Administración de suplementos de vitamina D para embarazadas
Resumen
Antecedentes
La administración de suplementos de vitamina D durante el embarazo puede ser necesaria para proteger contra resultados adversos del embarazo. La presente revisión es una actualización de una revisión publicada por primera vez en 2012 y posteriormente en 2016.
Objetivos
Examinar si la administración de suplementos de vitamina D a las embarazadas, sola o combinada con calcio u otras vitaminas y minerales, puede mejorar de forma segura los resultados maternos y neonatales.
Métodos de búsqueda
Para esta actualización, se realizaron búsquedas en el Registro de Ensayos del Grupo Cochrane de Embarazo y Parto (Cochrane Pregnancy and Childbirth's Trials Register) (12 de julio de 2018), se estableció contacto con organizaciones pertinentes (15 de mayo de 2018), y se examinaron las listas de referencias de los ensayos recuperados y los registros en clinicaltrials.gov, así como en la WHO International Clinical Trials Registry Platform (12 de julio de 2018). Se incluyeron los resúmenes si tenían suficiente información para extraer los datos.
Criterios de selección
Ensayos aleatorios y cuasialeatorios que evaluaron el efecto de la administración de suplementos de vitamina D sola o en combinación con otros micronutrientes para las mujeres durante el embarazo en comparación con placebo o ninguna intervención.
Obtención y análisis de los datos
Dos autores de la revisión, de forma independiente: i) evaluaron la elegibilidad de los ensayos según los criterios de inclusión, ii) extrajeron los datos de los ensayos incluidos y iii) evaluaron el riesgo de sesgo de los ensayos incluidos. La certeza de la evidencia se evaluó según los criterios GRADE.
Resultados principales
Se incluyeron 30 ensayos (7033 mujeres), se excluyeron 60 ensayos, se identificaron seis como ensayos en curso/no publicados y dos ensayos están a la espera de evaluación.
Administración de suplementos de vitamina D sola versus placebo/ninguna intervención
En esta comparación se incluyeron en total 22 ensayos con 3725 embarazadas; se evaluó que 19 ensayos tuvieron riesgo de sesgo de bajo a moderado para la mayoría de los dominios y tres ensayos se evaluaron como riesgo de sesgo alto para la mayoría de los dominios. La administración de suplementos de vitamina D sola durante el embarazo probablemente reduce el riesgo de preeclampsia (cociente de riesgos [CR] 0,48; intervalo de confianza [IC] del 95%: 0,30 a 0,79; cuatro ensayos, 499 mujeres; evidencia de certeza moderada) y diabetes gestacional (CR 0,51; IC del 95%: 0,27 a 0,97; cuatro ensayos, 446 mujeres; evidencia de certeza moderada); y probablemente reduce el riesgo de tener un recién nacido con bajo peso al nacer (menos de 2500 g) (CR 0,55; IC del 95%: 0,35 a 0,87); cinco ensayos, 697 mujeres, evidencia de certeza moderada) en comparación con las mujeres que recibieron placebo o ninguna intervención. La administración de suplementos de vitamina D puede hacer poca o ninguna diferencia con respecto al riesgo de tener un parto prematuro < 37 semanas en comparación con ninguna intervención o placebo (RR 0,66; IC del 95%: 0,34 a 1,30; siete ensayos, 1640 mujeres, evidencia de certeza baja). En cuanto a los eventos adversos maternos, la administración de suplementos de vitamina D puede reducir el riesgo de hemorragia posparto grave (RR 0,68; IC del 95%: 0,51 a 0,91; un ensayo, 1134 mujeres, evidencia de certeza baja). No hubo casos de hipercalcemia (un ensayo, 1134 mujeres, evidencia de certeza baja) y no se conoce con certeza si la vitamina D aumenta o disminuye el riesgo de síndrome nefrítico (RR 0,17; IC del 95%: 0,01 a 4,06; un ensayo, 135 mujeres, evidencia de certeza muy baja). Sin embargo, no es posible establecer conclusiones firmes debido a la escasez de datos en general sobre los eventos adversos maternos.
Administración de suplementos de vitamina D y calcio versus placebo/ninguna intervención
En esta comparación se incluyeron nueve ensayos con 1916 embarazadas; tres ensayos se evaluaron como bajo riesgo de sesgo para la asignación y el cegamiento, cuatro ensayos como alto riesgo de sesgo y dos con algunos componentes con bajo riesgo, alto riesgo o riesgo incierto. La administración de suplementos con vitamina D y calcio durante el embarazo probablemente reduce el riesgo de preeclampsia (RR 0,50; IC del 95%: 0,32 a 0,78; cuatro ensayos, 1174 mujeres, evidencia de certeza moderada). No está claro el efecto de la intervención sobre la diabetes gestacional (RR 0,33; IC del 95%: 0,01 a 7,84; un ensayo, 54 mujeres, evidencia de certeza muy baja); y bajo peso al nacer (menos de 2500 g) (RR 0,68; IC del 95%: 0,10 a 4,55; dos ensayos, 110 mujeres, evidencia de certeza muy baja) en comparación con las mujeres que recibieron placebo o ninguna intervención. La administración de suplementos de vitamina D y calcio durante el embarazo puede aumentar el riesgo de parto prematuro < 37 semanas en comparación con las mujeres que recibieron placebo o ninguna intervención (RR 1,52; IC del 95%: 1,01 a 2,28; cinco ensayos, 942 mujeres, evidencia de certeza baja). Ningún ensayo en esta comparación informó sobre los eventos adversos maternos.
Administración de suplementos con vitamina D + calcio + otras vitaminas y minerales versus calcio + otras vitaminas y minerales (pero sin vitamina D)
En esta comparación se incluyó un ensayo con 1300 participantes, el cual se evaluó como bajo riesgo de sesgo. No se evaluó la preeclampsia. La administración de suplementos con vitamina D + otros nutrientes puede hacer poca o ninguna diferencia con respecto al riesgo de parto prematuro < 37 semanas (RR 1,04; IC del 95%: 0,68 a 1,59; un ensayo, 1298 mujeres, evidencia de certeza baja), o bajo peso al nacer (menos de 2500 g) (RR 1,12; IC del 95%: 0,82 a 1,51; un ensayo, 1298 mujeres, evidencia de certeza baja). No está claro si hay alguna diferencia en el riesgo de diabetes gestacional (RR 0,42; IC del 95%: 0,10 a 1,73) o de eventos adversos maternos (sin eventos de hipercalcemia; RR de hipercalciuria 0,25; IC del 95%: 0,02 a 3,97; un ensayo, 1298 mujeres) porque se encontró que la certeza de la evidencia para ambos resultados fue muy baja.
Conclusiones de los autores
Se incluyeron 30 ensayos (7033 mujeres) en tres comparaciones separadas. Las evaluaciones mediante GRADE variaron de moderadas a muy bajas, y las decisiones de disminuir la calidad se basaron en las limitaciones en el diseño del estudio, la imprecisión y la falta de direccionalidad.
Administrar a las embarazadas suplementos de vitamina D sola probablemente reduzca el riesgo de preeclampsia, diabetes gestacional y bajo peso al nacer, y podría reducir el riesgo de hemorragia posparto grave. Puede tener una influencia escasa o nula en el riesgo de tener un parto prematuro de <37 semanas de gestación. La administración de suplementos de vitamina D y calcio a las embarazadas probablemente reduce el riesgo de preeclampsia, pero puede aumentar el riesgo de parto prematuro de < 37 semanas (estos resultados justifican estudios de investigación adicionales). La administración de suplementos de vitamina D y otros nutrientes a las embarazadas puede hacer poca o ninguna diferencia con respecto al riesgo de parto prematuro < 37 semanas de gestación o bajo peso al nacer (menos de 2500 g). Se necesitan otros ensayos aleatorios rigurosos, de alta calidad y más grandes para evaluar los efectos de la administración de suplementos de vitamina D durante el embarazo, en particular en relación con el riesgo de eventos adversos maternos.
PICO
Resumen en términos sencillos
La administración de suplementos de vitamina D, ¿es beneficiosa o perjudicial para las mujeres durante el embarazo?
¿Cuál es el problema?
No está claro si los suplementos de vitamina D, solos o combinados con calcio u otras vitaminas y minerales durante el embarazo tienen efectos beneficiosos o perjudiciales para la madre o su hijo.
¿Por qué es esto importante?
La vitamina D es esencial para la salud humana, en particular para los huesos, la contracción muscular, la conducción nerviosa y la función celular en general. Las bajas concentraciones de vitamina D en la sangre de las embarazadas se han asociado con complicaciones del embarazo. Se piensa que podría ser necesario incorporar vitamina D a través de suplementos durante el embarazo para proteger contra las complicaciones del embarazo.
¿Qué se estudió en la revisión?
La presente revisión es una actualización de una revisión publicada por primera vez en 2012 y actualizada posteriormente en 2016. Esta revisión evaluó el efecto de la administración de suplementos de vitamina D durante el embarazo, sola o combinada con otros micronutrientes, en comparación con placebo o ninguna intervención, independientemente de la dosis, la duración o el momento de inicio de la administración de suplementos o del tipo de suplementos (orales o inyectables).
¿Qué evidencia se encontró?
Se realizaron búsquedas de la evidencia (julio de 2018) y se encontraron 30 ensayos (con 7033 mujeres) para su inclusión en esta actualización.
La evidencia de 22 ensayos que incluyeron 3725 embarazadas indica que la administración de suplementos de vitamina D sola durante el embarazo probablemente reduce el riesgo de preeclampsia, diabetes gestacional y el riesgo de tener un recién nacido con bajo peso al nacer en comparación con placebo o ninguna intervención, y puede hacer poca o ninguna diferencia con respecto al riesgo de tener un parto prematuro. Puede reducir el riesgo de eventos adversos maternos como la hemorragia posparto grave, aunque debe tenerse en cuenta que este resultado fue inesperado y se basó en un único ensayo.
La evidencia de nueve ensayos con 1916 embarazadas indica que la administración de suplementos de vitamina D y calcio probablemente reduce el riesgo de preeclampsia, pero puede aumentar el riesgo de parto prematuro. Este ligero daño potencial merece consideración en el caso de las mujeres que reciben suplementos de calcio como parte de la atención prenatal.
La evidencia de un estudio en el que participaron 1300 embarazadas indica que la administración de suplementos de vitamina D más otros nutrientes puede hacer poca o ninguna diferencia con respecto al riesgo de la mayoría de los resultados evaluados.
En la mayoría de los ensayos faltaron datos sobre los eventos adversos maternos.
¿Qué significa esto?
El hecho de administrarles a las embarazadas suplementos de vitamina D sola probablemente reduce el riesgo de preeclampsia, diabetes gestacional, bajo peso al nacer y hemorragia posparto grave. Puede tener una influencia escasa o nula en el riesgo de tener un parto prematuro de <37 semanas de gestación. La administración de suplementos de vitamina D y calcio a las embarazadas probablemente reduce el riesgo de preeclampsia, pero puede aumentar el riesgo de parto prematuro de < 37 semanas (estos resultados justifican estudios de investigación adicionales). La administración de suplementos de vitamina D y otros nutrientes a las embarazadas puede hacer poca o ninguna diferencia con respecto al riesgo de parto prematuro o bajo peso al nacer (menos de 2500 g) y los efectos de la diabetes gestacional y los eventos adversos maternos no están claros. Se necesitan otros ensayos aleatorios rigurosos, de alta calidad y más grandes para evaluar los efectos de la administración de suplementos de vitamina D durante el embarazo, en particular en relación con el riesgo de eventos adversos maternos.
Conclusiones de los autores
Summary of findings
| Vitamin D supplementation compared to placebo/control for pregnancy and neonatal health outcomes | ||||||
| Patient or population: pregnant women and their infants. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo/control | Risk with vitamin D supplementation | |||||
| Pre‐eclampsia | Study population | RR 0.48 (0.30, 0.79) | 499 | ⊕⊕⊕⊝ | Included trials: Asemi 2013a; Naghshineh 2016; Sablok 2015; Sasan 2017 | |
| 168 per 1000 | 79 per 1000 | |||||
| Gestational diabetes | Study population | RR 0.51 | 446 | ⊕⊕⊕⊝ | Included trials: Asemi 2013a; Sablok 2015; Shahgheibi 2016; Tehrani 2014 | |
| 127 per 1000 | 65 per 1000 | |||||
| Maternal adverse events: severe postpartum haemorrhage | Study population | RR 0.68 | 1134 | ⊕⊕⊝⊝ | Included trial: Harvey 2012 | |
| 158 per 1000 | 106 per 1000 | |||||
| Maternal adverse event: nephritic syndrome | Study population | RR 0.17 (0.01 to 4.06) | 135 (1 RCT) | ⊕⊝⊝⊝ | Included trial: Yu 2008 | |
| 22 per 1000 | 4 per 1000 (0 to 90) | |||||
| Maternal adverse event: hypercalcaemia | Study population | Not estimable | 1134 | ⊕⊕⊝⊝ | Included trial: Harvey 2012 | |
| 0 per 1000 | 0 per 1000 | |||||
| Preterm birth (less than 37 weeks' gestation) | Study population | RR 0.66 (0.34 to 1.30) | 1640 | ⊕⊕⊝⊝ | Included trials: Asemi 2013a; Delvin 1986; Grant 2013; Harvey 2012; Mirghafourvand 2013; Roth 2010; Singh 2015 | |
| 87 per 1000 | 57 per 1000 | |||||
| Low birthweight (less than 2500 g) | Study population | RR 0.55 | 697 | ⊕⊕⊕⊝ | Included trials: Brooke 1980; Bhutta 2011; Marya 1988; Roth 2010; Sablok 2015 | |
| 136 per 1000 | 75 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We downgraded (1) level for serious limitations in study design due to one trial being assessed as high risk of bias for several domains and two trials having unclear allocation concealment. 2 We downgraded (1) level for serious limitations in study design due to one trial being assessed as high risk of bias for several domains. 3 We downgraded (2) levels for very serious limitations in study design due to one study being assessed as high risk of other bias because we do not know the impact of the participants who were allowed to continue taking their own multivitamin with 400 IU/d of vitamin D as this was not recorded. 4 We downgraded (1) level for serious limitations in study design due to one study being assessed as high risk of bias for performance and detection bias. 5 We downgraded (2) levels for very serious limitations in imprecision as only one small study, with a small number of events and wide 95% confidence intervals (CI) contributed data. 6 We downgraded (1) level for serious limitations in imprecision due to a single study with zero events contributing data. 7 We downgraded (1) level for serious limitations in study design due to two studies being at unclear risk of selection bias and one study being at high risk of other bias. 8 We downgraded (1) level for serious limitations in imprecision as the 95% confidence interval (CI) was wide and crossed the line of no effect. 9 We downgraded (1) level for serious limitations in study design due to two studies being at unclear risk of selection bias, one study being at high risk of bias for allocation concealment, and three studies being at high risk of attrition bias. | ||||||
| Vitamin D + calcium supplementation compared to placebo/control for pregnancy and neonatal health outcomes | |||||||
| Patient or population: pregnant women and their infants.. | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | ||
| Risk with placebo/control | Risk with vitamin D + calcium supplementation | ||||||
| Pre‐eclampsia | Study population | RR 0.50 | 1174 | ⊕⊕⊕⊝ | Included trials: Asemi 2012; Marya 1987; Samimi 2016; Taherian 2002 | ||
| 94 per 1000 | 47 per 1000 | ||||||
| Gestational diabetes | Study population | RR 0.33 | 54 | ⊕⊝⊝⊝ | Included trial: Asemi 2012 | ||
| 37 per 1000 | 12 per 1000 | ||||||
| Maternal adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | No trials reported on this outcome | |
| Preterm birth (less than 37 weeks' gestation) | Study population | RR 1.52 | 942 | ⊕⊕⊝⊝ | Included trials: Asemi 2012; Diogenes 2013, Mirghafourvand 2013, Samimi 2016; Taherian 2002; | ||
| 72 per 1000 | 110 per 1000 | ||||||
| Low birthweight (less than 2500 g) | Study population | RR 0.68 | 110 | ⊕⊝⊝⊝ | Included trials: Diogenes 2013; Samimi 2016 | ||
| 59 per 1000 | 40 per 1000 | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1 We downgraded (1) level for serious limitations in study design due to one study being at high risk of attrition and selection bias and three studies being at high risk of performance and detection bias. 2 We downgraded (1) level for serious limitations in study design due to one study being at high risk of performance and detection bias. 3 We downgraded (2) levels for very serious limitations in imprecision due to one small study, with a single event and wide 95% confidence intervals (CI) crossing the line of no effect contributing data. 4 We downgraded (1) level for serious limitations in study design due to three studies being at unclear risk of allocation concealment and three studies being at high risk of performance and detection bias. 5 We downgraded (1) level for serious limitations in imprecision due to wide 95% confidence intervals (CI). 6 We downgraded (1) level for serious limitations in study design due to one study being at unclear risk of allocation concealment and one study being at high risk of attrition bias. 7 We downgraded (2) levels for very serious limitations in imprecision due two small studies, with very few events and wide 95% confidence intervals (CI) crossing the line of no effect contributing data. | |||||||
| Vitamin D + calcium + other vitamins and minerals compared to calcium + other vitamins and minerals (but no vitamin D) for pregnancy and neonatal health outcomes | ||||||
| Patient or population: pregnant women and their infants.. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with calcium + other vitamins and minerals (but no vitamin D) | Risk with vitamin D + calcium + other vitamins and minerals | |||||
| Pre‐eclampsia | Study population | ‐ | (0 trials) | ‐ | No trials reported on this outcome | |
| see comment | see comment | |||||
| Gestational diabetes | Study population | RR 0.42 | 1298 | ⊕⊝⊝⊝ | Included trial: Roth 2013 | |
| 12 per 1000 | 5 per 1000 | |||||
| Maternal adverse event: hypercalcaemia | Study population | ‐ | 1298 | ⊕⊝⊝⊝ | Included trial: Roth 2013 | |
| 23 per 1000 | 64 per 1000 | |||||
| Maternal adverse event: hypercalciuria | Study population | 0.25 (0.02 to 3.97) | 1298 | ⊕⊝⊝⊝ | Included trial: Roth 2013 | |
| 4 per 1000 | 1 per 1000 (0 to 15) | |||||
| Preterm birth (less than 37 weeks' gestation) | Study population | RR 1.04 | 1298 | ⊕⊕⊝⊝ | Included trial: Roth 2013 | |
| 93 per 1000 | 96 per 1000 | |||||
| Low birthweight (less than 2500 g) | Study population | RR 1.12 | 1298 | ⊕⊕⊝⊝ | Included trial: Roth 2013 | |
| 162 per 1000 | 182 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We downgraded (2) levels for very serious limitations in imprecision with only one trial, with few events, and wide 95% confidence intervals (CI) crossing the line of no effect contributing data. 2 We downgraded (1) level for serious indirectness as there were multiple nutrient interventions in addition to vitamin D. 2 We downgraded (2) levels for very serious limitations in imprecision with only one trial, with zero events, and wide 95% confidence intervals (CI) crossing the line of no effect contributing data. 3 We downgraded (1) level for serious limitations in imprecision due to only one trial with wide 95% confidence intervals (CI) crossing the line of no effect contributing data. | ||||||
Antecedentes
Descripción de la afección
Metabolismo de la vitamina D
La vitamina D es una vitamina liposoluble que proviene principalmente de la exposición a la luz solar, y se encuentra de forma natural solo en unos pocos alimentos como los aceites de hígado de pescado, el pescado graso, las setas, las yemas de huevos y el hígado (Holick 2007a; Holick 2008). Hay dos formas fisiológicamente activas de la vitamina D, llamadas en conjunto calciferol: D2 y D3. La vitamina D2 (también llamada ergocalciferol) es sintetizada por las plantas, mientras que la vitamina D3 (también llamada colecalciferol) es producida por el tejido subcutáneo de los seres humanos a partir del 7‐dehidrocolecalciferol con la exposición a la radiación de la luz ultravioleta B (UVB) (DeLuca 2004). La vitamina D en los suplementos se encuentra como vitamina D2 o D3. Esta última puede ser tres veces más efectiva que la vitamina D2para aumentar las concentraciones séricas de vitamina D y mantener esos niveles durante más tiempo, particularmente durante los meses de invierno; además, sus metabolitos tienen una afinidad superior con las proteínas que se unen a la vitamina D en el plasma (Armas 2004; Logan 2013; McCullough 2007). Como la vitamina D tiene una vida media corta, la ingesta suficiente de vitamina D es necesaria para asegurar que se mantengan los niveles circulantes.
Las formas D2 y D3 comparten un metabolismo similar. Primero son hidroxiladas en el hígado para formar 25‐hidroxivitamina D (25[OH]D o calcidiol) y luego en el riñón pasa a 1,25‐dihidroxilo vitamina D (1,25 [OH]2 D o calcitriol) en respuesta a los niveles de la hormona paratiroidea (PTH). El calcitriol se considera una prehormona importante y sus metabolitos activos participan en procesos metabólicos que incluyen la integridad ósea y la homeostasis del calcio (Wagner 2008).
