Inmunoterapia con alérgenos específicos para el tratamiento del eccema atópico
Resumen
Antecedentes
La inmunoterapia con alérgenos específicos (IAE) es un tratamiento que puede mejorar la gravedad de la enfermedad en pacientes con eccema atópico (EA) al inducir la tolerancia inmunitaria al alérgeno pertinente. Ninguna revisión sistemática de calidad alta ha evaluado previamente la eficacia y la seguridad de este tratamiento.
Objetivos
Evaluar los efectos de la inmunoterapia con alérgenos específicos (IAE), que incluye las vías subcutánea, sublingual, intradérmica y oral, en comparación con placebo o un tratamiento estándar en pacientes con eccema atópico.
Métodos de búsqueda
Se hicieron búsquedas en las siguientes bases de datos hasta julio 2015: registro especializado del Grupo Cochrane de Piel (Cochrane Skin Group), CENTRAL en la Cochrane Library (número 7, 2015), MEDLINE (desde 1946), EMBASE (desde 1974), LILACS (desde 1982), Web of Science™ (desde 2005), Global Resource of EczemA Trials (GREAT database) y cinco bases de datos de ensayos. Se buscaron los resúmenes de reuniones europeas y norteamericanas recientes de alergias y se verificaron las referencias de los estudios y los artículos de revisión incluidos para referencias adicionales de ensayos relevantes.
Criterios de selección
Ensayos controlados aleatorios (ECA) de inmunoterapia con alérgenos específicos que utilizaron extractos estandarizados del alérgeno en pacientes con EA.
Obtención y análisis de los datos
Dos autores realizaron de forma independiente la selección de estudios, la extracción de datos (incluyendo efectos adversos), la evaluación del riesgo de sesgo, y los análisis. Se utilizaron los procedimientos metodológicos estándar previstos por la Colaboración Cochrane.
Resultados principales
Se identificaron 12 ECA para su inclusión en esta revisión; el número total de participantes fue de 733. Las intervenciones incluyeron IAE en niños y adultos alérgicos a los ácaros del polvo doméstico (diez ensayos), al polen del césped o a otros alérgenos inhalados (dos ensayos). Se administraron por vía subcutánea (seis ensayos), sublingual (cuatro ensayos), oral o intradérmica (dos ensayos). En general, el riesgo de sesgo fue moderado; las altas pérdidas durante el seguimiento y la falta de cegamiento fueron las principales inquietudes metodológicas.
Los resultados primarios fueron "Evaluación global informada por el participante o por los padres de la gravedad de la enfermedad al final del tratamiento"; "Síntomas específicos del eccema informados por el participante o los padres, mediante medidas subjetivas"; y "Eventos adversos, como episodios agudos de asma o anafilaxia". La SCORing Atopic Dermatitis (SCORAD) es la medida que se utilizó para medir el efecto de la dermatitis atópica por área (A); intensidad (B); y medidas subjetivas (C), como el prurito y el insomnio.
Para la "Evaluación global informada por el participante o por los padres de la gravedad de la enfermedad al final del tratamiento", un ensayo (20 participantes) encontró mejoría en 7/9 participantes (78%) tratados con IAE en comparación con 3/11 (27%) tratados con placebo (cociente de riesgos [CR] 2,85; intervalo de confianza [IC] del 95%: 1,02 a 7,96; P = 0,04). Otro estudio (24 participantes) no encontró diferencias: la gravedad global de la enfermedad mejoró en 8/13 participantes (62%) tratados con IAE en comparación con 9/11 (81%) tratados con placebo (CR 0,75; IC del 95%: 0,45 a 1,26; P = 0,38). No se realizó un metanálisis debido a la alta heterogeneidad entre estos dos estudios. La calidad de las pruebas era baja.
Para los "Síntomas específicos del eccema informados por el participante o los padres, mediante medidas subjetivas", dos ensayos (184 participantes) no encontraron que la IAE mejorara la parte C de la SCORAD (diferencia de medias [DM] ‐0,74; IC del 95%: ‐1,98 a 0,50) o los trastornos del sueño (DM ‐0,49; IC del 95%: ‐1,03 a 0,06) más que placebo. En la parte C de la SCORAD gravedad del prurito, estos dos ensayos (184 participantes) no encontraron que la IAE mejorara el prurito (DM ‐0,24; IC del 95%: ‐1,00 a 0,52). Otro estudio sin cegamiento (60 participantes) encontró que la IAE redujo el prurito en comparación con ningún tratamiento (DM ‐4,20; IC del 95%: ‐3,69 a ‐4,71) y redujo los síntomas generales de los participantes (p < 0,01), pero no fue posible agrupar estos tres estudios debido a la heterogeneidad alta. La calidad de las pruebas fue muy baja.
Siete ensayos informaron reacciones adversas sistémicas: 18/282 participantes (6,4%) tratados con IAE presentaron una reacción sistémica en comparación con 15/210 (7,1%) con ningún tratamiento (CR 0,78; IC del 95%: 0,41 a 1,49; la calidad de las pruebas fue moderada). Estos siete ensayos también informaron reacciones adversas locales: 90/280 participantes (32,1%) tratados con IAE presentaron una reacción local en comparación con 44/204 (21,6%) en el grupo de ningún tratamiento (CR 1,27; IC del 95%: 0,89 a 1,81). Como estos estudios tuvieron las mismas limitaciones, la calidad de las pruebas también se consideró moderada.
Entre los resultados secundarios hubo una mejoría significativa en la "Evaluación global calificada por el investigador o el médico de la gravedad de la enfermedad al final del tratamiento" (seis ensayos , 262 participantes; CR 1,48; IC del 95%: 1,16 a 1,88). Ninguno de los estudios informó el resultado secundario "Gravedad del eccema calificada por los padres o el participante evaluada mediante una escala publicada", pero dos estudios (n = 184) mencionados anteriormente utilizaron la parte C de la SCORAD, que se incluyó como el resultado primario de esta revisión "Síntomas específicos del eccema informados por el participante o los padres, mediante medidas subjetivas".
Los resultados generalmente no fueron concluyentes debido al escaso número de estudios. No fue posible determinar mediante análisis de subgrupos un tipo particular de alérgeno o una edad o nivel de gravedad de la enfermedad particulares donde la inmunoterapia con alérgenos fuera más exitosa. Tampoco fue posible determinar si la inmunoterapia sublingual se asoció con más reacciones adversas locales en comparación con la inmunoterapia subcutánea.
Conclusiones de los autores
En general, la calidad de las pruebas fue baja. La baja calidad se debió principalmente a los resultados diferentes entre los estudios, la falta de cegamiento en algunos estudios y los relativamente escasos estudios que informaron medidas de resultado centradas en el participante. Se encontraron pruebas limitadas de que la IAE pueda ser un tratamiento eficaz en los pacientes con EA. Los tratamientos utilizados en estos ensayos no se asociaron con un aumento en el riesgo de reacciones locales o sistémicas. Los estudios futuros deben utilizar formulaciones de alérgenos de alta calidad con registros previos comprobados en otras afecciones alérgicas y deben incluir medidas de resultado informadas por el participante.
PICO
Resumen en términos sencillos
Inmunoterapia con alérgenos específicos para el tratamiento del eccema atópico
Antecedentes
Al menos uno de siete niños y uno de 50 adultos presentan eccema atópico, una afección de la piel caracterizada por una erupción cutánea enrojecida que provoca picazón. Los pacientes con eccema atópico son alérgicos a cosas del ambiente como los ácaros del polvo doméstico, y la exposición a lo que son alérgicos puede provocar que el eccema empeore. La inmunoterapia con alérgenos específicos es un tratamiento que incluye un ciclo de inyecciones o gotas bajo la lengua que contienen la sustancia a la cual el paciente es alérgico. El tratamiento puede reducir la gravedad de la alergia de la persona y, por lo tanto, podría reducir los síntomas del eccema atópico. Se evaluó si la inmunoterapia con alérgenos específicos fue mejor o peor que un tratamiento estándar o placebo para mejorar la gravedad y los síntomas de la enfermedad según la evaluación de los participantes, los padres o los investigadores.
Pregunta de la revisión
¿La inmunoterapia con alérgenos específicos es un tratamiento eficaz para los pacientes con eccema atópico?
Características de los estudios
Las pruebas están actualizadas hasta julio 2015. Se encontraron 12 estudios, con 733 participantes, que incluyeron tanto a niños como adultos. Los estudios se realizaron en centros especializados en alergias en nueve países. La duración de los ensayos varió de cuatro meses a tres años. La inmunoterapia se administró a los participantes de cuatro maneras diferentes. Los fabricantes del alérgeno patrocinaron siete de los 12 estudios.
Resultados clave
En esta revisión no se encontraron pruebas de los estudios de que la IAE pudiera ser un tratamiento eficaz para el eccema atópico, según la calificación de los participantes o los padres de la gravedad y los síntomas de la enfermedad. Se encontraron pruebas limitadas de que la IAE puede mejorar la gravedad de la enfermedad calificada por el investigador. La inmunoterapia no provocó más efectos perjudiciales que un tratamiento estándar o placebo.
Calidad de la evidencia
En general, la calidad de las pruebas fue baja. La calidad se disminuyó principalmente debido a los resultados diferentes entre los estudios, la falta de cegamiento en algunos estudios y a que relativamente pocos estudios informaron resultados relevantes para los pacientes. Los estudios futuros deben utilizar formulaciones de alérgenos de alta calidad con registros previos comprobados en otras afecciones alérgicas y deben incluir medidas de resultado informadas por el participante.
