Inserción posparto inmediata versus diferida del implante anticonceptivo para la anticoncepción
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | A randomised controlled trial (RCT) conducted in large hospital in North Carolina, USA | |
| Participants | Adolescents and young women aged 14 to 24 years, gave birth to a healthy infant, spoke English or Spanish, and desired to use the contraceptive implant.
Most study participants were Hispanic/Latino (40% of women assigned to both immediate and delayed postpartum insertion groups). | |
| Interventions | Intervention arm: participants received etonogestrel contraceptive implant within 48 hours of delivery. | |
| Outcomes | Primary outcome: contraceptive implant use at 12 months postpartum. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned using computer‐generated random numbers in blocks of 4 and 6. |
| Allocation concealment (selection bias) | Low risk | The assignments were enclosed in sequentially numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) | Low risk | Although blinding of the participants was not possible, initiation rate was unlikely to be affect by the performance bias. |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of the participants was not possible which may affect the self‐reported side‐effects. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information available. |
| Incomplete outcome data (attrition bias) | High risk | There was a high rate of loss to follow‐up in both groups (23% (11/48) and 44% (21/48) in immediate insertion and delayed insertion groups respectively. |
| Selective reporting (reporting bias) | Low risk | The trial authors reported all potentially relevant outcomes. |
| Other bias | Unclear risk | Many outcomes in this study were not analysed following an intention‐to‐treat basis. |
| Methods | A RCT with non‐inferiority design conducted in a university hospital in Utah, USA | |
| Participants | Healthy peripartum women with healthy, term newborns who desired the etonogestrel implant for contraception. Exclusion criteria were onset of lactogenesis before randomisation, haemorrhage requiring transfusion, severe pregnancy‐induced hypertension, prolonged hospitalisation, coagulopathy, liver disease, undiagnosed genital bleeding, or other relative contraindication to etonogestrel implant insertion (known or suspected pregnancy; known, suspected, or history of breast cancer;or hypersensitivity to any of the components in the etonogestrel implant). Women taking inducers of hepatic enzymes were also excluded, including barbiturates, griseofulvin, rifampin, phenylbutazone, phenytoin, carbamazepine, felbamate, oxcarbazepine, topiramate, modafinil, protease inhibitors, and herbal products | |
| Interventions | Intervention arm: participants received etonogestrel contraceptive implant within 1 to 3 days after delivery. | |
| Outcomes | Primary outcome: time to lactogenesis stage II documented by maternal perception, lactational failure | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned using computer‐generated random numbers in blocks of varying sizes of 2, 4, and 6. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was ensured because the assignments were enclosed in sequentially numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) | Low risk | Although blinding of the participants was not possible, initiation rate was unlikely to be affect by the performance bias. |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of the participants was not possible which may affect the self‐reported side effects. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information was available. |
| Incomplete outcome data (attrition bias) | Low risk | There was a low rate of loss to follow‐up in both groups; (17% (6/35) and 17% (6/34) in the immediate insertion and delayed insertion groups respectively. |
| Selective reporting (reporting bias) | Low risk | The trial authors reported all potentially relevant outcomes. |
| Other bias | Unclear risk | The trial authors did not provide the definitions of abnormal vaginal bleeding patterns applied in this study, which was an outcome of interest in this review. |
| Methods | A RCT conducted in a large hospital in North Carolina, USA | |
| Participants | Peripartum women were offered inclusion in this trial if they intended to use contraceptive implant for contraception, had vaginal births, had predelivery gestational age of 34 weeks or more, had a spontaneous delivery of a grossly intact placenta, had a predelivery haemoglobin value of 8.0 g/dL or higher, and had no contraindications toNorplant insertion as listed by the manufacturer including acute liver disease, acute thromboembolic disorder, abnormal genital bleeding, pregnancy, breast cancer, or an allergy to silicone or levonorgestrel 250 non‐breastfeeding postpartum women who met the study inclusion were enrolled. Most women who participated in this study were black (75.2% and 72.5% of women in the immediate and delayed postpartum insertion groups, respectively) | |
