Inserción posparto inmediata versus diferida del implante anticonceptivo para la anticoncepción
Resumen
Antecedentes
El espaciamiento de los embarazos tiene una repercusión positiva sobre la salud materna y neonatal. El implante anticonceptivo de progestina, que es un método de anticoncepción reversible de acción prolongada, tiene una tasa de fracaso baja bien establecida que es compatible con la esterilización tubárica. La provisión estándar de métodos anticonceptivos en la primera visita posparto puede hacer que algunas mujeres estén en riesgo de un embarazo no intencional, ya sea debido a la pérdida durante el seguimiento o a que tienen relaciones sexuales antes de recibir anticoncepción. Por lo tanto, la administración inmediata de anticoncepción con una eficacia alta antes del egreso hospitalario puede mejorar la prevalencia anticonceptiva y prevenir el embarazo no intencional.
Objetivos
Comparar la tasa de iniciación, la efectividad y los efectos secundarios de la inserción posparto inmediata versus diferida del implante anticonceptivo para la anticoncepción.
Métodos de búsqueda
Se hicieron búsquedas de estudios elegibles hasta el 28 octubre 2016 en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL), MEDLINE, Embase y en POPLINE. Se examinaron artículos de revisión y se contactó con los investigadores. Para encontrar estudios potencialmente relevantes, también se verificaron los registros de ensayos clínicos en curso, las listas de referencias de los estudios incluidos, libros de texto clave, literatura gris y las revisiones sistemáticas anteriores.
Criterios de selección
Se buscaron los ensayos controlados aleatorios (ECA) que compararon la inserción posparto inmediata versus diferida del implante anticonceptivo para la anticoncepción.
Obtención y análisis de los datos
Dos autores de la revisión (JS, YW) seleccionaron de forma independiente títulos y resúmenes de los resultados de la búsqueda y evaluaron los artículos de texto completo de estudios potencialmente relevantes para su inclusión. Extrajeron los datos de los estudios incluidos, evaluaron el riesgo de sesgo, compararon los resultados y resolvieron los desacuerdos consultando a un tercer autor de la revisión (PL o SK). Cuando fue posible, se estableció contacto con los investigadores para solicitar datos adicionales. Se calculó el cociente de riesgos (CR) de Mantel‐Haenszel con el intervalo de confianza (IC) del 95% para los resultados binarios y la diferencia de medias (DM) con el IC del 95% para las variables continuas.
Resultados principales
Tres estudios que incluyeron a 410 participantes cumplieron los criterios de inclusión de esta revisión. No se identificó ningún ensayo en curso. Dos estudios incluidos tuvieron bajo riesgo de sesgos de selección, desgaste e informe, pero tuvieron alto riesgo de sesgos de realización y detección debido a la imposibilidad de cegar a las participantes a la intervención. Un estudio incluido tuvo alto riesgo de sesgo de desgaste. La calidad general de la evidencia de cada comparación varió de muy baja a moderada; las limitaciones principales fueron el riesgo de sesgo y la imprecisión.
La tasa de iniciación de los implantes anticonceptivos en la primera visita de chequeo posparto fue significativamente mayor en el grupo de inserción inmediata que en el grupo de inserción diferida (CR 1,41; IC del 95%: 1,28 a 1,55; tres estudios, 410 participantes; evidencia de calidad moderada).
Pareció haber poca o ninguna diferencia entre los grupos en la tasa de continuación del implante anticonceptivo utilizado a los seis meses después de la inserción (CR 1,02; IC del 95%: 0,93 a 1,11; dos estudios, 125 participantes; evidencia de baja calidad) o a los 12 meses después de la inserción (CR 1,04; IC del 95%: 0,81 a 1,34; un ensayo, 64 participantes, evidencia de calidad muy baja)
Las pacientes que recibieron una inserción posparto inmediata del implante anticonceptivo tuvieron un número medio mayor de días de hemorragia vaginal anormal en el transcurso de las seis semanas posparto (DM 5,80 días; IC del 95%: 3,79 a 7,81; un estudio, 215 participantes; evidencia de baja calidad) y una tasa mayor de otros efectos secundarios en las seis primeras semanas después del parto (CR 2,06; IC del 95%: 1,38 a 3,06; un estudio, 215 participantes; evidencia de baja calidad) que las que recibieron una inserción posparto diferida. Pareció haber poca o ninguna diferencia entre los grupos en la hemorragia vaginal irregular profusa o los dolores tipo cólicos graves asociados en el transcurso de 12 meses (CR 1,01; IC del 95%: 0,72 a 1,44; un estudio, 64 participantes; evidencia de muy baja calidad).
No estuvo claro si hubo alguna diferencia entre los grupos en las puntuaciones de satisfacción de la participante en una escala de 0 a 10 (DM ‐0,40; IC del 95%: ‐1,26 a 0,46; evidencia de baja calidad), o en las tasas de embarazo no intencional (CR 1,82; IC del 95%: 0,38 a 8,71; un ECA, 64 pacientes, evidencia de muy baja calidad) a los 12 meses, o en la tasa de lactancia materna a los seis meses (CR 2,01; IC del 95%: 0,72 a 5,63; un ECA, 64 pacientes, evidencia de muy baja calidad); la tasa no difirió de forma significativa entre los grupos.
Conclusiones de los autores
La evidencia de esta revisión indica que la tasa de iniciación del implante anticonceptivo en la primera visita de chequeo posparto fue mayor con la inserción posparto inmediata que con la inserción diferida. Pareció haber poca o ninguna diferencia entre los grupos en la tasa de continuación del uso del implante anticonceptivo a los seis meses. No estuvo claro si hubo una diferencia entre los grupos en la continuación del uso de anticonceptivos a los 12 meses o en la tasa de embarazo no intencional a los 12 meses.
PICO
Resumen en términos sencillos
Inserción de un implante anticonceptivo poco después del parto
Pregunta de la revisión
Los autores Cochrane compararon la tasa de iniciación, la efectividad y los efectos secundarios de la inserción de un implante anticonceptivo (implante para la regulación de la natalidad) poco después del parto versus la inserción diferida hasta la visita posparto.
Antecedentes
El espaciamiento de los embarazos tiene una repercusión positiva sobre la salud de las embarazadas y de los recién nacidos. El implante anticonceptivo es un método de regulación de la natalidad muy efectivo que sólo utiliza progestina. Habitualmente se proporciona un implante anticonceptivo en la primera visita posparto (por lo general a las seis semanas). Sin embargo, algunas mujeres no acuden o tienen relaciones sexuales antes de esta visita de chequeo. La inserción de un implante anticonceptivo después del parto pero antes del alta hospitalaria es digna de consideración; es conveniente en cuanto al momento y el lugar y puede aumentar el número de mujeres que utilizan este método.
