Primaquine at alternative dosing schedules for preventing relapse in people with Plasmodium vivax malaria
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012656Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 04 mayo 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Protocol
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Enfermedades infecciosas
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Patricia Graves (PMG), Paul Garner (CIDG Co‐ordinating Editor) and Rachael Milligan (RM) contributed to the conception of the research question.
All protocol authors contributed to the protocol design and approved the final version.
Sources of support
Internal sources
-
Liverpool School of Tropical Medicine, UK.
External sources
-
Department for International Development, UK.
Grant number: 5242
Declarations of interest
RM has no known conflicts of interest.
AD has no known conflicts of interest.
PMG has no known conflicts of interest.
Acknowledgements
We are grateful to Vittoria Lutje, the Information Specialist of the Cochrane Infectious Diseases Group (CIDG) for help with the literature search strategy. We thank Marty Richardson, CIDG statistician, for help with the data collection and analysis strategy. Rachael Milligan is supported by the Effective Health Care Research Consortium. This Consortium and the CIDG editorial base are funded by UK aid from the UK Government for the benefit of developing countries (Grant: 5242). The views expressed in this Cochrane protocol do not necessarily reflect UK government policy.
Version history
| Published | Title | Stage | Authors | Version |
| 2020 Aug 19 | Primaquine alternative dosing schedules for preventing malaria relapse in people with <i>Plasmodium vivax</i> | Review | Rachael Milligan, André Daher, Gemma Villanueva, Hanna Bergman, Patricia M Graves | |
| 2019 Jul 05 | Primaquine at alternative dosing schedules for preventing relapse in people with <i>Plasmodium vivax</i> malaria | Review | Rachael Milligan, André Daher, Patricia M Graves | |
| 2017 May 04 | Primaquine at alternative dosing schedules for preventing relapse in people with <i>Plasmodium vivax</i> malaria | Protocol | Rachael Milligan, André Daher, Patricia M Graves | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Antimalarials [administration & dosage, adverse effects, *therapeutic use];
- Drug Administration Schedule;
- Glucosephosphate Dehydrogenase;
- Malaria, Vivax [*drug therapy, enzymology];
- Primaquine [administration & dosage, adverse effects, *therapeutic use];
- Randomized Controlled Trials as Topic;
- Recurrence;
- Secondary Prevention;
Medical Subject Headings Check Words
Adult; Child; Humans;
PICO
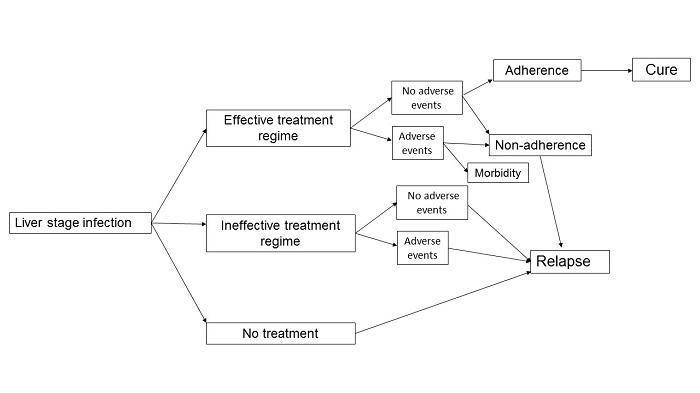
Logic framework: treatment outcome pathways in Plasmodium vivax liver hypnozoite infection
| Objective | Intervention | Control |
| Are higher doses more effective (0.5 mg/kg or 30 mg primaquine/day for 14 days), in all areas, or only in areas where they are standard treatment (Asia, Pacific)? | Blood‐stage antimalarial drug with primaquine 0.5 mg/kg (30 mg) per day for 14 days (total dose 420 mg) Both intervention and control groups must have received the same treatment: either CQ or ACT for the blood‐borne stage of infection. | Blood‐stage antimalarial drug with primaquine 0.25 mg/kg (15 mg) per day for 14 days (total dose 210 mg) Both intervention and control groups must have received the same treatment: either CQ or ACT for the blood‐borne stage of infection. |
| Are shorter, higher dose regimes of primaquine over 7 days as effective as treatment over 14 days (is the total dose rather than the length of treatment the important factor)? | Blood‐stage antimalarial drug with primaquine 0.5 mg/kg (30 mg) per day for 7 days (total dose 210 mg) Both intervention and control groups must have received the same treatment: either CQ or ACT for the blood‐borne stage of infection. | |
| Are weekly dosing regimens (0.75 mg/kg or 45 mg/week for 8 weeks) as effective? | Blood‐stage antimalarial drug with primaquine 0.75/kg (45 mg) per week for 8 weeks (total dose 360 mg) | |
| CQ = Chloroquine ACT = Artemisinin‐based combination therapy | ||

