Administración intrauterina de la gonadotrofina coriónica humana (hCG) para pacientes subfértiles sometidas a reproducción asistida
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Design: 2‐armed parallel RCT Location: Shariati Teaching Hospital, Tehran, Iran Period: January 2011 to July 2012 Power calculation: yes Funding: not mentioned Trial registration: not mentioned and not found Publication type: full text | |
| Participants | Number: 483 Women's age (mean years; experimental vs. control): 29.1 vs. 28.7 Inclusion criteria: all infertile women who were candidates for the first IVF/ICSI Exclusion criteria: aged > 40 years, history of percutaneous epididymal sperm aspiration, testicular sperm extraction, myomectomy, hydrosalpinx, presence of uterine fibroma with the pressure effect on endometrium, endometriosis, and azoospermia Ovarian controlled hyperstimulation: long GnRH agonist protocol Fertilisation: ICSI Stage of the embryo at transfer: cleavage Embryo processing: fresh Number of embryos transferred (mean; experimental vs. control): 2.8 vs. 2.9 | |
| Interventions | Experimental: hCG 500 IU in a volume of 50 μL tissue culture media (Vitrolife, Göteborg, Sweden) was injected into the uterus 5‐7 minutes prior to ET Control: 50 μL tissue culture media (Vitrolife, Göteborg, Sweden) instead of hCG | |
| Outcomes | Clinical pregnancy, miscarriage, live birth, intrauterine death | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list |
| Allocation concealment (selection bias) | Low risk | A technician, not belonging to the study personnel, prepared and coded the drugs according to the list |
| Blinding of participants and personnel (performance bias) | Low risk | All participants and clinical care providers were blinded to the list until the end of the study |
| Blinding of outcome assessment (detection bias) | Low risk | All participants and clinical care providers were blinded to the list until the end of the study |
| Incomplete outcome data (attrition bias) | Low risk | 0 women lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reported on all important outcomes |
| Other bias | Low risk | Similar baseline characteristics between groups after randomisation |
| Methods | Design: 2‐armed parallel RCT Location: Instituto Paulista de Ginecologia, Obstetricia e Medicinada Reproducao, Sao Paulo, Brazil Period: January to December 2012 Power calculation: no Funding: not mentioned Trial registration: not mentioned and not found Publication type: abstract | |
| Participants | Number: 44 Women's age (mean years; experimental vs. control): not mentioned Inclusion criteria: endometrial thickness > 7 mm on the day that the donor received hCG and at least 2 blastocysts on the day of ET Exclusion criteria: not mentioned Ovarian controlled hyperstimulation: donor oocytes, protocol not mentioned Fertilisation: not mentioned Stage of the embryo at transfer: blastocyst Embryo processing: fresh Number of embryos transferred: not mentioned (likely 2 from inclusion criteria) | |
| Interventions | Experimental: intrauterine injection of hCG 500 IU of 6 hours before the ET Control: ET without any pre‐intrauterine injection | |
| Outcomes | Clinical pregnancy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomisation |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) | Low risk | Not mentioned, but unlikely to induce bias |
| Incomplete outcome data (attrition bias) | Unclear risk | Very brief reporting of results |
| Selective reporting (reporting bias) | Unclear risk | No reporting on adverse events, miscarriage and live birth |
| Other bias | Unclear risk | No reporting on baseline characteristics between groups |
| Methods | Design: 2‐armed parallel RCT Location: Reproductive Medicine Associates of New Jersey, USA Period: August 2012 to December 2013 Power calculation: yes, but not met (778 embryos required, 473 embryos transferred) Funding: not mentioned Trial registration: NCT01643993 Publication type: full text | |
| Participants | Number: 300 Women's age (mean years; experimental vs. control): 35.0 vs. 35.1 Inclusion criteria: all participants undergoing fresh or frozen ET within the ART programme where the female partner was under 43 years of age Exclusion criteria: women could not be simultaneously participating in another prospective clinical trial at the centre, but there were no other inclusion/exclusion criteria Ovarian controlled hyperstimulation: not mentioned Fertilisation: not mentioned Stage of the embryo at transfer: blastocyst Embryo processing: fresh and frozen/thawed | |