Características de los estudios
Se realizaron búsquedas de estudios aleatoros hasta el 28 de octubre de 2016. Se analizó si la inserción del implante anticonceptivo poco después del parto o cuando las pacientes acuden para el primer chequeo posparto afectó el uso de este método de anticoncepción. Se incluyeron tres estudios con un total de 410 mujeres.
Resultados clave
El uso de un implante anticonceptivo fue mayor cuando se aplicó justo después del parto que cuando se aplicó a las cuatro a seis semanas después. Pareció haber poca o ninguna diferencia entre los grupos en la tasa de continuación del uso del implante anticonceptivo a los seis meses. No estuvo claro si hubo una diferencia entre los grupos en la continuación del uso de anticonceptivos a los 12 meses o en la tasa de embarazo no intencional a los 12 meses.
Aunque la hemorragia vaginal y otros efectos adversos en las primeras seis semanas después del parto que incluyen náuseas, alopecia, hirsutismo, cefalea y acné parecen ser mayores en las pacientes que reciben este método unos pocos días después del parto en lugar de a las cuatro a seis semanas después, este hallazgo, sin embargo, no se puede establecer definitivamente de manera concluyente ya que todas las participantes conocían la naturaleza de la intervención que recibieron (no se cegaron) y los informes de estos efectos adversos no se evaluaron de forma objetiva. No estuvo claro si hubo diferencia entre los grupos a los 12 meses en la hemorragia vaginal irregular profusa o los dolores tipo cólicos graves asociados, las tasas de embarazo no intencional, o en medidas de satisfacción de las participantes. Tampoco estuvo claro si hubo alguna diferencia en las tasas de lactancia materna a los seis meses. Los estudios incluidos se realizaron en los EE.UU. y la generalización de estos hallazgos a otra población o contextos se debe aplicar con cuidado.
Calidad de la evidencia
La calidad general de la evidencia se consideró moderada a muy baja. Las limitaciones principales fueron la imprecisión y el riesgo de sesgo (relacionado con la falta de cegamiento y el desgaste). Ensayos controlados aleatorios adicionales de buena calidad y bien diseñados proporcionarán información adicional.
Authors' conclusions
Summary of findings
| Immediate postpartum insertion compared with delayed insertion of contraceptive implant for contraception | ||||||
| Participant or population: postpartum women who desire a contraceptive implant for contraception Settings: hospitals in United States Intervention: immediate postpartum insertion Comparison: delayed insertion | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Delayed insertion | Immediate insertion | |||||
| Rate of initiation of contraceptive implants | 69 per 100 | 97 per 100 | RR 1.41 | 410 | ⊕⊕⊕⊝ | |
| Continuation rate at 6 months Continuation rate at 12 months | 90 per 100 | 92 per 100 | RR 1.02 | 125 | ⊕⊕⊝⊝ | |
| 78 per 100 | 81 per 100 (63 to 100) | RR 1.04 (0.81 to 1.34) | 64 (1 study) | ⊕⊕⊝⊝ | ||
| Side effects: Abnormal vaginal bleeding within 6 weeks post partum Side effects: Other side effects within 6 weeks Side effects: Heavy, irregular vaginal bleeding or associated severe cramping within 12 months | Mean 22.4 | Mean 5.80 higher | MD 5.80 | 215 | ⊕⊕⊝⊝ | |
| 23 per 100 | 47 per 100 | RR 2.06 | 215 | ⊕⊕⊝⊝ | ||
| 67 per 100 | 68 per 100 (48 to 96) | RR 1.01 (0.72 to 1.44) | 64 | ⊕⊕⊝⊝ | ||
| Participants' satisfaction at 12 months | Mean 8.7 | Mean 0.40 lower | MD ‐0.40 | 64 (1 study) | ⊕⊕⊝⊝ | |
| Unintended pregnancy rate at 12 months | 7 per 100 | 13 per 100 (3 to 61) | RR 1.82 | 64 (1 study) | ⊕⊕⊝⊝ | |
| Breastfeeding at six months postpartum | 15 per 100 | 30 per 100 (11 to 84) | RR 2.01 | 64 (1 study) | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level owing to serious imprecision (small number of participants) | ||||||
Background
Description of the condition
Traditionally, the first postpartum visit is scheduled to be at six weeks after childbirth (Speroff 2008). This visit is either to assess the recovery of the mother after childbirth or to address the contraceptive needs going forward. Although the postpartum visit is an ideal time to discuss and implement family planning services, there is a notably high default rate from postpartum appointments, particularly among adolescent mothers. This results in delayed or missed counselling opportunities about an appropriate contraception method (Moore 2015; Nkwabong 2015). In addition, nearly half of women have reported to have had unprotected vaginal sexual intercourse before attending a six‐week postpartum visit (Brito 2009; Chaovisitsaree 2012). Delayed initiation of contraception after childbirth and having unprotected sexual intercourse before attending postpartum clinic can lead to an unintended pregnancy (Wilson 2011). As unintended pregnancy may have a negative impact on newborn and maternal health (Fraser 1995; Singh 2010; Finer 2011), designing effective contraception practices for reducing unintended pregnancy among women who are at heightened risk is of utmost importance.
Description of the intervention
Provision of contraception usually occurs six weeks postpartum. However, a study conducted in the USA indicated that only 41% of women received contraceptives within 90 days after delivery (Thiel de Bocanegra 2013). This figure is similar to the reported results from low‐ and middle‐income countries (Moore 2015; Nkwabong 2015). Therefore immediate administration of effective contraception is worth considering.
Contraceptive implants are a long‐acting, reversible, progestin‐only method. Currently, two types of progestin are used as active ingredients in contraceptive implants, including levonorgestrel and etonogestrel. The primary mechanism of action of the implant is to suppress ovulation by altering the hypothalamic–pituitary–ovarian axis. The other mechanisms are to thicken cervical mucous and to alter the endometrial lining (ACOG 2011).
Contraceptive implants are extremely effective and safe. This contraceptive method offers up to three to five years of reliable pregnancy prevention once inserted. The typical‐use failure rate is only 0.05% (ACOG 2011). The satisfaction rate of the implant is reported to be high. Approximately 80% of users reported satisfaction with implant leading to a high continuation rate (Peipert 2011).