Los principales sitios de acción de la vitamina D incluyen la piel, el intestino, los huesos, la glándula paratiroidea, el sistema inmunológico y el páncreas, así como el intestino delgado y el colon en el feto humano (Theodoropoulos 2003). Además, la vitamina D ayuda a mantener los niveles normales de la glucosa en sangre por la unión y la activación del receptor de vitamina D en las células beta pancreáticas; además, regula la liberación de insulina en respuesta al nivel de glucosa circulante (Clifton‐Bligh 2008; Maghbooli 2008; Palomer 2008; Xuan 2013). La vitamina D también afecta de manera indirecta al metabolismo de la glucosa mediante la regulación de la homeostasis del calcio (Xuan 2013).
Hay una relación única entre la vitamina D y el calcio. La PTH se encarga de elevar la concentración de calcio en sangre mediante la resorción ósea, mientras el calcitriol inhibe la PTH y permite un aumento de la concentración sérica del calcio de fuentes diferentes del hueso. En presencia de calcitriol la absorción renal e intestinal de calcio y fósforo aumenta, lo que da lugar a un mejor estado del calcio.
Estado de la vitamina D
El calcidiol sérico o 25‐hidroxivitamina D se puede utilizar para evaluar el estado de la vitamina D, ya que refleja la suma de la vitamina D producida por la piel y la que se obtiene de los alimentos y de los suplementos (Jones 2008). Este metabolito es difícil de medir, con variaciones grandes entre los métodos y entre los laboratorios incluso cuando se utilizan los mismos métodos, lo que se puede explicar por las diferencias en las muestras antes del tratamiento o el sistema de extracción con disolvente utilizado (Hollis 2004; Lankes 2015).
Recientemente, el Institute of Medicine (IOM) definió el estado adecuado de la vitamina D como tener concentraciones séricas de 25‐hidroxivitamina D mayores que 50 nmol/l (o 20 ng/ml) en la población general y en las embarazadas (IOM 2011). Algunos investigadores proponen que las concentraciones alrededor de 80 nmol/l (32 ng/ml) son óptimas, ya que suprimen los niveles de PTH y dan lugar a una mayor absorción de calcio y a una mayor masa ósea, lo que reduce las tasas de pérdida ósea, las caídas y las fracturas (Dawson‐Hughes 2005; Dawson‐Hughes 2008). No está claro si estos niveles más altos propuestos para los adultos no embarazados también son adecuados para las embarazadas.
El estado de la vitamina D se ve afectado por factores que regulan su producción en la piel (es decir, la pigmentación de la piel, la latitud, los códigos para vestir, la estación del año, el envejecimiento, el uso de filtros solares y la contaminación del aire) y por factores que afectan su absorción o metabolismo (Holick 2007b; Maghbooli 2007). La melanina actúa como un filtro para los rayos ultravioletas (UV), por lo que reduce la producción de vitamina D por la piel. Las poblaciones hispanas y negras en los Estados Unidos pueden tener un mayor contenido de melanina y, por lo tanto, una reducción de la fotosíntesis de vitamina D (síntesis endógena por exposición a la luz solar) (Clemens 1982), lo que explica las variaciones en las concentraciones de vitamina D entre los grupos étnicos que viven en las mismas áreas geográficas (Brooke 1980; Egan 2008; Ganji 2012; Matsuoka 1991; Nesby‐O'Dell 2002; Rockell 2005). El fototipo de la piel de un individuo refleja el grado de quemadura solar versus el bronceado después de una exposición inicial moderada al sol, luego de un período prolongado de poca o ninguna exposición (Gilchrest 2008). Los fototipos I y II tienen una fotosíntesis rápida de vitamina D después de una dosis eritemática mínima (DEM). Por el contrario, el prototipo VI tiene una fotosíntesis pequeña de vitamina después de la misma DEM (Clemens 1982). También se ha mostrado que las diferencias en la latitud influyen en la concentración de vitamina D, y los individuos de los países en latitudes altas y bajas tienen niveles inferiores de vitamina D. La importancia de los rayos ultravioletas queda más en evidencia por la variación estacional en la concentración de vitamina D entre el verano y el invierno, con niveles más altos durante el verano en comparación con los meses de invierno (Holick 2007b; Levis 2005). El metabolismo de la vitamina D también está afectado en los individuos con obesidad, ya que la vitamina D se deposita en las reservas de grasa del cuerpo, haciéndola menos biodisponible (Arunabh 2003). Más recientemente, este bajo nivel de vitamina D en los individuos obesos se ha explicado por una simple dilución volumétrica de la vitamina D en la masa grasa (Drincic 2012), lo que da lugar a una mayor prevalencia de bajos niveles de 25‐hidroxivitamina D, que son más prevalentes entre los individuos con sobrepeso y obesos en comparación con los individuos de peso normal (Vilarrasa 2007; Vimaleswaran 2013; Wortsman 2000). En el mismo contexto, la actividad sedentaria también se asocia con niveles de vitamina D bajos, ya que puede estar vinculada con una menor exposición a la luz solar (Ohta 2009).
Magnitud de la deficiencia de vitamina D
La deficiencia de vitamina D puede ser un problema de salud frecuente en todo el mundo (Bandeira 2006; Palacios 2014; van Schoor 2011). Existe una alta prevalencia del estado de concentración baja de vitamina D en los lactantes, los niños, los adolescentes, los adultos y las personas de edad avanzada de todo el mundo, incluso en países con exposición al sol durante todo el año (Palacios 2014). La prevalencia más alta informada se encontró en el Oriente Medio, en particular en las niñas y las mujeres, aunque hay falta de datos de la mayoría de los países de América del Sur y de África.
En el embarazo, las bajas concentraciones de vitamina D en la sangre también son frecuentes. Una revisión que incluyó 17 ensayos en embarazadas y que lactaban (dos en América, seis en Europa, uno en África, siete en Asia, uno en Oceanía) (Palacios 2014) encontró una prevalencia de un estado bajo de vitamina D (definido como concentraciones inferiores a 50 nmol/l) del 33% en los EE.UU. y del 24% en embarazadas canadienses. En Europa, la prevalencia del estado de concentración baja de vitamina D fue del 45% en Bélgica, del 35% en el Reino Unido, del 44% en los Países Bajos, del 20% en España y del 77% en Alemania. Además, la prevalencia de la deficiencia de vitamina D (definida como < 30 nmol/l) fue del 12% en Bélgica, del 4% en Inglaterra y del 23% en los Países Bajos. El único estudio informado en África informó una prevalencia muy baja del estado bajo de vitamina D (1%) en una muestra de 139 embarazadas en Tanzania. En Asia, la prevalencia del estado bajo de vitamina D en las embarazadas fue muy alta: 90% en Turquía, 67% en Irán, el 72% en Pakistán, el 70% a 83% en Kuwait, el 96% en la India y el 69% en China. La prevalencia de deficiencia de vitamina D también fue muy alta: 50% en Turquía, 45% en Pakistán, 38% a 41% en Kuwait y el 60% en la India. En Australia se encontró un estado bajo de vitamina D en el 48% y se encontró deficiencia de vitamina D en el 15% de las embarazadas.
Más recientemente, una revisión que incluyó 13 ensayos de siete países encontró que la prevalencia de la deficiencia e insuficiencia de vitamina D varió entre el 39,4% y el 76,5% (van der Pligt 2018). También informaron solo sobre la deficiencia de vitamina D y encontraron la mayor prevalencia entre las mujeres chinas (100%), turcas (95,6%), iraníes (89,4%) y pakistaníes (89,0%).
La variación estacional aumenta el riesgo de bajas concentraciones de vitamina D en la sangre durante el embarazo, con una mayor prevalencia de bajas concentraciones de vitamina D en la sangre durante los meses de invierno en comparación con los meses de verano (Nicolaidou 2006; O'Riordan 2008). También se demostró que las diferencias en la latitud afectan la concentración de vitamina D en la mayoría de las embarazadas (Sloka 2009).
Estado de la vitamina D materna y resultados de salud
El estado de la vitamina D durante el embarazo es la etapa más importante del ciclo de vida, ya que el feto depende completamente de esta fuente durante este período para su desarrollo. Durante el embarazo, la 1,25‐dihidroxivitamina D aumenta de forma precoz durante el embarazo y este aumento continúa hasta el parto (Moller 2013). Este gran aumento de la 1,25‐dihidroxivitamina D al parecer depende de los niveles disponibles de 25‐dihidroxivitamina D, aunque es independiente del metabolismo del calcio, una característica única del embarazo que permite dichos niveles altos de 1,25‐dihidroxivitamina D (Pludowski 2013a). Por lo tanto, mantener niveles suficientemente altos de 25‐dihidroxivitamina D es fundamental para mantener niveles elevados de 1,25‐dihidroxivitamina D importantes durante el embarazo. Estos niveles aún no se han determinado, pero varios ensayos han demostrado que el estado de la vitamina D materna se asocia de manera significativa con el estado de vitamina D fetal y neonatal (El Koumi 2013; Sachan 2005), y que el estado de la vitamina D materna se asocia con los resultados de salud durante el embarazo y el desarrollo neonatal e infantil. Estas asociaciones se describirán a continuación.
Estado de la vitamina D y trastornos hipertensivos durante el embarazo
La deficiencia de vitamina D materna en el embarazo se ha asociado con un mayor riesgo de preeclampsia (hipertensión gestacional de reciente aparición y proteinuria después de las 20 semanas de gestación), una afección asociada con un aumento de la morbilidad y la mortalidad materna y perinatal (Bodnar 2007; Holick 2008; Li 2000b; MacKay 2001; Xiong 1999). Un metanálisis que incluyó ocho ensayos encontró una asociación significativa entre la deficiencia de vitamina D y el riesgo de preeclampsia, que fue más evidente en los ensayos que definieron la deficiencia de vitamina D como 25(OH)D de 50 nmol/l (20 ng/ml) y en los ensayos de los EE.UU. (Tabesh 2013). De manera similar, otro metanálisis que incluyó 31 ensayos también encontró un riesgo 78% mayor de preeclampsia en embarazadas con bajo nivel de vitamina D (odds ratio [OR] 1,79; intervalo de confianza [IC] del 95%: 1,25 a 2,58) (Aghajafari 2013). Una revisión sistemática más reciente que incluyó 13 ensayos de siete países también encontró que la deficiencia de vitamina D durante el embarazo se asoció con preeclampsia en tres de cuatro ensayos (van der Pligt 2018).
Las mujeres con preeclampsia tienen concentraciones más bajas de 25‐hidroxivitamina D en comparación con las mujeres con presión arterial normal (Díaz 2002; Frenkel 1991; Halhali 1995; Halhali 2000; Tolaymat 1994). Los bajos niveles de calcio urinario (hipocalciuria) en las pacientes con preeclampsia se pueden deber a una reducción en la absorción intestinal de calcio, afectada por los bajos niveles de vitamina D (August 1992; Halhali 1995). Además, la preeclampsia y las bajas concentraciones de vitamina D en la sangre se asocian directa e indirectamente a través de mecanismos biológicos como la disfunción inmunológica, la implantación de la placenta, la angiogénesis anormal, la inflamación excesiva y la hipertensión (Bodnar 2007; Cardus 2006; Evans 2004; Hewison 1992; Li 2002). La vitamina D puede afectar el desarrollo placentario precoz y, por lo tanto, el desarrollo de preeclampsia a través de su función en la regulación y la expresión de los genes; sin embargo, se necesitan más estudios para confirmar este hallazgo.
Estado de la vitamina D y otras afecciones maternas
Las bajas concentraciones de vitamina D en la sangre al comienzo del embarazo se han asociado con un aumento del riesgo de diabetes mellitus gestacional (Farrant 2009; Zhang 2008). Un metanálisis de 31 ensayos observacionales encontró que los bajos niveles de vitamina D aumentaron el riesgo de diabetes gestacional en el 49% (odds ratio [OR] 1,49; intervalo de confianza [IC] del 95%: 1,18 a 1,89) (Aghajafari 2013). Se hallaron resultados similares en otro metanálisis de 24 estudios observacionales (Wei 2013). El control deficiente de la diabetes materna durante el primer trimestre del embarazo guarda una relación inversa con el bajo contenido mineral óseo en los lactantes, al igual que con el estado de baja concentración de vitamina D materna (Namgunga 2003). La deficiencia de vitamina D (DVD) puede provocar un recambio óseo alto, pérdida ósea, osteomalacia (reblandecimiento de los huesos) y miopatía (debilidad muscular) en la madre, además de DVD neonatal e infantil (El Koumi 2013; Glerup 2000; Lips 2001).
Un estado adecuado de vitamina D también puede proteger contra otros resultados adversos en el embarazo. Por ejemplo, la deficiencia de vitamina D materna se ha relacionado con la cesárea (Merewood 2009; Scholl 2012), pero los mecanismos involucrados no están claros. Se ha indicado que la deficiencia de vitamina D durante el embarazo puede reducir la fuerza y el control muscular pelviano (Scholl 2012), pero es necesario confirmarlo.
Las concentraciones maternas prenatales y perinatales bajas de vitamina D pueden afectar la función de otros tejidos, lo que provoca un riesgo mayor de esclerosis múltiple, cáncer, diabetes mellitus dependiente de la insulina y esquizofrenia en etapas posteriores de la vida (McGrath 2001).
Estado de la vitamina D y parto prematuro y bajo peso al nacer
Se ha informado sobre una posible relación inversa entre el estado de la vitamina D materna y el parto prematuro (menos de 37 semanas de gestación) (Dawodu 2011; Morley 2006). Por el contrario, no todos los estudios muestran asociaciones significativas entre los niveles de calcidiol materno y cualquier medida del tamaño del niño al nacer ni durante los primeros meses de vida (Bodnar 2010; Farrant 2009; Gale 2008; Morley 2006).
Un metanálisis reciente de 24 estudios observacionales confirmó la asociación entre los niveles bajos de vitamina D (< 50 nmol/l) y un aumento en el riesgo de parto prematuro (OR 1,58; IC del 95%: 1,08 a 2,31) (Wei 2013). Además, dos metanálisis también encontraron asociaciones significativas entre el estado de baja concentración de vitamina D y el recién nacido pequeño para la edad gestacional (Theodoratou 2014; Wei 2013). Con respecto al peso al nacer, un metanálisis que incluyó tres estudios observacionales encontró una asociación positiva débil entre el estado de la vitamina D materna y el peso al nacer después de un ajuste por posibles factores de confusión (Harvey 2014), pero otro metanálisis que incluyó cuatro estudios observacionales sí encontró una asociación significativa entre estas variables (Theodoratou 2014). Una revisión sistemática más reciente que incluyó 13 estudios de siete países encontró que la deficiencia de vitamina D durante el embarazo se asoció con bajo peso al nacer en cuatro de siete estudios (van der Pligt 2018).
No hay mucha información sobre el estado de la vitamina D materna y el bajo peso al nacer o el parto prematuro en los niños nacidos de embarazadas con infección por VIH (Mehta 2009). Los estudios informaron una alta prevalencia de deficiencia de vitamina D entre las embarazadas con infección por VIH (Eckard 2013; Mave 2012).
Estado de la vitamina D y crecimiento posnatal
Algunos estudios observacionales indican que los niveles de vitamina D durante el embarazo afectan el desarrollo óseo fetal y el crecimiento de los niños (Bodnar 2010; Brooke 1980; Ioannou 2012; Mahon 2010; Morley 2006). Sin embargo, las asociaciones entre el estado de la vitamina D materna y el perímetro cefálico no son consistentes, como se encontró en una revisión sistemática de nueve estudios observacionales (Harvey 2014). Sin embargo, un estudio halló que el perímetro cefálico en niños de nueve años de edad se asoció de manera significativa con los niveles de calcidiol materno (Gale 2008). Con respecto al estado de la vitamina D materna y la masa ósea de los lactantes, los resultados tampoco son consistentes (Akcakus 2006; Harvey 2014; Javaid 2006; Viljakainen 2010).
No está claro si la deficiencia de vitamina D materna provoca raquitismo neonatal, ya que en general el raquitismo se identifica posteriormente en la niñez. Los estudios más antiguos indican un posible riesgo de raquitismo neonatal en los hijos de mujeres con osteomalacia, reblandecimiento anormal del hueso por deficiencia de fósforo, calcio o de vitamina D (Ford 1973). Los estudios más recientes encontraron que la deficiencia de vitamina D (concentraciones séricas menores de 25 nmol/l) se identificó en el 92% de los niños árabes con raquitismo y en el 97% de sus madres en comparación con el 22% de los niños sin raquitismo y el 52% de sus madres (Dawodu 2005). Se encontró una correlación positiva entre los niveles de vitamina D maternos e infantiles.
Además, los análisis que utilizan datos de embarazadas que participaron en la Southampton Women's Survey, un estudio longitudinal prospectivo, encontraron en los fetos de madres con bajo nivel de vitamina D una mayor área transversal metafisaria femoral y un mayor índice de separación femoral a las 19 y 34 semanas de gestación (Mahon 2010), así como una asociación significativa entre el volumen del fémur fetal y el estado de la vitamina D (Ioannou 2012), que se ha indicado que posiblemente se relaciona con el desarrollo de raquitismo temprano (Harvey 2014).
Estado de la vitamina D y respuesta inmunitaria
La vitamina D tiene efectos directos sobre los sistemas inmunológicos adaptativos e innatos (Miller 2010; Walker 2009). En los niños, la insuficiencia de vitamina D está vinculada con enfermedades autoinmunitarias como diabetes mellitus tipo 1, esclerosis múltiple, alergias y enfermedades atópicas (Bener 2009; Miller 2010; Pierrot‐Deseilligny 2010). Varios estudios también han demostrado que la deficiencia de vitamina D se asocia fuertemente con la tuberculosis, la neumonía y la fibrosis quística (Chocano‐Bedoya 2009; Hall 2010; Nnoaham 2008; Williams 2008), y la privación prenatal y perinatal de vitamina D podría influir en la morbilidad respiratoria en la primera infancia, ya que esta vitamina es importante para el crecimiento y desarrollo pulmonar (Devereux 2007; Litonjua 2009).
La vitamina D puede tener efectos positivos en el sistema inmunológico al aumentar la producción de péptidos antimicrobianos por parte de los macrófagos y las células endoteliales (Wang 2004), que pueden inactivar los virus y suprimir la inflamación (Cantorna 2008), y posteriormente reducir la gravedad de las infecciones.
Toxicidad de la vitamina D
El exceso de vitamina D provoca hipercalcemia (niveles de calcio de 10,5 mg/dl o mayores) e hipercalciuria (excreción urinaria de calcio superior a 250 mg/día en las mujeres), asociadas con cálculos renales y urinarios (Heaney 2008). La toxicidad en adultos aparece por lo general con dosis de vitamina D mayores de 10 000 unidades internacionales (UI)/d (250 µg/d), aunque la mayor parte de la evidencia se basa en exposiciones a corto plazo (menos de seis meses) (Hathcock 2007; Heaney 2008; IOM 2011; Vieth 1999). Los suplementos de dosis única que proporcionan 7,5 mg (300 000 UI) o más también pueden ser perjudiciales (Roth 2011a).
En algunos estudios en ratas y conejos se ha indicado la posibilidad de teratogénesis (defectos congénitos) inducida por la vitamina D y eventos adversos en la descendencia (p.ej. restricción del crecimiento, osificación retardada, hipoplasia craneofacial) (Ariyuki 1987; Chan 1979; Friedman 1969; Ornoy 1968; Ornoy 1969). Sin embargo, hay considerables limitaciones en la extrapolación de dichos resultados a los seres humanos, ya que supuestamente no han ocurrido efectos adversos fetales después de la ingestión materna de dosis de mantenimiento tan altas como 5 mg (200 000 UI) de vitamina D por día. En general, los estudios realizados en animales y en seres humanos indican que es poco probable que haya un exceso fetal de metabolitos de vitamina D cuando las concentraciones maternas se encuentran dentro de un rango normal (Roth 2011a).
Descripción de la intervención
La Organización Mundial de la Salud (OMS) actualmente no recomienda el suministro de suplementos de vitamina D durante el embarazo como parte de la atención prenatal habitual (OMS 2016), principalmente debido a la falta de evidencia y sólo en casos de DVD, lo que coincide con las guías del American Congress of Obstetricians and Gynecologists (ACOG 2015).
