaire étant la forme la plus courante. Le traitement standard de première ligne de la tuberculose pulmonaire pharmacosensible dure six mois et comprend l'isoniazide, la rifampicine, le pyrazinamide et l'éthambutol (HRZE) pendant deux mois, suivi de l’isoniazide, la rifampicine et l’éthambutol (HRE) (dans les zones de forte résistance aux médicaments antituberculeux) ou de l’isoniazide et de la rifampicine (HR), administrée pendant une phase de continuation de quatre mois. Beaucoup de personnes ne suivent pas ce cycle complet. Des schémas thérapeutiques raccourcis qui seraient tout aussi efficaces et sûrs pourraient améliorer le succès du traitement.
Objectifs
Évaluer l'efficacité et l'innocuité des schémas thérapeutiques raccourcis par rapport au schéma standard de six mois, pour les personnes atteintes de tuberculose pulmonaire pharmacosensible.
Stratégie de recherche documentaire
Nous avons effectué des recherches dans les bases de données suivantes jusqu'au 10 juillet 2019 : le registre spécialisé du groupe Cochrane sur les maladies infectieuses ; le registre des essais contrôlés Cochrane (CENTRAL), la Bibliothèque Cochrane ; MEDLINE (PubMed) ; Embase ; la Latin American Caribbean Health Sciences Literature (LILACS) ; le Science Citation Index‐Expanded ; l’Indian Medlars Center ; et la South Asian Database of Controlled Clinical Trials. Nous avons également fait des recherches dans le système d'enregistrement international des essais cliniques (ICTRP) de l’Organisation Mondiale de le Santé (OMS), sur le site ClinicalTrials.gov, dans l'unité des essais cliniques de l'Union internationale contre la tuberculose et les maladies respiratoires, dans l'unité des essais cliniques du Conseil de la recherche médicale du Royaume‐Uni et dans le registre des essais cliniques en Inde pour trouver les essais en cours. Nous avons vérifié les listes de référence des articles identifiés pour trouver d'autres études pertinentes.
Critères de sélection
Nous avons cherché des essais contrôlés randomisés (ECR) ou des quasi‐ECR qui comparaient des régimes de courte durée (moins de six mois) au régime standard de six mois pour des personnes de tous âges, sans tenir compte de leur statut sérologique VIH, qui venaient de recevoir un diagnostic de tuberculose pulmonaire par culture d'expectorations positives ou par GeneXpert, et dont la tuberculose était présumée ou prouvée pharmacosensible. Le critère de jugement principal était la rechute dans les deux ans suivant la fin du traitement antituberculeux (TAT).
Recueil et analyse des données
Deux auteurs de la revue ont, de manière indépendante, sélectionné les essais, extrait les données et évalué le risque de biais pour les essais inclus. Pour les résultats dichotomiques, nous avons utilisé des rapports de risque (RR) avec des intervalles de confiance (IC) de 95 %. Le cas échéant, nous avons regroupé les données des essais inclus dans des méta‐analyses. Nous avons évalué la qualité des données probantes à l'aide de l’outil GRADE.
Résultats principaux
Nous avons inclus cinq essais randomisés qui comparaient des schémas TAT de quatre mois contenant des fluoroquinolones, à des schémas TAT standard de six mois et avons recruté 5 825 adultes atteints de tuberculose pulmonaire pharmacosensible nouvellement diagnostiquée, dans 14 pays à forte transmission de tuberculose, en Asie, en Afrique et en Amérique latine. Trois d'entre eux étaient des essais multinationaux qui ont inclus un total de 572 personnes séropositives pour le VIH. Ces essais ont exclu les enfants, les femmes enceintes ou allaitantes, les personnes souffrant d'affections comorbides graves et celles atteintes de diabète sucré. Quatre essais avaient des bras de traitement multiples.
La moxifloxacine a remplacé l'éthambutol dans les schémas TAT standard de quatre mois, à raison d'une dose quotidienne ou de trois doses par semaine dans deux essais ; la moxifloxacine a remplacé l'isoniazide dans les schémas TAT de quatre mois dans deux essais, a été administrée quotidiennement dans un essai, et a été administrée avec de la rifapentine au lieu de la rifampicine, tous les jours pendant deux mois, et deux fois par semaine pendant deux mois dans un essai. La moxifloxacine a été ajoutée aux médicaments standard du TAT pendant trois à quatre mois dans un essai en cours qui a rapporté des résultats provisoires. La gatifloxacine a remplacé l'éthambutol dans les schémas TAT standard administrés quotidiennement ou trois fois par semaine, pendant quatre mois, dans deux essais. Le suivi s'est déroulé entre 12 et 24 mois après la fin du traitement pour la majorité des participantes.
Schémas TAT de quatre mois contenant de la moxifloxacine
Les schémas TAT de quatre mois contenant de la moxifloxacine, qui ont remplacé l'éthambutol ou l'isoniazide, ont probablement augmenté les proportions de personnes qui ont connu une rechute après un traitement réussi, par rapport aux schémas TAT standard (RR 3,56, IC à 95 % 2,37 à 5,37 ; 2 265 participants, 3 essais ; données probantes de qualité moyenne). Pour les décès toutes causes confondues, il y avait probablement peu ou pas de différence entre les deux schémas (2 760 participants, 3 essais ; données probantes de qualité moyenne). L'échec du traitement était rare, et il y avait probablement peu ou pas de différence dans les proportions d'échec du traitement entre les régimes TAT (2 282 participants, 3 essais ; données probantes de qualité moyenne). Aucun des participants ayant reçu des schémas contenant de la moxifloxacine n'a développé de résistance à la rifampicine, et ces schémas pourraient ne pas augmenter le risque de résistance acquise (2 282 participants, 3 essais ; données probantes de faible qualité). Les effets indésirables graves étaient probablement peu ou pas différents avec les schémas de quatre mois contenant de la moxifloxacine, qui ont remplacé l'éthambutol ou l'isoniazide, et avec les régimes de trois à quatre mois qui ont augmenté le TAT standard avec de la moxifloxacine, par rapport aux régimes TAT standard de six mois (3 548 participants, 4 essais ; données probantes de qualité moyenne).
Schémas TAT de quatre mois contenant de la gatifloxacine
Les schémas TAT de quatre mois contenant de la gatifloxacine, qui ont remplacé l'éthambutol, ont probablement augmenté les rechutes par rapport aux schémas TAT standard de six mois chez les adultes atteints de tuberculose pulmonaire pharmacosensible (RR 2,11, IC à 95 % 1,56 à 2,84 ; 1 633 participants, 2 essais ; données probantes de qualité moyenne). Le régime de quatre mois n'a probablement fait que peu ou pas de différence en termes de décès par rapport au régime de six mois (1 886 participants, 2 essais ; données probantes de qualité moyenne). L'échec du traitement était peu fréquent et différait probablement peu ou pas entre les régimes de quatre et six mois (1 657 participants, 2 essais ; données probantes de qualité moyenne). La résistance acquise à l'isoniazide ou à la rifampicine n'a pas été détectée chez les personnes ayant reçu le régime TAT raccourci contenant de la gatifloxacine, mais nous ne savons pas si la résistance acquise aux médicaments est différente entre les régimes de quatre et six mois (429 participants, 1 essai ; données probantes de très faible qualité). Les événements indésirables graves n'étaient probablement pas différents entre les deux schémas (1993 participants, 2 essais ; données probantes de qualité moyenne).
Conclusions des auteurs
Les données probantes recueillies jusqu'à présent n’appuient pas l'utilisation de schémas TAT raccourcis chez les adultes atteints de tuberculose pulmonaire pharmacosensible nouvellement diagnostiquée. Les schémas TAT de quatre mois, qui remplacent l'éthambutol par la moxifloxacine ou la gatifloxacine, ou l'isoniazide par la moxifloxacine, augmentent considérablement les rechutes par rapport aux schémas TAT standard de six mois, bien que le succès du traitement et les effets indésirables graves soient peu ou pas différents. Les résultats de six grands essais en cours aideront à décider si des schémas TAT raccourcis peuvent remplacer les schémas TAT standard de six mois.
PICOs
Résumé simplifié
Des schémas thérapeutiques plus courts pour les personnes atteintes de tuberculose pulmonaire
Objectif de la revue
Le but de cette revue Cochrane était de déterminer si la durée du traitement antituberculeux (TAT), chez les personnes nouvellement diagnostiquées pour tuberculose pulmonaire sensible aux médicaments, peut être réduite à moins de six mois. Les auteurs de la revue Cochrane ont recueilli et analysé toutes les études pertinentes pour répondre à cette question. Ils ont trouvé cinq études pertinentes.
Messages clés
Les schémas TAT raccourcis font probablement peu ou pas de différence en termes de décès, d'échec du traitement ou d'événements indésirables graves par rapport aux schémas TAT de six mois, mais ils augmentent probablement les rechutes de la tuberculose. Six grands essais en cours étudient cette question.
Quel était l’objet de la revue ?
La tuberculose est une maladie infectieuse, et la tuberculose affectant les poumons (tuberculose pulmonaire) est la présentation la plus courante de la tuberculose chez l'adulte. La tuberculose est un problème de santé publique majeur dans le monde entier, et parmi les maladies infectieuses, elle est la principale cause de décès.
Les personnes atteintes de tuberculose pulmonaire sont actuellement traitées par une combinaison de médicaments sur une durée de six mois qui comprend de l'isoniazide, de la rifampicine, de l'éthambutol et du pyrazinamide pendant deux mois, puis de l'isoniazide et de la rifampicine (avec ou sans éthambutol) pendant quatre mois. De nombreuses personnes ne terminent pas le traitement ou prennent les médicaments de façon irrégulière en raison de la longue durée du traitement ou des effets secondaires des médicaments. Un traitement incomplet ou irrégulier peut conduire à un échec thérapeutique et peut augmenter la rechute de la maladie. Un tel traitement peut également entraîner une résistance aux médicaments. Si de nouvelles combinaisons de médicaments administrées pendant moins de six mois s'avèrent aussi efficaces et sûres que les schémas TAT de six mois actuellement recommandés, un plus grand nombre de personnes pourraient adhérer au traitement et le terminer. Cela pourrait contribuer à réduire la résistance aux médicaments et à stopper l'infection par la tuberculose dans le monde entier.
Quels sont les principaux résultats de la revue ?
Les cinq essais inclus ont étudié 5 825 adultes nouvellement diagnostiqués pour la tuberculose pulmonaire sensible aux médicaments, dans 14 pays à forte transmission de tuberculose, en Asie, en Afrique et en Amérique latine. Trois essais ont inclus 572 personnes séropositives, mais tous ont exclu les personnes atteintes d'autres maladies graves et celles souffrant de diabète sucré. Cela a réduit l'applicabilité des résultats des études. Tous ont été financés par des organismes gouvernementaux ou internationaux.
Quatre études ont remplacé l'isoniazide ou l'éthambutol par la moxifloxacine ou la gatifloxacine dans les schémas TAT de quatre mois. Un suivi a été assuré pendant 12 à 24 mois après la fin du traitement. Dans une étude en cours, la moxifloxacine a été ajoutée au schéma TAT de quatre mois, mais les auteurs de l'étude ont fourni uniquement des résultats provisoires.
Cette revue montre les résultats suivants, lorsque des schémas TAT de quatre mois sont comparés à des schémas TAT standard de six mois.
‐ La rechute après un traitement réussi est probablement plus fréquente (données probantes de qualité moyenne).
‐ Le décès toutes causes confondues, l'échec du traitement et les événements indésirables graves sont probablement peu ou pas différents (données probantes de qualité moyenne).
‐ La résistance aux médicaments pourrait ne pas être augmentée avec les schémas de quatre mois contenant de la moxifloxacine (données probantes de faible qualité), mais nous ne savons pas si cela s'applique aux schémas contenant de la gatifloxacine (données probantes de très faible qualité).
Dans quelle mesure cette revue est‐elle à jour ?
Les auteurs de la revue ont cherché les études disponibles jusqu'au 10 juillet 2019.
Authors' conclusions
Summary of findings
| Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimen for drug‐sensitive pulmonary tuberculosis | ||||||
| Patient or population: adults with drug‐sensitive pulmonary tuberculosis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with 6‐month standard ATT | Risk with 4‐month moxifloxacin‐containing ATT | |||||
| Relapse | 32 per 1000 | 82 more relapses per 1000 | RR 3.56 | 2265 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably increases relapse compared to the 6‐month regimen |
| Death from any cause Follow‐up: range 18 to 24 months | 21 per 1000 | 2 more deaths per 1000 | RR 1.06 | 2760 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably makes little or no difference in death from any cause compared to the 6‐month regimen |
| Treatment failure | 16 per 1000 | 5 fewer treatment failures per 1000 | RR 0.71 | 2282 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably results in little or no difference in treatment failure compared to the 6‐month regimen |
| Acquired drug resistance | 7 per 1000 | 5 fewer with acquired drug resistance per 1000 (6 fewer to 2 more) | RR 0.33 | 2282 (3 RCTs)e | ⊕⊕⊝⊝ Due to indirectness and imprecision | The 4‐month regimen may be little or no different than the 6‐month regimen in the incidence of acquired drug resistance |
| Serious adverse events Follow‐up: range 18 to 24 months | 62 per 1000 | 2 fewer with serious adverse events per 1000 | RR 0.97 | 3548 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably results in little or no difference in serious adverse events compared to the 6‐month regimen |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aNo serious risk of bias: although Jawahar 2013 was at high risk of allocation bias, exclusion of this trial from the sensitivity analysis did not change the direction of effect. Not downgraded. | ||||||
| Gatifloxacin‐containing 4‐month ATT regimens compared to standard 6‐month ATT regimens for drug‐sensitive pulmonary tuberculosis | ||||||
| Patient or population: adults with drug‐sensitive pulmonary tuberculosis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with 6‐month standard ATT | Risk with gatifloxacin‐containing | |||||
| Relapse | 70 per 1000 | 77 more relapses per 1000 | RR 2.11 | 1633 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably increases relapse compared to the 6‐month regimen |
| Death from any cause | 29 per 1000 | 3 fewer deaths per 1000 | RR 0.90 | 1886 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably makes little or no difference in death compared to the 6‐month regimen |
| Treatment failure | 25 per 1000 | 1 less treatment failure per 1000 | RR 0.93 | 1657 | ⊕⊕⊝⊝ Due to indirectness | The 4‐month regimen probably makes little or no difference in treatment failure compared to the 6‐month regimen |
| Acquired drug resistance | 12 per 1000 | 9 fewer with acquired drug resistance per 1000 (12 fewer to 49 more) | RR 0.24 (0.01 to 5.01) | 301 (1 RCT)d | ⊕⊝⊝⊝ Due to indirectness, risk of bias, and imprecision | We do not know if acquired drug resistance is any different in the 4‐month and the 6‐month regimens |
| Serious adverse events | 24 per 1000 | 0 fewer serious adverse events per 1000 | RR 1.02 | 1993 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably results in little or no difference in serious adverse events compared to the 6‐month regimen |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. | ||||||
| aNo serious risk of bias: although Jawahar 2013 was assigned high risk of bias for allocation concealment, removal of this trial from the sensitivity analysis did not significantly alter the direction, magnitude, or precision of the effect estimate. Not downgraded. | ||||||
Background
Description of the condition