Based on its high efficacy and safety, contraceptive implants are suitable for nearly all women that need contraception (ACOG 2012; WHO 2015). The common side effects of contraceptive implants include amenorrhoea, irregular menstrual bleeding, spotting, sore breasts, headache, and weight gain. However, most side effects are mild, well‐tolerated, and self‐resolve within the first few months of use, and women should receive counselling in order to understand the side effects.
How the intervention might work
There is an increased risk of unintended pregnancy among women who have delayed initiation of contraception after childbirth or unprotected sexual intercourse before attending a postpartum clinic. Immediate postpartum provision of highly effective contraception, including intrauterine devices (IUDs) and contraceptive implant, has been proposed (ACOG 2011).
Based on a recent Cochrane systematic review, immediate post‐partum insertion of IUDs appears to be safe and effective (Lopez 2015). Advantages of this practice include high motivation and convenience for both postpartum women and providers. However, the expulsion rate of IUDs inserted immediately postpartum is slightly higher than in women that have deferred insertion of IUDs (Lopez 2015).
Immediate postpartum provision of the contraceptive implant has no significant negative impact on outcomes regarding maternal health and infant weight gain (Brito 2009). The World Health Organization's (WHO's) medical eligibility criteria for contraceptive use support contraceptive implant insertion prior to hospital discharge regardless of lactating status (Whaley 2015; WHO 2015). The side effects of immediate postpartum insertion of contraceptive implants also appear to be similar to that of interval insertion (Ireland 2014). Additionally, the three‐year continuation rate of this method is as high as 66% when contraceptive implants are inserted immediately postpartum, which emphasizes a high level of women's satisfaction with this practice (Wilson 2014).
Why it is important to do this review
Immediate postpartum insertion of a contraceptive implant could be a promising choice of timing of contraceptive method initiation. This is quite crucial for postpartum women who are at high risk of missing the six‐week postpartum visit, or of having early unprotected intercourse after childbirth, or both.
However, to our knowledge, there has been no systematic review to date that has evaluated the effectiveness and safety of immediate postpartum insertion of contraceptive implant for contraception.
Objectives
To compare the initiation rate, effectiveness, and side effects of immediate versus delayed postpartum insertion of implant for contraception.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), irrespective of blinding, language, publication status, or sample size. We did not include controlled clinical trials (CCTs) that used non‐random methods of assigning participants to treatment, such as by alternation, by birth date, or by medical record number, as they may be subject to high risk of bias.
Types of participants
Postpartum women who requested a reversible contraceptive method and were recruited before hospital discharge.
Types of interventions
Immediate postpartum insertion of contraceptive implant (after delivery to before hospital discharge) compared to delayed post‐partum insertion (during postpartum visit after hospital discharge), which is often referred to as standard or interval insertion.
Types of outcome measures
Primary outcomes
-
Rate of contraceptive implant initiation at the first postpartum check‐up visit.
Secondary outcomes
-
Continuation rate at 6, 12, and 24 months after delivery.
-
Side effects, such as abnormal vaginal bleeding.
-
Participant satisfaction.
-
Rate of pregnancy at 6, 12, and 24 months after delivery.
-
Unintended pregnancy rate at 6, 12, and 24 months after delivery.
-
Rapid repeat pregnancy (defined as pregnancy onset within two years of the previous pregnancy (Baldwin 2013)).
-
Breastfeeding at six months postpartum
Search methods for identification of studies
Electronic searches
We searched the following electronic databases;
-
The Cochrane Central Register of Controlled Trials (CENTRAL, 2016, issue 10);
-
MEDLINE (1946 to October week 4, 2016)
-
Embase (1980 to October 2016),
-
POPLINE (1970 to October 2016)
We have provided the search strategies in Appendix 1.
Searching other resources
We checked the citation lists of included studies, key textbooks, and systematic reviews for potentially relevant references. We searched the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (http://www.who.int/ictrp/en/) and ClinicalTrials.gov to identify ongoing trials. We applied modified versions of the same search strategy to check the following databases for grey literature: OpenGrey, GreyNet, Scirus, Social Care Online, National Research Register, NIHR portfolio database, and Index to theses.
Data collection and analysis
Selection of studies
Before examining the identified trials for possible inclusion, we developed and piloted a data collection form, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We downloaded all titles and abstracts retrieved by electronic searching to EndNote (EndNote 2015), a reference management database, and removed duplicates. Two review authors, JS and YW, independently screened the titles and abstracts of the remaining studies. We excluded studies that clearly did not meet the inclusion criteria. We obtained full‐text copies of potentially relevant studies. Two review authors, JS and YW, independently assessed the eligibility of the retrieved reports/publications. We resolved any disagreement through discussion or, if required, we consulted a third review author (PL). We identified and intended to collate multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Liberati 2009) and a 'Characteristics of excluded studies’ table.
Data extraction and management
Two review authors, JS and YW, independently extracted study characteristics and outcome data from included studies using a piloted data collection form. We noted in the 'Characteristics of included studies’ table if the included trials did not report outcome data in a usable way. We then contacted the trial authors for further information. We resolved disagreements by consensus or by involving a third review author (PL or SK). One review author, ML, entered data into Review Manager 5 (RevMan 5) (RevMan 2014). We double‐checked whether data is entered correctly by comparing the data presented in the systematic review with the study reports. A second review author (PL or SK) checked all study characteristics for accuracy against the trial report.
For included studies, we extracted the following data.
-
Author, year of publication, and journal citation (including language).
-
Country.
-
Setting.
-
Inclusion and exclusion criteria.
-
Study design and methodology.
-
Study population.
-
Study outcomes and their related summary statistics.
Assessment of risk of bias in included studies
We assessed and reported on the methodological risk of bias of included studies in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), which recommends the explicit reporting of the following individual elements for RCTs.
-
Selection bias: random sequence generation and allocation concealment.
-
Performance bias: blinding of participants and personnel (participants and treatment providers).
-
Detection bias: blinding of outcome assessors.
-
Attrition bias: incomplete outcome data.
-
Reporting bias: selective reporting of outcomes.
Two review authors, JS and YW, independently applied the 'Risk of bias’ tool to each included study and resolved differences by discussion or by consulting a third review author (PL or ML). We judged each item as at either 'high’, 'low’, or 'unclear’ risk of bias, as set out in the criteria provided by Higgins 2011, and provided a quote from the study report or a statement as justification for the judgement for each item in the 'Risk of bias’ table. We summarized the results in both a 'Risk of bias’ graph and a 'Risk of bias’ summary. When we interpreted treatment effects and meta‐analyses, we took into account the risk of bias for the studies that contributed to that outcome. Where information on risk of bias was related to unpublished data or correspondence with a trial author, we noted this in the ’Risk of bias’ table.