| Interventions | Experimental: endometrial infusion of 20 µL ET media (synthetic serum substitute and Medicult BlastAssist from Origio) laden with 500 IU of purified‐urinary placental hCG (Novarel, Ferring Pharmaceuticals) < 3 minutes before ET Control: endometrial infusion of 20 µL ET media only | |
| Outcomes | Miscarriage and clinical pregnancy (converted from ongoing pregnancy) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random number function was used to create variable blocks of 4‐8 with participants assigned to the 2 groups in a 1:1 allocation |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was achieved using sequentially numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Both the physician performing the transfer and the participants were blinded to the assigned treatment group throughout the entirety |
| Blinding of outcome assessment (detection bias) | Low risk | Not mentioned, but unlikely to induce bias |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No reports on live birth and adverse events |
| Other bias | Unclear risk | 25 participants declined to participate after randomisation for various reasons |
| Methods | Design: 3‐armed parallel RCT Location: Research and Clinical Center for Infertility, Shahid Sadoughi University of Medical Sciences, Yazd, Iran Period: not mentioned Power calculation: not mentioned Funding: not mentioned Trial registration: IRCT2012091310328N3 Publication type: abstract | |
| Participants | Number: 159 Women's age: not mentioned Inclusion criteria: women undergoing ART (from protocol) Exclusion criteria: aged > 40 and < 20 years, FSH > 12 mIU/mL, infertility causes except male or unexplained factor infertility, azoospermia, presence of uterine myoma, endometriosis, hydrosalpinges, previous IVF/ICSI trials (successful or unsuccessful), history of endocrine diseases (e.g. diabetes or thyroid dysfunction), women with previous history of hysteroscopic operation due to submucosal myoma or intrauterine synechia (from protocol) Ovarian controlled hyperstimulation: antagonist protocol Fertilisation: IVF or ICSI Stage of the embryo at transfer: cleavage (from protocol) Embryo processing: fresh (from protocol) Number of embryos transferred: 2 or 3 (from protocol) | |
| Interventions | Experimental: hCG 500 IU (40 µL) intrauterine injection 7 minutes before ET Experimental: hCG 1000 IU (40 µL) intrauterine injection 7 minutes before ET Control: nothing before ET | |
| Outcomes | Clinical pregnancy, miscarriage | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants divided into 3 groups using table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded (from protocol) |
| Blinding of outcome assessment (detection bias) | Low risk | Not mentioned, but unlikely to induce bias |
| Incomplete outcome data (attrition bias) | Unclear risk | Very brief reporting of results |
| Selective reporting (reporting bias) | Unclear risk | No reporting on live birth or adverse events |
| Other bias | Unclear risk | No reporting on baseline characteristics between groups |
| Methods | Design: 2‐armed parallel RCT Location: Genesis Athens Hospital, Centre for Human Reproduction, Athens, Greece Period: July 2012 to September 2013 Power calculation: no Funding: Genesis Athens Clinic Trial registration: not registered Publication type: abstract | |
| Participants | Number: 194 Women's age (years): > 40 Inclusion criteria: women aged > 40 years receiving donor eggs Exclusion criteria: not mentioned Ovarian controlled hyperstimulation: not mentioned Fertilisation: not mentioned Stage of the embryo at transfer: not mentioned Embryo processing: not mentioned Number of embryos transferred: not mentioned | |
| Interventions | Experimental: intrauterine hCG 500 IU injection 7 minutes before ET Control: no intrauterine injection | |
| Outcomes | Clinical pregnancy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed in a 1:1 fashion to 1 of 2 groups [...] prepared from a computer‐generated list |
| Allocation concealment (selection bias) | Low risk | Adequate allocation concealment was assured from sequentially numbered, opaque, sealed envelopes prepared from a computer‐generated list |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Not blinded, but unlikely to induce bias |
| Incomplete outcome data (attrition bias) | Unclear risk | Very brief reporting of results |
