Intubación orotraqueal en lactantes realizada con y sin estilete
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Objective: to determine whether paediatric trainees were more successful at neonatal orotracheal intubation when a stylet was used Study design: unblinded randomised controlled trial Object of randomisation: first intubation attempt; for infants who had more than 1 episode of intubation during admission, each episode of intubation was randomised and was treated as an independent event Recruitment: For emergency first intubations in the delivery room or within 24 hours of birth, a waiver of consent was used to enrol infants, and retrospective consent was obtained from parents as soon as possible after the intubation attempt. Infants who were intubated in the neonatal intensive care unit (NICU) after the first day were eligible if written parental consent had been obtained. Permission from parents was also sought to randomise future intubations Allocation: randomly assigned Total number of intubations: 713 Number of infants randomised: 232 Number of intubations randomised: 304 Method of analysis: Data are presented as means (standard deviations) for normally distributed continuous variables and medians (interquartile ranges) when the distribution is skewed. Clinical characteristics and outcome variables were analysed by using Student's t test for parametric comparisons, the Mann‐Whitney U test for non‐parametric comparisons of continuous variables, and X2 for categorical variables. P values were 2‐sided, and P values < 0.05 were considered statistically significant Follow‐up: No participants had tracheal or oesophageal perforation. Rates of blood‐stained aspirates within the first 24 hours were included as a secondary outcome. No information on follow‐up was provided beyond this | |
| Participants | Country: Australia Clinical setting: delivery room and neonatal intensive care unit Inclusion criteria: Eligible participants were newborn infants in the delivery room or NICU requiring endotracheal intubation Exclusion criteria: Infants who were intubated for suctioning of meconium from the trachea were not eligible owing to the difficulty of confirming correct endotracheal tube (ET) placement Age (weeks): mean gestational age of participants: stylet = 28.5 (standard deviation (SD) 5.0); no stylet = 28.7 (SD 5.2) Birth weight (grams): stylet = 925 (interquartile ratio (IQR) 689 to 1473); no stylet = 862 (IQR 714 to 1586) Gender: male infants: stylet = 86 (SD 58); no stylet = 92 (SD 60) Ethnicity: not stated Site of intubation: delivery room (DR): stylet n = 72; no stylet n = 74; NICU: stylet n = 77; NICU n = 79 Seniority of operator: fellow: stylet 33 (SD 11); no stylet 41 (SD 14); resident: stylet 116 (SD 38); no stylet 112 (SD 37) | |
| Interventions | Intervention arm: A stylet was used as an aid during orotracheal intubation of the newborn infant Control arm: orotracheal intubation of the newborn infant without the use of a stylet | |
| Outcomes | Primary outcome Intubation success rates on first attempt with use of stylet vs non‐use as indicated by detection of exhaled carbon dioxide Secondary outcomes • Duration of intubation attempt • Changes in heart rate and oxygen saturation from baseline • Presence of blood‐stained secretions after the procedure | |
| Notes | Trial registration: Australian and New Zealand Clinical Trials Register (ACTR identifier: 12607000186459) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Intervention was assigned by random sequence. Randomisation occurred in blocks of variable size stratified by site of intubation (delivery room (DR) or neonatal intensive care (NICU)) |
| Allocation concealment (selection bias) | Low risk | Upcoming allocations were concealed from those involved in enrolment of the trial. Sequentially numbered sealed opaque envelopes contained computer‐generated treatment groups, which the neonatal fellow on duty carried to the DR unopened to randomise the next eligible infant in the DR. Infants in the NICU were identifiable by a study label on the incubator |
| Blinding of participants and personnel (performance bias) | High risk | Study was unblinded with regards to intervention allocation. Owing to the nature of the intervention, it was not possible to mask hospital staff or parents/guardians of the infant to the allocation status |
| Blinding of outcome assessment (detection bias) | High risk | Assessors of outcomes were unblinded to intervention allocation |
| Incomplete outcome data (attrition bias) | Low risk | Reasons for excluded infants (n = 481): intubated for meconium/before fellow arrived (n = 102); forgot/team thought ineligible (n = 264); other reasons, e.g. emergencies, twins, nasal intubation, consultant intubation (n = 115). Eligible intubations that were excluded were accounted for and explained (n = 21). These were consented for prospective NICU intubations, but the team was unaware or had insufficient time owing to emergency intubation required |
| Selective reporting (reporting bias) | Low risk | Study protocol is available, and all prespecified primary and secondary outcomes have been reported in the prespecified way |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Prospective observational study | |
| Comparison of lighted vs regular stylet ‐ not of stylet vs no stylet | |
| Non‐experimental study: case report | |
| Randomised controlled trial comparing transillumination method vs main‐stem method |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 First intubation attempt success rate Show forest plot | 1 | 302 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.88, 1.32] |
| Analysis 1.1  Comparison 1 First intubation attempt success rate with use of stylet vs non‐use of stylet, Outcome 1 First intubation attempt success rate. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fellow: first intubation attempt success rate Show forest plot | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.69, 1.29] |
| Analysis 2.1  Comparison 2 Intubation success: professional category, Outcome 1 Fellow: first intubation attempt success rate. | ||||
| 2 Resident: first intubation attempt success rate Show forest plot | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.90, 1.52] |
| Analysis 2.2  Comparison 2 Intubation success: professional category, Outcome 2 Resident: first intubation attempt success rate. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intubations without premedication given to the infant Show forest plot | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.72, 1.32] |
| Analysis 3.1  Comparison 3 Intubation success: use of premedication, Outcome 1 Intubations without premedication given to the infant. | ||||
| 2 Intubations following premedication given to the infant Show forest plot | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.89, 1.55] |
| Analysis 3.2  Comparison 3 Intubation success: use of premedication, Outcome 2 Intubations following premedication given to the infant. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intubations just after birth in the delivery room: first intubation attempt success rate Show forest plot | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.72, 1.32] |
| Analysis 4.1 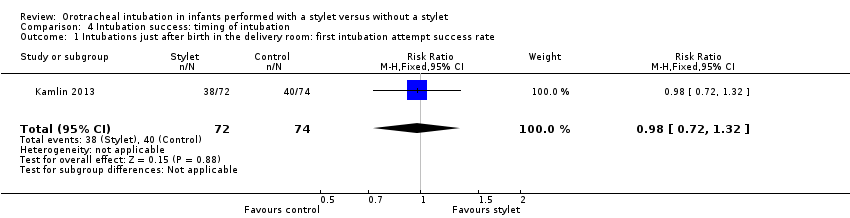 Comparison 4 Intubation success: timing of intubation, Outcome 1 Intubations just after birth in the delivery room: first intubation attempt success rate. | ||||
| 2 intubations following admission to NICU: first intubation attempt success rate Show forest plot | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.89, 1.55] |
| Analysis 4.2 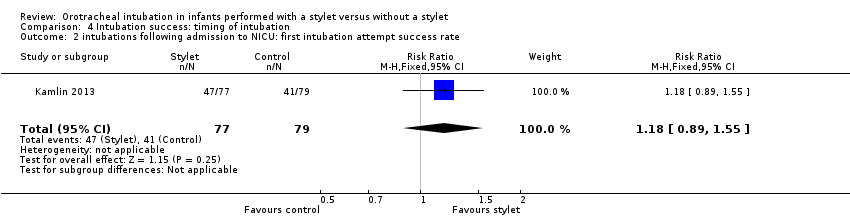 Comparison 4 Intubation success: timing of intubation, Outcome 2 intubations following admission to NICU: first intubation attempt success rate. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Weight < 1000 grams Show forest plot | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.67, 1.18] |
| Analysis 5.1  Comparison 5 Intubation success: weight at intubation, Outcome 1 Weight < 1000 grams. | ||||
| 2 Weight ≥ 1000 grams Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.97, 1.79] |
| Analysis 5.2  Comparison 5 Intubation success: weight at intubation, Outcome 2 Weight ≥ 1000 grams. | ||||
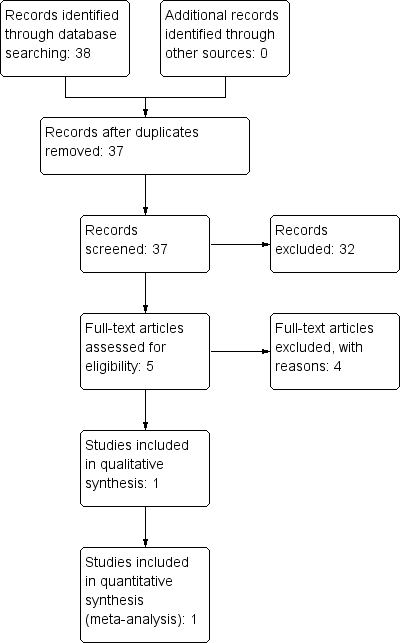
Study flow diagram.
