Corticosteroides orales de corta duración solos para la rinosinusitis crónica
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | 2‐arm, non‐blinded, parallel‐group RCT, with a 2‐week duration of treatment and follow‐up | |
| Participants | Location: Spain, 1 site Setting of recruitment and treatment: rhinology and smell clinic, department of otorhinolaryngology, hospital clinic, Barcelona Sample size: Number randomised: 92 (it is unclear if these were all randomised or if the 3 drop‐outs occurred prior to randomisation) Number completed: 67 in intervention group, 22 in comparison group Participant (baseline) characteristics:
Other important effect modifiers:
Inclusion criteria: diagnosis of bilateral nasal polyps was based on the EPOS criteria: "Presence of two or more nasal symptoms, one of which should be either nasal blockage or nasal discharge, and/or the reduction/loss of sense of smell, and/or facial pain for more than 12 weeks, and/or the presence of nasal polyps by nasal endoscopy or mucosal changes within the ostiomeatal complex, and/or paranasal sinuses by computed tomography (CT) scan" Exclusion criteria: none listed | |
| Interventions | Intervention (n = 67): oral prednisone (30 mg daily for 4 days followed by a 2‐day reduction of 5 mg) and intranasal budesonide 800 μg daily (400 μg twice daily) for 2 weeks Comparator group (n = 22): no corticosteroid treatment for 2 weeks Use of additional interventions (common to both treatment arm): both groups had a 4‐week washout period for intranasal and oral steroids. No other adjunct treatment is listed. | |
| Outcomes | Outcomes of interest in the review: Primary outcomes
Secondary outcomes
Other outcomes reported by the study:
| |
| Funding sources | "This article was partially sponsored by a research project from Fondo de Investigacion Sanitaria (FIS 99–0133), Instituto de Salud Carlos III." There was no information regarding the funding of the original study. | |
| Declarations of interest | No information is provided in the paper | |
| Notes | In the group receiving oral steroids, the treatment period was followed up by a 10‐week course of INCS. The "no steroids treatment" group were not followed up after 2 weeks and so these results have not been presented. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "…randomised…" Comment: no information about the randomisation procedures for the trial |
| Allocation concealment (selection bias) | Unclear risk | Comment: there is no information about the allocation concealment. Given the trial was not blinded, there is reason to believe that the study personnel may have been able to influence the groups into which participants were allocated. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: the trial does not appear to be blinded to patients or study personnel. The comparison group had no steroid treatment and did not receive placebo. |
| Blinding of outcome assessment (detection bias) | High risk | Comment: the main outcomes were subjective outcomes rated by patients. There was no mention of blinding of outcome assessors (for endoscopy) included in the paper. Since the control arm received no treatment, the risk of bias is high. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: paper indicates that 92 patients were recruited but only 89 patients are accounted for in the analysis. The 3 drop‐outs are identified but there is no indication if they dropped out prior to randomisation or after randomisation. The impact of the 3 drop‐outs (3%) is likely to be small. |
| Selective reporting (reporting bias) | Unclear risk | Comment: adverse event data are not presented in the paper. No protocol for the trial could be found. |
| Other bias | Unclear risk | Comment: only details of the validation of the smell test used in the study (BAST‐24) were provided. No details of validation for other outcomes (e.g. 0‐ to 3‐point Likert scale used to assess nasal congestion). |
| Methods | 2‐arm, non‐blinded, parallel‐group RCT, with 2‐week duration of treatment and follow‐up | |
| Participants | Location: Spain, 1 site Setting of recruitment and treatment: rhinology unit, ENT department, hospital clinic, Barcelona Sample size: 84 Number randomised: 63 in intervention group, 21 in comparison group Number completed: 63 in intervention group, 21 in comparison group Participant (baseline) characteristics:
Other important effect modifiers:
Inclusion criteria: diagnosis of severe nasal polyps based on nasal endoscopic examination (mean score of 2.7 over 3 using the Lildholdt score) Nasal polyps score: 0 – no polyps; 1 – mild polyposis; 2 – moderate polyposis; 3 – severe polyposis Exclusion criteria: patients with a steroid contraindication | |
| Interventions | Intervention (n = 63): oral prednisone, 30 mg daily for 4 days followed by 2‐day reduction of 5 mg, total duration 14 days Comparator group (n = 21): no steroid treatment Use of additional interventions (common to both treatment arms): asthmatic patients did not modify their treatment during the study. No patients were receiving treatment with leukotriene antagonists. (Note: both groups had a 4‐week washout period for intranasal and oral steroids) | |
| Outcomes | Outcomes of interest in the review: Primary outcomes:
Secondary outcomes
Other outcomes reported by the study:·
| |
| Funding sources | "Generalitat de Catalunya (2001SGR00384), Red RESPIRA (FIS, V‐2003‐REDC11D‐0) and Fondo de Investigaciones Sanitarias (99‐3121m PI020329)" | |
| Declarations of interest | No information provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: study design details that the study was "randomised" but no further details were provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details about allocation concealment were provided in the paper |
| Blinding of participants and personnel (performance bias) | High risk | Comment: the control group received no steroid treatment or placebo. No mention of blinding of participants or personnel was included in the paper. |
| Blinding of outcome assessment (detection bias) | High risk | Comment: the main outcomes were subjective outcomes rated by patients. There was no mention of blinding of outcome assessors (for endoscopy) included in the paper. Since the control arm received no treatment, the risk of bias is high. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: there was no mention of anyone who dropped out of the trial or had to discontinue for any reason. However, they also did not state how many patients were analysed for each outcome. |
| Selective reporting (reporting bias) | Unclear risk | Comment: adverse event data are not presented in the paper. No protocol for the trial could be found. |
| Other bias | Unclear risk | Comment: no information is provided about the validation of the symptom scores used |
| Methods | 2‐arm, double‐blind, parallel‐group RCT, with a 17‐day duration of oral steroid treatment | |
| Participants | Location: Turkey, 1 site Setting of recruitment and treatment: Department of Otorhinolaryngology ‐ Head and Neck Surgery, Dokuz Eylul University Hospital Sample size: 23 Number randomised: 11 in intervention group, 12 in comparison group Number completed: 10 in intervention group, 12 in comparison group Participant (baseline) characteristics:
Inclusion criteria: nasal polyps, age between 18 and 65 years, endoscopic stage II or III nasal polyposis patients Nasal polyps score: graded according to the following criteria: 0: no polyp; 1: mild polyposis (small polyps not reaching the upper edge of the inferior turbinate); 2: moderate polyposis (medium polyps between the upper and lower edges of the inferior turbinate); 3: severe polyposis (large polyps reaching the lower edge of the inferior turbinate, polyps from posterior ethmoidal sinuses, or both) Exclusion criteria: hypertension, type I or II diabetes mellitus, the signs of systemic infection, pregnancy or lactation, any type of M. tuberculosis infection, peptic ulcer, viral infection (measles, chicken pox or ocular herpes), myasthenia gravis, stage I nasal polyposis, aspirin intolerance, previous major head trauma | |