Conclusiones de los autores
Summary of findings
| Specific immunotherapy compared with no immunotherapy for atopic eczema | ||||||
| Patient or population: adults and children with atopic eczema and inhalant allergen sensitisation Settings: specialist allergy centres in the UK (2 trials), Italy (3 trials), USA, Germany, Belgium, Poland, Columbia, and China Intervention: specific allergen immunotherapy Comparison: no immunotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No immunotherapy | Specific allergen immunotherapy | |||||
| Participant‐ or parent‐reported global assessment of disease severity Follow‐up: 6 to 12 months | See comments | See comments | Not estimable | 44a | ⊕⊕⊝⊝ | Improvement in 7/9 participants (78%) in the immunotherapy group and 3/11 participants (27%) in the placebo group (RR 2.85, 95% CI 1.02 to 7.96; P = 0.04 (Warner 1978)) 8/13 participants (62%) in the immunotherapy group and 9/11 participants (81%) in the placebo group (RR 0.75, 95% CI 0.45 to 1.26; P = 0.38 (Glover 1992)) Due to unexplained statistical heterogeneity, we did not pool the data |
| Participant‐ or parent‐reported specific symptoms of eczema Follow‐up: 12 to 18 months SCORAD part C measured as a combination of 2 Visual Analogue Scales (1 for itch, 1 for sleep disturbance), each on a scale from 0, no specific symptoms, to 10, maximum specific symptoms | The mean SCORAD part C score ranged across control groups from 3.07 to 5.29 The mean SCORAD part C sleep severity score ranged across control groups from 0.8 to 2.31 | The mean SCORAD part C score in the immunotherapy group was on average 0.74 lower (95% CI ‐1.98 to 0.50) The mean SCORAD part C sleep severity score in the immunotherapy group was on average 0.49 lower (95% CI ‐1.03 to 0.06) | ‐ | 339a (6) | ⊕⊝⊝⊝ | Itch: SCORAD part C itch severity at the end of treatment: MD ‐0.24, 95% CI ‐1.00 to 0.52; I² = 0% for Di Rienzo 2014 and Novak 2012 Itch severity score: MD ‐4.20, 95% CI ‐3.69 to ‐4.71 for Sanchez 2012 Due to unexplained statistical heterogeneity, we did not pool the data |
| Adverse events ‐ any systemic reaction Follow‐up: 6 to 18 months | Low‐risk population | RR 0.78 (0.41 to 1.49) | 492a | ⊕⊕⊕⊝ | ‐ | |
| 0 per 1000 | 0 per 1000 | |||||
| Medium‐risk population | ||||||
| 71 per 1000 | 55 per 1000 | |||||
| High‐risk population | ||||||
| 163 per 1000 | 127 per 1000 | |||||
| Investigator‐ or physician‐rated global assessment of disease severity Follow‐up: 1 to 3 years | Low‐risk population | RR 1.48 (1.16 to 1.88) | 286a (7) | ⊕⊝⊝⊝ | ‐ | |
| 0 per 1000 | 0 per 1000 (0 to 10) | |||||
| Medium‐risk population | ||||||
| 471 per 1000 | 697 per 1000 (546 to 885) | |||||
| High‐risk population | ||||||
| 778 per 1000 | 1151 per 1000 (903 to 1462) | |||||
| Investigator‐ or physician‐rated eczema severity using a published scale Follow‐up: 12 to 18 months | The mean SCORAD score ranged across control groups from 26.7 to 32.6 | The mean SCORAD score in the immunotherapy group was on average 5.79 lower (95% CI ‐7.92 to ‐3.66) | ‐ | 435a (6) | ⊕⊝⊝⊝ | ‐ |
| Participant or parent‐rated eczema severity using a published scale Follow‐up: 12 to 18 months | See comment | See comment | Not estimable | 184a | ⊕⊕⊝⊝ | SCORAD part C used as the specific eczema symptom score (Di Rienzo 2014; Novak 2012) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Assumed risks are based on the total control group risk across all included studies (medium risk population) and the included studies with the lowest (low risk population) and highest (high risk population) control group risks. | ||||||
Antecedentes
En el glosario de términos en la Tabla 1 se enumeran los términos poco conocidos.
Descripción de la afección
El eccema atópico (EA) es una afección cutánea inflamatoria crónica que afecta del 15% al 30% de los niños y del 2% al 10% de los adultos en todo el mundo (Odhiambo 2009; Williams 2006). Los términos “eccema atópico” y “dermatitis atópica” son sinónimos. El prurito intenso y los parches de piel seca inflamada en varias ubicaciones según la edad del paciente caracterizan esta afección (Akdis 2006). En los neonatos, el EA generalmente se encuentra en las mejillas, la frente o el cuero cabelludo. En la niñez, el EA incluye generalmente las manos, los pies, las muñecas, los tobillos y los pliegues de los codos y el dorso de las rodillas(Akdis 2006). En los adultos, el EA provoca parches escamosos secos y placas grandes de piel engrosada (liquenificada) en los pliegues de flexión; la cara y el cuello; los brazos y la espalda; y el dorso de las manos, los pies, los dedos de la mano y los dedos de los pies (Akdis 2006). En sentido estricto, el término "eccema atópico" "solamente se debe referir a los individuos que tienen características físicas de eccema más pruebas de anticuerpos específicos inmunoglobulina E (IgE) contra alérgenos ambientales comunes como los ácaros del polvo doméstico" (Johansson 2004). Esta definición estricta se ha utilizado en toda la revisión a menos que se haya especificado lo contrario.
Varias observaciones indican que los alérgenos pueden ser causas importantes del eccema atópico. En primer lugar, la exposición directa de la piel a los alérgenos ambientales, que incluyen los alérgenos perennes como los ácaros del polvo doméstico y los alérgenos estacionales como el polen, ha mostrado aumentar la gravedad del eccema atópico (Capristo 2004; Purvis 2005; Schäfer 1999). En segundo lugar, otras enfermedades desencadenadas por los alérgenos son frecuentes en pacientes con eccema atópico. Por ejemplo, de los niños que desarrollan la afección durante los dos primeros años de vida, el 50% puede desarrollar asma durante años posteriores(Warner 2001). Finalmente, los pacientes con EA más grave tienen un mayor riesgo de asma y rinitis alérgica (Gustafsson 2000; Illi 2004).
A pesar del tratamiento tópico disponible actualmente con emolientes; corticosteroides; inhibidores de la calcineurina; y otros tratamientos como los antibióticos, los pacientes con eccema atópico a menudo no pueden mantener la afección completamente bajo control. En algunos casos, los fármacos administrados pueden provocar más efectos perjudiciales que beneficiosos (Akdis 2006). Por lo tanto, al considerar los antecedentes atópicos de la enfermedad y su posible correlación con factores desencadenados por alérgenos, se han propuesto algunos otros tipos de tratamiento que incluyen la inmunoterapia con alérgenos específicos (IAE) (Darsow 2012).
Descripción de la intervención
La inmunoterapia con alérgenos específicos (IAE) es un tratamiento para la enfermedad alérgica que incluye la administración de un alérgeno a dosis altas para inducir la tolerancia inmunitaria a ese alérgeno y aliviar los síntomas (Calderon 2007). Por ejemplo, en los pacientes con fiebre del heno que son alérgicos al polen del césped, la IAE puede incluir el tratamiento con inyecciones, gotas o comprimidos de polen del césped durante meses para aliviar los síntomas (Calderon 2007; Wilson 2005). La inmunoterapia con alérgenos específicos es el único tratamiento que ha mostrado proporcionar efectos beneficiosos a más largo plazo en las enfermedades alérgicas después que el tratamiento se ha interrumpido (Durham 1999). Ha mostrado ser un tratamiento eficaz para la rinitis alérgica y el asma alérgico, aunque el tratamiento conlleva un riesgo de reacción alérgica grave (Calderon 2007; CSM report 1986; Wilson 2005).
De qué manera podría funcionar la intervención
La inmunoterapia con alérgenos específicos funciona por la inducción de cambios en la respuesta inmunitaria al alérgeno pertinente para que en la enfermedad causada por una respuesta anormal a ese alérgeno pueda haber una mejoría en los síntomas (Allam 2006). Los cambios inmunitarios específicos causados por la IAE incluyen un aumento en la actividad de los componentes supresores del sistema inmunológico (linfocitos T reguladores) y un aumento en los anticuerpos (anticuerpos inmunoglobulina G [IgG]) al alérgeno (Bussmann 2007; Bussmann 2009; Maintz 2007). La presencia de sensibilización alérgica en los pacientes con EA y la relación entre EA y otras enfermedades alérgicas indican que las respuestas inmunitarias alérgicas son una parte importante del proceso de enfermedad en el EA(Gustafsson 2000; Illi 2004; Warner 2001). Por lo tanto, es creíble que la IAE quizás pueda reducir los síntomas en los pacientes con EA al inhibir las respuestas inmunitarias anormales a los alérgenos.
Por qué es importante realizar esta revisión
La inmunoterapia con alérgenos específicos es un tratamiento modificador de la enfermedad que reduce los síntomas en los pacientes con otras afecciones alérgicas: rinitis alérgica, conjuntivitis alérgica y asma Abramson 2003; Calderon 2007; Dahl 2006; Didier 2007; Penagos 2008). En consecuencia, la IAE podría ser potencialmente eficaz para reducir el EA. Una evaluación de los efectos sobre las manifestaciones cutáneas en el contexto de ensayos controlados aleatorios podría brindar un tratamiento alternativo para los pacientes con EA.
Los planes para esta revisión se publicaron como el protocolo "Inmunoterapia con alérgenos específicos para el tratamiento del eccema atópico" (Calderon 2010).
