Primaquina en esquemas de dosis alternativos para la prevención de la recurrencia en pacientes con paludismo por Plasmodium vivax
Resumen
Antecedentes
Los estadios hepáticos del Plasmodium vivax (hipnozoitos) pueden causar recurrencias, prolongando la morbilidad e impidiendo el control y la eliminación del paludismo. La Organización Mundial de la Salud (OMS) recomienda tres esquemas para la primaquina: 0,25 mg/kg/día (estándar) o 0,5 mg/kg/día (estándar alto) durante 14 días; o 0,75 mg/kg una vez por semana durante ocho semanas. Todos estos esquemas pueden ser difíciles de completar. Debido a que la primaquina puede causar hemólisis en pacientes con deficiencia de glucosa‐6‐fosfato deshidrogenasa (G6PD), los médicos pueden ser reacios a recetar primaquina sin realizar pruebas de G6PD, y las recomendaciones cuando se desconoce el estado de la G6PD se deben basar en una evaluación de los riesgos y beneficios de la prescripción de primaquina. Se necesitan regímenes alternativos seguros y eficaces.
Objetivos
Evaluar la eficacia y la seguridad de los regímenes alternativos de primaquina para la cura radical del paludismo por P vivax en comparación con los tratamientos de ciclo estándar o de estándar alto de 14 días.
Métodos de búsqueda
Se realizaron búsquedas en el Registro Especializado del Grupo Cochrane de Enfermedades Infecciosas (Cochrane Infectious Diseases Group); el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials, CENTRAL); MEDLINE (PubMed); Embase (Ovid); LILACS (BIREME); la Plataforma de Registro Internacional de Ensayos Clínicos de la OMS y ClinicalTrials.gov hasta el 2 de septiembre de 2019, y se comprobaron las listas de referencias de todos los estudios identificados.
Criterios de selección
Ensayos controlados aleatorizados (ECA) de adultos y niños con paludismo por P vivax que utilizaron el tratamiento combinado con cloroquina o artemisinina más primaquina a una dosis total para adultos de al menos 210 mg, en comparación con los regímenes recomendados por la OMS de 0,25 o 0,5 mg/kg/día durante 14 días.
Obtención y análisis de los datos
Dos autores de la revisión evaluaron de forma independiente la elegibilidad y la calidad de los ensayos y extrajeron los datos. Se calcularon los riesgos relativos (RR) con intervalos de confianza (IC) del 95% para los datos dicotómicos. Los datos de eficacia se agruparon según la duración del seguimiento, el fármaco asociado y el lugar del ensayo. Se analizaron los datos de seguridad cuando se incluyeron.
Resultados principales
0,5 mg/kg/día durante siete días versus estándar de 0,25 mg/kg/día durante 14 días
Puede haber poca o ninguna diferencia en las recurrencias del P vivax a los seis o siete meses cuando se utiliza la misma dosis total (dosis de 210 mg para adultos) durante siete días en comparación con 14 días (RR 0,96; IC del 95%: 0,66 a 1,39; cuatro ECA, 1211 participantes; evidencia de certeza baja). No se informaron eventos adversos graves. No se sabe si hay alguna diferencia en el número de eventos adversos que dan lugar a la interrupción del tratamiento con primaquina (RR 1,04; IC del 95%: 0,15 a 7,38; cinco ECA, 1427 participantes) o en la frecuencia de anemia (RR 3,00; IC del 95%: 0,12 a 72,91; un ECA, 240 participantes) entre los regímenes más cortos y más prolongados (evidencia de certeza muy baja). Tres ensayos excluyeron a los pacientes con deficiencia de G6PD y dos no aportaron esta información. Las mujeres embarazadas y que amamantaban se excluyeron o no se proporcionaron detalles.
Estándar alto de 0,5 mg/kg/día durante 14 días versus estándar de 0,25 mg/kg/día durante 14 días
Puede haber poca o ninguna diferencia en la recurrencias del P vivax a los seis meses con 0,5 mg/kg/día de primaquina durante 14 días en comparación con 0,25 mg/kg/día durante 14 días (RR 0,84; IC del 95%: 0,49 a 1,43; dos ECA, 677 participantes, evidencia de certeza baja). No se informaron eventos adversos graves. No se sabe si hay diferencia en los eventos adversos que dan lugar a la interrupción del tratamiento con la dosis de estándar alto (RR 4,19; IC del 95%: 0,90 a 19,60; un ECA, 778 participantes, evidencia de certeza muy baja). Se excluyeron los pacientes con deficiencia de G6PD y las mujeres embarazadas o en lactancia.
0,75 mg/kg/semana durante ocho semanas versus estándar alto de 0,5 mg/kg/día durante 14 días
No se sabe si la primaquina semanal aumenta o disminuye las recurrencias del P vivax en comparación con el estándar alto de 0,5 mg/kg/día durante 14 días, a los 11 meses de seguimiento (RR 3,18; IC del 95%: 0,37 a 27,60; un ECA, 122 participantes; evidencia de certeza muy baja). No se informó de eventos adversos graves ni de episodios de anemia. Los pacientes con deficiencia de G6PD no se asignaron al azar, sino que se incluyeron en el grupo de primaquina semanal (solo se detectó un paciente).
1 mg/kg/día durante siete días versus estándar alto de 0,5 mg/kg/día durante 14 días
Probablemente hay poca o ninguna diferencia en las recurrencias del P vivax a los 12 meses entre 1,0 mg/kg/día de primaquina durante siete días y el estándar alto de 0,5 mg/kg/día durante 14 días (RR 1,03; IC del 95%: 0,82 a 1,30; dos ECA, 2526 participantes; evidencia de certeza moderada). Puede haber un aumento moderado a grande de los eventos adversos graves con la primaquina de 1,0 mg/kg/día durante siete días, en comparación con el estándar alto de 0,5 mg/kg/día por 14 días, durante 42 días de seguimiento (RR 12,03; IC del 95%: 1,57 a 92,30; un ECA, 1872 participantes, evidencia de certeza baja). No se sabe si hay una diferencia entre 1,0 mg/kg/día de primaquina durante siete días y el estándar alto de 0,5 mg/kg/día durante 14 días en los eventos adversos que dieron lugar a la interrupción del tratamiento (RR 2,50; IC del 95%: 0,49 a 12,87; un ECA, 2526 participantes, evidencia de certeza muy baja), ni si hay una diferencia en la frecuencia de anemia a los 42 días (RR 0,93; IC del 95%: 0,62 a 1,41; dos ECA, 2440 participantes, evidencia de certeza muy baja). Se excluyó a los pacientes con deficiencia de G6PD.
Otros regímenes
Dos ECA evaluaron otras dosis de primaquina de uso poco frecuente, uno de los cuales tuvo una pérdida muy alta durante el seguimiento. No se informó de eventos adversos. Se excluyeron los pacientes con deficiencia de G6PD y las mujeres embarazadas o en lactancia.
Conclusiones de los autores
Los ensayos disponibles hasta la fecha no detectan una diferencia en la recurrencia entre los siguientes regímenes: 1) 0,5 mg/kg/día durante siete días versus estándar de 0,25 mg/kg/día durante 14 días; 2) estándar alto de 0,5 mg/kg/día durante 14 días versus estándar de 0,25 mg/kg/día durante 14 días; 3) 0,75 mg/kg/semana durante ocho semanas versus estándar alto de 0,5 mg/kg/día durante 14 días; 4) 1 mg/kg/día durante siete días versus estándar alto de 0,5 mg/kg/día durante 14 días. No se detectaron diferencias en los eventos adversos en las comparaciones 1, 2 o 3 pero es posible que haya eventos adversos más graves con el ciclo alto de siete días en la comparación 4.
El régimen más corto de 0,5 mg/kg/día durante siete días versus estándar de 0,25 mg/kg/día durante 14 días puede ser adecuado para los pacientes con G6PD normal. Los estudios de investigación adicionales ayudarán a aumentar la certeza de los hallazgos y la aplicabilidad en diferentes contextos.
PICO
Resumen en términos sencillos
Primaquina para la curación de personas con paludismo por Plasmodium vivax: comparación de esquemas de dosis
El paludismo por Plasmodium vivax a veces puede causar cuadros potencialmente mortales y la infección todavía afecta a muchas personas. La infección incluye una etapa hepática, que requiere primaquina para erradicarla y evitar que la infección se repita. Sin embargo, el esquema de dosis actual requiere 14 días de tratamiento diario.
¿Cuáles son las preocupaciones acerca de la primaquina?
La primaquina es el único medicamento actualmente recomendado para tratar los parásitos hepáticos en el paludismo por P. vivax. Puede causar anemia en los pacientes con deficiencia de la glucosa‐6‐fosfato deshidrogenasa (G6FD), que es un defecto genético de la coagulación relativamente frecuente. Los regímenes más cortos ayudarían a reducir el riesgo de incumplimiento con el régimen actual de dos semanas.
¿Qué dice la investigación?
Se resumieron los ensayos que compararon el régimen de primaquina recomendado por la Organización Mundial de la Salud (OMS) de 15 a 30 mg diarios durante 14 días, con dosis iguales o mayores de primaquina administradas durante diferentes períodos de tiempo, para determinar si los regímenes alternativos fueron tan efectivos como los tratamientos recomendados para prevenir episodios futuros de paludismo por P. vivax. Se buscaron ensayos hasta el 2 de septiembre de 2019 y en el análisis se incluyeron 11 ensayos controlados aleatorizados (estudios en que los participantes se asignan a uno de dos o más grupos de tratamiento mediante un método aleatorio).
Cuando se utilizan 30 mg de primaquina por día durante siete días en comparación con 15 mg por día durante 14 días, puede no haber diferencias en las recurrencias del P. vivax a los seis a siete meses (evidencia de certeza baja). No se informaron eventos adversos graves. No se sabe si hay diferencia en el número de eventos adversos que hacen que los pacientes dejen de tomar el fármaco (evidencia de certeza baja).
Cuando se utilizan dosis de primaquina de 30 mg por día en comparación con 15 mg por día durante 14 días, no se sabe si hay diferencias en las recurrencias del P. vivax a los seis meses (evidencia de certeza muy baja). No se informó de eventos adversos graves, pero no está claro si hay o no una diferencia entre las dosis en el caso de otros eventos adversos que hacen que los pacientes dejen de tomar el fármaco (evidencia de certeza muy baja).
Se desconoce si 45 mg de primaquina una vez por semana durante ocho semanas aumenta o disminuye las recurrencias del P vivax en comparación con el estándar alto de 30 mg por día durante 14 días, a los 11 meses de seguimiento (evidencia de certeza muy baja).
Es probable que haya poca o ninguna diferencia en la recurrencia cuando se utiliza una dosis alta de 60 mg por día durante siete días en comparación con el estándar alto de 30 mg por día durante 14 días, pero puede haber un aumento de los eventos adversos graves en el grupo de régimen de ciclo corto con dosis alta.
La realización de más ECA ayudará a aumentar el grado de certeza de la evidencia de los regímenes alternativos.
¿Cuál es el grado de actualización de esta revisión?
Los autores de la revisión buscaron estudios hasta el 2 de septiembre de 2019.
Authors' conclusions
Summary of findings
| 0.5 mg/kg primaquine/day for 7 days versus standard 0.25 mg/kg/day for 14 days for radical cure of P vivax malaria | ||||||
| Patient or population: adults and children with confirmed clinical and parasitological P vivax malaria | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with standard 14‐day course primaquine | Risk with 0.5mg/kg/day primaquine for 7 days | |||||
| Recurrence of P vivax parasitaemia | 84 per 1000 | 81 per 1000 | RR 0.96 | 1211 | ⊕⊕⊝⊝ due to risk of bias and imprecision | There may be little or no difference between 0.5 mg/kg/day primaquine for 7 days and the standard 14‐day course. |
| Serious adverse events | Not estimable (0 events in 723 participants) | Not estimable (0 events in 704 participants) | Not estimable | 1427 | — | No events reported. |
| Adverse events that result in the discontinuation of treatment | 3 per 1000 | 3 per 1000 | RR 1.04 | 1427 (5 RCTs) | ⊕⊝⊝⊝ due to risk of bias and serious imprecision | We do not know if there is any difference in adverse events that result in treatment discontinuation between 0.5 mg/kg/day primaquine for 7 days and the standard 14‐day course. |
| Anaemia or change in haemoglobin status | Not estimable (0 events in 120 participants) | Not estimable (1 event in 120 participants) | RR 3.0 (0.12 to 72.91) | 240 | ⊕⊝⊝⊝ due to risk of bias, indirectness, and serious imprecision | We do not know if the occurrence of anaemia differs between the 2 treatment regimens. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded once for risk of bias: Rajgor 2014 IND, which contributed the most weight to the meta‐analysis, was at high risk of selection bias due to no allocation concealment and high risk of attrition bias. Although Pareek 2015 IND was at risk of selection bias as well as other bias for being funded and carried out by drug company, it only contributed a small amount of weight to the meta‐analysis. | ||||||
| High standard 0.5 mg/kg primaquine /day for 14 days versus standard 0.25 mg/kg/day for 14 days for radical cure of P vivax malaria | ||||||
| Patient or population: adults and children with confirmed clinical and parasitological P vivax malaria | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with standard 14‐day course primaquine | Risk with high‐standard 14‐day course primaquine | |||||