| Interventions | Intervention (n = 11): prednisolone, oral, 60 mg/day (6 tablets per day) for 7 days, then reduced to 10 mg (1 tablet) taken every other day, stopping on day 17 Comparator group (n = 12): placebo, 6 tablets per day for 7 days, then reduced to 1 tablet every other day, stopping on day 17 Use of additional interventions (common to both treatment arms): none listed | |
| Outcomes | Outcomes of interest in the review: Primary outcomes
Other outcomes reported by the study:
| |
| Funding sources | "The authors have no funding, financial relationships, or conflicts of interest to disclose" | |
| Declarations of interest | "The authors have no funding, financial relationships, or conflicts of interest to disclose" | |
| Notes | The patients who met the inclusion criteria gave informed consent and were prescribed fluticasone nasal drops 1 x/day, 200 mg, for 6 weeks. Patients who did not respond to this medical treatment were evaluated for the study. All patients in the trial underwent surgery between the 15th and 17th day. Outcomes presented for this review are the pre‐operative results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Every eight boxes, including four study drug boxes and four placebo drug boxes, respectively, were assembled as a group by the pharmacy department." Comment: randomisation in blocks of 8 (pg 2042, col 1, para 6) |
| Allocation concealment (selection bias) | Low risk | Quote: "…The hospital pharmacy department performed the drug/placebo randomization, and the identity of the contents in the boxes was not disclosed to any clinicians interacting with patients throughout the study" Comment: pg 2042, col 1, para 6 |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "…the identity of the contents in the boxes was not disclosed to any clinicians interacting with patients throughout the study" "The surgeon was blinded to the patient treatment group" Comment: pg 2042, col 1, para 6/7. It is unclear whether the placebo tablets provide adequate masking in terms of taste, since prednisolone is bitter and may be recognisable to patients. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: from the flowchart on pg 2042 it appears that the codes were broken after all of the data had been collected |
| Incomplete outcome data (attrition bias) | Low risk | Comment: only 1 patient did not complete the study (4.3%). The drop‐out reason was that the patient was not given the study medication after surgery. No intention‐to‐treat analysis was completed. |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes appear to be reported well in the paper. No protocol for the trial could be found. |
| Other bias | Unclear risk | No information about the validation of any outcomes The mean age of participants in the oral steroid group was 45.6 ± 11.5 and the mean age in the control group was reported as 26.6 ± 43.6. This may be a reporting error since the range in the control group is 26 to 58 years. |
| Methods | 2‐arm, double‐blinded, parallel‐group RCT, with 14 days duration of treatment follow‐up | |
| Participants | Location: Australia, unclear number of sites Setting of recruitment and treatment: allergy outpatient clinics Sample size: Number randomised: 20 in intervention group, 21 in comparison group Number completed: 20 in intervention group, 20 in comparison group Participant (baseline) characteristics:
Other important effect modifiers:
Inclusion criteria: aged 18 to 65 years drawn mainly from allergy outpatient clinics who had symptomatic polyp disease diagnosed on nasoendoscopy Exclusion criteria: previous use of oral steroids, unstable asthma, recent sinus surgery, acute infection within 1 month of recruitment, polyps caused by cystic fibrosis or mucociliary disorders, diabetes mellitus, cataract, glaucoma, fungal sinusitis, contraindications for MRI scanning, or any other significant comorbid condition that contraindicated the use of systemic corticosteroids | |
| Interventions | Intervention (n = 20): prednisolone, 50 mg/day for 14 days Comparator group (n = 21): placebo, for 14 days Use of additional interventions (common to both treatment arms): participants were allowed to continue the use of regular antihistamines (13/40), topical corticosteroids (22/40), or both | |
| Outcomes | Outcomes of interest in the review: Primary outcomes
Secondary outcomes:
Other outcomes reported by the study:
| |
| Funding sources | No information provided | |
| Declarations of interest | "P. Wormwald receives royalties from Medtronic Xomed for instruments designed. The rest of the authors have declared that they have no conflict of interest." | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "… randomized by the hospital pharmacy to receive the study medication ..." Comment: pg 129, col 1, para 2 No information on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "These patients… were blinded to their treatment status"; "study personnel were not informed of the patient’s treatment status until all assessments were completed" Comment: pg 129, col 1, para 2. It is unclear whether the placebo tablets provide adequate masking in terms of taste, since prednisolone is bitter and may be recognisable to patients. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "study personnel were not informed of the patient's treatment status until all assessments were completed" Comment: pg 129, col 1, para 2 Blinding of nasoendoscopy scans (pg 129, col 2, para 3) were well documented when describing the outcomes |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 1 patient did not complete the study and their data were not included in the analysis |
| Selective reporting (reporting bias) | High risk | Comment: nasoendoscopy findings were reported inconsistently within the paper using differing criteria which had not been pre‐specified in the methods section. There was concern that the cut‐off points for reporting could have been chosen after the results were available. In addition, some of the scales used are unclear. No information is provided for the modified RSOM‐31 instrument or the RSOM‐31 nasal subscale. No protocol for the trial could be found |
| Other bias | High risk | The RSOM‐31 was modified, without further evidence of validation, and it is unclear whether the methods for measuring change in polyps size were validated |
| Methods | 4‐arm, unblinded, parallel‐group RCT, with unclear duration of treatment and 7‐day duration of follow‐up | |
| Participants | Location: Turkey, unclear number of sites (probably 1) Setting of recruitment and treatment: Department of Otorhinolaryngology, GATA medical faculty, Ankara Sample size:
Participant (baseline) characteristics: (based on all 4 groups)
Inclusion criteria: Nasal polyps diagnosed by computed tomography Exclusion criteria: past surgeries for nasal polyps, any glucocorticoid usage for any reason within 1 month, nasal polyp that was not eosinophilic nasal polyp according to the pathology study, fungal chronic sinusitis, age younger than 15 years, Churg‐Strauss syndrome, immunodeficiency, Kartagener's syndrome, Young's syndrome, cystic fibrosis, antrochoanal polyp and unilateral nasal polyp. Additional exclusion criteria were any contraindications for steroid treatment (such as glaucoma, peptic ulcer, acute psychosis, herpetic keratitis, chronic infections, severe osteoporosis, severe hypertension, uncontrolled diabetes mellitus, thromboembolic predisposition, newly formed bowel anastomosis, diverticulitis and Cushing's syndrome) | |