Tuberculosis (TB), a chronic infectious disease caused by air‐borne transmission of aerosolized droplets of Mycobacterium tuberculosis, is a major global public health problem (WHO 2018). An estimated 10 million new cases of tuberculosis and 1.6 million tuberculosis‐related deaths occurred in 2017, making tuberculosis one of the top 10 leading causes of death worldwide (WHO 2018). Among the new cases identified, 90% were adults, 58% were men, 10% were children, and 9% had HIV coinfection (WHO 2018). Among communicable diseases, tuberculosis is a major cause of mortality in the economically productive age group (15 to 49 years) (WHO 2017). The top eight countries in the world identified as having a high tuberculosis burden are India, China, Indonesia, the Philippines, Pakistan, Nigeria, Bangladesh, and South Africa (WHO 2018), and 87% of tuberculosis occurs in 30 high‐burden countries (WHO 2018). To add to the existing burden, 558,000 new cases of rifampicin‐resistant tuberculosis were diagnosed in 2017, and of these patients, 82% had multi‐drug resistant tuberculosis (MDR‐TB) (WHO 2018). Although tuberculosis‐related mortality fell by 23% between 2000 and 2017 worldwide, gaps in diagnosis and treatment persist (WHO 2018).
In May 2014, the World Health Assemby approved The 'End TB Strategy' of the World Health Organization (WHO), which aims to achieve a 95% reduction in mortality due to tuberculosis and a 90% reduction in the occurrence of new cases by the year 2035 compared with 2015 estimates (WHO 2015). This can result from a substantial decline in the numbers of tuberculosis cases and deaths in the years to come. However, the rate of decline in the incidence of tuberculosis was 1.9% from 2015 to 2016; to reach the 'End TB Strategy' targets, this rate of decline must increase to 4% to 5% yearly by 2020. Using the current standard WHO‐approved treatment regimen, the treatment success rate for individuals with new and relapsed cases of drug‐susceptible tuberculosis, as reported for the 2015 cohort, was 83% (WHO 2017a). Although this success rate is high when compared with that of individuals with MDR‐TB (success rate of 54%), poor outcomes such as failure to respond, death, and losses to follow‐up are of great concern, given that one of the targets of WHO's sustainable development goals for 2030 is to end the global tuberculosis epidemic (WHO 2015; WHO 2018).
The current standard WHO‐approved regimen consists of isoniazid, rifampicin, pyrazinamide, and ethambutol (HRZE) for two months (intensive phase), followed by isoniazid and rifampicin with ethambutol (HRE) in areas of high resistance, or without ethambutol (HR) for four months (continuation phase) (WHO 2010). This six‐month treatment duration can adversely impact patient adherence to therapy (Zumla 2014). Poor adherence leads to development of drug resistance and enhances the chance of relapse in such individuals (Ginsberg 2010; Ma 2010). Hence, new drug combinations are needed to shorten the course of treatment while maintaining high success rates and low relapse rates. Shortening the duration of treatment for individuals with drug‐sensitive or drug‐resistant tuberculosis is a global research priority and will certainly be highly beneficial for both patients and healthcare professionals. New tuberculosis drugs have begun to emerge from the clinical development pipeline, and shorter‐duration regimens containing new compounds could improve adherence to therapy while promoting infection control and leading to better disease management (Ma 2010).
Description of the intervention
The need for combination therapy for tuberculosis is a result of the distinctive cellular structure of M tuberculosis (a complex array of lipids, proteins, and glycolipids) and the tendency of the bacilli to develop resistance to monotherapy (Kerantzas 2017). Combinations of drugs are required to treat M tuberculosis: combining drugs with both bactericidal activity and sterilizing activity can help target the various bacterial subpopulations (actively dividing, slow growing, and dormant bacilli) present (Mitchison 1985). The bactericidal activity of a drug refers to its ability to kill metabolically active bacilli. An effective bactericidal drug prevents transmission of the bacilli and development of resistance to other drugs given as part of the regimen. The sterilizing activity of a drug refers to its ability to kill all viable bacilli, including the micro‐organisms tolerant to treatment with drugs. Drugs with good sterilizing capacity have the potential to shorten the duration of tuberculosis treatment (Ma 2010). In recent years, various drugs have been tried in differing combinations to shorten the standard six‐month treatment regimen, and these have shown promising preliminary results (Conde 2011).
Some of the desired characteristics of new anti‐tuberculosis drug compounds include the following (Ma 2010).
-
Effectiveness against both replicating and dormant tuberculosis bacilli.
-
Novel mechanism of action.
-
Improved safety profile (versus the standard treatment regimen).
-
Good oral bioavailability.
-
Low resistance development barrier.
-
Minimal interaction with cytochrome p450 enzymes.
-
Low cost.
Currently 10 compounds are in clinical development for the treatment of tuberculosis, six have been specifically developed, and four existing drugs have been re‐purposed. Drugs at the forefront of this quest include the fluoroquinolones (moxifloxacin, levofloxacin, and gatifloxacin), rifamycins (rifabutin and rifapentine), nitroimidazoles, diarylquinolines, oxazolidinediones, and ethylenediamines. These drugs have been investigated in clinical trials in combination with, or as substitutes for, one of the standard first‐line anti‐tuberculosis drugs, with the aim of shortening treatment duration (Lienhardt 2010). Second‐line anti‐tuberculosis drugs, including amoxicillin clavulanate, linezolid, carbapenems, and clofazimine, are also potential candidates for shorter‐duration anti‐tuberculosis regimens (D'Ambrosio 2015).
How the intervention might work
Fluoroquinolones
Fluoroquinolones possess good in vivo and in vitro bactericidal activity against M tuberculosis (Moadebi 2007). This class of drugs acts on the enzyme DNA gyrase, thereby preventing bacterial DNA synthesis (Lienhardt 2010). This mechanism of action is distinct from that of other anti‐tuberculosis drugs, raising the possibility of synergistic activity. Overall, the quinolones are well tolerated with minimal side effects on long‐term administration (Schluger 2013). Fluoroquinolones, when added to an anti‐tuberculosis treatment regimen, can enhance the sterilizing and bactericidal effects of combination therapy while increasing drug penetration into chronic tuberculosis lesions. Fluoroquinolones are better tolerated than standard first‐line drugs and can shorten treatment duration, hence improving patient adherence to treatment (Ginsburg 2003).
The main concern with quinolones is that they can prolong the QT interval, which may cause ventricular arrhythmias and sudden cardiac arrest (Schluger 2013). The frequency of torsades de pointes ‐ the type of arrhythmia induced by fluoroquinolones ‐ has been reported to be 1 per million with ciprofloxacin or levofloxacin, 3.8 per million with grepafloxacin, and 14.5 per million with sparfloxacin. The chance of arrhythmia is greater for individuals who have associated metabolic disorders such as hypokalaemia or cardiac disease, or who are taking other drugs that can prolong the QT interval (Rubinstein 2002). However, a pooled analysis of data from phase 2, 3, and 4 clinical trials comparing moxifloxacin with other antibiotics showed no clinically relevant differences in cardiac adverse effects between moxifloxacin and comparators (Haverkamp 2012).
Rifamycins
Rifapentine is a new‐generation rifamycin that acts by inhibiting the DNA‐dependent RNA polymerase of M tuberculosis. Like other rifamycins, rifapentine can (rarely) cause drug‐induced hepatitis and thrombocytopenia (Munsiff 2006). What makes rifapentine a good candidate for tuberculosis therapy shortening and dosage simplification is its long half‐life (10 to 15 hours for rifapentine versus two to three hours for rifampicin) and potency against M tuberculosis (Temple 1999). However, compared to rifampicin, rifapentine has poor penetrance into lung cavity lesions, particularly into liquefied caseous material that contains high concentrations of bacteria (Rifat 2018). Consequently, rifapentine requires considerably higher doses than those usually recommended to improve clinical outcomes in pulmonary tuberculosis; some patients with large lung cavitary lesions are less responsive to treatment even with high doses of rifapentine (Savic 2017). Given that currently recommended doses of rifampicin are less effective than higher rifampicin doses in achieving early culture conversion, if higher doses of rifampicin can be shown to reduce relapse rates, this could improve the efficacy of shortened ATT combinations (Boeree 2017).
Nitroimidazoles
Nitroimidazoles act against both multiplying and dormant bacilli, and thus may be suitable for potentially shortening the duration of tuberculosis therapy (Ma 2010). Two nitroimidazoles are currently being investigated in clinical trials for treatment of individuals with tuberculosis: pretomanid and delamanid. These agents are equally active against drug‐sensitive and drug‐resistant tuberculosis. They act on the bacilli through bioreduction of the nitroimidazole pharmacophore, generation of reactive oxygen species, and inhibition of mycolic acid synthesis (Matsumoto 2006). In phase 2 trials, QT prolongation was frequently seen in MDR‐TB patients who received delamanid (Gler 2012). The bactericidal activity of a novel drug combination of pyrazinamide, moxifloxacin, clofazimine, and pretomanid has been compared with that of the standard treatment regimen in individuals with drug‐sensitive and drug‐resistant tuberculosis. This new regimen was well tolerated and showed greater bactericidal activity than the standard regimen (Dawson 2015).
Diarylquinolines
One member of this class of drugs ‐ bedaquiline ‐ has been approved as an anti‐tuberculosis drug by the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) (Lessem 2015). Bedaquiline disrupts bacterial metabolism by affecting the synthesis of adenosine triphosphate (ATP) (Andries 2005). The drug is currently used for treatment of MDR‐TB, following the findings of a phase 2 trial that demonstrated rapid culture conversion of sputum and low rates of acquired resistance to coadministered drugs (Diacon 2014). Like the quinolones, bedaquiline can cause QT prolongation (Diacon 2012). Bedaqulline has potent late bactericidal properties that exceed those of rifampicin, especially during the second month of therapy, and may have superior sterilizing activity, particularly when combined with pyrazinamide, with the potential to shorten treatment duration for people with drug‐sensitive tuberculosis (Andries 2005).
Oxazolidinediones
Linezolid and sutezolid inhibit the initiation of bacterial protein synthesis by acting on the 50S ribosomal subunit. Linezolid, a re‐purposed drug, is effective in the management of drug‐resistant tuberculosis, but adverse effects such as myelosuppression and peripheral neuropathy restrict its long‐term use (Sotgiu 2012). A newer addition to this class ‐ sutezolid ‐ is gaining attention, as it has demonstrated greater potency as an anti‐tuberculosis drug than linezolid in murine models (Williams 2009). Phase 1 studies in humans have found sutezolid to be safe and well tolerated (Wallis 2010).
Ethylenediamines
The ethylenediamine, SQ109, inhibits protein synthesis by targeting the membrane transporter, MmpL3, in M tuberculosis, and is effective against drug‐susceptible and drug‐resistant tuberculosis. In vitro studies showed synergistic effects with bedaquiline and favourable interactions with sutezolid (D'Ambrosio 2015; Sacksteder 2012). However, SQ109 did not shorten time to culture conversion in clinical studies when used in place of ethambutol in anti‐tuberculosis regimens (Boeree 2017; Svensson 2018). Further research is required to determine the optimal dose and to identify drug combinations that could optimize the utility of SQ109, if considered for inclusion in treatment‐shortening regimens.
Why it is important to do this review
Novel drug regimens are needed to address the challenges associated with patient adherence to the current standard six‐month treatment regimen for tuberculosis (Ma 2010). Recent clinical trials have investigated the efficacy of newer regimens administered for less than six months for treatment of individuals with drug‐sensitive tuberculosis. A systematic review of these trials will help guide understanding of the efficacy and safety of these shorter regimens among individuals with drug‐sensitive pulmonary tuberculosis. A previous Cochrane Review ‐ Gelband 1999 ‐ concluded that longer periods of treatment (at least six months) resulted in higher success rates among individuals with active tuberculosis, but improvement was small when compared with regimens administered for less than six months. Another Cochrane Review on the use of fluoroquinolones for treatment of tuberculosis, published in 2013, concluded that evidence was insufficient to support conclusions, but noted that larger trials investigating short‐course fluoroquinolone‐based regimens were in progress (Ziganshina 2013). First‐line treatment with novel drug combinations administered for a shorter duration than the current standard six‐month treatment regimen could improve treatment outcomes, thereby reducing the chances of disease transmission and burden in this population.
Objectives
To evaluate the efficacy and safety of shortened treatment regimens versus the standard six‐month treatment regimen for individuals with drug‐sensitive pulmonary tuberculosis.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) and quasi‐RCTs.
Types of participants
Newly diagnosed individuals with pulmonary tuberculosis, as defined by positive sputum culture or a positive GeneXpert MTB/RIF, with presumed or proven drug‐sensitive tuberculosis, of all ages, irrespective of HIV status. Trials including people with extrapulmonary tuberculosis were eligible if such participants constituted less than 10% of participants, or if disaggregated data were available.
Types of interventions
Intervention
Treatment regimens of less than six months' duration including any anti‐tuberculosis drug(s) or combinations thereof (new drugs or standard anti‐tuberculosis drugs at higher than recommended doses).
Control
Standard first‐line therapy for pulmonary tuberculosis, defined as a regimen comprising two months of HRZE and four months of HR or HRE.
Types of outcome measures
Primary outcomes
-
Relapse of tuberculosis, defined as clinical or bacteriologic recurrence within two years of completion of anti‐tuberculosis therapy
Secondary outcomes
-
Death from any cause during anti‐tuberculosis therapy or within two years of end of treatment
-
Treatment discontinuation: rates of discontinuation of therapy at any time point during treatment
-
Positive sputum culture/smear at eight weeks: proportion of participants who remain smear or culture positive at the end of eight weeks of therapy
-
Treatment failure: persistent or recurrent positive sputum cultures at the time of treatment completion
-
Acquired drug resistance: development of secondary drug resistance to anti‐tuberculosis drugs, identified by drug susceptibility testing
Adverse events
-
Serious adverse events: adverse events that were fatal or life‐threatening, or that resulted in a change in treatment regimen
-
Other adverse events: other adverse events reported by trial authors, such as hepatitis, prolongation of the QT interval, hypersensitivity reactions, thrombocytopenia, peripheral neuropathy, ocular toxicity, and arthralgia
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
We searched the following databases up to 10 July 2019 using the search terms and strategy we have described in Appendix 1: the Cochrane Infectious Diseases Group Specialized Register; the Central Register of Controlled Trials (CENTRAL), in the Cochrane Library; MEDLINE (PubMed, from 1966); Embase (OVID, from 1947); the Latin American and Caribbean Health Science Information database (LILACS, from 1982); and Science Citation Index‐Expanded (Web of Science, from 1900). We also searched the website of the Indian Medlars Center (indmed.nic.in/, 10 July 2019) and the South Asian Database of Controlled Clinical Trials (cochrane‐sadcct.org/, 10 July 2019). We searched the WHO International Clinical Trials Registry Platform (who.int/ictrp/en/), ClinicalTrials.gov (clinicaltrials.gov/ct2/home), the Clinical Trials Unit of the International Union Against Tuberculosis and Lung Disease (theunion.org/what‐we‐do/research/clinical‐trials), the UK Medical Research Council Clinical Trials Unit (ctu.mrc.ac.uk/), and Clinical Trials Registry India (ctri.nic.in/) for trials in progress (all accessed on 10 July 2019).