| Interventions | Intervention arm: participants received levonorgestrel contraceptive implant within 48 hours of delivery. | |
| Outcomes | Primary outcome of interest was postpartum bleeding evaluated by the change of haemoglobin level and participants’ self‐reported of vaginal bleeding patterns All outcomes were evaluated during the visit scheduled at 4 to 6 weeks after delivery | |
| Notes | The trial investigators excluded 9 women after randomisation. The included study did not state about the definitions of abnormal vaginal bleeding patterns which were the primary safety concerns of this study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The allocation sequence was computer generated and sequences was numbered sequentially. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was ascertained by enclosing assignments in opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) | Low risk | Although blinding of the participants was not possible, initiation rate was unlikely to be affect by the performance bias. |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of the participants was not possible which may affect the self‐reported side effects. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information was available. |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up rates were 12% and 9% of participants assigned to immediate and delayed insertion groups, respectively. |
| Selective reporting (reporting bias) | Low risk | The trial authors reported all potentially relevant outcomes. |
| Other bias | Unclear risk | The trial investigators excluded 9 women after randomisation. In addition, no well‐defined terminology of abnormal vaginal bleeding was applied in this trial. |
Abbreviations: RCT: randomised controlled trial.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Did not compare immediate versus delayed contraceptive implant insertion. | |
| Did not compare immediate versus delayed contraceptive implant insertion. | |
| Did not compare immediate versus delayed contraceptive implant insertion. | |
| Not a RCT. | |
| Not a RCT. | |
| Did not compare immediate versus delayed contraceptive implant insertion. | |
| Did not compare immediate versus delayed contraceptive implant insertion. | |
| Not a RCT. | |
| Not a RCT. | |
| Not a RCT. |
Abbreviations: RCT: randomised controlled trial.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rate of initiation of contraceptive implants Show forest plot | 3 | 410 | Risk Ratio (IV, Fixed, 95% CI) | 1.41 [1.28, 1.55] |
| Analysis 1.1  Comparison 1 Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 1 Rate of initiation of contraceptive implants. | ||||
| 2 Continuation rate Show forest plot | 2 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 2 Continuation rate. | ||||
| 2.1 At 6 months postpartum | 2 | 125 | Risk Ratio (IV, Fixed, 95% CI) | 1.02 [0.93, 1.11] |
| 2.2 At 12 months postpartum | 1 | 64 | Risk Ratio (IV, Fixed, 95% CI) | 1.04 [0.81, 1.34] |
| 3 Side effect: mean days of abnormal vaginal bleeding Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3 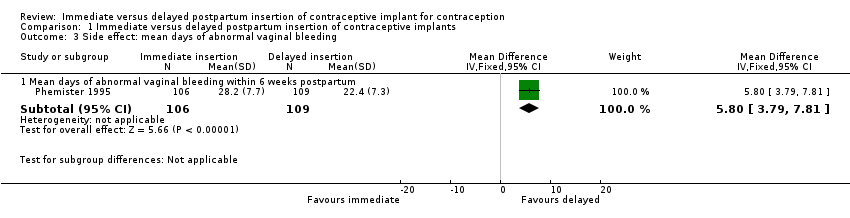 Comparison 1 Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 3 Side effect: mean days of abnormal vaginal bleeding. | ||||
| 3.1 Mean days of abnormal vaginal bleeding within 6 weeks postpartum | 1 | 215 | Mean Difference (IV, Fixed, 95% CI) | 5.80 [3.79, 7.81] |
| 4 Other side effects Show forest plot | 2 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 4 Other side effects. | ||||
| 4.1 Heavy, irregular bleeding or associated severe cramping within 12 months | 1 | 64 | Risk Ratio (IV, Fixed, 95% CI) | 1.01 [0.72, 1.44] |
| 4.2 Adverse effects other than abnormal vaginal bleeding | 1 | 215 | Risk Ratio (IV, Fixed, 95% CI) | 2.06 [1.38, 3.06] |
| 5 Participant's satisfaction Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 5 Participant's satisfaction. | ||||
| 5.1 At 12 months post partum | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.26, 0.46] |
| 6 Unintended pregnancy rate Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 6 Unintended pregnancy rate. | ||||
| 6.1 At 12 months after delivery | 1 | 64 | Risk Ratio (IV, Fixed, 95% CI) | 1.82 [0.38, 8.71] |
| 7 Breastfeeding at six months postpartum Show forest plot | 1 | 64 | Risk Ratio (IV, Fixed, 95% CI) | 2.01 [0.72, 5.63] |
| Analysis 1.7 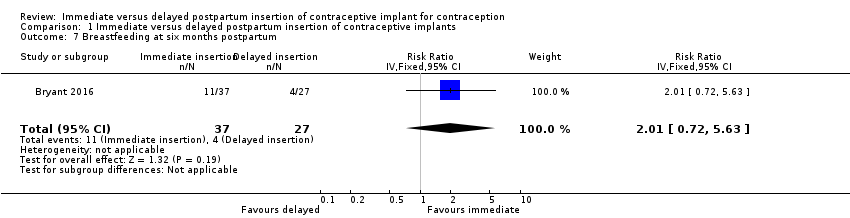 Comparison 1 Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 7 Breastfeeding at six months postpartum. | ||||

