Regímenes de tratamiento acortados versus régimen estándar para la tuberculosis pulmonar sensible a los fármacos
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: multi‐centre, randomized, parallel‐group, double‐blind (participant, care provider, investigator, outcomes assessor), 3‐armed, placebo‐controlled, non‐inferiority trial Study period: January 2008 to February 2014 Recruitment sites: 47 sites in 9 countries Countries where the trial was undertaken: South Africa, India, Tanzania, Kenya, Thailand, Malaysia, Zambia, China, Mexico Length of follow‐up: 18 months after randomization (1 year after treatment completion) | |
| Participants | No. of participants randomized: 1931 Interventions: 1291 (636 to ethambutol group; 655 to isoniazid group) Control: 640 . Age: > 35 years, 37% in isoniazid group, 39% in ethambutol group, and 40% in control group Gender: male 70% in ethambutol group, 68% in isoniazid group, 70% in control group Inclusion criteria:
Exclusion criteria:
Proportion with HIV seropositivity: 7% overall (and in intervention and control groups) Proportion with cavitation: 71% overall (69% and 70% in intervention groups and 72% in control groups) Baseline drug resistance: isoniazid: 7% overall (6% in control arm and 7% in each intervention arm); pyrazinamide: 2% overall (1% in each intervention arm) | |
| Interventions | Interventions: 4‐month (17‐week) ATT regimen Isoniazid group (moxifloxacin for 17 weeks substituting ethambutol): N = 655; 568 eligible, 514 completed (78% of those randomized; 91% of those eligible) 8 weeks of moxifloxacin, isoniazid, rifampicin, pyrazinamide, + ethambutol placebo administered daily, followed by 9 weeks of moxifloxacin, isoniazid, and rifampicin, followed by 9 weeks of isoniazid and rifampicin placebo Ethambutol group (moxifloxacin for 17 weeks substituting isoniazid): N = 636; 551 eligible, 524 completed (82% of those randomized; 91% of those eligible) 8 weeks of moxifloxacin, ethambutol, rifampicin, pyrazinamide + isoniazid placebo administered daily, followed by 9 weeks of moxifloxacin and rifampicin + isoniazid placebo daily, followed by 9 weeks of isoniazid and rifampicin placebo Control: 6‐month (26‐week) ATT regimen: N = 640; 555 eligible, 510 completed (80% of those randomized; 92% of those eligible) 8 weeks of isoniazid, rifampicin, ethambutol, pyrazinamide, and moxifloxacin placebo given daily, followed by 9 weeks of isoniazid, rifampicin, and moxifloxacin placebo given daily, followed by 9 weeks of isoniazid and rifampicin Dosage: Moxifloxacin 400 mg, isoniazid 300 mg; rifampicin, ethambutol, and pyrazinamide were dosed based on weight | |
| Outcomes | Outcomes reported and used in this review:
Outcomes sought but not reported:
Outcomes reported but not used in this review:
| |
| Notes | Funding: Global Alliance for TB Drug Development (supported by multiple international donor agencies and local agencies and institutions in participating countries). Bayer Healthcare donated moxifloxacin and Sanofi donated rifampicin Treatment supervision: treatment was given daily and was observed according to guidelines at the study site Follow‐up method: following screening and baseline visits, there were 8 weekly visits followed by 8 visits until 18 months after randomization. Safety analysis was performed at the screening visit and thereafter at weeks 2, 8, 12, and 17 Trial registration ID: NCT00864383 (retrospectively registered: registered March 2009; study start January 2008) Acronym: REMoxTB Comment: data from both moxifloxacin‐containing shorter regimens were combined and compared with data from the standard treatment regimen | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from report: "Randomization was performed with the use of lists with blocks of variable sizes that were stratified according to the patient weight group and study centre" |
| Allocation concealment (selection bias) | Low risk | Quote from report: "During randomization, patients were assigned a unique study number selected sequentially from the appropriate randomization list that corresponded to the treatment pack allocated" Quote from report: "Only statisticians who were responsible for preparing the reports for the independent data and safety monitoring committee and essential manufacturing and distribution staff members had access to the list of identifiers matched to the intervention" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote from study protocol: "This will be a blinded study with matching placebo for each of the study medicines except for pyrazinamide" Quote from trial registration document: "Masking: quadruple (participant, care provider, investigator, outcomes assessor)" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from trial registration document: "Masking: quadruple (participant, care provider, investigator, outcomes assessor)" |
| Incomplete outcome data (attrition bias): At the end of ATT (Treatment failure, positive sputum culture, treatment discontinuation, adverse events) | Low risk | Quote from report: "Of the 1931 patients who underwent randomization, 89% in the isoniazid group, 92% in the ethambutol group, and 89% in the control group met the requirements for treatment adherence, which was based on receipt of approximately 80% of the assigned regimen" Comment: the modified‐intention‐to‐treat analysis used included 87% of those randomized to the combined moxifloxacin‐containing ATT regimens and 87% of those randomized to standard regimens. A sensitivity analysis included 94% of those randomized to both regimens |
| Incomplete outcome data (attrition bias): At the end of follow‐up (Relapse, deaths) | Low risk | Of those randomized, 91% of those allotted to the 2 moxifloxacin combination therapy arms and 92% allotted to control treatment were included in the modified intention‐to‐treat analyses. Results of the per‐protocol and modified intention‐to‐treat analyses were consistent |
| Selective reporting (reporting bias) | Low risk | Although the trial was retrospectively registered, all pre‐stated outcomes listed in the trial registration document and protocol were published with no evidence of selective reporting |
| Other bias | Low risk | Quote from report: "Bayer Healthcare donated moxifloxacin, and Sanofi donated rifampin. Neither company had any role in the study design, data accrual, data analysis, or manuscript preparation. Representatives of Bayer Healthcare reviewed the manuscript but did not suggest revisions" |
| Methods | Study design: randomized, open‐label, parallel‐group, 3‐armed, active comparator, equivalence trial Study period: started May 2004 for an anticipated duration of 5 years; terminated early (between February and October 2006) due to high recurrence rates in the shorter treatment arms Recruitment sites: Chennai and Madurai Country where the trial was undertaken: India Length of follow‐up: 24 months after treatment completion | |
| Participants | No. of participants randomized: 429 (of 1200 anticipated) Intervention groups: 259 (gatifloxacin regimen 141; moxifloxacin regimen 118) Control group: 170 Age: < 40 years 72% (gatifloxacin 66%; moxifloxacin 77%; control 73%) Gender: male 74% (gatifloxacin 76%; moxifloxacin 72%; control 72%) Inclusion criteria:
Exclusion criteria:
Proportion with HIV seropositivity: nil (excluded) Proportion with cavitation: not reported Baseline drug resistance: isoniazid 7% overall (gatifloxacin 4%; moxifloxacin 1.2%; control 12%); rifampicin 0.2% overall (moxifloxacin 1%); ofloxacin 1.7% overall (gatifloxacin 2%; control 3%); isoniazid and ethambutol 0.4% overall (1% in each intervention arm); isoniazid and ofloxacin 0.2% overall (control 1%) | |
| Interventions | Interventions: 4‐month ATT regimens Gatifloxacin regimen (gatifloxacin replacing ethambutol): N = 141; 136 eligible, 131 completed (93% of those randomized; 96% of those eligible) 2 months of gatifloxacin, isoniazid, rifampicin, and pyrazinamide thrice weekly, followed by 2 months of gatifloxacin, isoniazid, and rifampicin thrice weekly Moxifloxacin regimen (moxifloxacin replacing ethambutol): N = 118; 115 eligible, 113 completed (96% of those randomized; 98% of those eligible) 2 months of moxifloxacin, isoniazid, rifampicin, and pyrazinamide thrice weekly, followed by 2 months of moxifloxacin, isoniazid, and rifampicin thrice weekly Control: 6‐month ATT regimen: N = 170; 165 eligible, 159 completed (94% of those randomized; 96% of those eligible) 2 months of ethambutol, isoniazid, rifampicin, and pyrazinamide thrice weekly, followed by 4 months of isoniazid and rifampicin thrice weekly Dosage: gatifloxacin or moxifloxacin 400 mg, rifampicin 450 or 600 mg, depending on body weight (< 60 kg or ≥ 60 kg), pyrazinamide 1500 mg, and isoniazid 600 mg | |
| Outcomes | Outcomes reported and used in this review:
| |