| Interventions | Intervention (n = 12): oral methylprednisolone (Prednol 16 mg tablet, Prednol 4 mg tablet; Mustafa Nevzat Pharmaceutical, Istanbul, Turkey), 1 mg/kg/day. The dose was applied for 3 days and tapered gradually, with a reduction rate of 8 mg/3 days. The duration of drug use varied for each patient changing according to his or her weight. Comparator group (n = 12): no medication was given Use of additional interventions (common to both treatment arm): no information | |
| Outcomes | No primary, secondary or adverse events were reported Other outcomes reported by the study:
| |
| Funding sources | "None" | |
| Declarations of interest | Competing interests "None"; sponsorships "None" | |
| Notes | 2 of the 4 groups within the study were not recorded in this data extraction. The interventions in the 2 additional groups were: intra‐polyp injection and INCS alone (for 30 days). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "…randomly assigned… " Comment: pg 564, col 1, para 3 |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) | High risk | Comment: no medication was given to the comparator group, implying there was no placebo used. It is assumed that the study is not blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: no information, but the study only measures objective outcomes |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no information. The paper implies that all patients completed treatment and were recorded in the outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no outcomes directly relevant to patients were reported. No protocol for the trial could be found. |
| Other bias | Unclear risk | Comment: no information about the validation of outcomes |
| Methods | 2‐arm, double‐blind, parallel‐group RCT, with 14 days oral steroid treatment duration and 12‐week follow‐up period | |
| Participants | Location: 1 site, Thailand Setting of recruitment and treatment: Allergy and Rhinology Clinic, Prince of Songkla University Sample size: 117 Number randomised: 69 in intervention group, 48 in comparison group Number completed: 67 in intervention group, 47 in comparison group Participant (baseline) characteristics:
Inclusion criteria: patients with benign bilateral nasal polyps diagnosed clinically and confirmed by nasal endoscopy Nasal polyps score: graded on a 4‐point scale (0 to 3): 0 ‐ no polyps, 1 – mild polyposis (small polyps, extending downward from the upper nasal cavity but not below the upper edge of the inferior turbinate, causing only slight obstruction), 2 – moderate polyposis (medium‐sized polyps, extending downward from the upper nasal cavity and reaching between the upper and lower edges of the inferior turbinate, causing troublesome obstruction), 3 – severe polyposis (large‐sized polyps, extending downward from the upper nasal cavity and reaching below the lower edge of the inferior turbinate, causing total or almost total obstruction). The total nasal polyps score was calculated as the sum of the polyps scores for each nostril. Exclusion criteria: patients with symptoms or physical signs suggestive of renal disease, hepatic disease, diabetes mellitus, cataract, glaucoma, cardiovascular disease, unstable asthma, cystic fibrosis, mucociliary disorders, immunocompromise, severe septal deviation or acute infection within the previous 2 months. Patients who had used nasal, inhaled or systemic steroids within 2 months; an antihistamine within 2 to 7 days; and/or a decongestant within 2 days or had had previous sinonasal surgery were also excluded. | |
| Interventions | Intervention (n = 67): oral prednisolone 50 mg daily for 14 days Comparator group (n = 47): placebo tablet daily for 14 days Use of additional interventions (common to both treatment arms): at the end of the "test treatment" stage all patients were then treated with administration of mometasone furoate nasal spray (MFNS) at 200 µg twice daily for 10 weeks Medications for rhinitis or allergy or nasal saline irrigation were not allowed during their participation in the study | |
| Outcomes | Primary outcomes
Secondary outcomes
Other outcomes reported by the study:
| |
| Funding sources | "Funded by the Faculty of Medicine, Prince of Songkla University" | |
| Declarations of interest | "The authors have no conflicts of interest to declare pertaining to this article" | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned at a 3:2 ratio" Comment: pg 456, col 1, last para. There is no information regarding the method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The study personnel were not informed of the treatment modality of the patients until all assessments were completed" Comment: no details on how the patients were allocated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "... prednisolone or placebo", "… were blinded to their treatment regimen." (pg 455, col 2, last para) "The study personnel were not informed of the treatment modality of the patients until all assessments were completed" (pg 456, col 2, para 1) Comment: it is unclear whether the placebo tablets provide adequate masking in terms of taste, since prednisolone is bitter and may be recognisable to patients |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The study personnel were not informed of the treatment modality of the patients until all assessments were completed" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: low numbers of patients dropped out – unlikely to affect the overall result |
| Selective reporting (reporting bias) | Low risk | Comment: appears that all of the outcomes listed were adequately reported in the paper. No protocol for the trial could be found. |
| Other bias | Unclear risk | Comment: no information about the validation of the "total nasal symptom score" |
| Methods | 2‐arm, double‐blind, parallel‐group RCT, with 2‐week duration of oral steroid treatment, followed by a 6‐month duration of intranasal steroid treatment and duration of follow‐up | |
| Participants | Location: Scotland, 1 site Setting of recruitment and treatment: speciality referral clinic Sample size: Number randomised: 30 in intervention group, 30 in comparison group Number completed: 27 in intervention group, 24 in comparison group Participant (baseline) characteristics:
Other important effect modifiers
Inclusion criteria: diagnosis of CRS with nasal polyposis made on the basis of the EPOS 2007 criteria Inclusion criteria were the presence on nasoendoscopy of bilateral moderate‐sized to large nasal polyps (grade > 1) according to the Lildholdt scale and at least 2 of anterior or posterior nasal discharge, nasal obstruction or decreased sense of smell for more than 12 weeks Lildholdt nasal polyps scale: 0, no nasal polyps; 1, small polyps confined to the middle meatus; 2, moderate sized polyps not crossing the lower edge of the inferior turbinate; 3, large polyps crossing the lower edge of the inferior turbinate Exclusion criteria: exclusion criteria included treatment with an oral corticosteroid in the past 3 months, sinus surgery in the past year, recent upper respiratory tract infection, mechanical nasal airway obstruction of more than 50% due to septal deviation, or pregnancy or lactation | |
| Interventions | Intervention (n = 30): prednisolone tablets, 25 mg/day, 2 weeks Comparator group (n = 30): placebo tablets, daily, 2 weeks Use of additional interventions (common to both treatment arm): All patients underwent a 2‐week 'run‐in' period prior to the trial during which therapy for CRS with nasal polyps was stopped. After the 2‐week oral steroid treatment period both study arms received fluticasone propionate nasal drops, 400 µg twice daily, for 8 weeks then fluticasone propionate nasal spray, 200 µg twice daily for a further 18 weeks No other rhinitis medications were permitted, including antihistamines, leukotriene receptor antagonists, intranasal corticosteroids or nasal decongestants. No antibiotics were permitted during the study. | |