| Recurrence of P vivax parasitaemia | 82 per 1000 | 69 per 1000 | RR 0.84 | 677 | ⊕⊕⊝⊝ due to risk of bias and imprecision | There may be little or no difference in P vivax recurrences between high‐standard or standard 14‐day courses of primaquine given with chloroquine or an ACT. |
| Serious adverse events | Not estimable (0 events in 398 participants) | Not estimable (0 events in 380 participants) | Not estimable | 778 | — | No events reported. |
| Adverse events that result in the discontinuation of treatment | 5 per 1000 | 21 per 1000 | RR 4.19 | 778 | ⊕⊝⊝⊝ due to indirectness, risk of bias, and imprecision | We do not know if there is any difference in adverse events resulting in treatment discontinuation between high‐standard or standard 14‐day courses of primaquine. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: ACT: artemisinin‐based combination therapy; CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded once for risk of bias: one study was open‐label with no allocation concealment (risk of selection bias) and risk of attrition bias due to high percentage not completing six months' follow‐up with minimal explanation; the other study had no blinding and a high rate of loss to follow‐up. | ||||||
| 0.75 mg/kg primaquine /week for 8 weeks versus high‐standard 0.5 mg/kg/day for 14 days for radical cure of P vivax malaria | ||||||
| Patient or population: adults and children with confirmed clinical and parasitological P vivax malaria | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with high‐standard 14‐day course primaquine | Risk with once‐weekly 0.75 mg/kg primaquine for 8 weeks | |||||
| Recurrence of P vivax malaria | 19 per 1000 | 59 per 1000 | RR 3.18 | 122 | ⊕⊝⊝⊝ due to risk of bias and serious imprecision | We do not know if weekly primaquine reduces the risk of malaria recurrences when compared to the high‐standard 14‐day course. |
| Serious adverse events | Not estimable (0 events in 55 participants) | Not estimable (0 events in 74 participants) | Not estimable | 129 | — | No events reported. |
| Anaemia (haemoglobin < 7 g/dL) | Not estimable (0 events in 55 participants) | Not estimable (0 events in 74 participants) | Not estimable | 129 | — | No events reported. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded once for risk of bias: Leslie 2008 PAK was at high risk of bias for randomization process, allocation concealment, and incomplete outcome data. | ||||||
| 1.0 mg/kg primaquine /day for 7 days versus high‐standard 0.5 mg/kg/day for 14 days for radical cure of P vivax malaria | ||||||
| Patient or population: adults and children with confirmed clinical and parasitological P vivax malaria Settings: Afghanistan, Ethiopia, Indonesia, Thailand, and Vietnam Intervention: 1.0 mg/kg/day primaquine for 7 days (adult dose 60 mg/day; total dose 420mg) Comparison: high‐standard 14‐day course primaquine (0.5 mg/kg/day, adult dose 30 mg/day; total dose 420mg) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of Participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with high‐standard 14‐day course primaquine | Risk with 1.0 mg/kg/day primaquine for 7 days | |||||
| Recurrence of P vivax parasitaemia | 104 per 1000 | 107 per 1000 | RR 1.03 (0.82 to 1.30) | 2526 (2 RCTs) | ⊕⊕⊕⊝a due to risk of bias | There is probably little or no difference between 1.0 mg/kg/day primaquine for 7 days and the high‐standard 0.5 mg/kg/day for 14 days course |
| Serious adverse events Follow‐up: up to 42 days | 1 per 1000 | 13 per 1000 (2 to 99) | RR 12.03 (1.57 to 92.30) | 1872 (1 RCT) | ⊕⊕⊝⊝b,c LOW due to indirectness and imprecision | There may be a moderate to large increase in serious adverse events in the 1.0 mg/kg/day primaquine for 7 days compared with the high‐standard 0.5 mg/kg/day Chu 2019 THA provides overall narrative results only, see Effects of interventions text. |
| Adverse events the resulted in discontinuation of treatment | 2 per 1000 | 4 per 1000 | RR 2.50 (0.49 to 12.87) | 2526 (2 RCTs) | ⊕⊝⊝⊝a,b,d due to risk of bias, indirectness and serious imprecision | We do not know if there is any difference in adverse events resulting in treatment discontinuation between 1.0 mg/kg/day primaquine for 7 days and the high‐standard 0.5 mg/kg/day for 14 days course. |
| Anaemia Follow‐up: between 3 and 42 days follow‐up | 35 per 1000 | 33 per 1000 (22 to 50) | RR 0.93 (0.62 to 1.41) | 2440 | ⊕⊝⊝⊝a,b,e due to risk of bias, indirectness and imprecision | We do not know if there is any difference in anaemia between 1.0 mg/kg/day primaquine for 7 days and the high‐standard 0.5 mg/kg/day for 14 days course. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded once for risk of bias: Chu 2019 THA was an open‐label trial with high risk of performance and detection bias; although drop‐outs were balanced between groups the proportion of drop‐outs after one year was high in both trials (30‐40%). | ||||||
Background
Malaria is a potentially life‐threatening disease caused by the Plasmodium parasite, which is transmitted by the bite of an infected female Anopheles mosquito. Five species of Plasmodium malaria parasites can cause malaria disease in humans, of which Plasmodium vivax and Plasmodium falciparum are the most important (WHO 2016). In 2018, an estimated 228 million cases of malaria occurred worldwide and an estimated 405,000 people died from the disease (WHO 2019). The World Health Organization (WHO) aims to reduce malaria case load and mortality by at least 90% by 2030 (WHO 2016).
Historically, P vivax infection was thought to be a milder form of malaria, and researchers have focused on P falciparum due to the high number of deaths it causes (Bassat 2016). In recent years, it has been shown that the morbidity and mortality of P vivax have been underestimated, with evidence of direct fatality and contribution to mortality in patients who have other comorbidities, such as malnutrition, HIV, or coexisting infections (Baird 2013; Bhattacharjee 2013; Rizvi 2013; Singh 2013; Battle 2014; Douglas 2014; Kochar 2014; Arévalo‐Herrera 2015; Baird 2015b). Repeated P vivax infections through childhood and adulthood also affect personal well‐being, development, and education and can thus negatively impact economic development, both for the individual and the community (Mendis 2001). P vivax malaria in pregnancy is associated with maternal anaemia, spontaneous abortion, stillbirth, and low birthweight, with especially poor pregnancy outcomes for women with severe infection (McGready 2012; Rijken 2012; Brutus 2013).
Description of the condition
P vivax infection caused an estimated 7.5 million cases of malaria in 2018 (WHO 2019). The geographical distribution of P vivax malaria is more widespread than any of the other forms of human malaria, with around 35% of the world’s population thought to be at risk (Howes 2016). Co‐infection with P falciparum is also common in many regions (Kumar 2007; Mueller 2009). As malaria control accelerates, the P vivax proportion in co‐endemic areas tends to rise compared to that of P falciparum, which highlights the importance and challenge of this infection (John 2012).
P vivax is important because as many countries progress towards malaria elimination, the parasite becomes a roadblock to eradication (Cibulskis 2015; Bassat 2016). Despite a reported 45% reduction in P vivax malaria cases between 2010 and 2016 (WHO 2017), the parasite has several characteristics that enable it to evade control (Newby 2016). The early appearance of gametocytes in the blood, often prior to symptoms of malaria, increases the chance of onward transmission by mosquitoes (Mendis 2001). P vivax differs from P falciparum in that as well as having a blood‐stage infection, hypnozoites develop in the liver that can be dormant for weeks to months before developing into an infection (White 2011). What triggers these relapses is not well‐understood. There is difficulty in distinguishing between relapse (hypnozoite activation), recrudescence (subpar treatment of the initial blood‐stage infection), and re‐infection (new infection with P vivax) (Imwong 2007). A study in Papua New Guinea suggested that relapses cause four‐fifths of P vivax infections (Robinson 2015), reinforcing the importance of relapse in sustaining transmission (White 2011). Parasites show high genetic diversity, even in countries that are at malaria elimination stage (Koepfli 2015). P vivax is likely underestimated worldwide, as the dormant liver stage is not detected in routine surveys (Gething 2012). Submicroscopic infections and asymptomatic infection reservoirs may also lead to underdiagnosis or misdiagnosis. A systematic review showed that across all study sites, the polymerase chain reaction (PCR) prevalence of P vivax was significantly higher than that identified by light microscopy (Cheng 2015). The effect this may have on P vivax malaria studies is unclear.
There are different strains of P vivax according to geographical region/endemicity areas, with relapse patterns that vary by latency (time to first relapse), likelihood of relapse, and frequency of relapses, which further complicates the assessment of efficacy of drugs on relapses (Battle 2014; White 2016). Strains commonly found in Southeast Asia and Oceania (including the ‘Chesson’ strain isolated from an individual infected in Papua New Guinea) have the shortest latency time to relapse, starting as early as three weeks after first infection (if untreated with a hypnozoiticidal drug) (Ehrman 1945). These areas correspond to zones 10 and 12 in Battle 2014. Indian and Pakistan strains (zone 8) exhibit heterogeneity in relapse latency, incidence, and frequency, while South American strains (zone 3) have a pattern of short latency to first relapse and less frequent relapses than in zones 10 and 12 (Battle 2014). The temperate strains (which include those from Korea in zone 11) relapse much more slowly (John 2012; Battle 2014). Strains of the type in zones 10 and 12, referred to here as 'East Asia and Oceania', are recommended to receive higher doses of primaquine (the high‐standard course of 0.5 mg/kg/day rather than standard 0.25 mg/kg/day for 14 days) to prevent relapses (WHO 2015), apparently based on research done in the 1950s and 1960s (Coatney 1953; Jones 1953; Vivona 1961; Maffi 1971; Clyde 1977), although not all these studies were done with strains from the targeted geographic area.
Primaquine, an 8‐aminoquinoline, has until very recently been the only drug available on the market for treating the hypnozoite stage of infection (Ashley 2014). One of the main barriers in P vivax treatment is the reluctance to use primaquine due to it potentially causing haemolysis in patients with glucose‐6‐phosphate dehydrogenase (G6PD) deficiency. G6PD deficiency is the most common enzyme deficiency worldwide and affects red blood cells by leading to their premature lysis (Nkhoma 2009). G6PD deficiency is common in countries where P vivax malaria is endemic, with an estimated population prevalence of 8% (Howes 2012). Within G6PD deficiency, there are differing phenotypes, meaning some people may be mildly sensitive to primaquine, while others may be very sensitive and experience life‐threatening haemolysis (Baird 2015a), which explains the varying responses to primaquine. In many areas where P vivax is predominant, testing for G6PD deficiency is not available locally (Baird 2015b). In 2018 the US Food and Drug Administration (FDA) approved a newer alternative, another 8‐aminoquinoline known as tafenoquine (MMV 2018), which has shown promise in reducing relapses, but there are increased safety concerns in patients with undiagnosed G6PD deficiency compared to primaquine, due to its longer half‐life (Rajapakse 2015).
Description of the intervention
People with P vivax malaria require treatment with an antimalarial drug to treat the blood‐stage infection, and a drug to treat the hypnozoite stage (radical cure). The WHO recommends treatment with either chloroquine or an artemisinin‐based combination therapy (ACT) for the blood‐stage infection, with 0.25 to 0.5 mg/kg/day primaquine for 14 days for the liver stages (WHO 2015). Artemisinin‐based combination therapies and chloroquine have been shown to be effective and comparable in treating the blood‐stage infection of P vivax malaria (Gogtay 2013).
A previous Cochrane Review showed that primaquine regimens of five days or fewer had similar recurrence rates to placebo or no primaquine. Of the comparisons included in the review, a regimen of 0.25 mg/kg/day (15 mg) a day of primaquine for 14 days had the lowest recurrence rates of P vivax infection (Galappaththy 2013). There were no trials at that time that compared higher doses of primaquine at 14 or 7 days.
Primaquine was first made available to North American soldiers in the 1950s (Baird 2004). Its mechanism and metabolism are not widely understood, but it has a broad spectrum of activity against the Plasmodium parasite. As well as preventing relapse of P vivax malaria by targeting the latent and developing hypnozoites in the liver, it is also used in malaria prophylaxis (Baird 2003) and is gametocytocidal (Graves 2018). It is absorbed from the gastrointestinal tract, has a half‐life of about four to nine hours, and crosses the placenta in pregnancy (Baird 2004). New advancements in studying P vivax in humanized mice may lead to a greater understanding of the mechanism of action of the drug (Mikolajczak 2015).
