Inserción posparto inmediata versus diferida del implante anticonceptivo para la anticoncepción
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011913.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 22 abril 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Regulación de la fertilidad
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Jen Sothornwit initiated the review topic. Jen Sothornwit, Yuthapong Werawatakul, Srinaree Kaewrudee, Malinee Laopaiboon and Pisake Lumbiganon drafted the review. All authors reviewed and approved the final version of the review.
Sources of support
Internal sources
-
Department of Obstetrics and Gynecology, Faculty of Medicine, Khon Kaen University, Thailand.
-
Department of Epidemiology and Biostatistics, Faculty of Public Health, Khon Kaen University, Thailand.
External sources
-
Thailand Research Fund (Distinguished Professor Awards), Thailand.
-
Cochrane Thailand, Thailand.
Declarations of interest
Jen Sothornwit: none known
Yuthapong Werawatakul: none known
Srinaree Kaewrudee: none known
Pisake Lumbiganon: none known
Malinee Laopaiboon: none known
Acknowledgements
We thank Anja Helmerhorst, Frans M Helmerhorst, and Helen Nagels for their contributions to the editorial process and clinical advice, Carol Manion for designing the search strategies and running the search, and the referees for their useful suggestions and comments during the prepublication editorial process. We also thank Chumnan Kietpeerakool for his input in the revision of the review.
Version history
| Published | Title | Stage | Authors | Version |
| 2022 Oct 27 | Immediate versus delayed postpartum insertion of contraceptive implant and IUD for contraception | Review | Jen Sothornwit, Srinaree Kaewrudee, Pisake Lumbiganon, Porjai Pattanittum, Sarah H Averbach | |
| 2017 Apr 22 | Immediate versus delayed postpartum insertion of contraceptive implant for contraception | Review | Jen Sothornwit, Yuthapong Werawatakul, Srinaree Kaewrudee, Pisake Lumbiganon, Malinee Laopaiboon | |
| 2015 Oct 25 | Immediate versus delayed postpartum insertion of contraceptive implant for contraception | Protocol | Jen Sothornwit, Yuthapong Werawatakul, Srinaree Kaewrudee, Pisake Lumbiganon, Malinee Laopaiboon | |
Differences between protocol and review
Primary review outcome
In our protocol the primary outcome was 'Postpartum contraceptive prevalence at the first postpartum check‐up visit'. This has been re‐worded as 'Rate of contraceptive implant initiation at the first postpartum check‐up visit'.
Unit of analysis issues
We intended to include studies where individual postpartum women were randomised and cluster‐randomized studies where, for example, the hospital was the unit of randomisation. However, we did not find any related cluster‐randomised study. In future updates of this review, for studies that use a cluster‐randomised design but did not have any information related to the design effect, we will estimate the design effect based on a fairly large assumed intra‐cluster correlation of 0.10. We will base this assumption by analogy on studies about implementation research (Ukoumunne 1999; Campbell 2000).
Assessment of reporting biases
As only three RCTs met the inclusion criteria, we were unable to construct funnel plots to determine the possibility of publication bias as previously stated in the protocol (Sothornwit 2015). In future updates of this review, we will construct funnel plots that correspond to the findings of the meta‐analysis of the primary outcome that this review needs to address if we identify a sufficient number of included studies (i.e. more than 10). We will also perform sensitivity analyses to investigate the effect on the pooled results of the funnel plots (Sterne 2011).
Subgroup analysis and investigation of heterogeneity
We did not perform subgroup analyses, as we stated in the Cochrane protocol, due to the limited number of included studies. All included studies were conducted in the USA. In future versions of this review, we will perform subgroup analysis to investigate heterogeneity, if feasible, and we will employ the following methods for subgroup analysis.
-
We will consider whether an overall summary is meaningful, and if it is, use a random‐effects analysis to produce it.
-
We plan to perform subgroup analyses according to age of participants (teenagers versus non‐teenagers) and population differences (high‐income versus low‐ and middle‐income countries).
-
We will assess subgroup differences by interaction tests available within RevMan 5 (RevMan 2014). We will report the results of subgroup analyses quoting the Chi² statistic and P value, the interaction test, and I² statistic value.
Sensitivity analysis
Only three published RCTs were available for this review. which were at low risk of selection bias. Furthermore, we did not find any unpublished studies. Thus we were unable to perform sensitivity analyses as we stated in the Cochrane protocol (Sothornwit 2015). In future versions of the review we will perform sensitivity analyses, if feasible, in order to determine the impact of the following factors on effect size.
-
Repeating the analysis by excluding unpublished studies (if any).
-
Repeating the analysis by excluding trials rated as at 'high’ or ’unclear’ risk of bias for allocation concealment.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Breast Feeding [statistics & numerical data];
- Contraception [instrumentation, *methods];
- *Contraceptive Devices, Female [adverse effects, statistics & numerical data];
- Patient Satisfaction [statistics & numerical data];
- *Postpartum Period;
- Pregnancy, Unplanned;
- Randomized Controlled Trials as Topic;
- Time Factors;
- Uterine Hemorrhage [epidemiology, etiology];
Medical Subject Headings Check Words
Female; Humans; Pregnancy;
PICO