En la actualidad hay controversia respecto de los niveles de 25‐hidroxivitamina D que se consideran suficientes u óptimos para la salud general. El Institute of Medicine de los EE.UU. determinó que las concentraciones mayores de 50 nmol/l o 20 ng/ml son suficientes según los estudios actuales disponibles (IOM 2011), aunque muchos investigadores consideran que los niveles óptimos deben ser mayores (mayor de 75 nmol/l o 30 ng/ml) (Dawson‐Hughes 2005; Hollick 2009). Las recomendaciones de vitamina D para mantener niveles adecuados de 25‐hidroxivitamina D también varían entre las diferentes organizaciones. La ingesta de nutrientes recomendada (INR) establecida por la Organización de las Naciones Unidas para la Agricultura y la Alimentación (FAO) es de 200 UI/día (5 mcg/día) de vitamina D para las embarazadas (OMS 2004). La European Food Safety Authority (EFSA) y el Institute of Medicine de los EE.UU. recomiendan 600 UI/día (15 mcg/día) de vitamina D para las embarazadas (EFSA 2016; IOM 2011). El Royal College of Obstetricians and Gynaecologists recomienda 400 UI/día (10 mcg/día) para todas las embarazadas (RCOG 2014). Para las mujeres de alto riesgo (piel oscura, exposición reducida a la luz solar, o las que están socialmente excluidas u obesas), recomiendan al menos 1000 UI/día (25 mcg/día). Además, para las mujeres con alto riesgo de preeclampsia recomiendan al menos 800 UI/día (20 mcg/día), en combinación con calcio. Un panel de expertos en Europa Central recomendó 1500 a 2000 IU/día (37,5 a 50,0 mcg/día) (Pludowski 2013b).
Las recomendaciones sobre el uso de suplementos de vitamina D durante el embarazo también varían, y van de 200 a 400 UI/día (5 a 10 mcg/día) (Canadian Paediatric Society 2007; UK Department of Health 2009). La American Academy of Pediatrics (Wagner 2008) indica que los profesionales sanitarios que proporcionen atención obstétrica deben considerar monitorizar el estado de vitamina D materna mediante la medición de las concentraciones en las embarazadas. Diferentes investigadores han indicado que una dosis suplementaria de vitamina D de 1000 a 1600 UI (25 a 40 mcg/día) podría ser necesaria para lograr el nivel óptimo de esta vitamina en el cuerpo (Dawson‐Hughes 2005). Se espera que esta dosis aumente la cantidad de 25‐hidroxivitamina D en suero en 1,2 nmol/l por cada mcg (40 UI) de vitamina 3 administrada por vía oral a individuos con niveles bajos de 25‐hidroxivitamina D; los que tenían concentraciones iniciales más altas tendrían incrementos más pequeños con la misma dosis (Dawson‐Hughes 2005). Otros han indicado que se pueden necesitar dosis de alrededor de 1000 UI/día para que las embarazadas mantengan una concentración sanguínea de vitamina D de más de 50 nmol/l (20 ng/ml) (Heaney 2003; Hollis 2004; Hollis 2007; Vieth 2001). También se han indicado dosis más altas, como dosis semanales de 5000 IU (125 mcg/semana) (Utiger 1998) o una dosis única de 200 000 IU (5 mg) o más (Mallet 1986; Sahu 2009; Yu 2009).
Debido a que la vitamina D también puede ser sintetizada por la piel con la exposición a la luz solar, se ha recomendado un aumento eventual de la exposición al sol para alcanzar concentraciones séricas óptimas (Holick 2002). Sin embargo, como la radiación ultravioleta excesiva es un carcinógeno, quizás valga la pena obtener vitamina D adicional de los alimentos o los suplementos.
De qué manera podría funcionar la intervención
La administración de suplementos de vitamina D ha demostrado mejorar el estado de la vitamina D materna durante el embarazo en algunos estudios (Delvin 1986; Yu 2009), lo que a su vez puede tener una influencia directa en el suministro fetal y neonatal de vitamina D (Brooke 1980). Se ha indicado un posible efecto de la administración de suplementos de vitamina D durante el embarazo para prevenir el parto prematuro (menos de 37 semanas de gestación) y el bajo peso al nacer (menos de 2500 g) (Maxwell 1981), aunque hay información limitada sobre el efectos beneficioso adicional de la administración de suplementos de vitamina D sobre otras intervenciones nutricionales durante el embarazo, como la administración de suplementos de hierro y ácido fólico, sobre el riesgo de bajo peso al nacer (Christian 2003). También hay un posible efecto de la administración materna de suplementos de vitamina D sobre el crecimiento neonatal (Marya 1988). La administración de suplementos de vitamina D durante el embarazo puede ser necesaria para asegurar las concentraciones suficientes de vitamina D en la leche materna durante la lactancia (Butte 2002). Sin embargo, es importante señalar que es posible observar efectos beneficiosos si la administración de suplementos comienza temprano en el embarazo, ya que hay evidencia que indica que el estado de vitamina D en las primeras etapas del embarazo es un determinante importante de los resultados de la salud materna y neonatal (Karras 2018).
Por qué es importante realizar esta revisión
Actualmente la mayoría de los países no incluyen la administración de suplementos de vitamina D como parte de su atención prenatal habitual. Según lo establecido por el Grupo de Trabajo convocado por el Sackler Institute for Nutrition Science en la New York Academy of Sciences y la Bill & Melinda Gates Foundation (en coordinación con un comité organizador científico para evaluar la prevalencia mundial y la carga de morbilidad de la deficiencia de vitamina D), la vitamina D afecta los resultados del embarazo y el parto, pero la evidencia es contradictoria (Roth 2018).
La presente revisión actualiza la revisión Cochrane anterior sobre la administración de suplementos de vitamina D en el embarazo (De‐Regil 2016). La revisión de 2016 incluyó 15 ensayos (2833 mujeres) y concluyó que la administración de suplementos de vitamina D a las embarazadas puede reducir el riesgo de preeclampsia, bajo peso al nacer y parto prematuro. Sin embargo, cuando se combinaron la vitamina D y el calcio puede aumentar el riesgo de parto prematuro. La presente revisión incorpora nueva evidencia de ensayos que evalúan los efectos y la seguridad de la administración de suplementos de vitamina D en el embarazo para el bienestar de la madre y el recién nacido. Los resultados de la presente revisión podrían contribuir a establecer guías prácticas a nivel poblacional.
También se necesita información sobre la dosis más eficaz y segura, el régimen de dosis óptimo (dosis diarias, intermitentes o únicas), el momento de inicio de la administración de suplementos de vitamina D y el efecto de la vitamina D cuando se combina con otras vitaminas y minerales para informar la elaboración de políticas. De hecho, se encuentra en curso otra revisión sistemática que compara las dosis de vitamina D y sus efectos sobre los resultados del embarazo y del lactante (Palacios 2018).
Objetivos
Examinar si la administración de suplementos de vitamina D a las embarazadas, sola o combinada con calcio u otras vitaminas y minerales, puede mejorar de forma segura los resultados maternos y neonatales.
Métodos
Criterios de inclusión de estudios para esta revisión
Tipos de estudios
Se planificó incluir ensayos aleatorios y cuasialeatorios con asignación al azar a nivel individual o grupal, pero solamente se encontraron ensayos controlados aleatorios con asignación al azar individual. En este metanálisis no se incluyeron ensayos cruzados (cross‐over) ni otros diseños observacionales (p.ej. estudios de cohortes o estudios de casos y controles), pero esta evidencia se tuvo en cuenta en la discusión, cuando fue relevante. Se incluyeron los resúmenes si tenían suficiente información para extraer los datos.
Tipos de participantes
Embarazadas de cualquier edad gestacional o cronológica, paridad (número de partos) y número de fetos, residentes en cualquier país. Se excluyeron las embarazadas con afecciones preexistentes.
Tipos de intervenciones
Administración de suplementos de vitamina D durante el embarazo, independientemente de la dosis, la duración o la hora de inicio de la administración de suplementos o el tipo de administración de suplementos (oral o inyectable). Se incluyeron los ensayos que analizaron la vitamina D sola o combinada con otros micronutrientes, siempre que los grupos control y de intervención se trataran de manera similar. Se evaluaron especialmente las siguientes comparaciones.
-
Administración de suplementos de vitamina D sola versus placebo o ninguna intervención (sin vitaminas ni minerales)
-
Administración de suplementos de vitamina D + calcio versus placebo o ninguna intervención (sin vitaminas ni minerales)
-
Administración de suplementos de vitamina D + calcio más otras vitaminas y minerales versus administración de suplementos de calcio + otras vitaminas y minerales (pero sin vitamina D)
-
Administración de suplementos de vitamina D + calcio versus administración de suplementos de calcio (pero sin vitamina D)
-
Administración de suplementos de vitamina D + calcio + otras vitaminas y minerales versus administración de suplementos de otras vitaminas y minerales (pero sin vitamina D + calcio)
Tipos de medida de resultado
Resultados prenatales clínicos y de laboratorio maternos y resultados clínicos y de laboratorio de los lactantes, como se describe a continuación.
Resultados primarios
Maternal
-
Preeclampsia (como la definieron los autores de los ensayos).
-
Diabetes gestacional (como la definieron los autores de los ensayos).
-
Eventos adversos (p.ej. hipercalcemia, cálculos renales).
Del recién nacido
-
Parto prematuro (menos de 37 semanas de gestación).
-
Bajo peso al nacer (menos de 2500 g).
Resultados secundarios
Maternal
-
Intolerancia a la glucosa (como la definieron los autores de los ensayos).
-
Cesárea.
-
Hipertensión gestacional (como la definieron los autores de los ensayos).
-
Muerte materna (muerte durante el embarazo o dentro de los 42 días de finalizado el embarazo).
-
Concentración de vitamina D al término (25‐hidroxivitamina D en nmol/l).
Del recién nacido
-
Talla al nacer (cm).
-
Perímetro cefálico al nacer (cm).
-
Peso al nacer (g).
-
Ingreso a cuidados especiales (incluidos cuidados intensivos) durante el período neonatal (en el transcurso de 28 días después del parto).
-
Mortinato (como lo definieron los autores de los ensayos).
-
Muerte neonatal (en el transcurso de 28 días después del parto).
-
Puntuación de Apgar menor a siete a los cinco minutos.
-
Infección neonatal (p.ej. infecciones respiratorias en el transcurso de 28 días después del parto).
-
Parto muy prematuro (de menos de 32 semanas de gestación).
Métodos de búsqueda para la identificación de los estudios
The following search methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Búsquedas electrónicas
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (12 July 2018).
The Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE (Ovid);
-
weekly searches of Embase (Ovid);
-
monthly searches of CINAHL (EBSCO);
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Studies awaiting classification; Ongoing studies).
We also searched the registries at ClinicalTrials.gov and WHO‐hosted International Clinical Trials Registry Platform (ICTRP) for any ongoing or planned trials (12 July 2018) (see:Appendix 1).
Búsqueda de otros recursos
For the identification of ongoing and unpublished studies, we contacted on different institutions including the WHO Departments of Reproductive Health and Research and Nutrition for Health and Development, the WHO regional offices, the United Nations Children's Fund (UNICEF), Nutrition International (NI), the Global Alliance for Improved Nutrition (GAIN) and the US Centers for Disease Control and Prevention (CDC) (15 May 2018)
We did not apply any date or language restrictions.
Obtención y análisis de los datos
For methods used in the previous version of this review, see De‐Regil 2016.
For this update, the following methods were used for assessing the reports that were identified as a result of the updated search.
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selección de los estudios
Two review authors (LK, CP) independently assessed for inclusion all the references identified through the search. All the papers were assessed in duplicate and we resolved any disagreements through discussion or, if required, we consulted the third review author (JP).
If studies were published only as abstracts, or study reports contained little information on methods, we attempted to contact the authors to obtain further details of study design and results. We were able to screen all the potentially eligible studies.
Extracción y manejo de los datos
We designed a form to extract data. For included studies, all review authors extracted the data using the agreed form. CP entered data into Review Manager software (RevMan 2014), and JP and LK checked for accuracy.
We analysed dichotomous data in terms of average risk ratio and we analysed continuous data in terms of mean difference. There was no need to use the standard mean difference as trials did not report outcomes in different scales.
Evaluación del riesgo de sesgo de los estudios incluidos
Two review authors (CP, LK) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion and consulted the third author (JP).
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
-
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes);
-
unclear.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
We classified blinding as 'high risk of bias' if the blinding status of a trial was unclear or the trial was open.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
-
low risk of bias;
-
high risk of bias;
-
unclear.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We assessed losses to follow‐up and post‐randomisation exclusions systematically for each trial.
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. We assessed methods as:
-
low risk of bias;
-
high risk of bias;
-
unclear.
We considered follow‐up to be 'low risk of bias' if more than 80% of participants initially randomised in a trial were included in the analysis and any loss was balanced across groups, unclear if the percentage of initially randomised participants included in the analysis was unclear, and 'high risk of bias' if less than 80% of those initially randomised were included in the analysis or if loss was imbalanced in different treatment groups.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
-
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
-
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(6) Other sources of bias
We assessed whether each study was free of other problems that could put it at risk of bias: We noted for each included study any important concerns we had about other possible sources of bias:
-
low risk of further bias;
-
high risk of further bias;
-
unclear whether there is a risk of further bias.
(7) Overall risk of bias
We summarised the risk of bias at two levels: within studies (across domains) and across studies.
For the first, we made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and for primary outcomes, we explored the impact of the level of bias through undertaking a Sensitivity analysis.
Assessment of the certainty of the evidence using the GRADE approach
For the assessment across studies, the main findings of the review are set out in the Summary of findings table 1; Summary of findings table 2 and Summary of findings table 3, prepared using GRADE profiler (GRADEpro) Guideline Development Tool (GRADEpro 2015). The primary outcomes for each comparison are listed with estimates of relative effects along with the number of participants and studies contributing data for those outcomes, where available. For each outcome, two review authors independently assessed the certainty of the evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Handbook (GRADE Handbook), which involves consideration of within‐study risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias; this results in one out of four levels of certainty (high, moderate, low or very low). This assessment was limited only to the trials included in this review.
Medidas del efecto del tratamiento
Dichotomous data
For dichotomous data, we present results as average risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference as the outcomes were measured in the same way between trials; there was no need to use the standardised mean difference to combine trials.
Cuestiones relativas a la unidad de análisis
Cluster‐randomised trials
We planned to include cluster‐randomised trials in the analyses along with individually‐randomised trials, but we did not find eligible studies with this design. We planned to adjust the standard errors of the results from cluster‐randomised studies using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), if sufficient information was available to allow for this. We planned to use an estimate of the intra cluster correlation co‐efficient (ICC) derived from the trial (if possible), or from another source. If ICCs from other sources were used, we planned to report this and to conduct sensitivity analyses to investigate the effect of variation in the ICC.
If we had identified both cluster‐randomised trials and individually‐randomised trials, we would have combined the results from both if there was little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit would be considered as unlikely.
Studies with more than two treatment groups
For studies with more than two intervention groups (multi‐arm studies), we combined groups to create a single pair‐wise comparison (Higgins 2011) and included the disaggregated data in the corresponding subgroup category. When the control group was shared by two or more study arms, we divided the control group (events and total population) over the number of relevant subgroup categories to avoid double counting the participants. The details are described in the Characteristics of included studies tables.
Cross‐over trials
We did not consider cross‐over trials eligible for inclusion.
Manejo de los datos faltantes
For included studies, we noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and analysed all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes are known to be missing.
Evaluación de la heterogeneidad
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either the Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Evaluación de los sesgos de notificación
We investigated reporting biases (such as publication bias) by using funnel plots for the primary outcomes with 10 or more studies. We assessed funnel plot asymmetry visually.
Síntesis de los datos
We carried out statistical analysis using the Review Manager software (RevMan 2014). We intended to use fixed‐effect meta‐analysis for combining data where it would be reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
Since we detected substantial heterogeneity, we used random‐effects meta‐analysis to produce an overall summary of an average treatment effect across trials. We treated the random‐effects summary as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials.
As we used random‐effects analyses, we present the results as the average treatment effect with its 95% confidence interval, and the estimates of Tau² and I².
Análisis de subgrupos e investigación de la heterogeneidad
We planned to investigate any substantial heterogeneity on the primary outcomes by using subgroup analyses as follows:
-
by start of supplementation: less than 20 weeks versus 20 weeks of pregnancy or more versus unknown/mixed;
-
by pre‐gestational body mass index (BMI) (kg/m2): underweight (lower than 18.5) versus normal weight (18.5 to 24.9) versus overweight (25 or higher) versus unknown/mixed;
-
by supplementation scheme/regimen: single versus daily versus weekly versus unknown/mixed;
-
by skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988): three or less versus four or more versus mixed/unknown;
-
by latitude: between the Tropics of Cancer and Capricorn versus north of the Tropic of Cancer or south of the Tropic of Capricorn versus unknown/mixed;
-
by season at the start of pregnancy: summer versus winter versus mixed/unknown/unreported.
Pragmatically, we decided not to conduct subgroup analyses in those outcomes with three or less trials.
We assessed subgroup differences by interaction tests available within RevMan (RevMan 2014). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Análisis de sensibilidad
We intended to conducted a sensitivity analysis based on the quality of the studies, however, as only one study was considered of high quality we did not perform this analysis. We considered a study to be of high quality if it was assessed as having low risk of bias in both the randomisation and allocation concealment and additionally a low risk of bias in either blinding or losses to follow‐up. In future updates, we will carry out sensitivity analysis to investigate the effect of the randomisation unit (if appropriate).
Results
Description of studies
Results of the search
We received a total of 111 new reports (after removing duplicates) from the search of Cochrane Pregnancy and Childbirth’s Trials Register, 13 reports from our additional search and we also reassessed the 23 ongoing trials (26 reports) and 27 excluded trials (46 reports) from the previous version of the review (De‐Regil 2016). See: Figure 1.