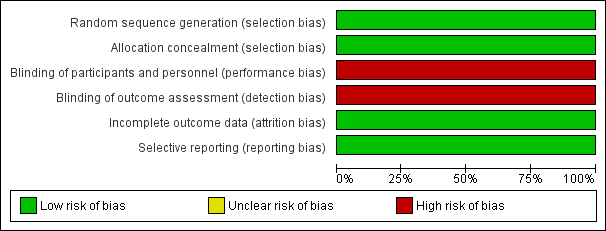
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
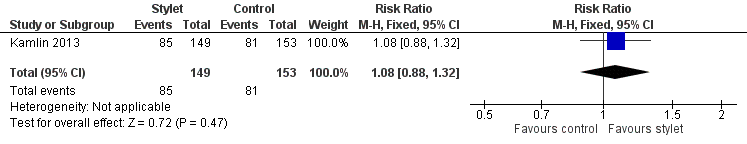
Forest plot of comparison: 1 First intubation attempt success rate with use of stylet versus non‐use of stylet, outcome: 1.1 First intubation attempt success rate.
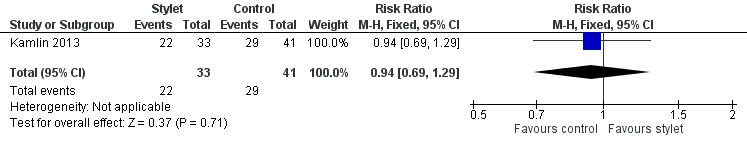
Forest plot of comparison: 2 Intubation success: Professional category, outcome: 2.1 Fellow: first intubation attempt success rate.
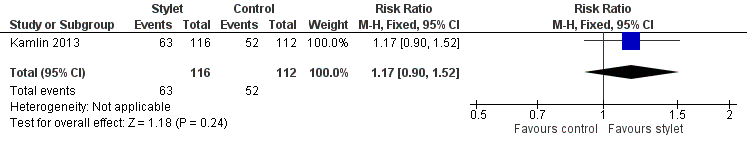
Forest plot of comparison: 2 Intubation success: Professional category, outcome: 2.2 Resident: first intubation attempt success rate.
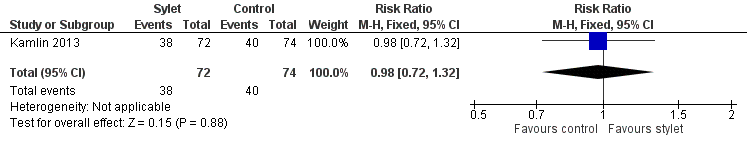
Forest plot of comparison: 3 Intubation success: use of premedication, outcome: 3.1 Intubations without premedication given to the infant.
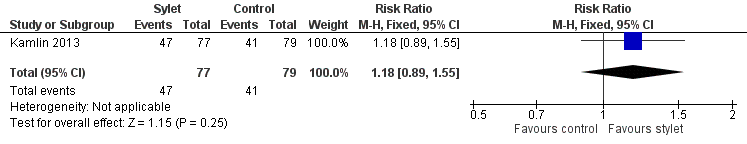
Forest plot of comparison: 3 Intubation success: use of premedication, outcome: 3.2 Intubations following premedication given to the infant.
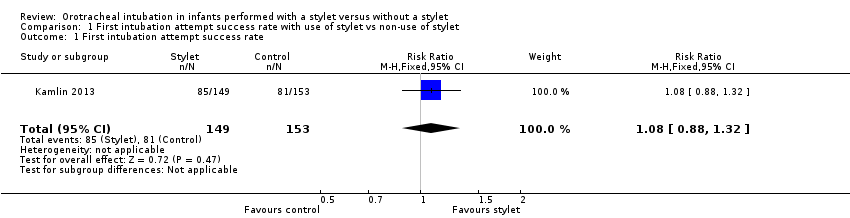
Comparison 1 First intubation attempt success rate with use of stylet vs non‐use of stylet, Outcome 1 First intubation attempt success rate.
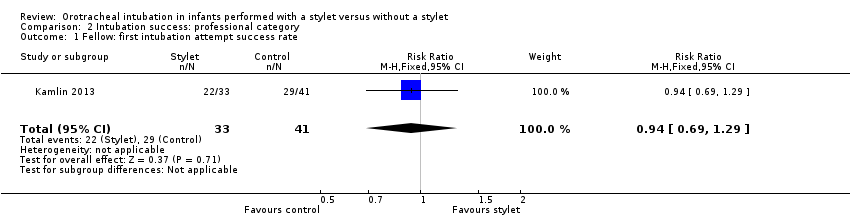
Comparison 2 Intubation success: professional category, Outcome 1 Fellow: first intubation attempt success rate.