Study flow diagram.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.

Forest plot of comparison: 1 Immediate versus delayed postpartum insertion of contraceptive implants, outcome: 1.1 Rate of initiation of contraceptive implants.

Forest plot of comparison: 1 Immediate versus delayed postpartum insertion of contraceptive implants, outcome: 1.2 Continuation rate.
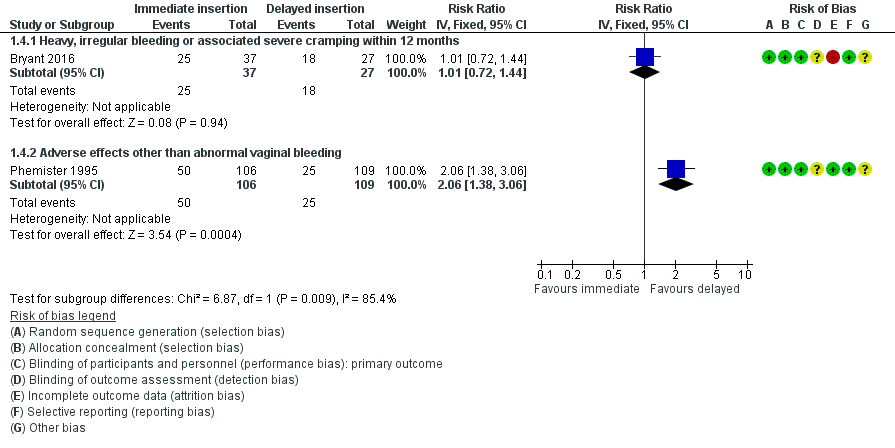
Forest plot of comparison: 1 Immediate versus delayed postpartum insertion of contraceptive implants, outcome: 1.4 Other side effects.

Comparison 1 Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 1 Rate of initiation of contraceptive implants.

Comparison 1 Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 2 Continuation rate.
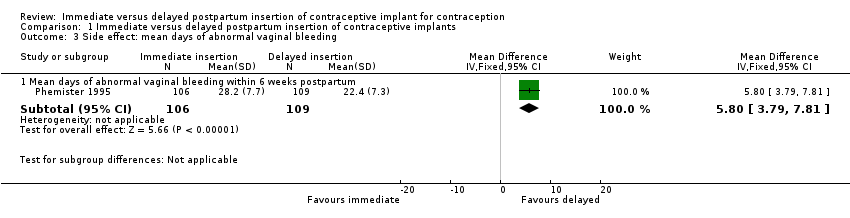
Comparison 1 Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 3 Side effect: mean days of abnormal vaginal bleeding.

Comparison 1 Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 4 Other side effects.
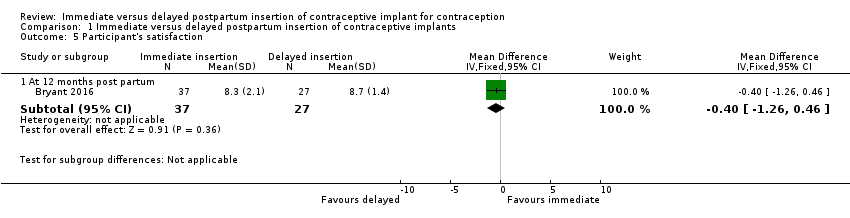
Comparison 1 Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 5 Participant's satisfaction.
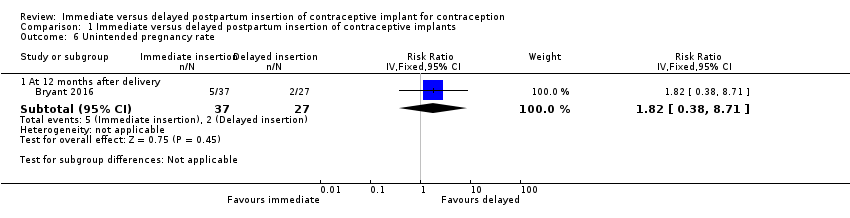
Comparison 1 Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 6 Unintended pregnancy rate.