| Selective reporting (reporting bias) | Unclear risk | No reporting on live birth and adverse events |
| Other bias | Unclear risk | No reporting on baseline characteristics between groups |
| Methods | Design: 2‐armed parallel RCT Location: IPGO, Sao Paulo, Brazil Period: January to December 2012 Power calculation: no Funding: not mentioned Trial registration: not mentioned and not found Publication type: abstract | |
| Participants | Number: 36 Women's age: not mentioned Inclusion criteria: women with 2 previous failures in IVF cycles with ET Exclusion criteria: not mentioned Ovarian controlled hyperstimulation: not mentioned Fertilisation: not mentioned Stage of the embryo at transfer: not mentioned Embryo processing: not mentioned Number of embryos transferred: not mentioned | |
| Interventions | Experimental: intrauterine injection of hCG 500 IU 6 hours before the ET Control: women were forwarded straight to ET | |
| Outcomes | Clinical pregnancy | |
| Notes | Abstract presented as poster at 5th IVI International Congress, Seville, Spain, 2013 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation mentioned without any details |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) | Low risk | Not mentioned, but unlikely to induce bias |
| Incomplete outcome data (attrition bias) | Unclear risk | Very brief reporting of results |
| Selective reporting (reporting bias) | Unclear risk | No reporting on adverse events, miscarriage and live birth |
| Other bias | High risk | Participants number in each arm was not reported, but deduced based on percentages and previous study by the same team |
| Methods | Design: 2 RCTs within the same study analysed as 4‐armed parallel RCT Location: The Egyptian IVF‐ET Center, Cairo, Egypt Period: January 2010 to January 2011 Power calculation: yes, but not met Funding: The Egyptian IVF‐ET Center Trial registration: NCT01030393 Publication type: full text | |
| Participants | Number: 280 + 215 = 495 Women's age (mean years; experimental 100, 200 vs. control; 500 vs. control): 29 vs. 28.5 vs. 29.1; 28.3 vs. 28.4 Inclusion criteria: women aged < 40 years old with infertility due to male factor Exclusion criteria: previous IVF/ICSI trials, including a successful trial, azoospermia, uterine myoma or previous myomectomy, endometriosis, or the presence of hydrosalpinges Ovarian controlled hyperstimulation: not mentioned Fertilisation: ICSI Stage of the embryo at transfer: cleavage Embryo processing: fresh Number of embryos transferred (mean; experimental 100, 200 vs. control; 500 vs. control): 2.9 vs. 2.8 vs. 2.9; 2.9 vs. 2.8 | |
| Interventions | Experimental 100: 40 µL of tissue culture medium (G‐2 plus ref. 10132, Vitrolife) containing hCG 100 IU injected intrauterine approximately 7 minutes before ET Experimental 200: 40 µL of tissue culture medium (G‐2 plus ref. 10132, Vitrolife) containing hCG 200 IU injected intrauterine approximately 7 minutes before ET Experimental 500: 40 µL of tissue culture medium (G‐2 plus ref. 10132, Vitrolife) containing hCG 500 IU injected intrauterine approximately 7 minutes before ET Control: no intrauterine hCG injection prior to ET | |
| Outcomes | Live birth, miscarriage, clinical pregnancy, ectopic pregnancy | |
| Notes | Live birth rate established by personal communication with authors, June 2015. Study publication reported number of deliveries, which included six women who had stillbirths (3 in each group). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using sealed dark envelopes into 2 groups |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not mentioned. Could explain different withdrawal rates between groups |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Not blinded, but unlikely to induce bias |
| Incomplete outcome data (attrition bias) | Unclear risk | Women lost to follow‐up live birth (similar numbers between groups) |
| Selective reporting (reporting bias) | Low risk | Reported on all important outcomes |
| Other bias | High risk | Interim analysis with change of protocol and premature ending of study. Relatively high live birth rate in control group, reasons unclear. |
| Methods | Design: 2‐armed parallel RCT Location: Reproductive Medicine Centre PROCREA, Mexico City Period: August 2011 to November 2012 Power calculation: yes Funding: PROCREA Trial registration: not mentioned and not found Publication type: full text | |