Adverse events observed with primaquine include production of methaemoglobin, an oxidated state of haemoglobin that cannot transport oxygen to tissues. Methaemoglobinaemia (an abnormal buildup of methaemoglobin) can result in cyanosis when levels exceed 10% of the usual haemoglobin level (Vale 2009). As described above, primaquine causes haemolysis in people with G6PD deficiency, which leads to anaemia (Ashley 2014). When taken on an empty stomach it can cause abdominal pain and gastrointestinal upset (Vale 2009). Safe use of primaquine during pregnancy has not been established. The radical cure with primaquine can be delayed until after pregnancy. With regard to breastfeeding patients, a recent study showed that the levels of primaquine in breast milk may not be sufficient to cause haemolysis even in a G6PD‐deficient baby (Gilder 2018), but it is not recommended at this time.
How the intervention might work
The WHO advises that 0.25 mg (standard) to 0.5 mg/kg/day (high standard) of primaquine for 14 days (total dose 210 mg or 420 mg) should be used for radical cure of P vivax malaria in patients over six months old, excluding people with G6PD deficiency and those who are pregnant or breastfeeding (WHO 2015). Citing the review previous Cochrane Review of this topic (Galappaththy 2013), the WHO notes that the standard regimen reduced relapses during 15 months of follow‐up by about 40% compared to placebo or no primaquine (high‐quality evidence), and reduced relapses during six months follow‐up by over 50% compared to seven days primaquine (low‐quality evidence) (WHO 2015). The increased dosing in the high standard 0.5 mg/kg/day regimen was previously recommended in East Asia and Oceania based on suggestion of failure of the standard regimen of 0.25 mg/kg/day for 14 days for strains of P vivax in these areas (including the Chesson strain). The guidelines note that "no direct comparison has been made of higher doses (0.5 mg/kg bw for 14 days) with the standard regimen (0.25 mg/kg bw for 14 days)". Given that the 15 trials included in the WHO assessment excluded G6PD‐deficient persons (12 trials) or did not comment on their exclusion, WHO guidelines also stated "in the absence of evidence to recommend alternatives, the guidelines development group consider 0.75 mg/kg bw primaquine given once weekly for 8 weeks to be the safest regimen for people with mild to moderate G6PD deficiency", but no trials of this regimen were included in the WHO guidance (WHO 2015).
The 14‐day course of primaquine at any dose, as well as the eight‐week course, can lead to treatment adherence issues, as well as to safety concerns about haemolysis in places where G6PD testing is not available, meaning that shorter courses of primaquine are desirable. Failure to treat the hypnozoite stage of P vivax malaria leads to repeated relapses, morbidity, and persistent infection.
It has long been suggested that it may be the total dose of primaquine that is important in the treatment of the hypnozoite stage rather than the length of the course (Schmidt 1977). If a higher dose of primaquine could be administered safely over a shorter period of time, it may improve adherence rates, thus reducing relapse rates and morbidity and mortality resulting from P vivax infection. There are small trials from the 1970s that suggest that shorter, higher‐dose regimens were as efficacious as the 14‐day courses (Clyde 1977; Saint‐Yves 1977). At the time of the previous Cochrane Review (Galappaththy 2013), there were no recent large high‐quality trials that had investigated the use of the same total dose as the standard regimen (210 mg), or higher total doses, given over either shorter or longer periods. We planned to include any such trials in this Cochrane Review.
Why it is important to do this review
The use of primaquine for radical cure of P vivax malaria continues to pose a therapeutic dilemma for healthcare providers in areas without adequate screening for G6PD status. Clinicians must either choose to give primaquine and risk haemolysis if the patient is G6PD‐deficient, or withhold treatment and accept the complications of ongoing parasite infection and relapses. This is why when clinicians choose to treat with primaquine they prefer a lower dose over a more prolonged period, which then risks difficulties with adherence and thus reduced effectiveness.
We know from the previous Cochrane Review on primaquine with chloroquine for radical cure that the standard 14‐day regimen of 0.25 mg/kg/day (15 mg per day or 210 mg total dose) is better than shorter regimens of similar daily doses and placebo (Galappaththy 2013). In fact, the regimen of 0.25 mg/kg/day for five days of primaquine did not reduce recurrences compared to treating with chloroquine alone.
A major problem with the radical cure of P vivax is difficulty with the adherence to the 14‐day course of primaquine, which has led to many countries shortening the regimen. Peru was one such example, although a study revealed that patients often still discontinued the therapy after around three days, when they started to feel better (Grietens 2010). A study that compared directly observed therapy (DOT) for 14 days of primaquine versus non‐DOT primaquine found that the P vivax recurrence rate was significantly lower in the DOT group (Takeuchi 2010). These problems have led to a more urgent call for shorter treatment regimens. Various trials are investigating regimens that revise dosing and duration of treatment in order to improve adherence and reduce the potential for incomplete treatment and development of resistance. As mentioned previously, the significance of the total cumulative primaquine dose given, rather than the length of the course, is one avenue of investigation. In areas where G6PD screening is present, using higher dosing regimens over shorter time periods, if at least similarly efficacious, could improve adherence and reduce morbidity associated with P vivax parasitaemia.
World Health Organization guidelines suggest a higher dosing regimen of primaquine for the tropical, frequent‐relapsing strains of P vivax in East Asia and Oceania (WHO 2015), although the previous Cochrane Review, Galappaththy 2013, did not find any trials assessing this. Investigating the evidence base for this is therefore important. The 2015 WHO guidelines also suggest an alternate dosing regimen of weekly primaquine, which may be safer in patients with G6PD deficiency. As the previous Cochrane Review included data from only one trial assessing this, it is useful to investigate whether there is further evidence to substantiate this guidance.
In this Cochrane Review, we have excluded comparisons between blood‐stage drug (chloroquine/ACT) with and without primaquine, as the rationale for primaquine use has been sufficiently demonstrated in a previous Cochrane Review (Galappaththy 2013). Similarly, we have not included comparisons between different blood‐stage drugs in which the same dose of primaquine was used; an update to an existing Cochrane Review, Gogtay 2013, is in progress and will address this. However, we planned to stratify our results according to partner drug, as there is increasing evidence that primaquine is metabolized via the cytochrome P450 2D6 (CYP2D6) pathway (Bennett 2013), and efficacy may thus be affected if the blood‐stage antimalarial drug is a CYP2D6 inhibitor (Baird 2018). This review excluded comparisons that do not use the standard (0.25 mg/kg/day) or high‐standard (0.5 mg/kg/day) regimens of 14 days of primaquine in the control groups. Also, it did not include comparisons of primaquine regimens of 0.25 mg/kg/day for less than 14 days, as Galappaththy 2013 has already assessed these shorter regimens at this dose.
There is currently a lack of consensus among studies as to what the minimum time frame for follow‐up of relapse in P vivax malaria should be. The WHO guidance on clinical trials in malaria sets out standard follow‐up for blood (or schizontal) stage infection as 28 days after treatment commencement, but has no clear definition on the follow‐up period for radical cure in primaquine studies. It states that "follow up varies from three months to a year in the literature, and should be adapted to regional parasite characteristics" (WHO 2009). In a recent review, John 2012 described relapse of the tropical frequently relapsing strain of P vivax as typically three weeks, but this varies according to blood‐stage treatment: "three weeks following quinine therapy" and "six to eight weeks following chloroquine" (White 2011). With exposure to primaquine ‐ even if radical cure is not achieved ‐ relapses may occur at longer intervals (Sutanto 2013). In the Cochrane Review (Galappaththy 2013), the follow‐up period started 30 days after completing primaquine treatment. Relapse is frequently defined as the presence of P vivax parasites more than 28 to 30 days after the full course of primaquine in people living in a non‐endemic area (Looareesuwan 1997). Due to the varying lengths of treatment and relapse time in P vivax malaria, 28 days from treatment completion may not allow true assessment of radical cure. It also makes assessment of the weekly primaquine regimen difficult, as the follow‐up time should start before the eight‐week treatment course has finished. In this Cochrane Review we planned to assess parasitaemia at three, six, and 12 months' follow‐up, in keeping with WHO guidance. We intended to describe the length of follow‐up across studies, and then group them into meaningful lengths of follow‐up, depending on the regimen.
Attention is needed to the problems of lack of completion of long treatment courses and potential insufficient dosing in some geographical areas, while maintaining daily doses within a safe range. We compare the efficacy and safety of alternative schedules to those currently recommended, or those with insufficient evidence in current recommendations. Specifically, we intended to answer the following questions by comparing alternative regimens with total adult dose of over 210 mg to the standard 14‐day regimen of primaquine (0.25 mg/kg/day,15 mg adult daily dose, total dose 210 mg), or the high‐standard 14‐day regimen (0.5 mg/kg/day,30 mg adult daily dose, total dose 420 mg).
-
Is a shorter, higher daily dose regimen with same or higher total dose, given over seven days, as (or more) efficacious and safe as the standard or high standard regimens given over 14 days? (Comparisons 1 and 4)
-
Is the high‐standard 14‐day regimen, with higher daily and total dose, as (or more) efficacious and safe as the standard 14‐day course, in all areas and/or where it was formerly recommended (East Asia and Oceania)? (Comparison 2)
-
Is a weekly dosing regimen with higher daily dose given one day a week and with either higher or lower total dose, as (or more) efficacious and safe as the standard or high‐standard 14‐day regimens? (Comparison 3)
Objectives
To assess the efficacy and safety of alternative primaquine regimens with total adult dose >210 mg for radical cure of P vivax malaria compared to the standard or high‐standard 14 days of primaquine (0.25 mg/kg/day or 0.5 mg/kg/day, total adult dose 210 mg or 420 mg), as well as comparison of these two WHO‐recommended regimens.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs). We excluded quasi‐RCTs.
Types of participants
Adults and children with confirmed clinical and parasitological (light microscopy or PCR, or both) diagnosis of P vivax malaria. We included trials that excluded people with G6PD deficiency and trials that included populations that had or had not been screened for G6PD deficiency. People with mixed malaria infections were excluded.
Types of interventions
Intervention
Any regimen of either chloroquine or an artemisinin‐based combination therapy (ACT) plus primaquine at total adult dose >210 mg in any of the following categories.
Daily doses higher than 0.25 mg/kg/day (15 mg daily adult dose, total dose 210 mg) for 14 days.
-
Shorter regimens with the same or higher total dose than the regimen they are being compared to i.e. standard or high standard regimen.
-
Weekly dosing regimens.
Control
WHO‐defined standard regimen of 14 days of primaquine at 0.25 mg/kg/day (15 mg daily adult dose, total dose 210 mg), or high‐standard regimen of 0.5 mg/kg/day for 14 days (30 mg adult daily dose, total dose 420 mg), plus either chloroquine or an ACT as the treatment for blood‐borne infection. Where possible, we stratified by the blood schizonticidal agent.
Types of outcome measures
Primary outcomes
-
P vivax parasitaemia (detected by light microscopy or PCR, or both) at 3, 6, and 12 months' follow‐up. We planned to describe this as recurrences of P vivax malaria due to the previously mentioned difficulties in distinguishing between relapse and re‐infection.