Measures of treatment effect
We used the following measures of the effect of treatment.
-
For dichotomous outcomes, such as the rate of contraceptive implant initiation, we used number of events and number of participants assessed for both the intervention and comparison groups to calculate the risk ratio (RR) and 95% confidence interval (CI).
-
For continuous outcomes, such as satisfaction rate, we used mean, standard deviation (SD), and the number of participants assessed for both the intervention and comparison groups to calculate mean difference (MD) with 95% CI.
Unit of analysis issues
We intended to include studies where individual postpartum women were randomised and cluster‐randomised studies where, for example, the hospital was the unit of randomisation. However, we did not find any cluster randomised studies that met the review inclusion.
Dealing with missing data
We did not impute any missing outcome data and we tried to contact the trial authors for missing data.
Assessment of heterogeneity
We assessed heterogeneity by visual inspection of the forest plots. We also assessed statistical heterogeneity in each meta‐analysis using the I² statistic and Chi² test. We regarded heterogeneity as substantial if the I² statistic value was greater than 50%, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity (Deeks 2001).
Assessment of reporting biases
As only three RCTs met the review inclusion criteria, we were unable to construct funnel plots to determine the possibility of publication bias.
Data synthesis
We undertook meta‐analysis for all the outcomes where suitable data were available. We described in narrative skewed data reported as medians and interquartile ranges.
Using RevMan 5 we performed statistical analyses (RevMan 2014). We used a fixed‐effect model to combine data where it is reasonable to assume that studies were estimating the same underlying treatment effect i.e. where studies were examining the same intervention, and we judged the studies' populations and methods to be sufficiently similar. If there was unexplained clinical heterogeneity sufficient to expect that the underlying treatment effects differed between studies, or if substantial statistical heterogeneity sufficient to expect that the underlying treatment effects differed between studies, or if we detected substantial statistical heterogeneity, we used a random‐effects meta‐analysis to produce an overall summary if we considered an average treatment effect across trials to be clinically meaningful. We used the random‐effects meta‐analysis, treated the pooled treatment effect as the average range of possible treatment effects, and discussed the clinical implications of treatment effects differing between studies. If the average treatment effect was not clinically meaningful, we did not intend to combine study results. If we used random‐effects analyses, we presented the results as the pooled treatment effect with 95% CIs, and the estimates of the T² and I² statistic (DerSimonian 1986). We prepared a 'Summary of findings' table to present the results of meta‐analysis, based on the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011). For the data that we were unable to pool for meta‐analysis, we conducted a narrative synthesis of the results.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses according to age of participants (teenagers versus non‐teenagers) and population differences (high‐income versus low‐ and middle‐income countries). We did not perform these subgroup analyses due to the limited number of included studies.
Sensitivity analysis
We planned to perform sensitivity analyses in order to determine the impact of the following factors on effect size:
-
Repeating the analysis excluding unpublished studies (if any).
-
Repeating the analysis excluding trials rated as 'high' or 'unclear' for risk of selection bias
There were too few studies to perform these sensitivity analyses.
Overall quality of the evidence: 'Summary of findings' table
Two review authors working independently prepared a ’Summary of findings’ table, with disagreements were resolved by consensus. The following outcomes were reported on the table: rate of initiation of contraceptive implants, continuation rate at 6 months, continuation rate at 12 months, side‐effects: abnormal vaginal bleeding within 6 weeks postpartum, side effects other than abnormal vaginal bleeding, participant's satisfaction, unintended pregnancy rate and breastfeeding at six months postpartum.
We used the GRADE approach (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the evidence (GRADEproGDT 2014). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We justified all decisions to downgrade the quality of evidence, using footnotes.
Results
Description of studies
Results of the search
We identified 817 references from the combined searches. After we removed 323 duplicated references, we screened the titles and abstracts of 494 references and discarded 481 references as they obviously did not meet the review inclusion criteria. Of the 13 studies that potentially met the review inclusion criteria, we excluded 10 studies after we reviewed the full texts of these studies (see the 'Characteristics of excluded studies' table), thereby leaving three studies that met the inclusion criteria of this review (Phemister 1995; Gurtcheff 2011; Bryant 2016); see the 'Characteristics of included studies' table). We checked the reference lists of included studies, key textbooks, and systematic reviews for potentially relevant references but found no relevant studies. We did not find any ongoing trials. Figure 1 displays the PRISMA flow diagram.
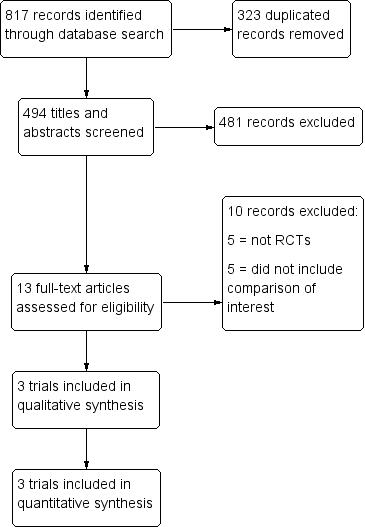
Study flow diagram.
Included studies
We included three randomised controlled trials (RCTs) (Phemister 1995; Gurtcheff 2011; Bryant 2016), and presented the details of the included studies in the 'Characteristics of included studies' table.
Phemister 1995 was conducted in the USA from June 1992 to February 1993. Participants were women who intended to use a contraceptive implant for postpartum contraception, had vaginal births at gestational age of 34 weeks or more, and did not have anaemia. Most women who participated in this study were black (understood to be African‐American). Participants in the intervention group received immediate postpartum insertion of the levonorgestrel contraceptive implant (Norplant®, Wyeth‐Ayerst, Philadelphia) between day 1 to day 3 after delivery. Participants in the control group received the same contraceptive method between four to six weeks after delivery. Reported outcomes included postpartum bleeding evaluated by the change of haemoglobin level and vaginal bleeding reported in the participant diary; postpartum contraceptive prevalence; and side effects that included nausea, hair loss, hirsutism, headache, and acne. The outcomes were evaluated during the visit scheduled at four to six weeks after delivery.
Gurtcheff 2011 was conducted in the USA from January to October 2009. Most participants were HIspanic. This study recruited 69 healthy peripartum women with healthy term newborns, who intended to breast feed, and desired a contraceptive implant. The contraceptive implant used was a single‐rod etonogestrel (Implanon®, Schering‐Plough). Participants allocated to the intervention group received immediate insertion of the etonogestrel contraceptive implant within three days after delivery. Participants assigned to the control group received the same contraceptive method four to eight weeks after delivery. The study outcomes were time to lactogenesis, lactational failure, postpartum contraceptive prevalence, participant‐reported vaginal bleeding, and breast milk creamatocrit.
