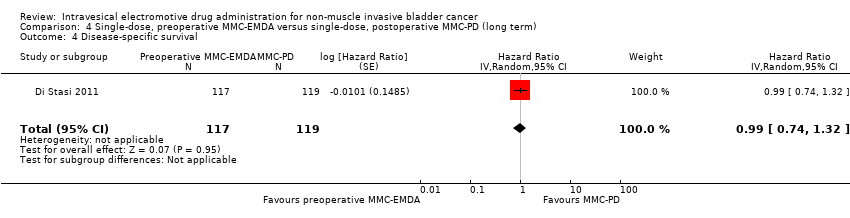
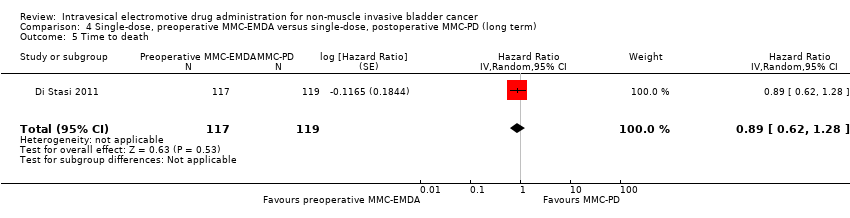
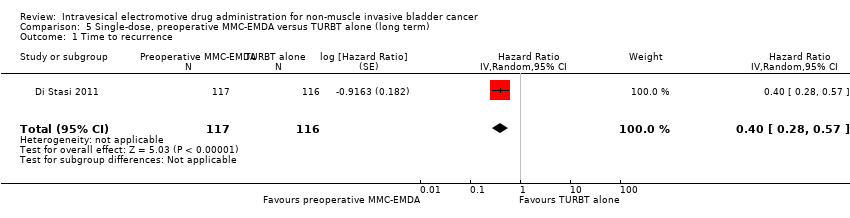
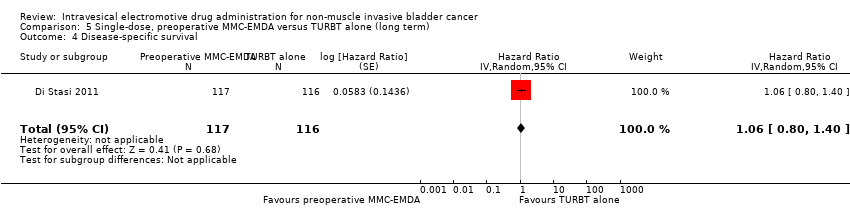
Contenido relacionado
Revisiones y protocolos relacionados
Andrew RH Shepherd, Emily Shepherd, Nicholas R Brook | 8 marzo 2017
Mi Ah Han, Philipp Maisch, Jae Hung Jung, Jun Eul Hwang, Vikram Narayan, Anne Cleves, Eu Chang Hwang, Philipp Dahm | 14 junio 2021
Pan Feng Shang, Joey Kwong, Zhi Ping Wang, Jinhui Tian, Lei Jiang, KeHu Yang, Zhong Jin Yue, Jun Qiang Tian | 11 mayo 2011
Stefanie Schmidt, Frank Kunath, Bernadette Coles, Desiree Louise Draeger, Laura‐Maria Krabbe, Rick Dersch, Samuel Kilian, Katrin Jensen, Philipp Dahm, Joerg J Meerpohl | 8 enero 2020
Mike Shelley, Anne Cleves, Timothy J Wilt, Malcolm Mason | 13 abril 2011
Mike Shelley, J B Court, Howard G Kynaston, Timothy J Wilt, Bernadette Coles, Malcolm Mason | 7 noviembre 2015
Philipp Maisch, Alex Koziarz, Jon Vajgrt, Vikram Narayan, Myung Ha Kim, Philipp Dahm | 1 diciembre 2021
Frank Kunath, Stefanie Schmidt, Laura‐Maria Krabbe, Arkadiusz Miernik, Philipp Dahm, Anne Cleves, Mario Walther, Nils Kroeger | 9 mayo 2017
Niranjan J Sathianathen, Makinna C Oestreich, Sarah Jane Brown, Shilpa Gupta, Badrinath R Konety, Philipp Dahm, Frank Kunath | 12 diciembre 2020
Lillian Y Lai, Sean M Tafuri, Emily C Ginier, Lindsey A Herrel, Philipp Dahm, Philipp Maisch, Giulia Ippolito Lane | 8 abril 2022