| Notes | Funding: Tuberculosis Research Centre of the Indian Council of Medical Research Follow‐up method: a physician examined the patient every month and recorded adherence to treatment, any adverse drug reactions, and the clinical response. Sputum specimens were examined every month by microscopy and culture: 2 (2 overnight and 1 spot) during the treatment phase, and 2 (1 overnight and 1 spot) during the follow‐up phase Treatment supervision: directly observed, thrice‐weekly treatment in all arms Trial registration ID: CTRI/2012/10/003060; retrospectively registered (trial commenced May 2004; trial registered 15/10/2012, after termination) Comment: the data safety monitoring board recommended termination of both intervention arms in 2006 due to high tuberculosis recurrence rates in the 2 arms compared to the standard 6‐month regimen | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from report: "Restricted random allocation sequences were generated by a biostatistician using random number tables, separately for the two strata and sealed envelopes were used to assign regimens" Quote from trial registration document: "Stratified block randomization" |
| Allocation concealment (selection bias) | High risk | Quote from report: "Patients were enrolled by the physicians, and when ready for allocation, the biostatistician drew the regimen from sealed envelopes. Allocation was stratified on sputum smear grading and extent of lesions in chest x ray" Quote from report: "The study design envisaged enrolling 400 patients in each arm in a 1∶1:1 ratio. However, due to the non‐availability of one of the test drugs (M), patients were enrolled initially in a 1∶1 ratio in the G and control regimen arms commencing in May 2004. Subsequently, when M became available (May 2005), patients were enrolled to the G, M, and control regimen arms in a 1∶2:1 ratio to compensate for the delay in recruiting to the moxifloxacin arm at the onset" Quotes from correspondence with study authors: "When the first patient on Moxifloxacin was allocated, there were 110 patients randomised to the Gatifloxacin regimen and 110 to the Control regimen. The last patient was allocated to the Gatifloxacin regimen on 3 February 2006" Comment: alteration of recruitment ratios raises serious concerns that allocation concealment was compromised. Even though biostatisticians implemented allocation after clinicians confirmed eligibility, by the time the first patient was recruited to the moxifloxacin regimen in May 2005, 110 allocated to the gatifloxacin regimen (80% of 136 eligible among those finally recruited), and 110 allocated to the control regimen (67% of the 165 eligible) had already been recruited. This would have alerted investigators that most of those to be recruited over the following year would be allocated to the moxifloxacin regimen. In addition, premature termination of the trial, combined with the alteration in allocation ratios, appears to have led to imbalance in the numbers recruited to the gatifloxacin (141), moxifloxacin (118), and control (170) regimens that is not explained, given that block randomization was used. There were also baseline imbalances in proportions resistant at baseline to any of the anti‐tuberculosis drugs tested (6%, 3%, and 16% respectively) |
| Blinding of participants and personnel (performance bias) | Low risk | This was an open‐label trial; given the likelihood that allocation concealment was compromised, treating personnel may have had knowledge of allocation. However, it is unlikely that this led to performance bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from report: "Sputum specimens were given identification laboratory numbers, and bacteriological investigations were carried out by technicians who were blinded to the clinical status of the patient and the regimen. ECG was done every month" Comment: although bacteriologic outcomes were done blind to treatment allocation, clinical efficacy and safety outcomes were undertaken by study personnel. But results at the time of termination favouring the standard and not experimental interventions suggest that detection bias was unlikely |
| Incomplete outcome data (attrition bias): At the end of ATT (Treatment failure, positive sputum culture, treatment discontinuation, adverse events) | Low risk | In the combined fluoroquinolone arms, 228 of 259 (88%) completed treatment compared to 152 of 170 (89%) in the control arms. Study results did not differ between per‐protocol and modified intention‐to‐treat analyses (that excluded only late‐screening failures). The modified‐ITT analysis included 97%, 98%, and 97% of those in the gatifloxacin, moxifloxacin, and standard ATT arms, respectively. The results of both analyses were consistent |
| Incomplete outcome data (attrition bias): At the end of follow‐up (Relapse, deaths) | Low risk | In the combined fluoroquinolone arms, 230 of 259 (89%) were assessed for tuberculosis recurrence compared to 154 of 170 (91%) in the control arm. Per‐protocol and modified intention‐to‐treat analyses did not significantly alter the results. Early termination led to recruitment of only a third of the estimated 1200 participants required to prove equivalence, but although this reduces the power of the trial to detect equivalence, lack of differential attrition, with similar reasons for exclusion, is unlikely to affect the reported relative effect estimates |
| Selective reporting (reporting bias) | Low risk | Although the trial was retrospectively registered, stated outcomes in trial registry documents and in the online study protocol were available in the trial report and do not suggest selective reporting |
| Other bias | Low risk | The trial was terminated early at the recommendation of the Data Safety Monitoring Board after an interim analysis showed high recurrence rates in the fluoroquinolone arms compared to the control arm. Because this was a planned interim analysis, it is unlikely to have introduced bias, other than that discussed under allocation concealment |
| Methods | Study design: randomized, multi‐centre, parallel‐group, open‐label, 3‐arm, active‐controlled, equivalence trial Study period: August 15, 2008, and August 1, 2011 Recruitment sites: Worcester, Johannesburg, Harare, Marondera, Francistown, and Macha Countries where the trial was undertaken: Botswana, South Africa, Zambia, and Zimbabwe Length of follow‐up: 18 months after randomization in 86%; in Botswana and South Africa, 6% of those randomized in the last 6 months of enrolment were followed up for 12 to 15 months and 8% for 15 to 18 months | |
| Participants | No. of participants randomized: 827 (of the estimated sample of 1095) Interventions: 275 in 4‐month regimen (277 in 6‐month regimen) Control group: 275 in the control regimen Age: 18 to 34 years 61% in the control regimen and 68% in the 4‐month regimen Gender: male 64% in the control regimen, 63% in the 4‐month regimen Inclusion criteria:
Exclusion criteria:
Proportion with HIV seropositivity: 32% in the control regimen and 28% in the 4‐month regimen Proportion with cavitation: 67% in the control regimen and 65% in the 4‐month regimen Baseline drug resistance: excluded people resistant to isoniazid, rifampicin, or moxifloxacin | |
| Interventions | Interventions: 4‐month (17‐week) ATT regimen: moxifloxacin replacing isoniazid throughout with twice‐weekly administration in continuation phase + rifapentine twice weekly replacing rifampicin in continuation phase: N = 275 randomized; 239 eligible, 165 completed (60% of those randomized; 69% of those eligible) 2 months (8 weeks) of ethambutol, moxifloxacin, rifampicin, and pyrazinamide administered daily, followed by 2 months (9 weeks) of moxifloxacin and rifapentine administered twice weekly 6‐month (26‐week) ATT regimen: moxifloxacin replacing isoniazid throughout with weekly rifapentine + weekly moxifloxacin in the 4‐month continuation phase 2 months (8 weeks) of ethambutol, moxifloxacin, rifampicin, and pyrazinamide administered daily, followed by 4 months (18 weeks) of rifapentine and moxifloxacin once a week Control: 6‐month (26‐week) ATT regimen: N = 275 randomized; 240 eligible, 163 completed (59% of those randomized; 68% of those eligible) 2 months (8 weeks) of isoniazid, rifampicin, ethambutol, and pyrazinamide administered daily, followed by 18 weeks of isoniazid and rifampicin daily Dosage: moxifloxacin 400 mg; rifapentine 900 mg in the 4‐month treatment arm (and 1200 mg in the 6‐month arm). All doses given were based on the weight of the patient | |
| Outcomes | Outcomes reported and used in this review:
Outcomes sought for this review and not reported:
Outcomes reported and not used in this review:
| |
