Trihexifenidilo para la distonía en la parálisis cerebral
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012430.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 15 mayo 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Problemas de desarrollo, psicosociales y de aprendizaje
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Adrienne R Harvey informed the protocol design, applied eligibility criteria, assessed studies, extracted data, and led the review write‐up.
Louise B Baker conceived and designed the review protocol and assisted with the review write‐up.
Dinagh Susan Reddihough informed the protocol design and assisted with the review write‐up.
Adam Scheinberg informed the protocol design, applied eligibility criteria, assessed studies, extracted data and assisted with the review write‐up.
Katrina Williams informed the protocol design, acted as the third reviewer and assisted with the review write‐up.
Sources of support
Internal sources
-
Department of Paediatrics, The University of Melbourne, Australia.
Work on this review was completed by AH during office hours whilst employed by The University of Melbourne
External sources
-
None, Other.
Declarations of interest
Adrienne R Harvey is an Editor with the Cochrane Developmental, Psychosocial and Learning Problems Group (CDPLPG). She is funded through a Melbourne Children's Campus Career Development Award.
Louise B Baker was supported by the Lorenzo and Pamela Galli Charitable Trust during the early stages of this Cochrane Review, which was during the early stages of her Fellowship.
Dinah Susan Reddihough has received a grant from the Flack Trust, a charitable trust, for previous pilot work on the use of medications (benzhexol hydrochloride) for dystonia. The Flack Trust have no interest in the review’s findings that might lead to a real or perceived conflict of interest.
Adam Scheinberg ‐ none known.
Katrina Williams (KW) is an Editor with CDPLPG. KW declares that her position as APEX Australia Chair of Developmental Medicine is funded jointly by AFRID (APEX Foundation for Research into Intellectual Disability) and the Royal Children’s Hospital Foundation.
None of the authors were involved in the editorial processes associated with the publication of this review.
Acknowledgements
The review team acknowledge the invaluable advice and support of the Cochrane Developmental, Psychosocial and Learning Problems Group (CDPLPG), including Geraldine Macdonald (Cochrane Co‐ordinating Editor), Joanne Duffield (Managing Editor), and the statistician and external peer reviewers. We also thank Margaret Anderson, the Information Specialist with CDPLPG for her assistance with designing the search strategy and conducting the search.
In addition, we acknowledge the contributions of Sarah Arnup who assisted with data analysis and interpretation, and Dr Kristine Egberts, who contributed significantly to the development of the protocol (Baker 2017).
Version history
| Published | Title | Stage | Authors | Version |
| 2018 May 15 | Trihexyphenidyl for dystonia in cerebral palsy | Review | Adrienne R Harvey, Louise B Baker, Dinah Susan Reddihough, Adam Scheinberg, Katrina Williams | |
| 2016 Nov 16 | Trihexyphenidyl for dystonia in cerebral palsy | Protocol | Louise B Baker, Adrienne R Harvey, Kristine J Egberts, Dinah Susan Reddihough, Adam Scheinberg, Katrina Williams | |
Differences between protocol and review
-
General
-
Throughout the review, we have reworded the outcomes of ‘increased activity’, ‘ improved participation in activities of daily living’, ‘reduced pain’ and ‘improved quality of life’ to the following, more neutral formulations, to reflect that we are assessing the variable rather than improvement in the variable: activity, participation, pain and quality of life.
-
We are using the most recent chapters of the Cochrane Handbook for Systematic Reviews of Interventions.
-
-
Selection of studies
-
The protocol, Baker 2017, stated that LBB and ARH would independently screen the titles and abstracts of the citations identified from the search and obtain the full texts of those studies that met, or seemed likely to have met, the inclusion criteria and assess them for relevance. However, ARH and AS did this.
-
The protocol stated that the other members of the review team (KJE, DSR, AS, KW) would act as arbiters in the event of dispute; however, KW was the arbiter.
-
-
Data extraction and management
-
The protocol stated that LBB and ARH would independently extract data from the included studies using a data extraction form designed and piloted for this review, and that disagreements would be resolved through consultation with the other authors (KJE, DSR, AS, KW). ARH and AS performed the data extraction, resolving disagreements in consultation with KW.
-
The protocol stated that LBB would enter data into RevMan 2014 and that ARH or KJE would check it for accuracy. ARH performed data entry, and KW checked it for accuracy.
