Administración intrauterina de la gonadotrofina coriónica humana (hCG) para pacientes subfértiles sometidas a reproducción asistida
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Group (CGF) Specialised Register search strategy
PROCITE Platform
From inception to 10 November 2015
Keywords CONTAINS "IVF" or "in vitro fertilization" or "in‐vitro fertilisation" or "ICSI" or "intracytoplasmic sperm injection" or "ET" or "Embryo" or "in‐vitro fertilization" or "Embryo Transfer" or "Embryo Transfer‐uterine" or "blastocyst transfer" or Title CONTAINS "IVF" or "in vitro fertilization" or "in‐vitro fertilisation" or "ICSI" or "intracytoplasmic sperm injection" or "Embryo" or "in‐vitro fertilization" or "ET" or "Embryo" or "in‐vitro fertilization" or "Embryo Transfer" or "Embryo Transfer‐uterine" or "blastocyst transfer"
AND
Keywords CONTAINS "HCG " or "human chorionic gonadotrophin" or "human chorionic gonadotropin" or "recombinant HCG" or "rhCG" or Title CONTAINS "HCG " or "human chorionic gonadotrophin" or "human chorionic gonadotropin" or "recombinant HCG" or "rhCG"
AND
Keywords CONTAINS "intrauterine human chorionic gonadotrophin" or "intrauterine" or "Intrauterine injection" or "intrauterine instillation "or "uterine cavity injection" or "endometrial" or "Endometrium" or "uterine" or Title CONTAINS "intrauterine human chorionic gonadotrophin" or "intrauterine" or "Intrauterine injection" or "intrauterine instillation "or "uterine cavity injection" or "Endometrium" or "uterine" (17 hits)
Appendix 2. CENTRAL search strategy
OVID Platform
From inception to 10 November 2015
1 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ (1756)
2 embryo transfer$.tw. (1200)
3 in vitro fertili?ation.tw. (1610)
4 ivf‐et.tw. (324)
5 (ivf or et).tw. (13581)
6 icsi.tw. (992)
7 intracytoplasmic sperm injection$.tw. (538)
8 (blastocyst adj2 transfer$).tw. (130)
9 or/1‐8 (15067)
10 exp Chorionic Gonadotropin/ad, tu, th [Administration & Dosage, Therapeutic Use, Therapy] (22)
11 (Human Chorionic Gonadotrop?in adj7 intrauter$).tw. (33)
12 (Human Chorionic Gonadotrop?in adj7 uter$).tw. (8)
13 (Human Chorionic Gonadotrop?in adj7 intra‐uter$).tw. (2)
14 ((endometri$ adj2 infusion$) and chorionic).tw. (3)
15 ((endometri$ adj2 ?instillation) and chorionic).tw. (0)
16 ((intra?uter$ adj2 infusion$) and chorionic).tw. (0)
17 ((intra?uter$ adj2 ?instillation) and chorionic).tw. (2)
18 ((endometri$ adj2 injection$) and chorionic).tw. (0)
19 ((intra?uter$ adj2 injection$) and chorionic).tw. (9)
20 ((intra?uter$ adj2 administration) and chorionic).tw. (5)
21 ((endometri$ adj2 administration) and chorionic).tw. (3)
22 (intrauter$ adj7 ?hcg).tw. (39)
23 (intra‐uter$ adj7 ?hcg).tw. (4)
24 (uter$ adj7 ?hcg).tw. (25)