| Notes | Funding: European and Developing Countries Clinical Trials Partnership, Wellcome Trust. Some of the trial medications were donated by Sanofi, Genus Pharmaceuticals, and Sandoz Follow‐up method Patients were followed‐up monthly up to 12 months after randomization and thereafter once in 3 months until 18 months. Two sputum samples were collected before treatment initiation for smear and culture, and 1 sample was collected monthly for 12 months and then again at 15 months and 18 months of follow‐up Treatment supervision: treatment was directly observed in all participants in the intensive treatment phase. Drugs were taken under the supervision of a relative or another designated person in the 18‐week continuation phase in the control arm. Moxifloxacin and rifapentine treatment was supervised at the treatment facility twice weekly for the 9‐week continuation phase Trial registration ID: ISRCTN44153044 (prospectively registered) Acronym: RIFAQUIN Comment: data from the 6‐month moxifloxacin intervention arm were not used in this study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from report: "A randomized allocation sequence was generated for each study centre with the use of blocks of varying size by an independent statistician based at the MRC CTU" |
| Allocation concealment (selection bias) | Low risk | Quote from updated protocol: "Sealed opaque envelopes containing the treatment allocation slips will be held by the pharmacist. When a patient is found to be eligible their details will be entered on the enrolment log by the designated member of the clinic team against the next available study number. These patient details and the study number will be entered on to the patient’s prescription. This will be taken to the pharmacy and the patient details entered onto the pharmacy register by the pharmacist against the next study number which will act as a check that the correct (next available) study number had been used. The pharmacist will then take the envelope corresponding to the study number and reveal the treatment allocation which will be written on the allocation slip. This will then be attached to the prescription and kept in the patient’s Trial folder or other appropriate place and the designated member of the clinic team made aware of the treatment allocation" |
| Blinding of participants and personnel (performance bias) | Low risk | This was an open‐label trial. The treating team was aware of allocated treatments. However, this does not seem to have influenced drug administration or use of co‐interventions |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from report: "Apart from the statisticians reporting to the data and safety monitoring committee, the staff at St. George’s and at the MRC CTU were unaware of treatment assignment except when a lack of awareness would have been unethical (e.g., in some discussions of serious adverse events). Participating laboratories were unaware of treatment assignment throughout the study" Comment: although treatment allocation before the start of the trial was concealed, the clinical team evaluating participants for efficacy and safety outcomes was aware of treatment allocation. However, laboratory assessments were objective and clinical outcomes were mostly based on objective assessments |
| Incomplete outcome data (attrition bias): At the end of ATT (Treatment failure, positive sputum culture, treatment discontinuation, adverse events) | Low risk | Although overall attrition was over 30%, there was no differential attrition in the 4‐month arm (31%) versus the control arm (32%). In the sensitivity analysis in the supplementary table, S1 attrition was 13% in each arm. We do not think this is likely to alter the estimates of relative effects |
| Incomplete outcome data (attrition bias): At the end of follow‐up (Relapse, deaths) | Low risk | Modifed intention‐to‐treat and per‐protocol analyses presented in Table 2 of the main report and in the sensitivity analyses in Table S1 in the online supplementary appendix do not indicate that bias due to differential attrition is likely to have affected the estimates of relative effects |
| Selective reporting (reporting bias) | Low risk | This trial was prospectively registered, and protocol amendments and reporting of results do not indicate selective reporting |
| Other bias | Low risk | Quote from report: "Some of the trial medications were donated by Sanofi, Genus Pharmaceuticals, and Sandoz, and a representative of Sanofi was a non‐voting observer at meetings of the steering committee, but none of these companies had any role in the study design, data accrual, data analysis, or manuscript preparation" |
| Methods | Study design: randomized, multi‐centre, open‐label, parallel‐group, active‐controlled, non‐inferiority trial Study period: June 2005 to April 2011 Recruitment sites: Conakry, Cotonou, Dakar, Durban, Nairobi Countries where the trial was undertaken: Benin, Guinea, Kenya, Senegal, South Africa Length of follow‐up: 24 months | |
| Participants | No of participants randomized: 1836 Intervention: 917 Control: 919 Age: mean age intervention 30.9 years, control 30.6 years Gender: male: Intervention 73%, control 72% Inclusion criteria:
Exclusion criteria:
Proportion with HIV seropositivity: 19% in the control regimen and 18% in the 4‐month regimen Proportion with cavitation: 50% in the control regimen and 52% in the 4‐month regimen Baseline drug resistance: excluded people with rifampicin resistance and MDR‐TB; isoniazid resistance: (gatifloxacin 8.5%; control 6.6%) | |
| Interventions | Intervention: 4‐month ATT regimen Gatifloxacin: gatifloxacin replacing ethambutol in intensive and continuation phases: N = 917 randomized; 791 eligible, 651 included in per‐protocol analysis (71% of those randomized, 82% of those eligible) 2 months of gatifloxacin, isoniazid, rifampicin, and pyrazinamide given daily, followed by 2 months of gatifloxacin, isoniazid, and rifampicin Control: 6‐month ATT regimen (N = 919 randomized; 784 eligible, 601 included in per‐protocol analysis (65% of those randomized; 77% of those eligible) 2 months of ethambutol, isoniazid, rifampicin, and pyrazinamide, followed by 4 months of isoniazid and rifampicin Dosage: Fixed‐dose combination tablets of isoniazid–rifampin, isoniazid–rifampin–pyrazinamide, or isoniazid–rifampin–pyrazinamide–ethambutol were used wherever needed. Gatifloxacin was given at a dose of 400 mg. Other drugs were given in weight‐based doses (< 50 kg, ≥ 50 kg) | |
| Outcomes | Outcomes reported and used in this review:
Outcomes sought for this review but not reported:
Outcomes reported and not used in this review:
| |
| Notes | Funding: Institut de Recherche pour le Développement (IRD) (on behalf of the OFLOTUB Consortium), World Health Organization (WHO). Lupin Pharmaceuticals provided study medicines Follow‐up method: trial drugs were administered orally under supervision 6 days a week during the intensive phase and were provided every 2 weeks in the continuation phase. Two sputum samples were obtained for smear examination, solid culture, and drug sensitivity tests at baseline and at all subsequent visits. Electrocardiograms (ECGs) were done at baseline, between 1 and 5 hours after drug intake, at 4 weeks, at 8 weeks, and at end of treatment Treatment supervision: trial drugs were given orally, 6 days a week under direct observation supervision during intensive phase and were provided every 2 weeks in the continuation phase to a supervisor who ensured treatment was taken. Adherence was assessed by pill count that remained in the weekly treatment boxes Trial Registration ID: NCT00216385 (retrospectively registered: September 2005) Acronym: OFLOTUB/gatifloxacin | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from report: "Patients were randomly assigned, in a 1:1 ratio with stratification according to country, to either a gatifloxacin‐containing regimen (experimental group) or the 6‐month standard treatment (control group)" Quote from protocol: "Randomization lists, stratified by study site and indicating a randomization number and which treatment is to be given, will be produced prior to the start of the trial by the medical statistician in London" |
| Allocation concealment (selection bias) | Low risk | Quote from protocol:"The Code for each individual will be provided in separate sealed envelopes and assigned to individuals in the order in which they are enrolled in the study" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote from protocol: "It must be noted that management of patients cannot be blinded, because of the difference in treatment length, but steps will be taken to ensure equal management and follow‐up of both treatment arms" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from protocol: "Lastly, when patients recruited in the trial come to the clinic with a suspicion of relapse, the treatment they received will be blinded to the physician examining them" Quote from protocol: "Laboratory technicians will be blinded to the origin of each sample, ensuring unbiased assessment of endpoints" |
| Incomplete outcome data (attrition bias): At the end of ATT (Treatment failure, positive sputum culture, treatment discontinuation, adverse events) | Low risk | Althought 35% of those eligible after randomization in the control arm and 29% in the intervention arm were excluded from the per‐protocol analyses, modified intention‐to‐treat analyses included 86% of those randomized to each arm. Results of modified intention‐to treat analyses and per‐protocol analyses were consistent and did not suggest that differential attrition significantly biased the relative estimates of effects |