Searching other resources
We searched the following conference proceedings for abstracts of relevant trials: World Congress on TB, World Lung Conferences of the International Union Against Tuberculosis Lung Disease (2004‐2018), American Thoracic Society Meeting Proceedings (2009 to 2019), and the British Society for Antimicrobial Therapy (2010‐2019). We contacted relevant organizations, including the Global Partnership to Stop TB and the WHO, for ongoing or completed but unpublished trials. We contacted researchers and experts in the field of clinical trials to identify any additional eligible studies. We checked the references of all included studies to identify additional relevant trials.
Data collection and analysis
Selection of studies
Two review authors (AG and AM) independently screened all citations and abstracts identified by the search strategy for inclusion. After eliminating duplicates, we scrutinized each report to ensure that multiple publications from the same trial were linked. If eligibility was not clear, or if we noted discrepancies, we resolved them through discussion or through consultation with another review author (SJ or JT). AG and AM obtained and scrutinized full texts of potentially eligible studies for inclusion and exclusion. Another review author (PT) independently screened the selected trials and the potentially eligible trials. We listed the excluded studies and tabulated reasons for their exclusion. We presented the study selection process in a PRISMA flow diagram.
Data extraction and management
Two review authors (AG and AM) independently extracted data using a pre‐tested data extraction form. We resolved discrepancies in the extracted data through discussion and by referring to the original articles.
We extracted the following data from the included studies.
-
Trial details: publication year, country where the trial was undertaken, study authors, year in which the study was done, study design, number of participants recruited, inclusion criteria, exclusion criteria, recruitment sites.
-
Baseline characteristics of participants: age, gender, nutritional status, comorbid illnesses including HIV, sputum smear grading, disease severity, chest X‐ray findings.
-
Intervention and control arms: numbers allocated to each arm, numbers completing the trial, description of the drugs used in the trial, drug dosage, route and frequency of administration, duration of treatment in the intensive and continuation phases.
-
Outcomes: we extracted data for the primary and secondary outcomes as defined above.
For each outcome, we extracted information on the number of participants randomized. For dichotomous outcomes, we extracted the number of participants who experienced the event and the number of people assessed for the event.
Two other review authors (PT and RK) independently verified all extracted data.
Assessment of risk of bias in included studies
Two review authors (AG and AM) independently assessed risk of bias in the trials included in this review using Cochrane’s ‘Risk of bias’ tool in Review Manager 5 (RevMan 5) (Review Manager 2014). We assessed each of the included trials for risk of bias in seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment (assessed at end of treatment and at end of follow‐up), incomplete outcome data, selective outcome reporting, and other potential biases. We resolved disagreements through discussion with a third review author (JT or SS). For each domain in the 'Risk of bias' assessment, we judged the risk of bias as low, high, or unclear. Another review author (PT) independently verified all assessments. We recorded our judgements and support for these judgements in 'Risk of bias' tables accompanying the characteristics of each included study, and we summarized our findings in a 'Risk of bias' summary and graph.
Measures of treatment effect
All outcomes were dichotomous, and we compared them using risk ratios and presented these with their 95% confidence intervals.
Unit of analysis issues
The included studies were parallel‐group RCTs. For trials with multiple intervention arms, we undertook pair‐wise comparisons of only relevant interventions and when possible combined the results of trial arms with similar ATT regimens. When adverse events were reported as the numbers of events (counts) as well as the numbers of participants experiencing adverse events (rates), we recorded both but used the latter for data synthesis.
Dealing with missing data
When data for outcomes were missing from the primary trial report, we sought these in supplementary data provided in appendices or related publications. When trials reported intention‐to‐treat (ITT) or modified intention‐to‐treat (m‐ITT) or per‐protocol analyses (available case analyses), we recorded the numbers excluded from analyses from among those randomized and allocated to each arm before and during treatment and during follow‐up. We also noted the reasons for their exclusion. Post‐randomization exclusions are not uncommon in trials comparing newer ATT regimens. One type occurs when sputum smear positive participants are randomized, but when sputum culture and drug susceptibility results become available, they may not confirm tuberculosis or may indicate infection with other mycobacteria, or the presence of drug resistance. These ineligible participants are excluded from the trials (late screening failures). Modified‐ITT analysis in such situations excludes late screening failures from ITT analyses, and all other participants are analysed in their allocated arms. In this deviation from the standard ITT analysis, post‐randomization exclusions are unrelated to compliance, withdrawals, or losses to follow‐up, or to the likelihood of getting the intervention; when ineligible participants do not represent populations to which trial results are likely to be applied, the risk of bias may not differ from traditional ITT analysis (Fergusson 2002). However, if m‐ITT analyses exclude participants post‐randomization for reasons other than late screen failure, this can lead to overestimation of treatment effects compared to standard ITT analyses (Abraha 2015). For this review, we used the data provided in ITT or m‐ITT analysis of the included trials for our main analysis, because this analysis included more eligible participants than were included in the reported per‐protocol analyses and it did not require us to make assumptions about missing data. When ITT or m‐ITT analyses reported in the trials differed from standard interpretations, we assessed the impact of missing data by performing sensitivity analysis for the review's primary outcome of relapse. In imputing missing data, we had intended to perform the commonly used 'best‐worst case' analysis, in which the ‘best‐case’ scenario is that all participants with missing outcomes in the experimental intervention group had good outcomes (no relapse), and all those with missing outcomes in the control intervention group had poor outcomes (relapse); the ‘worst‐case’ scenario is the converse. However, these are extreme assumptions, especially with rare outcomes such as relapse. Instead, we used relapse proportions in the treatment and control arms from per‐protocol analysis in these trials to impute relapse rates for the missing population.
Assessment of heterogeneity
We assessed clinical heterogeneity by looking at variability among trial participants, interventions, outcomes, and trial methods, including risk of bias. We assessed statistical heterogeneity by inspecting forest plots for non‐overlapping confidence intervals, and we used the Chi² test with a 10% level of statistical significance to denote that the inconsistency is not due to random error. We used the I² statistic, with a value of 50% or greater to generally denote moderate heterogeneity (the proportion of intertrial inconsistency that exceeds random error). However, we acknowledge that absolute thresholds for interpretation of I² can be misleading. Therefore we interpreted I² between 0% and 40% as possibly unimportant; from 30% to 60% as possibly representing moderate heterogeneity; from 50% to 90% as representing substantial heterogeneity; and from 75% to 100% as showing considerable heterogeneity, depending on the magnitude and direction of effects and the strength of evidence for heterogeneity (e.g. P value from the Chi² test) (Deeks 2011).
Assessment of reporting biases
We intended to evaluate the possibility of publication bias by evaluating funnel plots for asymmetry, but because we included fewer than 10 trials, this was not possible.
Data synthesis
We used risk ratios (RRs) with 95% confidence intervals (CIs) as summary effect estimates for dichotomous outcomes, and we synthesized data using RevMan 5 (Review Manager 2014). We conducted meta‐analyses using a fixed‐effect model when heterogeneity was low and a random‐effects model when heterogeneity was moderate (see Assessment of heterogeneity section). However, if heterogeneity was moderate and inconsistency was due to trials with large and small effects favouring an intervention, this need not necessarily denote imprecision of clinical importance (Guyatt 2011c). In such instances, if using a random‐effects model in sensitivity analyses also resulted in 95% CIs indicating appreciable effects of the intervention (see Sensitivity analysis), we used the fixed‐effect model in meta‐analysis but also reported random‐effects estimates in the results. If random‐effects meta‐analysis had resulted in imprecision (as indicated by the 95% CI including non‐appreciable benefits) or had changed the direction of effect, we would have retained the random‐effects model in meta‐analysis. If heterogeneity was substantial but could be explained in subgroup analyses (see below), we provided effect estimates for the subgroups without an overall pooled effect estimate.
Certainty of the evidence
We assessed the certainty of evidence by using the GRADE approach for the primary outcome of relapse and for the secondary outcomes important for clinical decision‐making, that is, death due to any cause, treatment failure, development of drug resistance, and serious adverse events (Guyatt 2011a). For each of these outcomes, we assessed how certain we were that pooled effect estimates were true (Balshem 2011), and that their 95% CIs represented the range of effects that were plausible and likely to be useful (Hultcrantz 2017). Certainty of evidence for each outcome is influenced by risk of bias in the studies contributing to pooled effect estimates for each outcome, as well as other factors such as unexplained inconsistency, indirectness, imprecision, and publication bias (Balshem 2011). Pooled effect estimates from RCTs are generally considered to provide high‐certainty evidence, but if there were serious or very serious concerns that any of the above‐mentioned factors may have compromised the certainty of effect estimates, we rated down the certainty for that outcome by one or two levels. In making these assessments, we used the overall guidance provided in Schünemann 2011 and Schünemann 2013. We also used guidance provided in Guyatt 2011b to assess the impact of imprecision on the certainty of evidence for each outcome. According to this guidance, precision is considered adequate if the 95% CI excludes an RR of 1.0, and the total number of events or patients in the total sample size is large enough to satisfy or exceed that required for an adequately powered individual trial (optimal information size, or OIS). However, when event rates are very low, as is likely with trials comparing shortened versus standard ATT regimens that were designed to assess equivalence or non‐inferiority within prespecified non‐inferiority margins, CIs around relative effects may be wide but CIs around absolute effects will be narrow. In such instances, rating down for imprecision may be inappropriate (Guyatt 2011b). For rating inconsistency, we used guidance provided in Guyatt 2011c, particularly when heterogeneity was moderate in fixed‐effect meta‐analysis but inconsistency in results was due to trials with large and small effects favouring an intervention. In such instances, if using a random‐effects model did not result in 95% CIs that now included non‐appreciable effects or no benefit associated with the intervention, we did not rate down for imprecision. We incorporated the ratings on certainty of evidence for effect estimates for each outcome along with relative and absolute measures of effect in 'Summary of findings' tables for each comparison in this review, using the GRADEpro Guideline Development Tool (GRADEpro GDT).
Subgroup analysis and investigation of heterogeneity
When we considered heterogeneity to be moderate or substantial, we explored potential causes in subgroup analyses based on categories of shortened treatment regimens. We subgrouped four‐month regimens according to whether they replaced components of standard ATT drugs or augmented them in comparison with standard six‐month ATT regimens.
Sensitivity analysis
We re‐analysed data using a random‐effects model in sensitivity analysis if fixed‐effect meta‐analysis revealed moderate heterogeneity but inconsistency in results of the trials was due to differences in the magnitude of effect favouring an intervention, rather than to differences in the direction of effects. Moderate inconsistency need not necessarily reduce our confidence in the pooled estimate if inconsistency is largely due to differences between large and small effects favouring an intervention (Guyatt 2011c). Thus, when we judged heterogeneity to be moderate but inconsistency in results was due to large and small effects favouring an intervention, we assessed the robustness of the results by changing from a fixed‐effect to a random‐effects meta‐analysis. If pooled effect estimates in random‐effects meta‐analysis continued to favour the intervention, and if both limits of the 95% CI continued to indicate appreciable benefit, we used the fixed‐effect model in the analysis but reported both fixed‐effect and random‐effects meta‐analysis in the results. We retained the fixed‐effect model in meta‐analysis in such instances to avoid compromising grading of imprecision in evaluating certainty of the evidence while summarizing the findings. Random‐effects meta‐analyses provide pooled estimates of the range of possible effects, with point estimates representing the mean of their distribution; this inherently denotes imprecision. Using the random‐effects model under such circumstances would warrant rating down for imprecision while assessing the certainty of evidence when this is not warranted.
We also assessed the impact of risk of bias on effect estimates of the primary outcome in sensitivity analysis by excluding studies judged to be at high risk of bias. We explored the effects of missing outcome data for the primary outcome of relapse in sensitivity analysis comparing results of the main analysis with results of the per‐protocol analysis, and including all randomized participants (excluding late screening failures, treatment failures, and deaths), and we imputed missing data using relapse rates from available data.
Results
Description of studies
Results of the search
We identified 1489 articles through database screening and two articles by searching other sources. After screening the 1491 titles and abstracts, we excluded 1457 records that were not relevant. We retrieved 34 full‐text records of potentially eligible studies (Figure 1). We excluded 11 records of RCTs that did not fulfil the inclusion criteria for the review (see Characteristics of excluded studies). We identified 23 relevant records for inclusion that reported on 11 RCTs. Eight of these records pertained to six ongoing studies that are detailed in Characteristics of ongoing studies. The remaining 15 records related to five RCTs that met criteria for selection to this review. No studies await assessment.

