MRI pathway and TRUS‐guided biopsy for detecting clinically significant prostate cancer
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012663Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 24 mayo 2017see what's new
- Tipo:
-
- Diagnostic
- Etapa:
-
- Protocol
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Urología
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Frank‐Jan H Drost (FD), Ivo G Schoots (IS) and Monique J Roobol (MR) all initiated the review and wrote the protocol. FD and IS will conduct the literature search, review abstracts and full‐text studies for inclusion, and perform quality assessment, data extraction, analysis and writing of the review. MR will assist with the selection of studies and quality assessment and will contribute to the writing of the review. Daan Nieboer will perform the statistical analysis. Chris H Bangma, Ewout W Steyerberg and MG Myriam Hunink critically evaluated the protocol and will provide general advice on the review.
Declarations of interest
Ivo G Schoots: None known.
Monique J Roobol: None known.
Frank‐Jan Hendrik H Drost: None known.
Chris H Bangma: None known.
Ewout W Steyerberg reports the following relevant financial activities outside the submitted work: receives royalties from Springer for the textbook entitled Clinical Prediction Models.
MG Myriam Hunink reports the following relevant financial activities outside the submitted work: receives royalties from Cambridge University Press for the textbook entitled, Decision Making in Health and Medicine; received travel support from the European Society of Radiology (ESR), the International Society for Strategic Studies in Radiology (ISSSR), and the European Institute for Biomedical Imaging Research (EIBIR).
Acknowledgements
The authors thank Mr. WM Bramer, information specialist, Medical Library, Erasmus University Medical Center, Rotterdam, for conducting the systematic literature search.
Version history
| Published | Title | Stage | Authors | Version |
| 2019 Apr 25 | Prostate MRI, with or without MRI‐targeted biopsy, and systematic biopsy for detecting prostate cancer | Review | Frank‐Jan H Drost, Daniël F Osses, Daan Nieboer, Ewout W Steyerberg, Chris H Bangma, Monique J Roobol, Ivo G Schoots | |
| 2017 May 24 | MRI pathway and TRUS‐guided biopsy for detecting clinically significant prostate cancer | Protocol | Frank‐Jan H Drost, Monique J Roobol, Daan Nieboer, Chris H Bangma, Ewout W Steyerberg, M G Myriam Hunink, Ivo G Schoots | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans; Male;
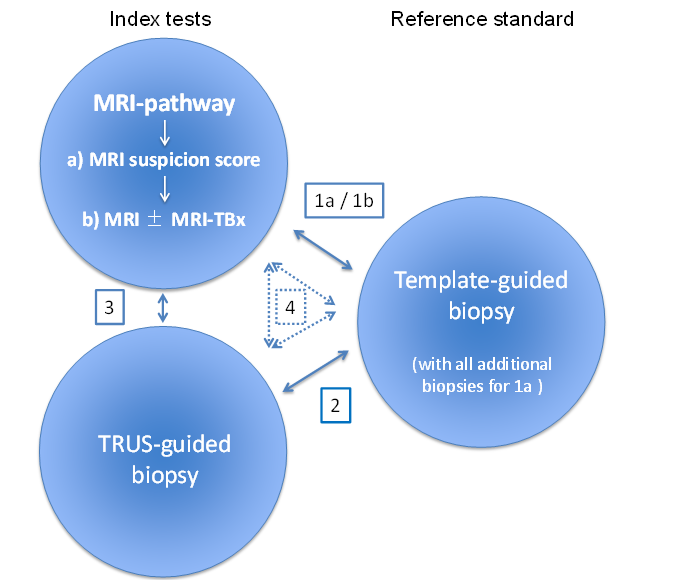
The MRI pathway comprises two components: MRI (suspicion score) and MRI‐targeted biopsy in the presence of suspicious lesions for clinically significant prostate cancer:
-
a) MRI suspicion score: refers to only the first component of the MRI pathway.
-
b) MRI±MRI‐TBx: refers to both components of the MRI pathway.
Review design
Meta‐analysis of studies reporting on:
The MRI pathway and the reference standard: ·
-
Comparison 1a: MRI suspicion score vs reference standard and all additional biopsies (e.g. MRI‐targeted biopsies).
-
Comparison 1b: MRI±MRI‐TBx vs reference standard.
TRUS‐guided biopsy and reference standard: ·
-
Comparison 2
MRI pathway B) MRI±MRI‐TBx and TRUS‐guide biopsy:
-
Comparison 3
A combination of all tests:
-
Comparison 4: comparing both index tests, either directly with the reference standard or indirectly as a randomised trial of test accuracy.
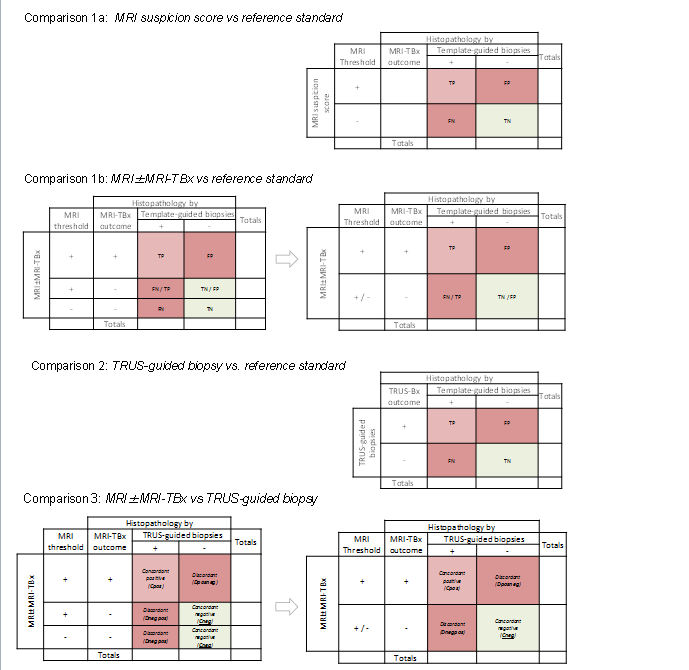
Two‐by‐two contingency tables for target condition.
Comparison 1a: MRI suspicion score vs reference standard (if MRI‐targeted biopsies are performed these will serve as additional reference biopsies).
Comparison 1b: MRI±MRI‐TBx vs reference standard.
Comparison 2: TRUS‐guided biopsy vs reference standard.
Comparison 3: MRI±MRI‐TBx vs TRUS‐guided biopsy.
For comparison 1b and comparison 3, a negative test can result in two ways:
-
a negative MRI (thus no MRI‐targeted biopsies are taken).
-
a positive MRI but negative MRI‐targeted biopsy result.
Both negative outcomes should be merged, creating a two‐by‐two from a three‐by‐two contingency table (first and second column).
In comparison 3 the TRUS‐guided biopsy is not a proper reference standard. Therefore we will focus on the number of cancers identified and the concordance and disconcordance between both index tests.
TP = true positive; FP = false positive; TN = true negative; FN = false negative; Dposneg = Discordant MRI positive + targeted biopsies positive and TRUS‐guided biopsies negative; Dnegpos = Discordant MRI positive/negative + targeted biopsies negative and TRUS‐guided biopsies positive.