| Incomplete outcome data (attrition bias): At the end of follow‐up (Relapse, deaths) | Low risk | Quote from report: "The cumulative percentage of patients retained in the experimental and control groups, respectively, was 93.5% and 93.4% by 52 weeks, 91.0% and 89.6% by 78 weeks, and 87.5% and 82.7% by 94 weeks" Comment: there was differential attrition over 24 months, but results of the per‐protocol and intention‐to‐treat analyses were consistent |
| Selective reporting (reporting bias) | Low risk | The trial was retrospectively registered. However all outcomes in the registration documents and changes in the protocol were documented and reported adequately, and did not indicate selective reporting |
| Other bias | Low risk | Quote from report: "Lupin Pharmaceuticals had no role in the conduct of the trial, the analysis of the data, or the preparation of the manuscript" |
| Methods | Study design: randomized, open‐label, parallel‐group, 5‐arm, active‐controlled trial Study period: patient recruitment commenced in May 2007 (interim results of an ongoing trial) Recruitment sites: Chennai, Madurai Country where the trial was undertaken: India Length of follow‐up: 24 months after treatment completion | |
| Participants | No. of participants randomized: 801 (of the 1650 anticipated) Combined intervention arms: 3 to 4 months moxifloxacin: N = 629 Control: 6 months ATT: N = 172 Age: < 35 years: moxifloxacin 52%; control 52% Gender: male: moxifloxacin 74%; control 77% Inclusion criteria:
Exclusion criteria:
Proportion with HIV seropositivity: nil (excluded) Proportion with cavitation: moxifloxacin 36%; control 41% Baseline drug resistance: isoniazid (moxifloxacin 7%; control 8%); ofloxacin (moxifloxacin 5%; control 7%); rifampicin, ethambutol, isoniazid, and ethambutol; isoniazid and ofloxacin (< 1% in both groups) | |
| Interventions | Interventions: 3‐ and 4‐month moxifloxacin regimens: moxifloxacin added to standard ATT drugs N = 629 randomized; 13 exclusions; 616 (98%) evaluated of those randomized
Control (6‐month regimen): N = 172 randomized: 8 exclusions; 164 (95%) evaluated of those randomized
Dosage: rifampicin 450 (< 60 kg) or 600 mg (> 60 kg); isoniazid 300 mg (daily) and 600 mg (thrice weekly); pyrazinamide 1500 mg; ethambutol 800 mg (daily) and 1200 mg (thrice weekly); moxifloxacin 400 mg | |
| Outcomes | Outcomes reported and used in this review:
Outcomes sought for the review but not reported:
| |
| Notes | Funding: Indian Council of Medical Research Follow‐up method: over the 24‐month follow‐up period, participants had monthly clinical examination and adherence and adverse events recording; monthly sputum microscopy and culture (and on days 15 and 45) and monthly drug susceptibility testing for 1 positive culture; monthly ECG, haemogram, liver and kidney functions, random blood sugars, and HIV ELISA tests. Chest X‐ray after the 2‐month intensive phase. Adverse events were assessed monthly for the duration of treatment (3 to 6 months) Treatment supervision: during the daily phase, treatment was under direct observation on 5 of 7 days of the week, whereas 2 doses were self‐administered. All thrice‐weekly phase doses were directly observed. Patients who missed treatment visits were visited at home and were motivated to attend the clinic for treatment Trial registration ID: CTRI 2008/091/000024 (retrospectively registered on 09/05/2008) Comment: results for sputum conversion at 2 months presented are the combined results of the 4 moxifloxacin regimens. Results for adverse events include up to the end of the 3‐ or 4‐month moxifloxacin regimens and over 6 months in the control regimen | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from report: "Restricted random allocation sequences generated using random number tables,separately for the 6 strata, were used to assign the regimens" |
| Allocation concealment (selection bias) | Low risk | Quote from trial registration document: "Sequentially numbered, sealed, opaque envelopes" |
| Blinding of participants and personnel (performance bias) | Low risk | This was an open‐label study but all treatment arms had supervised treatment and scheduled assessments for efficacy and safety outcomes |
| Blinding of outcome assessment (detection bias) | Low risk | The outcomes reported are sputum culture results at 2 months and drug adverse events that were assessed for all participants at specified time points. Sputum culture results and ECG reports are objective outcomes, and the likelihood of detection bias influencing the reporting of other adverse events is low |
| Incomplete outcome data (attrition bias): At the end of ATT (Treatment failure, positive sputum culture, treatment discontinuation, adverse events) | Low risk | 590 of 616 (96%) on moxifloxacin regimens and 151 of 162 (93%) on the control regimen had sputum cultures reported at 2 months. The 3% differential attrition is unlikely to have influenced the difference in proportions with negative sputum cultures at 2 months of 14.6% (95% CI 8.8% to 21.8%) |
| Incomplete outcome data (attrition bias): At the end of follow‐up (Relapse, deaths) | Low risk | Not reported, as the trial is ongoing and this report includes only interim outcomes |
| Selective reporting (reporting bias) | Low risk | This is an interim report of an ongoing trial. The outcomes presented were pre‐stated in the trial registration document |
| Other bias | Low risk | No other sources of bias were detected |
Abbreviations: ALT: alanine aminotransferase; ART: antiretroviral therapy; ARV: antiretroviral; AST: aspartate aminotransferase; ATT: anti‐tuberculosis treatment; CrCl: creatinine clearance; DM: diabetes mellitus; DMID: Division of Microbiology and Infectious Disease; ECG: electrocardiogram; ELISA: enzyme‐linked immunosorbent assay; IUCD: intrauterine contraceptive device; M tuberculosis: Mycobacterium tuberculosis; MDR‐TB: multi‐drug‐resistant tuberculosis; NIDDM: non‐insulin‐dependent diabetes mellitus; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| RCT comparing 3 months of rifampicin + isoniazid + ofloxacin versus a standard 6‐month regimen in people diagnosed with smear negative pulmonary tuberculosis Diagnosis of pulmonary tuberculosis was not confirmed by culture or GeneXpert | |
| Factorial RCT comparing 5 days a week versus 3 days a week treatment with moxifloxacin substituting ethambutol in the intensive phase of treatment with isoniazid, rifampicin, and pyrazinamide Not designed to compare treatments less than 6 months versus standard 6‐month regimen (phase 2b trial) | |
| RCT comparing moxifloxacin versus ethambutol in the intensive phase of treatment with rifampicin, isoniazid, and pyrazinamide Not designed to compare treatments less than 6 months versus standard 6‐month regimen (phase 2b trial) | |
| RCT comparing rifapentine plus moxifloxacin or rifampin plus ethambutol daily for 8 weeks, along with isoniazid and pyrazinamide Not designed to compare treatments less than 6 months versus standard 6‐month regimen (phase 2b trial) | |
| RCT comparing moxifloxacin versus isoniazid in the intensive phase of treatment with rifampicin, ethambutol, and pyrazinamide Not designed to compare treatments less than 6 months versus standard 6‐month regimen (phase 2b trial) | |
| RCT comparing levofloxacin added for the first 2 months to the standard 6‐month ATT regimen versus 6 to 9 months of standard ATT regimen No comparison with a regimen shorter than 6 months | |
| RCT in adults with newly diagnosed, sputum‐ or culture‐confirmed, non‐cavitary pulmonary tuberculosis who were culture negative after 4 months of daily treatment with 2HRZE + 2HR to stop treatment (4‐month treatment arm) or continue HR for 2 months Participants had already taken 4 months of ATT before randomization. Only those who were sputum‐negative were randomized, After randomization, participants received either no treatment or only 2 more months of HR | |
| Contolled trial comparing ofloxacin, rifampicin, and isoniazid with the regimen of ethambutol, rifampicin, and isoniazid given daily for 9 months No control arm with 2HRZE + 4HR (or 4HRE) or treatment arm with shorter regimens | |
| RCT (phase 2) comparing three 6‐month regimens with gatifloxacin, moxifloxacin, or ofloxacin given along with rifampicin, isoniazid, and pyrazinamide for the first 2 months followed by 4 months of isoniazid and rifampicin versus standard 2HRZE + 4HR regimens No arm with shorter regimens | |
| Controlled trial comparing 3 and 5 months of streptomycin, isoniazid, and pyrazinamide, with or without rifampicin No control arm with 2HRZE + 4HR (or 4HRE) | |
| RCT comparing 4 different regimens of ofloxacin, isoniazid, rifampicin, and pyrazinamide No control arm with 2HRZE + 4HR (or 4HRE) |