| Outcomes | Primary outcomes
Secondary outcomes
Other outcomes reported by the study:
| |
| Funding sources | "Chief Scientist Office, Scotland; National Health Service Tayside Small Grants Scheme; and an Anonymous Trust grant from University of Dundee." | |
| Declarations of interest | The link from the paper to the website does not appear to list any declarations of interests | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An independent, off‐site clinical trials pharmacist (Pharmacy Production Unit, Western Infirmary, Glasgow, United Kingdom) used a computer‐generated random allocation sequence to randomize the trial, using block randomization with a block size of 4." Comment: pg 294, col 2, para 5 |
| Allocation concealment (selection bias) | Low risk | Quote: "Tablets were distributed in sealed opaque envelopes at the research unit, in sequential order, by a laboratory technician who was not directly involved with the study." Comment: pg 294, col 2, para 5 |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "The same pharmacist masked and blinded the 25‐mg prednisolone tablet and an identical placebo tablet to double‐blind the study from the investigator and participants." (pg 294, col 2, para 5) "Three patients in the prednisolone group and 4 in the placebo group had previously received oral steroids" (pg 297, col 2) Comment: it is unclear whether the placebo tablets provide adequate masking in terms of taste, since prednisolone is bitter and may be recognisable to patients |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Standard video sequences were stored on a computer and viewed by 2 independent observers, who were blinded to patient, treatment, and sequence. Disagreements were resolved by discussion." Comment: pg 295, col 2, para 1 Patients and the main outcome assessors should remain adequately blinded throughout. The other outcomes were assessed by 2 blinded outcome assessors. |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "… We included all patients who received the allocated intervention in the analysis" Comment: pg 295, col 2, para 3 5 patients discontinued after the 2‐week treatment with oral corticosteroids; 4 of these were in the oral steroids group. Another 2 dropped out from each group by the 3‐month follow‐up (total 9/60, 15% overall; 10% in treatment group, 20% in control group) The results table gives different numbers of participants included in each analysis, which are closer to the number of patients available, rather than patients randomised. It is unclear why there is a discrepancy. |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes were reported in the results as described in the methods section. The methods for collecting data for adverse events (other than biological assays) were not reported. The paper reports that no oral steroid‐specific adverse events were reported, but it is unclear whether the patients were specifically asked. The protocol document was available (NCT00788749) and the outcomes appear to be consistent between the protocol and the paper. |
| Other bias | Unclear risk | Comment: there is no information about the total symptom score (e.g. validation) Study used the RQLQ (Rhinoconjunctivitis Quality of Life Questionnaire), which is validated for patients with allergy. Many of the items are not relevant for CRS patients, while items related to smell and sinonasal pain were not included. |
| Methods | 3‐arm, double‐blind, multicentre, parallel‐group RCT, with 20 days duration of treatment and 12 weeks duration of follow‐up | |
| Participants | Location: 5 sites in Belgium, Germany, Holland and Australia Setting of recruitment and treatment: not given Sample size: 47 Number randomised: 14 in oral steroids, 19 in placebo Number completed: 14 in oral steroids, 12 in placebo Participant (baseline) characteristics:
Other important effect modifiers:
Inclusion criteria: Participants had to be at least 18 years with a diagnosis of bilateral nasal polyps at screening and baseline that have recurred after surgical resection or nasal polyps that are grades 3 or 4 in both nares using the polyp scoring system. Women of childbearing potential had to use a medically acceptable form of birth control as defined by the study. Male participants had to agree to use an adequate form of birth control for the duration of the study as defined by the study. Participants with concurrent asthma had to be maintained on no more than 1000 µg/day beclomethasone dipropionate or the equivalent. Nasal polyp score: 0 ‐ no polyp; 1 ‐ small polyps in the middle meatus not reaching below the inferior border of the middle concha; 2 ‐ polyps reaching below the lower border of the middle turbinate; 3 ‐ large polyps reaching the lower border of the inferior turbinate or polyps medial to the middle concha, 4 ‐ large polyps causing almost complete congestion/obstruction of the inferior meatus Exclusion criteria: the following are exclusion criteria for the study: pregnancy, breast‐feeding or premenarcheal; oral corticosteroids within the 3 months before screening; systemic fungoid infections; known allergic reaction on methylprednisolone or tetracyclines; hypertension; diabetes (type 1 and 2); glaucoma; tuberculosis; herpes infection; zona ophthalmica; antineutrophil cytoplasmic antibodies such as Wegener granulomatosis, Churg‐Strauss syndrome and microscopic polyangiitis Participants with acute sinusitis or concurrent nasal infection or participants who have had a nasal or upper respiratory tract infection within 2 weeks of the screening visit; cystic fibrosis, primary ciliary dysfunction or Kartagener syndrome by history; those diagnosed with a parasitic infection; HIV‐positive or positive to hepatitis B surface antigen or C antibodies. Participants must not have had an acute asthmatic attack requiring admission to a hospital (excluding emergency department visits that resulted in direct discharge without hospitalisation) within the 4 weeks before screening Participants must not have received immunotherapy within the previous 3 months | |
| Interventions | Intervention (n = 14): oral methylprednisolone (32 mg/day on days 1 to 5; 16 mg/day on days 6 to 10; 8 mg/day on days 11 to 20) Control (n = 19): placebo, unlabelled lactose capsules, 20 days Use of additional interventions (common to all treatment arm): Systemic or local corticosteroids or antibiotics were not allowed; if necessary nasal corticosteroids were permitted as rescue medication 2 months after dosing with the study medication | |
| Outcomes | Primary outcomes:
Secondary outcomes:
Other outcomes reported by the study:
| |
| Funding sources | "Supported by a grant from the Flemish Scientific Research Board, FWO Nr. A12/5‐HBKH 3 (holder of a Fundamenteel Klinisch Mandaat), by a postdoctoral grant from the Research Foundation Flanders (FWO), and by postdoctoral mandate from the Research Foundation Flanders (FWO)." | |
| Declarations of interest | "Disclosure of potential conflict of interest: P. J. Wormald has received royalties from Medtronic ENT, is a consultant for NeilMed, and has received research support from the Garnett Passe and Rodney Williams Foundation. W. Fokkens has received research support from GlaxoSmithKline and Stallergenes. A. Beule has received research support from the European Union. The rest of the authors have declared that they have no conflict of interest." | |