Bryant 2016 was conducted in USA from August 2012 to April 2015. Most participants were Hispanic or Latino. The trial included postpartum women aged 14 to 24 years who desired to use the etonogestrel contraceptive implant. Participants allocated to intervention group received the contraceptive implant prior to discharge from the hospital. Participants in control group received the same contraceptive method at four to six weeks postpartum visit. The study outcomes were contraceptive prevalence at the first postpartum check‐up visit, rate of contraceptive implant use at 12 months, participants' satisfaction; abnormal bleeding patterns, breastfeeding, and unintended pregnancy rate.
Excluded studies
After assessing the full texts of 13 potentially eligible studies, we excluded 10 studies because of the following main reasons (see the 'Characteristics of excluded studies' table).
-
Six studies were not RCTs or CCTs (Shabaan 1985; Taneepanichkul 2001; Tocce 2012; Ireland 2014; Wilson 2014; Gariepy 2015).
-
Two studies compared immediate postpartum insertion of contraceptive implant as compared to no treatment (Brito 2012; Braga 2015).
-
Two studies compared immediate postpartum insertion of contraceptive implant as compared with depot medroxyprogesterone acetate (Brito 2009; Pentickly 2013).
Risk of bias in included studies
Figure 2 and Figure 3 summarize the 'Risk of bias' items presented as percentages across all included studies.
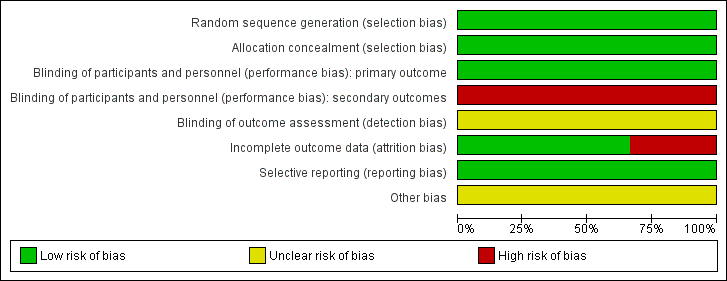
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
Allocation
Random sequence generation and allocation concealment
The three included studies randomly allocated participants to the comparison groups using adequate methods for sequence generation and concealment of allocation (Phemister 1995; Gurtcheff 2011; Bryant 2016). We therefore judged all three studies as at low risk of this bias.
Blinding
Although the timing of contraceptive implant insertion could not be blinded, we judged all three included studies as at low risk of performance and detection biases for the rate of initiation of contraceptive implant, which was the primary outcome of this review, because blinding is unlikely to affect this outcome measure. However, as knowledge of group assignment may affect self‐report of side effects, we therefore considered this to indicate high risk of bias for an assessment of treatment‐related side effects.
Incomplete outcome data
Two included studies had rates of incomplete outcome data of less than 20% (Phemister 1995; Gurtcheff 2011). Thus, we judged both studies as at low risk of attrition bias (Figure 3). Bryant 2016 had a 33% rate of incomplete outcome data, so we determined that this study was at high risk of attrition bias.
Selective reporting
Only two study protocols of the included studies were available for assessing the outcomes of interest (Gurtcheff 2011; Bryant 2016). However, the trial investigators of all included studies reported all relevant outcomes for their objectives, so we judged this domain as at low risk of selection bias.
Other potential sources of bias
In Phemister 1995, the trial investigators excluded nine women after randomisation. The trial investigators of all included studies did not state the definitions of abnormal vaginal bleeding patterns applied in their studies, which was the outcome of interest of this review (Phemister 1995; Gurtcheff 2011; Bryant 2016). We therefore assessed this domain as at unclear risk of bias in each of the included studies.
Effects of interventions
See 'Summary of findings' table 1 (summary of findings Table for the main comparison).
PRIMARY OUTCOME
Rate of initiation of contraceptive implant
All included studies reported the rate of initiation of contraceptive implant at the first postpartum check‐up visit (four to six weeks postpartum in Phemister 1995 and Bryant 2016 and four to eight weeks postpartum in Gurtcheff 2011). In Phemister 1995, 121 of 125 (96.8%) women assigned to the immediate postpartum insertion of contraceptive implant received contraceptive implant insertion as per the study protocol, while only 86 of 120 (71.7%) women assigned to the delayed postpartum insertion received implant insertion between four to six weeks postpartum at the time of the scheduled postpartum follow‐up visit. These findings were in line with that noted in Gurtcheff 2011, which observed that the rate of initiation of contraceptive implants was remarkably high among women assigned to immediate postpartum insertion when compared to women in the delayed postpartum insertion group (97.1% versus 67.6%, respectively). In Bryant 2016, all 48 women assigned to immediate insertion group received contraceptive implant insertion compared to only 32 of 48 women (67%) allocated to delayed insertion group. There was a higher rate of contraceptive implant initiation among women assigned to immediate insertion group compared with those in the delayed insertion group (risk ratio (RR) 1.41, 95% confidence interval (CI) 1.28 to 1.55; three studies, 410 participants; moderate quality evidence; Analysis 1.1; Figure 4; summary of findings Table for the main comparison).

Forest plot of comparison: 1 Immediate versus delayed postpartum insertion of contraceptive implants, outcome: 1.1 Rate of initiation of contraceptive implants.
SECONDARY OUTCOMES
Continuation rate
Continuation rate at six months after delivery
Gurtcheff 2011 and Bryant 2016 reported the continuation rate of contraceptive implant used at six months. The six‐month continuation rate of contraceptive implant use in Gurtcheff 2011 was 97.1% for women allocated to the immediate postpartum insertion, which was comparable to the rate of 95.7% reported for women in the delayed postpartum insertion group. In Bryant 2016, the continuation rate among women who received an immediate insertion was similar to that in women assigned to delayed insertion group (88% versus 86%, respectively). The meta‐analysis using fixed‐effects model suggested little or no difference between the groups in the rate of continuation of contraceptive implant use at six months following delivery (RR 1.02, 95% CI 0.93 to 1.11; two studies, 125 participants;low quality evidence; Analysis 1.2; Figure 5; summary of findings Table for the main comparison).