Abbreviations: ATT: anti‐tuberculosis treatment; E: ethambutol; H: isoniazid; R: rifampicin; RCT: randomized controlled trial; Z: pyrazinamide.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Randomized clinical trial to study the efficacy and tolerability of 3‐ and 4‐month regimens containing moxifloxacin in the treatment of patients with sputum smear and culture positive pulmonary tuberculosis |
| Methods | Randomized, open‐label, parallel‐group, 5‐arm, active‐controlled trial |
| Participants | Inclusion criteria:
Exclusion criteria:
Anticipated sample size: 1650 |
| Interventions | Intervention(s): Moxifloxacin arm: 4 regimens of 3 to 4 months 3 RHZEM 2 RHZEM/2 RHM 2 RHZEM/2 RHM thrice weekly 2 RHZEM/2 RHEM thrice weekly Dose: rifampicin 450 mg; isoniazid 300 mg (daily), 600 mg (thrice weekly); pyrazinamide 1500 mg; ethambutol 800 mg (daily), 1200 mg (thrice weekly); moxifloxacin 400 mg Control: 2 RHZE thrice weekly/4 RH thrice weekly (for 6 months) Dose: rifampicin 450 mg; isoniazid 600 mg; pyrazinamide 1500 mg; ethambutol 1200 mg |
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Starting date | 30 May 2007; anticipated study end date: May 2015; no results posted (last modified 06/02/2013) |
| Contact information | Dr MS Jawahar, Tuberculosis Research Centre, Mayor VR Ramanathan Road, Chetput, Chennai TAMIL NADU 600031 India. Tel: +91‐44‐28369500; Email: [email protected] |
| Notes | Study locations: Chennai and Madurai in India Registration number:CTRI/2008/091/000024 Primary sponsor: Indican Council of Medical Research Comment: the first author confirmed that this is completed, has been analysed, written up, submitted for publication, and will be re‐submitted after peer review |
| Trial name or title | Shorter treatment for minimal tuberculosis in children (SHINE study) |
| Methods | Parallel‐group, randomized, non‐inferiority, open‐label, 2‐arm, phase 3 clinical trial |
| Participants | Inclusion criteria:
Exclusion criteria:
Anticipated sample size: 1200 |
| Interventions | Intervention: 4‐month standard ATT regimen 8 weeks intensive Isoniazid (H), rifampicin (R), pyrazinamide (Z) with or without ethambutol (E) according to local practice, HRZ(E), followed by 8 weeks of continuation HR Control: 6‐month standard ATT regimen 8 weeks intensive HRZ(E), followed by 6 weeks of continuation HR |
| Outcomes | Primary outcome measures: • Efficacy: unfavourable outcome, defined by the composite endpoint of tuberculosis treatment failure, relapse (or re‐infection), or death • Safety: grade 3/4 adverse events Secondary outcome measures: • Mortality • Adverse drug reactions up to 30 days of completing treatment • Unfavourable outcome in those with definite tuberculosis • Suppressed HIV viral load at 24 and 48 weeks in HIV‐infected children starting ART, measured centrally on stored samples • Adherence and acceptability • Bacterial infection Anciliary studies will evaluate pharmacokinetics; cost/cost‐effectiveness implications of treatment shortening, and a nested qualitative substudy will investigate the ways in which health workers manage implementation of dose and weight band recommendations, particularly in children taking anti‐tuberculosis drugs and ARVs |
| Starting date | April 2015; anticipated end date: April 2019 (no longer recruiting) |
| Contact information | SHINE Trial Management Team MRC Clinical Trials Unit at UCL Institute of Clinical Trials and Methodology Aviation House, 125 Kingsway, London WC2B 6NH, UK Ph: +44 (0) 20 7670 4700 Email: [email protected] |
| Notes | Study locations: South Africa, India, Uganda, Zambia Registration number:ISRCTN63579542 Primary sponsors: University College London, Joint Global Health Trials Scheme: Department for International Development, the Wellcome Trust, the Medical Research Council, and Svizera Ltd |
| Trial name or title | Shortening treatment by advancing novel drugs (STAND) |
| Methods | Phase 3 open‐label, partially randomized, controlled clinical trial |
| Participants | Inclusion criteria:
Non‐childbearing potential:
Effective birth control methods:
Willing to continue practising birth control methods and not planning to conceive throughout treatment and for 12 weeks (male participants) or 1 week (female participants) after last dose of trial medication or discontinuation from trial medication in case of premature discontinuation Exclusion criteria:
Recruited sample size = 1500 |
| Interventions | Interventions:
Control:
(Additional MDR‐TB arm: moxifloxacin 400 mg + PA‐824 200 mg + pyrazinamide 1500 mg for 26 weeks) |
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Starting date | February 2015; completed May 2018 (no results posted) |
| Contact information | Stephen H Gillespie, MD School of Medicine, University of St. Andrews, North Haugh, St. Andrews KY16 9TF, United Kingdom Email: shg3@st‐andrews.ac.uk |
| Notes | Study locations: Georgia, Kenya, Malaysia, Philippines, South Africa, Tanzania, Uganda, Zambia Registration number:NCT02342886 Sponsors: Global Alliance for TB Drug Development |
| Trial name or title | Rifapentine‐containing tuberculosis treatment shortening regimens |
| Methods | Randomized, open‐label, parallel‐assignment, controlled phase 3 clinical trial |
| Participants | Inclusion criteria:
Exclusion criteria:
Anticipated sample size: 2500 |
| Interventions | Interventions: Standard ATT drugs: 4‐month (17 weeks) regimen 8 weeks of daily treatment with rifapentine, isoniazid, pyrazinamide, and ethambutol, followed by 9 weeks of daily treatment with rifapentine and isoniazid Moxifloxin combination: 4‐month (17 weeks) regimen: moxifloxacin for 4 months substituting ethambutol in intensive phase 8 weeks of daily treatment with rifapentine, isoniazid, pyrazinamide, and moxifloxacin, followed by 9 weeks of daily treatment with rifapentine, isoniazid, and moxifloxacin Control: standard 6‐month (26‐week) ATT regimen 8 weeks of daily treatment with rifampin, isoniazid, pyrazinamide, and ethambutol, followed by 18 weeks of daily treatment with rifampin and isoniazid Dosing: All drugs are administered orally, 7 days/week, directly observed by a healthcare worker at least 5 of the 7 days each week. Pyridoxine (vitamin B6), 25 or 50 mg, is administered with each study dose Study drug doses: rifampin 600 mg; isoniazid 300 mg; pyrazinamide < 55 kg 1000 mg, ≥ 55 to 75 kg 1500 mg, > 75 kg 2000 mg; ethambutol < 55 kg 800 mg, ≥ 55 to 75 kg 1200 mg, > 75 kg 1600 mg |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
Solid and liquid media considered separately:
Among participants with HIV infection receiving efavirenz‐based antiretroviral therapy, these values will be used to estimate steady state efavirenz PK parameters including mid‐dosing interval concentration |
| Starting date | January 2016; estimated study completion date: December 2019 |
| Contact information | Stefan Goldberg, Centers for Disease Control and Prevention. Ph: 404‐639‐5339; Email: [email protected] |
| Notes | Study locations: 38 including Brazil, China, India, and Malawi Registration number:NCT02410772 Sponsor: Centers for Disease Control and Prevention Collaborator: AIDS Clinical Trials Group |
| Trial name or title | An international multi‐centre controlled clinical trial to evaluate 1200 mg and 1800 mg rifampicin daily in the reduction of treatment duration for pulmonary tuberculosis from 6 months to 4 months (RIFASHORT) |
| Methods | Study type: interventional, phase 3; study design: open‐label 3‐arm trial |
| Participants | Inclusion criteria:
Exclusion criteria:
Anticipated sample size: 654 |
| Interventions | Interventions: 4‐month regimens Rifampicin 1200 mg combination ATT regimen 2 months of daily isoniazid and rifampicin Supplement of 450 mg (weight bands 35 to 39 kg and 40 to 54 kg) or 600 mg (weight band 55 to 69 kg and 70 and more kg) of rifampicin will be given throughout the 4 months (2EHR 1200Z/2HR1200) Rifampicin 1800 mg combination ATT regimen 2 months of daily isoniazid and rifampicin Supplement of 450 mg (weight bands 35 to 39 kg and 40 to 54 kg) or 600 mg (weight band 55 to 69 kg and 70 and more kg) of rifampicin will be given throughout the 4 months (2EHR1800Z/2HR1800) Control: Standard 6‐month ATT regimen 2 months of the standard regimen of isoniazid, pyrazinamide, and ethambutol plus 10 mg/kg rifampicin for the initial 8 weeks, followed by 4 months of isoniazid and rifampicin (at the same dose size) for an additional 4 months (2HRZE/4HR). |
| Outcomes | Primary outcomes:
Secondary outcomes
|
| Starting date | March 2017; estimated study completion date: December 2020; status: recruiting |
| Contact information | Eduardo Rómulo Chávez Ticona. Calle Rio Huaura Nro. 319, Pueblo Libre, Lima, Peru Pueblo Libre Lima LIMA Perú. Ph: 993560268; Email: [email protected] |
| Notes | Study locations: Bolivia, Botswana, Peru, Uganda Registration number:NCT02581527 Primary sponsor: St Georges University of London |