-
-
Asessment of risk of bias in included studies
-
The protocol stated that LBB and ARH would independently assess the risk of bias in the included studies and that they would resolve disagreements by consulting with the other review authors (KJE, DSR, AS, KW). ARH and AS performed the 'Risk of bias' assessment, with KW resolving disagreements.
-
-
Measures of treatment effect
-
The protocol stated that we would use an odds ratio (OR) for dichotomous data. However, adverse effects (the only dichotomous outcome in this review) were frequent in the included trial, so the risk ratio and odds ratio differ markedly. We reported the risk ratio (RR), as we believe it to be the more interpretable statistic.
-
The protocol stated that for continuous data we would use final values unless some of the studies used change scores, and that we would combine studies that reported final values with studies that reported only change scores in the same meta‐analysis, provided that the studies used the same rating scale. However, due to the one included study using change scores, we performed an ANCOVA to estimate the change score, as recommended in section 9.4.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017).
-
-
Summary of findings
-
There was inconsistency within the Methods section of the protocol around which outcomes we would use to populate the 'Summary of findings' table. The 'Types of outcome measures' section stated that we would use the outcomes marked with an asterisk, which was inconsistent with the 'Summary of findings' section. We have chosen to adhere to what is stated in the Types of outcome measures section, as this was our intention.
-
-
Search methods
-
In addition to Ovid MEDLINE, which is updated weekly, we searched Ovid MEDLINE In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE Epub Ahead of Print, which are updated daily.
-
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adolescent; Child; Child, Preschool; Female; Humans; Male;
PICO
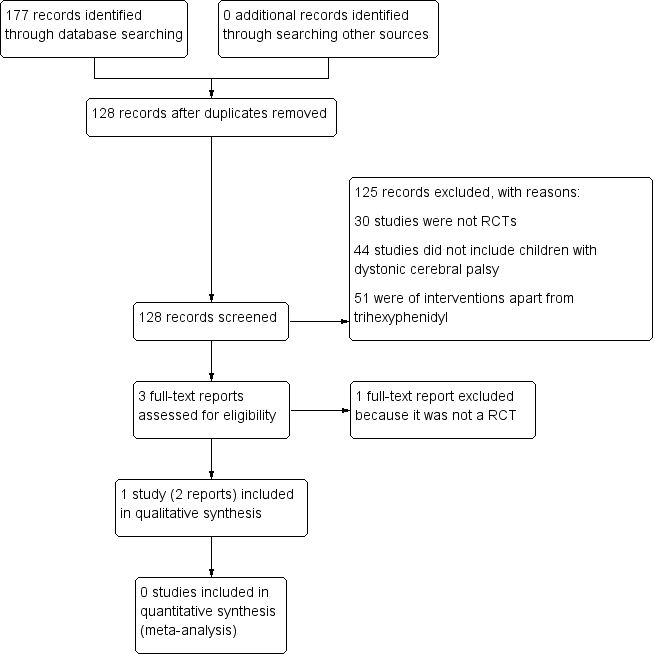
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
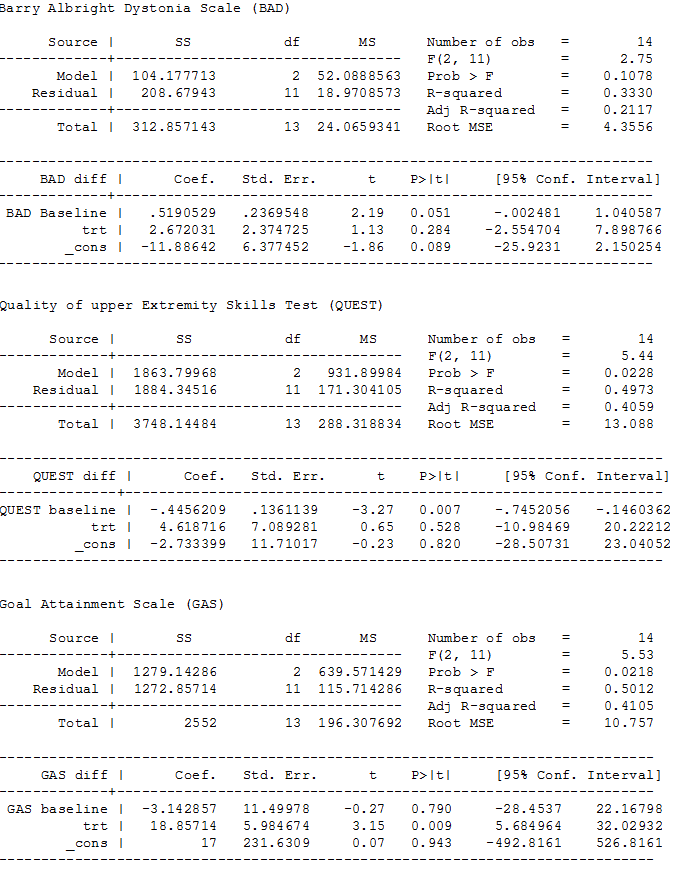
ANCOVA analyses, page 1
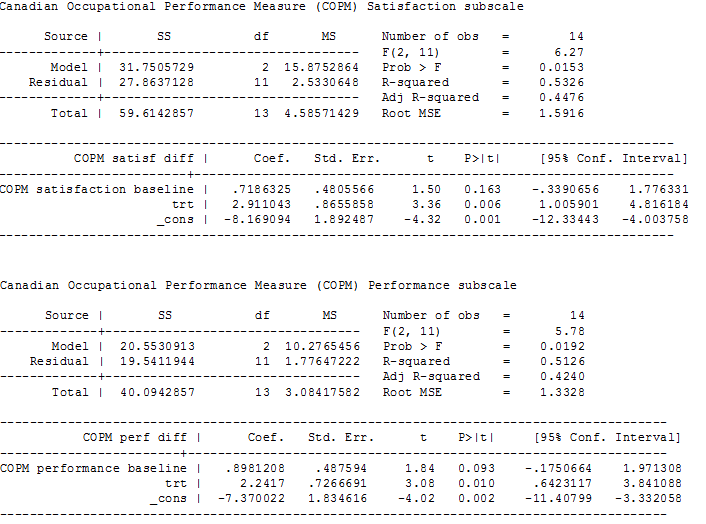
ANCOVA analyses, page 2

Comparison 1 Trihexphenidyl versus placebo, Outcome 1 Adverse effects.
| Trihexyphenidyl compared with placebo for dystonia in cerebral palsy | |||||
| Patient or population: children with dystonic cerebral palsy Settings: one tertiary care hospital Intervention: trihexyphenidyl Comparison: placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Placebo | Trihexyphenidyl | ||||
| Change in dystonia from baseline Measured by: BADS (eight body regions assessed for dystonia on a five‐point scale (0 = none to 4 = severe), minimum score 0 to maximum score 32; higher score = greater severity of dystonia) Follow‐up: 12 weeks | The mean follow‐up score in the control group was 15.50 points | The mean follow‐up score in the intervention group was 2.67 points higher (2.55 lower to 7.90 higher) | — | 16 | ⊕⊕⊝⊝ Lowa |
| Adverse effectsb (mood disturbance, irritability, behavioural change, constipation) Measured by: counts of number and type Follow‐up: various (includes data assessed at both 12 and 28 weeks) | 375 per 1000 | 1000 per 1000 | RR 2.54 (1.38 to 4.67) | 16 | ⊕⊕⊝⊝ |