| Participants | Number: 210 Women's age (mean years; experimental vs. control): 36.4 vs. 37.3 Inclusion criteria: infertile women aged < 40 years who had an indication for an IVF/ICSI Exclusion criteria: azoospermia Ovarian controlled hyperstimulation: indicated based on individual participant characteristics Fertilisation: IVF or ICSI Stage of the embryo at transfer: cleavage Embryo processing: fresh and frozen/thawed Number of embryos transferred (mean): 2.1 | |
| Interventions | Experimental: 20 μL of embryo culture medium (G‐2, Vitrolife) that contained hCG 500 IU was administered intrauterine before ET Control: no intrauterine hCG was administered | |
| Outcomes | Clinical pregnancy, ectopic pregnancy | |
| Notes | Authors mention "prospective observational study", but the design was in fact RCT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A simple randomisation sample and assignment was generated in a computer‐based program |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) | Low risk | Not mentioned, but unlikely to induce bias |
| Incomplete outcome data (attrition bias) | Low risk | All women followed up till pregnancy test/ultrasound scan |
| Selective reporting (reporting bias) | Unclear risk | No reporting on live birth and miscarriage despite mentioning follow‐up |
| Other bias | Low risk | Similar baseline characteristics between groups after randomisation |
| Methods | Design: 2‐armed parallel RCT Location: Bhopal Test Tube Baby Centre, Infertility, Bhopal, India Period: 2006‐2013 Power calculation: not mentioned Funding: Bhopal Test Tube Baby Centre Trial registration: BTTB/2006/19 (?) Publication type: abstract | |
| Participants | Number: 216 Women's age (mean years; experimental vs. control): 35 vs. 34.5 (from ESHRE 2014 oral presentation) Inclusion criteria: infertile women aged < 42 years, with from recurrent implantation failure Exclusion criteria: not mentioned Ovarian controlled hyperstimulation: based on individual participant characteristics (from ESHRE 2014 oral presentation) Fertilisation: ICSI Stage of the embryo at transfer: cleavage Embryo processing: not mentioned Number of embryos transferred (mean; experimental vs. control): 2.7 vs. 2.5 (from ESHRE 2014 oral presentation) | |
| Interventions | Experimental: intrauterine administration of rhCG 500 IU in 40 μL 5 minutes before ET Control: culture medium only administered before ET (from ESHRE 2014 oral presentation) | |
| Outcomes | Clinical pregnancy, miscarriage, live birth (from ESHRE 2014 oral presentation) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly divided into 2 groups using computer‐generated list |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) | Low risk | Not mentioned, but unlikely to induce bias |
| Incomplete outcome data (attrition bias) | Low risk | 0 women lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reported on all important outcomes |
| Other bias | Low risk | Similar baseline characteristics between groups after randomisation |
| Methods | Design: 4‐armed parallel RCT (same intervention on day 3 or 5) Location: IVF Centers Prof. Zech, Bregenz, Austria Period: February 2013 to February 2014 Power calculation: only met for day 5 administration Funding: not mentioned Trial registration: not mentioned and not found Publication type: full text | |
| Participants | Number: 182 + 1004 = 1186 Women's age (mean years; experimental vs. control): 36.1 vs. 35.5; 37.1 vs. 36.7 Inclusion criteria: fresh autologous blastocyst transfer on day 5 and woman age ≤ 43 years Exclusion criteria: oocyte donation cycles and women with reported recurrent implantation failure (≥ 3 negative IVF cycles) Ovarian controlled hyperstimulation: GnRH agonist long protocol Fertilisation: IVF or IMSI Stage of the embryo at transfer: blastocyst Embryo processing: fresh Number of embryos transferred: 1 or 2 | |
| Interventions | Experimental (day 3): intrauterine hCG 500 IU (Pregnyl, ORGANON, Netherlands) dissolved in 40 μL embryo culture medium G‐2 PLUS (Vitrolife, Sweden) administered on day 3 (2 days before ET) Control (day 3): administration of 40 μL culture medium without hCG on day 3 (2 days before ET) Experimental (day 5): intrauterine hCG 500 IU (Pregnyl, ORGANON, Netherlands) dissolved in 40 μL embryo culture medium G‐2 PLUS (Vitrolife, Sweden) administered on day 5 (3 minutes before ET) Control (day 5): administration of 40 μL culture medium without hCG on day 3 (3 minutes before ET) | |