25 or/10‐24 (115)
26 9 and 25 (45)
Appendix 3. MEDLINE search strategy
OVID Platform
From inception to 10 November 2015
1 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ (34811)
2 embryo transfer$.tw. (9012)
3 in vitro fertili?ation.tw. (18370)
4 ivf‐et.tw. (1958)
5 (ivf or et).tw. (200229)
6 icsi.tw. (6135)
7 intracytoplasmic sperm injection$.tw. (5460)
8 (blastocyst adj2 transfer$).tw. (638)
9 or/1‐8 (226616)
10 exp Chorionic Gonadotropin/ad, tu, th [Administration & Dosage, Therapeutic Use, Therapy] (4588)
11 (Human Chorionic Gonadotrop?in adj7 intrauter$).tw. (64)
12 (Human Chorionic Gonadotrop?in adj7 uter$).tw. (136)
13 (Human Chorionic Gonadotrop?in adj7 intra‐uter$).tw. (0)
14 ((endometri$ adj2 infusion$) and chorionic).tw. (1)
15 ((endometri$ adj2 ?instillation) and chorionic).tw. (0)
16 ((intra?uter$ adj2 infusion$) and chorionic).tw. (2)
17 ((intra?uter$ adj2 ?instillation) and chorionic).tw. (5)
18 ((endometri$ adj2 injection$) and chorionic).tw. (4)
19 ((intra?uter$ adj2 injection$) and chorionic).tw. (10)
20 ((intra?uter$ adj2 administration) and chorionic).tw. (9)
21 ((endometri$ adj2 administration) and chorionic).tw. (7)
22 (intrauter$ adj7 ?hcg).tw. (154)
23 (intra‐uter$ adj7 ?hcg).tw. (13)
24 (uter$ adj7 ?hcg).tw. (304)
25 or/10‐24 (5100)
26 9 and 25 (1371)
27 randomised controlled trial.pt. (415727)
28 controlled clinical trial.pt. (92036)
29 randomized.ab. (337724)
30 randomised.ab. (68893)
31 placebo.tw. (174138)
32 clinical trials as topic.sh. (179636)
33 randomly.ab. (243672)
34 trial.ti. (148881)
35 (crossover or cross‐over or cross over).tw. (66446)
36 or/27‐35 (1054341)
37 exp animals/ not humans.sh. (4140674)
38 36 not 37 (972043)
39 26 and 38 (284)
Appendix 4. EMBASE search strategy
OVID Platform
From inception to 10 November 2015
1 exp embryo transfer/ or exp fertilization in vitro/ or exp intracytoplasmic sperm injection/ (58694)
2 embryo$ transfer$.tw. (14774)
3 in vitro fertili?ation.tw. (22851)
4 ivf‐et.tw. (2625)
5 icsi.tw. (11364)
6 intracytoplasmic sperm injection$.tw. (7117)
7 (blastocyst adj2 transfer$).tw. (1412)
8 (ivf or et).tw. (563610)
9 or/1‐8 (601227)
10 (Human Chorionic Gonadotrop?in adj7 intrauter$).tw. (96)
11 (Human Chorionic Gonadotrop?in adj7 uter$).tw. (133)
12 (intrauter$ adj7 ?hcg).tw. (230)
13 chorionic gonadotropin/dt, ut [Drug Therapy, Intrauterine Drug Administration] (4564)
14 (uter$ adj3 ?hcg).tw. (116)
15 ((endometri$ adj2 infusion$) and chorionic).tw. (2)
16 ((endometri$ adj2 ?instillation) and chorionic).tw. (0)
17 ((intra?uter$ adj2 infusion$) and chorionic).tw. (3)
18 ((intra?uter$ adj2 ?instillation) and chorionic).tw. (5)
19 ((endometri$ adj2 injection$) and chorionic).tw. (5)
