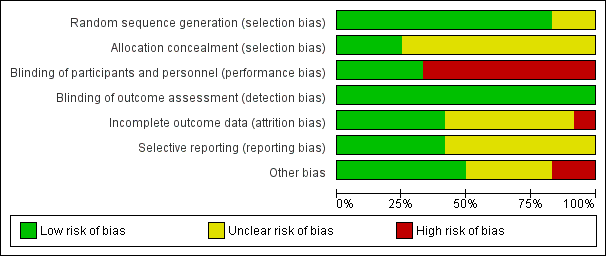
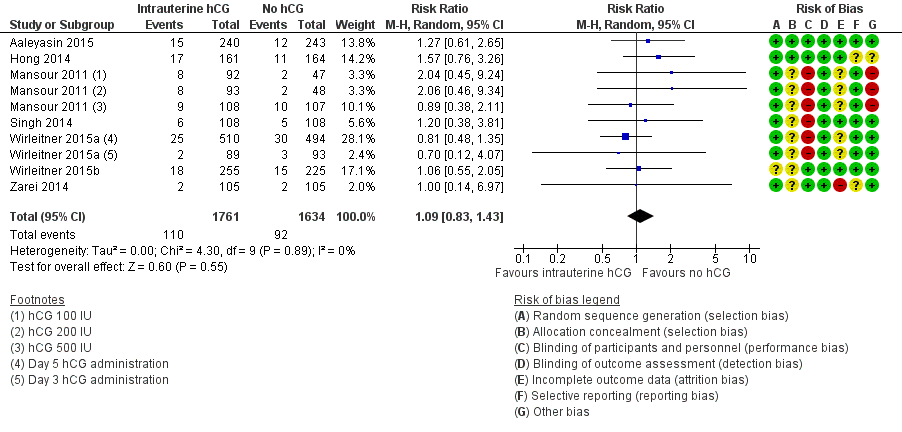
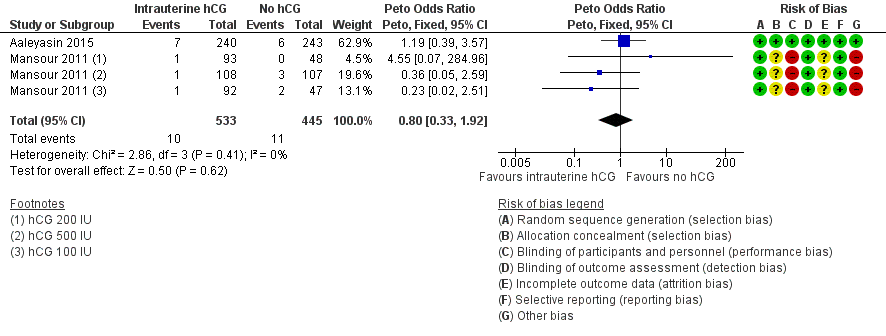
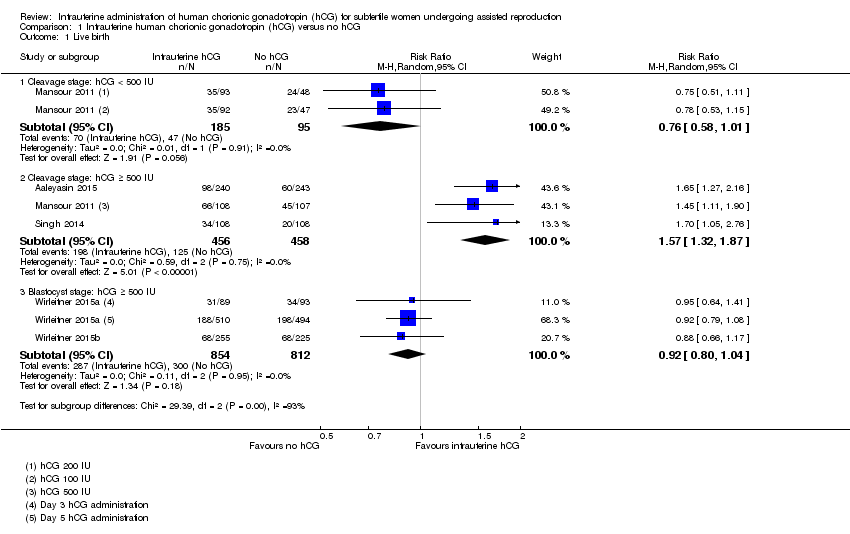
Contenido relacionado
Revisiones y protocolos relacionados
Laurentiu Craciunas, Nikolaos Tsampras, Nick Raine‐Fenning, Arri Coomarasamy | 20 octubre 2018
Wellington P Martins, Andrea DD Vieira, Jaqueline BP Figueiredo, Carolina O Nastri | 28 marzo 2013
Jane Reavey, Katy Vincent, Timothy Child, Ingrid E Granne | 16 noviembre 2016
Charalampos S Siristatidis, Eleni Sertedaki, Vasilios Karageorgiou, Dennis Vaidakis | 14 agosto 2020
Arianna D'Angelo, Nazar N Amso, Rudaina Hassan | 23 mayo 2017
Demián Glujovsky, Romina Pesce, Carlos Sueldo, Andrea Marta Quinteiro Retamar, Roger J Hart, Agustín Ciapponi | 28 octubre 2020
Michelle van der Linden, Karen Buckingham, Cindy Farquhar, Jan AM Kremer, Mostafa Metwally | 7 julio 2015
Mohan S Kamath, Abha Maheshwari, Siladitya Bhattacharya, Kar Yee Lor, Ahmed Gibreel | 2 noviembre 2017
Cindy Farquhar, Luk Rombauts, Jan AM Kremer, Anne Lethaby, Reuben Olugbenga Ayeleke | 25 mayo 2017
Salim Daya, Joanne L Gunby, Joseph Ogah | 16 julio 2008
Respuestas clínicas Cochrane
Sera Tort, George Salamalekis | 2 diciembre 2016