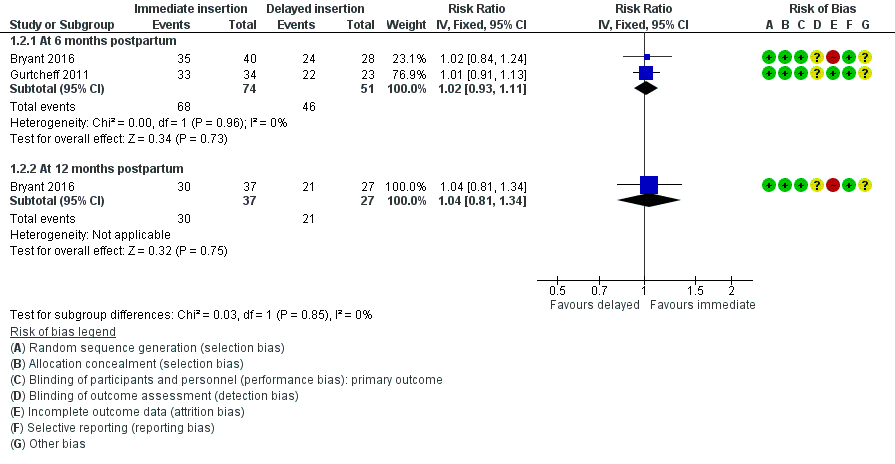
Forest plot of comparison: 1 Immediate versus delayed postpartum insertion of contraceptive implants, outcome: 1.2 Continuation rate.
Continuation rate at 12 months after delivery
Only Bryant 2016 reported the continuation rate of contraceptive implant use at 12 months after delivery. The 12‐month continuation rate of contraceptive implant use among women receiving immediate insertion was 81.1%, and it was 77.8% in women receiving delayed insertion. It was unclear whether there was any difference between the groups (RR 1.04; 95% CI 0.81 to 1.34; one study, 64 participants;very low quality evidence; Analysis 1.2; Figure 5; summary of findings Table for the main comparison).
Continuation rate at 24 months after delivery
No studies reported this outcome.
Side effects
Mean days of abnormal vaginal bleeding within six weeks postpartum
One included study reported the duration of abnormal vaginal bleeding (Phemister 1995). Duration of vaginal bleeding among women in the immediate insertion group was longer than that noted among women receiving delayed insertion of contraceptive implant (mean difference (MD) 5.80 days, 95% CI 3.79 to 7.81; one study, 215 participants;low quality evidence; Analysis 1.3; summary of findings Table for the main comparison). However, this difference might not constitute a clinically important benefit as there was no distinction of follow‐up haemoglobin level between these two comparison groups (Phemister 1995).
Heavy, irregular vaginal bleeding or associated severe cramping within 12 months
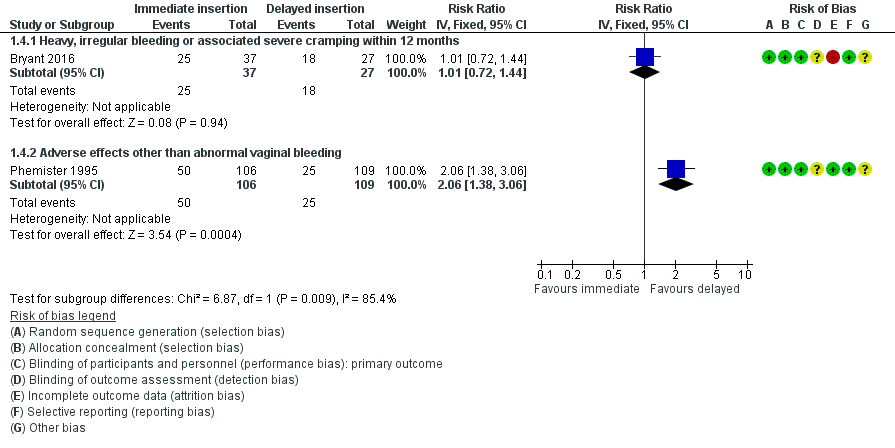
Bryant 2016 the rate of heavy, irregular bleeding, or associated cramping evaluated at 12 months among women receiving immediate insertion compared to women receiving delayed insertion of contraceptive implant. It was unclear whether there was any difference between the groups (RR 1.01, 95% CI 0.72 to 1.44; one study, 64 participants; very low quality evidence; Analysis 1.4; Figure 6).

Forest plot of comparison: 1 Immediate versus delayed postpartum insertion of contraceptive implants, outcome: 1.4 Other side effects.
Other side effects
Phemister 1995 reported that women in the immediate insertion group experienced a significantly higher rate of adverse effects other than vaginal bleeding including nausea, hair loss, hirsutism, headache, and acne than reported among women receiving delayed insertion (RR 2.06; 95% CI 1.38 to 3.06; one study, 215 participants; low quality evidence; Analysis 1.4; Figure 6; summary of findings Table for the main comparison).
Participant satisfaction
In Bryant 2016, the trial investigators asked their participants to rate the satisfaction score from zero to 10 during the one year follow‐up. There was little or no difference between the groups in participant satisfaction score (MD −0.40, 95% CI −1.26 to 0.46; one study, 64 participants; low quality evidence; Analysis 4.1; summary of findings Table for the main comparison).
Unintended pregnancy rates
Unintended pregnancy rates at 12 months after delivery
Bryant 2016 reported that the rates of unintended pregnancy occurring within one year after delivery were 13.5% and 7.0% among participants allocated to immediate insertion and those who were assigned to delayed insertion group, respectively. It was unclear whether there was any difference between the groups (RR 1.82, 95% CI 0.38 to 8.71; one study, 64 participants;low quality evidence; Analysis 1.6; summary of findings Table for the main comparison).
Unintended pregnancy rates at 24 months after delivery.
No studies reported this outcome.
Rapid repeat pregnancy (defined as pregnancy onset within two years of the previous pregnancy)
No studies reported this outcome.
Breastfeeding at six months postpartum
Gurtcheff 2011 and Bryant 2016 reported breastfeeding rate at six months. Gurtcheff 2011 presented Kaplan‐Meier curves to evaluate the impact of immediate postpartum insertion of contraceptive implant on exclusive or any breastfeeding compared to that of the delayed postpartum insertion. The rates between the two groups were not significantly different at any time periods. However, numerical data regarding this outcome were insufficient to determine the relative effect at each time point. Attempts to obtain additional data from the investigators were unsuccessful since the trial authors did not reply our requests.
Bryant 2016 reported rates of breastfeeding at six months after delivery among women receiving immediate contraceptive implant insertion compared to women allocated to delayed insertion, which were 30.0% versus 15.0%, respectively. It was unclear whether there was any difference between the groups (RR 2.01, 95% CI 0.72 to 5.63; one study, 64 participants;low quality evidence; Analysis 1.7; summary of findings Table for the main comparison).