20 ((intra?uter$ adj2 injection$) and chorionic).tw. (29)
21 ((intra?uter$ adj2 administration) and chorionic).tw. (22)
22 ((endometri$ adj2 administration) and chorionic).tw. (12)
23 or/10‐22 (5050)
24 9 and 23 (2018)
25 Clinical Trial/ (852930)
26 Randomized Controlled Trial/ (388340)
27 exp randomization/ (68781)
28 Single Blind Procedure/ (21262)
29 Double Blind Procedure/ (124741)
30 Crossover Procedure/ (45104)
31 Placebo/ (266177)
32 Randomi?ed controlled trial$.tw. (126646)
33 Rct.tw. (18757)
34 random allocation.tw. (1466)
35 randomly allocated.tw. (23611)
36 allocated randomly.tw. (2073)
37 (allocated adj2 random).tw. (741)
38 Single blind$.tw. (16599)
39 Double blind$.tw. (156489)
40 ((treble or triple) adj blind$).tw. (502)
41 placebo$.tw. (223655)
42 prospective study/ (313729)
43 or/25‐42 (1520537)
44 case study/ (34667)
45 case report.tw. (294447)
46 abstract report/ or letter/ (944413)
47 or/44‐46 (1266917)
48 43 not 47 (1480399)
49 24 and 48 (631)
Appendix 5. CINAHL search strategy
EBSCO Platform
From inception to 10 November 2015
| # | Query | Results |
| S15 | S8 AND S14 | 41 |
| S14 | S9 OR S10 OR S11 OR S12 OR S13 | 1,464 |
| S13 | TX(Chorionic Gonadotrop?in N7 intrauter*) | 0 |
| S12 | TX(Chorionic Gonadotrop?in N7 uter*) | 2 |
| S11 | TX(Human Chorionic Gonadotrop?in N7 intrauter*) | 967 |
| S10 | TX(Human Chorionic Gonadotrop?in N7 intrauter*) | 0 |
| S9 | (MM "Gonadotropins, Chorionic") | 496 |
| S8 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 | 3,690 |
| S7 | TX embryo* N3 transfer* | 754 |
| S6 | TX ovar* N3 hyperstimulat* | 334 |
| S5 | TX ovari* N3 stimulat* | 243 |
| S4 | TX IVF or TX ICSI | 1,234 |
| S3 | (MM "Fertilization in Vitro") | 1,435 |
| S2 | TX vitro fertilization | 2,821 |
| S1 | TX vitro fertilisation | 265 |
Appendix 6. PsycINFO search strategy
OVID Platform
From inception to 10 November 2015
1 exp reproductive technology/ (1380)
2 in vitro fertili?ation.tw. (567)
3 icsi.tw. (50)
4 intracytoplasmic sperm injection$.tw. (42)
5 (blastocyst adj2 transfer$).tw. (4)
6 (embryo$ adj2 transfer$).tw. (122)
7 or/1‐6 (1591)
8 exp Gonadotropic Hormones/ (3783)
9 (Human Chorionic Gonadotrop?in adj7 intrauter$).tw. (0)
10 (Human Chorionic Gonadotrop?in adj7 uter$).tw. (0)
11 (intrauter$ adj7 ?hcg).tw. (0)
12 (uter$ adj7 ?hcg).tw. (0)
13 or/8‐12 (3783)
14 7 and 13 (7)

Study flow diagram.
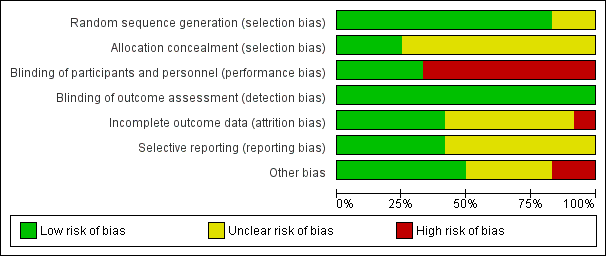
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, outcome: 1.1 Live birth.
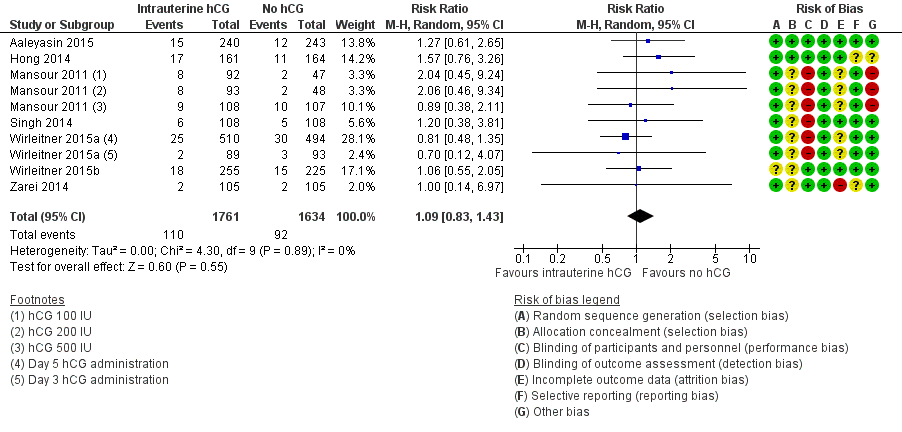
Forest plot of comparison: 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, outcome: 1.2 Miscarriage.

Forest plot of comparison: 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, outcome: 1.4 Clinical pregnancy.
