MRI pathway and TRUS‐guided biopsy for detecting clinically significant prostate cancer
Appendices
Appendix 1. QUADAS‐2 tool for assessing methodological quality of included studies
| Domain 1: PATIENT SELECTION | |
| Signalling question (SQ) 1: Was a consecutive or random sample of patients enrolled? | Yes/No/Unclear |
| SQ 2: Did the study avoid inappropriate exclusions? The study:
| Yes/No/Unclear |
| RISK OF BIAS – Could the selection of patients have introduced bias? Low risk if ‘Yes’ for all SQs. High risk if ‘No’ for at least one SQ. Unclear risk if ‘Unclear’ for at least one SQ. | Risk: Low/High/Unclear |
| CONCERNS FOR APPLICABILITY – Is there concern that the included patients do not match the review question? a) The cohort is comprised of patients tested for primary diagnosis or patients with a previous negative prostate biopsy. b) The number of previous prostate biopsies is limited to 3, with none being a TTMB or TSB with more than 19 cores. | Concern: Low/High/Unclear Low: a) and b) Yes High: a) or b) No Unclear: a) or b) Unclear |
| Domain 2: INDEX TESTS | |
| SQ 1: If applicable, was the MRI assessed without knowledge of the results of the reference standard? | Yes/No/Unclear |
| SQ 2: If applicable, was the MRI‐targeted biopsy performed without knowledge of the results nor influenced by the performance of the reference standard (i.e. influenced by template‐guided biopsy trajectory)? | Yes/No/Unclear |
| SQ 3: If applicable, was the TRUS‐guided biopsy performed without knowledge of the results nor influenced by the performance of the reference standard? | Yes/No/Unclear |
| SQ 4: When comparing the index tests, was the TRUS‐guided biopsy performed without knowledge of MRI results or MRI‐targeted biopsy core trajectory? | Yes/No/Unclear |
| SQ 5: If applicable, are the MRI score threshold for MRI‐targeted biopsy and the definition of clinically significant prostate cancer prespecified? | Yes/No/Unclear |
| RISK OF BIAS – Could the conduct or interpretation of the index test have introduced bias? Low risk if ‘Yes’ for all applicable SQs. High risk if ‘No’ for at least one applicable SQ. Unclear risk if ‘Unclear’ for at least one applicable SQ. | Risk: Low/High/Unclear |
| CONCERNS FOR APPLICABILITY ‐ Are there concerns that the index tests, their conduct or their interpretation differ from the review question? a) An extended sextant TRUS‐guided biopsy is performed, with 8 to 19 cores, distributed appropriately to sample the peripheral zone. b) The median number of MRI‐guided biopsy cores taken is between 2 and 5. | Concern: Low/High/Unclear Low: Yes High: No Unclear: Unclear |
| Domain 3: REFERENCE STANDARD | |
| SQ1: Is the reference standard likely to correctly classify the target condition? (i.e. Is histological diagnosis made from appropriately sampled tissue?)
| Yes/No/Unclear |
| SQ2: Was the reference standard performed without knowledge of the results of the index test? | Yes/No/Unclear |
| RISK OF BIAS ‐ Could the reference standard, its conduct, or its interpretation have introduced bias? Low risk if ’Yes’ for all SQs. High risk if ’No’ for at least one of the three SQs. Unclear risk if ’Unclear’ for at least one SQ. | Risk: Low/High/Unclear |
| CONCERNS FOR APPLICABILITY ‐ Are there concerns that the reference standard, its conduct or its interpretation differ from the review question? Low: Yes, the reference standard is performed in a way that it is commonly done in clinical practice. High: No, the reference standard is not performed in a way that it is commonly done in clinical practice. Unclear: Unclear how the reference standard is performed. | Concern: Low/High/Unclear |
| Domain 4: FLOW AND TIMING | |
| SQ1: Was the interval between the tests shorter than six months? (Short enough to be reasonably sure that the target condition did not change during the interval?) | Yes/No/Unclear |
| SQ2: Did all patients receive the same reference standard, regardless of the index test outcome? | Yes/No/Unclear |
| SQ3: Were all patients who underwent testing included in the analysis? | Yes/No/Unclear |
| RISK OF BIAS ‐ Could the patient flow have introduced bias? Low risk if ’Yes’ for all SQs. High risk if ’No’ for at least one SQ. Unclear risk if ’Unclear’, for at least one SQ. | Risk: Low/High/Unclear |
Appendix 2. MEDLINE search strategy
("Prostatic Neoplasms"/ OR prostate/ OR (prostat*).ab,ti,kf.) AND (exp biopsy/ OR (biops*).ab,ti.) AND ("Magnetic Resonance Imaging"/ OR "Diffusion Magnetic Resonance Imaging"/ OR Magnetic resonance spectroscopy/ OR Image guided biopsy/ OR ("magnetic resonance" OR mri OR ((mr OR nmr OR perfusion OR multiparamet* OR multimodal*) ADJ6 imag*) OR template* OR saturat* OR mapping OR T2‐weighted OR t2w OR Diffusion‐weighted OR dwi OR dynamic‐contrast‐enhanced OR dce OR Spectroscop*).ab,ti,kf.)