Secondary outcomes
-
P vivax parasitaemia (detected by light microscopy or PCR, or both) at one to three months' follow‐up.
Adverse events
-
Serious adverse events (fatal, life‐threatening, or requiring hospitalization).
-
Adverse events that result in discontinuation of treatment.
-
Anaemia or change in haemoglobin status.
-
Other adverse events.
Search methods for identification of studies
We attempted to identify all relevant studies regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
We searched the following databases using the search terms and strategy described in Appendix 1: the Cochrane Infectious Diseases Group Specialized Register (2 September 2019); the Cochrane Central Register of Controlled Trials (CENTRAL, 2018, Issue 12, published in the Cochrane Library); MEDLINE (PubMed, 1946 to 2 September 2019); Embase (Ovid, 1947 to 2 September 2019); and LILACS (Bireme, 1982 to 2 September 2019). We also searched the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP; www.who.int/ictrp/), and ClinicalTrials.gov (clinicaltrials.gov/ct2/home), for trials in progress, on 2 September 2019, using "primaquine" and "vivax" as search terms.
Searching other resources
We checked the reference lists of all studies identified by the above methods for additional potentially relevant studies. We contacted researchers working in the field and the WHO for unpublished and ongoing trials. We also searched the reference lists and included studies of the Cochrane Review Galappaththy 2013.
Data collection and analysis
Selection of studies
Two review authors independently screened the titles and abstracts of the search results to identify potentially eligible trials, coding the articles as either 'retrieve' or 'do not retrieve'. We obtained the full‐text reports of potentially eligible trials and assessed them for inclusion in the review using a predesigned eligibility form based on the inclusion criteria. Any discrepancies were resolved through discussion or by consulting a third review author if necessary. Where necessary, we contacted the trial authors for clarification of trial methods. We listed the excluded trials and the reasons for their exclusion in a Characteristics of excluded studies table. Where there were multiple reports relating to the same trial, we planned to include all reports and collate data. We detailed the trial selection process in a PRISMA diagram.
Data extraction and management
Two review authors independently extracted data from the included trials using a data extraction form designed specifically for this review, in keeping with Cochrane guidance (Higgins 2011).
For each included trial we extracted a minimum of the following data where available.
-
Study design.
-
Endemicity/population demographics.
-
G6PD status of participants (known/unknown).
-
CYP2D6 status (if available).
-
Blood‐stage antimalarial drug choice.
-
Dose/duration/timing of treatment arms.
-
Supervised or non‐supervised therapy.
-
Duration of follow‐up.
-
Adverse events.
-
Reported outcomes.
Any differences in data extraction were resolved through discussion or by consulting a third review author if necessary. We entered the extracted data into Review Manager 5 (RevMan 5) (Review Manager 2014). Where necessary, we contacted the authors of primary trials regarding missing data or methodological details of the trial. We noted any limitations in the included studies.
We grouped comparisons as illustrated in Table 1.
| Objective | Intervention | Control |
|---|---|---|
| Are higher doses (0.5 mg/kg/day or 30 mg/day primaquine for 14 days) more effective in all areas, or only in areas where they are standard treatment (East Asia and Oceania)? | Blood‐stage antimalarial drug with primaquine 0.5 mg/kg/day (adult dose 30 mg) for 14 days (total dose 420 mg). Both intervention and control groups must have received the same treatment for the blood‐borne stage of infection, that is, either CQ or ACT. | Blood‐stage antimalarial drug with standard 14‐day course primaquine (0.25 mg/kg/day, adult dose 15 mg, total dose 210 mg). Both intervention and control groups must have received the same treatment for the blood‐borne stage of infection, that is, either CQ or ACT. |
| Are shorter, higher‐dose regimens of primaquine over 7 days as effective as treatment over 14 days (is the total dose rather than the length of treatment the important factor)? | Blood‐stage antimalarial drug with primaquine 0.5 mg/kg/day (adult dose 30 mg) for 7 days (total dose 210 mg) or 1 mg/kg/day (adult dose 60 mg) for 7 days (total dose 420 mg). Both intervention and control groups must have received the same treatment for the blood‐borne stage of infection, that is, either CQ or ACT. | Blood‐stage antimalarial drug with standard 14‐day course primaquine (0.25 mg/kg/day, adult dose 15 mg, total dose 210 mg) or high‐standard 14‐day course primaquine (0.5 mg/kg/day, adult dose 30 mg, total dose 420 mg). Both intervention and control groups must have received the same treatment for the blood‐borne stage of infection, that is, either CQ or ACT. |
| Are weekly dosing regimens (0.75 mg/kg/week or 45 mg/week for 8 weeks) as effective? | Blood‐stage antimalarial drug with primaquine 0.75/kg (45 mg) per week for 8 weeks (total dose 360 mg) | Blood‐stage antimalarial drug with standard 14‐day course primaquine (0.25 mg/kg/day, adult dose 15 mg, total dose 210 mg) or high‐standard 14‐day course primaquine (0.5 mg/kg/day, adult dose 30 mg, total dose 420 mg). Both intervention and control groups must have received the same treatment for the blood‐borne stage of infection, that is, either CQ or ACT. |
Abbreviations: ACT = artemisinin‐based combination therapy; CQ = chloroquine.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias of each included trial using the Cochrane 'Risk of bias' assessment tool, discussing any differences of opinion. In the case of missing or unclear information, we contacted the trial authors for clarification. We summarized the results in the 'Risk of bias' tables in the Characteristics of included studies tables (Higgins 2011).
Measures of treatment effect
For dichotomous data, we compared interventions using risk ratios (RRs) to measure treatment effect. Where trial authors presented data as odds ratios, we recalculated the effect. We defined statistical significance as P < 0.05 and calculated 95% confidence intervals (CIs) for all results. For comparable trials, we performed meta‐analyses if there were sufficient data.
We also extracted measures of rate ratio and hazard ratio when reported by trials, and summarized these in appendices.
Unit of analysis issues
We split trials that included more than two comparison groups and analysed them as individual pair‐wise comparisons. If there was a shared control group, we split the control group so that participants were only counted once in the overall meta‐analysis.
Dealing with missing data
We analysed missing data using available‐case analysis if we judged the trial to be at low risk of bias for incomplete outcome data. We attempted to contact trial authors to obtain missing or unclear data. If the missing data rendered the result uninterpretable, we excluded the data from meta‐analyses and clearly stated the reason for exclusion. If the missing data meant that results were interpretable but likely to be at high risk of bias, we planned to use imputation methods to investigate the impact of the missing data. We analysed extracted data on an intention‐to‐treat basis where there were no missing data.
Assessment of heterogeneity
We inspected forest plots for overlapping CIs. We also applied the Chi² test as a statistical test for the presence of heterogeneity, with a P value of 0.10 used to indicate statistical significance, and we computed the I² statistic to quantify the percentage of the variability in effect estimates that was due to heterogeneity rather than sampling error (chance). We investigated possible causes of heterogeneity by subgroup analysis. If substantive heterogeneity persisted, defined as an I² statistic value of greater than 50%, we used a random‐effects meta‐analysis.
Assessment of reporting biases
We planned to examine the likelihood of reporting bias using funnel plots, however the number of included trials was insufficient to permit this.
Data synthesis
We analysed the data using RevMan 5 (Review Manager 2014). We assessed the certainty of the evidence for each outcome measure using the GRADE approach, and we constructed 'Summary of findings' tables using GRADEpro GDT (GRADEpro GDT 2015). We grouped the analyses by drug regimen and stratified results according to blood‐stage partner drug (if different blood‐stage antimalarials were used). Length of follow‐up varied with regimens and between studies. We conducted an inventory of length of follow‐up against each drug regimen and then grouped P vivax parasitaemia recurrence by appropriate groupings for length of follow‐up and stratified data accordingly.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses according to geographical region/endemicity and directly observed therapy (DOT) or non‐DOT. We had planned to perform a subgroup analysis according to CYP2D6 status, however data were insufficient to permit this.
Sensitivity analysis
We planned to assess the risk of bias of studies that contributed data to the meta‐analyses for the prespecified outcomes with sensitivity analyses against concealment of allocation.
Results
Description of studies
Results of the search
Our database update search, conducted up to 2 September 2019, identified 44 studies. We excluded 38 articles during abstract screening, and selected six studies for full‐text review. We excluded four studies with reasons provided, and included one new reference with outcome data for a previously ongoing study (Taylor 2019 MULTI) ,and one new reference with outcome data for an already included study that had not provided any data in the previous version of this review (Chu 2019 THA). Following this update search, this review now contains 11 included studies, 29 excluded studies and one ongoing study. The search results and screening process for this update are presented in a PRISMA diagram in Figure 1.
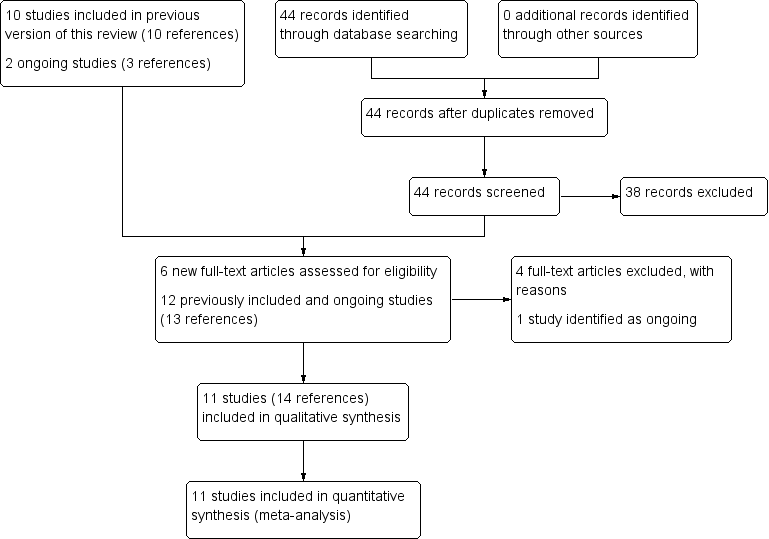
Study flow diagram.
Included studies
We included 11 studies (of 11 trials) in our quantitative analysis.
One trial was multinational and conducted in Africa (Ethiopia) and Asia (Afghanistan, Indonesia, and Vietnam) (Taylor 2019 MULTI). Four trials were conducted in South America: one in Colombia (Carmona‐Fonseca 2009 COL), one in Brazil (Abdon 2001 BRA), and two in Peru (Solari‐Soto 2002 PER; Durand 2014 PER). Six trials were conducted in Asia: one in Pakistan (Leslie 2008 PAK), two in Thailand (Bunnag 1994 THA; Chu 2019 THA), and three in India (Rajgor 2014 IND; Pareek 2015 IND; Saravu 2018 IND).
Eight trials were funded by not for profit organizations, government agencies, or academia. One trial was funded by the drug manufacturer (Pareek 2015 IND), and two trials did not report their source of funding (Bunnag 1994 THA; Abdon 2001 BRA).
All 11 trials included data for adults, and six trials included children under the age of 10 years (Solari‐Soto 2002 PER; Leslie 2008 PAK; Carmona‐Fonseca 2009 COL; Durand 2014 PER; Chu 2019 THA; Taylor 2019 MULTI). Two trials (Chu 2019 THA; Taylor 2019 MULTI) included children from the age of six months.
Nine trials excluded pregnant women, and two trials did not specify whether or not pregnant women were included (Bunnag 1994 THA; Solari‐Soto 2002 PER). Eight trials specified that lactating women were excluded, while the remaining three trials did not provide details regarding this (Bunnag 1994 THA; Solari‐Soto 2002 PER; Carmona‐Fonseca 2009 COL). Only one trial included people with G6PD deficiency (Leslie 2008 PAK). Eight trials excluded people with G6PD deficiency (Bunnag 1994 THA; Carmona‐Fonseca 2009 COL; Durand 2014 PER; Rajgor 2014 IND; Pareek 2015 IND; Saravu 2018 IND; Chu 2019 THA; Taylor 2019 MULTI), and two trials did not specify whether or not people with G6PD deficiency were included (Abdon 2001 BRA; Solari‐Soto 2002 PER).
All of the trials used microscopy for diagnosis of parasitaemia. Four trials carried out polymerase chain reaction (PCR) genotyping of vivax parasitaemia as well (Durand 2014 PER; Rajgor 2014 IND; Pareek 2015 IND; Saravu 2018 IND).
Two trials used different doses or regimens of chloroquine within trial arms, but as both confirmed that parasitaemia had resolved following treatment, we still included them in the review (see Characteristics of included studies) (Bunnag 1994 THA; Abdon 2001 BRA). None of the included trials described the CYP2D6 status of participants.
Excluded studies
We excluded 29 studies during full‐text screening; see details in Characteristics of excluded studies.
Risk of bias in included studies
A summary of the 'Risk of bias' assessments is presented in Figure 2. Full details are shown in the Characteristics of included studies tables.