| Outcomes | Clinical pregnancy, miscarriage, live birth | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done electronically with a random number generator |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | High risk | Participants blinded, but not the personnel |
| Blinding of outcome assessment (detection bias) | Low risk | Not blinded, but unlikely to induce bias |
| Incomplete outcome data (attrition bias) | Unclear risk | 19 participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reports on all relevant outcomes |
| Other bias | Low risk | Baseline characteristics of the participants were comparable between 2 study groups |
| Methods | Design: 2‐armed parallel RCT Location: IVF‐Centers Prof. Zech, Bregenz, Austria Period: not mentioned Power calculation: yes Funding: funded by hospital/clinic(s) ‐ this study was not externally funded Trial registration: CRT:355 Publication type: abstract | |
| Participants | Number: 480 Women's age (mean years; experimental vs. control): 40.3 vs. 40.4 Inclusion criteria: women aged 38‐43 years Exclusion criteria: recurrent implantation failure Ovarian controlled hyperstimulation: GnRH agonist long protocol Fertilisation: IMSI Stage of the embryo at transfer: blastocyst Embryo processing: fresh Number of embryos transferred: 1 or 2 | |
| Interventions | Experimental: intrauterine hCG 500 IU dissolved in 40 μL embryo culture medium administered 3 minutes before ET Control: administration of 40 μL culture medium without hCG 3 minutes before ET | |
| Outcomes | Clinical pregnancy, miscarriage, live birth | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was mentioned without further details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | Low risk | Participants were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Not blinded, but unlikely to induce bias |
| Incomplete outcome data (attrition bias) | Low risk | All participants were followed up |
| Selective reporting (reporting bias) | Low risk | Reports on all relevant outcomes |
| Other bias | Low risk | Baseline characteristics of the participants were comparable between 2 study groups |
| Methods | Design: 2‐armed parallel RCT Location: Reproductive Medicine Center of Mother and Child Hospital, Shiraz, Iran Period: December 2011 to November 2012 Power calculation: yes Funding: Shiraz University of Medical Sciences Trial registration: IRCT2012121711790N1 Publication type: full text | |
| Participants | Number: 210 Women's age (mean years; experimental vs. control): 29.9 vs. 31.2 Inclusion criteria: 18‐40‐year‐old women with infertility Exclusion criteria: women with from autoimmune disorders, endocrinopathies, who had previous successful IVF/ICSI trials, endometriosis, azoospermia and hydrosalpinges Ovarian controlled hyperstimulation: not mentioned Fertilisation: ICSI Stage of the embryo at transfer: cleavage Embryo processing: not mentioned (likely fresh) Number of embryos transferred (mean; experimental vs. control): 6.1 vs. 5.7 | |
| Interventions | Experimental: rhCG 250 μg (0.5 mL, 6500 IU) (Ovitrelle, Merck Serono, France) through intrauterine injection 12 minutes before ET Control: intrauterine injection of normal saline (0.5 mL) 12 minutes before ET | |
| Outcomes | Clinical pregnancy, miscarriage, ectopic pregnancy, still birth | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The participants were randomly assigned to 2 study groups using a computerised random digit generator based on their registration number in order of referral |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | Low risk | The syringes with volume of 0.5 mL from each group were prepared by fellowship student and injected blinded by the attending gynaecologist |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blinding mentioned (? women ? outcome assessors ‐ in addition to gynaecologists performing the transfer), unlikely to induce bias |
| Incomplete outcome data (attrition bias) | High risk | 23/105 participants in intrauterine rhCG group and 7/105 participants in placebo group were lost to follow‐up after receiving the allocated treatment (unclear why) |
| Selective reporting (reporting bias) | Unclear risk | No report on live birth |
| Other bias | Low risk | Baseline characteristics of the participants were comparable between 2 study groups |
ART: assisted reproductive technology; ET: embryo transfer; ESHRE: European Society of Human Reproduction and Embryology; FSH: follicle‐stimulating hormone; GnRH: gonadotropin‐releasing hormone; hCG: human chorionic gonadotropin; ICSI: intracytoplasmic sperm injection; IMSI: intracytoplasmic morphologically selected sperm injection; IU: international unit; IVF: in vitro fertilisation; RCT: randomised controlled trial; rhCG: recombinant human chorionic gonadotropin.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Retrospective | |