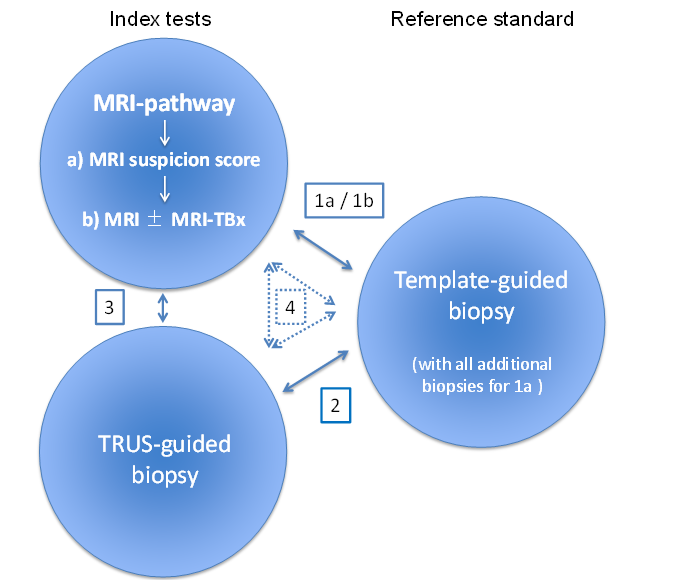
The MRI pathway comprises two components: MRI (suspicion score) and MRI‐targeted biopsy in the presence of suspicious lesions for clinically significant prostate cancer:
-
a) MRI suspicion score: refers to only the first component of the MRI pathway.
-
b) MRI±MRI‐TBx: refers to both components of the MRI pathway.
Review design
Meta‐analysis of studies reporting on:
The MRI pathway and the reference standard: ·
-
Comparison 1a: MRI suspicion score vs reference standard and all additional biopsies (e.g. MRI‐targeted biopsies).
-
Comparison 1b: MRI±MRI‐TBx vs reference standard.
TRUS‐guided biopsy and reference standard: ·
-
Comparison 2
MRI pathway B) MRI±MRI‐TBx and TRUS‐guide biopsy:
-
Comparison 3
A combination of all tests:
-
Comparison 4: comparing both index tests, either directly with the reference standard or indirectly as a randomised trial of test accuracy.
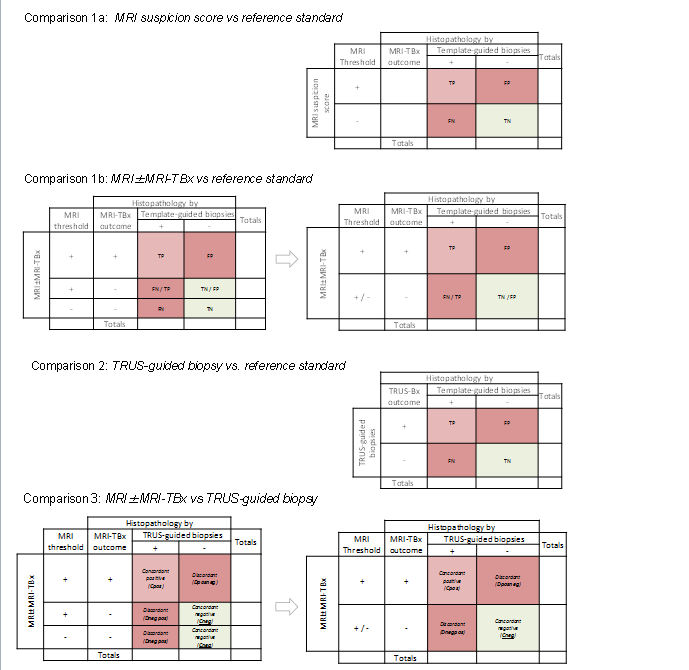
Two‐by‐two contingency tables for target condition.
Comparison 1a: MRI suspicion score vs reference standard (if MRI‐targeted biopsies are performed these will serve as additional reference biopsies).
Comparison 1b: MRI±MRI‐TBx vs reference standard.
Comparison 2: TRUS‐guided biopsy vs reference standard.
Comparison 3: MRI±MRI‐TBx vs TRUS‐guided biopsy.
For comparison 1b and comparison 3, a negative test can result in two ways:
-
a negative MRI (thus no MRI‐targeted biopsies are taken).
-
a positive MRI but negative MRI‐targeted biopsy result.
Both negative outcomes should be merged, creating a two‐by‐two from a three‐by‐two contingency table (first and second column).
In comparison 3 the TRUS‐guided biopsy is not a proper reference standard. Therefore we will focus on the number of cancers identified and the concordance and disconcordance between both index tests.
TP = true positive; FP = false positive; TN = true negative; FN = false negative; Dposneg = Discordant MRI positive + targeted biopsies positive and TRUS‐guided biopsies negative; Dnegpos = Discordant MRI positive/negative + targeted biopsies negative and TRUS‐guided biopsies positive.