| Participation in activities of daily living:individual goal setting Measured by: GAS (up to 5 functional goals scored on a 5‐point scale (−2 = much less than expected to +2 = much more than expected); higher score = better than expected outcome) Follow‐up: 12 weeks | The mean follow‐up score in the control group was 27.63 points | The mean follow‐up score in the intervention group was 18.86 points higher (5.68 higher to 32.03 higher) | — | 16 | ⊕⊕⊝⊝ |
| Participation in activities of daily living:satisfaction with individual goals Measured by: satisfaction subscale of the COPM (satisfaction with performance in up to 5 problem areas scored on a 10‐point scale (1 = not satisfied at all to 10 = extremely satisfied); higher score = greater satisfaction) Follow‐up: 12 weeks | The mean follow‐up score in the control group was 2.96 points | The mean follow‐up score in the intervention group was 2.91 points higher (1.01 higher to 4.82 higher) | — | 16 | ⊕⊕⊝⊝ |
| Participation in activities of daily living:performance of individual goals Measured by: performance subscale of the COPM (up to five problem areas scored on a 10‐point scale (1 = not able to do to 10 = able to do extremely well; higher score = better performance) Follow‐up: 12 weeks | The mean follow‐up score in the control group was 3.14 points | The mean follow‐up score in the intervention group was2.24 points higher (0.64 higher to 3.84 higher) | — | 16 | ⊕⊕⊝⊝ |
| Quality of life | Not measured | ||||
| *The basis for the assumed risk is provided in the footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded two levels due to imprecision; small sample size from one study only. | |||||
| Method | Unused methods |
| Measures of treatment effect | Continuous data For continuous outcomes we will calculate the MD and corresponding 95% CI if studies use the same rating scales. We will calculate the SMD with 95% CIs if studies use different scales to measure the same outcomes. As recommended in section 9.4.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017), we will focus on final values unless some of the studies use change scores. We will combine studies that report final values with studies that report only change scores in the same meta‐analysis, provided that the studies use the same rating scale. We will conduct the analysis according to age, as children and adults respond differently to medication. We will combine the data from all groups in studies that have trihexyphenidyl in more than one group (i.e. different frequencies) and then separate these when performing the subgroup analysis to see how the different frequencies influence the results (see item four in the Subgroup analysis and investigation of heterogeneity section). |
| Multiple outcomes If studies provide multiple, interchangeable measures of the same construct at the same point in time, we will calculate the average SMD across the outcomes and the average estimated variances, as recommended in section 16.1.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). | |
| Unit of analysis issues | Cluster‐RCTs If included trials use cluster randomisation, we will extract an ICC and use this to reanalyse the data. Where no ICC is given and a unit of analysis error appears to exist, we will contact the trial authors and ask them to provide either an ICC or the raw data to enable calculation of an ICC. Where no ICC is made available, we will search for similar studies from which we can impute an ICC, or seek statistical advice to obtain an estimate of the ICC. |