| Not randomised | |
| Not randomised | |
| Not randomised | |
| Meta‐analysis |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Design: 2‐armed parallel RCT Location: Avicenna Infertility Clinic, Tehran, Iran Period: not mentioned Power calculation: not mentioned Funding: not mentioned Trial registration: not mentioned and not found Publication type: abstract |
| Participants | Number: 80 Women's age (mean years; experimental vs. control): 29.5 vs. 29.3 Inclusion criteria: women undergoing ICSI Exclusion criteria: not mentioned Ovarian controlled hyperstimulation: not mentioned Fertilisation: ICSI Stage of the embryo at transfer: not mentioned Embryo processing: not mentioned Number of embryos transferred (mean; experimental vs. control): 2.9 vs. 2.8 |
| Interventions | Experimental: intrauterine injection of hCG 500 IU dissolved in 40 μL of ET media 10 minutes before ET Control: 40 μL of ET media 10 minutes before ET |
| Outcomes | Implantation rate defined as positive pregnancy test at 2 weeks after ET (biochemical pregnancy) |
| Notes | We emailed the authors in February 2016 for more information on study design and outcomes |
| Methods | Design: 2‐armed parallel RCT Location: Radhakrishna Multispecialty hospital and IVF Centre in Bengaluru in Southern India Period: April 2013 to March 2014 Power calculation: not mentioned Funding: none Trial registration: Not mentioned and not found. Publication type: full text |
| Participants | Number: 32 Women's age (mean years; experimental vs. control): 29.6 vs. 29.6 Inclusion criteria: women undergoing IVF Exclusion criteria: not mentioned Ovarian controlled hyperstimulation: not mentioned Fertilisation: IVF or ICSI Stage of the embryo at transfer: cleavage Embryo processing: fresh and frozen/thawed Number of embryos transferred (mean; experimental vs. control): 2.9 vs. 2.9 |
| Interventions | Experimental: intrauterine administration of hCG 500 IU 7 minutes before ET Control: ET without hCG |
| Outcomes | Fertilisation rate |
| Notes | We emailed the authors in February 2016 for more information on study design and outcomes. No reply has yet been received. |
ET: embryo transfer; hCG: human chorionic gonadotropin; ICSI: intracytoplasmic sperm injection; IU: international unit; IVF: in vitro fertilisation; RCT: randomised controlled trial.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, Outcome 1 Live birth. | ||||
| 1.1 Cleavage stage: hCG < 500 IU | 1 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.58, 1.01] |
| 1.2 Cleavage stage: hCG ≥ 500 IU | 3 | 914 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [1.32, 1.87] |
| 1.3 Blastocyst stage: hCG ≥ 500 IU | 2 | 1666 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.80, 1.04] |
| 2 Miscarriage Show forest plot | 7 | 3395 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.83, 1.43] |
| Analysis 1.2  Comparison 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, Outcome 2 Miscarriage. | ||||
| 3 Miscarriage per clinical pregnancy Show forest plot | 7 | 1450 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.77, 1.30] |
| Analysis 1.3  Comparison 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, Outcome 3 Miscarriage per clinical pregnancy. | ||||
| 4 Clinical pregnancy Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, Outcome 4 Clinical pregnancy. | ||||
| 4.1 Cleavage stage: hCG < 500 IU | 1 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.70, 1.10] |
| 4.2 Cleavage stage: hCG ≥ 500 IU | 7 | 1414 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [1.25, 1.58] |
| 4.3 Blastocyst stage: hCG ≥ 500 IU | 3 | 1991 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.86, 1.06] |
| 5 Complications: intrauterine death Show forest plot | 2 | 978 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.33, 1.92] |
| Analysis 1.5  Comparison 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, Outcome 5 Complications: intrauterine death. | ||||

Study flow diagram.
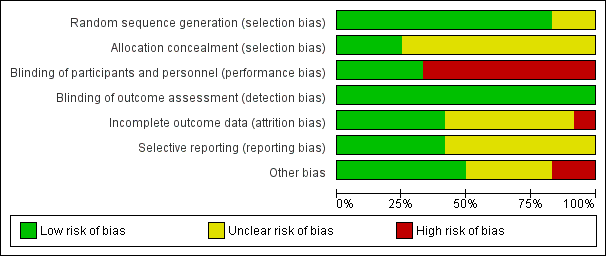
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, outcome: 1.1 Live birth.
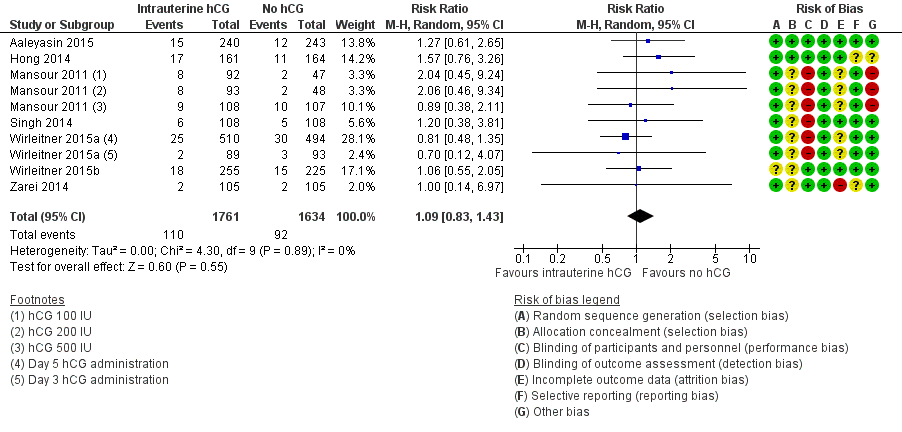
Forest plot of comparison: 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, outcome: 1.2 Miscarriage.