Study flow diagram.
Included studies
We included five RCTs that randomized a total of 5825 participants (Gillespie 2014; Jawahar 2013; Jindani 2014; Merle 2014; Velayutham 2014). Refer to Characteristics of included studies for a summary of included trial characteristics. Table 1 provides additional descriptive details.
| Study ID (Acronym) | (REMoxTB) | (RIFAQUIN) | (OFLOTUB) | |||||||
| Setting | Multiple sites in Africa (Kenya, South Africa, Tanzania, Zambia), Asia (China, India, Malaysia Thailand), Latin America (Mexico) | 6 sites in 2 cities in India | 6 cities in 4 countries in Africa (Botswana, South Africa, Zambia, Zimbabwe) | 5 countries in Africa (Benin, Guinea, Kenya, Senegal, South Africa) | 2 cities in India | |||||
| Participants | ||||||||||
| Number randomized | 1931 | 429 | 827 | 1836 | 801 | |||||
| Age | Adults (> 18 years) | Adults (> 18 years) | Adults (> 18 years) | Adults (18 to 65 years) | Adults (> 18 years) | |||||
| HIV infection | Included (if CD4 count > 250 cells/μL and not on ART); 110 (7%) | Excluded | Included (if CD4 count > 150/mm³ and not on ART; 158 (27%) | Included if not stage 3 or 4 disease and not on ART; 304 (17%) | Excluded | |||||
| Diagnosis of TB | Positive sputum smears on 2 occasions Culture‐confirmed susceptibility to rifampicin, isoniazid, pyrazinamide, and moxifloxacin | Newly diagnosed pulmonary TB with at least 2 positive sputum cultures. Confirmed by culture and MDR‐TB excluded, susceptibility to ofloxacin (as proxy for moxifloxacin) | 2 sputum samples that were positive for tubercle bacilli on direct smear microscopy No resistance to isoniazid, rifampicin, or moxifloxacin | Acid‐fast bacilli in 2 consecutive sputum smears; confirmed by culture (solid medium) and drug sensitivity tests to rifampicin, isoniazid, ethambutol, streptomycin, and gatifloxacin | 2 positive sputum smear smears for tuberculosis. Culture‐confirmed and MDR‐TB ruled out; susceptible to ofloxacin (as proxy for moxifloxacin) | |||||
| Intervention(s) and comparator | ||||||||||
| Duration of ATT | 4 monthsa | 6 months | 4 monthsb | 6 months | 4 months | 6 monthsc | 4 months | 6 months | 4 monthsa | 6 months |
| Regimens | 2HRZM/2HRM + 2MRZE/2MR | 2HRZE/4HR | 2(HRZG)₃/ 2 (HRG)₃ 2(HRZM)₃/2(HRM)₃ | 2(HRZE)₃ /4(HR)₃ | 2MRZE/ 2P₂M₂ | 2HRZE/ 4HR | 2HRZG/ 2HRG | 2HRZE/4HR | 3HRZM + 2HRZM/ 2RHM + 2HRZM/ 2(RHM)₃ + 2HRZM/ 2(RHEM)₃ | 2(HRZE)₃/ 4(HR)₃ |
| Number allocated | 655 + 636 = 1291 | 640 | 141 118 | 170 | 275 | 275 | 917 | 919 | 629 | 172 |
| Late screening failures excluded after allocation | 38 + 32 = 70 | 40 | 5 3 | 5 | 36 | 35 | 62 | 51 | 13 | 8 |
| Number eligible | 1231 | 600 | 136 115 | 165 | 239 | 240 | 852 | 868 | 616 | 164 |
| Number analysed in m‐ITT analysis (% of those allocated) | 568 + 551 = 1119 (87) | 555 (87) | 136 (97) 115 (98) | 165 (97) | 193 (70) | 188 (68) | 791 (86) | 794 (86) | 590 (94) | 151 (88) |
| Number analysed in per‐protocol analysis (% of those allocated) | 514 + 524 = 1038 (80) | 510 (80) | 131 (93) 113 (96) | 159 (94) | 165 (60) | 163 (59) | 651 (71) | 601 (65) | As above | |
| Number analysed in ancillary analysis (ITT) (% of those allocated) | 617 + 604 = 1221 (94) | 600 (94) | Not done | 239 (87) | 240 (87) | Not reported | Not reported | |||
| Outcomes reported | ||||||||||
| Relapse | Relapse within 18 months after randomization in those with negative culture with treatment. Relapse strains were those shown to be identical on 24‐locus MIRU analysis LJ solid media and MGIT liquid media used for culture | Recurrence of TB over 24 months after treatment in those with a favourable response with treatment: either bacteriologic recurrence (LJ solid media) or clinical/radiologic recurrence | Relapse within 12 to 18 months after treatment. Two positive cultures within a period of 4 months without an intervening negative culture). Re‐infections differentiated from relapse through genotyping (MIRU‐VNTRs) LJ solid media used for culture in some centres, MGIT liquid media in others, and both in some centres | Recurrence of TB over 24 months after treatment proven bacteriologically (2 consecutive positive sputum samples a day apart) or clinically Genotyping (MIRU‐VNTRs) results available for only 70/140 (55%) of those with culture confirmed recurrence. Most were relapses | Not reported | |||||
| Deaths | All deaths TB deaths | Reported (only non‐TB deaths occurred) | All deaths TB deaths | Death during treatment Death after treatment | Not reported | |||||
| Treatment discontinuation | Includes those who did not complete treatment, relocated, or withdrew consent | Includes those who did not complete treatment and those lost to follow‐up | Includes change in treatment due to adverse events, loss to follow‐up, and other treatment changes | Includes those who withdrew consent during treatment and dropouts | Reported but disaggregated data for each group not available | |||||
| Positive smear/ sputum culture at 2 months | Reported using LJ solid media (used in this review) and MGIT liquid media for all randomized participants excluding late screening failures | Reported using LJ solid media for all randomized participants excluding late screening failures | Reported but disaggregated data for moxifloxacin 4‐month and 6‐month treatment groups not available Data also not available for all participants from LJ media | Reported for 752 in the 4‐month and 759 in the 6‐month regimens (88% and 87% of those eligible, respectively) Culture using LJ solid media | Reported for 590 (94%) in the 4‐month and 151 (88%) in the 6‐month regimens | |||||
| Acquired drug resistance | Reported | Reported | Reported | Not reported | Not reported | |||||
| Treatment failure | Includes culture confirmed and not confirmed | Includes culture confirmed and unconfirmed | Culture confirmed | Includes culture confirmed failure | Not reported | |||||
| Serious adverse events | Reported for all randomized participants excluding late screening failures. Grade 3 and 4 severity (DAIDS 2009) | Deduced from adverse events reported for all randomized participants excluding late screening failures. Not graded | Reported for all participants randomized who took 1 dose and assessed as severe or life‐threatening during and 2 weeks after treatment. grade 3 and 4 severity (DAIDS 2009) | Reported for 1692 (92%) of all randomized participants. grade 3 and 4 severity (DAIDS 2009) | Deduced from adverse events reported. Not graded | |||||
| Other adverse events | Not reported | Reported | Not reported | QT prolongation Hyperglycaemic episodes | Reported | |||||
Abbreviations: ART: anti‐retroviral treatment; ATT: anti‐tuberculosis treatment; E: ethambutol; G: gatifloxacin; H: isoniazid; ITT: intention‐to‐treat; LJ: Löwenstein‐Jensen; M: moxifloxacin; MGIT: mycobacterial growth indicator tube; MIRU‐VNTRs: mycobacterial interspersed repetitive unit–variable number tandem repeats; m‐ITT: modified intention‐to‐treat; P: rifapentine; R: rifampicin; Z: pyrazinamide.
Leading numbers in regimens indicate duration in months. Drugs were administered daily, except when given thrice weekly as indicated by subscripts.
aData from moxifloxacin‐containing shortened regimens combined for data synthesis.
bData from the 2 shortened regimens compared separately with standard 6‐month regimens.
cData from an additional arm evaluating moxifloxacin‐containing 6‐month regimen not included.
Setting
Three of the included trials were multi‐country trials. Gillespie 2014 (REMoxTB study) included participants from multiple sites in nine countries: four in Africa (Kenya, South Africa, Tanzania, Zambia), four in Asia (China, India, Malaysia, Thailand), and one in Latin America (Mexico). Jindani 2014 (RIFAQUIN trial) recruited participants from six cities in four countries in Africa (Botswana, South Africa, Zambia, Zimbabwe). Merle 2014 (OFLOTUB/Gatifloxacin) included participants from five cities in five countries in Africa (Benin, Guinea, Kenya, Senegal, South Africa). The other two trials were conducted in two cities in south India (Jawahar 2013; Velayutham 2014).
Study participants
The five trials recruited only adults (> 18 years of age). Most participants were male, ranging from 64% to 74% across the five trials. Two trials excluded HIV‐positive participants (Jawahar 2013; Velayutham 2014). Gillespie 2014 included 110 HIV‐positive participants (7% in each arm) whose CD4 counts were > 250 cells/μL, and who were not receiving antiretroviral treatment (ART). Merle 2014 included 304 (18.1%) individuals with HIV who were not in stage 3 or 4 disease and were not receiving ART (17.4% in the shortened regimen, 18.7% in the standard regimen). Jindani 2014 included the largest proportion of HIV‐positive participants (158; 27%) after excluding those with CD4 count < 150/mm³ and those on ART; 28% were allocated to the shortened regimen and 29% to the six‐month regimen.
All five trials included patients with lung cavitation. In Gillespie 2014, this accounted for 71% overall (69% and 70% in the intervention groups, 72% in the control group). In Jindani 2014, 67% given the control regimen and 65% receiving the shortened regimen had cavitation. Merle 2014 included 50% in the control regimen and 52% in the shortened regimen with cavitation. Velayutham 2014 reported that cavitation was present in 36% of those allocated to the shortened regimen and in 41% of those given the control regimen. Jawahar 2013 did not provide numerical data about proportions with lung cavitation.
Gillespie 2014 and Jindani 2014 excluded those with body weight less than 35 kg; in Gillespie 2014 and in Jindani 2014, 9% to 11% and 4% to 5% of included participants, respectively, had body weight < 40 kg. Jawahar 2013 and Velayutham 2014 excluded participants who weighed < 30 kg. In Jawahar 2013, mean body weight ranged from 43.7 kg to 44.2 kg in the shortened treatment arms and was 43 kg in the control arm. In Velayutham 2014, 53% in the shortened‐treatment arms and 54% in the standard treatment arm weighed > 42 kg. Merle 2014 required participants to weigh between 38 kg and 80 kg; mean weight was 53.8 kg in the intervention arm and 54.2 kg in the control arm.
The diagnosis was made by using two positive sputum samples and was confirmed by culture in all trials. Gillespie 2014 required culture‐confirmed susceptibility to rifampicin, isoniazid, pyrazinamide, and moxifloxacin; Jindani 2014 additionally required susceptibility to isoniazid; and Merle 2014 required susceptibility to ethambutol and gatifloxacin. All trials excluded people with MDR‐TB (Table 1).
Shorter ATT regimens
The five included studies evaluated shorter regimens involving two fluoroquinolones (moxifloxacin and gatifloxacin) given to 3512 participants compared to 2176 participants given standard six‐month ATT regimens. We did not find trials evaluating other fluoroquinolones, nitroimidazoles, diarylquinolines, oxazolidinediones, or ethylenediamines in shortened ATT regimens compared to standard ATT regimens. We also did not find eligible trials that included other candidate drugs for shorter regimens, such as amoxicillin clavulanate, linezolid, carbapenems, or clofazimine.
Comparision 1. Moxifloxacin‐containing four‐month ATT regimens
Four trials compared moxifloxacin‐containing shortened ATT regimens (three to four months) versus standard six‐month ATT regimens.They differed in whether moxifloxacin was used to replace one of the standard ATT drugs in the four‐month ATT arm (Gillespie 2014; Jawahar 2013; Jindani 2014), or to augment them (Velayutham 2014). Treatments were supervised in all trials.
Moxifloxacin replacing standard ATT drugs
Gillespie 2014 (REMoxTB study) randomized 1931 participants to three arms. Two arms compared moxifloxacin‐containing daily regimens for four months (17 weeks) versus a control intervention for six months (26 weeks) of a daily ATT regimen. One arm (isoniazid group, where moxifloxacin (M) (400 mg) replaced ethambutol (E); N = 655) received eight weeks of M with isoniazid, rifampicin, and pyrazinamide (HRZ) plus E placebo administered daily, followed by nine weeks of MHR, followed by nine weeks of H and R placebo. The second intervention arm (ethambutol group, where moxifloxacin (400 mg) replaced isoniazid; N = 636) received eight weeks of MRZE plus H placebo administered daily, followed by nine weeks of MR plus H placebo daily, followed by nine weeks of H and R placebo. The control arm (N = 640) received eight weeks of HRZE and M placebo given daily, followed by nine weeks of HR and M placebo given daily, followed by nine weeks of HR. Results of the two moxifloxacin arms did not differ significantly. We combined the data for these two intervention arms compared to the six‐month regimen in data synthesis for our primary analysis.
Jawahar 2013 randomized 429 participants to three arms. In the two intervention arms, gatifloxacin (G) or moxifloxacin (M) replaced ethambutol in the shortened regimen. The moxifloxacin arm (N = 118) received two months of moxifloxacin (400 mg) and HRZ thrice weekly, followed by two months of MHR thrice weekly. The control arm (N = 170) received two months of HRZE thrice weekly, followed by four months of HR thrice weekly. This trial was stopped early by the data safety monitoring board at a planned interim analysis, after it had recruited only a third of the 1200 estimated sample, due to higher relapse rates in the intervention arms.
Jindani 2014 (RIFAQUIN trial) also had three arms randomizing 827 participants (of the estimated sample size of 1095). In two intervention arms, moxifloxacin (400 mg) replaced isoniazid throughout, and high‐dose (900 mg) rifapentine (P) replaced rifampicin in the continuation phase. We did not include one of these arms in data synthesis because the four‐month continuation phase resulted in a six‐month ATT regimen. In the other arm, 275 participants were given eight weeks of MRZE administered daily, followed by nine weeks of MP administered twice weekly. In the control arm, 275 participants were given eight weeks of HRZE administered daily, followed by 18 weeks of HR daily.
Moxifloxacin augmenting standard ATT drugs
Velayutham 2014 is the interim report of an ongoing trial ‐ CTRI/2008/091/000024 ‐ that compared four different regimens in which moxifloxacin (400 mg) was added to HRZE in shortened courses. The four arms randomized 629 participants to receive HRZEM daily for three months, or daily for two months followed by RHM daily for two months, or daily for two months followed by RHM thrice weekly for two months, or daily for two months followed by RHEM thrice weekly for two months. The standard six‐month (2HRZE/4HR) regimen was given thrice weekly to 172 participants. The report presented planned interim outcomes and final results are awaited.
Comparison 2. Gatifloxacin‐based four‐month ATT regimens
Gatifloxacin replacing standard ATT drugs
Merle 2014 (OFLOTUB/gatifloxacin) randomized 1836 participants, of whom 917 were given two months of gatifloxacin (400 mg; replacing ethambutol) and HRZ daily, followed by two months of daily HRG. In the control arm, 919 participants were given the standard daily six‐month (2HRZE/4HR) regimen.
In Jawahar 2013, the gatifloxacin arm replaced ethambutol in 141 participants who received two months of HRZG thrice weekly, followed by two months of HRG thrice weekly. The 170 participants in the control arm received 2HRZE/2HR given thrice weekly.
Follow‐up
Participants in three of the included trials were followed for a period of 24 months after end of treatment (Jawahar 2013; Merle 2014; Velayutham 2014). Gillespie 2014 and Jindani 2014 followed‐up participants for a period of 18 months after randomization (12 months after treatment). However, 14% of participants in Jindani 2014 who were randomized in the last six months of enrolment received follow‐up for 12 or 15 months after randomization. All trials reported regular scheduled assessments for efficacy and safety outcomes for participants in the intervention and control arms (see Characteristics of included studies).