‘Risk of bias' summary: review authors' judgements about each ‘Risk of bias' item for each included study.
Allocation
Six trials described adequate methods of treatment randomization and were judged to be at low risk of selection bias (Durand 2014 PER; Rajgor 2014 IND; Pareek 2015 IND; Saravu 2018 IND; Chu 2019 THA; Taylor 2019 MULTI). We assessed one trial as being at high risk of bias as it used two different methods of randomization depending on location, using house numbers or sequential patient numbers (Leslie 2008 PAK). Four trials did not detail the randomization process (Bunnag 1994 THA; Abdon 2001 BRA; Solari‐Soto 2002 PER; Carmona‐Fonseca 2009 COL).
Two trials used sealed envelopes to conceal allocation (Durand 2014 PER; Pareek 2015 IND) and an independent statistician held the group assignments in one trial (Taylor 2019 MULTI), so these three trials were assessed as being at low risk of bias. We assessed two trials with no concealment of treatment allocation as at high risk of bias (Leslie 2008 PAK; Rajgor 2014 IND), while six trials provided no information on whether or not allocation concealment was used (Bunnag 1994 THA; Abdon 2001 BRA; Solari‐Soto 2002 PER; Carmona‐Fonseca 2009 COL; Saravu 2018 IND; Chu 2019 THA).
Blinding
Eight trials were open‐label and were assessed as at high risk of performance bias (Abdon 2001 BRA; Solari‐Soto 2002 PER; Leslie 2008 PAK; Carmona‐Fonseca 2009 COL; Durand 2014 PER; Rajgor 2014 IND; Saravu 2018 IND; Chu 2019 THA). The remaining three trials reported blinding of participants and personnel, and were classified as being at low risk of performance bias (Bunnag 1994 THA; Pareek 2015 IND; Taylor 2019 MULTI). FIve trials were at high risk of detection bias (Abdon 2001 BRA; Carmona‐Fonseca 2009 COL; Durand 2014 PER; Saravu 2018 IND; Chu 2019 THA); they were all open‐label trials that did not report any details of blinding outcome assessment. Three trials were at unclear risk of detection bias. They were either double‐blind trials that did not report any details as to whether microscopy was blinded or whether there was double reading of smears (Bunnag 1994 THA; Pareek 2015 IND), or an open‐label trial that mentioned double‐checking of smears but did not clarify whether outcome assessment was blinded (Solari‐Soto 2002 PER). Three trials were at low risk of detection bias; they reported blinding of the microscopists who read the slides (Leslie 2008 PAK; Rajgor 2014 IND; Taylor 2019 MULTI).
Incomplete outcome data
Five trials had low rates of attrition with losses accounted for and so were judged as at low risk of attrition bias (Abdon 2001 BRA; Solari‐Soto 2002 PER; Carmona‐Fonseca 2009 COL; Durand 2014 PER; Pareek 2015 IND). We assessed four trials as at high risk of attrition bias. Bunnag 1994 THA had unexplained, significant loss to follow‐up (more than three‐quarters of participants by the end of the trial), making the results uninterpretable. Leslie 2008 PAK had a higher loss to follow‐up in the intervention group compared to the control group (6% loss versus 1% loss). Rajgor 2014 IND had a high percentage of missing results at six months. Saravu 2018 IND had a high percentage of loss to follow‐up in both arms by six months. Two trials were assessed as at unclear risk of attrition bias (Chu 2019 THA; Taylor 2019 MULTI), both had high rates of dropouts, but this was after one year and rates were balanced between groups and reasons for dropping out were provided.
Selective reporting
We judged six trials to have adequately reported on either prespecified or expected outcomes (Abdon 2001 BRA; Leslie 2008 PAK; Durand 2014 PER; Saravu 2018 IND; Chu 2019 THA; Taylor 2019 MULTI). Risk of reporting bias was unclear for four trials as no protocols were available (Bunnag 1994 THA; Solari‐Soto 2002 PER; Carmona‐Fonseca 2009 COL; Rajgor 2014 IND). We assessed Pareek 2015 IND as being at high risk of reporting bias because compliance was added as an outcome, primaquine levels were not reported as planned, and PCR results were not well‐detailed.
Other potential sources of bias
We assessed eight trials as at low risk of other bias (Solari‐Soto 2002 PER; Leslie 2008 PAK; Carmona‐Fonseca 2009 COL; Durand 2014 PER; Rajgor 2014 IND; Saravu 2018 IND; Chu 2019 THA; Taylor 2019 MULTI). We assessed Pareek 2015 IND to be at unclear risk of other bias as it was funded by the drug company that manufactured the primaquine preparations, and the authors were employees of the company. Another two trials, for which funding was not detailed, were also assessed as at unclear risk of other bias (Bunnag 1994 THA; Abdon 2001 BRA).
Effects of interventions
See: Summary of findings 1 Summary of findings table 1 (main comparison); Summary of findings 2 Summary of findings table 2; Summary of findings 3 Summary of findings table 3; Summary of findings 4 Summary of findings table 4
Comparison 1: 0.5 mg/kg/day for seven days versus standard 0.25 mg/kg/day for 14 days
This comparison aimed to investigate whether a shorter, higher‐dose regimen of primaquine over seven days is as efficacious as standard treatment over 14 days to determine whether the total dose rather than the length of treatment is an important factor (total dose 210 mg).
Five trials in India and South America compared 0.5 mg/kg/day of primaquine for seven days versus the standard (0.25 mg/kg/day) 14‐day regimen (same total dose 210 mg) (Abdon 2001 BRA; Solari‐Soto 2002 PER; Durand 2014 PER; Rajgor 2014 IND; Pareek 2015 IND). Pareek 2015 IND used a sustained‐release preparation of primaquine in two of the study arms (0.5 mg/kg/day sustained release and 0.25 mg/kg/day sustained release) and standard primaquine at 0.25 mg/kg/day in a third arm. We included the 0.5 mg/kg/day sustained release in the analysis and combined the results with the standard preparation at the same dose used for the other trials, but used only the standard‐preparation group of 0.25 mg/kg/day in the study as the control group and did not include the arm of 0.25 mg/kg/day sustained release preparation.
Three trials excluded people with G6PD deficiency, while two trials did not provide this information (Bunnag 1994 THA; Solari‐Soto 2002 PER). All but one trial excluded women who were pregnant or lactating (Solari‐Soto 2002 PER did not provide details). Participants were a mixture of adults and children over one‐year old. All trials used microscopy for diagnosis, and only Pareek 2015 IND did not use supervised treatment. Two trials gave chloroquine and primaquine courses simultaneously (Abdon 2001 BRA; Durand 2014 PER), while the other three trials administered primaquine following the chloroquine course. No trials stratified by age, so results were combined.
Efficacy
There was minimal difference in the number of malaria recurrences between groups at six to seven months' follow‐up (risk ratio (RR) 0.96, 95% confidence interval (CI) 0.66 to 1.39; 4 trials, 1211 participants; low‐certainty evidence; Analysis 1.1). One trial only followed participants for two months (Solari‐Soto 2002 PER), and so was not part of the main analysis.
We had planned to perform a sensitivity analysis based on risk of bias for allocation concealment (which would have involved removing Rajgor 2014 IND from the meta‐analysis), but we decided that as the remaining trials were all at high risk of bias for blinding and thus quality was generally low, we would not conduct a sensitivity analysis but address these issues in our GRADE assessment.
Two trials PCR‐adjusted their results to differentiate between relapses and new infections at six to seven months' follow‐up. In Durand 2014 PER, PCR‐adjusted results showed a 31% reduction in recurrence (24% reduction with light microscopy) with the regimen of 0.5 mg/kg/day for seven days compared with the standard 14‐day course, while in Rajgor 2014 IND, PCR‐adjusted results showed a 159% increase in recurrence (25% increase in recurrence with light microscopy) with the regimen of 0.5 mg/kg/day for seven days compared to the standard 14‐day regimen (Analysis 1.2). We decided that these results could not be combined in a meta‐analysis, as PCR techniques can differ, and there were high levels of heterogeneity.
We performed a subgroup analysis according to geographic region (Analysis 1.3). For trials in South America, the regimen of 0.5 mg/kg/day for seven days led to a 30% reduction in P vivax recurrences compared to a 19% increase in recurrences for trials in Asia, although CIs were wide and included no effect for both subgroups (South America: RR 0.70, 95% CI 0.39 to 1.26; 2 trials, 397 participants; Asia: RR 1.19, 95% CI 0.73 to 1.94; 2 trials, 814 participants). Only one trial did not use directly observed therapy (DOT) (Pareek 2015 IND). Subgroup analysis (Analysis 1.4) showed that with DOT there was minimal difference in recurrences at six to seven months between treatment regimens (RR 0.98, 95% CI 0.67 to 1.43; 1017 participants) compared to a reduction of about half of recurrences with the regimen of 0.5 mg/kg/day for seven days when treatment was not supervised (RR 0.48, 95% CI 0.04 to 5.20; 194 participants).
Adverse events
No serious adverse events were reported in either group (5 trials, 1427 participants, Analysis 1.5). The number of participants experiencing adverse events leading to discontinuation of treatment was similar in both groups (very low‐certainty evidence, RR 1.04, 95% CI 0.15 to 7.38; 5 trials, 1427 participants; Analysis 1.6). Only one study reported on adverse events during chloroquine administration (Rajgor 2014 IND), with more occurring in the group receiving 0.5 mg/kg/day for seven days than the standard 14‐day group (RR 9.40, 95% CI 0.51 to 174.01; one trial, 779 participants; Analysis 1.7). There was no difference in adverse events occurring during primaquine administration (RR 1.64, CI 0.75 to 3.57; 2 trials,1019 participants; Analysis 1.8). There was no difference between arms in other adverse events (RR 0.56, 95% CI 0.23 to 1.36; 2 trials,135 participants; Analysis 1.9).
One trial reported on change in haemoglobin status (Pareek 2015 IND), with one participant out of 120 in the group receiving 0.5 mg/kg/day for seven days becoming anaemic, versus no participants out of 120 in the standard 14‐day regimen group (very low‐certainty evidence, RR 3.00, 95% CI 0.12 to 72.91; 240 participants; Analysis 1.10).
Durand 2014 PER noted that the arms with higher daily primaquine dose did not present significantly higher frequency of the five symptoms (fever, chills, headache, muscular pain, and dark urine) monitored during treatment.
Details on the nature of the adverse events are reported in Appendix 2.
Comparison 2: high‐standard 0.5 mg/kg/day for 14 days versus standard 0.25 mg/kg/day for 14 days
The World Health Organization (WHO) recommends higher doses of primaquine (0.5 mg/kg/day) for 14 days in East Asia and Oceania. We intended to examine whether this high‐standard regimen was more efficacious in areas where it is currently recommended (East Asia and Oceania), as well as in all other areas where it has been used due to perceived resistance or strain differences.
Two trials compared the high‐standard 14‐day course with the standard (0.25 mg/kg/day) 14‐day course, both carried out in adults in India (Rajgor 2014 IND; Saravu 2018 IND). Both trials excluded pregnant/lactating women and G6PD‐deficient patients. In Rajgor 2014 IND, participants were treated with chloroquine, with the primaquine regimen (which was supervised) given after completion of the chloroquine course. In Saravu 2018 IND, participants were treated with either chloroquine or an artemisinin‐based combination therapy (ACT) (artesunate with doxycycline or artemether‐lumefantrine), and (unsupervised) primaquine was given after completion of the blood‐stage treatment. We planned to stratify results according to blood‐stage treatment; however, Saravu 2018 IND combined the results for both blood‐stage treatments, so we were unable to separate results according to partner drug. Only the blood‐stage drugs given to participants who had recurrences were described. Results from the two studies are presented separately in subgroups, but are also combined.
Efficacy
The combined estimate for both trials suggests little or no difference between the arms: (RR 0.84, 95% CI 0.49 to 1.43; 2 trials, 677 participants, low‐certainty evidence, test for subgroup differences, I2 = 0%).
In Rajgor 2014 IND, 21 participants out of 317 in the high‐standard 14‐day group had a recurrence of vivax malaria compared with 26 out of 322 in the standard 14‐day group at six‐month follow‐up, giving an 18% reduction in recurrence of parasitaemia in the high‐standard group (RR 0.82, 95% CI 0.47 to 1.43; 639 participants; Analysis 2.1). P vivax malaria recurrences were also investigated by PCR to determine whether they were true relapses or new infections. After this adjustment, results showed an 83% increase in P vivax malaria cases in the high‐standard group (RR 1.83, 95% CI 0.62 to 5.40; Analysis 2.2). Rajgor 2014 IND was at high risk of bias for allocation concealment.
Saravu 2018 IND was a small open‐label pilot trial in which participants were given either chloroquine or ACT depending on clinician's judgement of severity. 78% of participants were given chloroquine (76% in the standard arm and 80% in the high‐standard arm). In Saravu 2018 IND, two out of 18 participants in the high‐standard 14‐day group had a recurrence of P vivax malaria compared to two out of 20 in the standard 14‐day group at six months' follow‐up (RR 1.11, 95% CI 0.17 to 7.09; Analysis 2.1). Polymerase chain reaction (PCR) genotyping suggested that all four participants had true relapses of infection.
Adverse events
In Rajgor 2014 IND there were no serious adverse events reported in either study arm (778 participants). In the high‐standard 14‐day group, eight out of 380 participants discontinued treatment due to adverse events (one participant discontinued chloroquine and seven participants discontinued primaquine), compared to two out of 398 in the standard 14‐day group (both participants discontinued primaquine) (RR 4.19, 95% CI 0.90 to 19.60; 778 participants; Analysis 2.4). In the high‐standard arm during chloroquine treatment, four out of 380 participants experienced adverse events compared to zero out of 398 in the standard group (RR 9.43, 95% CI 0.51 to 174.47; 778 participants; Analysis 2.5). In the high‐standard arm during primaquine treatment, 13 out of 380 participants experienced adverse events known to occur with primaquine, compared to five out of 398 in the standard arm (RR 2.72, 95% CI 0.98 to 7.57; 778 participants; Analysis 2.5). These results could suggest a trend towards higher occurrence of adverse events in the high‐standard 14‐day regimen. Details on the nature of the adverse events are reported in Appendix 2.
No significant adverse events were noted in either group in Saravu 2018 IND.
Comparison 3: 0.75 mg/kg/week for eight weeks versus high‐standard 0.5 mg/kg/day for 14 days
This comparison aimed to investigate whether a higher once‐weekly dosing regimen, which may be more beneficial for people with G6PD deficiency, was as efficacious as the high‐standard 14‐day regimen.
One trial compared weekly 0.75 mg/kg primaquine (45 mg adult dose) for eight weeks with the high‐standard 14‐day regimen (0.5 mg/kg/day) (Leslie 2008 PAK). G6PD‐deficient participants were not randomized but were included in the weekly group, although there only was one G6PD‐deficient person included. Pregnant and lactating women were excluded. Treatment was supervised. It was not specified whether chloroquine and primaquine were given concurrently.
Efficacy
Recurrences were more common in the weekly group at eight months' follow‐up (RR 7.00, 95% CI 0.38 to 127.32; 126 participants; Analysis 3.1). Recurrences remained more common in the weekly group at 11 months' follow‐up (RR 3.18, 95% CI 0.37 to 27.60; 122 participants; very low‐certainty evidence; Analysis 3.1). Leslie 2008 PAK was at high risk of bias for allocation concealment, but a sensitivity analysis could not be done as it was the only trial found for this comparison.
Adverse events
No serious adverse events (Analysis 3.2) or notable non‐serious adverse events (Analysis 3.4) were reported in either study arm. No participants had anaemia defined as haemoglobin less than 7 g/dL (Analysis 3.3).
Comparison 4: 1 mg/kg/day for seven days versus high‐standard 0.5 mg/kg/day for 14 days
This comparison aimed to investigate whether shorter, higher doses of primaquine over seven days are as effective as the high‐standard 14‐day regimen; i.e. to determine whether the total dose rather than the length of treatment is the important factor (total dose 420 mg primaquine). Two trials conducted in Ethiopia, Afghanistan, Indonesia, Thailand, and Vietnam compared 1 mg/kg/day (adult dose 60 mg) of primaquine for seven days with the high‐standard 14‐day course (0.5 mg/kg/day, adult dose 30 mg/day) (Chu 2019 THA; Taylor 2019 MULTI), administering the regimen with either chloroquine or an ACT (dihydroartemisinin‐piperaquine (DHA‐PPQ). We stratified the results accordingly. Both trials excluded people with G6PD deficiency, however, both trials included those with G6PD deficiency in parallel observational cohorts. Women who were pregnant or lactating were excluded. Participants were a mixture of adults and children over six months old. Both trials used microscopy for diagnosis, and both trials used supervised treatment. Taylor 2019 MULTI gave chloroquine or DHA‐PPQ and primaquine courses simultaneously, while Chu 2019 THA did not specify whether primaquine was given concurrently, before, or after blood‐stage drug. No trials stratified by age, so results were combined.
Efficacy
Primary outcome
Little or no difference was detected in recurrence of P vivax malaria after 12 months between 1 mg/kg/day for seven days compared with the high‐standard 14‐day regimen in two trials (RR 1.03, 95% CI 0.82 to 1.30; 2526 participants, moderate‐certainty evidence, Analysis 4.1). Rate ratios and hazard ratios support the RR estimate and are reported in Appendix 3 The results were similar for the chloroquine subgroup (RR 0.91, 95% CI 0.67 to 1.22; 1404 participants) and for the DHA‐PPQ subgroup (RR 1.24, 95% CI 0.87 to 1.77; 1122 participants). Results were also similar for subgroups by geographical region (Analysis 4.2).