Study flow diagram.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.

Forest plot of comparison: 1 Immediate versus delayed postpartum insertion of contraceptive implants, outcome: 1.1 Rate of initiation of contraceptive implants.

Forest plot of comparison: 1 Immediate versus delayed postpartum insertion of contraceptive implants, outcome: 1.2 Continuation rate.
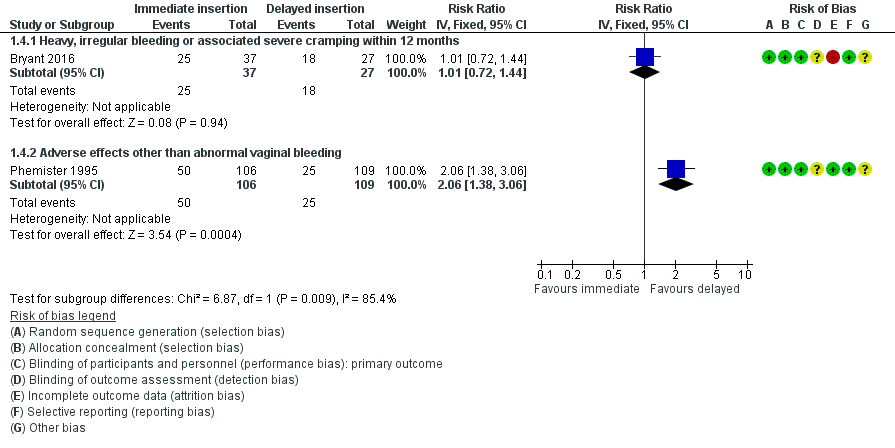
Forest plot of comparison: 1 Immediate versus delayed postpartum insertion of contraceptive implants, outcome: 1.4 Other side effects.

Comparison 1 Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 1 Rate of initiation of contraceptive implants.

Comparison 1 Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 2 Continuation rate.
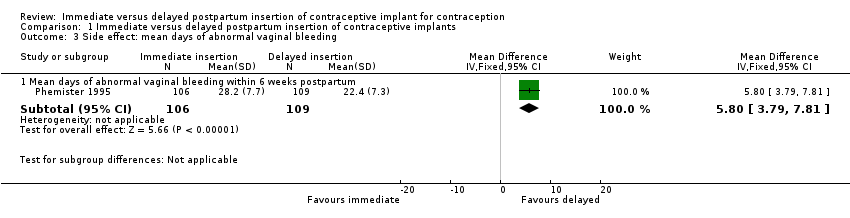
Comparison 1 Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 3 Side effect: mean days of abnormal vaginal bleeding.

Comparison 1 Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 4 Other side effects.
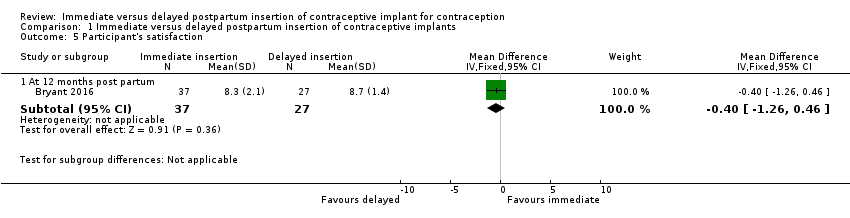
Comparison 1 Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 5 Participant's satisfaction.
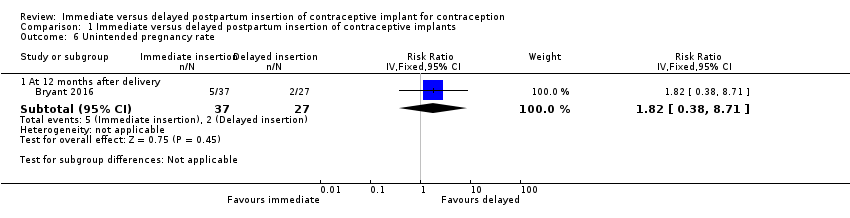
Comparison 1 Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 6 Unintended pregnancy rate.