| Notes | 3rd arm of the study was antibiotics (doxycycline). Results for this arm of the study are included in Head 2016b. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eligible patients were randomly assigned to 3 groups by individuals not involved in the study." Comment: pg 1070, col 1, para 3. No information was provided about how the sequence was generated. The number randomised was small and there is a risk that it was not balanced (14 versus 19) |
| Allocation concealment (selection bias) | Unclear risk | Quote: "… patients were randomly assigned to 3 groups by individuals not involved in the study" Comment: pg 1070, col 1, para 3 |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "… double‐blind… " "... Placebo (lactose) in unlabelled capsules" Comment: pg 1069, abstract: methods, pg 1070 methods Details of blinding not clear within the paper and it does not detail whether the placebo (and antibiotic) medications were given on the same dosing schedule with medication in an identical form |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Study participants and personnel were blind during the duration of the study. Randomisation codes were revealed to researchers after recruitment,data collection, and data entry" Comment: details of blinding not clear within the paper and it is not clear whether the oral steroids and antibiotic medications were given on the same dosing schedule and were an identical form, which could compromise blinding |
| Incomplete outcome data (attrition bias) | High risk | Comment: only 7/47 patients dropped out of the study (14.9%) but all were from the placebo group 7/19 (36.8%). This is an imbalance in drop‐out rate and the reasons for drop‐out include "unsatisfactory therapeutic effects", "withdrawal of consent" and "serious adverse events (asthma attack)". Patients who dropped out were still included in the analysis using the last observed carried forward. This may have had an effect on the overall results and no sensitivity analysis appears to have been completed to identify the impact. |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes in the methods section were reported in the full paper, although many of them were presented graphically, without providing values at key time periods. The data were not reported in a way that is sufficient to be included in the meta‐analysis of the review. The protocol document was available (NCT00480298) and the outcomes appear to be consistent between the protocol and the paper |
| Other bias | Unclear risk | Comment: details of the scales used to measure symptoms were not provided in the paper and there is no information on validation of the outcomes There was an imbalance in the number of participants with "allergy" (oral steroids: 35.7; placebo: 57.9%; antibiotics: 14.3%) and "aspirin intolerant" in the baseline characteristics (oral steroids: 14.3%; placebo: 26.3%; antibiotics: 7.1%). This was not a statistical difference between the groups due to the study size being small. A sensitivity analysis was completed by the study authors to determine if this affected the results. |
CRS: chronic rhinosinusitis
CT: computerised tomography
ENT: ear, nose and throat
EPOS: European Position Paper on Rhinosinusitis and Nasal Polyps 2012
F: female
INCS: intranasal corticosteroids
M: male
MFNS: mometasone furoate nasal spray
MRI: magnetic resonance imaging
RCT: randomised controlled trial
RSOM‐31: Rhinosinusitis Outcome Measures‐31
SD: standard deviation
SEM: standard error of the mean
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| INTERVENTION: oral steroids versus surgery | |
| INTERVENTION: surgery | |
| INTERVENTION: combined medical and surgical treatment | |
| STUDY DESIGN: not randomised | |
| STUDY DESIGN: not randomised | |
| STUDY DESIGN: not randomised | |
| INTERVENTION: both arms of the study received intranasal steroids | |
| STUDY DESIGN: not randomised | |
| INTERVENTION: oral steroid (12 days) + INCS versus oral steroid (20 days) + INCS | |
| STUDY DESIGN: review of previous oral steroids trials | |
| STUDY DESIGN: not randomised | |
| STUDY DESIGN: not randomised | |
| STUDY DESIGN: not randomised | |
| INTERVENTION: endoscopic polypectomy with ethmoidectomy | |
| INTERVENTION: surgical removal versus systemic corticosteroids | |
| INTERVENTION: surgical polypectomy followed by continuous topical steroid treatment versus a single dose of depot steroid | |
| INTERVENTION: oral steroid versus intranasal steroids; both arms of the study received antibiotics Ongoing study | |
| STUDY DESIGN: not randomised | |
| INTERVENTION: oral steroids versus placebo; all patients in both arms received antibiotics | |
| INTERVENTION: medical versus surgical treatment | |
| STUDY DESIGN: not randomised | |
| STUDY DESIGN: not randomised | |
| STUDY DESIGN: not randomised | |
| INTERVENTION: oral steroid versus INCS; duration of follow‐up less than 3 months | |
| POPULATION: allergic fungal sinusitis | |
| STUDY DESIGN: surgical outcomes paper | |
| STUDY DESIGN: not randomised | |
| STUDY DESIGN: not randomised | |
| STUDY DESIGN: not randomised | |
| STUDY DESIGN: not randomised |
INCS: intranasal corticosteroids
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | ChiCTRTRC11001323: Research on clinical efficacy of oral glucocorticoid in the treatment of eosinophilic nasal polyps and non‐eosinophilic nasal polyps |
| Methods | Randomised, parallel‐group, controlled trial |
| Participants | Chronic sinusitis with nasal polyps |
| Interventions | Oral prednisolone tablets versus placebo |
| Outcomes | VAS score, Lanza‐Kennedy nasal endoscopy score |
| Starting date | January 2011 |
| Contact information | Ming Zeng, Department of Otorhinolaryngology Head and Neck Surgery, Tongji Hospital of Tongji Medical College of Email: [email protected] |
| Notes | No response from study authors |
| Trial name or title | NCT00841802: Chronic rhinosinusitis with or without nasal polyps steroid study |
| Methods | Open‐label, parallel‐group randomised controlled trial |
| Participants | Diagnosis of chronic rhinosinusitis with or without nasal polyps and undergoing sinonasal surgery for this condition No diagnosis of CRS and NP and undergoing nasal surgery (septoplasty/rhinoplasty, nasal fracture repair, etc.) |
| Interventions | Prednisone versus no intervention |
| Outcomes | Alterations of inflammatory cells, levels of key antibodies and cytokines, and expression of key epithelial genes |
| Starting date | February 2009 |
| Contact information | Robert P Schleimer, PhD; email: [email protected] Kathleen E Harris, BS; email: keharris@ northwestern.edu |
| Notes | Authors responded to enquiry to say that the study is still in the process of being completed |
| Trial name or title | NCT02367118: Prednisone in chronic rhinosinusitis without nasal polyps |
| Methods | Double‐blind, parallel‐group randomised controlled trial |
| Participants | Diagnosis of CRSsNP as recommended by European Position Paper on Rhinosinusitis and Nasal Polyps 2012 |
| Interventions | Prednisone (30 mg for 7 days then 15 mg for 7 days then 5 mg for 7 days) versus placebo (21 days) |
| Outcomes | Changes in symptoms as measured by SNOT‐22 questionnaire and visual analogue scale at 6 months Change in olfactory function as measured by "Sniffin' Sticks 12 tests" at 6 months Change in nasal patency as measured by acoustic rhinometry and rhinomanometry at 6 months Changes in nasal endoscopy findings as measured by Lund‐Kennedy score at 6 months |
| Starting date | June 2015 |
| Contact information | Constanza J Valdes, MD; email: [email protected] Marcela A Veloz, MD; email: [email protected] |
| Notes | Authors responded to our enquiry to say that the study is still in the process of being completed |
CRS: chronic rhinosinusitis
CRSsNP: chronic rhinosinusitis without nasal polyps
NP: nasal polyps
SNOT‐22: Sino‐Nasal Outcome Test‐22
VAS: visual analogue scale