Discussion
Summary of main results
Only three randomised controlled trials (RCTs) met the inclusion criteria of this review. We considered the overall review evidence to be at low risk of bias. Evidence from this review demonstrates that immediate postpartum contraceptive implant insertion significantly increases the rate of postpartum contraceptive implant initiation at the first postpartum check‐up visit. The continuation rate of contraceptive implant use at six months between the two groups was comparable. The mean duration of vaginal bleeding among women receiving immediate insertion of contraceptive implant was longer than that noted among women receiving delayed insertion; however, this difference might not constitute a clinically important benefit as there was no distinction of follow‐up haemoglobin level between these two comparison groups. Additionally, rates of abnormal vaginal bleeding between the two comparison groups were comparable regardless of the severity of bleeding. According to the inability to blind the intervention assigned, the difference in the rate of self‐reported side effects between the immediate and delayed postpartum insertion of contraceptive implant remains inconclusive. Based on the limited data available from a small single study, there were no significant differences between the two groups in terms of participant satisfaction, unintended pregnancy rate at 12 months, and rate of breastfeeding at six months postpartum.
Overall completeness and applicability of evidence
This review included three RCTs that evaluated immediate versus delayed postpartum insertion of contraceptive implant for contraception in 410 participants in the USA. The study participants were black and Hispanic white young‐adult women. Additionally, the study' settings were large hospitals in the USA. None of the included studies were conducted in low‐ or middle‐income countries, which often have low postpartum contraceptive prevalence (UN 2013). Thus generalization of these findings to other population or settings should be applied with caution.
All included studies reported the initiation rate of contraceptive implant at the first postpartum check‐up visit, which was the primary objective of this review. However, only two included studies reported the continuation rate at six months after delivery and adverse effects. Gurtcheff 2011 and Bryant 2016 reported data regarding the impact of the insertion type of contraceptive implant on breastfeeding at six months postpartum. However, we could not determine its relative effect from the only included study that reported this outcome because it lacked data regarding the actual number of participants in each comparison group (Gurtcheff 2011). Data regarding long‐term continuation rate, rate of unintended pregnancy or repeat pregnancy, and participant satisfaction were limited as a single included study reported these data.
Quality of the evidence
Although there was an inability to blind the type of intervention received, it was unlikely to affect the rate of initiation of contraceptive implant and continuation rate. However, knowledge of the intervention assigned may affect self‐reporting of treatment‐related side effects, thus indicating a high risk of performance and assessment bias for this outcome measures. This compromises the internal validity of an included study when determining the subjective outcomes, such as side effects and participant satisfaction, since an assessment of these events was based on the outcomes retrieved from participants’ self‐reported data. The higher risk of side effects among women who received an immediate postpartum contraceptive implant insertion versus those who received a delayed insertion should be cautiously viewed; it is unknown whether this is because of knowledge of which intervention had been administered, or the type of contraceptive implant insertion itself, affects outcomes. We extracted data regarding the rates of continuation and unintended pregnancy, participant satisfaction, and rate of breastfeeding at six months from a small single study that had a high default rate.
We determined the quality of evidence using the GRADE approach for each outcome (see summary of findings Table for the main comparison). We downgraded the quality of the evidence to moderate quality for rate of initiation of contraceptive implants. We downgraded the evidence to low or very low quality for the continuation rate of contraceptive implant, duration of abnormal vaginal bleeding, adverse effects other than vaginal bleeding, participant satisfaction, and rates of unintended pregnancy and breastfeeding at six months (see summary of findings Table for the main comparison).
Thus the quality of the evidence ranged from moderate to very low. The main limitations were imprecision and risk of bias (related to lack of blinding and to attrition).
Potential biases in the review process
With assistance from the Information Specialist, Cochrane Fertility Regulation Group, we made every attempt to include all potential studies including a thorough search of the standard databases, grey literature, conference proceedings, and ongoing trials. However, since only three studies met the inclusion criteria, we cannot exclude the possibility of publication bias.
None of the review authors have any links to drug companies or a financial interest in the prescription of the drugs under assessment, nor were they involved in the conduct of the included studies. Thus, there were no issues related to bias secondary to conflicts of interests in this review.
Agreements and disagreements with other studies or reviews
To date there are no systematic reviews that have compared immediate versus delayed postpartum contraceptive implant insertion. A Cochrane review that assessed the effectiveness of immediate postplacental (within 10 minutes of placenta delivery) insertion of intrauterine device (IUD) for contraception compared to early postpartum insertion (10 minutes to 48 hours) and standard insertion (during the postpartum visit) demonstrated that there was no significant difference in the rates of IUD use among women who received an immediate insertion compared to those who received an early postpartum insertion (odds ratio (OR) 0.46, 95% confidence interval (CI) 0.04 to 5.75) (Lopez 2015). However, IUD use at six months was more likely with immediate insertion of IUD than with standard insertion (OR 2.04, 95% CI 1.01 to 4.09) (Lopez 2015). In this review, the meta‐analysis of both included studies revealed the significant higher rate of postpartum contraceptive implant initiation among women assigned to receive immediate insertion compared with women receiving delayed insertion (risk ratio (RR) 1.41, 95% CI 1.28 to 1.55; three studies, 410 participants). These findings therefore indicate that immediate postpartum provision of long‐acting reversible contraceptive method can increase the uptake rate of postpartum contraception. Regarding pregnancy rate, Lopez 2015 reported no difference between immediate postplacental and standard insertion, which is consistent with the results of this review.
The inability to blind the intervention assigned in the included studies in this review precludes a reliable assessment of the difference in the rate of self‐reported side effects between the immediate and delayed postpartum insertion of contraceptive implant. A two‐year observational study conducted in the USA with 414 women showed no difference in removal rate secondary to abnormal vaginal bleeding among women who received immediate and delayed postpartum insertion of contraceptive implant (19.3% versus 18.4%, respectively) (Ireland 2014). In the prospective cohort from Thailand that evaluated the safety of immediate postpartum insertion of contraceptive implant among 88 asymptomatic HIV type 1‐positive women, 62.5% of participants reported to have irregular bleeding at six months, which appeared to be comparable to women who received a standard insertion (Taneepanichkul 2001). In this Cochrane review, although duration of vaginal bleeding among women in the immediate insertion was statistically significant longer than that noted among women who received delayed insertion of contraceptive implant (mean difference (MD) 5.80 days; 95% CI 3.79 to 7.81; one study, 215 participants), this difference might not reach a clinically important level since as there was no distinction of follow‐up haemoglobin level between these two comparison groups.