| Dealing with missing data | We will contact trial investigators to request missing data. If the trialists provide missing data, we will conduct a meta‐analysis according to intention‐to‐treat principles using all data and keeping participants in the treatment group to which they were originally randomised, regardless of the treatment they actually received, as recommended in section 16.1.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). If missing data are not provided, we will analyse only the available data. If there is concern regarding a high level of missing data, such that data could not be included in a meta‐analysis, we will include a qualitative summary in the text of the review. We will document missing data and attrition in the 'Risk of bias' tables, and we will explore how missing data might affect the interpretation of the results by conducting a sensitivity analysis. |
| Assessment of heterogeneity | We will assess clinical heterogeneity by comparing the between‐trials distribution of participant characteristics (e.g. children versus adults) and intervention characteristics (e.g. treatment type and dose), and assess methodological heterogeneity by comparing trial characteristics (e.g. cross‐over versus parallel design). We will evaluate statistical heterogeneity using the I2 statistic and the Chi2 test of heterogeneity, with statistical significance set at P value < 0.10. As recommended in section 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017), we will consider I2 values as follows.
We will report Tau2 as an estimate of the between‐study variance when reporting the results from the random‐effects model. |
| Assessment of reporting biases | If we identify 10 or more studies, we will use funnel plots to investigate the relationship between intervention effect and study size. We will explore possible reasons for any asymmetry found. We will analyse the funnel plot of the data to ascertain asymmetry. Asymmetry of a funnel plot may indicate, among other things, publication bias or poor methodological quality (Egger 1997). |
| Data synthesis | We will synthesise results in a meta‐analysis using a fixed‐effect model when studies are similar enough with regard to the intervention, population and methods, to assume that the same treatment effect is estimated. We will synthesise results in a meta‐analysis using a random‐effects model when statistical heterogeneity is found or when studies differ enough with regard to the intervention, population, and methods, to assume that different yet related treatment effects are estimated, and when it is deemed to be clinically relevant, as recommended in section 9.4.3.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017). |
| Subgroup analysis and investigation of heterogeneity | We will conduct the subgroup analyses listed below.
We will also look at the number of participants per study to determine if this is sufficient to perform a subgroup analysis. |
| Sensitivity analysis | We will conduct sensitivity analyses to investigate the effect on the overall results of excluding trials that meet the criteria described below.
We will also conduct a sensitivity analysis for studies with very low risk of bias. In addition, we will conduct a sensitivity analysis using a range of ICCs to assess the impact on treatment effect. |
| CI: confidence intervals; ICC: intraclass correlation coefficient; MD: mean difference; SMD: standardised mean difference. | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