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Disease‐specific health‐related quality of life ‐ no pooling (2 to 3 weeks) Show forest plot | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Oral steroids versus no treatment/placebo, Outcome 1 Disease‐specific health‐related quality of life ‐ no pooling (2 to 3 weeks). | ||||
| 1.1 Severity score of RSOM | 1 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.24 [‐1.92, ‐0.56] |
| 1.2 Mini‐RQLQ | 1 | 58 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.79 [‐1.32, ‐0.25] |
| 2 Disease‐specific health‐related quality of life ‐ RQLQ (3 to 6 months) Show forest plot | 1 | 50 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.59 [‐1.16, ‐0.02] |
| Analysis 1.2  Comparison 1 Oral steroids versus no treatment/placebo, Outcome 2 Disease‐specific health‐related quality of life ‐ RQLQ (3 to 6 months). | ||||
| 3 Disease severity (patient‐reported total symptom score) Show forest plot | 4 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3 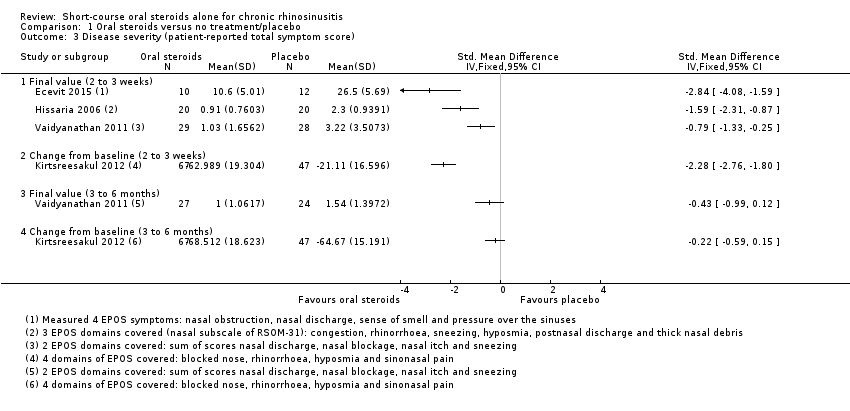 Comparison 1 Oral steroids versus no treatment/placebo, Outcome 3 Disease severity (patient‐reported total symptom score). | ||||
| 3.1 Final value (2 to 3 weeks) | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Change from baseline (2 to 3 weeks) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Final value (3 to 6 months) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Change from baseline (3 to 6 months) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Individual symptoms: nasal obstruction (final value) Show forest plot | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐4.5 [‐6.42, ‐2.58] |
| Analysis 1.4  Comparison 1 Oral steroids versus no treatment/placebo, Outcome 4 Individual symptoms: nasal obstruction (final value). | ||||
| 5 Individual symptoms: nasal obstruction (change from baseline) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5 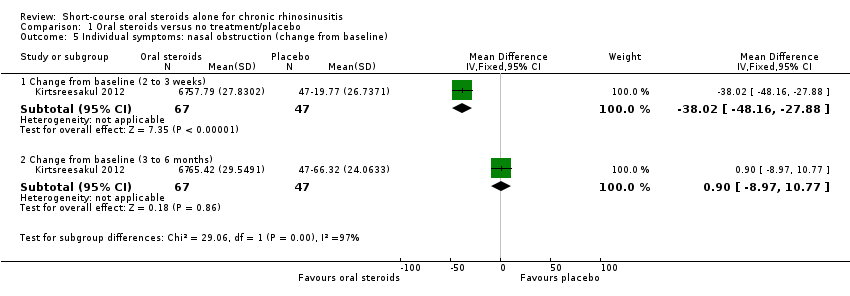 Comparison 1 Oral steroids versus no treatment/placebo, Outcome 5 Individual symptoms: nasal obstruction (change from baseline). | ||||
| 5.1 Change from baseline (2 to 3 weeks) | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐38.02 [‐48.16, ‐27.88] |
| 5.2 Change from baseline (3 to 6 months) | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐8.97, 10.77] |
| 6 Individual symptoms: nasal discharge (final value) Show forest plot | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐4.7 [‐6.79, ‐2.61] |
| Analysis 1.6 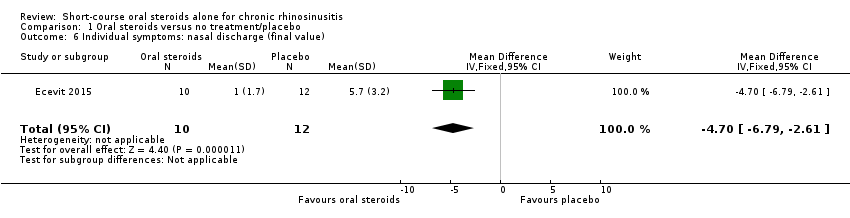 Comparison 1 Oral steroids versus no treatment/placebo, Outcome 6 Individual symptoms: nasal discharge (final value). | ||||
| 7 Individual symptoms: nasal discharge (change from baseline) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Oral steroids versus no treatment/placebo, Outcome 7 Individual symptoms: nasal discharge (change from baseline). | ||||
| 7.1 2 to 3 weeks | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐55.57 [‐69.23, ‐41.91] |
| 7.2 3 to 6 months | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐1.83 [‐13.46, 9.81] |
| 8 Individual symptoms: facial pressure (final value) Show forest plot | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐3.7 [‐6.02, ‐1.38] |
| Analysis 1.8  Comparison 1 Oral steroids versus no treatment/placebo, Outcome 8 Individual symptoms: facial pressure (final value). | ||||
| 9 Individual symptoms: facial pressure (change from baseline) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.9 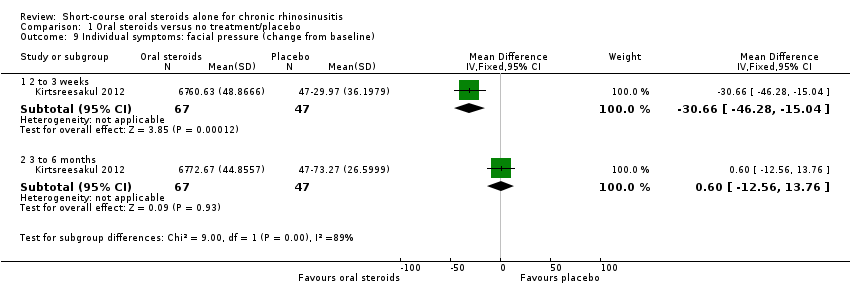 Comparison 1 Oral steroids versus no treatment/placebo, Outcome 9 Individual symptoms: facial pressure (change from baseline). | ||||
| 9.1 2 to 3 weeks | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐30.66 [‐46.28, ‐15.04] |
| 9.2 3 to 6 months | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐12.56, 13.76] |
| 10 Individual symptoms: loss of sense of smell (final value) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.10  Comparison 1 Oral steroids versus no treatment/placebo, Outcome 10 Individual symptoms: loss of sense of smell (final value). | ||||
| 10.1 2 to 3 weeks | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐2.79 [‐4.11, ‐1.47] |
| 10.2 3 to 6 months | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐2.68, 0.28] |
| 11 Individual symptoms: loss of sense of smell (change from baseline) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.11  Comparison 1 Oral steroids versus no treatment/placebo, Outcome 11 Individual symptoms: loss of sense of smell (change from baseline). | ||||
| 11.1 2 to 3 weeks (after treatment) | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐44.35 [‐57.31, ‐31.39] |
| 11.2 3 to 6 months | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐15.05 [‐29.69, ‐0.41] |
| 12 Adverse events ‐ significant mood disturbance Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.55, 11.41] |
| Analysis 1.12  Comparison 1 Oral steroids versus no treatment/placebo, Outcome 12 Adverse events ‐ significant mood disturbance. | ||||
| 13 Adverse events ‐ gastrointestinal disturbance Show forest plot | 3 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.45 [1.11, 10.78] |
| Analysis 1.13  Comparison 1 Oral steroids versus no treatment/placebo, Outcome 13 Adverse events ‐ gastrointestinal disturbance. | ||||
| 14 Adverse events ‐ insomnia Show forest plot | 3 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.63 [1.10, 11.95] |
| Analysis 1.14  Comparison 1 Oral steroids versus no treatment/placebo, Outcome 14 Adverse events ‐ insomnia. | ||||
| 15 Endoscopy score ‐ nasal polyps (final value) Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.15 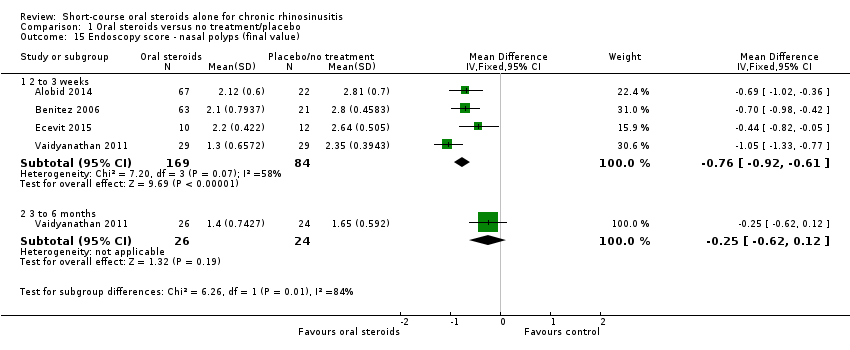 Comparison 1 Oral steroids versus no treatment/placebo, Outcome 15 Endoscopy score ‐ nasal polyps (final value). | ||||
| 15.1 2 to 3 weeks | 4 | 253 | Mean Difference (IV, Fixed, 95% CI) | ‐0.76 [‐0.92, ‐0.61] |
| 15.2 3 to 6 months | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.62, 0.12] |
| 16 Endoscopy score ‐ nasal polyps score (change from baseline) Show forest plot | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.16  Comparison 1 Oral steroids versus no treatment/placebo, Outcome 16 Endoscopy score ‐ nasal polyps score (change from baseline). | ||||
| 16.1 2 to 3 weeks | 2 | 146 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.77 [‐2.16, ‐1.38] |
| 16.2 3 to 6 months | 1 | 114 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐0.90, ‐0.14] |
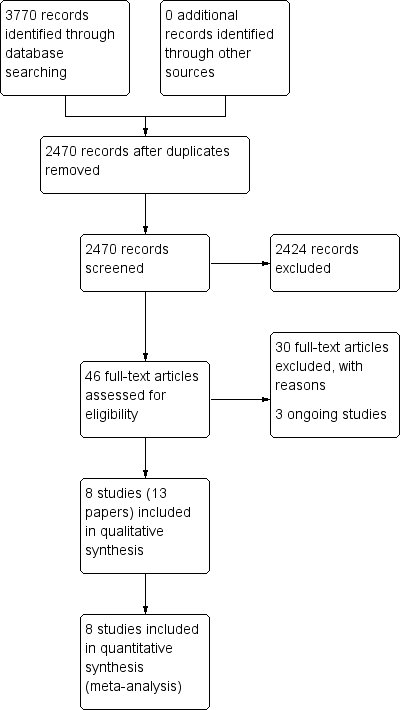
Process for sifting search results and selecting studies for inclusion.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Forest plot of comparison: 1 Oral steroids versus no treatment/placebo, outcome: 1.1 Disease‐specific health‐related quality of life ‐ no pooling (2 to 3 weeks).

Forest plot of comparison: 1 Oral steroids versus no treatment/placebo, outcome: 1.3 Disease severity (patient‐reported total symptom score).

Comparison 1 Oral steroids versus no treatment/placebo, Outcome 1 Disease‐specific health‐related quality of life ‐ no pooling (2 to 3 weeks).

Comparison 1 Oral steroids versus no treatment/placebo, Outcome 2 Disease‐specific health‐related quality of life ‐ RQLQ (3 to 6 months).

Comparison 1 Oral steroids versus no treatment/placebo, Outcome 3 Disease severity (patient‐reported total symptom score).
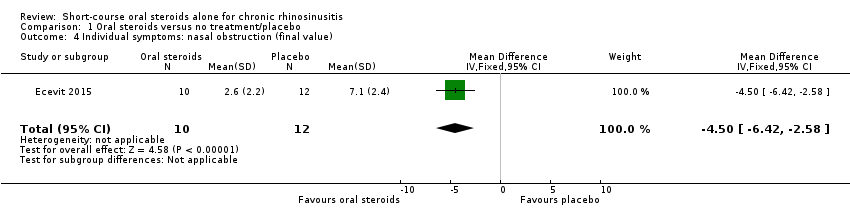
Comparison 1 Oral steroids versus no treatment/placebo, Outcome 4 Individual symptoms: nasal obstruction (final value).

Comparison 1 Oral steroids versus no treatment/placebo, Outcome 5 Individual symptoms: nasal obstruction (change from baseline).

Comparison 1 Oral steroids versus no treatment/placebo, Outcome 6 Individual symptoms: nasal discharge (final value).