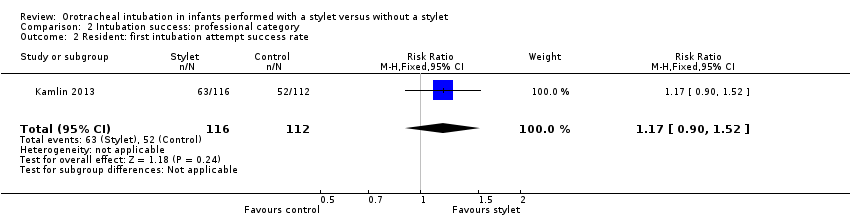
Comparison 2 Intubation success: professional category, Outcome 2 Resident: first intubation attempt success rate.
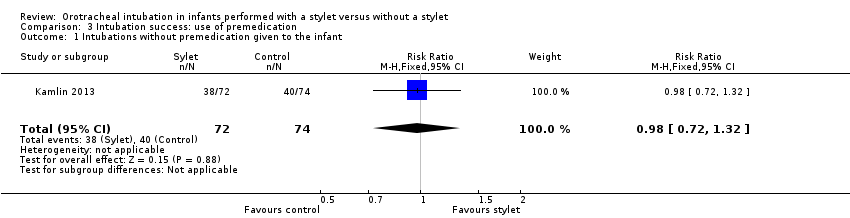
Comparison 3 Intubation success: use of premedication, Outcome 1 Intubations without premedication given to the infant.
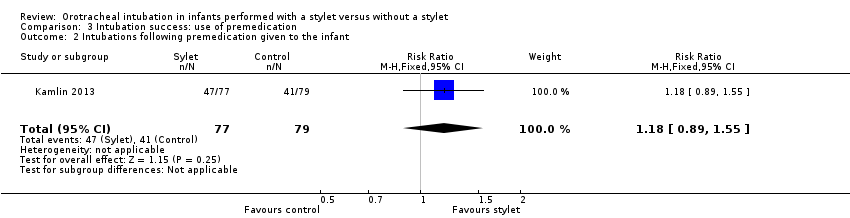
Comparison 3 Intubation success: use of premedication, Outcome 2 Intubations following premedication given to the infant.
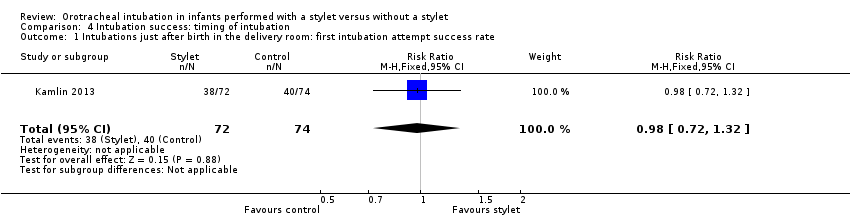
Comparison 4 Intubation success: timing of intubation, Outcome 1 Intubations just after birth in the delivery room: first intubation attempt success rate.
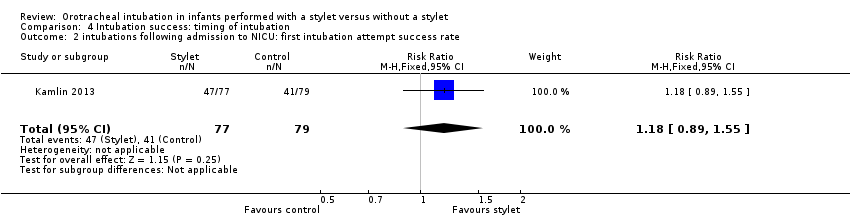
Comparison 4 Intubation success: timing of intubation, Outcome 2 intubations following admission to NICU: first intubation attempt success rate.
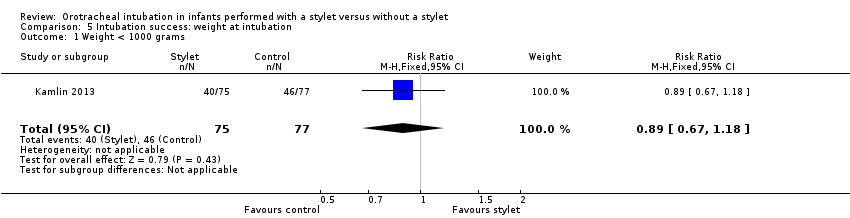
Comparison 5 Intubation success: weight at intubation, Outcome 1 Weight < 1000 grams.