Objetivos
Evaluar los efectos de la inmunoterapia con alérgenos específicos (IAE), que incluye las vías subcutánea, sublingual, intradérmica y oral, en comparación con placebo o un tratamiento estándar en pacientes con eccema atópico.
Métodos
Criterios de inclusión de estudios para esta revisión
Tipos de estudios
Ensayos controlados aleatorios (ECA).
Tipos de participantes
Adultos y niños con eccema atópico (EA) y sensibilización alérgica a un alérgeno inhalado o dietético. "Era necesario comprobar la alergia mediante una prueba objetiva como una prueba cutánea positiva o niveles circulantes altos del anticuerpo IgE específico al alérgeno detectado por un análisis de sangre específico para la alergia llamado prueba radioalergoabsorbente. Se excluyeron los ensayos que se centraron en la rinitis alérgica o el asma sin eccema" (Calderon 2011). Cuando los ensayos incluyeron participantes con y sin EA, el ensayo solamente se incluyó si los resultados de los participantes con EA se presentaron por separado.
Tipos de intervenciones
Inmunoterapia a dosis alta con extractos estandarizados del alérgeno para un único alérgeno o alérgenos mixtos administrados por vía sublingual (debajo de la lengua), subcutánea (debajo de la piel), intradérmica (en la piel) u oral en comparación con placebo o un tratamiento estándar como emolientes, corticosteroides tópicos o inhibidores tópicos de la calcineurina. Se consideraron todos los alérgenos apropiados a todas las dosis y en todas las duraciones del tratamiento.
Tipos de medida de resultado
Resultados primarios
-
Evaluación global informada por el participante o por los padres de la gravedad de la enfermedad al final del tratamiento, es decir, proporción con mejoría buena o excelente en ese momento como se informó en los ensayos (si el tratamiento se proporcionó durante uno, dos o tres años, u otra duración).
-
Síntomas específicos de eccema informados por el participante o los padres, mediante medidas subjetivas como el prurito o el trastorno del sueño (SCORing Atopic Dermatitis [SCORAD] parte C).
-
Eventos adversos, como episodios agudos de asma o anafilaxia.
Resultados secundarios
-
Evaluación global calificada por el investigador o el médico de la gravedad de la enfermedad al final del tratamiento, es decir, proporción con mejoría buena o excelente en ese momento como se informó en los ensayos (si el tratamiento se proporcionó durante uno, dos o tres años, u otra duración).
-
Gravedad del eccema calificada por los padres o el participante, evaluada mediante una escala publicada (p.ej. Patient Oriented Eczema Measure [POEM]).
-
Gravedad del eccema calificada por el investigador o el médico, evaluada mediante una escala publicada (p.ej. SCORAD).
-
Administración de otra medicación para el tratamiento del eccema durante el período de intervención (p.ej. corticosteroides tópicos / sistémicos, inhibidores de la calcineurina o antihistamínicos orales).
-
Puntuaciones validadas de calidad de vida relacionada con el eccema (p.ej. Dermatitis Family Impact Questionnaire, Children's Dermatology Life Quality Index)(Lewis‐Jones 1995).
Results
Description of studies
See the 'Characteristics of included studies', 'Characteristics of excluded studies', 'Characteristics of studies awaiting classification', and 'Characteristics of ongoing studies' tables.
Results of the search
The search identified 1550 references from electronic databases and six additional reports from other sources (three from screening references of review articles and three from ongoing trials registries), which gave a total of 1556 records (see the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram in Figure 1). We excluded 1465 references based on titles and abstracts. MC or HT and RB selected 91 records for which they screened the full text. We excluded 64 records and listed one as an ongoing study. Overall, 26 reports of 12 separate studies met the inclusion criteria (Di Rienzo 2014; Galli 1994; Glover 1992; Kaufman 1974; Leroy 1993; Luna‐Pech 2013; Novak 2012; Pajno 2007; Qin 2014; Sanchez 2012; Silny 2006; Warner 1978). We contacted the authors of all of the 12 included trials for original data and clarification of methods; we received further details from the authors or their collaborators for four trials (Di Rienzo 2014; Novak 2012; Sanchez 2012; Warner 1978).
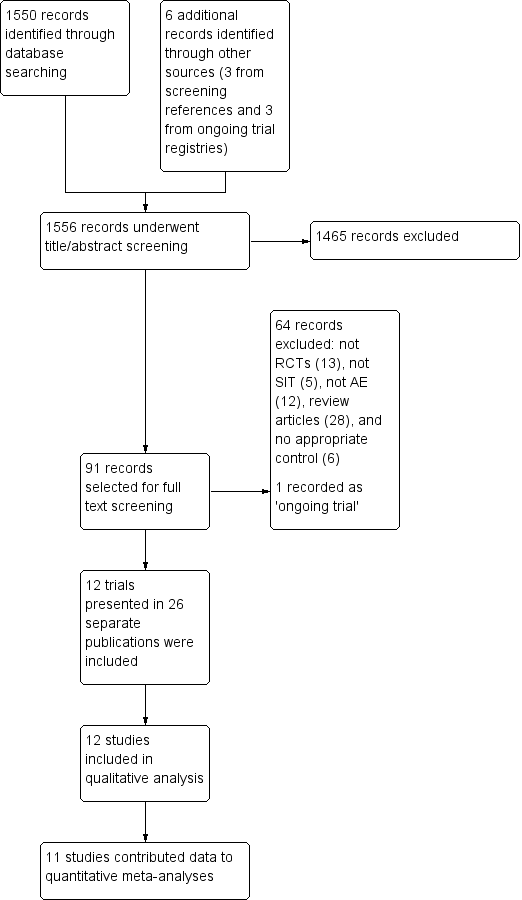
PRISMA flow diagram
Included studies
We included 12 studies, with a total of 733 participants.
Setting
Studies were conducted in specialist allergy centres in the UK (Glover 1992; Warner 1978), Italy (Di Rienzo 2014; Galli 1994; Pajno 2007), the USA (Kaufman 1974), Germany (Novak 2012), Belgium (Leroy 1993), Poland (Silny 2006), Columbia (Sanchez 2012), Mexico (Luna‐Pech 2013), and China (Qin 2014).
Participants
Two trials studied adults (Novak 2012; Qin 2014), six studied children (Di Rienzo 2014; Galli 1994; Glover 1992; Luna‐Pech 2013; Pajno 2007; Warner 1978), and four studied both children and adults (Kaufman 1974; Leroy 1993; Sanchez 2012; Silny 2006). Ten studies were restricted to people allergic to Dermatophagoides pteronyssinus or Dermatophagoides farinae (house dust mites) or both (Di Rienzo 2014; Galli 1994; Glover 1992; Leroy 1993; Luna‐Pech 2013; Novak 2012; Pajno 2007; Qin 2014; Sanchez 2012; Warner 1978), one study was restricted to people allergic to house dust mites or grass pollen (Silny 2006), and one study was restricted to people allergic to a group of unspecified inhalant antigens (Kaufman 1974).
Interventions
The 12 included studies were all of specific allergen immunotherapy (SIT). Of these, six trials studied subcutaneous immunotherapy (SCIT) (Glover 1992; Kaufman 1974; Novak 2012; Sanchez 2012; Silny 2006; Warner 1978), four studied sublingual immunotherapy (SLIT) (Di Rienzo 2014; Luna‐Pech 2013; Pajno 2007; Qin 2014), one studied intradermal immunotherapy (Leroy 1993), and one studied oral immunotherapy (Galli 1994).
Eight trials compared the intervention with a placebo (Glover 1992; Kaufman 1974; Leroy 1993; Luna‐Pech 2013; Novak 2012; Pajno 2007; Silny 2006; Warner 1978), and four compared the intervention with a standard treatment (Di Rienzo 2014; Galli 1994; Qin 2014; Sanchez 2012). The duration of treatment was less than a year in one trial, Leroy 1993, and at least a year in Di Rienzo 2014, Galli 1994, Glover 1992, Kaufman 1974, Luna‐Pech 2013, Novak 2012, Pajno 2007, Qin 2014, Sanchez 2012, Silny 2006, and Warner 1978.
Outcomes
With regard to our prespecified primary outcomes, two studies reported 'Participant‐ or parent‐reported global assessment of disease severity at the end of treatment' (Glover 1992; Warner 1978), six studies reported 'Participant‐ or parent‐reported specific symptoms of eczema, by subjective measures' (Di Rienzo 2014; Glover 1992; Leroy 1993; Novak 2012; Pajno 2007; Sanchez 2012), and seven studies reported 'Adverse events' (Di Rienzo 2014; Glover 1992; Novak 2012; Pajno 2007; Qin 2014; Sanchez 2012; Silny 2006).
With regard to our prespecified secondary outcomes, seven studies reported 'Investigator‐ or physician‐rated global assessment of disease severity at the end of treatment' (Di Rienzo 2014; Galli 1994; Kaufman 1974; Leroy 1993; Qin 2014; Sanchez 2012; Silny 2006), two studies reported 'Parent‐ or participant‐rated eczema severity assessed using a published scale' in the form of SCORing Atopic Dermatitis (SCORAD) part C (Di Rienzo 2014; Novak 2012), six studies reported 'Investigator‐ or physician‐rated eczema severity assessed using a published scale' (Di Rienzo 2014; Luna‐Pech 2013; Novak 2012; Qin 2014; Pajno 2007; Sanchez 2012), eight studies reported 'Use of other medication for treatment of eczema during the intervention period' (Glover 1992; Kaufman 1974; Luna‐Pech 2013; Novak 2012; Pajno 2007; Qin 2014; Sanchez 2012; Silny 2006), and one study reported 'Validated eczema‐related quality of life scores' (Novak 2012).