Forest plot of comparison: 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, outcome: 1.4 Clinical pregnancy.
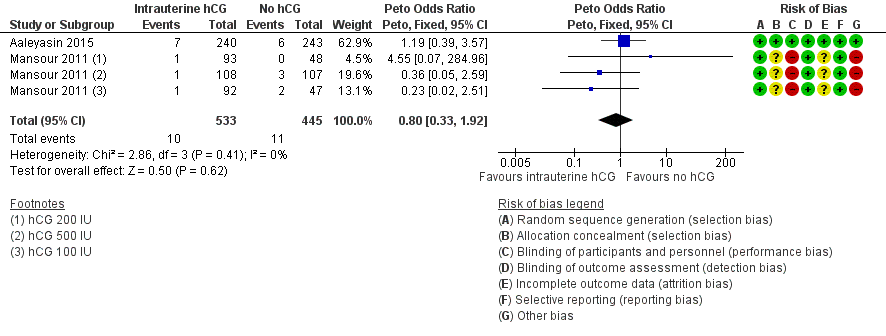
Forest plot of comparison: 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, outcome: 1.5 Complications: intrauterine death.
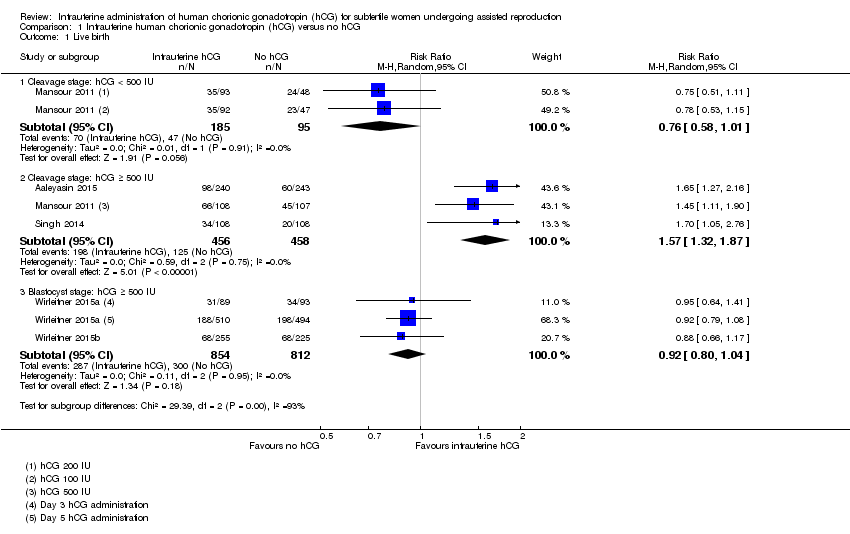
Comparison 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, Outcome 1 Live birth.
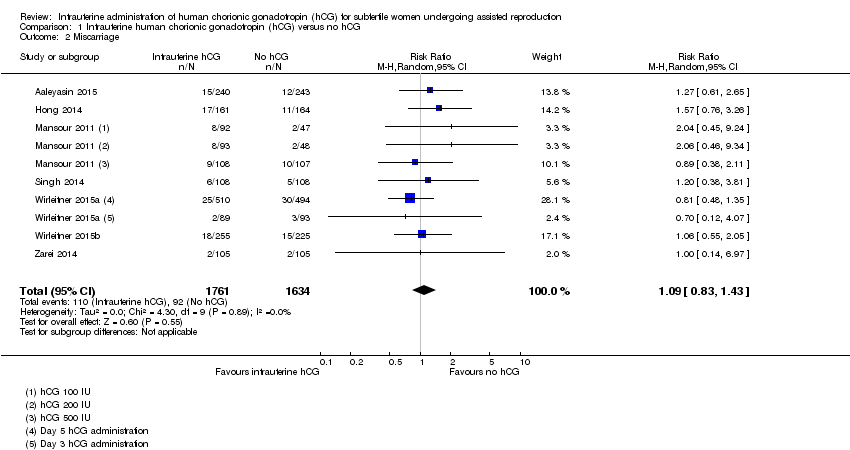
Comparison 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, Outcome 2 Miscarriage.