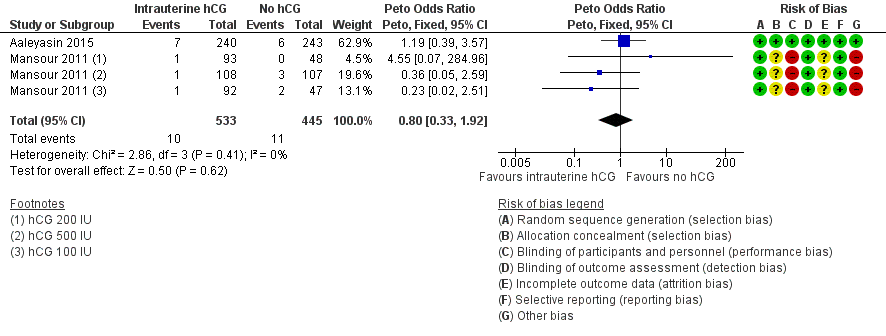
Forest plot of comparison: 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, outcome: 1.5 Complications: intrauterine death.
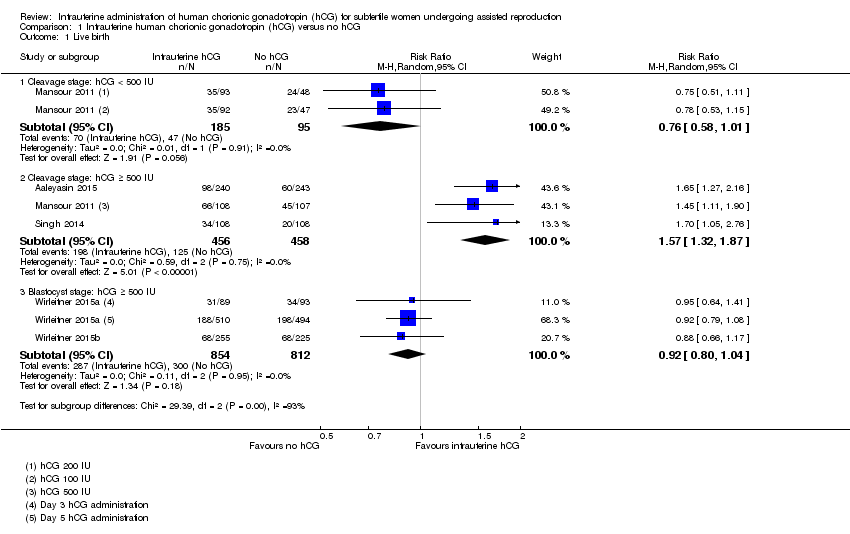
Comparison 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, Outcome 1 Live birth.
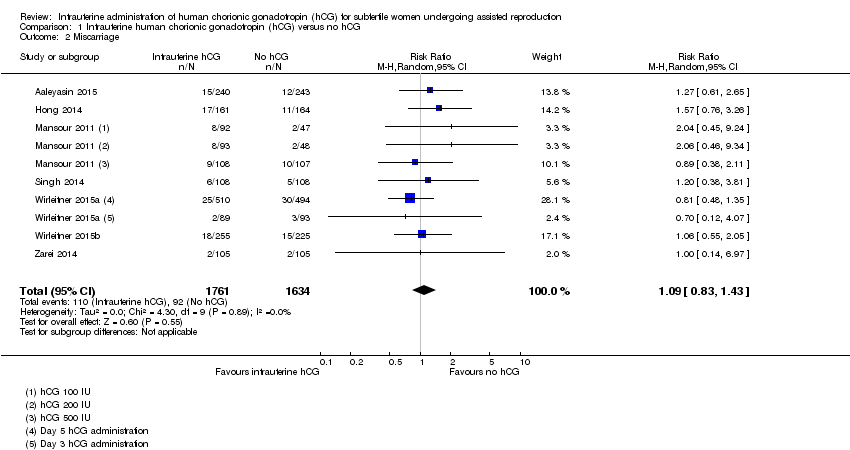
Comparison 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, Outcome 2 Miscarriage.