Only one trial (Chu 2019 THA) reported risk ratios at six and three months. There was little or no difference detected in recurrence of P vivax malaria after six months (RR 1.10, 95% CI 0.61 to 1.97; 1 RCT, 474 participants; Analysis 4.3) and after three months (RR 0.94, 95% CI 0.41 to 2.14; 1 RCT, 522 participants; Analysis 4.4). Results subgrouped by blood‐stage drug were similar, but results were imprecise due to wide 95% CIs.
Taylor 2019 MULTI did not report risk ratios at six and three months that could be pooled with the main results. Rate ratios from this study are presented in Appendix 3, and they also show little or no difference in recurrences between the two arms.
Secondary outcome
Taylor 2019 MULTI reported on short‐term follow‐up of P vivax parasitaemia. Little or no difference was detected for P vivax parasitaemia after 28 days (RR 0.67, 95% CI 0.11 to 3.99; 1872 participants; Analysis 4.5), and after 42 days (RR 1.00, 95% CI 0.35 to 2.85; 1872 participants; Analysis 4.5). The estimates are uncertain due to very wide 95% CIs.
Adverse events
There may be moderate to large increase in serious adverse events in the 1.0 mg/kg/day primaquine for seven days group compared with the high‐standard 0.5 mg/kg/day at 42 days (RR 12.03, 95% CI 1.57 to 92.30; 1872 participants; low‐certainty evidence; Analysis 4.6), and at one year follow‐up (RR 3.61, 95% CI 1.35 to 9.68; 1872 participants; Analysis 4.6). The absolute numbers reported by Taylor 2019 MULTI at 42 days and one year were 12 and 18 serious adverse events in the primaquine 1 mg/kg/day for seven days group among 935 participants compared to one and five in the control arm among 937 participants, respectively. Of the 12 serious adverse events reported at 42 days in the 1 mg/kg/day group, nine were regarded as being possibly, probably, or definitely related to primaquine (details of each event from Taylor 2019 MULTI supplementary files are given in Appendix 2). At one year, an additional six serious adverse events deemed unrelated to primaquine were reported in the 1 mg/kg/day group. Only one of the five serious adverse events in the high‐standard 14 day regimen (observed by 42 days) was deemed probably related to primaquine (Appendix 2).
Chu 2019 THA only provides a narrative summary and does not report serious adverse events per group. The authors noted that there were 30 serious adverse events reported; most common were methaemoglobinaemia (n = 10), haemolysis (n = 3), and presumed bacterial infection (n = 10). Four deaths occurred. None was considered related to the study drugs (follow‐up, up to 42 days).
There is probably no difference in adverse events leading to discontinuation of treatment between both groups (RR 2.50, 95% CI 0.49 to 12.87; 2 trials, 2526 participants; very low‐certainty evidence; Analysis 4.7). However, the evidence is very uncertain due to risk of bias and wide 95% CIs.
Taylor 2019 MULTI reported 1819 adverse events among the 935 participants in the seven‐day primaquine group and 1732 events among the 937 participants in the 14‐day primaquine group; Chu 2019 THA reported 169 events among the 327 participants in the seven‐day primaquine group and 173 events among the 327 participants in the 14‐day primaquine group (Analysis 4.8).
Full details on the nature of adverse events are reported in Appendix 2.
There is probably no difference in haemoglobin status between both groups (RR 0.93, CI 95% 0.62 to 1.41; 2 trials, 2440 participants; very low‐certainty evidence; Analysis 4.9). However, the evidence is very uncertain due to risk of bias and wide 95% CIs.
Comparisons 5 and 6: other regimens
0.375 mg/kg/day for 14 days versus standard 14‐day regimen
Bunnag 1994 THA compared 0.375 mg/kg/day (adult dose 22.5 mg) primaquine daily for 14 days with the standard regimen of 0.25 mg/kg/day for 14 days. There was a high loss to follow‐up, with 167 participants enrolled and only 38 completing 18 months' follow‐up, although the loss was equal in both groups at the end of follow‐up. At six months' follow‐up there were no episodes of P vivax in the experimental group (0/40) and two recurrences in the standard‐regimen group (2/33) (RR 0.17, 95% CI 0.01 to 3.34; 73 participants; Analysis 5.1), although only about half of enrolled participants were followed up at this time point. No further recurrences were described in either group up to the end of follow‐up at 18 months, but as described, the high level of unexplained dropout makes interpretation difficult.
No formal assessment of adverse events was reported, but it is mentioned in the study narrative that patients tolerated the medication well and no serious adverse effect was seen in either group.There was no drop in haematocrit, or haemoglobinuria in either group.
1.17 mg/kg/day for three days versus standard 14‐day regimen
One trial delivered the total dose of primaquine (1.17 mg/kg/day or 70 mg adult dose, total dose 210 mg) over three days versus the standard (0.25 mg/kg/day) 14‐day regimen (Carmona‐Fonseca 2009 COL). Recurrences of P vivax malaria were more common in the group receiving 1.17 mg/kg/day for three days than in the standard 14‐day group at 4 months' follow‐up (RR 3.88, 95% CI 2.11 to 7.11; 129 participants; Analysis 6.1).
Adverse events were not reported, although it was noted that there were no serious adverse events from co‐administering primaquine and chloroquine.
Discussion
Summary of main results
Comparison 1 (main comparison): 0.5 mg/kg/day for seven days versus standard 0.25 mg/kg/day for 14 days
See summary of findings Table 1
We included five randomised controlled trials (RCTs) that compared 0.5 mg/kg/day (adult dose 30 mg) primaquine for seven days with the standard 14‐day regimen (0.25 mg/kg/day). There may be little or no difference in P vivax recurrences at six to seven months when using the same total dose (210 mg) over seven days as compared to 14 days (low‐certainty evidence). No serious adverse events were reported. There may be little or no difference in the number of adverse events during primaquine treatment when using the shorter regimen as compared to the longer regimen.
We do not know whether there is any difference in the frequency of anaemia or discontinuation of treatment between groups (very low‐certainty evidence). Three trials excluded people with G6PD deficiency, and two did not provide this information, so we do not know the effect of the higher daily‐dose regimen in this group. Pregnant and lactating women were either excluded or this information was not provided.
Comparison 2: high‐standard 0.5 mg/kg for 14 days versus standard 0.25 mg/kg/day for 14 days
See summary of findings Table 2
We included two RCTs that compared 0.5 mg/kg/day primaquine (daily adult dose 30 mg) for 14 days with 0.25 mg/kg/day (daily adult dose 15 mg) for 14 days, both conducted in India. The total dose differed between arms, being 420 mg in the high standard arm and 210 mg in the standard arm. People with G6PD deficiency and pregnant or lactating women were excluded. One trial did not account for whether participants were given chloroquine or an artemisinin‐based combination therapy (ACT) for blood‐stage treatment.
There may be little or no difference in P vivax recurrences at six months with the high‐standard 14‐day course compared to the standard 14‐day course when given with chloroquine or an ACT. No differences were observed when primaquine was given either with chloroquine, or with chloroquine or ACT.
No serious events were reported in either trial. We do not know whether there is a difference in adverse events leading to discontinuation between the high‐standard 14‐day course and the standard 14‐day course (very low‐certainty evidence).
Comparison 3: 0.75 mg/kg/week for eight weeks versus high‐standard 0.5 mg/kg/day for 14 days
See summary of findings Table 3
We included one RCT that compared 0.75 mg/kg (daily adult dose 45 mg) weekly primaquine for eight weeks with the high‐standard 14‐day regimen (0.5 mg/kg/day, daily adult dose 30 mg). The total dose was 360 mg in the weekly arm versus 420 mg in the high‐standard arm. G6PD‐deficient participants were not randomized but were included in the weekly primaquine group. Only one G6PD‐deficient participant was detected during the trial and was included in the weekly group.
We do not know whether weekly primaquine reduces recurrence of P vivax compared to the high‐standard 14‐day regimen at eight to 11 months' follow‐up (very low‐certainty evidence).
No serious adverse events and no episodes of anaemia were reported.
Comparison 4: 1.0 mg/kg/day for seven days versus high‐standard 0.5 mg/kg/day for 14 days
See summary of findings Table 4
We included two RCTS (one a large multicentre trial) that compared a new high dose of 1 mg/kg/day for seven days with the high‐standard course. The total dose of 420 mg was the same for both arms. G6PD‐deficient participants were excluded from one trial; in the other trial, patients with G6PD deficiency were excluded from the randomised trial, but were enrolled into a parallel observational group receiving weekly primaquine.
There is probably little or no difference in recurrences between 1.0 mg/kg/day primaquine for seven days and the high‐standard 0.5 mg/kg/day for 14 days course at 12 months follow‐up (moderate‐certainty evidence). No differences were observed when primaquine was given with chloroquine or dihydroartemisinin‐piperaquine (DHA‐PPQ).
There may be moderate to large increase in serious adverse events with 1.0 mg/kg/day primaquine for seven days compared with the high‐standard 0.5 mg/kg/day, as reported by one trial (Taylor 2019 MULTI) at 42 days and one‐year follow‐up (low‐certainty evidence); the other trial reported narrative summary only (Chu 2019 THA). We do not know if there is difference in anaemia at 42 days follow‐up or in adverse events leading to discontinuation (very low‐certainty evidence).
Comparisons 5 and 6: other regimens
Some other included trials evaluated alternative regimens and doses of primaquine, but these regimens have not been widely used, and the evidence available from stand‐alone trials was limited.
Overall completeness and applicability of evidence
Although the evidence is currently of low certainty, it appears from Comparison 1 that using 0.5 mg/kg/day with the same total dose (210 mg) over seven days may be non‐inferior to the regimen of 0.25 mg/kg/day for 14 days. It is likely that this shorter regimen would promote course completion. No serious adverse events were reported in the five trials in this comparison. However, this seven‐day regimen was not tested in G6PD‐deficient patients in any of the RCTs meeting our inclusion criteria. This remains a concern in settings where testing is not available.
We initially planned to evaluate whether the high‐standard 14‐day regimen (0.5 mg/kg/day) was more effective in areas where recommended by the World Health Organization (WHO) due to reported resistance or strain differences (East Asia and Oceania), as well as in other areas (WHO 2015). However, the two randomized clinical trials that compared the efficacy of high‐standard to the standard regimen were both conducted in India (Rajgor 2014 IND; Saravu 2018 IND), where the high‐standard regimen is not recommended. A recent retrospective case review in French Guiana (also an area where the high‐standard regimen is not currently recommended) found that recurrences were similar in both standard and high‐standard 14‐day regimens (Valdes 2018). We did not find any RCTs that evaluated whether the high‐standard 14‐day regimen was more effective compared to the standard 14‐day regimen for the tropical, frequently relapsing strain of P vivax in East Asia and Oceania, so we are unable to comment on its efficacy.
Only one included RCT investigated the weekly primaquine regimen that is currently recommended by WHO for G6PD‐deficient individuals, and only one G6PD‐deficient participant was actually included in the trial.
We found two trials of a new high dose 1.0 mg/kg/day for seven days compared to the high standard 0.5 mg/kg/day for 14 days. There is probably no difference in frequency of recurrences between these two regimens. However one of these trials reported increased serious adverse events in the seven‐day arm.
A difficulty encountered in including and comparing studies was the variation in dosing and length of follow‐up in studies. In general, there were few well‐conducted RCTs that used an evidence‐based standard primaquine regimen (0.25 mg/kg/day for 14 days) as a comparator. Some trials used the high‐standard 0.5 mg/kg/day for 14 days regimen, which is recommended by the WHO in East Asia and Oceania, as a comparator. However, as noted above, there is limited clear evidence in this review that the high‐standard 0.5 mg/kg/day 14‐day regimen is better than the standard 0.25 mg/kg/day for 14 days.
We found that trials continue to be conducted where placebo is used instead of an alternative primaquine regimen, which is contrary to the evidence available demonstrating its superiority for reducing recurrences (Galappaththy 2013). This may be because there is continued reluctance to use primaquine in some national programmes.
We excluded studies where individuals had mixed malaria infections so as to assess the efficacy of treatment on P vivax malaria alone. Areas endemic for P vivax malaria may also be co‐endemic for Plasmodium falciparum or Plasmodium ovale infection, or both. However, it should be noted that as part of our screening process we did not identify any studies where participants with mixed malaria were included, so we do not think that narrowing our search criteria impacted the directness of our results.
There was a lack of detailed safety data for some trials, despite the fact that safety is a particular concern with primaquine use. Although more recent trials have paid more attention to this issue, they do not all report the adverse events by arm.
Certainty of the evidence
The overall certainty of evidence for most of the outcomes was either low or very low. Most results were downgraded for imprecision due to wide confidence intervals (CIs) in the meta‐analyses performed. The exception is the recent Comparison 4 using high dose 1 mg/kg/day versus high‐standard course for the efficacy outcomes, where evidence for non‐inferiority was moderate certainty.
The efficacy comparison for Comparison 2 (high‐standard 14‐day regimen versus the standard 14‐day regimen) was also downgraded for indirectness. Results were based on two trials in adults in India (Rajgor 2014 IND; Saravu 2018 IND). Rajgor 2014 IND was at risk of bias as there was no allocation concealment and unexplained loss to follow‐up; this study also contributed most to the meta‐analysis for the comparison of 0.5 mg/kg/day for 7 days versus standard 14‐day regimen, so this study was also downgraded. Saravu 2018 IND was a small pilot study where participants were given either chloroquine or an ACT for the blood stage, and which blood‐stage treatment they were given was not stated by outcome (although appeared balanced between arms). Saravu 2018 IND was downgraded for imprecision, indirectness, and risk of bias (not blinded and high rate of loss to follow‐up).
We downgraded the Comparison 3 of 0.75 mg/kg weekly primaquine versus high‐standard 14‐day regimen for indirectness as it was based on just one study conducted in Pakistan (Leslie 2008 PAK), with only one G6PD‐deficient patient participating. Leslie 2008 PAK was at risk of bias due to the randomization process used, lack of allocation concealment, and incomplete outcome data. We downgraded efficacy outcomes for this comparison for serious imprecision due to few events and very wide CIs.
Comparison 4 of high‐dose 1 mg/kg for seven days versus high‐standard 14‐day regimen was downgraded because one of the two trials was open‐label (Chu 2019 THA).
Potential biases in the review process
The strictness of our inclusion criteria to not include trials where the total dose was less than the total dose of the standard regimen, and the necessity of having the comparison arm be one of the WHO‐recommended regimens, may have meant that some relevant comparisons were excluded.
We changed the protocol to include the high‐standard 14‐day regimen that WHO recommends in East Asia and Oceania as a control regimen, as we realized that some trials had used this as the comparator, and we felt that these comparisons were useful. However, this may have introduced bias, as the evidence base for RCTs (including our results) showing the relative efficacy of this regimen is limited.
The difficulty in determining between relapse and re‐infection with P vivax remains a recognized challenge for assessing the efficacy of drugs for radical cure.
Agreements and disagreements with other studies or reviews
Three previous meta‐analyses (John 2012; Carmona‐Fonseca 2015; Zuluaga‐Idarraga 2015) of this topic have been published. John 2012 did not explicitly compare short and long schedules, and none of the reviews included the recent high‐dose 1 mg/kg/day seven‐day course trials which are evaluated here.
Our findings that 210 mg over seven days may be as efficacious as the standard course of 210 mg over 14 days (Comparison 1) are consistent with findings of other systematic reviews that examined both randomized and non‐randomized studies (Carmona‐Fonseca 2015; Zuluaga‐Idarraga 2015). The other reviews also confirm the lack of comparative evidence for the high‐standard versus standard regimens (Comparison 2), whether in the recommended geographical area or not. However we included additional recent RCTs using the standard and high‐standard schedules that were not included in Carmona‐Fonseca 2015 or Zuluaga‐Idarraga 2015. Only Carmona‐Fonseca 2015 included weekly schedules, and like us identified only a single study (with one G6PD deficient participant) evaluating 0.75 mg/kg/day once a week for eight weeks compared to high‐standard 14‐day regimen (Comparison 3). Comparison 4 includes trials that are not in the other reviews.
Other reviews also commented on the difficulty of comparing results due to the varying treatment regimens and length of follow‐up used in clinical trials.
The systematic review and individual patient data analysis of Commons 2019 showed that the adverse haematological effects of primaquine (when given with chloroquine) may be outweighed by the effect of preventing anaemia due to malaria relapses by day 42. We have not been able to investigate such a trade‐off using the studies in this review.