Three studies measured other outcomes: one measured total serum immunoglobulin E (IgE) levels, specific IgE levels, and skin prick test results (Glover 1992); another measured specific IgE levels and other serum inflammatory parameters associated with either allergic inflammation or its suppression, including eosinophilic cationic protein (ECP), soluble interleukin 2 receptor (sIL‐2R), interferon gamma (IFN‐gamma), or interleukins 4, 5, and 10 (Silny 2006); and a third measured specific serum IgG4 levels (Qin 2014).
Only two of the five publications that reported outcomes from the Pajno 2007 study contributed data to the review, because the other three publications did not report atopic eczema outcomes.
Excluded studies
We rejected the other 64 titles for the following reasons: not a randomised controlled trial (RCT) (13), not SIT (five), not atopic eczema (AE) (12), review articles (28), and no appropriate control (six). The reason we included these articles for the full text review stage is that from the title or abstract we could not exclude the possibility that they were RCTs of adults or children with AE and allergic sensitisation, but after assessment of the full text, we excluded them.
Studies awaiting classification
There were no studies awaiting classification.
Ongoing studies
There was one ongoing trial with no outcome data available at the time of review (see the 'Characteristics of ongoing studies' table). The contacts for the trial NCT00310492 did not respond to our request for further information.
Risk of bias in included studies
Full details are shown in the 'Characteristics of included studies' tables. Please see the 'Risk of bias' summary (review authors' judgements about each 'Risk of bias' item for each included study, Figure 2).
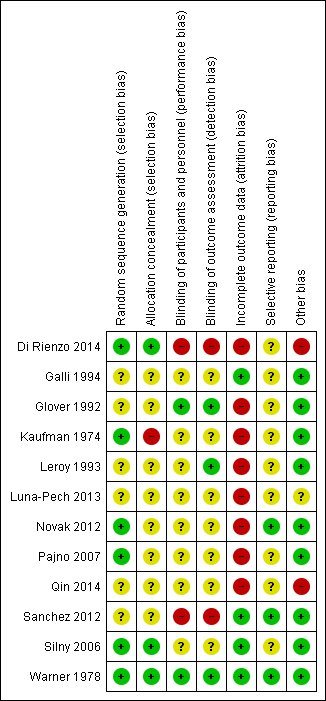
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
Random sequence generation
There was a low risk of bias related to generation of randomisation sequence concealment in six studies, Di Rienzo 2014, Kaufman 1974, Novak 2012, Pajno 2007, Silny 2006, Warner 1978, and unclear risk in the following six studies: Galli 1994, Glover 1992, Leroy 1993, Luna‐Pech 2013, Qin 2014, and Sanchez 2012.
Allocation
There was a low risk of bias related to allocation concealment in three studies (Di Rienzo 2014; Silny 2006; Warner 1978), high risk in one study (Kaufman 1974), and unclear risk in eight studies due to insufficient details provided (Galli 1994; Glover 1992; Leroy 1993; Luna‐Pech 2013; Novak 2012; Pajno 2007; Sanchez 2012; Qin 2014).
Blinding
There was a low risk of bias related to blinding of participants and personnel in two studies (Glover 1992; Warner 1978), which were either double blinded or triple blinded; high risk in two studies (Di Rienzo 2014; Sanchez 2012), which were open label; and unclear risk in eight studies due to insufficient details provided (Galli 1994; Kaufman 1974; Leroy 1993; Luna‐Pech 2013; Novak 2012; Pajno 2007; Qin 2014; Silny 2006).
There was a low risk of bias related to blinding of outcome assessors in three studies (Glover 1992; Leroy 1993; Warner 1978); high risk in two studies (Di Rienzo 2014; Sanchez 2012), which were open label; and unclear risk in seven studies (Galli 1994; Kaufman 1974; Luna‐Pech 2013; Novak 2012; Pajno 2007; Qin 2014; Silny 2006), four of which were unclear regarding whether they included outcome assessors in the double blinding (Kaufman 1974; Novak 2012; Pajno 2007; Silny 2006).
Incomplete outcome data
There was a low risk of bias related to incomplete outcome data in four studies, Galli 1994, Sanchez 2012, Silny 2006, and Warner 1978, where loss to follow‐up rates were low, and high risk in eight studies where loss to follow up rates were high (up to 51%) or postrandomisation exclusions were noted: Di Rienzo 2014, Glover 1992, Kaufman 1974, Leroy 1993, Luna‐Pech 2013, Qin 2014, Novak 2012, and Pajno 2007.
Selective reporting
There was a low risk of bias related to selective reporting in three studies where the specified outcomes in the methodology were reported in the results, Novak 2012, Sanchez 2012, Warner 1978, and unclear risk in nine studies: Di Rienzo 2014, Galli 1994, Glover 1992, Kaufman 1974, Leroy 1993, Luna‐Pech 2013, Pajno 2007, Qin 2014, and Silny 2006.
Other potential sources of bias
There was low risk of bias related to other sources in nine studies (Galli 1994; Glover 1992; Kaufman 1974; Leroy 1993; Novak 2012; Pajno 2007; Sanchez 2012; Silny 2006; Warner 1978), high risk in two studies where the manufacturer funded the study either partly or wholly and the authors were affiliated with the manufacturer (Di Rienzo 2014; Qin 2014), and unclear risk in one study where it was unclear whether the authors were affiliated with the manufacturer (Luna‐Pech 2013).
Effects of interventions
See: Summary of findings for the main comparison Specific allergen immunotherapy versus no immunotherapy
See summary of findings Table for the main comparison for the main comparison 'specific allergen immunotherapy versus no immunotherapy'.
Primary outcomes
1. Participant‐ or parent‐reported global assessment of disease severity at the end of treatment
One study, Warner 1978, measured this outcome as whether the eczema was improved, there was no change, or it was worse as rated by the participants or parents. These data were available for 20 participants at the end of the treatment (nine active, 11 placebo), with improvement in 7/9 (78%) of the immunotherapy group and 3/11 (27%) in the placebo group (risk ratio (RR) 2.85, 95% confidence interval (CI) 1.02 to 7.96). Another study, Glover 1992, measured this outcome as whether the eczema was better, the same, or worse as rated by parents. These data were available for 24 participants, with improvement in 8/13 (62%) of those in the active treatment group and 9/11 (81%) in the placebo group (RR 0.75, 95% CI 0.45 to 1.26). We did not perform meta‐analysis because of high heterogeneity between the two studies (I² = 83%). The high loss to follow‐up rate and as‐treated analysis in the study by Glover 1992 may have contributed to the significant heterogeneity. The quality of the evidence was low.
2. Participant‐ or parent‐reported specific symptoms of eczema, by subjective measures
We used original data shared by the authors of two studies, Di Rienzo 2014 and Novak 2012, to calculate SCORing Atopic Dermatitis (SCORAD) part C scores at the end of treatment, and the components of SCORAD part C, which are itch measured by Visual Analogue Scales (VAS) and sleep disturbance measured by VAS, each on a scale from 0 to 10. Meta‐analysis, with a total of 184 participants, showed no significant difference in SCORAD part C (mean difference (MD) ‐0.74, 95% CI ‐1.98 to 0.50; I² = 0%; Analysis 1.1) or severity of sleep disturbance (MD ‐0.49, 95% CI ‐1.03 to 0.06; I² = 0%; Analysis 1.1).
The authors of Sanchez 2012 provided original data that showed subjective symptom scores at the end of the treatment on a scale of 0 to 100, where higher scores meant more symptoms, and a component of the symptom score, which measured itching severity on a scale of 0 to 10, where higher scores also mean more symptoms. These data were available for 60 participants at the end of the treatment (31 active, 29 placebo), with a mean overall severity score of 37.3 (95% CI 32.4 to 42.1) in the immunotherapy group and 80.8 (95% CI 75.8 to 85.7) in the control group (P < 0.001) and a mean itch severity score of 3.2 (95% CI 2.3 to 4.0) in the immunotherapy group and 7.5 (95% CI 6.9 to 8.0) in the control group (P < 0.001). The difference between groups in change in itch severity score from baseline was also statistically significant (MD ‐4.20, 95% CI ‐3.69 to ‐4.71).
For itch severity, we did not meta‐analyse data from these three studies because of extreme heterogeneity (I² = 98%), which was attributable to the open label study of Sanchez 2012. When we excluded this study from meta‐analysis, combined data from Novak 2012 and Di Rienzo 2014 showed no significant difference in SCORAD part C itch severity (MD ‐0.24, 95% CI ‐1.00 to 0.52; I² = 0%).
One study, Glover 1992, reported symptoms in the form of itch score presented graphically that showed no significant difference between the active and placebo groups. One study, Leroy 1993, reported a mean itch score of 2.2 (or 33% reduction from baseline) after immunotherapy compared with 2.6 (or 19% reduction from baseline) in the control group. The authors did not comment on whether this difference was statistically significant and did not respond to our request for further data.
Other studies reported insufficient data, such as Pajno 2007, or did not measure this outcome, such as Galli 1994, Kaufman 1974, Luna‐Pech 2013, Qin 2014, Silny 2006, and Warner 1978.
3. Adverse events
Seven studies reported local or systemic reactions to treatment (Di Rienzo 2014; Glover 1992; Novak 2012; Pajno 2007; Qin 2014; Sanchez 2012; Silny 2006).