| Trial name or title | Shortened regimens for drug‐susceptible pulmonary tuberculosis |
| Methods | Multi‐centre, randomized, non‐inferiority, open‐label, controlled phase 3 clinical trial |
| Participants | Patients with newly diagnosed drug‐susceptible pulmonary TB who fulfil the inclusion and exclusion criteria Inclusion criteria:
Exclusion criteria:
Anticipated sample size: 3900 (1300 in each group) |
| Interventions | Interventions: Levofloxacin + ethambutol 4.5‐month combination ATT regimen 4.5 months of isoniazid, rifampin, pyrazinamide, ethambutol, and levofloxacin Dosage: isoniazid 300 mg (given once daily), rifampin 450 mg (less than 50 kg given once daily) or 600 mg (more than 50 kg given once daily), pyrazinamide 1500 mg ((less than 50 kg given once daily) or 30 mg/kg (more than 50 kg once daily), ethambutol 750 mg (less than 50 kg once daily) or 1000 mg (more than 50 kg once daily), levofloxacin 600 mg (less than 50 kg given once daily) or 800 mg (more than 50 kg once daily) Ethambutol 4.5‐month combination ATT regimen 4.5 months of isoniazid, rifampin, pyrazinamide, and ethambutol. Dosage of isoniazid, rifampin, pyrazinamide, and ethambutol is same as that of control regimen Control: Standard 6‐month ATT regimen 2 months of isoniazid, rifampin, pyrazinamide, and ethambutol, followed by 4 months of isoniazid and rifampin Dosage: isoniazid 300 mg (given once daily), rifampin 450 mg (less than 50 kg given once daily) or 600 mg (more than 50 kg given once daily), pyrazinamide 1500 mg (less than 50 kg given once daily) or 30 mg/kg (more than 50 kg once daily), ethambutol 750 mg (less than 50 kg once daily), or 1000 mg (more than 50 kg, once daily) |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | August 2016; estimated study completion date: December 2018 |
| Contact information | Shenjie Tang, MD. Beijing Chest Hospital Email: [email protected] |
| Notes | Study location: China Registration number:NCT02901288 Primary sponsors: Beijing Chest Hospital, Hubei Provincial Center for Disease Control and Prevention |
Abbreviations: AFB: acid‐fast bacilli; ALP: alkaline phosphatase; ALT: alanine aminotransferase; ART: antiretroviral therapy; ARV: antiretroviral; AST: aspartate aminotransferase; ATT: anti‐tuberculosis treatment; CrCl: creatinine clearance; E: ethambutol; ECG: electrocardiogram; FSH: follicle‐stimulating hormone; H: isoniazid; HbA1c: glycosylated haemoglobin; IUCD: intrauterine coil device; LH: luteinizing hormone; M: moxifloxacin; MDR‐TB: multi‐drug‐resistant tuberculosis; MGIT: mycobacterial growth indicator tube; MIC: minimum inhibitory concentration; MTB: Mycobacterium tuberculosis; PK: pharmacokinetics; R: rifampicin; TB: tuberculosis; TEAE: treatment‐emergent adverse event; TTP: time to positivity; U‐HCG: urine human chorionic gonadotropin; ULN: upper limit of normal; WHO: World Health Organization; XDR: extensively drug‐resistant; Z: pyrazinamide.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse Show forest plot | 3 | 2265 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.56 [2.37, 5.37] |
| Analysis 1.1  Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 1 Relapse. | ||||
| 2 Relapse: subgroup analysis Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 2 Relapse: subgroup analysis. | ||||
| 2.1 Moxifloxacin replacing ethambutol | 2 | 1386 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.74 [1.69, 4.43] |
| 2.2 Moxifloxacin replacing isoniazid | 2 | 1424 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.89 [3.02, 7.92] |
| 3 Relapse: sensitivity analysis accounting for missing data Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 3 Relapse: sensitivity analysis accounting for missing data. | ||||
| 3.1 Modified‐ITT analysis | 3 | 2265 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.56 [2.37, 5.37] |
| 3.2 Per‐protocol analysis | 3 | 2135 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.79 [2.48, 5.78] |
| 3.3 Imputing missing data | 3 | 2524 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.83 [2.58, 5.70] |
| 4 Death from any cause Show forest plot | 3 | 2760 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.65, 1.75] |
| Analysis 1.4  Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 4 Death from any cause. | ||||
| 5 Treatment discontinuation Show forest plot | 3 | 2335 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.78, 1.61] |
| Analysis 1.5  Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 5 Treatment discontinuation. | ||||
| 6 Positive sputum culture/smear at 8 weeks Show forest plot | 3 | 2828 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.22, 1.13] |
| Analysis 1.6  Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 6 Positive sputum culture/smear at 8 weeks. | ||||
| 6.1 Moxifloxacin replacing isoniazid or ethambutol in 4‐month ATT regimen | 2 | 2087 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.45, 1.20] |
| 6.2 Moxifloxacin augmenting standard 6‐month ATT regimen | 1 | 741 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.15, 0.39] |
| 7 Treatment failure Show forest plot | 3 | 2282 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.33, 1.52] |
| Analysis 1.7  Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 7 Treatment failure. | ||||
| 8 Acquired drug resistance Show forest plot | 3 | 2282 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.08, 1.31] |
| Analysis 1.8  Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 8 Acquired drug resistance. | ||||
| 9 Serious adverse events Show forest plot | 4 | 3548 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.74, 1.27] |
| Analysis 1.9  Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 9 Serious adverse events. | ||||
| 9.1 Moxifloxacin replacing standard drugs in 4‐month ATT regimens | 3 | 2760 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.72, 1.26] |
| 9.2 Moxifloxacin augmenting standard 6‐month ATT regimens | 1 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.45, 3.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse Show forest plot | 2 | 1633 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [1.56, 2.84] |
| Analysis 2.1  Comparison 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 1 Relapse. | ||||
| 2 Relapse: sensitivity analysis accounting for missing data Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 2 Relapse: sensitivity analysis accounting for missing data. | ||||
| 2.1 Modified‐ITT analysis | 2 | 1633 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [1.56, 2.84] |
| 2.2 Per‐protocol analysis | 2 | 1525 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [1.55, 2.87] |
| 2.3 Modified‐ITT analysis (all eligible participants ‐ imputing missing data) | 2 | 1851 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [1.53, 2.63] |
| 3 Death from any cause Show forest plot | 2 | 1886 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.53, 1.53] |
| Analysis 2.3  Comparison 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 3 Death from any cause. | ||||
| 4 Treatment discontinuation Show forest plot | 2 | 1657 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.46, 1.08] |
| Analysis 2.4  Comparison 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 4 Treatment discontinuation. | ||||
| 5 Positive sputum culture at 8 weeks Show forest plot | 2 | 1818 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.80, 1.23] |
| Analysis 2.5  Comparison 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 5 Positive sputum culture at 8 weeks. | ||||
| 6 Treatment failure Show forest plot | 2 | 1657 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.51, 1.70] |
| Analysis 2.6  Comparison 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 6 Treatment failure. | ||||
| 7 Acquired drug resistance Show forest plot | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.01, 5.01] |
| Analysis 2.7  Comparison 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 7 Acquired drug resistance. | ||||
| 8 Serious adverse events Show forest plot | 2 | 1993 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.58, 1.77] |
| Analysis 2.8  Comparison 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 8 Serious adverse events. | ||||
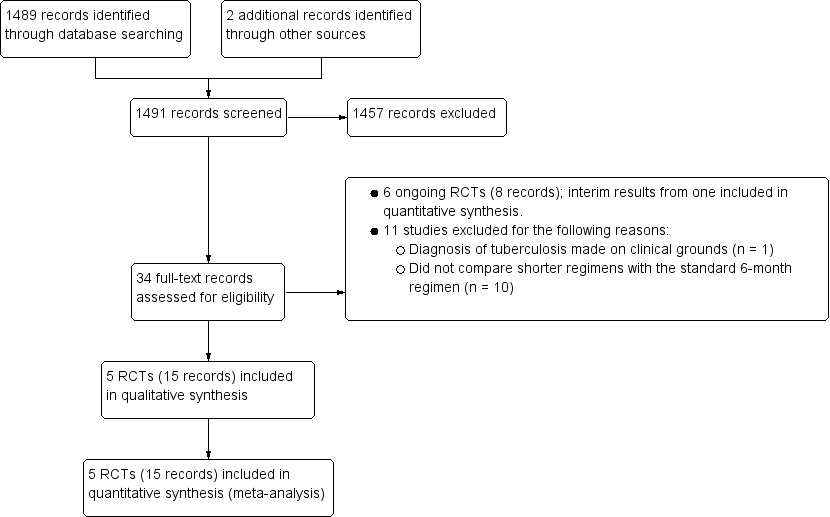
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
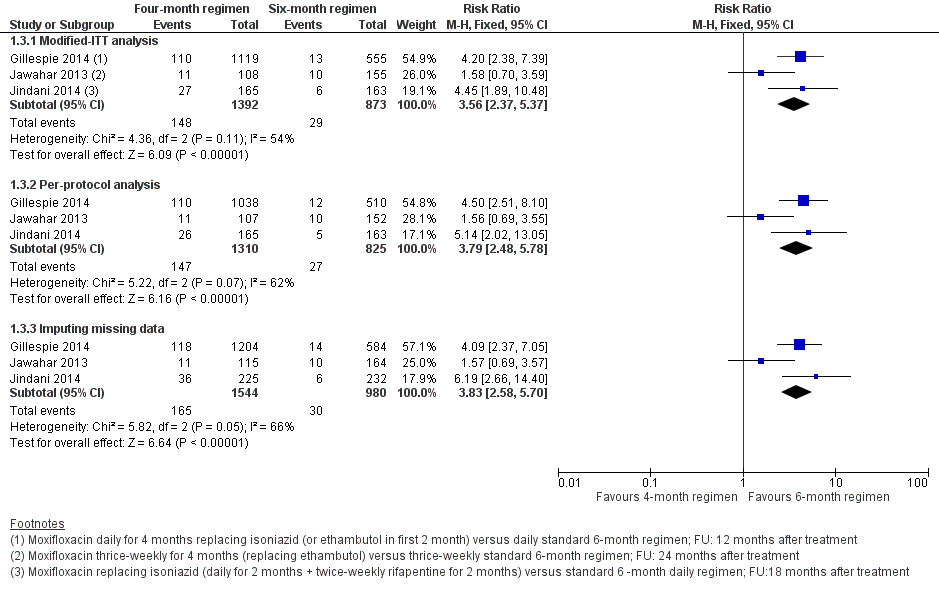
Forest plot of comparison: 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, outcome: 1.3 Relapse: sensitivity analysis accounting for missing data.

Forest plot of comparison: 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, outcome: 2.2 Relapse: sensitivity analysis accounting for missing data.
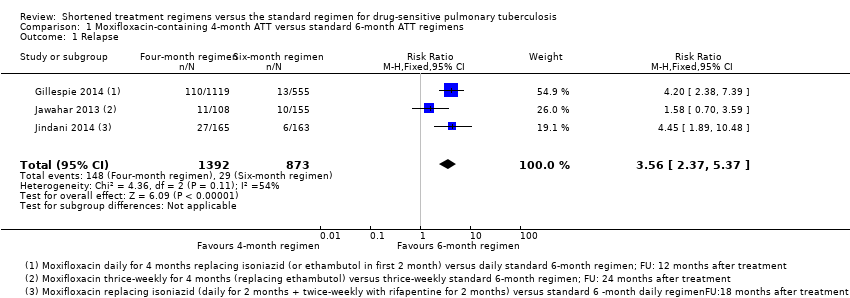
Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 1 Relapse.

Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 2 Relapse: subgroup analysis.
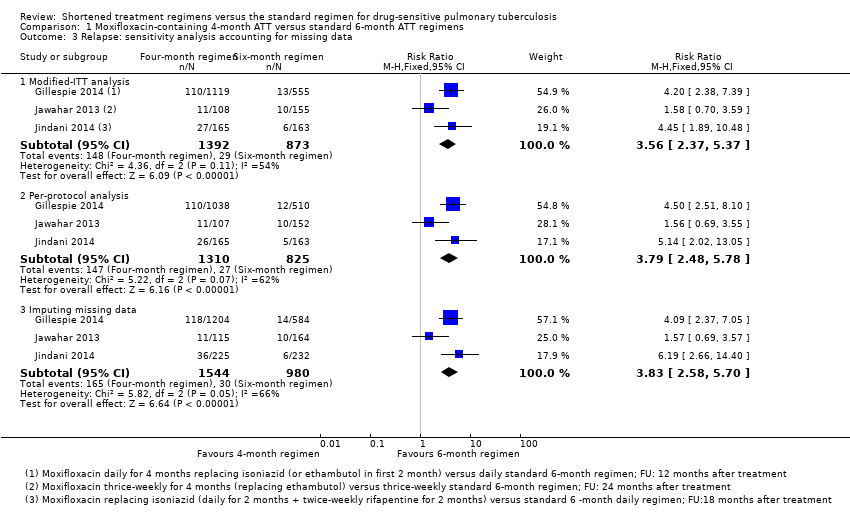
Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 3 Relapse: sensitivity analysis accounting for missing data.

Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 4 Death from any cause.

Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 5 Treatment discontinuation.

Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 6 Positive sputum culture/smear at 8 weeks.
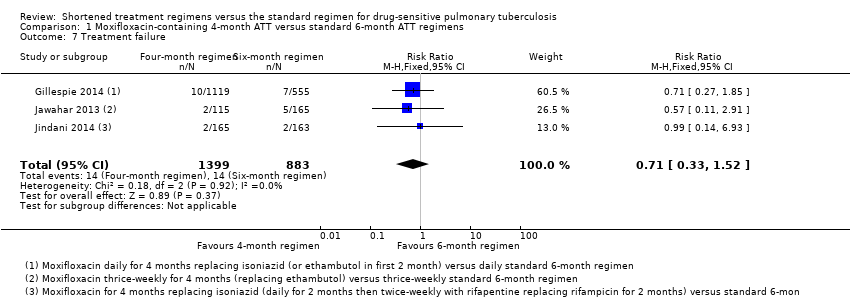
Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 7 Treatment failure.

Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 8 Acquired drug resistance.

Comparison 1 Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 9 Serious adverse events.

Comparison 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 1 Relapse.

Comparison 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 2 Relapse: sensitivity analysis accounting for missing data.

Comparison 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 3 Death from any cause.

Comparison 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 4 Treatment discontinuation.

Comparison 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 5 Positive sputum culture at 8 weeks.

Comparison 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 6 Treatment failure.

Comparison 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 7 Acquired drug resistance.
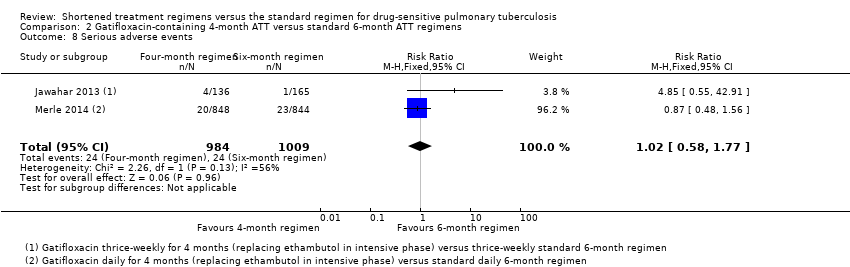
Comparison 2 Gatifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimens, Outcome 8 Serious adverse events.
| Moxifloxacin‐containing 4‐month ATT versus standard 6‐month ATT regimen for drug‐sensitive pulmonary tuberculosis | ||||||
| Patient or population: adults with drug‐sensitive pulmonary tuberculosis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with 6‐month standard ATT | Risk with 4‐month moxifloxacin‐containing ATT | |||||
| Relapse | 32 per 1000 | 82 more relapses per 1000 | RR 3.56 | 2265 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably increases relapse compared to the 6‐month regimen |
| Death from any cause Follow‐up: range 18 to 24 months | 21 per 1000 | 2 more deaths per 1000 | RR 1.06 | 2760 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably makes little or no difference in death from any cause compared to the 6‐month regimen |
| Treatment failure | 16 per 1000 | 5 fewer treatment failures per 1000 | RR 0.71 | 2282 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably results in little or no difference in treatment failure compared to the 6‐month regimen |
| Acquired drug resistance | 7 per 1000 | 5 fewer with acquired drug resistance per 1000 (6 fewer to 2 more) | RR 0.33 | 2282 (3 RCTs)e | ⊕⊕⊝⊝ Due to indirectness and imprecision | The 4‐month regimen may be little or no different than the 6‐month regimen in the incidence of acquired drug resistance |
| Serious adverse events Follow‐up: range 18 to 24 months | 62 per 1000 | 2 fewer with serious adverse events per 1000 | RR 0.97 | 3548 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably results in little or no difference in serious adverse events compared to the 6‐month regimen |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aNo serious risk of bias: although Jawahar 2013 was at high risk of allocation bias, exclusion of this trial from the sensitivity analysis did not change the direction of effect. Not downgraded. | ||||||
| Gatifloxacin‐containing 4‐month ATT regimens compared to standard 6‐month ATT regimens for drug‐sensitive pulmonary tuberculosis | ||||||
| Patient or population: adults with drug‐sensitive pulmonary tuberculosis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with 6‐month standard ATT | Risk with gatifloxacin‐containing | |||||
| Relapse | 70 per 1000 | 77 more relapses per 1000 | RR 2.11 | 1633 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably increases relapse compared to the 6‐month regimen |
| Death from any cause | 29 per 1000 | 3 fewer deaths per 1000 | RR 0.90 | 1886 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably makes little or no difference in death compared to the 6‐month regimen |
| Treatment failure | 25 per 1000 | 1 less treatment failure per 1000 | RR 0.93 | 1657 | ⊕⊕⊝⊝ Due to indirectness | The 4‐month regimen probably makes little or no difference in treatment failure compared to the 6‐month regimen |
| Acquired drug resistance | 12 per 1000 | 9 fewer with acquired drug resistance per 1000 (12 fewer to 49 more) | RR 0.24 (0.01 to 5.01) | 301 (1 RCT)d | ⊕⊝⊝⊝ Due to indirectness, risk of bias, and imprecision | We do not know if acquired drug resistance is any different in the 4‐month and the 6‐month regimens |
| Serious adverse events | 24 per 1000 | 0 fewer serious adverse events per 1000 | RR 1.02 | 1993 | ⊕⊕⊕⊝ Due to indirectness | The 4‐month regimen probably results in little or no difference in serious adverse events compared to the 6‐month regimen |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. | ||||||
| aNo serious risk of bias: although Jawahar 2013 was assigned high risk of bias for allocation concealment, removal of this trial from the sensitivity analysis did not significantly alter the direction, magnitude, or precision of the effect estimate. Not downgraded. | ||||||
| Study ID (Acronym) | (REMoxTB) | (RIFAQUIN) | (OFLOTUB) | |||||||
| Setting | Multiple sites in Africa (Kenya, South Africa, Tanzania, Zambia), Asia (China, India, Malaysia Thailand), Latin America (Mexico) | 6 sites in 2 cities in India | 6 cities in 4 countries in Africa (Botswana, South Africa, Zambia, Zimbabwe) | 5 countries in Africa (Benin, Guinea, Kenya, Senegal, South Africa) | 2 cities in India | |||||
| Participants | ||||||||||
| Number randomized | 1931 | 429 | 827 | 1836 | 801 | |||||
| Age | Adults (> 18 years) | Adults (> 18 years) | Adults (> 18 years) | Adults (18 to 65 years) | Adults (> 18 years) | |||||
| HIV infection | Included (if CD4 count > 250 cells/μL and not on ART); 110 (7%) | Excluded | Included (if CD4 count > 150/mm³ and not on ART; 158 (27%) | Included if not stage 3 or 4 disease and not on ART; 304 (17%) | Excluded | |||||
| Diagnosis of TB | Positive sputum smears on 2 occasions Culture‐confirmed susceptibility to rifampicin, isoniazid, pyrazinamide, and moxifloxacin | Newly diagnosed pulmonary TB with at least 2 positive sputum cultures. Confirmed by culture and MDR‐TB excluded, susceptibility to ofloxacin (as proxy for moxifloxacin) | 2 sputum samples that were positive for tubercle bacilli on direct smear microscopy No resistance to isoniazid, rifampicin, or moxifloxacin | Acid‐fast bacilli in 2 consecutive sputum smears; confirmed by culture (solid medium) and drug sensitivity tests to rifampicin, isoniazid, ethambutol, streptomycin, and gatifloxacin | 2 positive sputum smear smears for tuberculosis. Culture‐confirmed and MDR‐TB ruled out; susceptible to ofloxacin (as proxy for moxifloxacin) | |||||
| Intervention(s) and comparator | ||||||||||
| Duration of ATT | 4 monthsa | 6 months | 4 monthsb | 6 months | 4 months | 6 monthsc | 4 months | 6 months | 4 monthsa | 6 months |
| Regimens | 2HRZM/2HRM + 2MRZE/2MR | 2HRZE/4HR | 2(HRZG)₃/ 2 (HRG)₃ 2(HRZM)₃/2(HRM)₃ | 2(HRZE)₃ /4(HR)₃ | 2MRZE/ 2P₂M₂ | 2HRZE/ 4HR | 2HRZG/ 2HRG | 2HRZE/4HR | 3HRZM + 2HRZM/ 2RHM + 2HRZM/ 2(RHM)₃ + 2HRZM/ 2(RHEM)₃ | 2(HRZE)₃/ 4(HR)₃ |
| Number allocated | 655 + 636 = 1291 | 640 | 141 118 | 170 | 275 | 275 | 917 | 919 | 629 | 172 |
| Late screening failures excluded after allocation | 38 + 32 = 70 | 40 | 5 3 | 5 | 36 | 35 | 62 | 51 | 13 | 8 |
| Number eligible | 1231 | 600 | 136 115 | 165 | 239 | 240 | 852 | 868 | 616 | 164 |
| Number analysed in m‐ITT analysis (% of those allocated) | 568 + 551 = 1119 (87) | 555 (87) | 136 (97) 115 (98) | 165 (97) | 193 (70) | 188 (68) | 791 (86) | 794 (86) | 590 (94) | 151 (88) |
| Number analysed in per‐protocol analysis (% of those allocated) | 514 + 524 = 1038 (80) | 510 (80) | 131 (93) 113 (96) | 159 (94) | 165 (60) | 163 (59) | 651 (71) | 601 (65) | As above | |
| Number analysed in ancillary analysis (ITT) (% of those allocated) | 617 + 604 = 1221 (94) | 600 (94) | Not done | 239 (87) | 240 (87) | Not reported | Not reported | |||
| Outcomes reported | ||||||||||