Outcomes
Four trials provided data on relapse ‐ the primary outcome of this review (Gillespie 2014; Jawahar 2013; Jindani 2014; Merle 2014). In Gillespie 2014 and Jindani 2014, relapse was differentiated from re‐infection through genotyping of patients with culture‐confirmed recurrence. In Merle 2014, genotyping results were available for only 77 of 140 (55%) of those with culture‐confirmed recurrence. However, 79% of the 77 with genotyping results were confirmed as relapses. In Jawahar 2013, relapse was not differentiated from re‐infection but most recurrences occurred within six months after treatment, suggesting that these were instances of relapse.
Again, four trials provided data on death from any cause, including tuberculosis, that occurred on treatment and during follow‐up (Gillespie 2014; Jawahar 2013; Jindani 2014; Merle 2014). No deaths were reported in the interim analysis provided in Velayutham 2014. Rates of treatment discontinuation and treatment failure were reported in four trials (Gillespie 2014; Jawahar 2013; Jindani 2014; Merle 2014), with different definitions used to compute these outcomes (Table 1).
Four trials reported the outcome of sputum culture positivity at eight weeks (Gillespie 2014; Jawahar 2013; Merle 2014; Velayutham 2014). In Velayutham 2014, data for this outcome were presented for all participants allocated to four groups combined, but because participants in the four groups had received identical regimens for the first two months, we used these data in the meta‐analysis. In the fifth trial (Jindani 2014), these results were presented as combined data for the four‐month and six‐month moxifloxacin arms, and disaggregated data for sputum positivity at two months were not available. Gillespie 2014, Jawahar 2013, and Jindani 2014 provided data on acquired drug resistance. Merle 2014 and Velayutham 2014 did not report on this.
Acquired drug resistance was assessed and reported in three trials (Gillespie 2014; Jawahar 2013; Jindani 2014), which assessed drug susceptibility at baseline as well as in those who were culture positive at end of treatment, or who experienced relapse/recurrence. Resistance results were missing for isoniazid in 24 patients and for pyrazinamide in 27 patients at baseline in Gillespie 2014, and the cases of acquired drug resistance reported were only probable and were not unequivocal in the absence of whole genome sequencing. Jawahar 2013 did not directly assess susceptibility to moxifloxacin and gatifloxacin but used susceptibility to ofloxacin as a proxy indicator. Merle 2014 assessed drug susceptibility at baseline and performed indirect drug susceptibility tests during follow‐up but did not report acquired drug resistance.
Serious adverse events experienced by trial participants were reported in all trials or could be deduced from the adverse events reported. Gillespie 2014 and Jindani 2014 did not report adverse events other than serious adverse events. Merle 2014 also reported the proportions of participants with QT prolongation and with hyperglycaemic episodes.
Excluded studies
We excluded 11 studies for reasons detailed under Characteristics of excluded studies. One trial, Alavi 2009, studied the effects of rifampicin, isoniazid, and ofloxacin in people with smear negative pulmonary tuberculosis, diagnosed solely on the basis of clinical criteria. Five were phase 2b trials with no six‐month standard ATT comparator arm (Burman 2006; Conde 2009; Conde 2016; Dorman 2009; Rustomjee 2008). These trials, along with El‐Sadr 1998 which we excluded because it compared levofloxacin added for the first two months of the standard six‐month ATT regimen versus six to nine months of standard ATT regimens, are included in an earlier Cochrane Review (Ziganshina 2013). We excluded three other trials because they lacked comparisons with a standard six‐month ATT arm (Kohno 1992; Tuberculosis Research Centre 1986; Tuberculosis Research Centre 2002). Johnson 2009 evaluated the effects of four months of standard ATT drugs versus six months of standard ATT but randomized only those who were sputum negative after four months of treatment to receive no further treatment or two more months of ATT.
Risk of bias in included studies
Please refer to Figure 2 for the summary of 'Risk of bias' assessments for each included study, and to Figure 3 for a risk of bias graph regarding each item presented as percentages across all included trials. Please also see 'Risk of bias' tables for individual trials under Characteristics of included studies for supporting evidence on the judgement of risk of bias for the included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
All included studies were judged to be at low risk of bias for generating the random sequence. All but one ‐ Jawahar 2013 ‐ were judged as having low risk of bias for allocation concealment. Jawahar 2013 was judged to be at high risk of bias because recruitment ratios were altered during the course of the trial, thus likely compromising concealment of allocation. In conjunction with premature termination of the trial following a planned interim analysis, this led to baseline imbalance in some prognostic indicators.
Blinding
In Jawahar 2013, participants and care providers were not blinded to the interventions, and allocation concealment was likely to have been compromised. Jindani 2014 was an open‐label trial and treating physicians were aware of the treatment allocated. However, we believe this did not increase the risk of performance bias in these trials because we found no evidence that this influenced the administration of interventions or co‐interventions differentially between four‐month and six‐month regimens. We judged the other three trials to have low risk of performance bias, and we judged the five included trials as having low risk for detection bias.
Incomplete outcome data
We judged the five trials to be at low risk of attrition bias for outcomes assessed at the end of ATT and at the end of follow‐up. These trials had low attrition (Jawahar 2013; Velayutham 2014), or, if attrition exceeded 10% (Gillespie 2014; Jindani 2014; Merle 2014), differential attrition was not substantial and the results of per‐protocol analysis, modified intention‐to‐treat analyses, and other sensitivity analyses reported in the trials were consistent. In Jawahar 2013, although the power of the trial to prove equivalence was reduced due to early termination, we judged this study as having low risk of attrition bias, as attrition was low with similar reasons for exclusion, and this was unlikely to have altered the relative estimates of effects.
Selective reporting
The five studies reported all outcomes stated in the methods sections of their trial publications, or their protocols, or their clinical trial registry documents, and we judged them to be at low risk of reporting bias.
Other potential sources of bias
In three trials (Gillespie 2014; Jindani 2014; Merle 2014), study drugs were provided by their manufacturers, but we judged these studies to be at low risk of bias because the trial publications provided explicit statements that the manufacturers had no role in the study nor in the publication of results. We did not detect any other sources of bias.
Effects of interventions
See: Summary of findings for the main comparison Moxifloxacin‐containing 4‐month ATT regimens versus standard 6‐month ATT regimen for drug‐sensitive pulmonary tuberculosis; Summary of findings 2 Gatifloxacin‐containing 4‐month ATT regimens compared to standard 6‐month ATT regimens for drug‐sensitive pulmonary tuberculosis
Comparison 1. Moxifloxacin‐containing four‐month ATT regimens versus standard six‐month ATT regimens
Primary outcome
Relapse
Three trials provided data on relapse over 12 to 24 months following treatment in people with drug‐sensitive pulmonary tuberculosis (Gillespie 2014; Jawahar 2013; Jindani 2014). Two trials differentiated relapse from re‐infection using molecular methods (Gillespie 2014; Jindani 2014). Of 2769 participants randomized to the three regimens, 2265 participants (82%) were culture negative at the end of treatment and were evaluated for relapse or recurrence. Relapse proportions for the two regimens compared in the three trials are shown in Table 2.
| Primary outcome: relapse | ||||||
| Trial ID | ||||||
| Regimens | 4 months | 6 months | 4 months | 6 months | 4 months | 6 months |
| aModified‐ITT analysis (primary analysis) | 110/1119 (9.8%) | 13/555 (2.3%) | 11/108 (10.1%) | 10/155 (6.5%) | 27/165 (16.4%) | 6/163 (3.7%) |
| aPer‐protocol analysis | 110/1038 (10.6%) | 12/510 (2.4%) | 11/107 (10.1%) | 10/152 (6.6%) | 26/165 (15.8%) | 5/163 (3.1%) |
| bSensitivity analysis imputing missing data | 126/1184 (10.7%) | 14/577 (2.4%) | 11/114 (9.7%) | 10/159 (6.3%) | 36/225 (16.0%) | 71/232 (2.6%%) |
Abbreviations: ATT: anti‐tuberculosis treatment; ITT: intention‐to‐treat.
aAs reported in trial reports.
bIncludes in the denominators for each trial arm all randomized participants minus those excluded post randomization due to ineligibility (not confirmed TB, or drug resistant), those who died, and those who experienced treatment failure. The difference in this denominator and the denominator in per‐protocol analyses are missing data. Relapse rates for missing people were imputed from rates in the per‐protocol analysis for each trial arm.
Overall, 177 (5.2%) in the two groups included in the primary modified ITT analysis experienced a recurrence; most cases (156/178; 88%) were confirmed as relapse through genotyping; and 17 of 21 (81%) tuberculosis recurrences in Jawahar 2013 occurred in the first six months after treatment, suggesting that they were relapses rather than re‐infections. Relapse in the six‐month ATT arm varied from 2.3% of 555 participants in Gillespie 2014, to 6.5% of 155 participants in Jawahar 2013 and 3.7% of 163 participants in Jindani 2014. The corresponding incidence of relapse in the moxifloxacin‐containing shorter ATT regimens was 9.8% of 1119 in Gillespie 2014, 10.1% of 108 in Jawahar 2013, and 16.4% of 165 in Jindani 2014. Meta‐analysis showed that risk of relapse was thrice more common with moxifloxacin‐containing four‐month ATT regimens than with the standard six‐month regimen (RR 3.56, 95% CI 2.37 to 5.37; 2265 participants, 3 trials; Analysis 1.1). Results showed some heterogeneity (I² = 54%), but inconsistency between large and small effects favoured the six‐month ATT regimen, with considerable overlap in the 95% CI of the effect estimates. Re‐analysing data in sensitivity analysis using a random‐effects model did not introduce imprecision into estimates of appreciable benefit with the six‐month regimen (RR 3.18, 95% CI 1.69 to 5.97).
In the main analysis, we combined modified‐ITT data from the two moxifloxacin‐containing intervention arms in Gillespie 2014. We subgrouped data according to whether moxifloxacin was used in the four‐month regimen to replace ethambutol in the intensive phase (one of the intervention arms in Gillespie 2014 and the moxifloxacin arm in Jawahar 2013), or to replace isoniazid in the four‐month regimen (the other intervention arm in Gillespie 2014, and the moxifloxacin with high‐dose rifapentine arm in Jindani 2014). Results again favoured the six‐month ATT regimen (Analysis 1.2), irrespective of whether moxifloxacin replaced isoniazid in four‐month ATT regimens (RR 2.74, 95% CI 1.69 to 4.43; 747 participants, 3 trials; Analysis 1.2: subgroup 1), or whether moxifloxacin replaced ethambutol (RR 4.89, 95% CI 3.02 to 7.92; 1424 participants, 2 trials; Analysis 1.2: subgroup 2). We did not undertake subgroup analysis based on HIV status as there were only three trials, and one excluded HIV‐positive people (Jawahar 2013). However, Gillespie 2014 and Jindani 2014 reported no significant interaction effects between HIV status and unfavourable outcomes in subgroup analyses.
Jawahar 2013 was at high risk of bias for allocation concealment, and we explored the impact of this in sensitivity analysis by removing this study's data. Pooled estimates from the two studies without high risk of bias also show that the four‐month regimen increases relapse compared to the standard six‐month regimen (RR 4.26, 95% CI 2.65 to 6.84; 2002 participants, 2 trials; I² = 0%).
We used data for relapse from m‐ITT analyses reported in the three trials for the main meta‐analysis in this review. However results did not differ substantially when we performed sensitivity analyses using data from the per‐protocol analyses in the three trials in meta‐analysis (Table 2; Analysis 1.3: subgroup 2). When we explored the impact of missing data for all randomized participants (excluding late screening failures, treatment failures, and deaths) and imputed relapse rates for missing participants using relapse proportions reported in per‐protocol analyses of individual trials, results were consistent with the main meta‐analysis (Table 2; Analysis 1.3: subgroup 3; Figure 4).
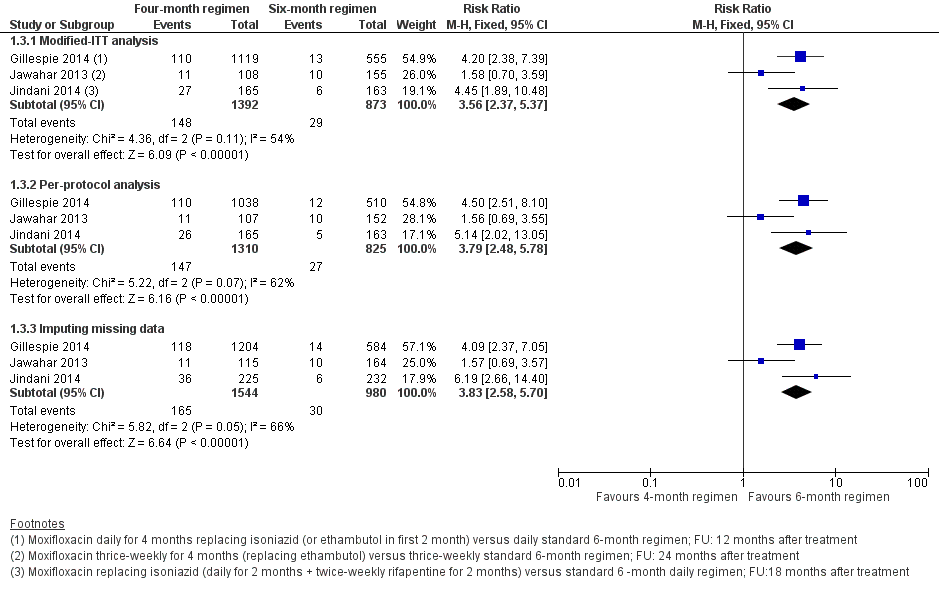
Forest plot of comparison: 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, outcome: 1.3 Relapse: sensitivity analysis accounting for missing data.
Secondary outcomes
Death from any cause
Three trials reported 62 deaths (Gillespie 2014; Jawahar 2013; Jindani 2014). Gillespie 2014 reported 27 deaths with four‐month ATT; 19 (70%) were tuberculosis‐related deaths, and 11 of 16 (69%) deaths with six‐month ATT were tuberculosis‐related. The one death (non‐tuberculosis) in Jawahar 2013 occurred with six‐month ATT. Jindani 2014 reported 2 of 12 (16%) deaths as tuberculosis‐related with the four‐month regimen, and 1 of 6 (16%) as tuberculosis‐related deaths with six‐month ATT. Pooled estimates of the risk of death due to any cause did not significantly differ between four‐month and six‐month ATT regimens (2760 participants, 3 trials; Analysis 1.4).
Treatment discontinuation
Of 2335 evaluable participants in three trials (Gillespie 2014; Jawahar 2013; Jindani 2014), 121 (5.2%) discontinued treatment for different reasons (Table 1). In meta‐analysis, treatment discontinuation showed little or no difference between the two groups (2335 participants, 3 studies; Analysis 1.5).
Sputum culture/smear positivity at eight weeks
Data for sputum culture conversion at the end of the intensive phase of ATT treatment were reported by all trials in the review; however, Jindani 2014 reported only combined sputum conversion data for the four‐month and six‐month moxifloxacin‐containing ATT arms of the trial. Disaggregated data for the four‐month moxifloxacin arm were not available for inclusion in meta‐analysis.
The pooled point estimate from the three trials with usable data for sputum culture/smear positivity at eight weeks favoured the four‐month moxifloxacin‐containing ATT regimen (Gillespie 2014; Jawahar 2013; Velayutham 2014), but the 95% CI did not rule out a small benefit for the standard six‐month ATT regimen, and heterogeneity was substantial (I² = 91%; 2828 participants, 3 trials; Analysis 1.6). We explored heterogeneity by subgrouping the data according to whether moxifloxacin replaced isoniazid or ethambutol in four‐month ATT regimens (Gillespie 2014; Jawahar 2013), or augmented standard ATT drugs in four‐month ATT regimens in random‐effects meta‐analysis(Velayutham 2014). Four‐month moxifloxacin‐containing regimens that replaced isoniazid or ethambutol were not unequivocally better than standard six‐month ATT regimens in achieving sputum culture conversion at eight weeks (2087 participants, 2 trials; Analysis 1.6: subgroup 1). However, moxifloxacin augmentation of standard ATT drugs in four‐month regimens was more effective than standard six‐month ATT in sterilizing sputum (sputum positivity at eight weeks 4.6% versus 19.2%; RR 0.24, 95% CI 0.15 to 0.39, 741 participants; Analysis 1.6: subgroup 2) (Velayutham 2014). The test for subgroup differences confirmed that moxifloxacin augmentation rather than substitution of standard ATT drugs achieves better sputum conversion at eight weeks compared to standard six‐month ATT regimens (P = 0.001; I² = 90.2%; Analysis 1.6).