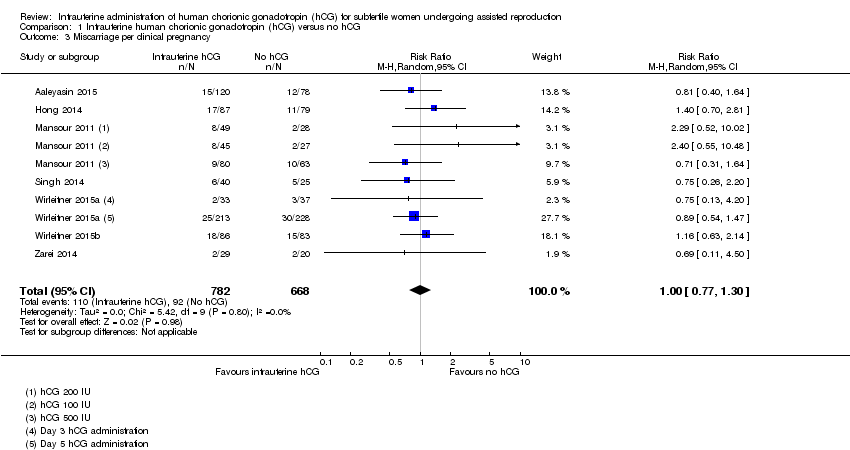
Comparison 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, Outcome 3 Miscarriage per clinical pregnancy.

Comparison 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, Outcome 4 Clinical pregnancy.

Comparison 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, Outcome 5 Complications: intrauterine death.
| Intrauterine administration of hCG for women undergoing assisted reproduction | |||||
| Population: women undergoing assisted reproduction | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Control | Intrauterine administration of hCG | ||||
| Live birth ‐ cleavage stage: hCG < 500 IU | 495 per 1000 | 376 per 1000 | RR 0.76 | 280 | ⊕⊝⊝⊝ |
| Live birth ‐ cleavage stage: hCG ≥ 500 IU | 247 per 1000 | 388 per 1000 | RR 1.57 | 914 | ⊕⊕⊕⊝ |
| Live birth ‐ blastocyst stage: hCG ≥ 500 IU | 366 per 1000 | 337 per 1000 | RR 0.92 | 1666 | ⊕⊕⊕⊝ |
| Pregnancy ‐ cleavage stage: hCG < 500 IU | 579 per 1000 | 509 per 1000 (405 to 637) | RR 0.88 (0.70 to 1.10) | 280 | ⊕⊝⊝⊝ |
| Pregnancy ‐ cleavage stage: hCG ≥ 500 IU | 321 per 1000 | 453 per 1000 (401 to 507) | RR 1.41 (1.25 to 1.58) | 1414 (7 studies) | ⊕⊕⊕⊝ |
| Pregnancy ‐ blastocyst stage: hCG ≥ 500 IU | 430 per 1000 | 408 per 1000 (370 to 455) | RR 0.95 (0.86 to 1.06) | 1991 (3 studies) | ⊕⊕⊕⊝ |
| Miscarriage Follow‐up: mean 40 weeks | 48 per 1000 | 52 per 1000 (40 to 68) | RR 1.09 (0.83 to 1.43) | 3395 (7 studies) | ⊕⊝⊝⊝ |
| Other complications | Other complications reported in the included studies were ectopic pregnancy (3 studies, n = 915, 3 events overall), heterotopic pregnancy (1 study, n = 495, 1 event), intrauterine death (2 studies, n = 978, 21 events) and triplets (1 study, n = 48, 3 events). There were too few events to allow any conclusions to be drawn | ⊕⊝⊝⊝ | |||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Downgraded two levels due to very serious risk of bias: lack of blinding of participants and personnel, no clear description of allocation concealment and premature termination of the study following interim analysis. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Cleavage stage: hCG < 500 IU | 1 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.58, 1.01] |
| 1.2 Cleavage stage: hCG ≥ 500 IU | 3 | 914 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [1.32, 1.87] |
| 1.3 Blastocyst stage: hCG ≥ 500 IU | 2 | 1666 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.80, 1.04] |
| 2 Miscarriage Show forest plot | 7 | 3395 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.83, 1.43] |
| 3 Miscarriage per clinical pregnancy Show forest plot | 7 | 1450 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.77, 1.30] |
| 4 Clinical pregnancy Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Cleavage stage: hCG < 500 IU | 1 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.70, 1.10] |
| 4.2 Cleavage stage: hCG ≥ 500 IU | 7 | 1414 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [1.25, 1.58] |
| 4.3 Blastocyst stage: hCG ≥ 500 IU | 3 | 1991 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.86, 1.06] |
| 5 Complications: intrauterine death Show forest plot | 2 | 978 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.33, 1.92] |

