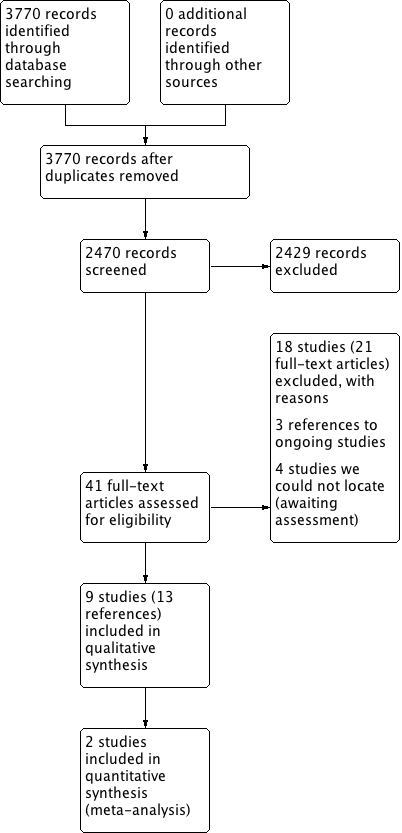
| High‐dose versus low‐dose intranasal corticosteroids for chronic rhinosinusitis |
| Patient or population: chronic rhinosinusitis (all studies recruited patients with bilateral polyps)
Setting: studies mostly conducted in Europe/North America about 10 years ago, in secondary care settings
Intervention: high‐dose intranasal corticosteroids
Comparison: low‐dose intranasal corticosteroids |
| Disease‐specific health‐related quality of life | Not measured | Impact unknown |
| Disease severity ‐ overall symptoms, measured as average change from baseline at 4 months |
| All 4 EPOS domains | No information available | |
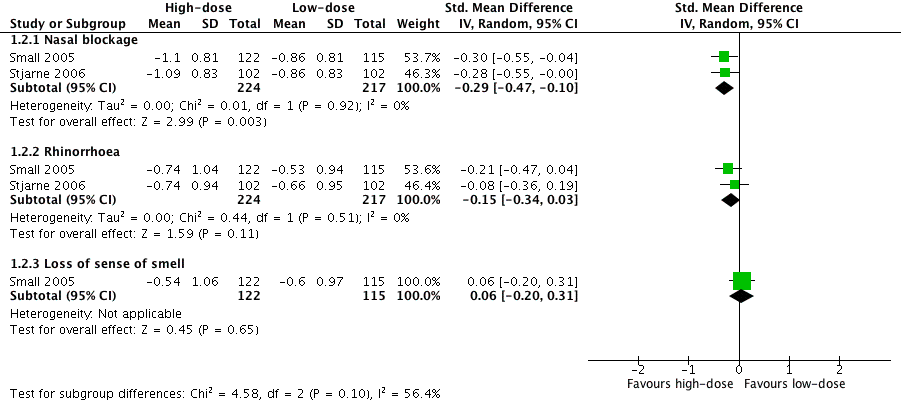
| 3 domains (nasal blockage, rhinorrhoea, loss of sense of smell) Range 0 to 3, lower score = less severe № of participants: 237
(1 RCT) | — | The mean disease severity ‐ overall symptoms, measured as average change from baseline at 4 months (range 0 to 3) ‐ average symptom score (3 domains) without high‐dose was ‐0.66 points | — | MD 0.13 points lower (0.37 lower to 0.11 more) than low‐dose group | ⊕⊕⊝⊝
LOW 1 2 3 | The average score for 3 types of symptoms seems to be similar between the high‐dose and low‐dose groups. |
| (2 domains: nasal blockage, rhinorrhoea)
Range 0 to 3, lower score = less severe № of participants: 441
(2 RCTs) | — | The mean disease severity ‐ overall symptoms, measured as average change from baseline at 4 months (range 0 to 3) ‐ average symptom score (2 domains) without high‐dose was ‐0.73 points | — | MD 0.19 points lower (0.36 lower to 0.02 lower) than low‐dose group | ⊕⊕⊝⊝
LOW 1 2 3 | The average score for 2 types of symptoms seems to be slightly lower for the high‐dose group. The clinical significance of this reduction is unclear. |
| Disease severity ‐ measured as average change from baseline at 4 months (range 0 to 3) |
| № of participants: 441
(2 RCTs) | — | The mean disease severity ‐ individual symptoms, measured as average change from baseline at 4 months (range 0 to 3) ‐ nasal blockage without high‐dose was ‐0.86 points | — | MD 0.24 points lower (0.39 lower to 0.08 lower) than low‐dose group | ⊕⊕⊝⊝
LOW 1 2 3 | The nasal blockage score seems to be slightly lower in the high‐dose group. The clinical significance of this reduction is unclear. |
| № of participants: 441 (2 RCTs) | — | The mean disease severity ‐ individual symptoms, measured as average change from baseline at 4 months (range 0 to 3) ‐ rhinorrhoea without high‐dose was ‐0.6 points | — | MD 0.15 points lower (0.33 lower to 0.03 higher) than low‐dose group | ⊕⊕⊝⊝
LOW 1 2 3 | The average score for rhinorrhoea seems to be similar between the high‐dose and low‐dose groups. |
| № of participants: 237
(1 RCT) | — | The mean disease severity ‐ individual symptoms, measured as average change from baseline at 4 months (range 0 to 3) ‐ loss of sense of smell without high‐dose was ‐0.6 points | — | MD 0.06 points higher (0.2 lower to 0.32 higher) than low‐dose group | ⊕⊕⊝⊝
LOW 1 2 3 | The average score for loss of sense of smell seems to be very similar between the high‐dose and low‐dose groups. |
| Adverse effects: epistaxis № of participants: 637
(4 RCTs) | RR 2.06
(1.20 to 3.54) | Study population | ⊕⊕⊕⊝
MODERATE 4 5 | The risk of epistaxis is likely to be higher in the higher‐dose groups. However, the studies included very minor nosebleeds, such as blood stains in the mucus, and most of these events are not likely to be severe. |
| 57 per 1000 | 118 per 1000
(69 to 202) | 61 more per 1000 (11 more to 145 more) |
| Moderate |
| 60 per 1000 | 124 per 1000
(72 to 214) | 64 more per 1000 (12 more to 153 more) |
| Adverse effects: local irritation № of participants: 542
(3 RCTs) | RR 0.97
(0.28 to 3.31) | Study population | ⊕⊕⊝⊝
LOW 4 6 7 | The risk of local irritation seems to be similar between groups, but the overall risks are underestimated due to the way the data were reported. |
| 19 per 1000 | 18 per 1000
(5 to 62) | 10 fewer per 1000 (13 fewer to 43 more) |
| Moderate |
| 17 per 1000 | 17 per 1000
(5 to 58) | 10 fewer per 1000 (13 fewer to 40 more) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: confidence interval; EPOS: European Position Paper on Rhinosinusitis and Nasal Polyps 2012;MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; SD: standard deviation |
| GRADE Working Group grades of evidence
High quality: We are very confident that the true effect lies close to that of the estimate of the effect
Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect
Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect |