Intubación orotraqueal en lactantes realizada con y sin estilete
Resumen
Antecedentes
La intubación endotraqueal neonatal es una intervención frecuente que tiene la posibilidad de salvar vidas. Es una habilidad obligatoria para los pasantes de neonatología, pero es difícil de dominar y mantener. Las oportunidades de intubación para los pasantes han disminuido, por lo que han descendido las tasas de éxito. El uso de un estilete puede ayudar a la intubación y mejorar el éxito. Sin embargo, se debe considerar la posibilidad de efectos perjudiciales asociados.
Objetivos
Comparar los efectos beneficiosos y perjudiciales de la intubación orotraqueal neonatal con estilete versus la intubación orotraqueal neonatal sin estilete.
Métodos de búsqueda
Se realizaron búsquedas en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials, CENTRAL) en la Cochrane Library; MEDLINE; Embase; el Cumulative Index to Nursing and Allied Health Literature (CINAHL), y en revisiones anteriores. También se buscaron las referencias cruzadas, se estableció contacto con fuentes expertas, se realizaron búsquedas manuales en revistas y se examinaron las actas de congresos. Se realizaron búsquedas de ensayos actuales y recientemente completados en registros de ensayos clínicos. La búsqueda más reciente se realizó en abril 2017.
Criterios de selección
Todos los ensayos aleatorizados, cuasialeatorizados y controlados aleatorizados grupales que compararon el uso versus el no uso de un estilete en la intubación orotraqueal neonatal.
Obtención y análisis de los datos
Dos autores de la revisión de manera independiente evaluaron los resultados de las búsquedas contra los criterios de inclusión predeterminados, evaluaron el riesgo de sesgo y extrajeron los datos. Se utilizaron los métodos estándar de la Colaboración Cochrane, como se documenta en el Manual Cochrane de Revisiones Sistémicas de Intervenciones (Cochrane Handbook for Systemic Reviews of Interventions) y en el Grupo Cochrane de Neonatología (Cochrane Neonatal Review Group).
Resultados principales
Se incluyó un ensayo controlado aleatorizado no cegado de un centro único que informó 302 intentos de intubación en 232 lactantes. La mediana de edad gestacional de los lactantes incorporados fue 29 semanas. Los residentes de pediatría y los becarios realizaron las intubaciones. Se determinó que el riesgo de sesgo general de los estudios fue incierto. Los investigadores compararon las tasas de éxito de la intubación en el primer intento con y sin el uso de un estilete e informaron tasas de éxito similares entre los grupos de estilete y ningún estilete (57% y 53%) (P = 0,47). Las tasas de éxito no difirieron entre los grupos en los análisis de subgrupos según el nivel de adiestramiento del profesional sanitario y el peso de los lactantes. Los resultados no mostraron diferencias en los resultados secundarios de la revisión que incluyen la duración de la intubación, el número de intentos, la inestabilidad del participante durante el procedimiento y el traumatismo local de las vías respiratorias. Sólo el 25% de todas las intubaciones demoraron menos de 30 segundos para ser realizadas. Los autores del estudio no informaron la morbilidad neonatal ni la mortalidad. La calidad de la evidencia se consideró baja según el análisis GRADE debido a que sólo se identificó un estudio no cegado.
Conclusiones de los autores
La evidencia actual disponible indica que el uso de un estilete durante la intubación orotraqueal neonatal no mejora significativamente la tasa de éxito entre los pasantes de pediatría. Sin embargo, sólo se ha probado una marca de estilete y una marca de tubo endotraqueal y los investigadores realizaron todas las intubaciones en los lactantes en un contexto hospitalario. Por lo tanto, estos resultados no se pueden generalizar más allá de estas limitaciones.
PICOs
Resumen en términos sencillos
Tasas de intubación exitosa en lactantes realizada con un estilete comparadas con las tasas de intubación exitosa realizada sin un estilete
Pregunta de la revisión: ¿El uso de un estilete aumenta las tasas de éxito de la intubación del recién nacido sin riesgo de que aumente el daño?
Antecedentes: La intubación consiste en la colocación de un tubo respiratorio (tubo endotraqueal) en la tráquea del recién nacido para mantener una vía respiratoria abierta. Es un procedimiento frecuente que se puede necesitar en el momento del parto y en la unidad de cuidados intensivos neonatales si el recién nacido no es capaz de respirar bien por sí mismo. Los médicos pasantes deben aprender esta habilidad difícil y a veces deben hacer más de un intento para conseguir colocar el tubo en el lugar adecuado. El tubo respiratorio es un tubo estrecho, plástico y flexible. Se puede insertar un estilete, que es un alambre de metal maleable recubierto de plástico, en el tubo de respiración para hacerlo más rígido; esto podría facilitar colocar el tubo en el lugar correcto en el primer intento. Sin embargo, el uso de un estilete puede aumentar el riesgo de daño al paciente durante el procedimiento.
Características de los estudios: En las búsquedas bibliográficas actualizadas en abril de 2017, se encontró un ensayo controlado aleatorizado (302 intubaciones) que cumplió los criterios de inclusión de esta revisión.
Resultados: Las tasas de intubación exitosa en el primer intento con o sin el uso de un estilete como ayuda fueron similares, del 57% y el 53%, respectivamente. La tasa de éxito con y sin el uso de un estilete no difirió entre los lactantes de diferentes pesos, ni entre los médicos pediatras pasantes con diferentes niveles de experiencia. El período de tiempo que demoró intubar y el número de intentos hechos antes de que la intubación tuviera éxito fueron equivalentes entre los grupos. La incidencia de una disminución en el nivel de oxígeno del paciente y en la frecuencia cardíaca fue equivalente entre los grupos, al igual que la incidencia informada de traumatismo de las vías respiratorias asociado con el procedimiento.
Calidad de la evidencia: La calidad de la evidencia fue baja. El nivel se disminuyó porque sólo se incluyó un estudio no cegado.
Authors' conclusions
Summary of findings
| Stylet compared with no stylet for neonatal intubation | ||||||
| Patient or population: neonates requiring endotracheal intubation Settings: neonatal intensive care unit or delivery room or theatre Intervention: a stylet inserted into the endotracheal tube Comparison: no stylet inserted into the endotracheal tube | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of intubations | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Stylet | |||||
| First intubation attempt success rate (outcome achieved at time of intubation attempt and not followed up) | 529 per 1000 | 570 per 1000 | RR 1.08 | 302 | ⊕⊕⊕⊝a,b | Unblinded trial with no blinded outcome assessment Single study |
| Gestational age of the infant | no data | no data | no data | no data | absence of evidence | |
| Professional category of the intubator ‐ fellow: first intubation attempt success rate (outcome achieved at time of intubation attempt and not followed up) | 707 per 1000 | 667 per 1000 | RR 0.94 | 74 | ⊕⊕⊝⊝a,b | Unblinded trial with no blinded outcome assessment Single study |
| Professional category of the intubator ‐ resident: first intubation attempt success rate (outcome achieved at time of intubation attempt and not followed up) | 464 per 1000 | 543 per 1000 | RR 1.17 | 228 | ⊕⊕⊕⊝a,b | Unblinded trial with no blinded outcome assessment Single study |
| Level of experience of the intubator | no data | no data | no data | no data | absence of evidence | |
| Premedication given ‐ no premedication given: first intubation attempt success rate (outcome achieved at time of intubation attempt and not followed up) | 540 per 1000 | 528 per 1000 | RR 0.98 | 146 | ⊕⊕⊕⊝a,b | Unblinded trial with no blinded outcome assessment Single study |
| Premedication given ‐ no premedication given: first intubation attempt success rate (outcome achieved at time of intubation attempt and not followed up) | 519 per 1000 | 610 per 1000 | RR 1.18 | 156 | ⊕⊕⊕⊝a,b | Unblinded trial with no blinded outcome assessment Single study |
| Timing of intubation ‐ just after birth in the delivery room: first intubation attempt success rate (outcome achieved at time of intubation attempt and not followed up) | 540 per 1000 | 528 per 1000 | RR 0.98 | 146 | ⊕⊕⊕⊝a,b | Unblinded trial with no blinded outcome assessment Single study |
| Timing of intubation ‐ following admission to NICU: first intubation attempt success rate (outcome achieved at time of intubation attempt and not followed up) | 519 per 1000 | 610 per 1000 | RR 1.18 | 156 | ⊕⊕⊕⊝a,b | Unblinded trial with no blinded outcome assessment Single study |
| Type of stylet | no data | no data | no data | no data | absence of evidence | |
| Weight < 1000 g (outcome achieved at time of intubation attempt and not followed up) | 597 per 1000 | 533 per 1000 | RR 0.89 | 152 | ⊕⊕⊕⊝a,b | Unblinded trial with no blinded outcome assessment Single study |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aHigh risk of detection bias (due to lack of blinding of caregivers and outcome assessors) bSerious imprecision (due to small number of events and small sample sizes; 95% CIs include null effects) | ||||||
Background
Description of the condition
Neonatal endotracheal intubation refers to placement of an endotracheal tube (ETT; breathing tube) within an infant's airway. This intervention is commonly needed and may be life‐saving for infants after birth and during neonatal intensive care. Indications for intubation during neonatal resuscitation include ineffective or prolonged positive‐pressure ventilation delivered via face mask; need to secure the airway when cardiac compressions are performed; intratracheal administration of medications; and special resuscitation circumstances such as congenital diaphragmatic hernia or endotracheal suctioning for meconium (ILCOR 2005; Perlman 2010). Endotracheal intubation is necessary when neonatal intensive care is provided for infants in respiratory failure, despite non‐invasive respiratory support, as well as for administration of surfactant, for treatment of resistant apnoea of prematurity, and for preparation of infants undergoing surgery. Intubation can be performed by the nasotracheal (through the nose) or orotracheal (through the mouth) route. This review will focus solely on orotracheal intubation; whenever intubation is mentioned, we will be referring to orotracheal intubation. We will not consider nasal intubation here, as it is not possible to use a stylet safely during nasal intubation.
Endotracheal intubation is a mandatory competency for neonatal trainees. However, it is a difficult skill to learn and maintain, and initial attempts are often unsuccessful. Successful intubation relies on the ability of the intubator to perform laryngoscopy (using a laryngoscope inserted into the patient's mouth to obtain a view of the infant’s airway) and to recognise the anatomy displayed. Opportunities for neonatal trainees to acquire and maintain proficiency in endotracheal intubation are decreasing (Leone 2005), likely owing to increased use of non‐invasive respiratory support in neonatal intensive care, reduced working hours for trainees, increased numbers of trainees, and changes in clinical recommendations, such as to discontinue routine intubation of babies delivered through meconium‐stained liquor.
Studies evaluating success rates for neonatal endotracheal intubation report that more than one attempt is frequently required for successful intubation. An Australian study (O'Donnell 2006) reported that 62% of total first intubation attempts were successful, but the success rate was only 24% among the most inexperienced trainees. In a study conducted in the United States (Falck 2003), paediatric residents successfully intubated neonates on the first or second attempt at rates of 50%, 55%, and 62% for first‐, second‐, and third‐year residents, respectively. None of these residents met the study authors’ definition of procedural competence for intubation (successful at first or second attempt 80% or more of the time) over a two‐year period. Another American study examining intubation success rates over a 10‐year period (Leone 2005) reported median success rates of 33% for first‐year residents, 40% for second‐ or third‐year residents, and 68% for neonatal fellows. Success rates were significantly different between groups (P < 0.001), but success rates for paediatric residents were not significantly different for delivery room (DR) non‐meconium intubations than for neonatal intensive care unit (NICU) intubations (36% vs 36.5%). The most recent US study examining endotracheal intubation success rates (Haubner 2013) reported an overall success rate of 44%. Investigators again found significant differences between experienced and inexperienced providers – residents 20%, fellows 72%, and attending physicians 70%. Researchers observed that participant characteristics of birth weight and gestation did not impact success rates. Studies of intubation performed at US tertiary academic centres by neonatologists, fellows, residents, and respiratory therapists, in which detection of exhaled carbon dioxide was used to confirm correct tube placement, suggest that oesophageal intubation is not infrequent (Roberts 1995; Aziz 1999; Repetto 2001; Lane 2004). Inability to successfully perform ETT placement, or delayed recognition of unsuccessful placement, can cause death or severe hypoxic injury. Multiple intubations or traumatic intubations increase the risk of serious glottic, subglottic, and tracheal injury (Meneghini 2000; Wei 2011).
The current Neonatal Resuscitation Program 7th Edition (AAP 2016) recommends that intubation attempts should be limited to 30 seconds. This has been expanded from the 20‐second recommendation provided in the 5th Edition (Kattwinkel 2006) following a study of delivery room intubations performed mainly by residents and fellows (Lane 2004), which found that a more realistic time needed for intubation was 30 seconds without apparent adverse effects.
Studies have demonstrated that premedicating infants with various types of induction agents increases the speed of successful intubation and reduces the likelihood of associated adverse sequelae (Marshall 1984; McAuliffe 1995; Cook‐Sathler 1998). Premedication has been shown to improve intubating conditions significantly and to reduce the number of attempts required for successful intubation and risk of intubation‐related airway trauma.(Dempsey 2006; Roberts 2006; Carbajal 2007; Ghanta 2007; Silva 2007; Lemyre 2009).
Strategies for improving training are being developed to compensate for the reduced clinical experience of practitioners. Airway trainers, animal models, and cadaveric specimens are useful for demonstrating the anatomy (Haubner 2013). Simulation is a tool that is used increasingly in medical education. However, studies that examined the role of simulation in teaching intubation (Nishiasaki 2010; Finan 2012) did not report improved clinical performance. Videolaryngoscopy (use of a laryngoscope to transmit images from the tip of the blade to a nearby monitor) allows the teacher to share the view of the trainee intubator and may be useful for improving intubation success.
Description of the intervention
As small‐diameter ETTs are flexible, intubation may be performed with or without a stylet inserted into the lumen (hollow centre of the ETT) and secured. A neonatal stylet is a 6 French (2‐mm diameter) malleable aluminium wire covered with lubricated plastic, which extends beyond the tip (Rusch Flexi‐Slip™ Stylet, Teleflex Medical, Research Triangle Park, NC, USA; Satin‐Slip Stylet, Mallinckrodt Medical, Athlone, Ireland). Available stylets are suitable for use with tubes of 2.5‐mm internal diameter and greater. The stylet is positioned so that its tip does not extend beyond the tip of the tube. The proximal (top) end of the ETT is attached to a plastic adapter that connects to the ventilator. The stylet is threaded through the adapter into the ETT and is positioned so that the tip of the stylet does not extend beyond the tip of the tube. The proximal end of the stylet is then bent over the rim of the adapter to prevent further slipping of the stylet. Endotracheal tubes for neonates are made of pliable plastic and have a small internal diameter of 2.0 mm to 4.0 mm. They become increasingly flexible with decreasing internal diameter, especially if exposed to the heat of an overhead radiant warmer. A stylet may increase the rigidity and curvature of the tube, perhaps making it easier to navigate between vocal cords. Current guidelines (Richmond 2011; AAP 2016) do not recommend routine use of a stylet for orotracheal intubation but rather classify it as an optional instrument. Some operators may prefer the rigidity and curvature afforded by this technique and may achieve higher success rates. However, this rigidity could provide a disadvantage and may cause airway damage. Published case reports have described shearing off of the stylet sheath, causing acute airway obstruction (Cook 1985; Zmyslowski 1989; Bhargava 1998; Rabb 1998; Boyd 1999; Chiou 2007). Stylet costs are similar to those of an endotracheal tube.
How the intervention might work
A stylet increases the rigidity of the ETT and may facilitate placement within the airway.
Why it is important to do this review
Neonatal intubation is a commonly needed life‐saving intervention. Success rates, especially among inexperienced trainees, are suboptimal. If use of a stylet could improve intubation success, then it should be recommended for routine use. However, if use of a stylet does not improve success, or if its use may cause harm, it should not be recommended.
Objectives
To compare the benefits and harms of neonatal orotracheal intubation with a stylet versus neonatal orotracheal intubation without a stylet.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), quasi‐RCTs, and cluster RCTs.
Types of participants
We defined our population as infants of 44 weeks' postmenstrual age or less who required endotracheal intubation. Infants who were intubated on more than one occasion were included again for subsequent intubation episodes, and we included only the first intubation attempt per episode. We excluded studies that enrolled infants with craniofacial or airway anomalies and those that enrolled infants born through meconium‐stained liquor who were intubated for tracheal suctioning, owing to difficulty confirming ETT placement within the trachea.
Types of interventions
Orotracheal intubation performed with a stylet versus without a stylet.
Types of outcome measures
Primary outcomes
-
Rate of successful first attempt at orotracheal intubation
-
An attempt was defined as introduction of the ETT into the infant's mouth after laryngoscopy. Successful placement within the tracheobronchial tree was confirmed immediately post intubation attempt, objectively, through a predetermined method, for example, by observation of colour change on an exhaled colorimetric carbon dioxide detector, misting within the ETT, or auscultation of the chest.
-
Secondary outcomes
-
Duration of the intubation in seconds
-
This measures time from insertion until removal of the laryngoscope
-
-
Number of intubation attempts
-
Patient instability during the procedure, as measured by:
-
heart rate (HR) < 100 during the procedure; and
-
desaturation to < 70% (with 100% showing full oxygen saturation).
-
-
Local trauma to the airway or surrounding soft tissue diagnosed by the presence of blood‐stained endotracheal aspirates or oral sections over the 24 hours after the attempt (number per thousand infant population)
-
Evidence of airway damage, for example, post‐extubation stridor, subglottic stenosis, or vocal cord paralysis (number per thousand infant population)
Search methods for identification of studies
Electronic searches
Two review authors independently searched electronic databases, including the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 3) in the Cochrane Library; MEDLINE (1966 to April 2017); Embase (1980 to April 2017); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to April 2017). We also searched previous reviews including cross‐references, contacted expert informants, and handsearched journals. We searched MEDLINE, Embase, and CINAHL for relevant articles, using the following search terms: (intubation AND stylet) OR (intubation (explode) [MeSH heading] AND stylet) plus database specific limiters for neonates and randomised controlled trials (see Appendix 1). We applied no language restrictions.
Searching other resources
The search strategy included communication with expert informants and searches of bibliographies of systematic reviews and trials for references to other trials. We examined previous reviews, including cross‐references, abstracts, and conferences, and symposium proceedings of the Perinatal Society of Australia and New Zealand and of the Pediatric Academic Societies (American Pediatric Society, Society for Pediatric Research, and European Society for Pediatric Research) from 1990 to 2015. If we were to identify any unpublished trial, we planned to contact study author to request information. We considered unpublished studies and studies reported only as abstracts as eligible for inclusion in the review if study authors reported final trial data and did not perform an interim analysis. We planned to contact the authors of identified RCTs to ask for additional study data when needed. We searched clinical trial registries to April 2017 for current and recently completed trials (clinicaltrials.gov; controlled‐trials.com; who.int/ictrp), as well as the Australia and New Zealand Clinical Trials Register (ANZCTR).
Data collection and analysis
We used the standard methods of the Cochrane Collaboration, as documented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), and of the Cochrane Neonatal Review Group (CNRG).
Selection of studies
Two review authors independently assessed all studies identified via the search strategy for possible inclusion in the review. We planned to resolve disagreements through discussion or, if required, through consultation with a Cochrane review arbiter.
Specifically, we performed the following tasks.
-
Merged search results by using reference management software and removed duplicate records of the same report.
-
Examined titles and abstracts to remove irrelevant reports.
-
Retrieved full texts of potentially relevant reports.
-
Linked multiple reports of the same study.
-
Examined full‐text reports for study compliance with eligibility criteria.
-
Corresponded with investigators, when appropriate, to clarify study eligibility.
-
Noted reasons for inclusion and exclusion of articles at all stages (we resolved disagreements through consensus, or sought assistance with arbitration from the editorial base of the CNRG, if needed).
-
Made final decisions on study inclusion and proceeded to data collection.
-
Resolved all discrepancies through a consensus process.
Data extraction and management
Two review authors independently extracted data from full‐text articles using a specially designed spreadsheet to manage the information. We resolved discrepancies through discussion, or, if required, we planned to consult a review arbiter. We entered data into Review Manager software (RevMan 2014) and checked them for accuracy. When information regarding any of the above was missing or unclear, we attempted to contact authors of the original reports to clarify and provide additional details.
Assessment of risk of bias in included studies
We used the standardised review methods of the CNRG (http://neonatal.cochrane.org/en/index.html) to assess the methodological quality of included studies. Review authors independently assessed study quality and risk of bias using the criteria documented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). See Appendix 2 for the 'Risk of bias' tool.
Measures of treatment effect
We analysed the results of included studies using the statistical package Review Manager software (RevMan 2014). We used the standard method of the CNRG and applied a fixed‐effect model for meta‐analysis (Deeks 2011).
Unit of analysis issues
The unit of analysis is an intubation attempt. We included the first attempt for each intubation episode. We excluded further attempts by the same intubator or by other intubators. A participant who had more than one intubation episode could be included more than once; however, we would treat each intubation as a separate study event and would randomise it separately. We planned to combine cluster‐RCTs and individually randomised RCTs in a single meta‐analysis using the generic inverse variance method. We planned to adjust cluster‐RCTs for their intracluster correlation coefficient.
Assessment of heterogeneity
We planned to use RevMan 5.3 (RevMan 2014) to assess the heterogeneity of treatment effects between trials. We planned to use the two formal statistics described below.
-
Chi2 test for homogeneity. We planned to calculate whether statistical heterogeneity was present by performing the Chi2 test for homogeneity (P < 0.1). As this test has low power when the number of studies included in the meta‐analysis is small, we set probability at the 10% level of significance (Deeks 2011).
-
I2 statistic to ensure that pooling of data was valid (Higgins 2003). We planned to quantify the impact of statistical heterogeneity by using I2 statistics available in RevMan 2014, which describe the percentage of total variation across studies due to heterogeneity rather than to sampling error. We planned to grade the degree of heterogeneity as follows: < 25% no heterogeneity, 25% to 49% low heterogeneity, 50% to 74% moderate heterogeneity, and ≥ 75% high heterogeneity.
When we found evidence of apparent or statistical heterogeneity, we planned to assess the source of the heterogeneity by performing sensitivity and subgroup analyses to look for evidence of bias or methodological differences between trials.
Data synthesis
We performed statistical analyses according to the recommendations of CNRG (http://neonatal.cochrane.org/en/index.html). We analysed all infants randomised on an intention‐to‐treat (ITT) basis. We planned to analyse treatment effects in individual trials and planned to use a fixed‐effect model for meta‐analysis in the first instance to combine data. When we noted substantial heterogeneity, we planned to examine the potential cause of heterogeneity by performing subgroup and sensitivity analyses. If we judged meta‐analysis to be inappropriate, we planned to analyse and interpret individual trials separately. For estimates of typical risk ratio (RR) and risk difference (RD), we planned to use the Mantel‐Haenszel (MH) method (Mantel 1959; Greenland 1985). For measured quantities, we planned to use the inverse variance method. When assessing treatment effects, we used RR and RD, with 95% confidence intervals (CIs), for dichotomous outcomes. When the RD was statistically significant, we calculated the number needed to treat for an additional beneficial outcome (NNTB) and the number needed to treat for an additional harmful outcome (NNTH) (1/RD). For outcomes measured on a continuous scale, we used mean difference (MD) with 95% CI.
Quality of evidence
We used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: first intubation attempt success rate; first attempt success rate for intubations without premedication; first attempt success rate for intubations with premedication; first attempt success rate for experienced intubators; first attempt success rate for inexperienced intubators; and first attempt success rate for intubations in infants weighing less than 1 kilogram.
We considered evidence from RCTs as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations according to the following: design (risk of bias), consistency across studies, directness of evidence, precision of estimates, and presence of publication bias.
The GRADE approach provides an assessment of the quality of a body of evidence according to one of four grades.
-
High: We are very confident that the true effect lies close to the estimate of effect.
-
Moderate: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different.
-
Low: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect.
-
Very low: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
Two review authors independently assessed the quality of the evidence for each of the outcomes above. We used the GRADEpro GDT Guideline Development Tool to create a ‘Summary of findings’ table to report evidence quality.
Subgroup analysis and investigation of heterogeneity
We carried out the following subgroup analyses.
-
Gestational age: < 28 weeks, 28 to 37 weeks, ≥ 37 weeks.
-
Professional category of person performing intubation: neonatologists, neonatal fellows, resident doctors, respiratory therapists, nurses, and neonatal nurse practitioners.
-
Level of experience of intubators: < 1 year, 1 to 4 years, ≥ 5 years.
-
Premedications: intubations for which premedication is given; intubations performed without premedications.
-
Timing of intubation: during resuscitation following birth; during neonatal intensive care stay.
-
Type of stylet used: a plastic‐coated malleable wire inserted into the ETT; any other type of stylet.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
For this review, we found and assessed 38 titles and abstracts in electronic format after we had removed duplicates. Of the 38 titles and abstracts screened, we assessed five as relevant, and one study met the inclusion criteria (Figure 1, Study flow diagram).
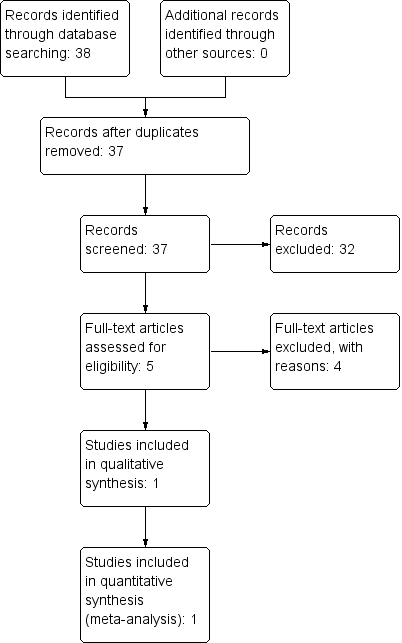
Study flow diagram.
Included studies
Kamlin 2013 is a single‐centred RCT conducted at an Australian tertiary neonatal unit between July 2006 and January 2009. The study included 304 first intubation attempts in 232 infants.
Intervention: Investigators randomised intubations to use of a stylet inserted into the ETT lumen or no stylet inserted. ETTs used were sterile, single‐use, uniform internal diameter (ID), plastic ETTs (Mallinckrodt Medical, Athlone, Ireland) of appropriate ID based on infants' actual or estimated birth weight; the stylet used was a Satin Slip intubation stylet (Malinckrodt Medical, Athlone, Ireland). Researchers confirmed correct ETT placement by using a colourimetric exhaled carbon dioxide detector (Pedicap, Nellcor Puritan Bennett, Pleasanton, CA, USA). Infants admitted to the NICU had a chest radiograph to confirm ETT position. Study authors recorded the level of experience of the operator, as well as the operator's preference (i.e. stylet, no stylet, no preference).
Investigators randomised the first attempted intubation by a single operator. If unsuccessful, the operator was free to choose his or her preferred method for subsequent attempts. Doctors performed all intubations. In general, residents had no previous intubation experience, whereas fellows had at least 12 months' experience in neonatal intensive care. Researchers defined an attempted intubation as laryngoscopy followed by introduction of the ETT past the lips. They defined the duration of an attempt, timed by a digital stop watch, as the interval from introduction of the laryngoscope blade into the mouth to its removal. Intubation attempts were limited by the infant's heart rate (> 100 beats per minute deemed acceptable) rather than by a time limit. Study authors obtained baseline readings for heart rate and pulse oxygen saturations by using a pulse oximeter and recorded the lowest heart rate and oxygen saturations during the attempt.
Investigators did not use premedication for emergency intubations following delivery. They used premedication with morphine or fentanyl, atropine, and suxamethonium for elective intubations within the NICU. During the course of the study, researchers updated hospital guidelines and replaced morphine with fentanyl.
Participants: Infants requiring orotracheal intubation were eligible for study inclusion. Excluded infants had facial or airway anomalies or were briefly intubated for suctioning of meconium from the trachea, as tube placement was difficult to confirm. The first attempted intubation of each intubation episode was eligible for randomisation. Therefore, if an infant was intubated again later during the inpatient course, researchers could randomise further intubations.
Outcomes: The primary outcome was intubation success on first attempt indicated by detection of exhaled carbon dioxide. Secondary outcomes included duration of the intubation attempt, changes in heart rate and oxygen saturation from baseline, and the presence of blood‐stained secretions after the procedure. Prespecified subgroup analyses examined the effects of gestation, birth weight, premedication, and level of experience of the operator on intubation success.
Excluded studies
We excluded four potentially relevant studies from this original review because study design did not meet the criteria for included studies. We excluded two studies that did not randomise infants to the assigned treatment – one that was a case series (Shukry 2005), and another that was a prospective observational trial (Fisher 1997). We excluded two other RCTs, as the comparisons did not match our criteria: MacNab 1998 compared three different types of stylets but did not include a 'no‐stylet' arm; Yamashita 2015 compared two different methods of confirming that the ETT was in the trachea ‐ not the main‐stem bronchus.
Risk of bias in included studies
We deemed the included study to be at low risk of bias overall. See the risk of bias graph (Figure 2) and summary (Figure 3).
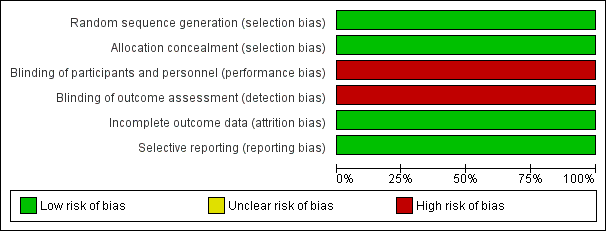
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Investigators performed randomisation in blocks of variable size, stratified by site of intubation (delivery room or NICU) (low risk of bias for generation of random sequence).
Researchers concealed allocation by using sequentially numbered sealed opaque envelopes containing computer‐generated treatment groups (low risk of bias). The neonatal fellow on duty would bring an unopened sealed envelope to the delivery room to randomise the next eligible infant. Infants in the NICU were identified by a study label placed on the incubator.
Blinding
This unblinded trial did not perform blinded outcome assessment (high risk of bias).
Incomplete outcome data
Researchers presented a complete flow chart for all intubations performed during the study period. They accounted for all exclusions and missed eligibles and for two post‐randomisation exclusions (low risk of bias).
Selective reporting
The study protocol is available, and study authors reported all prespecified primary and secondary outcomes (low risk of bias).
Other potential sources of bias
We identified no other sources of bias.
Effects of interventions
See: Summary of findings for the main comparison
Primary outcomes
Rate of successful first attempt at orotracheal intubation (Analysis 1.1)
Intubation was successful on the first attempt in 57% of the stylet group and in 53% of the no‐stylet group (P = 0.47; RR 1.08, 95% CI 0.88 to 1.32) (Figure 4).
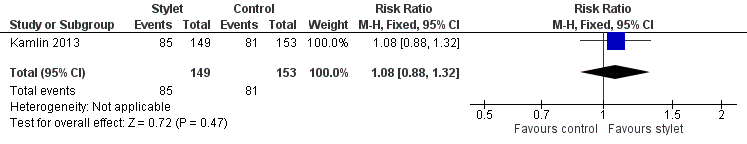
Forest plot of comparison: 1 First intubation attempt success rate with use of stylet versus non‐use of stylet, outcome: 1.1 First intubation attempt success rate.
Subgroup analyses
-
Gestational age: < 28 weeks, 28 to 37 weeks, ≥ 37 weeks; analysis was not possible owing to lack of data
-
Professional category of person performing intubation
-
-
Success by fellows was 67% with a stylet and 71% without a stylet (RR 0.94, 95% CI 0.69 to 1.29) (Analysis 2.1;Figure 5)
-
Success by residents was 54% with a stylet and 46% without a stylet (RR 1.17, 95% CI 0.9 to 1.52) (Analysis 2.2;Figure 6)
-
Doctors carried out all intubations in Kamlin 2013
-
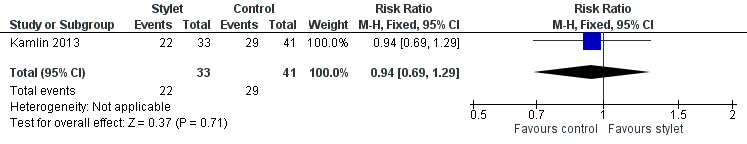
Forest plot of comparison: 2 Intubation success: Professional category, outcome: 2.1 Fellow: first intubation attempt success rate.
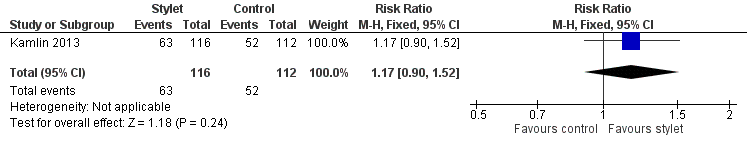
Forest plot of comparison: 2 Intubation success: Professional category, outcome: 2.2 Resident: first intubation attempt success rate.
-
Level of experience of intubators ‐ analysis was not possible owing to lack of data
-
Effect of premedication
-
-
Success rate without premedication was 53% with a stylet and 54% without a stylet (RR 0.98, 95% CI 0.72 to 1.32) (Analysis 3.1Figure 7)
-
Success rate with premedication was 61% with a stylet and 52% without a stylet (RR 1.18, 95% CI 0.89 to 1.55) (Analysis 3.2Figure 8)
-
-
Timing of intubation.
-
Success rate during resuscitation following birth was 53% with a stylet and 54% without a stylet (RR 0.98, 95% CI 0.72 to 1.32) (Analysis 4.1)
-
Success rate during neonatal intensive care stay was 61% with a stylet and 52% without a stylet (RR 1.18, 95% CI 0.89 to 1.55) (Analysis 4.2)
-
-
Type of stylet
-
Success rate with Satin Slip intubation stylet was 57% in the stylet group and 53% in the no‐stylet group (P = 0.47; RR 1.08, 95% CI 0.88 to 1.32) (Analysis 1.1; Figure 4)
-
-
Weight of infant at the time of intubation
-
Success in infants weighing less than 1 kilogram at the time of intubation was 53% with a stylet and 60% without a stylet (RR 0.89, 95% CI 0.67 to 1.18) (Analysis 5.1)
-
Success in infants weighing 1 kilogram or more at the time of intubation was 61% with a stylet and 46% without a stylet (RR 1.32, 95% CI 0.97 to 1.79) (Analysis 5.2)
-
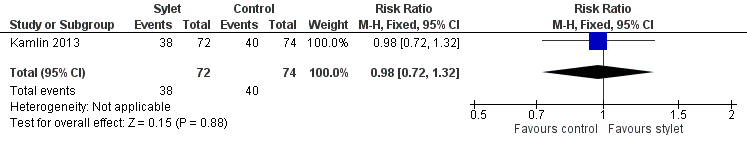
Forest plot of comparison: 3 Intubation success: use of premedication, outcome: 3.1 Intubations without premedication given to the infant.
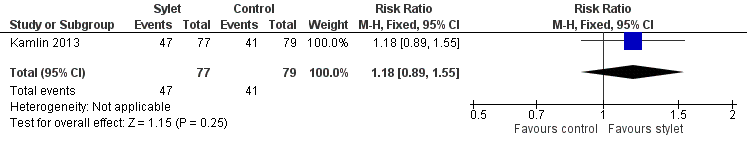
Forest plot of comparison: 3 Intubation success: use of premedication, outcome: 3.2 Intubations following premedication given to the infant.
Secondary outcomes
Duration of the intubation in seconds
The median duration of intubation attempts was similar in the two groups: 43 (interquartile ratio (IQR) 30 to 60) and 38 (IQR 27 to 57) seconds for stylet and no‐stylet groups (P = 0.23), respectively. Only 25% of all intubations took less than 30 seconds.
Number of intubation attempts
The median number of intubation attempts reported per infant before an ETT was successfully passed was one (range 1 to 5). Difficult airways appear to have been equally represented, with eight randomisations in each of the stylet and no‐stylet groups requiring four or more attempts before successful intubation.
Participant instability during the procedure
Investigators measured participant instability during the procedure by assessing:
-
heart rate (HR) < 100 during the procedure; and
-
desaturation to < 70% (with 100% indicating full oxygen saturation).
In Kamlin 2013, trial pulse oximetry data were available for 277 intubation attempts in 215 infants (121 in DR, 156 in NICU). Investigators reported no significant differences between groups in lowest recorded oxygen saturation and heart rate during randomised attempts in the DR and the NICU, respectively. The mean lowest heart rate recorded for the stylet group was 128 beats per minute (standard deviation (SD) 36) compared with 121 (SD 37) for the non‐stylet group. Only one infant in the trial received chest compressions. This infant had an antenatal diagnosis of tricuspid atresia and was randomised to the no‐stylet group. No published data were available with regards to lowest oxygen saturation for the stylet group versus the non‐stylet group during intubation attempts.
Local trauma to the airway or surrounding soft tissue
Researchers diagnosed local trauma to the airway or surrounding soft tissue by the presence of blood‐stained endotracheal aspirates or oral sections during the 24 hours following the attempt (number per thousand infant population). Rates of blood‐stained aspirates within the first 24 hours were 10% and 13% (P = 0.49) in stylet and no‐stylet groups, respectively.
Evidence of airway damage
As some infants were randomised more than once (8% of infants) and were allocated to both groups, Kamlin 2013 did not report neonatal morbidity and mortality data. Of note, no participants were reported to have had tracheal or oesophageal perforation following intubation attempts.
Discussion
Summary of main results
Of 38 titles screened, we included one study with a total of 304 first intubation attempts in 232 infants (Kamlin 2013). This study, an unblinded randomised controlled trial (RCT) carried out in an Australian tertiary perinatal centre, compared use of a stylet as an aid during intubation of the newborn infant versus intubation without use of a stylet. The included trial assessed the primary outcome and most of the secondary outcomes of this review, while excluding assessment of airway damage. The salient result from this included trial suggests that using a stylet did not significantly improve the success rate of paediatric trainees in performing neonatal orotracheal intubation when compared with intubation performed without using a stylet. Results reported were consistent across subgroups according to site of intubation and birth weight of the infant. Investigators reported no serious side effects resulting from intubation with the use of a stylet.
Overall completeness and applicability of evidence
The effectiveness of stylet use during intubation has been evaluated in only one study, which evaluated the use of one particular make of stylet (Stain Slip intubation stylet, Malinckrodt Medical, Athlone, Ireland), one brand of endotracheal tube, in one country, by doctors with a minimum of six months' neonatal experience, among a population of newborn infants. Thus, results cannot be generalised beyond this population and use of this particular make of stylet in a hospital setting.
Quality of the evidence
We assessed the quality of evidence using GRADE (Grades of Recommendation, Assessment, Development, and Evaluation) methods (Guyatt 2008). We judged the included study to be at low risk of bias overall. We stratified randomisation in blocks of variable size by site of intubation (delivery room or neonatal intensive care unit (NICU)). In terms of allocation concealment, researchers used sequentially numbered sealed opaque envelopes containing computer‐generated treatment groups to determine allocation status. Study authors provided no evidence of incomplete outcome data. Researchers accounted for infants and eligible intubations that were excluded and provided reasons for these exclusions. Exclusions after randomisation were minimal. The study protocol was available, and all prespecified outcomes were reported as intended.
One limitation of this study is that the trial was unblinded. Hospital staff and family members were unblinded to the intervention, and no evidence suggests that a blinded outcome assessment was conducted. It is unclear if the trial would have been improved by blinding of outcome assessment because of the objective nature of measured outcomes. The study is also limited in that investigators tested one brand of stylet and one brand of endotracheal tube. Endotracheal tubes likely have different degrees of rigidity. A more rigid tube may hold its shape better, and practitioners may note less benefit with use of a stylet, whereas a more floppy flexible tube may not hold its shape, and use of a stylet may be beneficial. Results show no differences in the incidence of blood‐stained endotracheal aspirates between groups. However, if the initial attempt was unsuccessful, a stylet was used for subsequent attempts, at the clinician's discretion. This result should be interpreted cautiously. Another limitation is that some infants were randomised more than once, and some were included in both study arms. This makes assessment of longer‐term outcomes impossible. In addition, inclusion of the same participant more than once leads to reduced power of the trial because of lack of independence of each intubation studied. This is somewhat ameliorated by the fact that premature infants are an atypical population that changes rapidly as the result of rapid growth (thereby posing different challenges for the operator) and changes to the upper airway resulting from each intubation and perhaps from steroid therapy. Therefore, a later intubation may be considered an independent event. Data were also derived from a single study with a moderately small number of participants.
We downgraded the quality of evidence to low for these reasons.
Potential biases in the review process
We conducted a thorough search of the literature and did not apply language restrictions to minimise selection bias. We conducted the review robustly, according to good systematic review standards. It is unlikely that we have overlooked relevant high‐quality large studies examining use versus non‐use of a stylet during intubation of the newborn infant. Therefore, we believe that the probability of bias in the review process is low.
A potential source of bias in the review as a whole is that three of the contributing authors of this Cochrane review and protocol are authors of the included study.
Agreements and disagreements with other studies or reviews
No other neonatal studies have examined whether a stylet can increase intubation success rates.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 First intubation attempt success rate with use of stylet versus non‐use of stylet, outcome: 1.1 First intubation attempt success rate.

Forest plot of comparison: 2 Intubation success: Professional category, outcome: 2.1 Fellow: first intubation attempt success rate.

Forest plot of comparison: 2 Intubation success: Professional category, outcome: 2.2 Resident: first intubation attempt success rate.

Forest plot of comparison: 3 Intubation success: use of premedication, outcome: 3.1 Intubations without premedication given to the infant.

Forest plot of comparison: 3 Intubation success: use of premedication, outcome: 3.2 Intubations following premedication given to the infant.

Comparison 1 First intubation attempt success rate with use of stylet vs non‐use of stylet, Outcome 1 First intubation attempt success rate.

Comparison 2 Intubation success: professional category, Outcome 1 Fellow: first intubation attempt success rate.

Comparison 2 Intubation success: professional category, Outcome 2 Resident: first intubation attempt success rate.

Comparison 3 Intubation success: use of premedication, Outcome 1 Intubations without premedication given to the infant.

Comparison 3 Intubation success: use of premedication, Outcome 2 Intubations following premedication given to the infant.
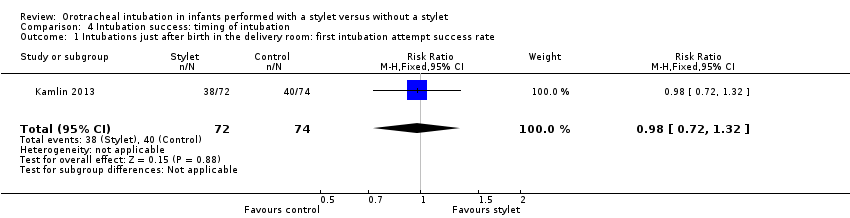
Comparison 4 Intubation success: timing of intubation, Outcome 1 Intubations just after birth in the delivery room: first intubation attempt success rate.
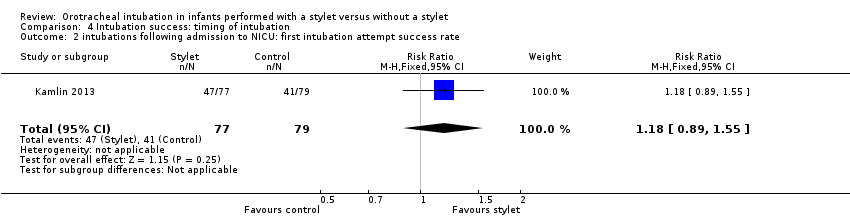
Comparison 4 Intubation success: timing of intubation, Outcome 2 intubations following admission to NICU: first intubation attempt success rate.

Comparison 5 Intubation success: weight at intubation, Outcome 1 Weight < 1000 grams.

Comparison 5 Intubation success: weight at intubation, Outcome 2 Weight ≥ 1000 grams.
| Stylet compared with no stylet for neonatal intubation | ||||||
| Patient or population: neonates requiring endotracheal intubation Settings: neonatal intensive care unit or delivery room or theatre Intervention: a stylet inserted into the endotracheal tube Comparison: no stylet inserted into the endotracheal tube | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of intubations | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Stylet | |||||
| First intubation attempt success rate (outcome achieved at time of intubation attempt and not followed up) | 529 per 1000 | 570 per 1000 | RR 1.08 | 302 | ⊕⊕⊕⊝a,b | Unblinded trial with no blinded outcome assessment Single study |
| Gestational age of the infant | no data | no data | no data | no data | absence of evidence | |
| Professional category of the intubator ‐ fellow: first intubation attempt success rate (outcome achieved at time of intubation attempt and not followed up) | 707 per 1000 | 667 per 1000 | RR 0.94 | 74 | ⊕⊕⊝⊝a,b | Unblinded trial with no blinded outcome assessment Single study |
| Professional category of the intubator ‐ resident: first intubation attempt success rate (outcome achieved at time of intubation attempt and not followed up) | 464 per 1000 | 543 per 1000 | RR 1.17 | 228 | ⊕⊕⊕⊝a,b | Unblinded trial with no blinded outcome assessment Single study |
| Level of experience of the intubator | no data | no data | no data | no data | absence of evidence | |
| Premedication given ‐ no premedication given: first intubation attempt success rate (outcome achieved at time of intubation attempt and not followed up) | 540 per 1000 | 528 per 1000 | RR 0.98 | 146 | ⊕⊕⊕⊝a,b | Unblinded trial with no blinded outcome assessment Single study |
| Premedication given ‐ no premedication given: first intubation attempt success rate (outcome achieved at time of intubation attempt and not followed up) | 519 per 1000 | 610 per 1000 | RR 1.18 | 156 | ⊕⊕⊕⊝a,b | Unblinded trial with no blinded outcome assessment Single study |
| Timing of intubation ‐ just after birth in the delivery room: first intubation attempt success rate (outcome achieved at time of intubation attempt and not followed up) | 540 per 1000 | 528 per 1000 | RR 0.98 | 146 | ⊕⊕⊕⊝a,b | Unblinded trial with no blinded outcome assessment Single study |
| Timing of intubation ‐ following admission to NICU: first intubation attempt success rate (outcome achieved at time of intubation attempt and not followed up) | 519 per 1000 | 610 per 1000 | RR 1.18 | 156 | ⊕⊕⊕⊝a,b | Unblinded trial with no blinded outcome assessment Single study |
| Type of stylet | no data | no data | no data | no data | absence of evidence | |
| Weight < 1000 g (outcome achieved at time of intubation attempt and not followed up) | 597 per 1000 | 533 per 1000 | RR 0.89 | 152 | ⊕⊕⊕⊝a,b | Unblinded trial with no blinded outcome assessment Single study |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aHigh risk of detection bias (due to lack of blinding of caregivers and outcome assessors) bSerious imprecision (due to small number of events and small sample sizes; 95% CIs include null effects) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 First intubation attempt success rate Show forest plot | 1 | 302 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.88, 1.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fellow: first intubation attempt success rate Show forest plot | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.69, 1.29] |
| 2 Resident: first intubation attempt success rate Show forest plot | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.90, 1.52] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intubations without premedication given to the infant Show forest plot | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.72, 1.32] |
| 2 Intubations following premedication given to the infant Show forest plot | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.89, 1.55] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intubations just after birth in the delivery room: first intubation attempt success rate Show forest plot | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.72, 1.32] |
| 2 intubations following admission to NICU: first intubation attempt success rate Show forest plot | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.89, 1.55] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Weight < 1000 grams Show forest plot | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.67, 1.18] |
| 2 Weight ≥ 1000 grams Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.97, 1.79] |