In addition to individual studies, meta‐analysis, with a total of 484 participants, showed no statistically significant increase in risk of local reactions (RR 1.27, 95% CI 0.89 to 1.81; I² = 25%; Analysis 1.2). Data from seven of the 12 studies contributed to this effect estimate (Di Rienzo 2014; Glover 1992; Novak 2012; Pajno 2007; Qin 2014; Sanchez 2012; Silny 2006).
In addition to individual studies, meta‐analysis with a total of 492 participants showed no statistically significant increase in risk of systemic reactions (RR 0.78, 95% CI 0.41 to 1.49; I² = 0%; Analysis 1.2), with 18 events observed in the immunotherapy group and 15 in the control group. Data from four of 12 studies contributed to this effect estimate (Glover 1992; Novak 2012; Pajno 2007; Qin 2014). However, there were no systemic reactions reported in three studies (Di Rienzo 2014; Sanchez 2012; Silny 2006).
One study, Pajno 2007, with 48 participants, measured other adverse reactions and showed no statistically significant increase in risk of tiredness (RR 5.08, 95% CI 0.66 to 39.02; Analysis 1.2) or headache (RR 2.56, 95% CI 0.11 to 59.75; Analysis 1.2).
Secondary outcomes
1. Investigator‐ or physician‐rated global assessment of disease severity at the end of treatment
Six studies reported investigator‐ or physician‐rated global assessment of disease severity (Di Rienzo 2014; Galli 1994; Kaufman 1974; Qin 2014; Sanchez 2012; Silny 2006). Meta‐analysis, with 262 participants, showed significant improvement in disease severity (RR 1.48, 95% CI 1.16 to 1.88; I² = 19%; Analysis 1.3).
One study, Leroy 1993, with 24 participants, reported improvement in 70% of all of the participants that used an investigator‐rated index of disease severity at a threshold of 50% improvement. This was significant between the treatment and the placebo group (P < 0.003), but there were no separate data for the treatment and placebo group, so we could not include them in a meta‐analysis.
Other studies did not measure this outcome (Glover 1992; Luna‐Pech 2013; Novak 2012; Pajno 2007; Warner 1978).
2. Parent‐ or participant‐rated eczema severity assessed using a published scale
None of the studies reported participant‐ or parent‐rated eczema severity using a published scale, except for two studies that we have mentioned above, Di Rienzo 2014 and Novak 2012, which recorded SCORAD part C, which we included in this systematic review as a parent‐ or participant‐rated specific eczema symptom (MD ‐0.74, 95% CI ‐1.98 to 0.50; I² = 0%; Analysis 1.1).
Participant‐ or parent‐rated eczema severity assessed using a non‐published scale
Although this was not a prespecified outcome, we felt it important to include. Four studies measured participant‐ or parent‐rated eczema severity assessed using non‐published Visual Analogue Scales (VAS) on a scale of 0 to 10 (0 = no symptoms, 10 = maximal symptoms). Meta‐analysis of two studies (Di Rienzo 2014; Qin 2014), with a total of 158 participants, showed statistically significant lower end‐of‐treatment VAS scores (MD ‐1.12, 95% CI ‐1.92 to ‐0.32; I² = 0%; Analysis 1.4). We used original data shared by the authors of one study, Di Rienzo 2014, to conduct this analysis.
The other two studies only provided original data listed as illustrative text: Pajno 2007 reported a VAS that measured overall eczema symptoms with 10.7% improvement in the treatment group and 13.1% worsening in the placebo group (P = 0.07), but the study did not report absolute values. Leroy 1993 reported a VAS that measured participant general well‐being with a significant improvement in the treatment group (P = 0.008) but not in the control group, but again, did not report absolute values. Authors of the latter two studies did not respond to our requests for original data for inclusion in a meta‐analysis.
3. Investigator‐ or physician‐rated eczema severity assessed using a published scale
Six studies reported 'Investigator‐ or physician‐rated eczema severity assessed using a published scale' in the form of SCORAD (Di Rienzo 2014; Luna‐Pech 2013; Novak 2012; Pajno 2007; Qin 2014; Sanchez 2012). Authors of two studies supplied original data for end‐of‐treatment SCORAD (Novak 2012; Sanchez 2012). Meta‐analysis of three trials (Di Rienzo 2014; Novak 2012; Sanchez 2012), with 244 participants, showed significant improvement in end of treatment SCORAD (MD ‐5.79, 95% CI ‐7.92 to ‐3.66; I² = 0%; Analysis 1.5).
One study, Qin 2014, reported reduction ratios in SCORAD and classified scores as cure (greater than 90%), marked effect (60% to 89%), improvement (20% to 59%), and ineffective (less than 19%). The total efficacy (defined as percentage of participants with change in SCORAD ≥ 60%) was significantly greater in the specific allergen immunotherapy (SIT) group (77.78%) than in the control group (53.85%) (P < 0.05) and was included as a dichotomous 'Investigator‐ or physician‐rated global assessment of disease severity at the end of treatment' outcome in a meta‐analysis in this review (RR 1.48, 95% CI 1.16 to 1.88; I² = 19%; Analysis 1.3). Another study, Luna‐Pech 2013, found a significant change in SCORAD between immunotherapy (‐18.4 ± 6.5) and control (‐6.6 ± 4.1) (P = 0.008). This effect was greater for participants with severe eczema at baseline. A further study, Pajno 2007, suggested greater SCORAD improvement with the SIT than in controls in graphical data (P < 0.001), but no numerical data were available. No data for end of treatment SCORAD scores from these three studies were available for inclusion in a meta‐analysis.
One study, Glover 1992, reported no significant difference in a non‐published scale that measured erythema, lichenification, and surface damage between the immunotherapy and the placebo groups. Another study, Galli 1994, reported no significant difference between treatment groups, using a non‐published scale that measured severity of erythema, vesicles, fissuration, lichenification, and itching.
4. Use of other medication for treatment of eczema during the intervention period
One study, Silny 2006, with 20 participants, reported no statistically significant difference between the treatment groups in the use of topical steroids for mild to moderate flares of AE (RR 1.33, 95% CI 0.74 to 2.41; Analysis 1.6). Another study, Glover 1992, reported no significant difference in the use of topical steroids between the treatment groups. (There were no numerical data for meta‐analysis.) One study, Sanchez 2012, reported a significant reduction in the use of topical steroids and tacrolimus during one year of immunotherapy (P = 0.02), but there was no such reduction in the control group.
Two studies reported the use of systemic steroids for AE. One study, Kaufman 1974, with 26 participants, required the use of systemic steroids in 8/16 participants (50%) in the immunotherapy group and 4/10 participants (40%) in the placebo group (P = 0.70). Another study, Sanchez 2012, with 60 participants, reported a significant increase in systemic steroid use in 12/29 participants (41%) in the control group compared with 4/31 participants (13%) in the immunotherapy group (P = 0.02). We did not perform meta‐analysis because of the high heterogeneity (I² = 76%). The reason for high heterogeneity between these two studies was unclear.
Another study, Novak 2012, with 168 participants, reported a non‐significant 32% difference in the median AUC (area under the curve) of medication score, a culmination of topical medication and overall consumption of systemic medication (19,330 in the immunotherapy group and 28,420 in the placebo group; P = 0.08). These data were not in a format suitable for incorporation into a meta‐analysis.
One study, Pajno 2007, reported a significant decrease in the use of rescue medications (oral hydroxyzine and topical steroids, respectively) in the immunotherapy group. There were 171 occasions where rescue medications were used in the immunotherapy group compared with 346 occasions in the placebo group (P = 0.03). The rescue medications were used on 93 days in the immunotherapy group and 158 days in the placebo groups (P = 0.01).
One study, Luna‐Pech 2013, reported significantly less use of rescue medications (not defined) in the treatment group compared with the control group, but no details were provided.
Another study, Qin 2014, reported an average daily drug score (one point for symptomatic use of levocetirizine hydrochloride tablet, mometasone furoate cream, or mupirocin ointment each day; and six points for every six‐day course of clarithromycin for superinfection). Average daily drug score was lower in the treatment group (mean 0.5, standard deviation (SD) 0.4) than in the control group (mean 1.3, SD 0.7) (P < 0.01).
Other studies did not report this outcome (Di Rienzo 2014; Galli 1994; Leroy 1993; Warner 1978). None of the studies reported the use of oral antihistamines or calcineurin inhibitors as separate outcomes.
5. Validated eczema‐related quality of life scores
One study, Novak 2012, reported a validated eczema‐related quality of life score, the Dermatology Life Quality Index (DLQI), at the end of treatment. We used original data kindly provided by the trial authors to calculate DLQI at the end of treatment, which showed no difference between the treatment groups ‐ a median of 3 (interquartile range (IQR) 1.0 to 8.0) for immunotherapy and a median of 3.5 (IQR 1.0 to 10.5) for placebo (P = 0.525).
Subgroup analyses
We undertook 16 planned subgroup analyses where data were available. We did not undertake further sensitivity analyses because of the small number of trials that contributed data to the analyses.
-
Immunotherapy type: sublingual and subcutaneous.
-
Allergen type: seasonal inhalant, perennial inhalant, food, and microbial.
-
Age of participants: up to four years, five to 11, 12 to 17, and 18 or over.
-
Immunotherapy regimens to be subdivided empirically into low, intermediate, and high dose therapy according to content of major allergen per dose (e.g. Phleum p5 for grass, Bet v1 for birch pollen, Fel d1 for cat, etc.):
-
for subcutaneous immunotherapy, content of major allergen 1 mcg to 5 mcg, 6 mcg to 10 mcg, and greater than 11 mcg per four‐ to six‐weekly maintenance injection doses; and
-
for sublingual immunotherapy, content of major allergen 1 mcg to 5 mcg, 6 mcg to 10 mcg, and greater than 11 mcg per daily maintenance sublingual dose (or equivalent if taken less frequently).