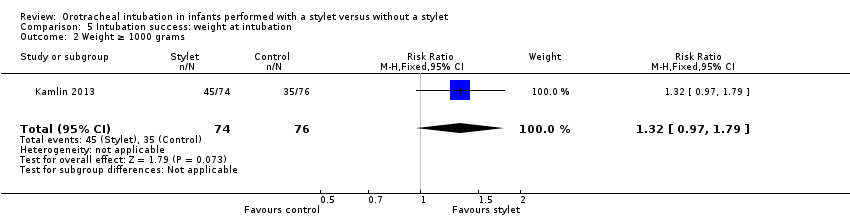
Comparison 5 Intubation success: weight at intubation, Outcome 2 Weight ≥ 1000 grams.
| Stylet compared with no stylet for neonatal intubation | ||||||
| Patient or population: neonates requiring endotracheal intubation Settings: neonatal intensive care unit or delivery room or theatre Intervention: a stylet inserted into the endotracheal tube Comparison: no stylet inserted into the endotracheal tube | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of intubations | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Stylet | |||||
| First intubation attempt success rate (outcome achieved at time of intubation attempt and not followed up) | 529 per 1000 | 570 per 1000 | RR 1.08 | 302 | ⊕⊕⊕⊝a,b | Unblinded trial with no blinded outcome assessment Single study |
| Gestational age of the infant | no data | no data | no data | no data | absence of evidence | |
| Professional category of the intubator ‐ fellow: first intubation attempt success rate (outcome achieved at time of intubation attempt and not followed up) | 707 per 1000 | 667 per 1000 | RR 0.94 | 74 | ⊕⊕⊝⊝a,b | Unblinded trial with no blinded outcome assessment Single study |
| Professional category of the intubator ‐ resident: first intubation attempt success rate (outcome achieved at time of intubation attempt and not followed up) | 464 per 1000 | 543 per 1000 | RR 1.17 | 228 | ⊕⊕⊕⊝a,b | Unblinded trial with no blinded outcome assessment Single study |
| Level of experience of the intubator | no data | no data | no data | no data | absence of evidence | |
| Premedication given ‐ no premedication given: first intubation attempt success rate (outcome achieved at time of intubation attempt and not followed up) | 540 per 1000 | 528 per 1000 | RR 0.98 | 146 | ⊕⊕⊕⊝a,b | Unblinded trial with no blinded outcome assessment Single study |
| Premedication given ‐ no premedication given: first intubation attempt success rate (outcome achieved at time of intubation attempt and not followed up) | 519 per 1000 | 610 per 1000 | RR 1.18 | 156 | ⊕⊕⊕⊝a,b | Unblinded trial with no blinded outcome assessment Single study |
| Timing of intubation ‐ just after birth in the delivery room: first intubation attempt success rate (outcome achieved at time of intubation attempt and not followed up) | 540 per 1000 | 528 per 1000 | RR 0.98 | 146 | ⊕⊕⊕⊝a,b | Unblinded trial with no blinded outcome assessment Single study |
| Timing of intubation ‐ following admission to NICU: first intubation attempt success rate (outcome achieved at time of intubation attempt and not followed up) | 519 per 1000 | 610 per 1000 | RR 1.18 | 156 | ⊕⊕⊕⊝a,b | Unblinded trial with no blinded outcome assessment Single study |
| Type of stylet | no data | no data | no data | no data | absence of evidence | |
| Weight < 1000 g (outcome achieved at time of intubation attempt and not followed up) | 597 per 1000 | 533 per 1000 | RR 0.89 | 152 | ⊕⊕⊕⊝a,b | Unblinded trial with no blinded outcome assessment Single study |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aHigh risk of detection bias (due to lack of blinding of caregivers and outcome assessors) bSerious imprecision (due to small number of events and small sample sizes; 95% CIs include null effects) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 First intubation attempt success rate Show forest plot | 1 | 302 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.88, 1.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fellow: first intubation attempt success rate Show forest plot | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.69, 1.29] |
| 2 Resident: first intubation attempt success rate Show forest plot | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.90, 1.52] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intubations without premedication given to the infant Show forest plot | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.72, 1.32] |
| 2 Intubations following premedication given to the infant Show forest plot | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.89, 1.55] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intubations just after birth in the delivery room: first intubation attempt success rate Show forest plot | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.72, 1.32] |
| 2 intubations following admission to NICU: first intubation attempt success rate Show forest plot | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.89, 1.55] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Weight < 1000 grams Show forest plot | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.67, 1.18] |
| 2 Weight ≥ 1000 grams Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.97, 1.79] |