Comparison 1 Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 7 Breastfeeding at six months postpartum.
| Immediate postpartum insertion compared with delayed insertion of contraceptive implant for contraception | ||||||
| Participant or population: postpartum women who desire a contraceptive implant for contraception Settings: hospitals in United States Intervention: immediate postpartum insertion Comparison: delayed insertion | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Delayed insertion | Immediate insertion | |||||
| Rate of initiation of contraceptive implants | 69 per 100 | 97 per 100 | RR 1.41 | 410 | ⊕⊕⊕⊝ | |
| Continuation rate at 6 months Continuation rate at 12 months | 90 per 100 | 92 per 100 | RR 1.02 | 125 | ⊕⊕⊝⊝ | |
| 78 per 100 | 81 per 100 (63 to 100) | RR 1.04 (0.81 to 1.34) | 64 (1 study) | ⊕⊕⊝⊝ | ||
| Side effects: Abnormal vaginal bleeding within 6 weeks post partum Side effects: Other side effects within 6 weeks Side effects: Heavy, irregular vaginal bleeding or associated severe cramping within 12 months | Mean 22.4 | Mean 5.80 higher | MD 5.80 | 215 | ⊕⊕⊝⊝ | |
| 23 per 100 | 47 per 100 | RR 2.06 | 215 | ⊕⊕⊝⊝ | ||
| 67 per 100 | 68 per 100 (48 to 96) | RR 1.01 (0.72 to 1.44) | 64 | ⊕⊕⊝⊝ | ||
| Participants' satisfaction at 12 months | Mean 8.7 | Mean 0.40 lower | MD ‐0.40 | 64 (1 study) | ⊕⊕⊝⊝ | |
| Unintended pregnancy rate at 12 months | 7 per 100 | 13 per 100 (3 to 61) | RR 1.82 | 64 (1 study) | ⊕⊕⊝⊝ | |
| Breastfeeding at six months postpartum | 15 per 100 | 30 per 100 (11 to 84) | RR 2.01 | 64 (1 study) | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level owing to serious imprecision (small number of participants) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rate of initiation of contraceptive implants Show forest plot | 3 | 410 | Risk Ratio (IV, Fixed, 95% CI) | 1.41 [1.28, 1.55] |
| 2 Continuation rate Show forest plot | 2 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At 6 months postpartum | 2 | 125 | Risk Ratio (IV, Fixed, 95% CI) | 1.02 [0.93, 1.11] |
| 2.2 At 12 months postpartum | 1 | 64 | Risk Ratio (IV, Fixed, 95% CI) | 1.04 [0.81, 1.34] |
| 3 Side effect: mean days of abnormal vaginal bleeding Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Mean days of abnormal vaginal bleeding within 6 weeks postpartum | 1 | 215 | Mean Difference (IV, Fixed, 95% CI) | 5.80 [3.79, 7.81] |
| 4 Other side effects Show forest plot | 2 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Heavy, irregular bleeding or associated severe cramping within 12 months | 1 | 64 | Risk Ratio (IV, Fixed, 95% CI) | 1.01 [0.72, 1.44] |
| 4.2 Adverse effects other than abnormal vaginal bleeding | 1 | 215 | Risk Ratio (IV, Fixed, 95% CI) | 2.06 [1.38, 3.06] |
| 5 Participant's satisfaction Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 At 12 months post partum | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.26, 0.46] |
| 6 Unintended pregnancy rate Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 At 12 months after delivery | 1 | 64 | Risk Ratio (IV, Fixed, 95% CI) | 1.82 [0.38, 8.71] |
| 7 Breastfeeding at six months postpartum Show forest plot | 1 | 64 | Risk Ratio (IV, Fixed, 95% CI) | 2.01 [0.72, 5.63] |