| Relapse | Relapse within 18 months after randomization in those with negative culture with treatment. Relapse strains were those shown to be identical on 24‐locus MIRU analysis LJ solid media and MGIT liquid media used for culture | Recurrence of TB over 24 months after treatment in those with a favourable response with treatment: either bacteriologic recurrence (LJ solid media) or clinical/radiologic recurrence | Relapse within 12 to 18 months after treatment. Two positive cultures within a period of 4 months without an intervening negative culture). Re‐infections differentiated from relapse through genotyping (MIRU‐VNTRs) LJ solid media used for culture in some centres, MGIT liquid media in others, and both in some centres | Recurrence of TB over 24 months after treatment proven bacteriologically (2 consecutive positive sputum samples a day apart) or clinically Genotyping (MIRU‐VNTRs) results available for only 70/140 (55%) of those with culture confirmed recurrence. Most were relapses | Not reported | |||||
| Deaths | All deaths TB deaths | Reported (only non‐TB deaths occurred) | All deaths TB deaths | Death during treatment Death after treatment | Not reported | |||||
| Treatment discontinuation | Includes those who did not complete treatment, relocated, or withdrew consent | Includes those who did not complete treatment and those lost to follow‐up | Includes change in treatment due to adverse events, loss to follow‐up, and other treatment changes | Includes those who withdrew consent during treatment and dropouts | Reported but disaggregated data for each group not available | |||||
| Positive smear/ sputum culture at 2 months | Reported using LJ solid media (used in this review) and MGIT liquid media for all randomized participants excluding late screening failures | Reported using LJ solid media for all randomized participants excluding late screening failures | Reported but disaggregated data for moxifloxacin 4‐month and 6‐month treatment groups not available Data also not available for all participants from LJ media | Reported for 752 in the 4‐month and 759 in the 6‐month regimens (88% and 87% of those eligible, respectively) Culture using LJ solid media | Reported for 590 (94%) in the 4‐month and 151 (88%) in the 6‐month regimens | |||||
| Acquired drug resistance | Reported | Reported | Reported | Not reported | Not reported | |||||
| Treatment failure | Includes culture confirmed and not confirmed | Includes culture confirmed and unconfirmed | Culture confirmed | Includes culture confirmed failure | Not reported | |||||
| Serious adverse events | Reported for all randomized participants excluding late screening failures. Grade 3 and 4 severity (DAIDS 2009) | Deduced from adverse events reported for all randomized participants excluding late screening failures. Not graded | Reported for all participants randomized who took 1 dose and assessed as severe or life‐threatening during and 2 weeks after treatment. grade 3 and 4 severity (DAIDS 2009) | Reported for 1692 (92%) of all randomized participants. grade 3 and 4 severity (DAIDS 2009) | Deduced from adverse events reported. Not graded | |||||
| Other adverse events | Not reported | Reported | Not reported | QT prolongation Hyperglycaemic episodes | Reported | |||||
| Abbreviations: ART: anti‐retroviral treatment; ATT: anti‐tuberculosis treatment; E: ethambutol; G: gatifloxacin; H: isoniazid; ITT: intention‐to‐treat; LJ: Löwenstein‐Jensen; M: moxifloxacin; MGIT: mycobacterial growth indicator tube; MIRU‐VNTRs: mycobacterial interspersed repetitive unit–variable number tandem repeats; m‐ITT: modified intention‐to‐treat; P: rifapentine; R: rifampicin; Z: pyrazinamide. Leading numbers in regimens indicate duration in months. Drugs were administered daily, except when given thrice weekly as indicated by subscripts. aData from moxifloxacin‐containing shortened regimens combined for data synthesis. | ||||||||||
| Primary outcome: relapse | ||||||
| Trial ID | ||||||
| Regimens | 4 months | 6 months | 4 months | 6 months | 4 months | 6 months |
| aModified‐ITT analysis (primary analysis) | 110/1119 (9.8%) | 13/555 (2.3%) | 11/108 (10.1%) | 10/155 (6.5%) | 27/165 (16.4%) | 6/163 (3.7%) |
| aPer‐protocol analysis | 110/1038 (10.6%) | 12/510 (2.4%) | 11/107 (10.1%) | 10/152 (6.6%) | 26/165 (15.8%) | 5/163 (3.1%) |
| bSensitivity analysis imputing missing data | 126/1184 (10.7%) | 14/577 (2.4%) | 11/114 (9.7%) | 10/159 (6.3%) | 36/225 (16.0%) | 71/232 (2.6%%) |
| Abbreviations: ATT: anti‐tuberculosis treatment; ITT: intention‐to‐treat. aAs reported in trial reports. | ||||||
| Primary outcome: relapse | ||||
| Trial ID | ||||
| Regimen | 4 months | 6 months | 4 months | 6 months |
| aModified‐ITT analysis (primary analysis) | 19/122 (15.6%) | 10/155 (6.5%) | 101/694 (14.6%) | 47/662 (7.1%) |
| aPer‐protocol analysis | 19/121 (15.7%) | 10/152 (6.6%) | 98/651 (15.1%) | 44/601 (7,3%) |
| bSensitivity analysis imputing missing data | 19/132 (14.4%) | 10/159 (6.3%) | 122/786 (15.5%) | 61/774 (7,9%) |
| Abbreviations: ATT: anti‐tuberculosis treatment; ITT: intention‐to‐treat. aAs reported in trial reports. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse Show forest plot | 3 | 2265 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.56 [2.37, 5.37] |
| 2 Relapse: subgroup analysis Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Moxifloxacin replacing ethambutol | 2 | 1386 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.74 [1.69, 4.43] |
| 2.2 Moxifloxacin replacing isoniazid | 2 | 1424 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.89 [3.02, 7.92] |
| 3 Relapse: sensitivity analysis accounting for missing data Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Modified‐ITT analysis | 3 | 2265 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.56 [2.37, 5.37] |
| 3.2 Per‐protocol analysis | 3 | 2135 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.79 [2.48, 5.78] |
| 3.3 Imputing missing data | 3 | 2524 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.83 [2.58, 5.70] |
| 4 Death from any cause Show forest plot | 3 | 2760 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.65, 1.75] |
| 5 Treatment discontinuation Show forest plot | 3 | 2335 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.78, 1.61] |
| 6 Positive sputum culture/smear at 8 weeks Show forest plot | 3 | 2828 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.22, 1.13] |
| 6.1 Moxifloxacin replacing isoniazid or ethambutol in 4‐month ATT regimen | 2 | 2087 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.45, 1.20] |
| 6.2 Moxifloxacin augmenting standard 6‐month ATT regimen | 1 | 741 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.15, 0.39] |
| 7 Treatment failure Show forest plot | 3 | 2282 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.33, 1.52] |
| 8 Acquired drug resistance Show forest plot | 3 | 2282 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.08, 1.31] |
| 9 Serious adverse events Show forest plot | 4 | 3548 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.74, 1.27] |
| 9.1 Moxifloxacin replacing standard drugs in 4‐month ATT regimens | 3 | 2760 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.72, 1.26] |
| 9.2 Moxifloxacin augmenting standard 6‐month ATT regimens | 1 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.45, 3.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse Show forest plot | 2 | 1633 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [1.56, 2.84] |
| 2 Relapse: sensitivity analysis accounting for missing data Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Modified‐ITT analysis | 2 | 1633 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [1.56, 2.84] |
| 2.2 Per‐protocol analysis | 2 | 1525 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [1.55, 2.87] |
| 2.3 Modified‐ITT analysis (all eligible participants ‐ imputing missing data) | 2 | 1851 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [1.53, 2.63] |
| 3 Death from any cause Show forest plot | 2 | 1886 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.53, 1.53] |
| 4 Treatment discontinuation Show forest plot | 2 | 1657 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.46, 1.08] |
| 5 Positive sputum culture at 8 weeks Show forest plot | 2 | 1818 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.80, 1.23] |
| 6 Treatment failure Show forest plot | 2 | 1657 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.51, 1.70] |
| 7 Acquired drug resistance Show forest plot | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.01, 5.01] |
| 8 Serious adverse events Show forest plot | 2 | 1993 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.58, 1.77] |