Study flow diagram for this update
A total of 30 trials were included in this update. Fifteen were already included in the previous update (Asemi 2012; Asemi 2013a; Brooke 1980; Delvin 1986; Diogenes 2013; Grant 2013; Li 2000a; Mallet 1986; Marya 1987; Marya 1988; Mazurkevich 2013; Roth 2010; Sablok 2015; Taherian 2002; Yu 2008). We identified nine new trials through our updated search (Kaur 1991, Naghshineh 2016; Sabet 2012; Samimi 2016; Samimi 2017; Sasan 2017; Shahgheibi 2016; Singh 2015; Vaziri 2016) and included six additional trials that were categorised as ongoing in the previous update (Benson 2009; Bhutta 2011; Harvey 2012; Mirghafourvand 2013; Roth 2013; Tehrani 2014).
We identified another study (Qian 2015) that raised concerns with the veracity of the information as there were several outcomes with the same results to another published study (Karamali 2015). We followed the guidelines from the Committee on Publication Ethics (COPE) to investigate the issue with the editors of both journals (Cope 2016) and the publication (Qian 2015) was retracted by the editors on 20 August 2018. Therefore, this trial was moved to excluded.
We excluded a total of 60 trials (125 reports). We identified six ongoing or unpublished trials (Baird 2016; Jelsma 2013; Judkins 2010; Lindqvist 2010; Mosalanejad 2016; Rasmussen 2009). There are two trials awaiting classification as they were available only in the abstract form with not enough information for data extraction (Bimson 2017; Das 2009).
Details of these trials are provided in: Characteristics of included studies; Characteristics of excluded studies; Studies awaiting classification tables.
Included studies
We included 30 trials (involving 7033 women and their infants) in this updated review (Asemi 2012; Asemi 2013a; Benson 2009; Bhutta 2011; Brooke 1980; Delvin 1986; Diogenes 2013; Grant 2013; Harvey 2012; Kaur 1991; Li 2000a; Mallet 1986; Marya 1987; Marya 1988; Mazurkevich 2013; Mirghafourvand 2013; Naghshineh 2016; Roth 2010; Roth 2013; Sabet 2012; Sablok 2015; Samimi 2016; Samimi 2017; Sasan 2017; Shahgheibi 2016; Singh 2015; Taherian 2002; Tehrani 2014; Vaziri 2016; Yu 2008). Details of these trials are provided in: Characteristics of included studies table.
Settings
The trials included in this review were carried from 1980s to 2015.
Trials were conducted in Australia (Benson 2009), Bangladesh (Roth 2010; Roth 2013), Brazil (Diogenes 2013), China (Li 2000a), France (Delvin 1986; Mallet 1986), India (Kaur 1991;Marya 1987; Marya 1988; Sablok 2015; Singh 2015), Iran (Asemi 2012; Asemi 2013a; Mirghafourvand 2013; Naghshineh 2016; Sabet 2012; Samimi 2016; Samimi 2017; Sasan 2017; Shahgheibi 2016; Taherian 2002; Tehrani 2014; Vaziri 2016), New Zealand (Grant 2013), Pakistan (Bhutta 2011), Russia (Mazurkevich 2013) and the UK (Brooke 1980; Harvey 2012; Yu 2008).
Latitude
Most trials were conducted either above or below the Tropics of Cancer and Capricorn (Asemi 2012; Asemi 2013a; Brooke 1980; Delvin 1986; Kaur 1991; Li 2000a; Mallet 1986; Marya 1987; Marya 1988; Mazurkevich 2013; Mirghafourvand 2013, Naghshineh 2016; Roth 2010; Roth 2013; Sablok 2015; Taherian 2002; Yu 2008; Sabet 2012; Samimi 2016; Samimi 2017; Sasan 2017; Shahgheibi 2016; Vaziri 2016; Benson 2009; Bhutta 2011; Tehrani 2014; Harvey 2012). Only two trials were conducted between the Tropics of Cancer and Capricorn (Grant 2013; Singh 2015), and one study was conducted just were the tropic of Capricorn lies (Diogenes 2013).
Seasonality
The seasons varied among trials with some trials occurring during the winter‐spring period (Delvin 1986); winter (Mallet 1986; Naghshineh 2016; Tehrani 2014); summer (Roth 2010; Yu 2008); spring‐summer period (Asemi 2013a), fall (Samimi 2016; Vaziri 2016), unknown/unreported in 13 trials (Asemi 2012; Benson 2009; Bhutta 2011; Kaur 1991; Li 2000a; Marya 1987; Marya 1988; Mazurkevich 2013; Sabet 2012; Sasan 2017; Shahgheibi 2016; Singh 2015; Taherian 2002) or mixed (Brooke 1980; Diogenes 2013; Grant 2013; Harvey 2012; Mirghafourvand 2013; Roth 2013; Sablok 2015; Samimi 2017).
Participants
The sample size from all the trials ranged between 40 women (Delvin 1986) and 1560 women (Roth 2013).
Pre‐gestational body‐mass index (kg/m2)
Pre‐gestational body mass index (BMI) of the participants was reported only in five trials (Asemi 2012; Asemi 2013a; Diogenes 2013; Sablok 2015; Taherian 2002). The rest of the trials did not report this. One study stratified for pre intervention BMI (in kg/m2; less than 30 and 30 or more) before randomisation (Asemi 2013a).
Skin pigmentation based on Fitzpatrick skin tone chart
None of the trials used the Fitzpatrick skin tone chart (Fitzpatrick 1988); however, several trials reported the ethnicity/race of participants. Most trials were among women from the Middle East (Asemi 2012; Asemi 2013a; Brooke 1980; Bhutta 2011; Tehrani 2014; Mirghafourvand 2013; Naghshineh 2016; Sabet 2012; Samimi 2016; Samimi 2017; Sasan 2017; Shahgheibi 2016; Taherian 2002; Vaziri 2016) or Asia (Kaur 1991; Li 2000a; Marya 1987; Marya 1988; Roth 2010; Roth 2013; Sablok 2015; Singh 2015). Two trials reported that participants were from mixed ethnicity (Benson 2009; Yu 2008), two trials were on whites (Harvey 2012; Mallet 1986), one among white women or black women (Diogenes 2013), and another among Pacific, European and Maori women (Grant 2013). Two trials did not report the characteristics of the participants in terms of ethnicity or origin (Delvin 1986; Mazurkevich 2013).
Interventions
A total of 22 trials compared provision of vitamin D supplement in comparison with placebo or no intervention (Comparison 1: Asemi 2013a; Benson 2009; Bhutta 2011; Brooke 1980; Delvin 1986; Grant 2013; Harvey 2012; Kaur 1991; Mallet 1986; Marya 1988; Mirghafourvand 2013; Naghshineh 2016; Roth 2010; Sabet 2012; Sablok 2015; Samimi 2017; Sasan 2017; Shahgheibi 2016; Singh 2015; Tehrani 2014; Vaziri 2016; Yu 2008).
A total of nine trials compared provision of oral vitamin D plus calcium supplements versus no intervention or placebo (Comparison 2: Asemi 2012; Asemi 2013a; Diogenes 2013; Li 2000a; Marya 1987; Mazurkevich 2013; Mirghafourvand 2013; Samimi 2016; Taherian 2002). The study by Mirghafourvand 2013 was included in both comparisons as they compared both vitamin D alone and vitamin D plus calcium with placebo.
Only one trial compared oral vitamin D plus calcium, iron and folic acid versus calcium, iron and folic acid but no vitamin D (Comparison 4: Roth 2013).
No trials evaluated the effects of either oral vitamin D plus calcium supplements versus calcium (Comparison 3), nor oral vitamin D + calcium + other vitamins and minerals supplements versus other oral vitamins and minerals supplements (but no vitamin D + calcium) (Comparison 5).
Start of supplementation
A total of seven trials started supplementation before week 20 (Benson 2009; Bhutta 2011; Harvey 2012; Naghshineh 2016; Samimi 2017; Singh 2015; Tehrani 2014). The rest of the trials started supplementation at 20 or more weeks' gestation (Asemi 2012; Asemi 2013a; Brooke 1980; Delvin 1986; Diogenes 2013; Grant 2013; Kaur 1991; Li 2000a; Mallet 1986; Marya 1987; Marya 1988; Mazurkevich 2013; Mirghafourvand 2013; Roth 2010; Roth 2013; Sabet 2012; Sablok 2015; Samimi 2016; Sasan 2017; Shahgheibi 2016; Taherian 2002; Vaziri 2016; Yu 2008).
Dose of vitamin D used
The dose of vitamin D provided varied in the included trials as well as the regimen.
Trials differed in the frequency of supplementation, with some trials using daily doses, weekly doses, monthly doses or single doses. Some trials had more than one group of vitamin D intervention.
For daily, weekly and monthly dosage, we calculated the total amount in international units (IU) per day. The daily doses used were 200 IU vitamin D in five trials (Asemi 2012; Diogenes 2013; Li 2000a; Mazurkevich 2013; Taherian 2002); 400 IU vitamin D in three trials (Asemi 2013a; Li 2000a; Samimi 2017); 600 IU vitamin D in two trials (Naghshineh 2016; Roth 2013); 800 IU vitamin D in another trial (Yu 2008); 1000 IU vitamin D in six trials (Brooke 1980; Delvin 1986; Grant 2013; Harvey 2012; Mirghafourvand 2013; Mallet 1986); 1200 IU vitamin D in two trials (Kaur 1991; Marya 1987); 2000 IU vitamin D in three trials (Grant 2013; Singh 2015; Vaziri 2016); 2400 IU vitamin D in one trial (Roth 2013); 3333 to 3500 IU vitamin D in five trials (Sabet 2012; Samimi 2016; Sasan 2017; Tehrani 2014); 4000 IU vitamin D in two trials (Bhutta 2011; Roth 2013), and 5000 IU vitamin D in one trial (Shahgheibi 2016). One study started supplementation at 2000 IU per day and if 25(ODH)‐D levels were below 75 nmol/L by week 28 of pregnancy, the dose was doubled to 4000 IU per day (Benson 2009). One study also provided to both groups a gel with 400 mg/day of vaginal progesterone (Samimi 2017). The study by Roth 2013 gave three different doses during pregnancy as mentioned above: 4200 IU per week or 600 IU/day; 16,800 IU per week or 2400 IU/day; 28,000 IU per week or 4000 IU/day. We combined the data from these groups, and on average, this group received 16,333 IU per week or 2333 IU/day.
For single‐dose supplementation of vitamin D, the dose varied from 200,000 IU vitamin D in a group in one study (Yu 2008); 600,000 IU vitamin D in one trial (Marya 1988); and 60,000 IU vitamin D two times (Kaur 1991). There was also one trial that used a monthly dose of 100,000 vitamin D (Sabet 2012); three trials that used a 50,000 dose every two weeks (Tehrani 2014; Samimi 2016; Sasan 2017); and one trial that used a dose of 35,000 IU vitamin D every week (Roth 2010). For the study by Sablok 2015, the dose depended upon the level of serum 25(OH)‐D levels at baseline; it varied from one dose of 60,000 IU (if serum 25(OH)‐D levels were > 50 nmol/L), two doses of 120,000 IU (if serum 25(OH)‐D levels were 25 to 50 nmol/L), or four doses of 120,000 IU (if serum 25(OH)‐D levels < 25 nmol/L).
Overall, the total provision of supplemental vitamin D provided throughout pregnancy varied. Sixteen trials provided 56,000 IU vitamin D or less (Asemi 2012; Asemi 2013a; Benson 2009; Delvin 1986; Diogenes 2013; Grant 2013; Harvey 2012Li 2000a; Mazurkevich 2013; Naghshineh 2016; Roth 2013; Sabet 2012; Samimi 2017; Singh 2015; Taherian 2002; Vaziri 2016); nine trials provided more than 56,000 to 200,000 IU vitamin D (Bhutta 2011;Brooke 1980; Kaur 1991; Mallet 1986; Marya 1987; Mirghafourvand 2013; Roth 2013; Sablok 2015; Yu 2008); and seven trials provided more than 200,000 IU of vitamin D (Marya 1988; Roth 2010; Roth 2013; Sablok 2015; Samimi 2016; Sasan 2017; Tehrani 2014) throughout pregnancy. One study did not specify when the supplementation started, therefore, we were not able to estimate this (Shahgheibi 2016).
Vitamin D form used
The vitamin D was provided in the form of cholecalciferol‐D3 in 20 trials (Asemi 2012; Asemi 2013a; Benson 2009; Delvin 1986; Diogenes 2013; Grant 2013; Harvey 2012; Kaur 1991; Li 2000a; Mazurkevich 2013; Roth 2010; Roth 2013; Sabet 2012; Sablok 2015; Samimi 2016; Samimi 2017; Sasan 2017; Singh 2015; Taherian 2002; Vaziri 2016) and as ergocalciferol‐D2 in three trials (Brooke 1980; Mallet 1986; Yu 2008). Seven trials did not report the vitamin D form used (Bhutta 2011; Marya 1987; Marya 1988; Mirghafourvand 2013; Naghshineh 2016; Shahgheibi 2016; Tehrani 2014).
Doses of calcium in the trials providing vitamin D and calcium supplementation
The doses of calcium provided along with the vitamin D ranged from 300 mg (Mirghafourvand 2013), 375 mg (Marya 1987); 500 mg (Asemi 2012; Asemi 2013a; Roth 2013; Taherian 2002); 600 mg calcium (Diogenes 2013; Li 2000a), 1000 mg (Samimi 2016) and 1250 mg (Mazurkevich 2013). All used calcium carbonate.
Health worker cadre
The trials were mostly carried out in the context of antenatal care and the administration of the supplements and the antenatal care was provided by the researchers themselves or through health allied personnel. The outcomes measurements were carried out by different groups according to the nature of the outcome, whether it was clinical, biochemical, anthropometric, or dietary assessments. A more detailed description of the health worker cadre is presented in Characteristics of included studies.
Laboratory methodology for the assessment of vitamin D status
Different laboratory methods were used to measure vitamin D status as serum 25‐hydroxyvitamin D concentrations. Five trials (Asemi 2012; Asemi 2013a; Sabet 2012; Sablok 2015; Samimi 2016) used immunoassay ELISA kit for their determinations; six trials used a chemiluminescent enzyme‐labelled immunometric assay (Benson 2009; Bhutta 2011; Diogenes 2013; Harvey 2012; Singh 2015; Vaziri 2016); another trial used isotope‐dilution liquid chromatography–tandem mass spectrometry (Grant 2013). Two trials used a competitive protein binding assay (Brooke 1980; Mallet 1986); one trial used a radioligand assay (Delvin 1986); and two trials used the Liebermann‐Burchard method (Sasan 2017; Shahgheibi 2016). Only two trials used high‐performance liquid chromatography tandem mass spectroscopy (LCMS/MS) (Roth 2010; Roth 2013). In two trials, the laboratory method was not reported (Samimi 2017; Yu 2008). The other trials did not report on this outcome (Kaur 1991; Li 2000a; Marya 1987; Marya 1988; Mazurkevich 2013; Mirghafourvand 2013; Naghshineh 2016; Samimi 2016; Tehrani 2014).
Funding sources
Trials were funded mainly by research grants from universities, health institutions and non‐government organisations; sometimes in combination. The Vice‐chancellor for research supported Asemi 2012 and Asemi 2013a. The Luke Proposch Perinatal Research Scholarship from the Australian and New Zealand College of Obstetrics and Gynaecology Research Foundation supported Benson 2009. The Pakistan Initiative for Mothers and Newborns (PAIMAN) supported Bhutta 2011. The pathological research fund, St George's Hospital Medical School, and the South‐west Thames Regional Health Authority funded Brooke 1980. Shriners of North America, the France‐Quebec Exchange Program, and INSERM funded Delvin 1986. The Conselho Nacional de Desenvolvimento Cientıfico e Tecnologico and the Fundacao Carlos Chagas Filho de Amparo a` Pesquisa do Estado do Rio de Janeiro supported Diogenes 2013. The Health Research Council of New Zealand and Cure Kids supported Grant 2013. The Arthritis Research UK, Medical Research Council, Bupa Foundation, and National Institute for Health Research supported Harvey 2012. Tabriz University of Medical Sciences funded Mirghafourvand 2013. Isfahan University of Medical Sciences supported Naghshineh 2016. The Thrasher Research Fund supported Roth 2010 and Bill & Melinda Gates Foundation supported Roth 2013. The Research Institute of Endocrine Sciences and the Shahid Beheshti University of Medical Sciences supported Sabet 2012. Kashan University of Medical Sciences funded both Samimi 2016 and Samimi 2017. Research Deputy of Isfahan University of Medical Sciences supported Taherian 2002. Tehrani 2014 did not report funding. The Research Vice‐chancellor of Shiraz University of Medical Sciences supported Vaziri 2016. The Institute of Obstetrics and Gynaecology Trust and the Wolfson and Weston Research Centre for Family Health supported Yu 2008. Sablok 2015 was self‐funded. For Kaur 1991, Li 2000a, Mallet 1986, Marya 1987, Marya 1988, Mazurkevich 2013, , Sasan 2017, and Singh 2015, funding was unknown/unreported. No trials had funding sources of concern, e.g. vitamin D manufacturers or similar. Shahgheibi 2016 reported that they received no funding.
Declarations of interest
The following trials reported that none of the authors had conflict of interests: Asemi 2013a; Benson 2009; Diogenes 2013; Grant 2013; Mirghafourvand 2013; Roth 2010; Roth 2013; Sabet 2012; Sablok 2015; Samimi 2016; Samimi 2017; Sasan 2017; Shahgheibi 2016; Tehrani 2014; Vaziri 2016.
The following trials did not include the conflict of interest statement in their publication: Asemi 2012; Bhutta 2011; Brooke 1980; Delvin 1986; Kaur 1991; Li 2000a; Mallet 1986; Marya 1987; Marya 1988; Mazurkevich 2013; Naghshineh 2016; Singh 2015; Taherian 2002; Yu 2008.
Only one trial reported conflict of interests: Harvey 2012.
SeeCharacteristics of included studies for a detailed description of the trials, including vitamin D doses used and regimens compared.
Excluded studies
We excluded 60 trials. The main reason for exclusion was that the comparisons were among different doses of vitamin D in 26 trials (Baqui 2009; Bhatia 2012; Bhatia 2010; Bisgaard 2009; Dawodu 2013; de Menibus 1984; Gerais 2015; Hashemipour 2014; Kachhawa 2014; Kiely 2015; Lalooha 2012; March 2010; Marya 1981; McLean 2012; Mojibian 2015; Mutlu 2013; Nausheen 2014; Rostami 2018; Shakiba 2013; Stephensen 2011; Thiele 2014; Wagner 2006a; Wagner 2006b; Wagner 2013; Weiss 2009; Yap 2014), without placebo or no treatment control. Also, three trials were excluded because the treatment groups differed in other nutrients given in the supplements, other than vitamin D (Asemi 2015; Azami 2017; Pandey 2015), and one trial had no group with vitamin D (Atkinson 2010). In addition, four trials were not randomised trials (Ala‐Houhala 1986; Cockburn 1980; Bhatia 2010; Ito 1994). Four trials (Czech‐Kowalska 2013; Niramitmahapanya 2017; Taheri 2014; von Hurst 2009) were conducted on non pregnant women; 12 trials were carried out in pregnant women with glucose intolerance or with gestational diabetes (Asemi 2013b; Asemi 2014; Jamilian 2016; Jamilian 2017; Karamali 2014; Li 2016; Mozzafari 2010; Razavi 2017; Simsek 2011; Valizadeh 2016; Yazdchi 2016; Zhang 2016) or other chronic conditions (Etemadifar 2015; Shi 2017; Sudfeld 2017), one trial was conducted only among postpartum women (Chandy 2016), and one trial was conducted among couples for fertility purposes (Kermack 2017). One reference referred to a trial registered in 1986 on the Oxford Database of Perinatal Trials and reports the recruitment and follow‐up completed in 1979, but there were no reports available and we were unable to locate the author who registered the trial (MacDonald 1986). One trial was excluded because treatment groups differed more than vitamin D supplementation (Hossain 2012). For more detailed descriptions of excluded trials along with the reasons for exclusion, seeCharacteristics of excluded studies.
Risk of bias in included studies
Allocation
Sequence generation
We assessed 21 trials as having adequate methods for generating the randomisation sequence. Ten trials used computer‐generated random number sequences (Asemi 2013a; Diogenes 2013; Grant 2013; Harvey 2012; Naghshineh 2016; Roth 2010; Roth 2013; Sablok 2015; Samimi 2016; Yu 2008), four used permuted block randomisation (Bhutta 2011; Mirghafourvand 2013; Samimi 2017; Vaziri 2016), and seven trials used a random numbers table (Asemi 2012; Benson 2009; Mallet 1986; Sasan 2017; Shahgheibi 2016; Taherian 2002; Tehrani 2014) to randomise the intervention groups. The other trials reported the trials as randomised but the methods used to generate the sequence were not described (Brooke 1980; Delvin 1986; Kaur 1991; Marya 1987; Marya 1988; Mazurkevich 2013; Sabet 2012; Singh 2015). One trial did not mention that participants were randomly allocated to the treatment groups (Li 2000a).
Allocation concealment
We judged that 13 trials had adequate methods of allocation concealment (Asemi 2013a; Asemi 2012; Benson 2009; Bhutta 2011; Grant 2013; Harvey 2012; Roth 2010; Roth 2013; Samimi 2016; Samimi 2017; Shahgheibi 2016; Tehrani 2014; Yu 2008). It is assumed that allocation concealment did not occur in the following trials as one of the groups did not receive any supplementation: Kaur 1991; Li 2000a; Mallet 1986; Marya 1987; Marya 1988; Mazurkevich 2013; Sablok 2015; Singh 2015; Taherian 2002. In Sablok 2015, the intervention dosage depended on the vitamin D status, therefore, there was a selection bias based on status of vitamin D at baseline. In the case of Harvey 2012, participants at 28 weeks had their serum 25‐hydroxyvitamin D measured and if below 75 nmol/L, the dose was doubled to 4000 IU. The others did not report the methods of concealment (Brooke 1980; Delvin 1986; Diogenes 2013; Mirghafourvand 2013; Naghshineh 2016; Sabet 2012; Sasan 2017; Vaziri 2016).
Blinding
Blinding of participants, staff and outcome assessors
Investigators in 15 trials reported that they used a double‐blinded design (Asemi 2013a; Bhutta 2011; Brooke 1980; Grant 2013; Harvey 2012; Mirghafourvand 2013; Naghshineh 2016; Roth 2010; Roth 2013; Sabet 2012; Samimi 2016; Samimi 2017; Sasan 2017; Shahgheibi 2016; Tehrani 2014). However, only 10 trials specified that both participants and those conducting the assessments were blinded (Asemi 2013a; Bhutta 2011; Grant 2013; Harvey 2012; Naghshineh 2016; Roth 2010; Roth 2013; Sasan 2017; Shahgheibi 2016; Tehrani 2014). Two trials were reported as single‐blinded, being blinded for participants only (Asemi 2012; Diogenes 2013). The trial by Vaziri 2016 reported that it was a single‐blinded study; however, depression was assessed by blinded nurse although the rest of the assessments were not clear if they were performed by a blinded staff. The rest of the trials reported being single‐blinded but since one of the groups received no supplementation, it was assumed that it was not blinded to participants but to the assessment team: Benson 2009, Delvin 1986; Kaur 1991; Li 2000a; Mallet 1986; Marya 1987; Marya 1988; Mazurkevich 2013; Sablok 2015; Singh 2015; Taherian 2002; Yu 2008.
Incomplete outcome data
Sixteen trials did not have incomplete data: Asemi 2012; Asemi 2013a; Grant 2013; Harvey 2012; Mirghafourvand 2013; Naghshineh 2016; Roth 2010; Roth 2013; Sabet 2012; Samimi 2016; Samimi 2017; Sasan 2017; Shahgheibi 2016; Taherian 2002; Tehrani 2014; Yu 2008). The others did not report on attrition, missing data and lack of intention‐to‐treat analyses.
Selective reporting
We did not have access to study protocols and therefore, formally assessing reporting bias was not possible. Insufficient trials contributed data to allow us to carry out exploration of possible publication bias by using funnel plots.
Other potential sources of bias
This varied in the different trials. For example, Harvey 2012 reported that participants were allowed to continue taking their own multivitamin but they did not specify who took those supplements and who did not take them during the study. The report from Li 2000a is very short, with most details of the methods not available. The trial by Mallet 1986 had groups with notoriously different sample sizes; it is unclear whether the numbers reflect the participants who finished the trial, a non randomised process, or a selection bias in which randomised participants did not receive the intervention. The trial by Shahgheibi 2016 reported different interventions in the abstract to what was described in methods.
We have also included figures that summarise our ’Risk of bias’ assessments (Figure 2; Figure 3).
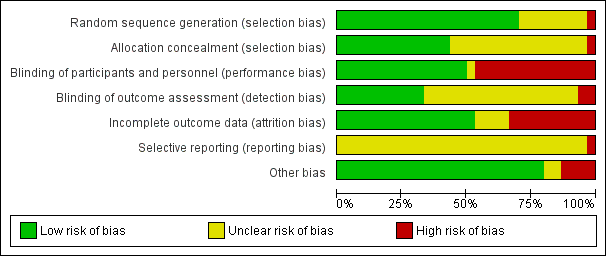
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