Study flow diagram.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.

Forest plot of comparison: 1 Immediate versus delayed postpartum insertion of contraceptive implants, outcome: 1.1 Rate of initiation of contraceptive implants.

Forest plot of comparison: 1 Immediate versus delayed postpartum insertion of contraceptive implants, outcome: 1.2 Continuation rate.
Forest plot of comparison: 1 Immediate versus delayed postpartum insertion of contraceptive implants, outcome: 1.4 Other side effects.

Comparison 1 Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 1 Rate of initiation of contraceptive implants.

Comparison 1 Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 2 Continuation rate.
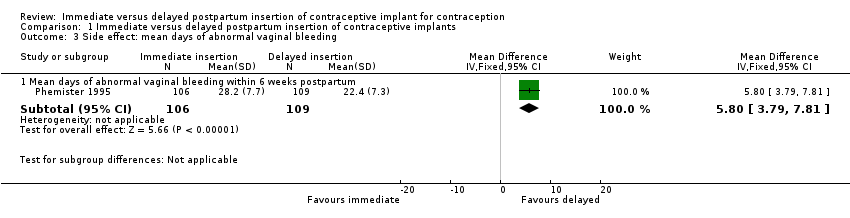
Comparison 1 Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 3 Side effect: mean days of abnormal vaginal bleeding.

Comparison 1 Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 4 Other side effects.
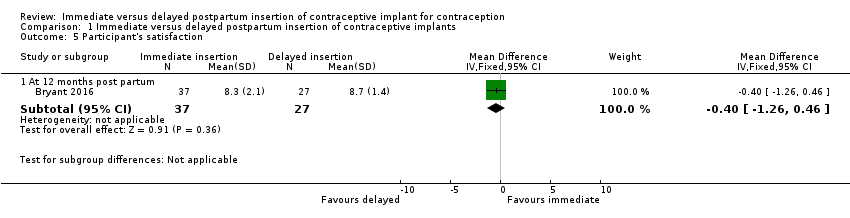
Comparison 1 Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 5 Participant's satisfaction.
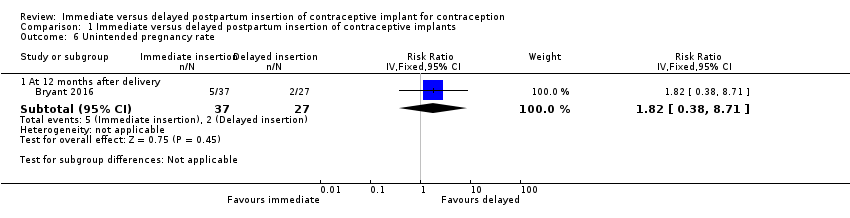
Comparison 1 Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 6 Unintended pregnancy rate.

Comparison 1 Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 7 Breastfeeding at six months postpartum.
| Immediate postpartum insertion compared with delayed insertion of contraceptive implant for contraception | ||||||
| Participant or population: postpartum women who desire a contraceptive implant for contraception Settings: hospitals in United States Intervention: immediate postpartum insertion Comparison: delayed insertion | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Delayed insertion | Immediate insertion | |||||
| Rate of initiation of contraceptive implants | 69 per 100 | 97 per 100 | RR 1.41 | 410 | ⊕⊕⊕⊝ | |
| Continuation rate at 6 months Continuation rate at 12 months | 90 per 100 | 92 per 100 | RR 1.02 | 125 | ⊕⊕⊝⊝ | |
| 78 per 100 | 81 per 100 (63 to 100) | RR 1.04 (0.81 to 1.34) | 64 (1 study) | ⊕⊕⊝⊝ | ||
| Side effects: Abnormal vaginal bleeding within 6 weeks post partum Side effects: Other side effects within 6 weeks Side effects: Heavy, irregular vaginal bleeding or associated severe cramping within 12 months | Mean 22.4 | Mean 5.80 higher | MD 5.80 | 215 | ⊕⊕⊝⊝ | |
| 23 per 100 | 47 per 100 | RR 2.06 | 215 | ⊕⊕⊝⊝ | ||
| 67 per 100 | 68 per 100 (48 to 96) | RR 1.01 (0.72 to 1.44) | 64 | ⊕⊕⊝⊝ | ||
| Participants' satisfaction at 12 months | Mean 8.7 | Mean 0.40 lower | MD ‐0.40 | 64 (1 study) | ⊕⊕⊝⊝ | |
| Unintended pregnancy rate at 12 months | 7 per 100 | 13 per 100 (3 to 61) | RR 1.82 | 64 (1 study) | ⊕⊕⊝⊝ | |
| Breastfeeding at six months postpartum | 15 per 100 | 30 per 100 (11 to 84) | RR 2.01 | 64 (1 study) | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level owing to serious imprecision (small number of participants) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rate of initiation of contraceptive implants Show forest plot | 3 | 410 | Risk Ratio (IV, Fixed, 95% CI) | 1.41 [1.28, 1.55] |
| 2 Continuation rate Show forest plot | 2 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At 6 months postpartum | 2 | 125 | Risk Ratio (IV, Fixed, 95% CI) | 1.02 [0.93, 1.11] |
| 2.2 At 12 months postpartum | 1 | 64 | Risk Ratio (IV, Fixed, 95% CI) | 1.04 [0.81, 1.34] |
| 3 Side effect: mean days of abnormal vaginal bleeding Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Mean days of abnormal vaginal bleeding within 6 weeks postpartum | 1 | 215 | Mean Difference (IV, Fixed, 95% CI) | 5.80 [3.79, 7.81] |
| 4 Other side effects Show forest plot | 2 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Heavy, irregular bleeding or associated severe cramping within 12 months | 1 | 64 | Risk Ratio (IV, Fixed, 95% CI) | 1.01 [0.72, 1.44] |
| 4.2 Adverse effects other than abnormal vaginal bleeding | 1 | 215 | Risk Ratio (IV, Fixed, 95% CI) | 2.06 [1.38, 3.06] |
| 5 Participant's satisfaction Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 At 12 months post partum | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.26, 0.46] |
| 6 Unintended pregnancy rate Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 At 12 months after delivery | 1 | 64 | Risk Ratio (IV, Fixed, 95% CI) | 1.82 [0.38, 8.71] |
| 7 Breastfeeding at six months postpartum Show forest plot | 1 | 64 | Risk Ratio (IV, Fixed, 95% CI) | 2.01 [0.72, 5.63] |