Study flow diagram.

‘Risk of bias' summary: review authors' judgements about each ‘Risk of bias' item for each included study.

Comparison 1: 0.5 mg/kg/day 7 days versus standard 0.25 mg/kg/day 14 days, Outcome 1: Recurrence by 6 to 7 months' follow‐up

Comparison 1: 0.5 mg/kg/day 7 days versus standard 0.25 mg/kg/day 14 days, Outcome 2: Recurrence by 6 to 7 months' follow‐up (PCR‐adjusted)

Comparison 1: 0.5 mg/kg/day 7 days versus standard 0.25 mg/kg/day 14 days, Outcome 3: Recurrence by 6 to 7 months subgrouped by geographical region

Comparison 1: 0.5 mg/kg/day 7 days versus standard 0.25 mg/kg/day 14 days, Outcome 4: Recurrence by 6 to 7 months subgrouped by directly observed therapy (DOT) versus non‐DOT

Comparison 1: 0.5 mg/kg/day 7 days versus standard 0.25 mg/kg/day 14 days, Outcome 5: Serious adverse events

Comparison 1: 0.5 mg/kg/day 7 days versus standard 0.25 mg/kg/day 14 days, Outcome 6: Adverse events that result in discontinuation of treatment

Comparison 1: 0.5 mg/kg/day 7 days versus standard 0.25 mg/kg/day 14 days, Outcome 7: Adverse events during chloroquine administration

Comparison 1: 0.5 mg/kg/day 7 days versus standard 0.25 mg/kg/day 14 days, Outcome 8: Adverse effects during primaquine administration

Comparison 1: 0.5 mg/kg/day 7 days versus standard 0.25 mg/kg/day 14 days, Outcome 9: Other adverse events

Comparison 1: 0.5 mg/kg/day 7 days versus standard 0.25 mg/kg/day 14 days, Outcome 10: Anaemia or change in haemoglobin status

Comparison 2: High‐standard 0.5 mg/kg/day 14 days versus standard 0.25 mg/kg/day 14 days, Outcome 1: Recurrence by 6 months' follow‐up

Comparison 2: High‐standard 0.5 mg/kg/day 14 days versus standard 0.25 mg/kg/day 14 days, Outcome 2: Recurrence by 6 months' follow‐up (PCR‐adjusted)

Comparison 2: High‐standard 0.5 mg/kg/day 14 days versus standard 0.25 mg/kg/day 14 days, Outcome 3: Serious adverse events

Comparison 2: High‐standard 0.5 mg/kg/day 14 days versus standard 0.25 mg/kg/day 14 days, Outcome 4: Adverse events that result in discontinuation of treatment

Comparison 2: High‐standard 0.5 mg/kg/day 14 days versus standard 0.25 mg/kg/day 14 days, Outcome 5: Other adverse events

Comparison 3: 0.75 mg/kg/week 8 weeks versus high‐standard 0.5 mg/kg/day 14 days, Outcome 1: Recurrence

Comparison 3: 0.75 mg/kg/week 8 weeks versus high‐standard 0.5 mg/kg/day 14 days, Outcome 2: Serious adverse events

Comparison 3: 0.75 mg/kg/week 8 weeks versus high‐standard 0.5 mg/kg/day 14 days, Outcome 3: Anaemia (haemoglobin < 7 g/dL)

Comparison 3: 0.75 mg/kg/week 8 weeks versus high‐standard 0.5 mg/kg/day 14 days, Outcome 4: Other adverse events

Comparison 4: 1.0 mg/kg/day primaquine 7 days versus high‐standard 0.5 mg/kg/day 14 days, Outcome 1: Recurrence by 12 months' follow‐up

Comparison 4: 1.0 mg/kg/day primaquine 7 days versus high‐standard 0.5 mg/kg/day 14 days, Outcome 2: Recurrence by 12 months' follow‐up subgrouped by geographical region

Comparison 4: 1.0 mg/kg/day primaquine 7 days versus high‐standard 0.5 mg/kg/day 14 days, Outcome 3: Recurrence by 6 months' follow‐up

Comparison 4: 1.0 mg/kg/day primaquine 7 days versus high‐standard 0.5 mg/kg/day 14 days, Outcome 4: Recurrence by 3 months' follow‐up

Comparison 4: 1.0 mg/kg/day primaquine 7 days versus high‐standard 0.5 mg/kg/day 14 days, Outcome 5: P vivax parasitaemia

Comparison 4: 1.0 mg/kg/day primaquine 7 days versus high‐standard 0.5 mg/kg/day 14 days, Outcome 6: Serious adverse events

Comparison 4: 1.0 mg/kg/day primaquine 7 days versus high‐standard 0.5 mg/kg/day 14 days, Outcome 7: Adverse events that resulted in discontinuation of treatment
| Other adverse events | ||
| Study | 1.0 mg/kg/day for 7 days | High‐standard 0.5 mg/kg/day for 14 days |
|---|---|---|
| Up to day 14 | ||
| Taylor 2019 MULTI | 1819 events in 935 participants | 1732 events in 937 participants |
| Chloroquine group, up to day 42 | ||
| Chu 2019 THA | 97 events in 165 participants | 91 events in 164 participants |
| DHA‐PPQ group, up to day 42 | ||
| Chu 2019 THA | 72 events in 162 participants | 82 events in 163 participants |
Comparison 4: 1.0 mg/kg/day primaquine 7 days versus high‐standard 0.5 mg/kg/day 14 days, Outcome 8: Other adverse events

Comparison 4: 1.0 mg/kg/day primaquine 7 days versus high‐standard 0.5 mg/kg/day 14 days, Outcome 9: Anaemia

Comparison 5: 0.375 mg/kg/day primaquine for 14 days versus standard 14‐day regimen, Outcome 1: Recurrence by 6 months' follow‐up