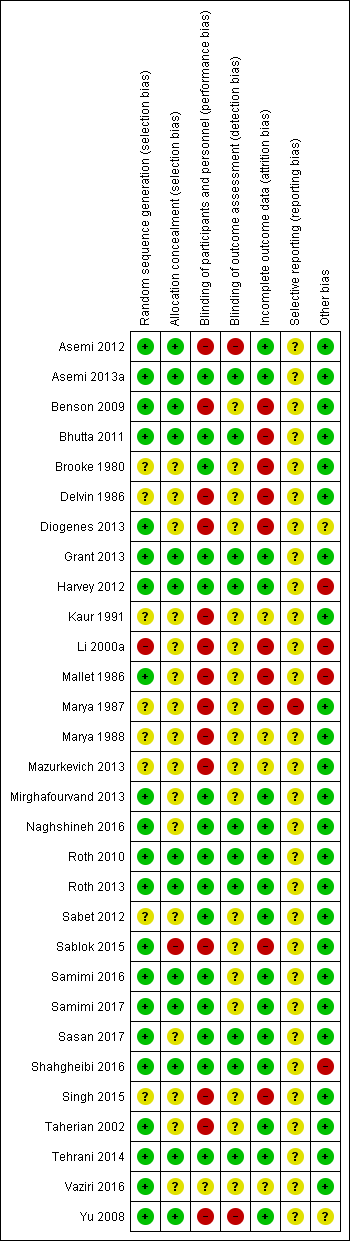
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Summary of findings for the main comparison Vitamin D supplementation compared to placebo or no intervention for pregnancy and neonatal health outcomes; Summary of findings 2 Vitamin D + calcium supplementation compared to placebo or no intervention for pregnancy and neonatal health outcomes; Summary of findings 3 Vitamin D + calcium + other vitamins and minerals compared to calcium + other vitamins and minerals (but no vitamin D) for pregnancy and neonatal health outcomes
In this updated review we included 30 trials assessing a total of 7033 women. We organised the summary results by comparison and by primary and secondary outcomes.
In the Data and analyses tables, we set up all four prespecified comparisons but outcome data were only available for two of these. We have not added outcomes to those comparisons without data (Comparisons three and four). For the comparisons with data, we set up tables for all primary outcomes (even where no data were available) not only to highlight gaps in the current research evidence, but also to be able to add any data that may become available in future updates.
SeeData and analyses for detailed results on primary and secondary outcomes.
For each of the comparisons, we have indicated the number of trials contributing data and the total number of women recruited in these trials. However, for some outcomes only one or two trials provided data and due to loss to follow‐up, denominators for particular outcomes may have been considerably less than the randomised sample. Therefore, we have indicated the number of trials contributing data and the number of women included in that analysis.
(1) Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals) (22 trials, 3725 women)
Twenty‐two trials involving 3725 pregnant women were included in this comparison (Asemi 2013a; Benson 2009; Bhutta 2011; Brooke 1980; Delvin 1986; Grant 2013; Harvey 2012; Kaur 1991; Mallet 1986; Marya 1988; Mirghafourvand 2013; Naghshineh 2016; Roth 2010; Sabet 2012; Sablok 2015; Samimi 2017; Sasan 2017; Shahgheibi 2016; Singh 2015; Tehrani 2014; Vaziri 2016; Yu 2008). Two trials contributed to both Comparisons 1 and 2 (Asemi 2013a; Mirghafourvand 2013).
The following trials were assessed as having low risk of bias for allocation and blinding: Asemi 2013a; Bhutta 2011; Grant 2013; Harvey 2012; Mirghafourvand 2013; Naghshineh 2016; Roth 2010; Samimi 2017; Shahgheibi 2016; Tehrani 2014. The following trials were assessed as having high risk of bias for allocation and blinding: Delvin 1986; Kaur 1991; Mallet 1986; Marya 1988; Sablok 2015; Singh 2015. Benson 2009 and Yu 2008 had high risk for blinding. Sabet 2012 did not report on random sequence generation or on blinding of outcome assessments. Brooke 1980 and Vaziri 2016 did not report on most of these issues, therefore, we could not judge on their risk of bias. In Sasan 2017 allocation concealment was not well described and so was assessed as being at unclear risk of bias.
Primary outcomes
Maternal
Pre‐eclampsia (as defined by trialists)
Data from four trials (Asemi 2013a; Naghshineh 2016; Sablok 2015; Sasan 2017) involving 499 women found that supplementation with vitamin D probably reduces the risk of pre‐eclampsia compared to no intervention or placebo (risk ratio (RR 0.48, 95% CI 0.30 to 0.79); moderate‐certainty evidence; Analysis 1.1). The data from Sablok 2015 were wrongly entered in the previous update (De‐Regil 2016), as it included data for both pre‐eclampsia and gestational hypertension together. We contacted the author and now the data only for pre‐eclampsia are reported here and the data for gestational hypertension are reported in that outcome. It is also important to note that all women in the Sasan 2017 trial had a history of pre‐eclampsia.
Gestational diabetes (as defined by trialists)
Data from four trials (Asemi 2013a;Tehrani 2014; Naghshineh 2016; Sablok 2015) involving 446 women found that supplementation with vitamin D probably reduces the risk of gestational diabetes compared to women receiving no intervention, or in the placebo group (RR 0.51, 95% CI 0.27 to 0.97; moderate‐certainty evidence; Analysis 1.2).
Maternal adverse events Analysis 1.3
One trial reported on severe postpartum haemorrhage in 1134 women (Harvey 2012); vitamin D supplementation appears to reduce the risk of severe postpartum haemorrhage (RR 0.68, 95% CI 0.51 to 0.91; 1 trial, 1134 women, low‐certainty evidence), although it should be noted that this result is based on a single trial and was an unexpected finding that has not been documented before by any other study. Another trial reported on nephritic syndrome in 135 women (Yu 2008); no clear differences were found between groups (just one event in the control group: RR 0.17, 95% CI 0.01 to 4.06; very low‐certainty evidence). In terms of hypercalcaemia, only one trial reported this outcome (Harvey 2012) and there were no cases of hypercalcaemia in any group. Given the scarcity of data in general for maternal adverse events, no firm conclusions can be drawn.
Infant
Preterm birth (less than 37 weeks' gestation)
Data from seven trials (Asemi 2013a; Delvin 1986; Harvey 2012; Grant 2013; Mirghafourvand 2013; Roth 2010; Singh 2015) involving 1640 women suggest that supplementation with vitamin D probably may make little or no difference in the risk of having a preterm birth compared to no intervention or placebo (RR 0.66, 95% CI 0.34 to 1.30; low‐certainty evidence; Analysis 1.4). It is important to note that in Singh 2015, participants in the intervention group had significantly lower 25‐hydroxyvitamin D levels at baseline compared to the control group, therefore, there was an imbalance in study groups. However, the result in lowering preterm birth risk in this trial was consistent with the other trials.
Low birthweight (less than 2500 g)
The data from five trials (Brooke 1980; Bhutta 2011; Marya 1988; Roth 2010; Sablok 2015) involving 697 women suggest that supplementation with vitamin D probably reduces the risk of low birthweight (< 2500 g) compared to no intervention or placebo (RR 0.55, 95% CI 0.35 to 0.87; moderate‐certainty evidence;Analysis 1.5).
Subgroup analysis
For preterm birth, results did not differ greatly whether supplementation started before week 20 of pregnancy (three trials, 1149 women) (RR 0.73, 95% CI 0.26 to 2.04), or after 20 weeks of pregnancy (four trials, 491 women) (RR 0.49, 95% CI 0.13 to 1.87; Analysis 1.18).
With respect to the other subgroup analyses, most only had one or two trials (Analysis 1.6, Analysis 1.7; Analysis 1.8; Analysis 1.9; Analysis 1.10; Analysis 1.11; Analysis 1.12; Analysis 1.13; Analysis 1.14; Analysis 1.15; Analysis 1.16; Analysis 1.17; Analysis 1.19; Analysis 1.20; Analysis 1.21; Analysis 1.22; Analysis 1.23; Analysis 1.24; Analysis 1.25; Analysis 1.26; Analysis 1.27; Analysis 1.28; Analysis 1.29).
Secondary outcomes
Maternal
Caesarean section
Ten trials including 1104 women reported on this outcome (Asemi 2013a; Delvin 1986; Mirghafourvand 2013; Naghshineh 2016; Roth 2010; Sablok 2015; Sasan 2017; Shahgheibi 2016; Singh 2015; Yu 2008). The data from this trial suggest that vitamin D supplementation probably makes little or no difference in the risk of caesarean section compared to no supplementation or placebo (RR 0.98, 95% CI 0.80 to 1.21; Analysis 1.30). The trial by Singh 2015 was the only trial detecting a lower risk of caesarean section with the intervention, however, participants in the intervention group had significantly lower 25‐hydroxyvitamin D levels at baseline compared to the control group, therefore, there was an imbalance in study groups. If this trial is removed from the analysis, the results did not change (RR 1.06, 95% CI 0.93 to 1.21).
Gestational hypertension
Two trials reported on this outcome in 1130 women (Harvey 2012; Sablok 2015). Vitamin D supplementation probably makes little or no difference in the risk of gestational hypertension compared to no intervention or placebo (RR 0.78, 95% CI 0.41 to 1.49; Analysis 1.31).
Maternal death
One trial reported on this outcome in 180 women (Sablok 2015). No maternal deaths were reported in any of the groups (Analysis 1.32).
Impaired glucose tolerance
No trial reported this outcome.
Maternal vitamin D concentration at term (25‐hydroxyvitamin D in nmol/L)
The data from 14 trials (Asemi 2013a; Benson 2009; Bhutta 2011; Brooke 1980; Delvin 1986; Grant 2013; Harvey 2012; Mallet 1986; Roth 2010; Sabet 2012; Sablok 2015; Samimi 2017; Singh 2015; Vaziri 2016) involving 2470 women show that supplementation with vitamin D probably results in a higher 25‐hydroxyvitamin D concentrations than those women who received no intervention or a placebo. The average mean difference (MD) between groups was 35.66 nmol 25‐hydroxyvitamin D per litre (95% CI 24.19 to 47.13; Analysis 1.33). This result should be interpreted cautiously as the response to supplementation was highly heterogeneous (Tau² = 437.5, I² = 99% and Chi² test for heterogeneity P < 0.00001) and ranged from 16.3 nmol 25‐hydroxyvitamin D per litre (95% CI 13.6 to 19.0) (Mallet 1986) to 152 nmol 25‐hydroxyvitamin D per litre (95% CI 127 to 177) (Brooke 1980). If the trial by Brooke 1980 is removed, heterogeneity remains similar. The trial by Singh 2015 found consistent results with the other trials, even though there was an imbalance in study groups in this outcome at baseline. However, the results are consistent between trials. We also detected funnel plot asymmetry in this outcome (Figure 4); the presence of funnel plot asymmetry suggested that publication bias was a likely source of this heterogeneity.
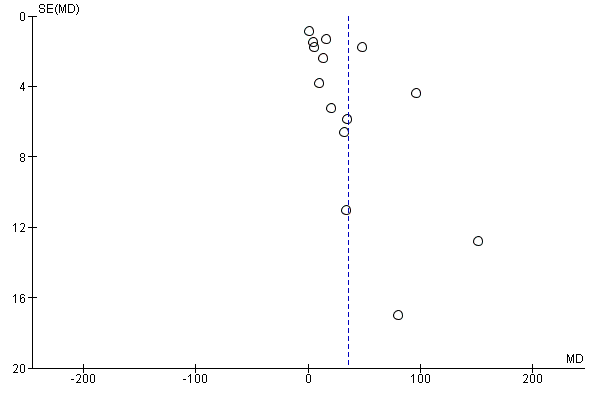
Funnel plot of comparison: 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), outcome: 1.15 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (ALL).
Infant
Length at birth (cm)
The data from eight trials (Asemi 2013a; Brooke 1980; Marya 1988; Mirghafourvand 2013; Roth 2010; Sabet 2012; Sablok 2015; Vaziri 2016) involving 931 women probably suggest a longer birth length among infants from women taking vitamin D supplementation during pregnancy compared to women in the no treatment or placebo group (MD 0.57, 95% CI 0.19 to 0.95; Analysis 1.34). There was heterogeneity in the response to the supplementation (Tau² = 0.16; I² = 63% and Chi² test for heterogeneity P = 0.008) and most of these studies did not report if outcome assessments were performed by blinded personnel (Brooke 1980; Marya 1988; Mirghafourvand 2013; Sabet 2012; Sablok 2015; Vaziri 2016). Taking this into consideration and the very small effect, these results should be interpreted with caution.
Head circumference at birth (cm)
Eight trials involving 1841 women (Asemi 2013a; Brooke 1980; Harvey 2012; Marya 1988; Mirghafourvand 2013; Roth 2010; Sablok 2015; Vaziri 2016) reported on this anthropometric measurement. Results suggest that supplementation with vitamin D probably makes little or no difference in head circumference at birth compared to no treatment or placebo (MD 0.11, 95% CI ‐0.21 to 0.44; Analysis 1.35). There was heterogeneity in the response to the supplementation (Tau² = 0.16; I² = 80% and Chi² test for heterogeneity P < 0.00001); therefore, results should be interpreted with caution.
Birthweight (g)
Seventeen trials involving 2828 women (Asemi 2013a; Bhutta 2011; Brooke 1980; Grant 2013; Harvey 2012; Kaur 1991; Mallet 1986; Marya 1988; Mirghafourvand 2013; Naghshineh 2016; Roth 2010; Sabet 2012; Sablok 2015; Shahgheibi 2016; Singh 2015; Vaziri 2016; Yu 2008) reported on this outcome. Results suggest that supplementation with vitamin D probably makes little or no difference in birthweight compared to no treatment or placebo (MD 80.30, 95% CI ‐14.40 to 175.00; Analysis 1.36). There was some substantial heterogeneity among trials in terms of the size of the treatment (Tau² = 32319; I² = 92% and Chi² test for heterogeneity P < 0.00001). However, when the trial by Mallet 1986 is excluded from the analysis, heterogeneity is reduced from 92% to 84% and results show that supplementation with vitamin D probably results in a higher birthweight (MD 99.27, 95% CI 16.22 to 182.32). The standard deviations for this study are very small and so we have concerns that these may not be reported correctly. Also, the trial by Singh 2015 found a very different result compared to the other trials and could be explained by the fact that women in the intervention group had significantly lower 25‐hydroxyvitamin D levels at baseline compared to the control group. When this trial is also removed from the analysis, heterogeneity is further reduced to 67%, with no significant differences between groups (MD 59.24, 95% CI ‐1.93 to 120.42).
Stillbirth (as defined by trialists)
Three trials (Grant 2013; Roth 2010; Yu 2008) including 584 women reported this outcome. Vitamin D supplementation probably makes little or no difference in the risk of stillbirth compared to no intervention or placebo (RR 0.35, 95% CI 0.06 to 1.98; Analysis 1.37). In particular, for this outcome, only one case of stillbirth out of 364 was reported in the vitamin D group and three cases out of 220 in the no intervention or placebo group.
Neonatal death (within 28 days after delivery)
Two trials (Roth 2010; Yu 2008) including 326 women reported this outcome. Vitamin D supplementation probably makes little or no difference in the risk of neonatal death compared to no intervention or placebo (RR 0.27, 95% CI 0.04 to 1.67; Analysis 1.38). Only one neonatal death out of 193 was reported in the vitamin D group and four neonatal deaths were reported out of 133 in the no intervention or placebo group. Given the scarcity of data for this outcome, no firm conclusions can be drawn.
Apgar score less than seven at five minutes
One study including 165 women did not find clear differences in Apgar scores between groups (RR 0.53, 95% CI 0.11 to 2.53; Analysis 1.39).
Other infant secondary outcomes
No trials reported on the other pre‐specified infant secondary outcomes: admission to special care (including intensive care) during the neonatal period (within 28 days after delivery); neonatal infection (e.g. respiratory infections) or very preterm birth (less than 34 weeks' gestation).
(2) Supplementation with vitamin D + calcium versus placebo/no intervention (no vitamin or minerals) (nine trials, 1916 women)
Nine trials involving 1916 women made this comparison (Asemi 2012; Asemi 2013a; Diogenes 2013; Li 2000a; Marya 1987; Mazurkevich 2013; Mirghafourvand 2013; Samimi 2016; Taherian 2002).
The following trials were assessed as having low risk of bias for allocation and blinding: Asemi 2013a; Mirghafourvand 2013; Samimi 2016, while four trials were assessed as having high risk of bias: Diogenes 2013; Li 2000a; Marya 1987; Mazurkevich 2013. The remaining two trials had mixed results, with some components having a low risk, high risk, or unclear risk: Asemi 2012; Taherian 2002.
Primary outcomes
Maternal
Pre‐eclampsia (as defined by trialists)
Four trials (Asemi 2012; Marya 1987; Samimi 2016; Taherian 2002) including 1174 women reported on this outcome. Supplementation with vitamin D probably reduces the risk of pre‐eclampsia compared to no intervention or placebo (RR 0.50, 95% CI 0.32 to 0.78;moderate‐certainty evidence; Analysis 2.1).
Gestational diabetes (as defined by trialists)
A single study including 54 women reported on this outcome (Asemi 2012). It is uncertain whether vitamin D supplementation makes any difference to the risk of gestational diabetes compared to no intervention or placebo (RR 0.33, 95% CI 0.01 to 7.84; very low‐certainty evidence; Analysis 2.2). The scarcity of data for this outcome and the wide CIs means no firm conclusions can be drawn.
Maternal adverse events
No trial reported this outcome.
Infant
Preterm birth (less than 37 weeks' gestation)
Five trials with 942 women reported on this outcome (Asemi 2012; Diogenes 2013; Mirghafourvand 2013; Samimi 2016; Taherian 2002). Supplementation with vitamin D may increase the risk of preterm birth compared to no intervention or placebo (RR 1.52, 95% CI 1.01 to 2.28; low‐certainty evidence; Analysis 2.3). These results are mostly driven by one trial, which recruited most of the patients and had most of the events (Taherian 2002).
Low birthweight (less than 2500 g)
Two trials with 110 women reported on this outcome (Diogenes 2013; Samimi 2016). We are uncertain whether supplementation with vitamin D makes any difference to the risk of low birthweight compared to no intervention or placebo (RR 0.68, 95% CI 0.10 to 4.55; very low‐certainty evidence; Analysis 2.4). The data were scarce for this outcome and the CIs wide and so, no firm conclusions can be drawn.
Secondary outcomes
Maternal
Impaired glucose tolerance
No trial reported this outcome.
Caesarean section
Two trials including 146 women reported on this outcome (Mirghafourvand 2013; Samimi 2016). Vitamin D supplementation probably makes little or no difference in the risk of caesarean section compared to no intervention or placebo (RR 1.16, 95% CI 0.87 to 1.54; Analysis 2.5).
Gestational hypertension
One trial reported on this outcome in 59 women (Li 2000a). Vitamin D supplementation probably makes little or no difference in the risk of gestational hypertension compared to no intervention or placebo (RR 0.26, 95% CI 0.06 to 1.12; Analysis 2.6).
Maternal death
No trial reported this outcome.
Maternal vitamin D levels at term (25‐hydroxyvitamin D in nmol/L)
A single study including 60 women reported on this outcome (Samimi 2016). The average MD between groups was 12.50 nmol 25‐hydroxyvitamin D per litre (95% CI 3.80 to 21.20; Analysis 2.7), but given the scarcity of data for this outcome and the wide CIs, no firm conclusions can be drawn.
Infant
Length at birth (cm)
The data from three trials (Diogenes 2013; Mirghafourvand 2013; Samimi 2016) involving 194 women show that vitamin D supplementation probably makes little or no difference in birth length compared to no intervention or placebo (MD ‐0.07, 95% CI ‐0.67 to 0.52; Analysis 2.8).
Head circumference at birth (cm)
Three trials involving 198 women (Diogenes 2013; Mirghafourvand 2013; Samimi 2016) reported on this anthropometric measurement. Vitamin D supplementation probably makes little or no difference in head circumference at birth compared to no intervention or placebo (MD ‐0.03, 95% CI ‐0.39 to 0.33; Analysis 2.9).
Birthweight (g)
Three trials involving 194 women (Diogenes 2013; Mirghafourvand 2013; Samimi 2016) reported on this outcome. Vitamin D supplementation probably makes little or no difference in birthweight compared to no intervention or placebo (MD 42.39, 95% CI ‐86.96 to 171.74; Analysis 2.10).
Neonatal death (within 28 days after delivery)
One trial (Taherian 2002) reported on this outcomes with one death during the study period in the no intervention or placebo group (RR 0.20, 95% 0.01 to 4.15; one study, 660 women; Analysis 2.11).
Other infant secondary outcomes
No trials reported on the other pre‐specified infant secondary outcomes: length at birth (cm); head circumference at birth (cm); weight at birth (g); admission to special care (including intensive care) during the neonatal period (within 28 days after delivery); stillbirths (as defined by trialists); Apgar score less than seven at five minutes; neonatal infection (e.g. respiratory infections) or very preterm birth (less than 34 weeks' gestation).
(3) Supplementation with vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals (but no vitamin D) (one study, 1300 women)
One trial was included in this comparison (Roth 2013). It was assessed as having low risk of bias.
Primary outcomes
Maternal
Pre‐eclampsia (as defined by trialists)
The included study under this comparison did not report on this outcome.
Gestational diabetes (as defined by trialists)
It is unclear whether vitamin D supplementation with calcium and other vitamins and minerals makes any difference in the risk of gestational diabetes compared to no intervention or placebo (RR 0.42, 95% CI 0.10 to 1.73; 1 trial, 1298 women; very low‐certainty evidence;Analysis 3.1) because the certainty of the evidence was found to be very‐low.
Maternal adverse events
It is unclear whether vitamin D supplementation with calcium and other vitamins and minerals makes any difference in the risk of maternal hypercalciuria (confirmed cases) compared to no intervention or placebo (RR 0.25, 95% CI 0.02 to 3.97; 1 trial, 1298 women; very low‐certainty evidence;Analysis 3.2) because the certainty of the evidence was found to be very‐low. No confirmed cases were reported for maternal hypercalcaemia.
Infant
Preterm birth (less than 37 weeks' gestation)
Vitamin D supplementation may make little or no difference in the risk of preterm birth compared to no intervention or placebo (RR 1.04, 95% CI 0.68 to 1.59; 1 trial, 1298 women; low‐certainty evidence;Analysis 3.3).
Low birthweight (less than 2500 g)
Vitamin D supplementation probably may make little or no difference in the risk of low birthweight compared to no intervention or placebo (RR 1.12, 95% CI 0.82 to 1.51; 1 trial, 1298 women; low‐certainty evidence;Analysis 3.4).
Secondary outcomes
Maternal
Caesarean section
Vitamin D supplementation probably makes little or no difference in the risk of C‐section compared to no intervention or placebo (RR 1.10, 95% CI 0.95 to 1.27; 1 trial, 1298 women; Analysis 3.5).
Gestational hypertension
Vitamin D supplementation probably makes little or no difference in the risk of gestational hypertension compared to no intervention or placebo (RR 0.93, 95% CI 0.31 to 2.79; 1 trial, 1298 women; Analysis 3.6).
Maternal death
Vitamin D supplementation probably makes little or no difference in the risk of maternal death compared to no intervention or placebo (RR 0.25, 95% CI 0.02 to 3.98; 1 trial, 1300 women; Analysis 3.7). Only one maternal death out of 1040 was reported in the vitamin D group and one maternal death was reported out of 260 in the no intervention or placebo group. Given the scarcity of data for this outcome, no firm conclusions can be drawn.
Maternal vitamin D concentration at term (25‐hydroxyvitamin D in nmol/L)
The average MD between groups was 75.17 nmol 25‐hydroxyvitamin D per litre (95% CI 71.97 to 78.37; 1 trial, 635 women; Analysis 3.8) but given the scarcity of data for this outcome and the wide CIs, no firm conclusions can be drawn.
Infant
Stillbirth (as defined by trialists)
Vitamin D supplementation probably makes little or no difference in the risk of stillbirth compared to no intervention or placebo (RR 0.66, 95% CI 0.29 to 1.46; 1 trial, 1300 women; Analysis 3.12). A total of 21 stillbirths out of 1040 were reported in the vitamin D group and eight stillbirths were reported out of 260 in the no intervention or placebo group. Given the scarcity of data for this outcome, no firm conclusions can be drawn.
Neonatal death (within 28 days after delivery)