Treatment failure
In the three trials that reported this outcome (Gillespie 2014; Jawahar 2013; Jindani 2014), treatment failures were equally rare, with only 14 failures reported among 1399 participants evaluated in the four‐month arm and 14 among 883 participants evaluated in the six‐month arm (2282 participants, 3 trials; Analysis 1.7). Most of these were culture confirmed treatment failures.
Acquired drug resistance
Acquired drug resistance was evaluated in three of the four included trials among those who had treatment failure, or who suffered a relapse with the four‐month regimen and the six‐month regimen (Gillespie 2014; Jawahar 2013; Jindani 2014). Due to the greater proportion of relapses in the four‐month ATT arm, proportions assessed for acquired drug resistance differed between the four‐month regimen (162/1392; 11.7%) and the six‐month regimen (43/873; 4.9%). Overall, eight people were judged to have developed acquired drug resistance. Two persons in the four‐month moxifloxacin‐containing ATT regimens in the three trials were detected with acquired drug resistance ‐ one to moxifloxacin and one to isoniazid. The incidence of acquired drug resistance ranged from 0.83% (1/120) in Gillespie 2014 to 7.7% (1/13) in Jawahar 2013 to 0% (of 29 assessed) in Jindani 2014. Six people developed acquired drug resistance in the six‐month standard ATT arms ‐ three to isoniazid and three to rifampicin. The incidence ranged from 15% (3/20) in Gillespie 2014 to 13% (2/15) in Jawahar 2013 to 12.5% (1/8) in Jindani 2014. Results for the four people with acquired drug resistance in Gillespie 2014 were not unequivocal but were judged probable. We pooled the data for acquired drug resistance from these trials using numbers evaluated for treatment failure in each trial as a more appropriate denominator for assessing acquired drug resistance than only those who experienced treatment failure or relapse. The pooled effect estimate suggests that acquired drug resistance was less frequent with the four‐month moxifloxacin‐containing ATT regimen than with the standard six‐month ATT regimen, but events were rare and 95% CIs were imprecise (2282 participants, 3 trials; Analysis 1.8).
Adverse events
Serious adverse events
All five included studies reported serious adverse events (SAEs) that were fatal or life‐threatening, or required hospitalization or a change in treatment regimen. Gillespie 2014 reported that a total of 349 SAEs occurred in 173 participants, with 246 events occurring during the treatment period and 103 during follow‐up. Serious adverse events occurred in 62 of 655 (9%) in the isoniazid group and in 52 of 636 (8%) in the ethambutol group, compared with 59 of 639 (9%) in the control group. The incidence of adverse events, including seizures, clinically significant cardiac toxicity, hypoglycaemia or hyperglycaemia, and peripheral neuropathy, did not significantly differ. Jawahar 2013 noted only two SAEs ‐ a case of jaundice in a person on the six‐month regimen and QTc prolongation in a person on the moxifloxacin‐ATT regimen. Jindani 2014 reported 12 SAEs among 11 participants on the four‐month ATT regimen, four of which were considered possibly or probably related to study medicines. In the control arm, 16 events were reported among 12 participants, with six possibly or probably related to treatment. Velayutham 2014 reported QTc prolongation in five participants in the moxifloxacin group and in one on standard ATT, but all cases were reversible. Other SAEs included hepatitis (12 in the moxifloxacin arm and 2 in the control arm) and seizures (four in the moxifloxacin arm and two in the control arm).
The meta‐analysis did not show significant differences between treatment regimens in the incidence of SAEs among 3548 participants in the four trials (Analysis 1.9).
Other adverse events
In Jawahar 2013, the most common adverse events were gastrointestinal symptoms (nausea, vomiting, abdominal discomfort), which occurred in 25 of 115 (22%) in the moxifloxacin group and in 15 of 165 (9%) in the control group. Giddiness or dizziness was also more frequent with moxifloxacin‐containing regimens (17/115; 15%) than with standard ATT (5/165; 3%). Arthralgia attributable to pyrazinamide was seen in 3 of 115 (3%) in the four‐month regimen and in 4 of 165 (2%) in the six‐month regimen.
Velayutham 2014 also reported that arthralgia was significantly greater in the moxifloxacin group (25% of 616 participants) than in the control group (4% of 164 participants). Skin rash with or without pruritis occurred in 5% of 616 participants in the moxifloxacin arms and in 4% of 164 participants in the six‐month ATT arm. The other three trials did not report adverse events other than SAEs.
Comparison 2. Gatifloxacin‐based four‐month ATT regimens versus standard six‐month ATT regimens
Two trials provided data for this intervention. Jawahar 2013 was a three‐armed, open‐label, equivalence trial, one arm of which randomized 141 adults with drug‐sensitive pulmonary tuberculosis to two months of supervised gatifloxacin 400 mg (replacing ethambutol), isoniazid, rifampicin, and pyrazinamide thrice weekly, followed by two months of gatifloxacin, isoniazid, and rifampicin thrice weekly. The 170 participants in the control arm were administered thrice‐weekly supervised standard six‐month ATT. Merle 2014 was an open‐label, two‐arm, non‐inferiority trial that randomized 917 participants to a similar gatifloxacin‐containing regimen (also replacing ethambutol) but given daily and compared the effects with 919 participants given a daily, supervised, standard six‐month ATT regimen.
We did not find trials that used gatifloxacin to replace isoniazid or to augment standard ATT regimens.
Primary outcome
Relapse
Both trials reported on relapse after confirming culture conversion by Löwenstein–Jensen (LJ) solid media to confirm tuberculosis recurrence over 24 months after treatment in people who had become culture negative with treatment. Jawahar 2013 did not differentiate relapse from recurrence but reported that all 19 recurrences in the gatifloxacin‐containing ATT arm and 8 of 10 recurrences in the six‐month ATT arm occurred within six months after treatment (suggestive of relapse rather than re‐infection). In Merle 2014, of 140 participants with culture‐positive recurrence, 77 (55%) had strains genotyped by means of a 15‐locus mycobacterial interspersed repetitive unit–variable‐number tandem‐repeat analysis. Of these 77 patients, 15 of 20 (75%) in the gatifloxacin arm and 46 of 57 (81%) in the standard ATT arm had a verified relapse. Relapse was diagnosed in 6.5% of 155 participants given six months of ATT in Jawahar 2013 and in 7.1% of 662 people given six months of ATT in Merle 2014. Relapse was more common with the gatifloxacin‐containing regimens: 15.6% of 122 in Jawahar 2013, and 14.6% of 694 in Merle 2014. Meta‐analysis of the two trials showed that relapse was twice as common with the gatifloxacin‐containing four‐month regimen than with the six‐month ATT regimen (RR 2.11, 95% CI 1.56 to 2.84; 1633 participants, 2 trials; Analysis 2.1).
Jawahar 2013 was at high risk of bias for allocation concealment and excluded HIV‐positive individuals. However, meta‐analysis results did not reveal any inconsistency in the results. Merle 2014 included HIV‐positive participants and undertook subgroup analysis based on HIV status. No significant interaction effects were detected between HIV status and unfavourable outcomes.
As in the previous comparison, we used m‐ITT analysis data from both trials for meta‐analysis in this review. Sensitivity analyses comparing m‐ITT data and per‐protocol data showed similar results, as did meta‐analysis using all randomized participants (minus late screening failures, treatment failures, and deaths) with imputed relapse rates for missing participants from relapse proportions in the per‐protocol analyses reported in the two trials (Table 3; Analysis 2.2; Figure 5).
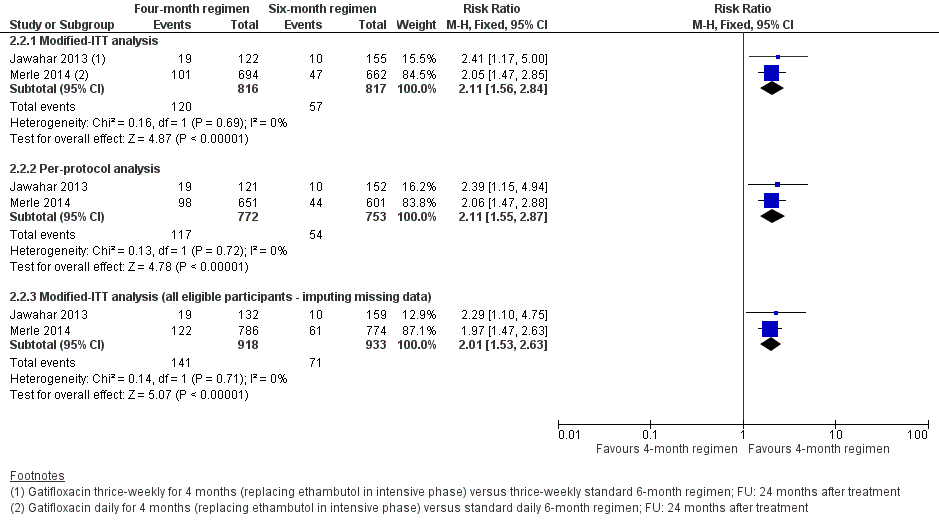
Forest plot of comparison: 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, outcome: 2.2 Relapse: sensitivity analysis accounting for missing data.
| Primary outcome: relapse | ||||
| Trial ID | ||||
| Regimen | 4 months | 6 months | 4 months | 6 months |
| aModified‐ITT analysis (primary analysis) | 19/122 (15.6%) | 10/155 (6.5%) | 101/694 (14.6%) | 47/662 (7.1%) |
| aPer‐protocol analysis | 19/121 (15.7%) | 10/152 (6.6%) | 98/651 (15.1%) | 44/601 (7,3%) |
| bSensitivity analysis imputing missing data | 19/132 (14.4%) | 10/159 (6.3%) | 122/786 (15.5%) | 61/774 (7,9%) |
Abbreviations: ATT: anti‐tuberculosis treatment; ITT: intention‐to‐treat.
aAs reported in trial reports.
bIncludes in the denominators for each trial arm all randomized participants minus those excluded post randomization due to ineligibility (not confirmed TB, or drug resistant), those who died, and those who experienced treatment failure. The difference in this denominator and the denominator in per‐protocol analyses are missing data. Relapse rates for missing people were imputed from rates in the per‐protocol analysis for each trial arm.
Secondary outcomes
Death from any cause
One non‐tuberculosis‐related death was reported in each of the four‐month and six‐month arms in Jawahar 2013, In Merle 2014, five deaths in the gatifloxacin arm and nine deaths in the six‐month ATT arm occurred during treatment. Two deaths in the gatifloxacin arm and three in the control arm were defined as SAEs. An additional 19 deaths in the gatifloxacin arm and 18 deaths in the standard ATT arm were reported after treatment. Meta‐analysis did not reveal significant differences in risk of death due to any cause between ATT regimens (1886 participants, 2 trials; Analysis 2.3).
Treatment discontinuation
In Jawahar 2013, seven people in each arm discontinued treatment (5,2% of 136 with gatifloxacin‐containing ATT, and 4.2% of 165 with standard ATT); in Merle 2014, 27 of 694 (3.9%) and 41 of 662 (6.2%) discontinued treatment. Although the control arm included more people who discontinued treatment in Merle 2014, and the reverse was seen in Jawahar 2013, risk of treatment discontinuation between the two ATT regimens was not appreciably different (1657 participants, 2 trials; Analysis 2.4).
Positive sputum culture at eight weeks
Gatifloxacin replacing ethambutol in ATT regimens did not offer any advantage over standard ATT in sterilizing sputum at the end of the intensive phase of anti‐tuberculosis treatment. At eight weeks 16% of 1818 participants on the two ATT regimens were sputum positive. Pooled data did not reveal that either intervention was better in sputum conversion at eight weeks (1818 participants, 2 trials; Analysis 2.5).
Treatment failure
Treatment failure was rare in both trials. In the gatifloxacin‐containing ATT arms, 19 of 830 in the two trials (2.1%) had positive sputum cultures at end of treatment. In the control arms, 21 of 827 (2.5%) participants experienced treatment failure. Pooled data did not show significant differences in treatment failure between the two ATT regimens (1657 participants, 2 trials; Analysis 2.6).
Acquired drug resistance
Of the two included trials evaluating gatifloxacin‐containing four‐month ATT regimens versus standard six‐month ATT regimens, only Jawahar 2013 reported on acquired drug resistance among 41 participants who experienced culture confirmed treatment failure, or who suffered a recurrence in the six‐month ATT arm. Rifampicin resistance developed in one participant and isoniazid resistance in another. None of the participants given the gatifloxacin‐containing four‐month ATT regimen was detected to have acquired drug resistance. Acquired drug resistance did not differ significantly between the two ATT regimens when the number of participants in each ATT regimen assessed for treatment failure was used as the denominator rather than only the number with treatment failure (301 participants; Analysis 2.7). However, susceptibility to gatifloxacin was not directly evaluated in this trial.
Serious adverse events
Five people in Jawahar 2013 had SAEs; with gatifloxacin‐containing ATT, three had seizures and one had QTc prolongation requiring termination of treatment; and with control ATT, one person had jaundice. In Merle 2014, 20 people in the gatifloxacin arm had 20 SAEs, of which 14 were considered unrelated to treatment; two of three SAEs considered treatment related were deaths. With control ATT, 23 people had 23 SAEs, of which 20 were considered unrelated to treatment; two of three considered treatment related were deaths. Pooled effect estimates were similar for both regimens (1993 participants, 2 trials; Analysis 2.8).
Other adverse events
In Jawahar 2013, nausea, vomiting, and abdominal discomfort (23%) and giddiness (18%) were more frequent among 136 participants given the four‐month regimen than among 165 participants on standard ATT (9% and 3%, respectively). Merle 2014 systematically assessed participants for QTc and blood sugar abnormalities and reported no differences in abnormal peak values of the QTc interval between ATT regimens, nor in episodes of high or low blood sugar, between ATT regimens.
Discussion
We included five trials that compared fluoroquinolone‐containing four‐month anti‐tuberculosis treatment (ATT) regimens versus standard six‐month ATT regimens, recruiting 5825 adults with drug‐sensitive pulmonary tuberculosis from 14 countries with high tuberculosis transmission in Asia, Africa, and Latin Ameria. Three were multi‐country trials that included a total of 572 HIV‐positive people who were not receiving antiretroviral treatment (ART).
Summary of main results
Moxifloxacin‐containing four‐month ATT regimens that substitute for ethambutol or isoniazid probably increase relapse following treatment in adults with drug‐sensitive pulmonary tuberculosis compared to standard six‐month ATT regimens (moderate‐certainty evidence; summary of findings Table for the main comparison). Compared to standard six‐month ATT, four‐month ATT regimens that substitute gatifloxacin for ethambutol probably increase relapse following treatment in adults with drug‐sensitive tuberculosis (moderate‐certainty evidence; summary of findings Table 2).
Compared to six‐month ATT, four‐month ATT regimens containing either moxifloxacin or gatifloxacin probably make little or no difference in treatment failure, death, or serious adverse events (moderate‐certainty evidence). Four‐month moxifloxacin‐containing regimens may not increase the incidence of acquired drug resistance (low‐certainty evidence). We are uncertain whether gatifloxacin‐containing four‐month ATT regimens increase the incidence of acquired drug resistance (very low‐certainty evidence). See summary of findings Table for the main comparison for moxifloxacin‐containing four‐month regimens, and summary of findings Table 2 for gatifloxacin‐containing four‐month regimens.
Overall completeness and applicability of evidence