Comparison 1 Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 7 Breastfeeding at six months postpartum.
| Immediate postpartum insertion compared with delayed insertion of contraceptive implant for contraception | ||||||
| Participant or population: postpartum women who desire a contraceptive implant for contraception Settings: hospitals in United States Intervention: immediate postpartum insertion Comparison: delayed insertion | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Delayed insertion | Immediate insertion | |||||
| Rate of initiation of contraceptive implants | 69 per 100 | 97 per 100 | RR 1.41 | 410 | ⊕⊕⊕⊝ | |
| Continuation rate at 6 months Continuation rate at 12 months | 90 per 100 | 92 per 100 | RR 1.02 | 125 | ⊕⊕⊝⊝ | |
| 78 per 100 | 81 per 100 (63 to 100) | RR 1.04 (0.81 to 1.34) | 64 (1 study) | ⊕⊕⊝⊝ | ||
| Side effects: Abnormal vaginal bleeding within 6 weeks post partum Side effects: Other side effects within 6 weeks Side effects: Heavy, irregular vaginal bleeding or associated severe cramping within 12 months | Mean 22.4 | Mean 5.80 higher | MD 5.80 | 215 | ⊕⊕⊝⊝ | |
| 23 per 100 | 47 per 100 | RR 2.06 | 215 | ⊕⊕⊝⊝ | ||
| 67 per 100 | 68 per 100 (48 to 96) | RR 1.01 (0.72 to 1.44) | 64 | ⊕⊕⊝⊝ | ||
| Participants' satisfaction at 12 months | Mean 8.7 | Mean 0.40 lower | MD ‐0.40 | 64 (1 study) | ⊕⊕⊝⊝ | |
| Unintended pregnancy rate at 12 months | 7 per 100 | 13 per 100 (3 to 61) | RR 1.82 | 64 (1 study) | ⊕⊕⊝⊝ | |
| Breastfeeding at six months postpartum | 15 per 100 | 30 per 100 (11 to 84) | RR 2.01 | 64 (1 study) | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level owing to serious imprecision (small number of participants) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rate of initiation of contraceptive implants Show forest plot | 3 | 410 | Risk Ratio (IV, Fixed, 95% CI) | 1.41 [1.28, 1.55] |
| 2 Continuation rate Show forest plot | 2 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At 6 months postpartum | 2 | 125 | Risk Ratio (IV, Fixed, 95% CI) | 1.02 [0.93, 1.11] |
| 2.2 At 12 months postpartum | 1 | 64 | Risk Ratio (IV, Fixed, 95% CI) | 1.04 [0.81, 1.34] |
| 3 Side effect: mean days of abnormal vaginal bleeding Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Mean days of abnormal vaginal bleeding within 6 weeks postpartum | 1 | 215 | Mean Difference (IV, Fixed, 95% CI) | 5.80 [3.79, 7.81] |
| 4 Other side effects Show forest plot | 2 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Heavy, irregular bleeding or associated severe cramping within 12 months | 1 | 64 | Risk Ratio (IV, Fixed, 95% CI) | 1.01 [0.72, 1.44] |
| 4.2 Adverse effects other than abnormal vaginal bleeding | 1 | 215 | Risk Ratio (IV, Fixed, 95% CI) | 2.06 [1.38, 3.06] |
| 5 Participant's satisfaction Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 At 12 months post partum | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.26, 0.46] |
| 6 Unintended pregnancy rate Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 At 12 months after delivery | 1 | 64 | Risk Ratio (IV, Fixed, 95% CI) | 1.82 [0.38, 8.71] |
| 7 Breastfeeding at six months postpartum Show forest plot | 1 | 64 | Risk Ratio (IV, Fixed, 95% CI) | 2.01 [0.72, 5.63] |