-
-
Severity of AE at randomisation: mild (SCORAD mean objective score of 0 to 15), moderate (SCORAD mean objective score of 16 to 40), and severe (SCORAD mean objective score of greater than 40).
First, we analysed our primary outcome measure 'Participant‐ or parent‐reported global assessment of disease severity at the end of treatment'. Two studies reported dichotomous outcomes that we did not combine in meta‐analyses because of significant heterogeneity (I² = 83%) (Glover 1992; Warner 1978). We did not perform subgroup analyses because both studies fell under the same subgroup categories (subcutaneous route, perennial allergen, and both children and adults). One study, Warner 1978, showed significant improvement in 7/9 participants (78%) in the immunotherapy group compared with 3/11 participants (27%) in the placebo group (P = 0.04). Another study, Glover 1992, showed significant improvement in 8/13 participants (62%) in the active group compared with 9/11 (81%) in the placebo group (P = 0.38).
Next, we analysed our primary outcome measure 'Participant‐ or parent‐reported specific symptoms of eczema, by subjective measures' in nine subgroup analyses. We found no evidence that this outcome differed according to the following.
-
Route of immunotherapy: SCORAD part C (subcutaneous: MD ‐0.62, 95% CI ‐2.18 to 0.93) (sublingual: MD ‐0.94, 95% CI ‐3.00 to 1.13) (test for subgroup differences: I² = 0%; Analysis 2.1). With regard to itch, meta‐analysis was not possible due to extreme heterogeneity (I² = 99%) attributable to the study of Sanchez 2012. Without this study in the analysis, the test for subgroup difference between sublingual and subcutaneous immunotherapies and their controls was not significant (I² = 0%) for sleep disturbance (subcutaneous: MD ‐0.42, 95% CI ‐1.24 to 0.40) (sublingual: MD ‐0.54, 95% CI ‐1.27 to 0.19) (test for subgroup differences: I² = 0%; Analysis 2.2).
-
Allergen type: SCORAD part C (seasonal inhalant: MD not estimable) (perennial inhalant: MD ‐0.74, 95% CI ‐1.98 to 0.50; Analysis 2.3) (food: MD not estimable) (microbial: MD not estimable). With regard to itch, meta‐analysis was not possible due to extreme heterogeneity (I² = 99%) attributable to the study of Sanchez 2012. Without this study in the analysis, the test for subgroup differences for seasonal inhalant and perennial inhalant immunotherapies was not significant (I² = 0%) for sleep disturbance (seasonal inhalant: MD not estimable) (perennial inhalant: MD ‐0.49, 95% CI ‐1.03 to 0.06; Analysis 2.4) (food: MD not estimable) (microbial: MD not estimable).
-
Participant age: SCORAD part C (up to four years: MD not estimable) (five to 11 years of age: MD not estimable) (12 to 17 years of age: MD not estimable) (18 years of age or over: (MD ‐0.62, 95% CI ‐2.18 to 0.93; Analysis 2.5); itch (up to four years of age: MD not estimable) (five to 11 years of age: MD not estimable) (12 to 17 years of age: MD not estimable) (18 years of age or over: MD ‐0.20, 95% CI ‐1.05 to 0.64; Analysis 2.6); or sleep disturbance (up to four years of age: MD not estimable) (five to 11 years of age: MD not estimable) (12 to 17 years of age: MD not estimable) (18 years of age or over: MD ‐0.42, 95% CI ‐1.24 to 0.40; Analysis 2.7).
-
Severity at randomisation using original data from one study for the outcomes itch and sleep disturbance (Novak 2012). In the moderate severity subgroup, data were available for 37 participants (23 in the immunotherapy group and 14 in the placebo group): itch did not differ significantly between groups ‐ with a median of 1.7 (IQR 0.3 to 3.5) for immunotherapy and 1.7 (IQR 0.5 to 3.7) for placebo (P = 0.96) ‐ nor did sleep disturbance ‐ with a median of 0.3 (IQR 0.1 to 2.8) for immunotherapy and 0.5 (IQR 0.3 to 1.5) for placebo (P = 0.53). In the severe subgroup, data were available for 109 participants (75 in the active group and 34 in the placebo group): itch did not differ significantly between groups ‐ with a median of 2.0 (IQR 0.7 to 4.1) for immunotherapy and 2.9 (IQR 1.3 to 5.4) for placebo (P = 0.22) ‐ nor did sleep disturbance ‐ with a median of 1.1 (IQR 0.4 to 3.3) for immunotherapy and 1.9 (IQR 0.6 to 5.1) for placebo (P = 0.14). During treatment, we also calculated the change in itch in the moderate (MD 1.01, 95% CI ‐1.31 to 3.33) and severe subgroups (MD 0.10, 95% CI ‐1.38 to 1.58; Analysis 2.8) and sleep disturbance in the moderate (MD 0.38, 95% CI ‐1.32 to 2.09) and severe subgroups (MD ‐0.31, 95% CI ‐1.66 to 1.04; Analysis 2.9). We found no significant difference between the immunotherapy and control groups.
Last, we analysed our primary outcome 'Adverse events' in six subgroup analyses. We found evidence that this outcome differed significantly according to the following:
-
route of immunotherapy: local reactions were greater in the immunotherapy group than the control group by the sublingual (RR 9.76, 95% CI 1.28 to 74.26) but not the subcutaneous route (RR 1.18, 95% CI 0.90 to 1.55) (test for subgroup differences: I² = 76%; Analysis 2.10).
We found no evidence that this outcome differed between the immunotherapy or control groups according to the following:
-
route of immunotherapy: systemic reactions (subcutaneous: RR 0.82, 95% CI 0.34 to 2.00) (sublingual: RR 0.74, 95% CI 0.29 to 1.89) (test for subgroup differences: I² = 0%; Analysis 2.11);
-
allergen type: local reactions (seasonal inhalant: RR not estimable) (perennial inhalant: RR 1.31, 95% CI 0.81 to 2.13; Analysis 2.12) (food: RR not estimable) (microbial: RR not estimable); systemic reactions (seasonal inhalant: RR not estimable) (perennial inhalant: RR 0.78, 95% CI 0.41 to 1.49; Analysis 2.13) (food: RR not estimable) (microbial: RR not estimable); and
-
participant age: local reactions (up to four years: RR not estimable) (five to 11: RR not estimable) (12 to 17: RR not estimable) (18 years or over: RR 1.37, 95% CI 0.44 to 4.23; Analysis 2.14); systemic reactions (up to four years: RR not estimable) (five to 11: RR not estimable) (12 to 17: RR not estimable) (18 years or over: RR 0.74, 95% CI 0.38 to 1.47; Analysis 2.15).
There were no data available for other subgroup analyses of our primary outcomes.
Discusión
Resumen de los resultados principales
Se identificaron 12 ensayos clínicos controlados aleatorios de inmunoterapia con alérgenos específicos (IAE) para el tratamiento del eccema atópico (EA) que incluyeron 733 participantes con eccema y sensibilización alérgica a un alérgeno inhalado. Los estudios se realizaron en participantes niños y adultos alérgicos a ácaros del polvo doméstico, el polen del césped y otros alérgenos inhalados; y la inmunoterapia se administró por las vías subcutánea, sublingual, oral e intradérmica. Se consideró que nueve estudios tuvieron un alto riesgo de sesgo debido a las altas tasas de pérdida durante el seguimiento o a las exclusiones posteriores a la asignación al azar, Di Rienzo 2014, Glover 1992, Kaufman 1974, Leroy 1993, Luna‐Pech 2013, Novak 2012, 2007,Pajno Qin 2014, o a la evaluación no cegada del resultado, Di Rienzo 2014, Sanchez 2012.
Para los resultados primarios preespecificados "Evaluación global informada por el participante o por los padres de la gravedad de la enfermedad al final del tratamiento" (dos estudios, 44 participantes, pruebas de calidad baja) y "Síntomas específicos del eccema informados por el participante o los padres, mediante medidas subjetivas" (seis estudios, 339 participantes, pruebas de calidad muy baja), la IAE no es un tratamiento eficaz para el EA (Resumen de los hallazgos para la comparación principal). Sin embargo, los hallazgos de los resultados secundarios "Evaluación global calificada por el investigador o el médico de la actividad de la enfermedad al final del tratamiento" (siete estudios, 286 participantes) y "Gravedad del eccema evaluada por el investigador o el médico mediante una escala publicada (p.ej. SCORing Atopic Dermatitis [SCORAD])" (seis estudios, 435 participantes) indicaron que la IAE fue eficaz, aunque la calidad de las pruebas fue baja y muy baja para estos dos resultados, respectivamente. Los otros resultados secundarios "Gravedad del eccema calificada por los padres o el participante evaluada mediante una escala publicada" (dos estudios, 184 participantes) y "Puntuaciones validadas de la calidad de vida relacionada con el eccema" (un estudio, 168 participantes) no mostraron diferencias con la IAE.
Para el resultado primario "eventos adversos", la IAE no se asoció con un mayor riesgo de reacciones adversas locales (siete estudios, 484 participantes) o sistémicas (siete estudios, 492 participantes, pruebas de calidad moderada). Además, la IAE no se asoció con una mayor necesidad de uso de corticosteroides tópicos (un estudio, 20 participantes) o sistémicos (dos estudios, 86 participantes) durante los estudios.