The trials that met our inclusion criteria evaluated only two of the third‐generation fluoroquinolones in use (moxifloxacin and gatifloxacin). Four of the five trials evaluated their effects in replacing ethambutol or isoniazid in shortened ATT regimens. Only one ongoing trial evaluated the effects of adding a fluoroquinolone (moxifloxacin) to standard ATT drugs in shortened regimens, and results for the clinically relevant outcomes of treatment failure and relapse are awaited. Available evidence from the studies in this review indicates that shortened regimens that replace ethambutol or isoniazid with moxifloxacin may not increase acquired drug resistance. We are uncertain whether gatifloxacin‐containing regimens will increase acquired drug resistance, as this was assessed in only one trial that used ofloxacin susceptibility as a proxy. Resistance to fluoroquinolones in people with newly diagnosed pulmonary tuberculosis, and in those undergoing re‐treatment, is increasingly recognized as a problem, particularly in parts of the world where fluoroquinolone use is widespread and is unregulated (Agarwal 2009; Devasia 2009; Selvakumar 2015). Fluoroquinolone‐related harms were systematically assessed in all five trials, particularly in the three multi‐country trials. Ongoing trials are comparing other four‐month regimens versus standard six‐month ATT (Characteristics of ongoing studies). Results of these studies will add to the available evidence to inform decisions on whether first‐line treatment for drug‐sensitive pulmonary tuberculosis can be shortened effectively without compromising safety or increasing relapse or acquired drug resistance.
Two of the three trials of moxifloxacin‐containing regimens differentiated relapse from re‐infection through genotyping. In the other two trials, most recurrences were considered relapse rather than re‐infection, although in a smaller proportion, re‐infection may have caused recurrence. ATT treatment primarily affects relapse rates ‐ not re‐infection rates; the latter would depend on other factors such as comorbid HIV infection and the intensity of tuberculosis transmission (Wood 2011). The trials in this review were conducted in high tuberculosis‐burden countries, where the pressure of tuberculosis transmission is high. These high‐burden countries account for 84% of the burden of tuberculosis worldwide (WHO 2018). Relapse and re‐infection are not usually differentiated in tuberculosis control programmes in these countries. It is reassuring to note that in this regard, one of the sensitivity analyses undertaken in Gillespie 2014 and Jindani 2014 included all re‐infections under unfavourable outcomes, and effect estimates did not substantially differ from meta‐analysis that excluded re‐infection.
The trials included in this review excluded children and pregnant or lactating women. They also excluded people with many comorbid conditions such as previous tuberculosis, those with HIV on ART or with low CD4 counts, and those with diabetes. Therefore, results of this review can be applied primarily to adults with drug‐sensitive pulmonary tuberculosis without serious comorbid conditions. This review provides evidence of moderate certainty that four‐month ATT regimens that substitute moxifloxacin or gatifloxacin for isoniazid or ethambutol are probably inferior to standard six‐month ATT regimens in preventing relapse (even though there is probably little or no difference in cure). It may be argued that relapse with the four‐month regimens would likely not be less in populations with serious comorbid conditions than was reported in the trials in this review.
Nevertheless, extrapolation of the results of this review to people with diabetes (many of whom may have other serious comorbid illnesses) may be more problematic. First, comorbid diabetes mellitus (DM) and tuberculosis are increasingly common, as people with DM have increased risk of developing active tuberculosis; most of this dual burden is found in low‐ and middle‐income, high tuberculosis‐burden countries (Al‐Rifai 2017; Jeon 2008; Tegegne 2018). People with DM and tuberculosis are more likely to have poorer treatment outcomes than people for whom DM is not comorbid with tuberculosis (Baker 2011). Diabetes increases the risk of treatment failure, death, relapse, and recurrence due to new infection among people with tuberculosis (Baker 2011). Diabetes also increases the odds of developing multi‐drug resistant tuberculosis (MDR‐TB) (Liu 2017; Tegegne 2018). Management of tuberculosis and DM also poses problems due to drug interactions between anti‐tuberculosis drugs, particularly rifampicin, and anti‐diabetic drugs, and adverse drug events are more frequent among those with tuberculosis and DM than in those with tuberculosis alone (Riza 2014).
Certainty of the evidence
We used the GRADE approach to judge the certainty of evidence for pre‐selected outcomes for each comparison in this review (Guyatt 2011a). One of the trials that contributed data to both comparisons in this review was judged at high risk of selection bias due to compromised allocation concealment (Jawahar 2013). However, removal of data from this trial in sensitivity analyses did not alter the direction of effect estimates, so we did not downgrade for risk of bias in the comparison of moxifloxacin‐containing four‐month versus standard six‐month ATT regimens. We downgraded all outcomes by one level for indirectness due to restricted inclusion criteria in all trials, particularly exclusion of people with DM and tuberculosis. People with tuberculosis and DM are four times more likely to relapse than those without DM (Baker 2011). However, they also are more likely to die than people without DM, and this can affect relapse estimates variably. These differences in vulnerability among people with comorbid DM and tuberculosis reduce our certainty in the effect estimates for relapse with shortened versus standard ATT regimens ascertained most often from people without comorbid DM recruited to the trials in this review. Results for this outcome in the comparison of moxifloxacin‐containing four‐month ATT versus standard ATT show inconsistency between large and small effects in favour of the six‐month regimen. We graded the certainty of evidence for the primary outcome of relapse in both comparisons as moderate (summary of findings Table for the main comparison; summary of findings Table 2).
We also graded the certainty of evidence for death due to any cause, treatment discontinuation, and serious adverse events in both comparisons as moderate, downgrading all by one level for indirectness due to restricted inclusion criteria, particularly for those with DM. For these outcomes, the 95% confidence intervals (CIs) for the risk ratios (RRs) were wide, but events were few and samples size was sufficiently large. The RR and the 95% CI around the RR were precise and indicated little or no difference in clinically appreciable effects with either treatment regimen. Moreover, the primary studies were designed as non‐inferiority trials, with the non‐inferiority margin set at 6%, and the 95% CIs for pooled absolute estimates of risk for the outcomes of death, treatment failure, and serious adverse events in both sets of comparisons were well within this margin. We therefore did not downgrade these outcomes for imprecision. We graded the certainty of evidence for the outcome of acquired drug resistance as low for the comparison of moxifloxacin‐based combination regimens, and as very low for the comparison of gatifloxacin‐based regimens, because in addition to indirectness, we downgraded these outcomes for imprecision, and additionally for high risk of bias for the comparison with the gatifloxacin‐containing regimen, because the sole trial reported baseline imbalance among the proportions with drug resistance (Jawahar 2013).
Potential biases in the review process
We used standard methods as provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The literature search covered multiple databases; in addition, we evaluated reference lists of included studies and of relevant systematic reviews for potentially eligible trials. We were unable to formally assess publication bias by using funnel plots because we identified only five relevant trials with outcomes pertinent to this review. We are aware of six ongoing studies that will inform updates of this review. At least two review authors independently screened studies for inclusion, and this was independently verified by a senior review author. Data extraction was done independently by two review authors and was verified independently by two other review authors.
We attempted to account for loss to follow‐up for the primary outcome of relapse by using in our main analysis data provided in each trial report's modified intent‐to‐treat (m‐ITT) analysis, because trials included more randomized participants than were included in their per‐protocol analyses. The three multi‐country trials had shown that the results of sensitivity analyses comparing per‐protocol and m‐ITT analyses were consistent. However, when data from trials are included in a meta‐analysis, pooled estimates can vary depending on how much information is missing for trial outcomes, as well as the magnitude and direction of effect estimates in individual trials. The series of sensitivity analyses that we carried out did not indicate that missing data for relapse influenced the overall results.
We excluded many trials that compared ATT regimens containing fluoroquinolones versus standard ATT regimens (see Characteristics of excluded studies) and reported data for sputum culture positivity at eight weeks ‐ a secondary outcome of this review. This review's inclusion criteria required comparison of shortened tuberculosis regimens versus standard six‐month tuberculosis regimens, and because these phase 2b trials were primarily designed to evaluate and report sputum conversion only at two months, they did not fulfil the review's inclusion criteria. On the other hand, we included Velayutham 2014, which reported sputum culture results at two months but did not provide data on treatment success or relapse. However, unlike the phase 2b trials that we excluded, this study was designed as a phase 3 trial that fulfilled this review's selection criteria. Sputum conversion data at eight weeks was a pre‐stated secondary outcome, and the interim report included adverse events during treatment, further justifying its inclusion. We also excluded data for sputum conversion from one of the trial arms in Jindani 2014, which used moxifloxacin in place of isoniazid but for six months. Data for sputum culture conversion at two months from the four‐month and six‐month moxifloxacin arms were combined and reported, and we could not use these data. However, we will review in the following section the data for sputum culture conversion from these trials for other published work to take into account the totality of trial evidence for this outcome.
Agreements and disagreements with other studies or reviews
An earlier Cochrane Review on fluoroquinolones for treating pulmonary tuberculosis found only ongoing trials of fluoroquinolone‐containing shortened regimens compared to six‐month standard ATT regimens that have now been included in this present review (Ziganshina 2013). Ziganshina 2013 included the phase 2b trials that were excluded from the present review. Other systematic reviews on fluoroquinolones for treating people with drug‐sensitive pulmonary tuberculosis also included these phase 2b trials (Lee 2016; Li 2016; Ruan 2016), as well as the phase 3 trials included in this review. Meta‐analyses of data for sputum positivity at eight weeks from the phase 2b and phase 3 trials (including combined data from the moxifloxacin‐containing four‐ and six‐month arms in Jindani 2014) included in these reviews showed similar results as this review for sputum conversion at eight weeks. These systematic reviews also reported similar results for the other outcomes reported in this review.
The effect of shortened treatment regimens for people with non‐cavitary disease was not an objective of this review. However, another systematic review sought to evaluate whether people with non‐cavitary tuberculosis may have better outcomes than those with cavitary disease with shorter regimens, because the bacterial load is less with non‐cavitary pulmonary tuberculosis. Only the three multi‐country trials included in this review met their inclusion criteria and provided data for participants with non‐cavitary disease (Gillespie 2014; Jindani 2014; Merle 2014). They used data in meta‐analysis from 1066 participants from the three trials who had non‐cavitary pulmonary tuberculosis. They had intended to study the effects of fluoroquinolone‐containing regimens on relapse and cure but could not find disaggregated data for these outcomes for people with non‐cavitary tuberculosis in the three trials. They used the composite 'unfavourable outcome' in these trials and used a margin of 6% in the risk ratio (RR) for pooled estimates to indicate non‐inferiority. The 95% confidence interval (CI) for the pooled RR for unfavourable outcomes using data from the three trials exceeded this margin, and the results were heterogeneous. In subgroup analyses of pooled data from trials using daily treatment (Gillespie 2014; Merle 2014), the results were homogeneous and the 95% CI for the RR was within the non‐inferiority margin (RR 1%, 95% CI ‐3% to 5%; 965 participants, 2 trials). Also, in subgroup analysis using pooled data from the arms of these two trials when fluoroquinolones were substituted for ethambutol and were compared to six‐month ATT, the 95% CI for the pooled RR for an unfavourable outcome was within the non‐inferiority margin (RR ‐1%, 95% CI ‐5% to 4%; 857 participants, 2 trials). Pooling data from the three trials for serious adverse events among participants with non‐cavitary disease also showed no difference for the flouroquinolone‐containing regimens versus the six‐month regimens, with the 95% CI for the RR clearly within the non‐inferiority margin (RR 0%, 95% CI ‐2% to 1%; 4811 participants, 3 trials). Alipanah 2016 concluded that four‐month daily regimens substituting ethambutol with gatifloxacin or moxifloxacin may be non‐inferior to standard therapy for patients with culture confirmed, non‐cavitary, drug‐susceptible pulmonary tuberculosis. These review authors acknowledged that these estimates may be prone to error because they had to use data from a mix of per‐protocol and intention‐to‐treat analysis data from the trials in their analysis.
The suggestion from the results in Alipanah 2016 that increased relapse proportions seen with moxifloxacin‐ and gatifloxacin‐containing regimens compared to the standard six‐month regimen in the present review may be due to inclusion of people with cavitary lung disease due to tuberculosis needs verification. Support for this observation comes from a pooled analysis of individual patient data‐sets of 3411 participants from Gillespie 2014,Jindani 2014, and Merle 2014 (Imperial 2018). This analysis identified two subgroups of participants that differed in their response to the four‐month regimens. A subgroup of patients with drug‐susceptible tuberculosis with low grades of sputum positivity or absence of cavitation at baseline assessments was at lower risk for unfavourable outcomes, and this population (with either of these low‐risk characteristics) constituted 47% of the 3405 participants in the three trials. The four‐month fluoroquinolone regimens in these trials were effective in reducing the risk of unfavourable outcomes in this population with "minimal disease". Another subgroup of participants with a smear grade of 3+ and the presence of cavitation on chest radiographs at baseline (34% of total sample) had unfavourable outcomes. Data from this pooled analysis suggest that this "hard‐to‐treat" population may require treatment for longer than those given the standard six‐month regimen to achieve optimal outcomes (Imperial 2018). These observations from Alipanah 2016 and Imperial 2018 have implications of heuristic value for the design and interpretation of future trials on this topic.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Forest plot of comparison: 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, outcome: 1.3 Relapse: sensitivity analysis accounting for missing data.