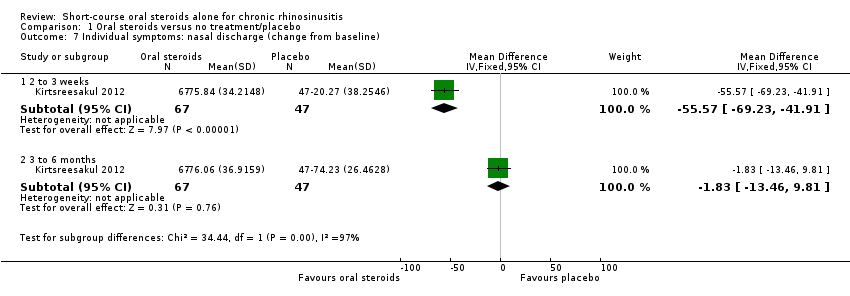
Comparison 1 Oral steroids versus no treatment/placebo, Outcome 7 Individual symptoms: nasal discharge (change from baseline).

Comparison 1 Oral steroids versus no treatment/placebo, Outcome 8 Individual symptoms: facial pressure (final value).

Comparison 1 Oral steroids versus no treatment/placebo, Outcome 9 Individual symptoms: facial pressure (change from baseline).
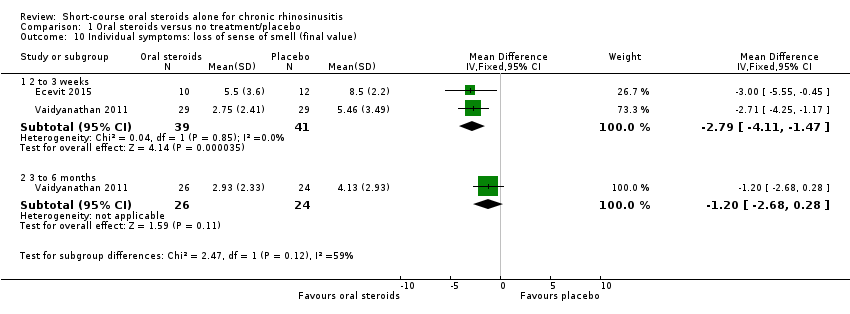
Comparison 1 Oral steroids versus no treatment/placebo, Outcome 10 Individual symptoms: loss of sense of smell (final value).
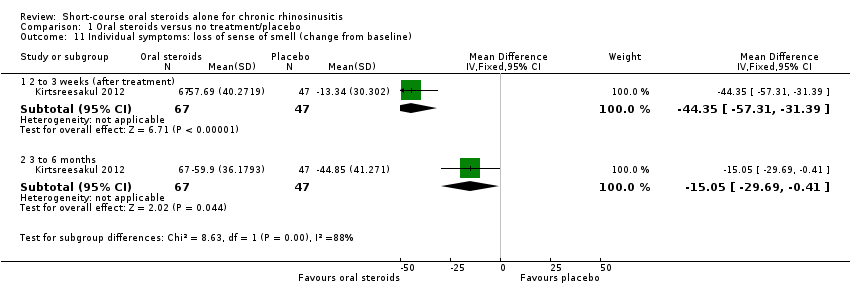
Comparison 1 Oral steroids versus no treatment/placebo, Outcome 11 Individual symptoms: loss of sense of smell (change from baseline).
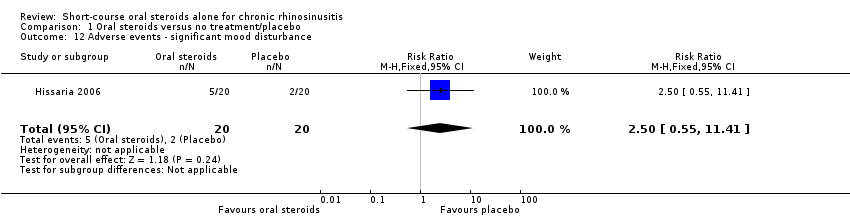
Comparison 1 Oral steroids versus no treatment/placebo, Outcome 12 Adverse events ‐ significant mood disturbance.
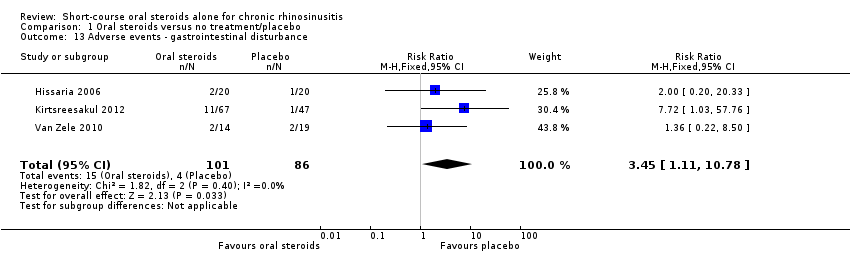
Comparison 1 Oral steroids versus no treatment/placebo, Outcome 13 Adverse events ‐ gastrointestinal disturbance.

Comparison 1 Oral steroids versus no treatment/placebo, Outcome 14 Adverse events ‐ insomnia.
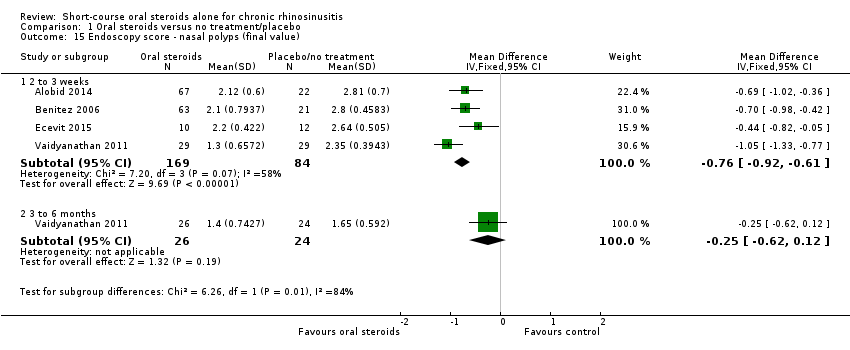
Comparison 1 Oral steroids versus no treatment/placebo, Outcome 15 Endoscopy score ‐ nasal polyps (final value).
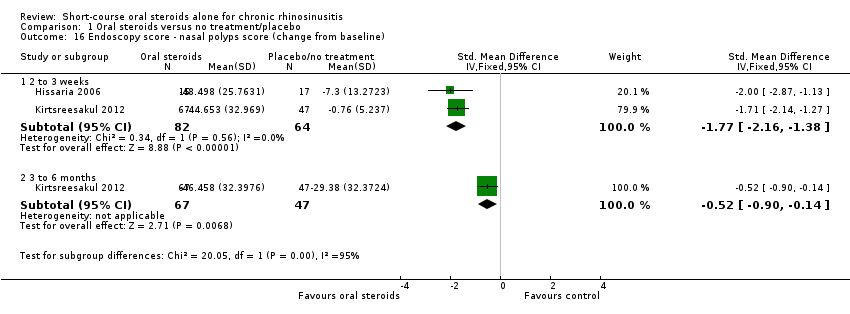
Comparison 1 Oral steroids versus no treatment/placebo, Outcome 16 Endoscopy score ‐ nasal polyps score (change from baseline).
| Short‐course oral corticosteroids compared with placebo/no treatment for chronic rhinosinusitis | ||||||
| Patient or population: chronic rhinosinusitis with nasal polyps | ||||||
| Outcomes № of participants | Relative effect | Anticipated absolute effects* (95% CI) | Quality | What happens | ||
| Without oral steroids | With oral steroids | Difference | ||||
| Disease‐specific health‐related quality of life measured by Follow‐up: 2 weeks № of participants: 40 | — | Not estimable | — | The mean disease‐specific health‐related quality of life in the intervention group was 1.24 standard deviations lower (1.92 lower to 0.56 lower) | ⊕⊕⊝⊝ | A lower score indicates reduced impairment. Treatment effect in favour of short‐course oral steroids.