Vitamin D supplementation probably makes little or no difference in the risk of neonatal death compared to no intervention or placebo (RR 0.69, 95% CI 0.22 to 2.14; 1 trial, 1298 women; Analysis 3.13). A total of 11 neonatal deaths out of 1039 were reported in the vitamin D group and four neonatal death were reported out of 259 in the no intervention or placebo group. Given the scarcity of data for this outcome, no firm conclusions can be drawn.
(4) Supplementation with vitamin D + calcium versus calcium (but no vitamin D) (no trials)
No trials were included in this comparison.
(5) Supplementation with vitamin D + calcium + other vitamins and minerals versus other vitamins and minerals (but no vitamin D + calcium) (no trials)
No trials were included in this comparison.
Discusión
Resumen de los resultados principales
Esta revisión evalúa los efectos de la administración de suplementos de vitamina D solos o en combinación con calcio y otras vitaminas y minerales durante el embarazo. Incluye 30 ensayos con 7033 mujeres, 22 de los cuales compararon vitamina D sola versus ningún tratamiento o placebo, nueve compararon vitamina D más calcio con ninguna intervención, y uno comparó vitamina D más calcio, hierro y ácido fólico con ninguna vitamina D. Ningún ensayo evaluó los efectos de la vitamina D + calcio + otras vitaminas y minerales versus otras vitaminas y minerales (pero sin vitamina D + calcio).
Administración de suplementos de vitamina D en comparación con ninguna intervención o placebo.
-
Probablemente reduce el riesgo de preeclampsia (cuatro ensayos), el riesgo de diabetes gestacional (cuatro ensayos) y el riesgo de tener un bebé con bajo peso al nacer (menos de 2500 g) (cinco ensayos).
-
Puede haber poca o ninguna diferencia con respecto al riesgo de parto prematuro (siete ensayos).
-
En cuanto a los eventos adversos maternos, la administración de suplementos de vitamina D puede reducir el riesgo de hemorragia posparto grave, aunque se debe tener en cuenta que este resultado se basa en los hallazgos de un único ensayo que además fue inesperado y no se había documentado antes en otro estudio. No se conocen con certeza los efectos sobre el riesgo de síndrome nefrítico. Ningún ensayo informó sobre casos de hipercalcemia. Sin embargo, no es posible establecer conclusiones firmes debido a la escasez de datos en general sobre los eventos adversos maternos.
Para los resultados secundarios, la administración de suplementos de vitamina D en comparación con ninguna intervención o con placebo probablemente da lugar a concentraciones más altas de 25‐hidroxivitamina D (14 ensayos). Este resultado se debe interpretar con precaución porque la respuesta a la administración de suplementos fue muy heterogénea. Lo anterior podría deberse a las diferentes dosis utilizadas en los ensayos (desde 200 UI/día hasta 4000 UI/día), las diferentes frecuencias de administración de los suplementos (es decir, diarios, semanales o en bolo), el diferente inicio de la administración de suplementos (es decir, antes o después de la semana 20), y también a las diferencias en los métodos para evaluar la 25‐hidroxivitamina D sérica. Este biomarcador es difícil y complejo, con una alta variabilidad en los resultados entre los métodos utilizados (Holick 2008). La cromatografía líquida de alta resolución con espectrometría de masas es el mejor método disponible (Holick 2005), pero sólo dos ensayos utilizaron este método. Por lo tanto, los resultados deben ser interpretados con cautela.
Administración de suplementos con vitamina D + calcio en comparación con ninguna intervención o placebo.
-
Probablemente reduce el riesgo de preeclampsia (cuatro ensayos);
-
Puede aumentar el riesgo de parto prematuro de < 37 semanas (cinco ensayos). Sin embargo, los resultados se basan principalmente en un solo ensayo; por lo tanto, los resultados se deben interpretarse con precaución;
-
No se conocen con certeza los efectos sobre el riesgo de diabetes gestacional (un ensayo) y el riesgo de tener un recién nacido con bajo peso al nacer (dos ensayos);
-
Ningún ensayo informó sobre eventos adversos maternos.
Solo un ensayo evaluó la administración de suplementos de vitamina D, calcio, hierro y ácido fólico en comparación con calcio, hierro y ácido fólico, pero sin vitamina D, con poca o ninguna diferencia en el riesgo de parto prematuro o bajo peso al nacer, y los hallazgos para otros resultados fueron inciertos debido a la evidencia de certeza muy baja.
En general se necesitan más datos para establecer conclusiones sobre el riesgo de eventos adversos maternos.
Compleción y aplicabilidad general de las pruebas
El objetivo de la presente revisión fue comparar los ensayos que proporcionaron cualquier dosis de suplementos de vitamina D durante el embarazo con placebo o ninguna intervención para mejorar los resultados del embarazo y neonatales. En esta actualización el número de ensayos incluidos se duplicó en comparación con la versión anterior (De‐Regil 2016), y algunos de los resultados parecen ser más consistentes. Sin embargo, todavía hay un número limitado de ensayos que informan sobre ciertos resultados maternos (eventos adversos, alteración de la tolerancia a la glucosa, hipertensión gestacional o muerte), así como sobre los resultados infantiles (muerte neonatal, ingreso a la atención especial en el período neonatal, puntuación de Apgar menor de siete a los cinco minutos, infección neonatal o parto muy prematuro).
En general, esta revisión mostró que la administración de suplementos de vitamina D probablemente reduce el riesgo de preeclampsia y diabetes gestacional y puede reducir el riesgo de bajo peso al nacer, pero puede hacer poca o ninguna diferencia con respecto al riesgo de tener un parto prematuro. Se necesita más información para determinar la seguridad de la intervención.
¿Qué le falta a la revisión global?
Se necesitan más ensayos para cada uno de los resultados principales, ya que la mayoría de los resultados sólo se informaron en unos pocos ensayos (de dos a cinco ensayos) y con tamaños de muestra más grandes. La mayoría de los ensayos fueron de tamaño pequeño a mediano; 14 ensayos incluyeron menos de 100 participantes (Asemi 2012; Asemi 2013a; Benson 2009; Bhutta 2011; Delvin 1986; Diogenes 2013; Kaur 1991; Li 2000a; Mallet 1986; Mazurkevich 2013; Sabet 2012; Samimi 2016; Samimi 2017; Shahgheibi 2016), 13 ensayos incluyeron de 100 a 500 participantes (Brooke 1980; Grant 2013; Marya 1987; Marya 1988; Mirghafourvand 2013; Naghshineh 2016; Roth 2010; Sablok 2015; Sasan 2017; Singh 2015; Tehrani 2014; Vaziri 2016; Yu 2008), y solo tres ensayos incluyeron más de 500 participantes (Harvey 2012; Roth 2013; Taherian 2002). Otro factor que no estuvo presente fue la falta de especificación del índice de masa corporal (IMC) pre‐gestacional y la pigmentación de la piel, dos determinantes importantes del estado de la vitamina D. Además, la mayoría de los ensayos no tuvieron en cuenta el estado de la vitamina D al comienzo del ensayo, lo cual es importante, ya que los efectos de la administración de suplementos de vitamina D pueden ser más profundos entre las mujeres con deficiencia de vitamina D. La mayoría de los ensayos proporcionaron vitamina D sola o con calcio. Solo un ensayo (Roth 2013) comparó la vitamina D con otros nutrientes, que es lo que en la práctica tomarían la mayoría de las mujeres. Es importante evaluar este hecho, ya que podría haber interacciones entre los nutrientes de los suplementos dietéticos que se deben probar. Además, se necesitan más ensayos que comiencen antes del embarazo, ya que solo siete ensayos comenzaron la administración de suplementos antes de la semana 20 (Benson 2009; Bhutta 2011; Harvey 2012; Naghshineh 2016; Samimi 2017; Singh 2015; Tehrani 2014). Los efectos de la vitamina D pueden ser más importantes si se comienza al inicio del embarazo, ya que la enzima 1‐alfa‐hidroxilasa, que cataliza la síntesis de 1,25‐dihidroxi vitamina D3, tiene el nivel más alto de expresión en el primer trimestre y se reduce hacia el tercer trimestre, lo que destaca su posible función al inicio del embarazo (Zehnder 2002).
Por lo tanto, se necesitan ensayos más grandes que comiencen la administración al inicio del embarazo y evalúen los efectos de la vitamina D en combinación con otros nutrientes sobre los resultados maternos e infantiles. Además, estos ensayos deben tener en cuenta el IMC previo al embarazo, la pigmentación de la piel y el estado de la vitamina D al inicio del estudio, ya que las pacientes con deficiencia de vitamina D se podrían beneficiar más. Aunque el ensayo realizado por Roth 2013 es el más grande hasta ahora (> 1500 participantes) y probó diferentes dosis de vitamina D en combinación con otros nutrientes entre mujeres con 64% de deficiencia de vitamina D, comenzó en el segundo trimestre del embarazo. Lo anterior podría explicar la falta de efectos significativos sobre estos resultados de salud. Como se mencionó anteriormente, la enzima 1‐alfa‐hidroxilasa tiene el nivel más alto de expresión en el primer trimestre, lo que destaca su posible función al comienzo del embarazo (Zehnder 2002).
Se identificaron 60 ensayos que luego se excluyeron, principalmente porque las comparaciones se realizaron entre diferentes dosis de vitamina D sin placebo o sin grupo de administración de suplementos o en embarazadas con intolerancia a la glucosa, diabetes gestacional u otras afecciones crónicas. No se incluyeron ensayos con diferentes dosis y sin placebo, ya que la mayoría de los países no tienen la política de incluir la vitamina D en sus guías de administración de suplementos prenatales. Por lo tanto, es importante determinar primero si la administración de suplementos de vitamina D es beneficiosa contra un grupo placebo o un grupo ninguna intervención.
Hasta donde se conoce, actualmente hay seis estudios en curso que, una vez publicados, aumentarán el conjunto de evidencia identificado para la actualización de esta revisión. Además, las actualizaciones podrían incluir la respuesta a la dosis de la administración de suplementos de vitamina D sobre resultados importantes del embarazo. De hecho, existe otra revisión que tendrá en cuenta los ensayos con diferentes dosis de vitamina D para determinar el mejor régimen a proporcionar durante el embarazo para mejorar los resultados de salud prenatal y neonatal (Palacios 2018).
Calidad de la evidencia
El riesgo de sesgo fue alto para la asignación o el cegamiento en 14 ensayos y para el desgaste en diez ensayos. Además, los resultados variaron de manera considerable entre los ensayos, lo que podría estar relacionado con la variabilidad en los regímenes de vitamina D utilizados. Por ejemplo, nueve ensayos utilizaron dosis de aproximadamente 200 a 600 UI de vitamina D por día. Aunque se podrían considerar dosis bajas, son las dosis recomendadas para el embarazo por varias organizaciones (EFSA 2016; IOM 2011; RCOG 2014; WHO 2004). Doce estudios utilizaron dosis medias de 800 a 2000 UI de vitamina D al día; y ocho ensayos utilizaron dosis > 2000 UI de vitamina D al día. También hubo una gran variabilidad en la frecuencia de administración de los suplementos; 20 ensayos proporcionaron vitamina D diariamente; seis de manera semanal o mensual; tres administraron una dosis de bolo una o dos veces y uno combinó la administración diaria y el bolo. Finalmente, como se mencionó antes, solo siete ensayos comenzaron la administración de suplementos antes de la semana 20. Los efectos de la vitamina D pueden ser más importantes si se comienza al inicio del embarazo, ya que la enzima 1‐alfa‐hidroxilasa, que cataliza la síntesis de 1,25‐dihidroxi vitamina D3, tiene el nivel más alto de expresión en el primer trimestre y se reduce hacia el tercer trimestre, lo que destaca su posible función al inicio del embarazo (Zehnder 2002). Estas diferencias pueden haber influido en los resultados observados.
Según el riesgo de sesgo y los resultados de los estudios, la certeza del conjunto de evidencia para los resultados primarios se evaluó con la metodología GRADE para la Comparación 1 (vitamina D sola versus placebo/ninguna intervención; Resumen de los hallazgos, tabla 1), la Comparación 2 (vitamina D + calcio versus placebo/ninguna intervención; Resumen de los hallazgos, tabla 2) y la Comparación 3 (vitamina D + calcio + otras vitaminas y minerales versus calcio + otras vitaminas y minerales versus calcio + otras vitaminas y minerales pero sin vitamina D; Resumen de los hallazgos, tabla 3). Se consideró que la inconsistencia o el sesgo de publicación fueron poco probables, pero el riesgo de sesgo de los ensayos y la imprecisión dio lugar a: evidencia de certeza moderada para la preeclampsia, la diabetes gestacional y el bajo peso al nacer; evidencia de certeza baja para la hemorragia posparto grave, la hipercalcemia y el parto prematuro; y evidencia de certeza muy baja para el síndrome nefrítico en la comparación de la administración de suplementos de vitamina D sola versus ninguna intervención o placebo. La certeza de la evidencia en los ensayos que evaluaron la administración de suplementos de vitamina D más calcio fue moderada para la preeclampsia, baja para el parto prematuro y muy baja para la diabetes gestacional y el bajo peso al nacer. La certeza de la evidencia en los ensayos que evaluaron la administración de suplementos de vitamina D + calcio + otras vitaminas y minerales fue baja para el parto prematuro y el bajo peso al nacer y muy baja para la diabetes gestacional y los eventos adversos maternos.
Sesgos potenciales en el proceso de revisión
Se identificaron varios sesgos potenciales en el proceso de revisión. Se redujeron al mínimo de dos maneras: (1) dos revisores evaluaron de forma independiente la elegibilidad para la inclusión y la extracción de los datos y (2) dos revisores también examinaron de forma independiente la evaluación del riesgo de sesgo y la introducción de los datos. Sin embargo, cuando se examinan las evaluaciones de elegibilidad y el riesgo de sesgo es necesario hacer varias valoraciones subjetivas. Otros pueden haber tomado decisiones diferentes con respecto a estas cuestiones. Se invita a los lectores a examinar las tablas de características de estudios incluidos para ayudar a la interpretación de los resultados.
Acuerdos y desacuerdos con otros estudios o revisiones
Esta revisión actualiza la revisión Cochrane anterior sobre la administración de suplementos de vitamina D en el embarazo (De‐Regil 2012; De‐Regil 2016). La revisión de 2012 incluyó seis ensayos con 1023 mujeres, excluyó ocho ensayos y seis aún estaban en curso, mientras que la revisión de 2016 incluyó 15 ensayos que evaluaron 2833 mujeres, excluyó 27 ensayos y 23 ensayos aún estaban en curso o no se habían publicado. En la revisión de 2016, los autores concluyeron que la administración de suplementos de vitamina D a las embarazadas aumentó de manera significativa la 25‐hidroxivitamina D sérica al término y puede reducir el riesgo de preeclampsia, bajo peso al nacer y parto prematuro. Sin embargo la combinación de vitamina D y calcio puede aumentar el riesgo de parto prematuro. En esta actualización los resultados son similares, pero están reforzados por un mayor número de ensayos que informan sobre cada resultado. La única diferencia observada fue que en la revisión de 2016 se observó una mayor circunferencia cefálica en los recién nacidos de mujeres que recibieron suplementos de vitamina D durante el embarazo, pero en esta actualización no se observó este hallazgo. Todavía no hay datos suficientes para confirmar los efectos sobre otros resultados de salud materna e infantil, ya que éstos no se informaron o solo se evaluaron en uno o dos ensayos.
Los resultados coinciden en parte con otras revisiones sistemáticas similares. Por ejemplo, Harvey 2014 comparó los efectos de la administración de suplementos de vitamina D con placebo o un nivel más bajo de vitamina D sobre los resultados de salud materna y neonatal. Entre los siete ensayos que evaluaron el peso al nacer, tres demostraron un peso al nacer significativamente mayor en los recién nacidos de madres que recibieron suplementos, mientras que los otros cuatro no encontraron un efecto significativo. Para la duración del parto se identificaron dos ensayos; uno halló que la administración de suplementos de vitamina D provocó una mayor duración del parto de los lactantes de pacientes que recibieron suplementos, mientras que el otro ensayo encontró que "no hubo una asociación significativa, aunque hubo una tendencia de una duración mayor del parto en el grupo que recibió suplementos" en comparación con el grupo control. Además, en los dos ensayos que evaluaron el perímetro cefálico de la descendencia, uno encontró un perímetro cefálico significativamente mayor, mientras que el otro encontró una tendencia no significativa hacia un mayor perímetro cefálico en las madres que recibieron suplementos. Solamente se identificó una intervención para la preeclampsia (ninguna diferencia en el riesgo entre los grupos) y no se identificaron intervenciones para el parto prematuro, el bajo peso al nacer, la diabetes gestacional y la cesárea. Otra revisión sistemática y metanálisis de 13 ensayos (n = 2299) comparó la administración de suplementos de vitamina D (con o sin calcio) con un grupo placebo, incluido el bajo nivel de vitamina D (400 UI/día) en el embarazo (Perez‐Lopez 2015). La 25‐hidroxivitamina D sérica al término fue significativamente mayor en el grupo que recibió suplementos en comparación con el grupo control (diferencia de medias: 66,5 nmol/l; IC del 95%: 66,2 a 66,7; diez ensayos, 1468 participantes), similar a la presente revisión. Sin embargo, contrario a la presente revisión, Perez‐Lopez 2015 encontró que la administración de suplementos de vitamina D no tuvo efectos sobre la preeclampsia (tres ensayos; 654 participantes), la diabetes mellitus gestacional (tres ensayos; 384 participantes), el bajo peso al nacer (cuatro ensayos; 496 participantes) y el parto prematuro (tres ensayos; 384 participantes), mientras que aumentó de manera significativa el peso al nacer (diez ensayos; 1489 participantes) y la duración del parto (seis ensayos; 866 participantes). De acuerdo con la presente revisión, la administración de suplementos de vitamina D no tuvo efectos sobre la cesárea (cuatro ensayos; 1028 participantes). Otra revisión evaluó el efecto de la administración de suplementos de vitamina D versus un grupo control, que también incluyó una dosis baja de vitamina D (400 UI/d) o ninguna intervención durante el embarazo para reducir el riesgo de preeclampsia (Hyppönen 2013). De los cuatro ensayos identificados, que incluyeron 5871 mujeres, dos estudios compararon 2000 UI/día versus 4000 UI/día, otro comparó 1200 UI/día versus ninguna vitamina D, un ensayo muy antiguo comparó 450 UI/día versus ninguna vitamina D y otro ensayo comparó varios suplementos con ninguna administración de suplementos. Encontraron que la intervención redujo de manera significativa el riesgo de preeclampsia en comparación con el grupo control (odds ratio [OR] 0,66; IC del 95%: 0,52 a 0,83); sin embargo, utilizaron ensayos muy diferentes. La revisión de Thorne‐Lyman 2012 también incluyó ensayos con dosis bajas de vitamina D (400I UI/día) en el grupo placebo o control. No se observaron efectos en la reducción del parto prematuro en los dos ensayos incluidos (529 participantes) con la administración de suplementos de vitamina D, aunque se observó un riesgo 60% menor de bajo peso al nacer con los suplementos (tres ensayos; 507 participantes). Una revisión sistemática más reciente realizada por Roth 2017 que incluyó 43 ensayos con 8406 participantes comparó la administración de suplementos de vitamina D versus un grupo placebo (con menos de 600 UI/día) o ninguna intervención. La administración de suplementos de vitamina D aumentó significativamente la cantidad de 25‐hidroxivitamina D materna a término, pero el efecto dosis‐respuesta fue débil. Además, las mujeres asignadas a la administración de suplementos de vitamina D tuvieron recién nacidos con un peso medio al nacer más alto en comparación con los controles, pero no se encontraron efectos sobre el riesgo de preeclampsia, diabetes gestacional, parto prematuro y bajo peso al nacer, contrario a lo que indicó la presente revisión. Sin embargo, de manera similar a esta revisión, Roth 2017 tampoco encontró efectos de la administración de suplementos de vitamina D sobre el riesgo de hipertensión gestacional, cesárea, ingreso hospitalario, muerte neonatal, mortinatalidad u otros eventos adversos. Este metanálisis también encontró que la mayoría de los ensayos tuvieron alto riesgo de sesgo. Es importante señalar que Roth 2017 incluyó ensayos con bajos niveles de vitamina D en el grupo control, mientras que en la presente revisión solo se incluyeron ensayos sin vitamina D o con ninguna intervención en el grupo control.
En general, las revisiones sistemáticas y los metanálisis disponibles mostraron de forma consistente que los niveles séricos de 25‐hidroxivitamina D mejoran significativamente con la administración de suplementos de vitamina D. Sin embargo, hay diferencias importantes en los resultados. Aunque esta revisión y otra (Hyppönen 2013) mostraron una reducción significativa en el riesgo de preeclampsia con la administración de suplementos de vitamina D, una revisión (Perez‐Lopez 2015; Roth 2017) no encontró esta reducción. Además, se encontraron diferencias en la reducción del riesgo de parto prematuro, diabetes gestacional y bajo peso al nacer. Las principales diferencias encontradas entre los resultados de la presente revisión y las revisiones mencionadas anteriormente son los criterios de inclusión de los ensayos. Solo se incluyeron ensayos que compararon cualquier dosis de vitamina D con un grupo placebo con 0 UI/día o ninguna intervención. Sin embargo, los ensayos descritos anteriormente compararon la administración de suplementos de vitamina D con un grupo placebo que podría tener un nivel bajo de vitamina D o ninguna intervención.
Con respecto a la seguridad, los ensayos que informaron resultados maternos e infantiles relacionados con la seguridad podrían indicar que la administración de suplementos de vitamina D durante el embarazo podría ser segura. Sin embargo, este tema se evaluó de forma diferente en los ensayos, por ejemplo, mediante la evaluación de la hemorragia, el síndrome nefrítico, la hipercalcemia y la hipercalciuria. Por lo tanto, la seguridad de esta intervención aún necesita de estudios adicionales. Además, ninguno de los ensayos informó la mayoría de los resultados definidos en esta revisión (muerte materna, ingreso del neonato a una unidad de cuidados intensivos, puntuación de Apgar menor que siete a los cinco minutos, infección neonatal o parto muy prematuro). El ensayo de Sablok 2015 informó sobre una puntuación de Apgar menor que tres o siete, sin diferencias entre los grupos con suplementos o con placebo. Se necesitan más ensayos para informar sobre estos resultados relacionados con la seguridad para establecer una conclusión definitiva.