Tres estudios presentaron más hallazgos positivos que los otros. Uno,Sanchez 2012, informó una mejoría marcada en los síntomas informados por el participante o los padres y mejorías más pequeñas pero estadísticamente significativas en la gravedad de eccema global informada por el investigador o el médico y la SCORAD total (una mejoría 5,8 puntos mayor) en comparación con los participantes sin tratar. Otro, Qin 2014, informó una gravedad global de la enfermedad calificada por el investigador o el médico significativamente mayor, definida como un cambio en la SCORAD ≥ 60%, con la IAE (77,78%) en comparación con el control (53,85%) (P < 0,05). Un estudio adicional, Luna‐Pech 2013, informó un cambio significativo en la gravedad global de la enfermedad calificada por el investigador o el médico mediante la evaluación SCORAD con la IAE (media ‐18,4; DE 6,5) en comparación con el control (media ‐6,6; DE 4,1) (P = 0,008), con un mayor efecto en los pacientes que presentaban eccema grave al inicio. No había datos originales disponibles para su inclusión en el metanálisis.
Los análisis de subgrupos identificaron una confianza baja en el efecto de que la inmunoterapia sublingual se asoció con más reacciones adversas locales en comparación con la inmunoterapia subcutánea. Otros análisis de subgrupos no identificaron un tipo de alérgeno, una edad del participante o una gravedad del EA al momento de la asignación al azar con una eficacia o un perfil de seguridad diferentes, aunque generalmente estos análisis no fueron concluyentes debido a los datos limitados disponibles.
Compleción y aplicabilidad general de las pruebas
En general, se encontraron pruebas de calidad baja de que la inmunoterapia con alérgenos específicos es eficaz en el tratamiento del eccema atópico. Las diferentes escalas de gravedad de la enfermedad y puntuaciones de los síntomas utilizadas en los ensayos en general limitaron los metanálisis. En los que tuvieron datos comparables algunos resultados fueron significativos. Los amplios intervalos de confianza para muchas medidas de resultado reflejaron los estudios relativamente pequeños y las variadas metodologías. Varios resultados se basaron en el análisis de un único ensayo, Novak 2012, con un gran número de participantes pero pérdidas altas durante el seguimiento. Tres ensayos, Di Rienzo 2014, Qin 2014, Sanchez 2012, tuvieron más hallazgos positivos que los otros y mostraron un efecto beneficioso claro sobre los síntomas de eccema informados por el participante o los padres o la gravedad global del eccema informada por el médico mediante SCORAD. No está claro por qué los resultados de estos ensayos difirieron, pero hubo riesgo de sesgo de detección debido a la falta de cegamiento de los participantes o los investigadores en al menos dos ensayos(Di Rienzo 2014; Sanchez 2012). Se encontró que las tasas de reacciones adversas no aumentaron significativamente con la inmunoterapia en los estudios incluidos, pero otras pruebas indican que la IAE conlleva un aumento significativo en el riesgo de reacciones alérgicas graves (Calderon 2007). Aunque lo anterior podría indicar que la sensibilización alérgica presente en los participantes del ensayo tiene poca relevancia clínica o que los extractos del alérgeno utilizados tuvieron baja potencia, también puede reflejar el número pequeño de ensayos y participantes que contribuyeron a los análisis de los eventos adversos.
Calidad de la evidencia
La valoración general de la calidad del grupo de pruebas que contribuyeron a los resultados de la revisión, mediante el enfoque Grading of Recommendations Assessment, Development and Education (GRADE) (Higgins 2011), fue baja. Los motivos para disminuirla fueron los relativamente pocos ensayos y participantes, la falta de cegamiento en al menos dos ensayos, los intervalos de confianza amplios, el riesgo moderado de sesgo con pérdidas altas durante el seguimiento como inquietud principal y la heterogeneidad significativa entre la estimación de los efectos del tratamiento para un resultado primario.
Sesgos potenciales en el proceso de revisión
Las fortalezas de esta revisión fueron el cumplimiento con el protocolo publicado y los esfuerzos repetidos para adquirir los datos originales de los autores de los estudios para maximizar las oportunidades para el metanálisis y aclarar las incertidumbres metodológicas. El número limitado de estudios incluidos no permitió la evaluación formal del sesgo de publicación. Se analizaron diferentes medidas de resultado como análisis separados, lo que limitó las oportunidades de agrupar los datos de diferentes estudios que utilizaron diferentes herramientas de evaluación de los resultados.
Acuerdos y desacuerdos con otros estudios o revisiones
Se han realizado otras tres revisiones sistemáticas de IAE en el tratamiento del EA. En una revisión (Bae 2013), los autores identificaron ocho de los 12 ensayos incluidos en esta revisión, pero analizaron los datos de manera diferente al agrupar resultados heterogéneos "medidos mediante cualquier sistema de calificación", lo que puede no ser apropiado (Tam 2013). A diferencia de la presente revisión, encontraron pruebas moderadas de que la IAE puede ser un tratamiento eficaz para el EA en todos los participantes estudiados (odds ratio [OR] para mejoría en el eccema 5,35; intervalo de confianza [IC] del 95%: 1,61 a 17,77) y en los análisis de subgrupos de los participantes con eccema grave al momento de la asignación al azar (OR 3,13; IC del 95%: 1,31 a 7,47) y los estudios que utilizaron inmunoterapia subcutánea (OR 4,27; IC del 95%: 1,36 a 13,39). Los diferentes resultados en esa revisión se deben probablemente a los enfoques no convencionales para extraer y combinar los datos de los ensayos incluidos. No hubo un protocolo registrado para esa revisión, por lo que no fue posible confirmar que los criterios de inclusión y las medidas de resultado se determinaran a priori.
En una revisión sistemática que utilizó las recomendaciones GRADE (Gendelman 2013), los autores identificaron cinco de los nueve ensayos incluidos en la presente revisión y dos adicionales que se excluyeron aquí (Ring 1982; Werfel 2006). La revisión no realizó metanálisis. Similar a la presente revisión, solamente encontraron recomendaciones débiles para la administración de la IAE en el tratamiento del EA. También informaron deficiencias metodológicas similares, incluidas las altas pérdidas durante el seguimiento.
En una revisión sistemática similar sobre inmunoterapia sublingual solamente que utilizó las recomendaciones GRADE(Gendelman 2015), los autores identificaron tres de los 12 ensayos incluidos en la presente revisión y dos adicionales que se excluyeron aquí(Cadario 2007; Mastrandrea 2000). La revisión no realizó metanálisis. Similar al presente estudio, solamente encontraron recomendaciones débiles para la administración de inmunoterapia sublingual en el tratamiento del EA, con un efecto placebo grande en dos estudios. También informaron deficiencias metodológicas similares que incluyeron falta de cegamiento, ausencia de control y ausencia de asignación al azar.

PRISMA flow diagram

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
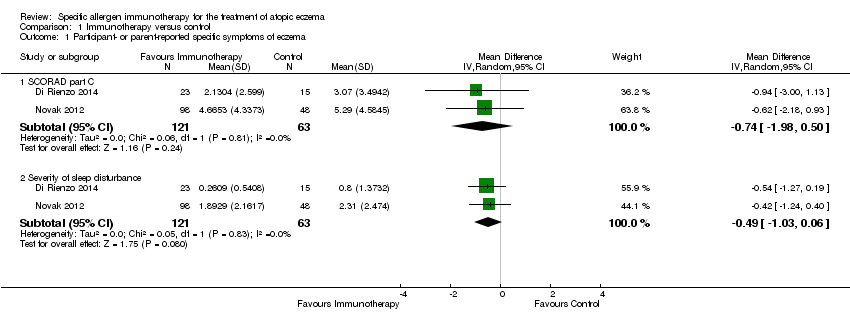
Comparison 1 Immunotherapy versus control, Outcome 1 Participant‐ or parent‐reported specific symptoms of eczema.

Comparison 1 Immunotherapy versus control, Outcome 2 Adverse events.

Comparison 1 Immunotherapy versus control, Outcome 3 Investigator‐ or physician‐rated global disease severity.
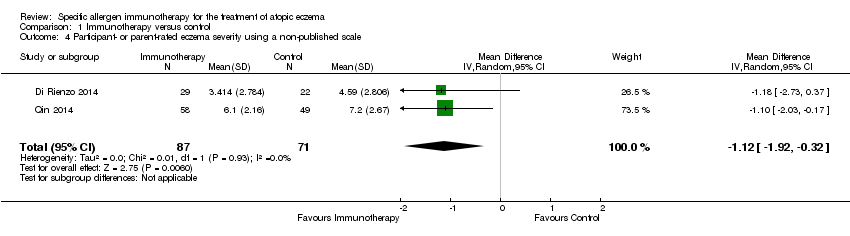
Comparison 1 Immunotherapy versus control, Outcome 4 Participant‐ or parent‐rated eczema severity using a non‐published scale.

Comparison 1 Immunotherapy versus control, Outcome 5 Investigator‐rated eczema severity assessed using a published scale.

Comparison 1 Immunotherapy versus control, Outcome 6 Use of other medications for eczema.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 1 Participant‐ or parent‐reported specific symptoms of eczema ‐ SCORAD part C by route of immunotherapy.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 2 Participant‐ or parent‐reported specific symptoms of eczema ‐ severity of sleep disturbance by route of immunotherapy.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 3 Participant‐ or parent‐reported specific symptoms of eczema ‐ SCORAD part C by allergen type.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 4 Participant‐ or parent‐reported specific symptoms of eczema ‐ severity of sleep disturbance by allergen type.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 5 Participant‐ or parent‐reported specific symptoms of eczema ‐ SCORAD part C by participant age.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 6 Participant‐ or parent‐reported specific symptoms of eczema ‐ itch severity by participant age.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 7 Participant‐ or parent‐reported specific symptoms of eczema ‐ severity of sleep disturbance by participant age.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 8 Participant‐ or parent‐reported specific symptoms of eczema ‐ itch severity by severity at randomisation.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 9 Participant‐ or parent‐reported specific symptoms of eczema ‐ severity of sleep disturbance by severity at randomisation.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 10 Adverse events: any local reaction by route of immunotherapy.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 11 Adverse events: any systemic reaction by route of immunotherapy.