Comparison 6: 1.17 mg/kg/day primaquine for 3 days versus standard 14‐day regimen, Outcome 1: Recurrence by 4 months' follow‐up
| 0.5 mg/kg primaquine/day for 7 days versus standard 0.25 mg/kg/day for 14 days for radical cure of P vivax malaria | ||||||
| Patient or population: adults and children with confirmed clinical and parasitological P vivax malaria | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with standard 14‐day course primaquine | Risk with 0.5mg/kg/day primaquine for 7 days | |||||
| Recurrence of P vivax parasitaemia | 84 per 1000 | 81 per 1000 | RR 0.96 | 1211 | ⊕⊕⊝⊝ due to risk of bias and imprecision | There may be little or no difference between 0.5 mg/kg/day primaquine for 7 days and the standard 14‐day course. |
| Serious adverse events | Not estimable (0 events in 723 participants) | Not estimable (0 events in 704 participants) | Not estimable | 1427 | — | No events reported. |
| Adverse events that result in the discontinuation of treatment | 3 per 1000 | 3 per 1000 | RR 1.04 | 1427 (5 RCTs) | ⊕⊝⊝⊝ due to risk of bias and serious imprecision | We do not know if there is any difference in adverse events that result in treatment discontinuation between 0.5 mg/kg/day primaquine for 7 days and the standard 14‐day course. |
| Anaemia or change in haemoglobin status | Not estimable (0 events in 120 participants) | Not estimable (1 event in 120 participants) | RR 3.0 (0.12 to 72.91) | 240 | ⊕⊝⊝⊝ due to risk of bias, indirectness, and serious imprecision | We do not know if the occurrence of anaemia differs between the 2 treatment regimens. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded once for risk of bias: Rajgor 2014 IND, which contributed the most weight to the meta‐analysis, was at high risk of selection bias due to no allocation concealment and high risk of attrition bias. Although Pareek 2015 IND was at risk of selection bias as well as other bias for being funded and carried out by drug company, it only contributed a small amount of weight to the meta‐analysis. | ||||||
| High standard 0.5 mg/kg primaquine /day for 14 days versus standard 0.25 mg/kg/day for 14 days for radical cure of P vivax malaria | ||||||
| Patient or population: adults and children with confirmed clinical and parasitological P vivax malaria | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with standard 14‐day course primaquine | Risk with high‐standard 14‐day course primaquine | |||||
| Recurrence of P vivax parasitaemia | 82 per 1000 | 69 per 1000 | RR 0.84 | 677 | ⊕⊕⊝⊝ due to risk of bias and imprecision | There may be little or no difference in P vivax recurrences between high‐standard or standard 14‐day courses of primaquine given with chloroquine or an ACT. |
| Serious adverse events | Not estimable (0 events in 398 participants) | Not estimable (0 events in 380 participants) | Not estimable | 778 | — | No events reported. |
| Adverse events that result in the discontinuation of treatment | 5 per 1000 | 21 per 1000 | RR 4.19 | 778 | ⊕⊝⊝⊝ due to indirectness, risk of bias, and imprecision | We do not know if there is any difference in adverse events resulting in treatment discontinuation between high‐standard or standard 14‐day courses of primaquine. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: ACT: artemisinin‐based combination therapy; CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded once for risk of bias: one study was open‐label with no allocation concealment (risk of selection bias) and risk of attrition bias due to high percentage not completing six months' follow‐up with minimal explanation; the other study had no blinding and a high rate of loss to follow‐up. | ||||||
| 0.75 mg/kg primaquine /week for 8 weeks versus high‐standard 0.5 mg/kg/day for 14 days for radical cure of P vivax malaria | ||||||
| Patient or population: adults and children with confirmed clinical and parasitological P vivax malaria | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with high‐standard 14‐day course primaquine | Risk with once‐weekly 0.75 mg/kg primaquine for 8 weeks | |||||
| Recurrence of P vivax malaria | 19 per 1000 | 59 per 1000 | RR 3.18 | 122 | ⊕⊝⊝⊝ due to risk of bias and serious imprecision | We do not know if weekly primaquine reduces the risk of malaria recurrences when compared to the high‐standard 14‐day course. |
| Serious adverse events | Not estimable (0 events in 55 participants) | Not estimable (0 events in 74 participants) | Not estimable | 129 | — | No events reported. |
| Anaemia (haemoglobin < 7 g/dL) | Not estimable (0 events in 55 participants) | Not estimable (0 events in 74 participants) | Not estimable | 129 | — | No events reported. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded once for risk of bias: Leslie 2008 PAK was at high risk of bias for randomization process, allocation concealment, and incomplete outcome data. | ||||||
| 1.0 mg/kg primaquine /day for 7 days versus high‐standard 0.5 mg/kg/day for 14 days for radical cure of P vivax malaria | ||||||
| Patient or population: adults and children with confirmed clinical and parasitological P vivax malaria Settings: Afghanistan, Ethiopia, Indonesia, Thailand, and Vietnam Intervention: 1.0 mg/kg/day primaquine for 7 days (adult dose 60 mg/day; total dose 420mg) Comparison: high‐standard 14‐day course primaquine (0.5 mg/kg/day, adult dose 30 mg/day; total dose 420mg) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of Participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with high‐standard 14‐day course primaquine | Risk with 1.0 mg/kg/day primaquine for 7 days | |||||
| Recurrence of P vivax parasitaemia | 104 per 1000 | 107 per 1000 | RR 1.03 (0.82 to 1.30) | 2526 (2 RCTs) | ⊕⊕⊕⊝a due to risk of bias | There is probably little or no difference between 1.0 mg/kg/day primaquine for 7 days and the high‐standard 0.5 mg/kg/day for 14 days course |
| Serious adverse events Follow‐up: up to 42 days | 1 per 1000 | 13 per 1000 (2 to 99) | RR 12.03 (1.57 to 92.30) | 1872 (1 RCT) | ⊕⊕⊝⊝b,c LOW due to indirectness and imprecision | There may be a moderate to large increase in serious adverse events in the 1.0 mg/kg/day primaquine for 7 days compared with the high‐standard 0.5 mg/kg/day Chu 2019 THA provides overall narrative results only, see Effects of interventions text. |
| Adverse events the resulted in discontinuation of treatment | 2 per 1000 | 4 per 1000 | RR 2.50 (0.49 to 12.87) | 2526 (2 RCTs) | ⊕⊝⊝⊝a,b,d due to risk of bias, indirectness and serious imprecision | We do not know if there is any difference in adverse events resulting in treatment discontinuation between 1.0 mg/kg/day primaquine for 7 days and the high‐standard 0.5 mg/kg/day for 14 days course. |
| Anaemia Follow‐up: between 3 and 42 days follow‐up | 35 per 1000 | 33 per 1000 (22 to 50) | RR 0.93 (0.62 to 1.41) | 2440 | ⊕⊝⊝⊝a,b,e due to risk of bias, indirectness and imprecision | We do not know if there is any difference in anaemia between 1.0 mg/kg/day primaquine for 7 days and the high‐standard 0.5 mg/kg/day for 14 days course. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded once for risk of bias: Chu 2019 THA was an open‐label trial with high risk of performance and detection bias; although drop‐outs were balanced between groups the proportion of drop‐outs after one year was high in both trials (30‐40%). | ||||||
| Objective | Intervention | Control |
|---|---|---|
| Are higher doses (0.5 mg/kg/day or 30 mg/day primaquine for 14 days) more effective in all areas, or only in areas where they are standard treatment (East Asia and Oceania)? | Blood‐stage antimalarial drug with primaquine 0.5 mg/kg/day (adult dose 30 mg) for 14 days (total dose 420 mg). Both intervention and control groups must have received the same treatment for the blood‐borne stage of infection, that is, either CQ or ACT. | Blood‐stage antimalarial drug with standard 14‐day course primaquine (0.25 mg/kg/day, adult dose 15 mg, total dose 210 mg). Both intervention and control groups must have received the same treatment for the blood‐borne stage of infection, that is, either CQ or ACT. |
| Are shorter, higher‐dose regimens of primaquine over 7 days as effective as treatment over 14 days (is the total dose rather than the length of treatment the important factor)? | Blood‐stage antimalarial drug with primaquine 0.5 mg/kg/day (adult dose 30 mg) for 7 days (total dose 210 mg) or 1 mg/kg/day (adult dose 60 mg) for 7 days (total dose 420 mg). Both intervention and control groups must have received the same treatment for the blood‐borne stage of infection, that is, either CQ or ACT. | Blood‐stage antimalarial drug with standard 14‐day course primaquine (0.25 mg/kg/day, adult dose 15 mg, total dose 210 mg) or high‐standard 14‐day course primaquine (0.5 mg/kg/day, adult dose 30 mg, total dose 420 mg). Both intervention and control groups must have received the same treatment for the blood‐borne stage of infection, that is, either CQ or ACT. |
| Are weekly dosing regimens (0.75 mg/kg/week or 45 mg/week for 8 weeks) as effective? | Blood‐stage antimalarial drug with primaquine 0.75/kg (45 mg) per week for 8 weeks (total dose 360 mg) | Blood‐stage antimalarial drug with standard 14‐day course primaquine (0.25 mg/kg/day, adult dose 15 mg, total dose 210 mg) or high‐standard 14‐day course primaquine (0.5 mg/kg/day, adult dose 30 mg, total dose 420 mg). Both intervention and control groups must have received the same treatment for the blood‐borne stage of infection, that is, either CQ or ACT. |
| Abbreviations: ACT = artemisinin‐based combination therapy; CQ = chloroquine. | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Recurrence by 6 to 7 months' follow‐up Show forest plot | 4 | 1211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.66, 1.39] |
| 1.2 Recurrence by 6 to 7 months' follow‐up (PCR‐adjusted) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.3 Recurrence by 6 to 7 months subgrouped by geographical region Show forest plot | 4 | 1211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.66, 1.39] |
| 1.3.1 South America | 2 | 397 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.39, 1.26] |
| 1.3.2 Asia | 2 | 814 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.73, 1.94] |
| 1.4 Recurrence by 6 to 7 months subgrouped by directly observed therapy (DOT) versus non‐DOT Show forest plot | 4 | 1211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.66, 1.39] |
| 1.4.1 DOT | 3 | 1017 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.67, 1.43] |
| 1.4.2 Non‐DOT | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.04, 5.20] |
| 1.5 Serious adverse events Show forest plot | 5 | 1427 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.6 Adverse events that result in discontinuation of treatment Show forest plot | 5 | 1427 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.15, 7.38] |
| 1.7 Adverse events during chloroquine administration Show forest plot | 1 | 779 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.40 [0.51, 174.01] |
| 1.8 Adverse effects during primaquine administration Show forest plot | 2 | 1019 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.75, 3.57] |
| 1.9 Other adverse events Show forest plot | 2 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.23, 1.36] |
| 1.10 Anaemia or change in haemoglobin status Show forest plot | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.12, 72.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Recurrence by 6 months' follow‐up Show forest plot | 2 | 677 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.49, 1.43] |
| 2.1.1 6 months (chloroquine blood‐stage treatment) | 1 | 639 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.47, 1.43] |
| 2.1.2 6 months (chloroquine or ACT blood‐stage treatment) | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.17, 7.09] |
| 2.2 Recurrence by 6 months' follow‐up (PCR‐adjusted) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.3 Serious adverse events Show forest plot | 1 | 778 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.4 Adverse events that result in discontinuation of treatment Show forest plot | 1 | 778 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.19 [0.90, 19.60] |
| 2.5 Other adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.5.1 AEs during chloroquine treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.5.2 AEs during primaquine treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Recurrence Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1.1 5 months | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.23 [0.28, 99.15] |
| 3.1.2 8 months | 1 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.00 [0.38, 127.32] |
| 3.1.3 11 months | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.18 [0.37, 27.60] |
| 3.2 Serious adverse events Show forest plot | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.3 Anaemia (haemoglobin < 7 g/dL) Show forest plot | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.4 Other adverse events Show forest plot | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Recurrence by 12 months' follow‐up Show forest plot | 2 | 2526 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.82, 1.30] |
| 4.1.1 Chloroquine blood‐stage treatment | 2 | 1404 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.67, 1.22] |
| 4.1.2 DHA‐PPQ blood‐stage treatment | 2 | 1122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.87, 1.77] |
| 4.2 Recurrence by 12 months' follow‐up subgrouped by geographical region Show forest plot | 2 | 2526 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.82, 1.29] |
| 4.2.1 Afghanistan | 1 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.44, 1.28] |
| 4.2.2 Ethiopia | 1 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.74, 2.30] |
| 4.2.3 Indonesia | 1 | 797 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.86, 2.03] |
| 4.2.4 Thailand | 1 | 654 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.57, 1.30] |
| 4.2.5 Vietnam | 1 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.43, 2.53] |
| 4.3 Recurrence by 6 months' follow‐up Show forest plot | 1 | 474 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.61, 1.97] |
| 4.3.1 Chloroquine blood‐stage treatment | 1 | 256 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.42, 1.86] |
| 4.3.2 DHA‐PPQ blood‐stage treatment | 1 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.60, 4.05] |
| 4.4 Recurrence by 3 months' follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.4.1 Chloroquine blood‐stage treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.4.2 DHA‐PPQ blood‐stage treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.5 P vivax parasitaemia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.5.1 Day 28 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.5.2 Day 42 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.6 Serious adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.6.1 Up to 42 days follow‐up | 1 | 1872 | Risk Ratio (M‐H, Fixed, 95% CI) | 12.03 [1.57, 92.30] |
| 4.6.2 Up to 1 year follow‐up | 1 | 1872 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.61 [1.35, 9.68] |
| 4.7 Adverse events that resulted in discontinuation of treatment Show forest plot | 2 | 2526 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.50 [0.49, 12.87] |
| 4.8 Other adverse events Show forest plot | 2 | Other data | No numeric data | |
| 4.8.1 Up to day 14 | 1 | Other data | No numeric data | |
| 4.8.2 Chloroquine group, up to day 42 | 1 | Other data | No numeric data | |
| 4.8.3 DHA‐PPQ group, up to day 42 | 1 | Other data | No numeric data | |
| 4.9 Anaemia Show forest plot | 2 | 2440 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.62, 1.41] |
| 4.9.1 Up to 3 days | 1 | 1786 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.38, 2.66] |
| 4.9.2 Up to 42 days follow‐up | 1 | 654 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.58, 1.44] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Recurrence by 6 months' follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1.1 6 months' follow‐up | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 3.34] |
| 5.1.2 12 months' follow‐up | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 5.1.3 18 months' follow‐up | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 Recurrence by 4 months' follow‐up Show forest plot | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.88 [2.11, 7.11] |