Forest plot of comparison: 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, outcome: 2.2 Relapse: sensitivity analysis accounting for missing data.

Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 1 Relapse.

Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 2 Relapse: subgroup analysis.

Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 3 Relapse: sensitivity analysis accounting for missing data.

Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 4 Death from any cause.

Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 5 Treatment discontinuation.

Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 6 Positive sputum culture/smear at 8 weeks.

Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 7 Treatment failure.

Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 8 Acquired drug resistance.

Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 9 Serious adverse events.

Comparison 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 1 Relapse.

Comparison 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 2 Relapse: sensitivity analysis accounting for missing data.

Comparison 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 3 Death from any cause.

Comparison 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 4 Treatment discontinuation.

Comparison 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 5 Positive sputum culture at 8 weeks.

Comparison 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 6 Treatment failure.

Comparison 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 7 Acquired drug resistance.

Comparison 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 8 Serious adverse events.
| Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimen for drug‐sensitive pulmonary tuberculosis | ||||||
| Patient or population: adults with drug‐sensitive pulmonary tuberculosis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with 6‐month standard ATT | Risk with 4‐month moxifloxacin‐containing ATT | |||||
| Relapse | 32 per 1000 | 82 more relapses per 1000 | RR 3.56 | 2265 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably increases relapse compared to the 6‐month regimen |
| Death from any cause Follow‐up: range 18 to 24 months | 21 per 1000 | 2 more deaths per 1000 | RR 1.06 | 2760 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably makes little or no difference in death from any cause compared to the 6‐month regimen |
| Treatment failure | 16 per 1000 | 5 fewer treatment failures per 1000 | RR 0.71 | 2282 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably results in little or no difference in treatment failure compared to the 6‐month regimen |
| Acquired drug resistance | 7 per 1000 | 5 fewer with acquired drug resistance per 1000 (6 fewer to 2 more) | RR 0.33 | 2282 (3 RCTs)e | ⊕⊕⊝⊝ Due to indirectness and imprecision | The 4‐month regimen may be little or no different than the 6‐month regimen in the incidence of acquired drug resistance |
| Serious adverse events Follow‐up: range 18 to 24 months | 62 per 1000 | 2 fewer with serious adverse events per 1000 | RR 0.97 | 3548 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably results in little or no difference in serious adverse events compared to the 6‐month regimen |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aNo serious risk of bias: although Jawahar 2013 was at high risk of allocation bias, exclusion of this trial from the sensitivity analysis did not change the direction of effect. Not downgraded. | ||||||
| Gatifloxacin‐containing 4‐month ATT regimens compared to standard 6‐month ATT regimens for drug‐sensitive pulmonary tuberculosis | ||||||
| Patient or population: adults with drug‐sensitive pulmonary tuberculosis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with 6‐month standard ATT | Risk with gatifloxacin‐containing | |||||
| Relapse | 70 per 1000 | 77 more relapses per 1000 | RR 2.11 | 1633 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably increases relapse compared to the 6‐month regimen |
| Death from any cause | 29 per 1000 | 3 fewer deaths per 1000 | RR 0.90 | 1886 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably makes little or no difference in death compared to the 6‐month regimen |
| Treatment failure | 25 per 1000 | 1 less treatment failure per 1000 | RR 0.93 | 1657 | ⊕⊕⊝⊝ Due to indirectness | The 4‐month regimen probably makes little or no difference in treatment failure compared to the 6‐month regimen |
| Acquired drug resistance | 12 per 1000 | 9 fewer with acquired drug resistance per 1000 (12 fewer to 49 more) | RR 0.24 (0.01 to 5.01) | 301 (1 RCT)d | ⊕⊝⊝⊝ Due to indirectness, risk of bias, and imprecision | We do not know if acquired drug resistance is any different in the 4‐month and the 6‐month regimens |
| Serious adverse events | 24 per 1000 | 0 fewer serious adverse events per 1000 | RR 1.02 | 1993 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably results in little or no difference in serious adverse events compared to the 6‐month regimen |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. | ||||||
| aNo serious risk of bias: although Jawahar 2013 was assigned high risk of bias for allocation concealment, removal of this trial from the sensitivity analysis did not significantly alter the direction, magnitude, or precision of the effect estimate. Not downgraded. | ||||||
| Study ID (Acronym) | (REMoxTB) | (RIFAQUIN) | (OFLOTUB) | |||||||
| Setting | Multiple sites in Africa (Kenya, South Africa, Tanzania, Zambia), Asia (China, India, Malaysia Thailand), Latin America (Mexico) | 6 sites in 2 cities in India | 6 cities in 4 countries in Africa (Botswana, South Africa, Zambia, Zimbabwe) | 5 countries in Africa (Benin, Guinea, Kenya, Senegal, South Africa) | 2 cities in India | |||||
| Participants | ||||||||||
| Number randomized | 1931 | 429 | 827 | 1836 | 801 | |||||
| Age | Adults (> 18 years) | Adults (> 18 years) | Adults (> 18 years) | Adults (18 to 65 years) | Adults (> 18 years) | |||||
| HIV infection | Included (if CD4 count > 250 cells/μL and not on ART); 110 (7%) | Excluded | Included (if CD4 count > 150/mm³ and not on ART; 158 (27%) | Included if not stage 3 or 4 disease and not on ART; 304 (17%) | Excluded | |||||
| Diagnosis of TB | Positive sputum smears on 2 occasions Culture‐confirmed susceptibility to rifampicin, isoniazid, pyrazinamide, and moxifloxacin | Newly diagnosed pulmonary TB with at least 2 positive sputum cultures. Confirmed by culture and MDR‐TB excluded, susceptibility to ofloxacin (as proxy for moxifloxacin) | 2 sputum samples that were positive for tubercle bacilli on direct smear microscopy No resistance to isoniazid, rifampicin, or moxifloxacin | Acid‐fast bacilli in 2 consecutive sputum smears; confirmed by culture (solid medium) and drug sensitivity tests to rifampicin, isoniazid, ethambutol, streptomycin, and gatifloxacin | 2 positive sputum smear smears for tuberculosis. Culture‐confirmed and MDR‐TB ruled out; susceptible to ofloxacin (as proxy for moxifloxacin) | |||||
| Intervention(s) and comparator | ||||||||||
| Duration of ATT | 4 monthsa | 6 months | 4 monthsb | 6 months | 4 months | 6 monthsc | 4 months | 6 months | 4 monthsa | 6 months |
| Regimens | 2HRZM/2HRM + 2MRZE/2MR | 2HRZE/4HR | 2(HRZG)₃/ 2 (HRG)₃ 2(HRZM)₃/2(HRM)₃ | 2(HRZE)₃ /4(HR)₃ | 2MRZE/ 2P₂M₂ | 2HRZE/ 4HR | 2HRZG/ 2HRG | 2HRZE/4HR | 3HRZM + 2HRZM/ 2RHM + 2HRZM/ 2(RHM)₃ + 2HRZM/ 2(RHEM)₃ | 2(HRZE)₃/ 4(HR)₃ |
| Number allocated | 655 + 636 = 1291 | 640 | 141 118 | 170 | 275 | 275 | 917 | 919 | 629 | 172 |
| Late screening failures excluded after allocation | 38 + 32 = 70 | 40 | 5 3 | 5 | 36 | 35 | 62 | 51 | 13 | 8 |
| Number eligible | 1231 | 600 | 136 115 | 165 | 239 | 240 | 852 | 868 | 616 | 164 |
| Number analysed in m‐ITT analysis (% of those allocated) | 568 + 551 = 1119 (87) | 555 (87) | 136 (97) 115 (98) | 165 (97) | 193 (70) | 188 (68) | 791 (86) | 794 (86) | 590 (94) | 151 (88) |
| Number analysed in per‐protocol analysis (% of those allocated) | 514 + 524 = 1038 (80) | 510 (80) | 131 (93) 113 (96) | 159 (94) | 165 (60) | 163 (59) | 651 (71) | 601 (65) | As above | |
| Number analysed in ancillary analysis (ITT) (% of those allocated) | 617 + 604 = 1221 (94) | 600 (94) | Not done | 239 (87) | 240 (87) | Not reported | Not reported | |||
| Outcomes reported | ||||||||||
| Relapse | Relapse within 18 months after randomization in those with negative culture with treatment. Relapse strains were those shown to be identical on 24‐locus MIRU analysis LJ solid media and MGIT liquid media used for culture | Recurrence of TB over 24 months after treatment in those with a favourable response with treatment: either bacteriologic recurrence (LJ solid media) or clinical/radiologic recurrence | Relapse within 12 to 18 months after treatment. Two positive cultures within a period of 4 months without an intervening negative culture). Re‐infections differentiated from relapse through genotyping (MIRU‐VNTRs) LJ solid media used for culture in some centres, MGIT liquid media in others, and both in some centres | Recurrence of TB over 24 months after treatment proven bacteriologically (2 consecutive positive sputum samples a day apart) or clinically Genotyping (MIRU‐VNTRs) results available for only 70/140 (55%) of those with culture confirmed recurrence. Most were relapses | Not reported | |||||
| Deaths | All deaths TB deaths | Reported (only non‐TB deaths occurred) | All deaths TB deaths | Death during treatment Death after treatment | Not reported | |||||
| Treatment discontinuation | Includes those who did not complete treatment, relocated, or withdrew consent | Includes those who did not complete treatment and those lost to follow‐up | Includes change in treatment due to adverse events, loss to follow‐up, and other treatment changes | Includes those who withdrew consent during treatment and dropouts | Reported but disaggregated data for each group not available | |||||
| Positive smear/ sputum culture at 2 months | Reported using LJ solid media (used in this review) and MGIT liquid media for all randomized participants excluding late screening failures | Reported using LJ solid media for all randomized participants excluding late screening failures | Reported but disaggregated data for moxifloxacin 4‐month and 6‐month treatment groups not available Data also not available for all participants from LJ media | Reported for 752 in the 4‐month and 759 in the 6‐month regimens (88% and 87% of those eligible, respectively) Culture using LJ solid media | Reported for 590 (94%) in the 4‐month and 151 (88%) in the 6‐month regimens | |||||
| Acquired drug resistance | Reported | Reported | Reported | Not reported | Not reported | |||||
| Treatment failure | Includes culture confirmed and not confirmed | Includes culture confirmed and unconfirmed | Culture confirmed | Includes culture confirmed failure | Not reported | |||||
| Serious adverse events | Reported for all randomized participants excluding late screening failures. Grade 3 and 4 severity (DAIDS 2009) | Deduced from adverse events reported for all randomized participants excluding late screening failures. Not graded | Reported for all participants randomized who took 1 dose and assessed as severe or life‐threatening during and 2 weeks after treatment. grade 3 and 4 severity (DAIDS 2009) | Reported for 1692 (92%) of all randomized participants. grade 3 and 4 severity (DAIDS 2009) | Deduced from adverse events reported. Not graded | |||||
| Other adverse events | Not reported | Reported | Not reported | QT prolongation Hyperglycaemic episodes | Reported | |||||
| Abbreviations: ART: anti‐retroviral treatment; ATT: anti‐tuberculosis treatment; E: ethambutol; G: gatifloxacin; H: isoniazid; ITT: intention‐to‐treat; LJ: Löwenstein‐Jensen; M: moxifloxacin; MGIT: mycobacterial growth indicator tube; MIRU‐VNTRs: mycobacterial interspersed repetitive unit–variable number tandem repeats; m‐ITT: modified intention‐to‐treat; P: rifapentine; R: rifampicin; Z: pyrazinamide. Leading numbers in regimens indicate duration in months. Drugs were administered daily, except when given thrice weekly as indicated by subscripts. aData from moxifloxacin‐containing shortened regimens combined for data synthesis. | ||||||||||
| Primary outcome: relapse | ||||||
| Trial ID | ||||||
| Regimens | 4 months | 6 months | 4 months | 6 months | 4 months | 6 months |
| aModified‐ITT analysis (primary analysis) | 110/1119 (9.8%) | 13/555 (2.3%) | 11/108 (10.1%) | 10/155 (6.5%) | 27/165 (16.4%) | 6/163 (3.7%) |
| aPer‐protocol analysis | 110/1038 (10.6%) | 12/510 (2.4%) | 11/107 (10.1%) | 10/152 (6.6%) | 26/165 (15.8%) | 5/163 (3.1%) |
| bSensitivity analysis imputing missing data | 126/1184 (10.7%) | 14/577 (2.4%) | 11/114 (9.7%) | 10/159 (6.3%) | 36/225 (16.0%) | 71/232 (2.6%%) |
| Abbreviations: ATT: anti‐tuberculosis treatment; ITT: intention‐to‐treat. aAs reported in trial reports. | ||||||
| Primary outcome: relapse | ||||
| Trial ID | ||||
| Regimen | 4 months | 6 months | 4 months | 6 months |
| aModified‐ITT analysis (primary analysis) | 19/122 (15.6%) | 10/155 (6.5%) | 101/694 (14.6%) | 47/662 (7.1%) |
| aPer‐protocol analysis | 19/121 (15.7%) | 10/152 (6.6%) | 98/651 (15.1%) | 44/601 (7,3%) |
| bSensitivity analysis imputing missing data | 19/132 (14.4%) | 10/159 (6.3%) | 122/786 (15.5%) | 61/774 (7,9%) |
| Abbreviations: ATT: anti‐tuberculosis treatment; ITT: intention‐to‐treat. aAs reported in trial reports. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse Show forest plot | 3 | 2265 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.56 [2.37, 5.37] |
| 2 Relapse: subgroup analysis Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Moxifloxacin replacing ethambutol | 2 | 1386 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.74 [1.69, 4.43] |
| 2.2 Moxifloxacin replacing isoniazid | 2 | 1424 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.89 [3.02, 7.92] |
| 3 Relapse: sensitivity analysis accounting for missing data Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Modified‐ITT analysis | 3 | 2265 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.56 [2.37, 5.37] |
| 3.2 Per‐protocol analysis | 3 | 2135 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.79 [2.48, 5.78] |
| 3.3 Imputing missing data | 3 | 2524 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.83 [2.58, 5.70] |
| 4 Death from any cause Show forest plot | 3 | 2760 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.65, 1.75] |
| 5 Treatment discontinuation Show forest plot | 3 | 2335 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.78, 1.61] |
| 6 Positive sputum culture/smear at 8 weeks Show forest plot | 3 | 2828 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.22, 1.13] |
| 6.1 Moxifloxacin replacing isoniazid or ethambutol in 4‐month ATT regimen | 2 | 2087 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.45, 1.20] |
| 6.2 Moxifloxacin augmenting standard 6‐month ATT regimen | 1 | 741 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.15, 0.39] |
| 7 Treatment failure Show forest plot | 3 | 2282 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.33, 1.52] |
| 8 Acquired drug resistance Show forest plot | 3 | 2282 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.08, 1.31] |
| 9 Serious adverse events Show forest plot | 4 | 3548 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.74, 1.27] |
| 9.1 Moxifloxacin replacing standard drugs in 4‐month ATT regimens | 3 | 2760 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.72, 1.26] |
| 9.2 Moxifloxacin augmenting standard 6‐month ATT regimens | 1 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.45, 3.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse Show forest plot | 2 | 1633 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [1.56, 2.84] |
| 2 Relapse: sensitivity analysis accounting for missing data Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Modified‐ITT analysis | 2 | 1633 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [1.56, 2.84] |
| 2.2 Per‐protocol analysis | 2 | 1525 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [1.55, 2.87] |
| 2.3 Modified‐ITT analysis (all eligible participants ‐ imputing missing data) | 2 | 1851 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [1.53, 2.63] |
| 3 Death from any cause Show forest plot | 2 | 1886 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.53, 1.53] |
| 4 Treatment discontinuation Show forest plot | 2 | 1657 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.46, 1.08] |
| 5 Positive sputum culture at 8 weeks Show forest plot | 2 | 1818 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.80, 1.23] |
| 6 Treatment failure Show forest plot | 2 | 1657 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.51, 1.70] |
| 7 Acquired drug resistance Show forest plot | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.01, 5.01] |
| 8 Serious adverse events Show forest plot | 2 | 1993 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.58, 1.77] |