Study flow diagram for this update

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
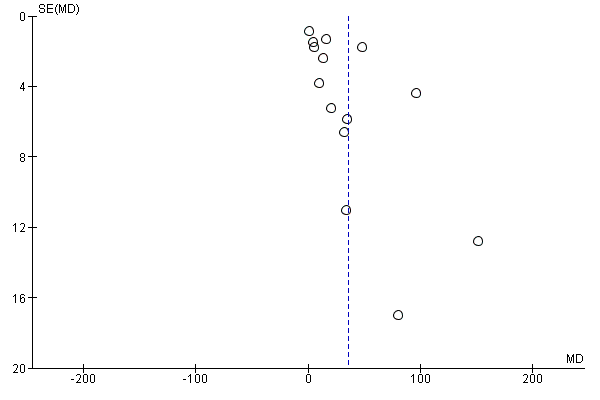
Funnel plot of comparison: 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), outcome: 1.15 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (ALL).
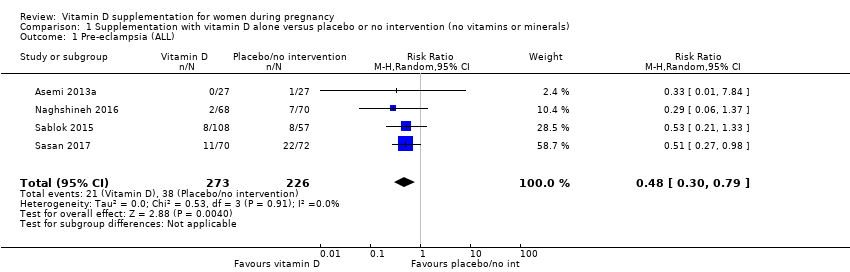
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 1 Pre‐eclampsia (ALL).
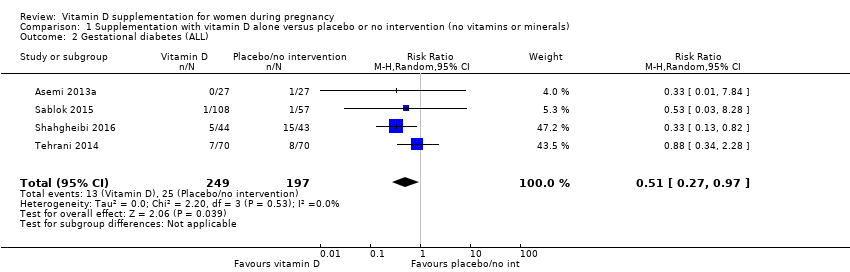
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 2 Gestational diabetes (ALL).
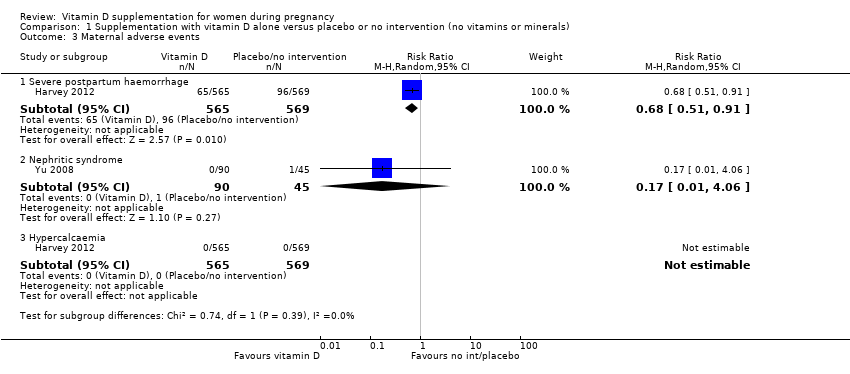
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 3 Maternal adverse events.
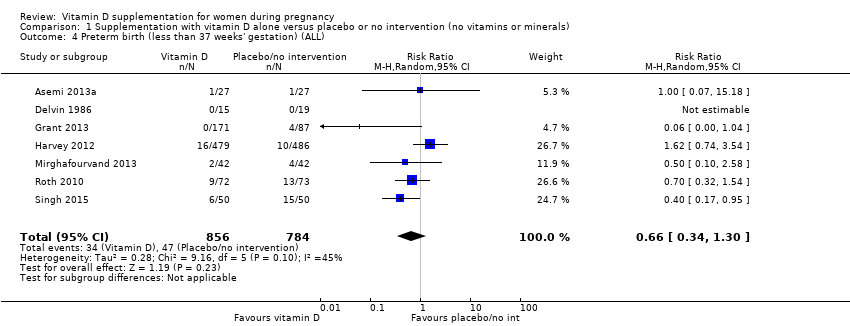
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 4 Preterm birth (less than 37 weeks' gestation) (ALL).
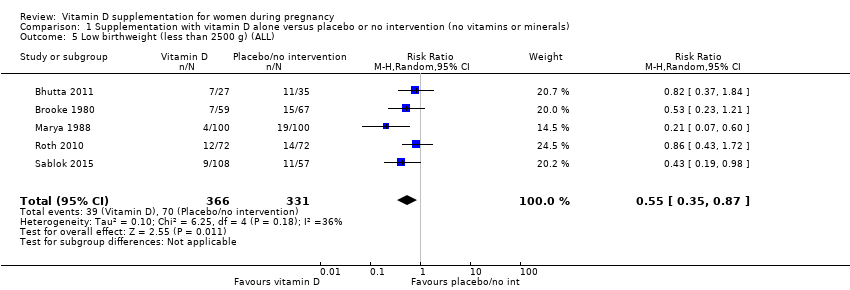
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 5 Low birthweight (less than 2500 g) (ALL).
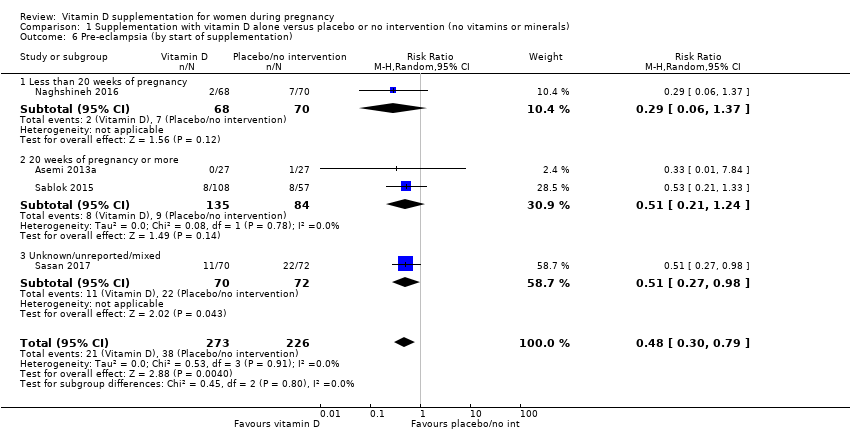
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 6 Pre‐eclampsia (by start of supplementation).

Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 7 Pre‐eclampsia (by pre‐gestational BMI).

Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 8 Pre‐eclampsia (by supplementation scheme/regimen).

Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 9 Pre‐eclampsia (by skin pigmentation based on Fitzpatrick skin tone chart).

Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 10 Pre‐eclampsia (by latitude).
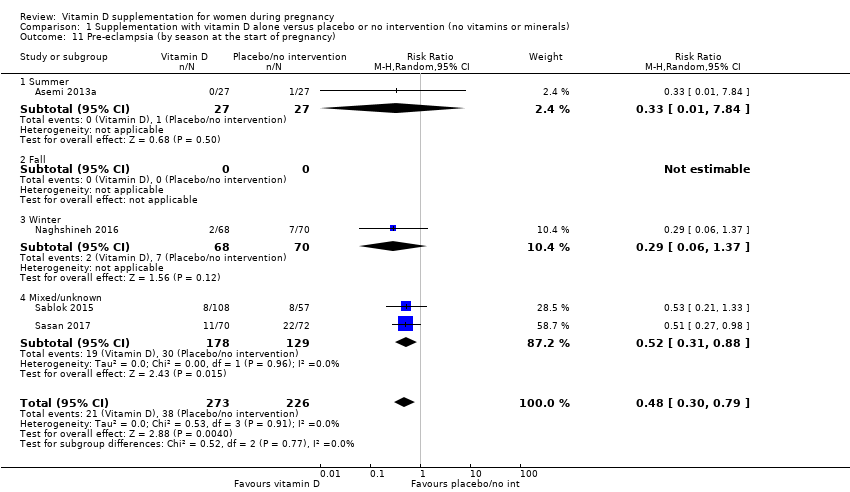
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 11 Pre‐eclampsia (by season at the start of pregnancy).

Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 12 Gestational diabetes (by start of supplementation).
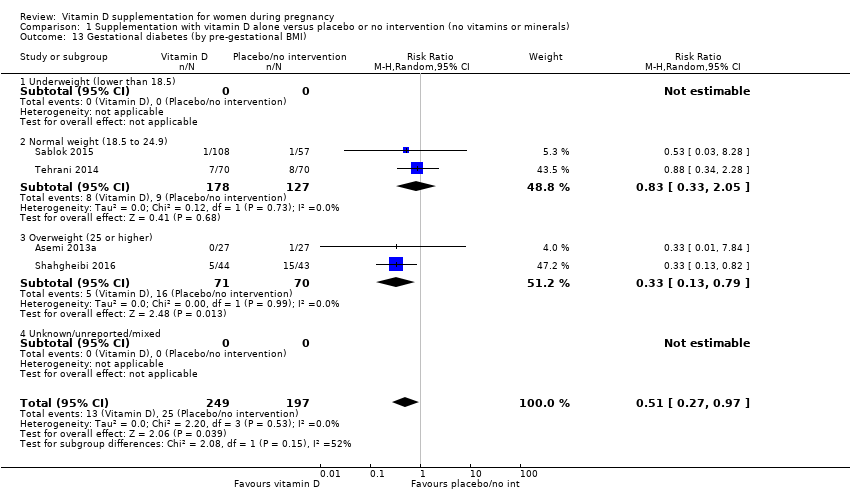
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 13 Gestational diabetes (by pre‐gestational BMI).

Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 14 Gestational diabetes (by supplementation scheme/regimen).

Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 15 Gestational diabetes (by skin pigmentation based on Fitzpatrick skin tone chart).
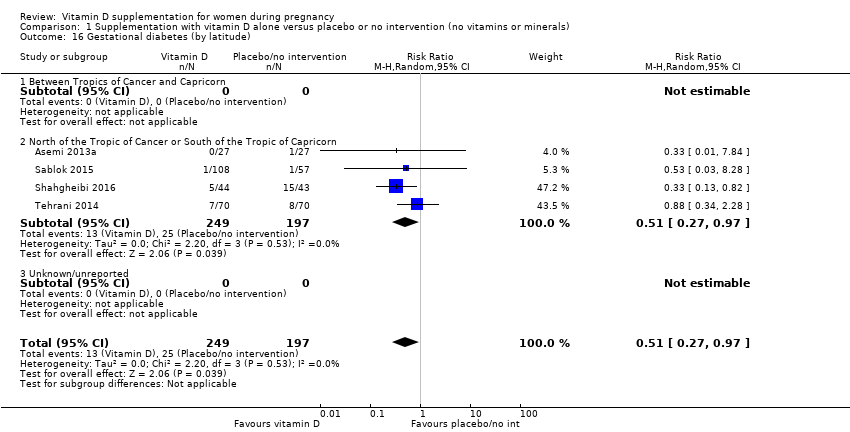
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 16 Gestational diabetes (by latitude).

Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 17 Gestational diabetes (by season at the start of supplementation).
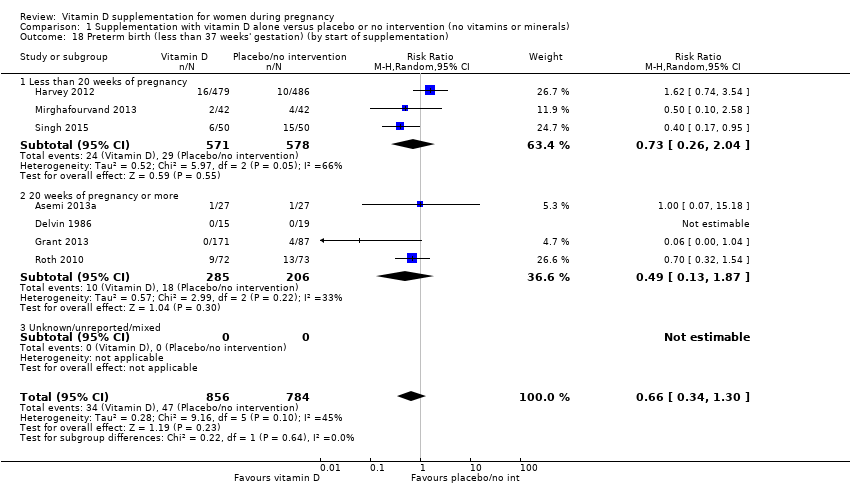
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 18 Preterm birth (less than 37 weeks' gestation) (by start of supplementation).

Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 19 Preterm birth (less than 37 weeks' gestation) (by pre‐gestational BMI).

Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 20 Preterm birth (less than 37 weeks' gestation) (by supplementation scheme/regimen).

Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 21 Preterm birth (less than 37 weeks' gestation) (by skin pigmentation based on Fitzpatrick skin tone chart).

Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 22 Preterm birth (less than 37 weeks' gestation) (by latitude).

Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 23 Preterm birth (less than 37 weeks' gestation) (by season at the start of supplementation).

Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 24 Low birthweight (less than 2500 g) (by start of supplementation).

Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 25 Low birthweight (less than 2500 g) (by pre‐gestational BMI).

Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 26 Low birthweight (less than 2500 g) (by supplementation scheme/regimen).
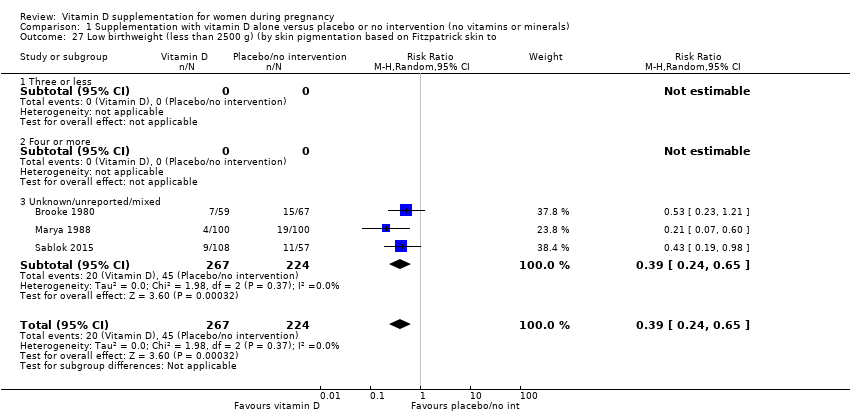
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 27 Low birthweight (less than 2500 g) (by skin pigmentation based on Fitzpatrick skin to.
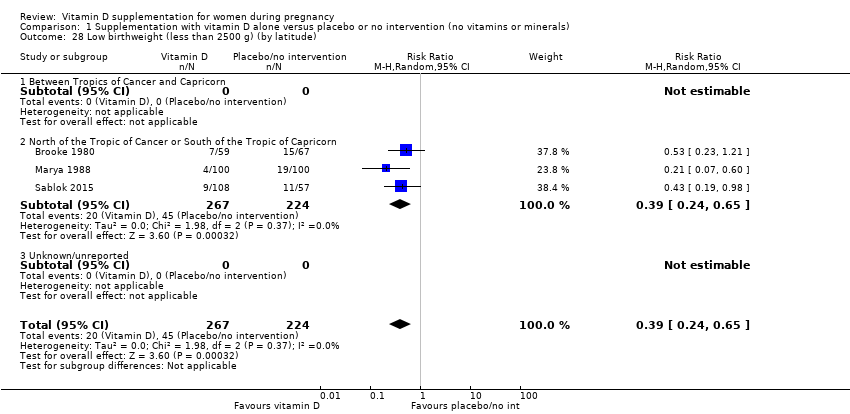
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 28 Low birthweight (less than 2500 g) (by latitude).

Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 29 Low birthweight (less than 2500 g) (by season at the start of pregnancy).
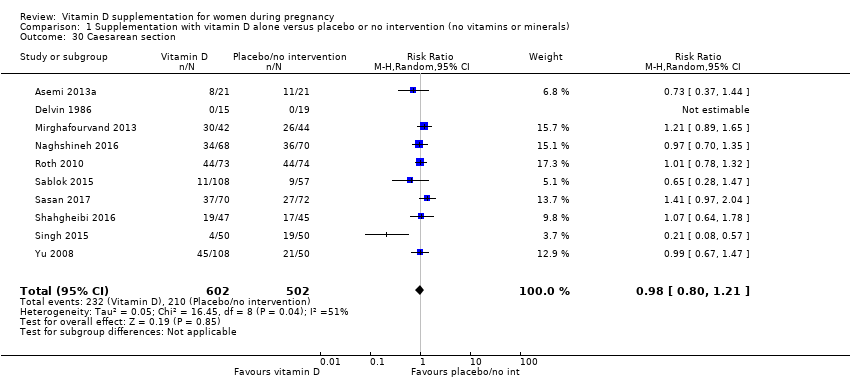
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 30 Caesarean section.
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 31 Gestational hypertension.

Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 32 Maternal death (death while pregnant or within 42 days of termination of pregnancy) (ALL).

Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 33 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (ALL).

Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 34 Birth length (cm).

Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 35 Head circumference at birth (cm).
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 36 Birthweight (g).

Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 37 Stillbirth.
Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 38 Neonatal death.

Comparison 1 Supplementation with vitamin D alone versus placebo or no intervention (no vitamins or minerals), Outcome 39 Apgar score less than seven at five minutes.
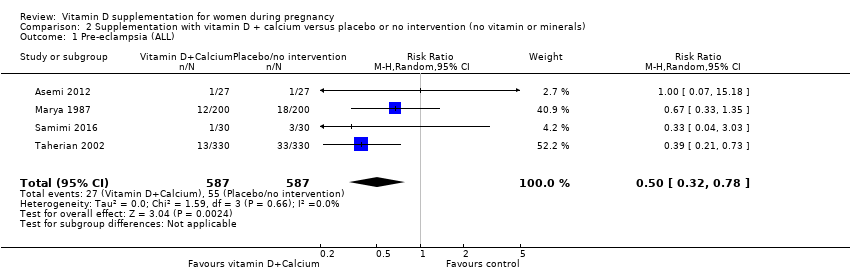
Comparison 2 Supplementation with vitamin D + calcium versus placebo or no intervention (no vitamin or minerals), Outcome 1 Pre‐eclampsia (ALL).

Comparison 2 Supplementation with vitamin D + calcium versus placebo or no intervention (no vitamin or minerals), Outcome 2 Gestational diabetes (ALL).
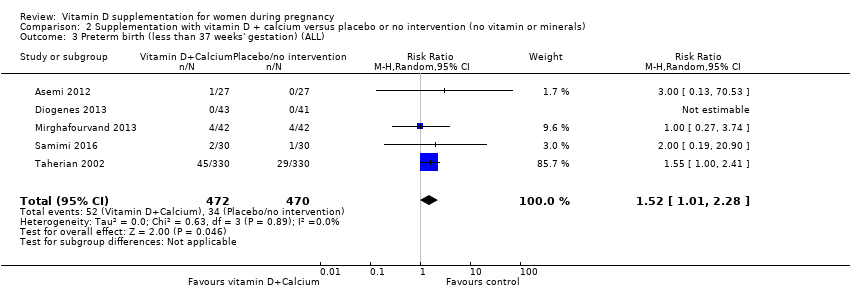
Comparison 2 Supplementation with vitamin D + calcium versus placebo or no intervention (no vitamin or minerals), Outcome 3 Preterm birth (less than 37 weeks' gestation) (ALL).
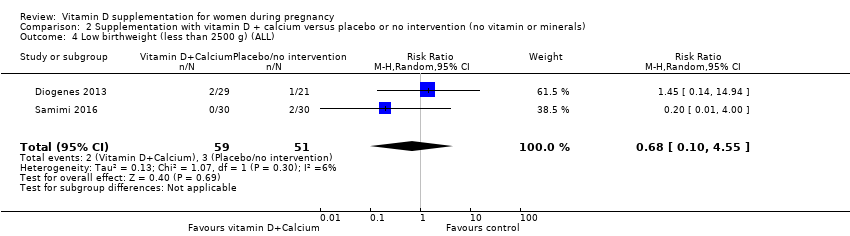
Comparison 2 Supplementation with vitamin D + calcium versus placebo or no intervention (no vitamin or minerals), Outcome 4 Low birthweight (less than 2500 g) (ALL).

Comparison 2 Supplementation with vitamin D + calcium versus placebo or no intervention (no vitamin or minerals), Outcome 5 Caesarean section.
Comparison 2 Supplementation with vitamin D + calcium versus placebo or no intervention (no vitamin or minerals), Outcome 6 Gestational hypertension.

Comparison 2 Supplementation with vitamin D + calcium versus placebo or no intervention (no vitamin or minerals), Outcome 7 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (ALL).

Comparison 2 Supplementation with vitamin D + calcium versus placebo or no intervention (no vitamin or minerals), Outcome 8 Birth length (cm).

Comparison 2 Supplementation with vitamin D + calcium versus placebo or no intervention (no vitamin or minerals), Outcome 9 Head circumference at birth (cm).
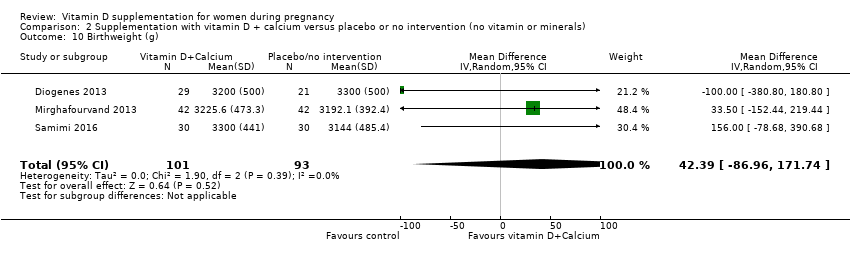
Comparison 2 Supplementation with vitamin D + calcium versus placebo or no intervention (no vitamin or minerals), Outcome 10 Birthweight (g).

Comparison 2 Supplementation with vitamin D + calcium versus placebo or no intervention (no vitamin or minerals), Outcome 11 Neonatal death.
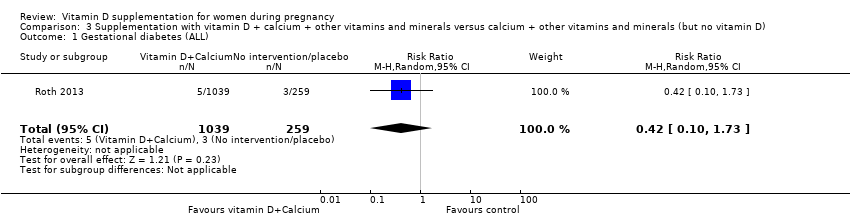
Comparison 3 Supplementation with vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals (but no vitamin D), Outcome 1 Gestational diabetes (ALL).
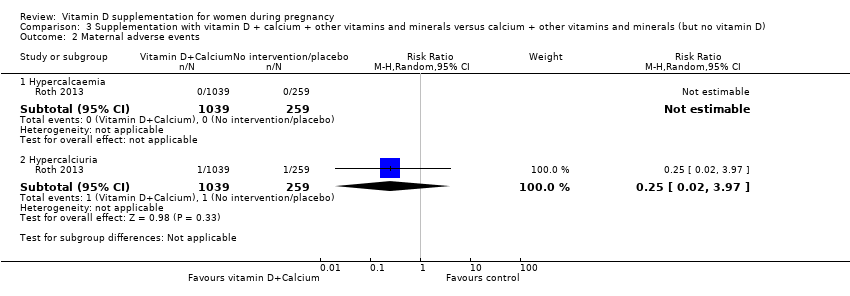
Comparison 3 Supplementation with vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals (but no vitamin D), Outcome 2 Maternal adverse events.

Comparison 3 Supplementation with vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals (but no vitamin D), Outcome 3 Preterm birth (less than 37 weeks' gestation) (ALL).

Comparison 3 Supplementation with vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals (but no vitamin D), Outcome 4 Low birthweight (less than 2500 g) (ALL).
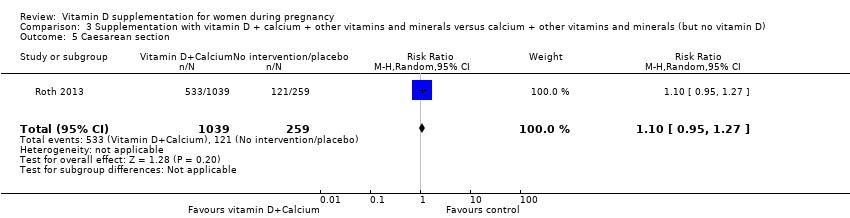
Comparison 3 Supplementation with vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals (but no vitamin D), Outcome 5 Caesarean section.