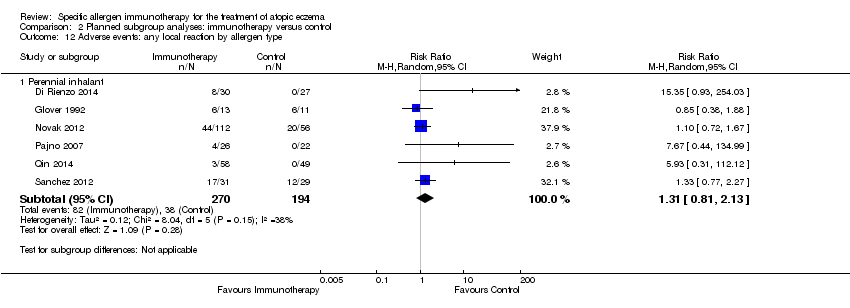
Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 12 Adverse events: any local reaction by allergen type.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 13 Adverse events: any systemic reaction by allergen type.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 14 Adverse events: any local reaction by participant age.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 15 Adverse events: any systemic reaction by participant age.
| Specific immunotherapy compared with no immunotherapy for atopic eczema | ||||||
| Patient or population: adults and children with atopic eczema and inhalant allergen sensitisation Settings: specialist allergy centres in the UK (2 trials), Italy (3 trials), USA, Germany, Belgium, Poland, Columbia, and China Intervention: specific allergen immunotherapy Comparison: no immunotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No immunotherapy | Specific allergen immunotherapy | |||||
| Participant‐ or parent‐reported global assessment of disease severity Follow‐up: 6 to 12 months | See comments | See comments | Not estimable | 44a | ⊕⊕⊝⊝ | Improvement in 7/9 participants (78%) in the immunotherapy group and 3/11 participants (27%) in the placebo group (RR 2.85, 95% CI 1.02 to 7.96; P = 0.04 (Warner 1978)) 8/13 participants (62%) in the immunotherapy group and 9/11 participants (81%) in the placebo group (RR 0.75, 95% CI 0.45 to 1.26; P = 0.38 (Glover 1992)) Due to unexplained statistical heterogeneity, we did not pool the data |
| Participant‐ or parent‐reported specific symptoms of eczema Follow‐up: 12 to 18 months SCORAD part C measured as a combination of 2 Visual Analogue Scales (1 for itch, 1 for sleep disturbance), each on a scale from 0, no specific symptoms, to 10, maximum specific symptoms | The mean SCORAD part C score ranged across control groups from 3.07 to 5.29 The mean SCORAD part C sleep severity score ranged across control groups from 0.8 to 2.31 | The mean SCORAD part C score in the immunotherapy group was on average 0.74 lower (95% CI ‐1.98 to 0.50) The mean SCORAD part C sleep severity score in the immunotherapy group was on average 0.49 lower (95% CI ‐1.03 to 0.06) | ‐ | 339a (6) | ⊕⊝⊝⊝ | Itch: SCORAD part C itch severity at the end of treatment: MD ‐0.24, 95% CI ‐1.00 to 0.52; I² = 0% for Di Rienzo 2014 and Novak 2012 Itch severity score: MD ‐4.20, 95% CI ‐3.69 to ‐4.71 for Sanchez 2012 Due to unexplained statistical heterogeneity, we did not pool the data |
| Adverse events ‐ any systemic reaction Follow‐up: 6 to 18 months | Low‐risk population | RR 0.78 (0.41 to 1.49) | 492a | ⊕⊕⊕⊝ | ‐ | |
| 0 per 1000 | 0 per 1000 | |||||
| Medium‐risk population | ||||||
| 71 per 1000 | 55 per 1000 | |||||
| High‐risk population | ||||||
| 163 per 1000 | 127 per 1000 | |||||
| Investigator‐ or physician‐rated global assessment of disease severity Follow‐up: 1 to 3 years | Low‐risk population | RR 1.48 (1.16 to 1.88) | 286a (7) | ⊕⊝⊝⊝ | ‐ | |
| 0 per 1000 | 0 per 1000 (0 to 10) | |||||
| Medium‐risk population | ||||||
| 471 per 1000 | 697 per 1000 (546 to 885) | |||||
| High‐risk population | ||||||
| 778 per 1000 | 1151 per 1000 (903 to 1462) | |||||
| Investigator‐ or physician‐rated eczema severity using a published scale Follow‐up: 12 to 18 months | The mean SCORAD score ranged across control groups from 26.7 to 32.6 | The mean SCORAD score in the immunotherapy group was on average 5.79 lower (95% CI ‐7.92 to ‐3.66) | ‐ | 435a (6) | ⊕⊝⊝⊝ | ‐ |
| Participant or parent‐rated eczema severity using a published scale Follow‐up: 12 to 18 months | See comment | See comment | Not estimable | 184a | ⊕⊕⊝⊝ | SCORAD part C used as the specific eczema symptom score (Di Rienzo 2014; Novak 2012) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Assumed risks are based on the total control group risk across all included studies (medium risk population) and the included studies with the lowest (low risk population) and highest (high risk population) control group risks. | ||||||
| Term | Definition |
| Anaphylaxis | A serious, life‐threatening allergic reaction |
| Fissuration | Formation of tears in the skin |
| Intradermally | Into the skin (dermis), below the epidermis |
| Lichenification | Thickening and hardening of the skin |
| Monovalent | 1 kind of antibody |
| Perennial | Long‐lasting continually |
| Photopheresis | A form of apheresis and photodynamic therapy |
| Sublingual | Under the tongue |
| Vesicles | Fluid‐filled cavities |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participant‐ or parent‐reported specific symptoms of eczema Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 SCORAD part C | 2 | 184 | Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐1.98, 0.50] |
| 1.2 Severity of sleep disturbance | 2 | 184 | Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐1.03, 0.06] |
| 2 Adverse events Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Any local reaction | 7 | 484 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.89, 1.81] |
| 2.2 Any systemic reaction | 7 | 492 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.41, 1.49] |
| 2.3 Tiredness | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 5.08 [0.66, 39.02] |
| 2.4 Headache | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 2.56 [0.11, 59.75] |
| 3 Investigator‐ or physician‐rated global disease severity Show forest plot | 6 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [1.16, 1.88] |
| 4 Participant‐ or parent‐rated eczema severity using a non‐published scale Show forest plot | 2 | 158 | Mean Difference (IV, Random, 95% CI) | ‐1.12 [‐1.92, ‐0.32] |
| 5 Investigator‐rated eczema severity assessed using a published scale Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Total SCORAD | 3 | 244 | Mean Difference (IV, Random, 95% CI) | ‐5.79 [‐7.92, ‐3.66] |
| 6 Use of other medications for eczema Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participant‐ or parent‐reported specific symptoms of eczema ‐ SCORAD part C by route of immunotherapy Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Subcutaneous immunotherapy | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Sublingual immunotherapy | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Participant‐ or parent‐reported specific symptoms of eczema ‐ severity of sleep disturbance by route of immunotherapy Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Subcutaneous immunotherapy | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Sublingual immunotherapy | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Participant‐ or parent‐reported specific symptoms of eczema ‐ SCORAD part C by allergen type Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Perennial inhalant | 2 | 184 | Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐1.98, 0.50] |
| 4 Participant‐ or parent‐reported specific symptoms of eczema ‐ severity of sleep disturbance by allergen type Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Perennial inhalant | 2 | 184 | Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐1.03, 0.06] |
| 5 Participant‐ or parent‐reported specific symptoms of eczema ‐ SCORAD part C by participant age Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 18 years or over | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Participant‐ or parent‐reported specific symptoms of eczema ‐ itch severity by participant age Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 18 years or over | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Participant‐ or parent‐reported specific symptoms of eczema ‐ severity of sleep disturbance by participant age Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 18 years or over | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Participant‐ or parent‐reported specific symptoms of eczema ‐ itch severity by severity at randomisation Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Moderate (SCORAD mean objective score 16 to 40) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Severe (SCORAD mean objective score > 40) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Participant‐ or parent‐reported specific symptoms of eczema ‐ severity of sleep disturbance by severity at randomisation Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Moderate (SCORAD mean objective score 16 to 40) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Severe (SCORAD mean objective score > 40) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Adverse events: any local reaction by route of immunotherapy Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Subcutaneous | 5 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.90, 1.55] |
| 10.2 Sublingual | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 9.76 [1.28, 74.26] |
| 11 Adverse events: any systemic reaction by route of immunotherapy Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Subcutaneous | 5 | 328 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.34, 2.00] |
| 11.2 Sublingual | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.29, 1.89] |
| 12 Adverse events: any local reaction by allergen type Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 Perennial inhalant | 6 | 464 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.81, 2.13] |
| 13 Adverse events: any systemic reaction by allergen type Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 Perennial inhalant | 6 | 472 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.41, 1.49] |
| 14 Adverse events: any local reaction by participant age Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 18 years or over | 2 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.44, 4.23] |
| 15 Adverse events: any systemic reaction by participant age Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15.1 18 years or over | 2 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.38, 1.47] |