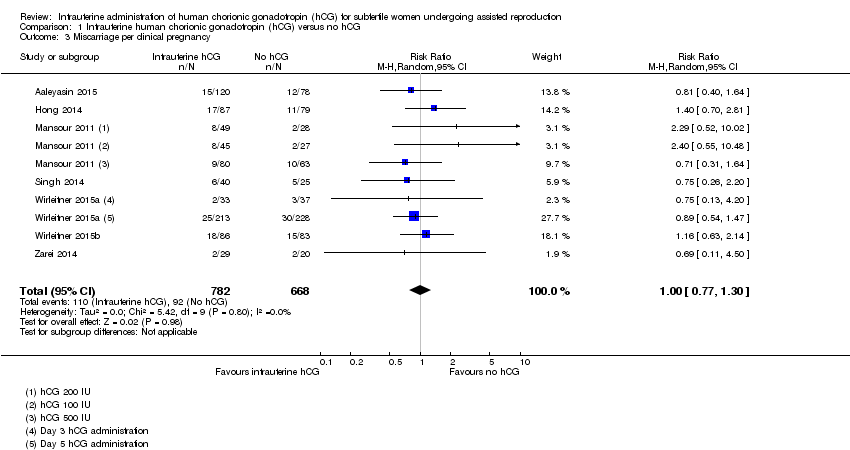
Comparison 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, Outcome 3 Miscarriage per clinical pregnancy.

Comparison 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, Outcome 4 Clinical pregnancy.

Comparison 1 Intrauterine human chorionic gonadotropin (hCG) versus no hCG, Outcome 5 Complications: intrauterine death.
| Intrauterine administration of hCG for women undergoing assisted reproduction | |||||
| Population: women undergoing assisted reproduction | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Control | Intrauterine administration of hCG | ||||
| Live birth ‐ cleavage stage: hCG < 500 IU | 495 per 1000 | 376 per 1000 | RR 0.76 | 280 | ⊕⊝⊝⊝ |
| Live birth ‐ cleavage stage: hCG ≥ 500 IU | 247 per 1000 | 388 per 1000 | RR 1.57 | 914 | ⊕⊕⊕⊝ |
| Live birth ‐ blastocyst stage: hCG ≥ 500 IU | 366 per 1000 | 337 per 1000 | RR 0.92 | 1666 | ⊕⊕⊕⊝ |
| Pregnancy ‐ cleavage stage: hCG < 500 IU | 579 per 1000 | 509 per 1000 (405 to 637) | RR 0.88 (0.70 to 1.10) | 280 | ⊕⊝⊝⊝ |
| Pregnancy ‐ cleavage stage: hCG ≥ 500 IU | 321 per 1000 | 453 per 1000 (401 to 507) | RR 1.41 (1.25 to 1.58) | 1414 (7 studies) | ⊕⊕⊕⊝ |
| Pregnancy ‐ blastocyst stage: hCG ≥ 500 IU | 430 per 1000 | 408 per 1000 (370 to 455) | RR 0.95 (0.86 to 1.06) | 1991 (3 studies) | ⊕⊕⊕⊝ |
| Miscarriage Follow‐up: mean 40 weeks | 48 per 1000 | 52 per 1000 (40 to 68) | RR 1.09 (0.83 to 1.43) | 3395 (7 studies) | ⊕⊝⊝⊝ |
| Other complications | Other complications reported in the included studies were ectopic pregnancy (3 studies, n = 915, 3 events overall), heterotopic pregnancy (1 study, n = 495, 1 event), intrauterine death (2 studies, n = 978, 21 events) and triplets (1 study, n = 48, 3 events). There were too few events to allow any conclusions to be drawn | ⊕⊝⊝⊝ | |||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Downgraded two levels due to very serious risk of bias: lack of blinding of participants and personnel, no clear description of allocation concealment and premature termination of the study following interim analysis. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Cleavage stage: hCG < 500 IU | 1 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.58, 1.01] |
| 1.2 Cleavage stage: hCG ≥ 500 IU | 3 | 914 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [1.32, 1.87] |
| 1.3 Blastocyst stage: hCG ≥ 500 IU | 2 | 1666 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.80, 1.04] |
| 2 Miscarriage Show forest plot | 7 | 3395 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.83, 1.43] |
| 3 Miscarriage per clinical pregnancy Show forest plot | 7 | 1450 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.77, 1.30] |
| 4 Clinical pregnancy Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Cleavage stage: hCG < 500 IU | 1 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.70, 1.10] |
| 4.2 Cleavage stage: hCG ≥ 500 IU | 7 | 1414 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [1.25, 1.58] |
| 4.3 Blastocyst stage: hCG ≥ 500 IU | 3 | 1991 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.86, 1.06] |
| 5 Complications: intrauterine death Show forest plot | 2 | 978 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.33, 1.92] |