Comparison 3 Supplementation with vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals (but no vitamin D), Outcome 6 Gestational hypertension.

Comparison 3 Supplementation with vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals (but no vitamin D), Outcome 7 Maternal death (death while pregnant or within 42 days of termination of pregnancy).

Comparison 3 Supplementation with vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals (but no vitamin D), Outcome 8 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (ALL).

Comparison 3 Supplementation with vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals (but no vitamin D), Outcome 9 Birth length (cm).
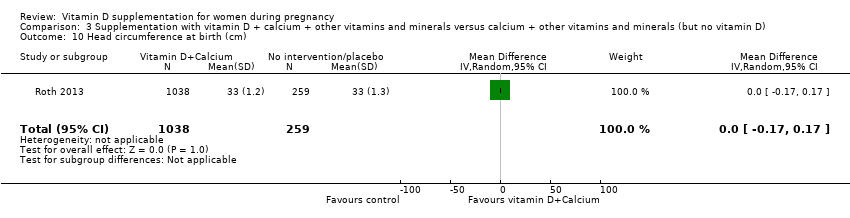
Comparison 3 Supplementation with vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals (but no vitamin D), Outcome 10 Head circumference at birth (cm).
Comparison 3 Supplementation with vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals (but no vitamin D), Outcome 11 Birthweight (g).

Comparison 3 Supplementation with vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals (but no vitamin D), Outcome 12 Stillbirth.
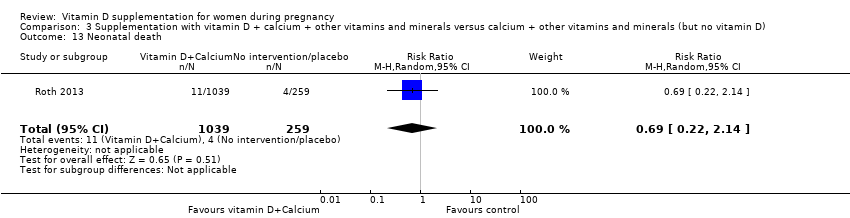
Comparison 3 Supplementation with vitamin D + calcium + other vitamins and minerals versus calcium + other vitamins and minerals (but no vitamin D), Outcome 13 Neonatal death.
| Vitamin D supplementation compared to placebo/control for pregnancy and neonatal health outcomes | ||||||
| Patient or population: pregnant women and their infants. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo/control | Risk with vitamin D supplementation | |||||
| Pre‐eclampsia | Study population | RR 0.48 (0.30, 0.79) | 499 | ⊕⊕⊕⊝ | Included trials: Asemi 2013a; Naghshineh 2016; Sablok 2015; Sasan 2017 | |
| 168 per 1000 | 79 per 1000 | |||||
| Gestational diabetes | Study population | RR 0.51 | 446 | ⊕⊕⊕⊝ | Included trials: Asemi 2013a; Sablok 2015; Shahgheibi 2016; Tehrani 2014 | |
| 127 per 1000 | 65 per 1000 | |||||
| Maternal adverse events: severe postpartum haemorrhage | Study population | RR 0.68 | 1134 | ⊕⊕⊝⊝ | Included trial: Harvey 2012 | |
| 158 per 1000 | 106 per 1000 | |||||
| Maternal adverse event: nephritic syndrome | Study population | RR 0.17 (0.01 to 4.06) | 135 (1 RCT) | ⊕⊝⊝⊝ | Included trial: Yu 2008 | |
| 22 per 1000 | 4 per 1000 (0 to 90) | |||||
| Maternal adverse event: hypercalcaemia | Study population | Not estimable | 1134 | ⊕⊕⊝⊝ | Included trial: Harvey 2012 | |
| 0 per 1000 | 0 per 1000 | |||||
| Preterm birth (less than 37 weeks' gestation) | Study population | RR 0.66 (0.34 to 1.30) | 1640 | ⊕⊕⊝⊝ | Included trials: Asemi 2013a; Delvin 1986; Grant 2013; Harvey 2012; Mirghafourvand 2013; Roth 2010; Singh 2015 | |
| 87 per 1000 | 57 per 1000 | |||||
| Low birthweight (less than 2500 g) | Study population | RR 0.55 | 697 | ⊕⊕⊕⊝ | Included trials: Brooke 1980; Bhutta 2011; Marya 1988; Roth 2010; Sablok 2015 | |
| 136 per 1000 | 75 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We downgraded (1) level for serious limitations in study design due to one trial being assessed as high risk of bias for several domains and two trials having unclear allocation concealment. 2 We downgraded (1) level for serious limitations in study design due to one trial being assessed as high risk of bias for several domains. 3 We downgraded (2) levels for very serious limitations in study design due to one study being assessed as high risk of other bias because we do not know the impact of the participants who were allowed to continue taking their own multivitamin with 400 IU/d of vitamin D as this was not recorded. 4 We downgraded (1) level for serious limitations in study design due to one study being assessed as high risk of bias for performance and detection bias. 5 We downgraded (2) levels for very serious limitations in imprecision as only one small study, with a small number of events and wide 95% confidence intervals (CI) contributed data. 6 We downgraded (1) level for serious limitations in imprecision due to a single study with zero events contributing data. 7 We downgraded (1) level for serious limitations in study design due to two studies being at unclear risk of selection bias and one study being at high risk of other bias. 8 We downgraded (1) level for serious limitations in imprecision as the 95% confidence interval (CI) was wide and crossed the line of no effect. 9 We downgraded (1) level for serious limitations in study design due to two studies being at unclear risk of selection bias, one study being at high risk of bias for allocation concealment, and three studies being at high risk of attrition bias. | ||||||
| Vitamin D + calcium supplementation compared to placebo/control for pregnancy and neonatal health outcomes | |||||||
| Patient or population: pregnant women and their infants.. | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | ||
| Risk with placebo/control | Risk with vitamin D + calcium supplementation | ||||||
| Pre‐eclampsia | Study population | RR 0.50 | 1174 | ⊕⊕⊕⊝ | Included trials: Asemi 2012; Marya 1987; Samimi 2016; Taherian 2002 | ||
| 94 per 1000 | 47 per 1000 | ||||||
| Gestational diabetes | Study population | RR 0.33 | 54 | ⊕⊝⊝⊝ | Included trial: Asemi 2012 | ||
| 37 per 1000 | 12 per 1000 | ||||||
| Maternal adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | No trials reported on this outcome | |
| Preterm birth (less than 37 weeks' gestation) | Study population | RR 1.52 | 942 | ⊕⊕⊝⊝ | Included trials: Asemi 2012; Diogenes 2013, Mirghafourvand 2013, Samimi 2016; Taherian 2002; | ||
| 72 per 1000 | 110 per 1000 | ||||||
| Low birthweight (less than 2500 g) | Study population | RR 0.68 | 110 | ⊕⊝⊝⊝ | Included trials: Diogenes 2013; Samimi 2016 | ||
| 59 per 1000 | 40 per 1000 | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1 We downgraded (1) level for serious limitations in study design due to one study being at high risk of attrition and selection bias and three studies being at high risk of performance and detection bias. 2 We downgraded (1) level for serious limitations in study design due to one study being at high risk of performance and detection bias. 3 We downgraded (2) levels for very serious limitations in imprecision due to one small study, with a single event and wide 95% confidence intervals (CI) crossing the line of no effect contributing data. 4 We downgraded (1) level for serious limitations in study design due to three studies being at unclear risk of allocation concealment and three studies being at high risk of performance and detection bias. 5 We downgraded (1) level for serious limitations in imprecision due to wide 95% confidence intervals (CI). 6 We downgraded (1) level for serious limitations in study design due to one study being at unclear risk of allocation concealment and one study being at high risk of attrition bias. 7 We downgraded (2) levels for very serious limitations in imprecision due two small studies, with very few events and wide 95% confidence intervals (CI) crossing the line of no effect contributing data. | |||||||
| Vitamin D + calcium + other vitamins and minerals compared to calcium + other vitamins and minerals (but no vitamin D) for pregnancy and neonatal health outcomes | ||||||
| Patient or population: pregnant women and their infants.. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with calcium + other vitamins and minerals (but no vitamin D) | Risk with vitamin D + calcium + other vitamins and minerals | |||||
| Pre‐eclampsia | Study population | ‐ | (0 trials) | ‐ | No trials reported on this outcome | |
| see comment | see comment | |||||
| Gestational diabetes | Study population | RR 0.42 | 1298 | ⊕⊝⊝⊝ | Included trial: Roth 2013 | |
| 12 per 1000 | 5 per 1000 | |||||
| Maternal adverse event: hypercalcaemia | Study population | ‐ | 1298 | ⊕⊝⊝⊝ | Included trial: Roth 2013 | |
| 23 per 1000 | 64 per 1000 | |||||
| Maternal adverse event: hypercalciuria | Study population | 0.25 (0.02 to 3.97) | 1298 | ⊕⊝⊝⊝ | Included trial: Roth 2013 | |
| 4 per 1000 | 1 per 1000 (0 to 15) | |||||
| Preterm birth (less than 37 weeks' gestation) | Study population | RR 1.04 | 1298 | ⊕⊕⊝⊝ | Included trial: Roth 2013 | |
| 93 per 1000 | 96 per 1000 | |||||
| Low birthweight (less than 2500 g) | Study population | RR 1.12 | 1298 | ⊕⊕⊝⊝ | Included trial: Roth 2013 | |
| 162 per 1000 | 182 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We downgraded (2) levels for very serious limitations in imprecision with only one trial, with few events, and wide 95% confidence intervals (CI) crossing the line of no effect contributing data. 2 We downgraded (1) level for serious indirectness as there were multiple nutrient interventions in addition to vitamin D. 2 We downgraded (2) levels for very serious limitations in imprecision with only one trial, with zero events, and wide 95% confidence intervals (CI) crossing the line of no effect contributing data. 3 We downgraded (1) level for serious limitations in imprecision due to only one trial with wide 95% confidence intervals (CI) crossing the line of no effect contributing data. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pre‐eclampsia (ALL) Show forest plot | 4 | 499 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.30, 0.79] |
| 2 Gestational diabetes (ALL) Show forest plot | 4 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.27, 0.97] |
| 3 Maternal adverse events Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Severe postpartum haemorrhage | 1 | 1134 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.51, 0.91] |
| 3.2 Nephritic syndrome | 1 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.01, 4.06] |
| 3.3 Hypercalcaemia | 1 | 1134 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Preterm birth (less than 37 weeks' gestation) (ALL) Show forest plot | 7 | 1640 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.34, 1.30] |
| 5 Low birthweight (less than 2500 g) (ALL) Show forest plot | 5 | 697 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.35, 0.87] |
| 6 Pre‐eclampsia (by start of supplementation) Show forest plot | 4 | 499 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.30, 0.79] |
| 6.1 Less than 20 weeks of pregnancy | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.06, 1.37] |
| 6.2 20 weeks of pregnancy or more | 2 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.21, 1.24] |
| 6.3 Unknown/unreported/mixed | 1 | 142 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.27, 0.98] |
| 7 Pre‐eclampsia (by pre‐gestational BMI) Show forest plot | 4 | 499 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.30, 0.79] |
| 7.1 Underweight (lower than 18.5) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Normal weight (18.5 to 24.9) | 1 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.21, 1.33] |
| 7.3 Overweight (25 or higher) | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.84] |
| 7.4 Unknown/unreported/mixed | 2 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.26, 0.86] |
| 8 Pre‐eclampsia (by supplementation scheme/regimen) Show forest plot | 4 | 499 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.30, 0.79] |
| 8.1 Single dose | 1 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.21, 1.33] |
| 8.2 Daily | 2 | 192 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.08, 1.20] |
| 8.3 Weekly/monthly | 1 | 142 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.27, 0.98] |
| 9 Pre‐eclampsia (by skin pigmentation based on Fitzpatrick skin tone chart) Show forest plot | 4 | 499 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.30, 0.79] |
| 9.1 Three or less | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Four or more | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Unknown/unreported/mixed | 4 | 499 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.30, 0.79] |
| 10 Pre‐eclampsia (by latitude) Show forest plot | 4 | 499 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.30, 0.79] |
| 10.1 Between Tropics of Cancer and Capricorn | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 North of the Tropic of Cancer or South of the Tropic of Capricorn | 4 | 499 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.30, 0.79] |
| 10.3 Unknown/unreported | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Pre‐eclampsia (by season at the start of pregnancy) Show forest plot | 4 | 499 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.30, 0.79] |
| 11.1 Summer | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.84] |
| 11.2 Fall | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Winter | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.06, 1.37] |
| 11.4 Mixed/unknown | 2 | 307 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.31, 0.88] |
| 12 Gestational diabetes (by start of supplementation) Show forest plot | 4 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.27, 0.97] |
| 12.1 Less than 20 weeks of pregnancy | 1 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.34, 2.28] |
| 12.2 20 weeks of pregnancy or more | 2 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.05, 3.45] |
| 12.3 Unknown/unreported/mixed | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.13, 0.82] |
| 13 Gestational diabetes (by pre‐gestational BMI) Show forest plot | 4 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.27, 0.97] |
| 13.1 Underweight (lower than 18.5) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Normal weight (18.5 to 24.9) | 2 | 305 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.33, 2.05] |
| 13.3 Overweight (25 or higher) | 2 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.13, 0.79] |
| 13.4 Unknown/unreported/mixed | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Gestational diabetes (by supplementation scheme/regimen) Show forest plot | 4 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.27, 0.97] |
| 14.1 Single dose | 1 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.03, 8.28] |
| 14.2 Daily | 2 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.13, 0.79] |
| 14.3 Weekly/monthly | 1 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.34, 2.28] |
| 15 Gestational diabetes (by skin pigmentation based on Fitzpatrick skin tone chart) Show forest plot | 4 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.27, 0.97] |
| 15.1 Three or less | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Four or more | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Unknown/unreported/mixed | 4 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.27, 0.97] |
| 16 Gestational diabetes (by latitude) Show forest plot | 4 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.27, 0.97] |
| 16.1 Between Tropics of Cancer and Capricorn | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 North of the Tropic of Cancer or South of the Tropic of Capricorn | 4 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.27, 0.97] |
| 16.3 Unknown/unreported | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Gestational diabetes (by season at the start of supplementation) Show forest plot | 4 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.27, 0.97] |
| 17.1 Summer | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.84] |
| 17.2 Fall | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 Winter | 1 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.34, 2.28] |
| 17.4 Mixed/unknown | 2 | 252 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.14, 0.82] |
| 18 Preterm birth (less than 37 weeks' gestation) (by start of supplementation) Show forest plot | 7 | 1640 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.34, 1.30] |
| 18.1 Less than 20 weeks of pregnancy | 3 | 1149 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.26, 2.04] |
| 18.2 20 weeks of pregnancy or more | 4 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.13, 1.87] |
| 18.3 Unknown/unreported/mixed | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Preterm birth (less than 37 weeks' gestation) (by pre‐gestational BMI) Show forest plot | 7 | 1640 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.34, 1.30] |
| 19.1 Underweight (lower than 18.5) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.2 Normal weight (18.5 to 24.9) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.3 Overweight (25 or higher) | 2 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.15, 2.46] |
| 19.4 Unknown/unreported/mixed | 5 | 1502 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.27, 1.54] |
| 20 Preterm birth (less than 37 weeks' gestation) (by supplementation scheme/regimen) Show forest plot | 7 | 1640 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.34, 1.30] |
| 20.1 Single dose | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 Daily | 6 | 1495 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.23, 1.58] |
| 20.3 Weekly/monthly | 1 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.32, 1.54] |
| 21 Preterm birth (less than 37 weeks' gestation) (by skin pigmentation based on Fitzpatrick skin tone chart) Show forest plot | 9 | 1943 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.30, 0.91] |
| 21.1 Three or less | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 Four or more | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.3 Unknown/unreported/mixed | 9 | 1943 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.30, 0.91] |
| 22 Preterm birth (less than 37 weeks' gestation) (by latitude) Show forest plot | 7 | 1640 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.34, 1.30] |
| 22.1 Between Tropics of Cancer and Capricorn | 2 | 358 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.05, 1.34] |
| 22.2 North of the Tropic of Cancer or South of the Tropic of Capricorn | 5 | 1282 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.59, 1.66] |
| 22.3 Unknown/unreported | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Preterm birth (less than 37 weeks' gestation) (by season at the start of supplementation) Show forest plot | 7 | 1640 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.34, 1.30] |
| 23.1 Summer | 2 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.34, 1.53] |
| 23.2 Winter | 1 | 34 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.3 Mixed/unknown | 4 | 1407 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.19, 1.66] |
| 24 Low birthweight (less than 2500 g) (by start of supplementation) Show forest plot | 3 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.24, 0.65] |
| 24.1 Less than 20 weeks of pregnancy | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 20 weeks of pregnancy or more | 3 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.24, 0.65] |
| 24.3 Unknown/unreported/mixed | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Low birthweight (less than 2500 g) (by pre‐gestational BMI) Show forest plot | 3 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.24, 0.65] |
| 25.1 Underweight (lower than 18.5) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 Normal weight (18.5 to 24.9) | 1 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.19, 0.98] |
| 25.3 Overweight (25 or higher) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.4 Unknown/unreported/mixed | 2 | 326 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.14, 0.88] |
| 26 Low birthweight (less than 2500 g) (by supplementation scheme/regimen) Show forest plot | 3 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.24, 0.65] |
| 26.1 Single dose | 2 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.16, 0.65] |
| 26.2 Daily | 1 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.23, 1.21] |
| 26.3 Weekly/monthly | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Low birthweight (less than 2500 g) (by skin pigmentation based on Fitzpatrick skin to Show forest plot | 3 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.24, 0.65] |
| 27.1 Three or less | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.2 Four or more | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.3 Unknown/unreported/mixed | 3 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.24, 0.65] |
| 28 Low birthweight (less than 2500 g) (by latitude) Show forest plot | 3 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.24, 0.65] |
| 28.1 Between Tropics of Cancer and Capricorn | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.2 North of the Tropic of Cancer or South of the Tropic of Capricorn | 3 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.24, 0.65] |
| 28.3 Unknown/unreported | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29 Low birthweight (less than 2500 g) (by season at the start of pregnancy) Show forest plot | 3 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.24, 0.65] |
| 29.1 Summer | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.2 Fall | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.3 Winter | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.4 Mixed/unknown | 3 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.24, 0.65] |
| 30 Caesarean section Show forest plot | 10 | 1104 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.80, 1.21] |
| 31 Gestational hypertension Show forest plot | 2 | 1130 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.41, 1.49] |
| 32 Maternal death (death while pregnant or within 42 days of termination of pregnancy) (ALL) Show forest plot | 1 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (ALL) Show forest plot | 14 | 2470 | Mean Difference (IV, Random, 95% CI) | 35.66 [24.19, 47.13] |
| 34 Birth length (cm) Show forest plot | 8 | 931 | Mean Difference (IV, Random, 95% CI) | 0.57 [0.19, 0.95] |
| 35 Head circumference at birth (cm) Show forest plot | 8 | 1841 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.21, 0.44] |
| 36 Birthweight (g) Show forest plot | 17 | 2828 | Mean Difference (IV, Random, 95% CI) | 80.30 [‐14.40, 175.00] |
| 37 Stillbirth Show forest plot | 3 | 584 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.06, 1.98] |
| 38 Neonatal death Show forest plot | 2 | 326 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.04, 1.67] |
| 39 Apgar score less than seven at five minutes Show forest plot | 1 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.11, 2.53] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pre‐eclampsia (ALL) Show forest plot | 4 | 1174 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.32, 0.78] |
| 2 Gestational diabetes (ALL) Show forest plot | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.84] |
| 3 Preterm birth (less than 37 weeks' gestation) (ALL) Show forest plot | 5 | 942 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [1.01, 2.28] |
| 4 Low birthweight (less than 2500 g) (ALL) Show forest plot | 2 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.10, 4.55] |
| 5 Caesarean section Show forest plot | 2 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.87, 1.54] |
| 6 Gestational hypertension Show forest plot | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.06, 1.12] |
| 7 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (ALL) Show forest plot | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 12.5 [3.80, 21.20] |
| 8 Birth length (cm) Show forest plot | 3 | 194 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.67, 0.52] |
| 9 Head circumference at birth (cm) Show forest plot | 3 | 198 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.39, 0.33] |
| 10 Birthweight (g) Show forest plot | 3 | 194 | Mean Difference (IV, Random, 95% CI) | 42.39 [‐86.96, 171.74] |
| 11 Neonatal death Show forest plot | 1 | 660 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.01, 4.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gestational diabetes (ALL) Show forest plot | 1 | 1298 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.10, 1.73] |
| 2 Maternal adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Hypercalcaemia | 1 | 1298 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Hypercalciuria | 1 | 1298 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.02, 3.97] |
| 3 Preterm birth (less than 37 weeks' gestation) (ALL) Show forest plot | 1 | 1298 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.68, 1.59] |
| 4 Low birthweight (less than 2500 g) (ALL) Show forest plot | 1 | 1298 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.82, 1.51] |
| 5 Caesarean section Show forest plot | 1 | 1298 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.95, 1.27] |
| 6 Gestational hypertension Show forest plot | 1 | 1298 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.31, 2.79] |
| 7 Maternal death (death while pregnant or within 42 days of termination of pregnancy) Show forest plot | 1 | 1300 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.02, 3.98] |
| 8 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (ALL) Show forest plot | 1 | 635 | Mean Difference (IV, Random, 95% CI) | 75.17 [71.97, 78.37] |
| 9 Birth length (cm) Show forest plot | 1 | 1297 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.28, 0.28] |
| 10 Head circumference at birth (cm) Show forest plot | 1 | 1297 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.17, 0.17] |
| 11 Birthweight (g) Show forest plot | 1 | 1297 | Mean Difference (IV, Random, 95% CI) | ‐7.0 [‐55.95, 41.95] |
| 12 Stillbirth Show forest plot | 1 | 1300 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.29, 1.46] |
| 13 Neonatal death Show forest plot | 1 | 1298 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.22, 2.14] |