|
| Disease severity, as measured by patient‐reported symptom score,
| — | — | — |
| ⊕⊕⊝⊝ ⊕⊕⊝⊝ ⊕⊕⊝⊝ | A lower score indicates milder symptoms in favour of short‐course oral steroids.
|
| Adverse events: significant mood disturbance № of participants: 40 | RR 2.50 | Study population | ⊕⊕⊝⊝ | It is uncertain whether there were more mood disturbance adverse events in the oral corticosteroids group. | ||
| 100 per 1000 | 250 per 1000 | 150 more per 1000 (45 fewer to 1041 more) | ||||
| Health‐related quality of life, using generic quality of life scores | This outcome was not reported in any of the studies | |||||
| Adverse events: gastrointestinal disturbance Follow‐up: 3 months № of participants:187 | RR 3.45 | Study population | ⊕⊕⊝⊝ | There were more gastrointestinal disturbance adverse events in the oral corticosteroids group. | ||
| 47 per 1000 | 160 per 1000 | 114 more per 1000 (5 more to 455 more) | ||||
| Adverse events: insomnia Follow‐up: 3 months № of participants:187 | RR 3.63 | Study population | ⊕⊕⊝⊝ | There were more insomnia adverse events in the oral corticosteroids group. | ||
| 23 per 1000 | 84 per 1000 | 61 more per 1000 (2 more to 255 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded to low quality due to limitations in study methodology and imprecision. Only the disease severity scale of the RSOM‐31 was used (unknown validity of this subscale and the range of scores is unclear). One small study (n = 40), which lacked information about the method of randomisation and allocation concealment. There is a also concern that the magnitude of improvement is not sustained; one study that used a non‐validated instrument reported smaller benefit at three to six months than at two to three weeks for health‐related quality of life. 2The individual symptoms measured were: nasal obstruction, nasal discharge, sense of smell and pressure over the sinuses. Scores for the individual symptoms (0 to 10 visual analogue scale (VAS)) were summed to find the total score.The effect size could be underestimated with this method. 3Downgraded to low quality due to imprecision. Results are from one very small study (n = 22) and the results were only measured at the end of treatment (17 days). There is a concern that the magnitude of improvement is not sustained. The outcome was not measured using a validated tool. 4Downgraded to low quality due to limitations in study methodology and imprecision. One small study (n = 40), which lacked information about the method of randomisation and allocation concealment. The definition of 'mood disturbance' is not provided in the paper. The results have large confidence intervals. 5Downgraded to low quality due to inconsistency and imprecision. The terminology between the papers for this outcome differed from "diarrhoea/GI disturbance" to "gastrointestinal disturbance" to "reflux and/or gastric pain". A low number of events were reported resulting in large confidence intervals. 6Downgraded to low quality due to inconsistency and imprecision. The definition of 'insomnia' is not provided in the papers. A low number of events were reported resulting in large confidence intervals. 7The individual symptoms measured were: blocked nose, rhinorrhoea, hyposmia and sinonasal pain. The results were measured as individual symptoms on a seven‐point Likert scale (0 = no symptoms) and presented as percentage change from baseline for each symptom, which was averaged across the four symptoms to create an average change from baseline. The effect size could be underestimated with this method. 8All patients in both groups received intranasal steroids at the end of the treatment period until the end of follow‐up (12 weeks). 9Downgraded to low quality due to limitations in study methodology and imprecision. Results are from one small study (n = 117) with unclear randomisation and allocation concealment. The results were measured at the end of treatment (two weeks). There is a concern that the results are not sustained. The outcome was not measured using a validated tool. 10Downgraded to low quality due to limitations in study methodology and imprecision. Results are from one small study (n = 117) with unclear randomisation and allocation concealment. There is a small effect size with large confidence intervals. The outcome was not measured using a validated tool. | ||||||
| System | Adverse events | Notes |
| Musculoskeletal | Osteoporosis | Largely limited to long‐term use Significantly increased risk of fractures with prolonged use |
| Osteonecrosis | Rare, appears to be dose‐dependent | |
| Endocrine | Hyperglycaemia | Common; dose‐dependent, usually reversible |
| Cardiovascular | Hypertension | Common; dose‐dependent, usually reversible |
| Dermatological | Striae, bruising | Dose‐dependent; occurs after > 1 month usage |
| Ophthalmological | Cataracts | Irreversible; largely related to long‐term usage |
| Glaucoma | High risk with pre‐existing disease | |
| Gastrointestinal tract | Peptic ulceration | Increased risk largely due to concomitant NSAIDs |
| Psychological | Psychosis | Common; increased risk with dosages > 40 mg/day |
| References: Da Silva 2006; Naber 1996; Stanbury 1998 NSAIDs: non‐steroidal anti‐inflammatory drugs | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Disease‐specific health‐related quality of life ‐ no pooling (2 to 3 weeks) Show forest plot | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Severity score of RSOM | 1 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.24 [‐1.92, ‐0.56] |
| 1.2 Mini‐RQLQ | 1 | 58 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.79 [‐1.32, ‐0.25] |
| 2 Disease‐specific health‐related quality of life ‐ RQLQ (3 to 6 months) Show forest plot | 1 | 50 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.59 [‐1.16, ‐0.02] |
| 3 Disease severity (patient‐reported total symptom score) Show forest plot | 4 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Final value (2 to 3 weeks) | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Change from baseline (2 to 3 weeks) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Final value (3 to 6 months) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Change from baseline (3 to 6 months) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Individual symptoms: nasal obstruction (final value) Show forest plot | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐4.5 [‐6.42, ‐2.58] |
| 5 Individual symptoms: nasal obstruction (change from baseline) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Change from baseline (2 to 3 weeks) | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐38.02 [‐48.16, ‐27.88] |
| 5.2 Change from baseline (3 to 6 months) | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐8.97, 10.77] |
| 6 Individual symptoms: nasal discharge (final value) Show forest plot | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐4.7 [‐6.79, ‐2.61] |
| 7 Individual symptoms: nasal discharge (change from baseline) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 2 to 3 weeks | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐55.57 [‐69.23, ‐41.91] |
| 7.2 3 to 6 months | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐1.83 [‐13.46, 9.81] |
| 8 Individual symptoms: facial pressure (final value) Show forest plot | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐3.7 [‐6.02, ‐1.38] |
| 9 Individual symptoms: facial pressure (change from baseline) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 2 to 3 weeks | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐30.66 [‐46.28, ‐15.04] |
| 9.2 3 to 6 months | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐12.56, 13.76] |
| 10 Individual symptoms: loss of sense of smell (final value) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 2 to 3 weeks | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐2.79 [‐4.11, ‐1.47] |
| 10.2 3 to 6 months | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐2.68, 0.28] |
| 11 Individual symptoms: loss of sense of smell (change from baseline) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 2 to 3 weeks (after treatment) | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐44.35 [‐57.31, ‐31.39] |
| 11.2 3 to 6 months | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐15.05 [‐29.69, ‐0.41] |
| 12 Adverse events ‐ significant mood disturbance Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.55, 11.41] |
| 13 Adverse events ‐ gastrointestinal disturbance Show forest plot | 3 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.45 [1.11, 10.78] |
| 14 Adverse events ‐ insomnia Show forest plot | 3 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.63 [1.10, 11.95] |
| 15 Endoscopy score ‐ nasal polyps (final value) Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 15.1 2 to 3 weeks | 4 | 253 | Mean Difference (IV, Fixed, 95% CI) | ‐0.76 [‐0.92, ‐0.61] |
| 15.2 3 to 6 months | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.62, 0.12] |
| 16 Endoscopy score ‐ nasal polyps score (change from baseline) Show forest plot | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 16.1 2 to 3 weeks | 2 | 146 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.77 [‐2.16, ‐1.38] |
| 16.2 3 to 6 months | 1 | 114 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐0.90, ‐0.14] |

