Specifična imunoterapija alergenima za liječenje atopijskog ekcema
Abstract
Background
Specific allergen immunotherapy (SIT) is a treatment that may improve disease severity in people with atopic eczema (AE) by inducing immune tolerance to the relevant allergen. A high quality systematic review has not previously assessed the efficacy and safety of this treatment.
Objectives
To assess the effects of specific allergen immunotherapy (SIT), including subcutaneous, sublingual, intradermal, and oral routes, compared with placebo or a standard treatment in people with atopic eczema.
Search methods
We searched the following databases up to July 2015: the Cochrane Skin Group Specialised Register, CENTRAL in the Cochrane Library (Issue 7, 2015), MEDLINE (from 1946), EMBASE (from 1974), LILACS (from 1982), Web of Science™ (from 2005), the Global Resource of EczemA Trials (GREAT database), and five trials databases. We searched abstracts from recent European and North American allergy meetings and checked the references of included studies and review articles for further references to relevant trials.
Selection criteria
Randomised controlled trials (RCTs) of specific allergen immunotherapy that used standardised allergen extracts in people with AE.
Data collection and analysis
Two authors independently undertook study selection, data extraction (including adverse effects), assessment of risk of bias, and analyses. We used standard methodological procedures expected by Cochrane.
Main results
We identified 12 RCTs for inclusion in this review; the total number of participants was 733. The interventions included SIT in children and adults allergic to either house dust mite (10 trials), grass pollen, or other inhalant allergens (two trials). They were administered subcutaneously (six trials), sublingually (four trials), orally, or intradermally (two trials). Overall, the risk of bias was moderate, with high loss to follow up and lack of blinding as the main methodological concern.
Our primary outcomes were 'Participant‐ or parent‐reported global assessment of disease severity at the end of treatment'; 'Participant‐ or parent‐reported specific symptoms of eczema, by subjective measures'; and 'Adverse events, such as acute episodes of asthma or anaphylaxis'. SCORing Atopic Dermatitis (SCORAD) is a means of measuring the effect of atopic dermatitis by area (A); intensity (B); and subjective measures (C), such as itch and sleeplessness, which we used.
For 'Participant‐ or parent‐reported global assessment of disease severity at the end of treatment', one trial (20 participants) found improvement in 7/9 participants (78%) treated with the SIT compared with 3/11 (27%) treated with the placebo (risk ratio (RR) 2.85, 95% confidence interval (CI) 1.02 to 7.96; P = 0.04). Another study (24 participants) found no difference: global disease severity improved in 8/13 participants (62%) treated with the SIT compared with 9/11 (81%) treated with the placebo (RR 0.75, 95% CI 0.45 to 1.26; P = 0.38). We did not perform meta‐analysis because of high heterogeneity between these two studies. The quality of the evidence was low.
For 'Participant‐ or parent‐reported specific symptoms of eczema, by subjective measures', two trials (184 participants) did not find that the SIT improved SCORAD part C (mean difference (MD) ‐0.74, 95% CI ‐1.98 to 0.50) or sleep disturbance (MD ‐0.49, 95% CI ‐1.03 to 0.06) more than placebo. For SCORAD part C itch severity, these two trials (184 participants) did not find that the SIT improved itch (MD ‐0.24, 95% CI ‐1.00 to 0.52). One other non‐blinded study (60 participants) found that the SIT reduced itch compared with no treatment (MD ‐4.20, 95% CI ‐3.69 to ‐4.71) and reduced the participants' overall symptoms (P < 0.01), but we could not pool these three studies due to high heterogeneity. The quality of the evidence was very low.
Seven trials reported systemic adverse reactions: 18/282 participants (6.4%) treated with the SIT had a systemic reaction compared with 15/210 (7.1%) with no treatment (RR 0.78, 95% CI 0.41 to 1.49; the quality of the evidence was moderate). The same seven trials reported local adverse reactions: 90/280 participants (32.1%) treated with the SIT had a local reaction compared with 44/204 (21.6%) in the no treatment group (RR 1.27, 95% CI 0.89 to 1.81). As these had the same study limitations, we deemed the quality of the evidence to also be moderate.
Of our secondary outcomes, there was a significant improvement in 'Investigator‐ or physician‐rated global assessment of disease severity at the end of treatment' (six trials, 262 participants; RR 1.48, 95% CI 1.16 to 1.88). None of the studies reported our secondary outcome 'Parent‐ or participant‐rated eczema severity assessed using a published scale', but two studies (n = 184), which have been mentioned above, used SCORAD part C, which we included as our primary outcome 'Participant‐ or parent‐reported specific symptoms of eczema, by subjective measures'.
Our findings were generally inconclusive because of the small number of studies. We were unable to determine by subgroup analyses a particular type of allergen or a particular age or level of disease severity where allergen immunotherapy was more successful. We were also unable to determine whether sublingual immunotherapy was associated with more local adverse reactions compared with subcutaneous immunotherapy.
Authors' conclusions
Overall, the quality of the evidence was low. The low quality was mainly due to the differing results between studies, lack of blinding in some studies, and relatively few studies reporting participant‐centred outcome measures. We found limited evidence that SIT may be an effective treatment for people with AE. The treatments used in these trials were not associated with an increased risk of local or systemic reactions. Future studies should use high quality allergen formulations with a proven track record in other allergic conditions and should include participant‐reported outcome measures.
PICO
Laički sažetak
Specifična imunoterapija alegenima za liječenje atopijskog ekcema
Dosadašnje spoznaje
Najmanje jedno od 7 djece i jedno od 50 odraslih boluju od atopijskog ekcema, kožne bolesti koju obilježava crveni osip i svrbež. Osobe koje boluju od atopijskog ekcema su alergične na tvari u svojoj okolini, kao što su grinje, a izlaganje alergenima može pogoršati njihovo stanje. Specifična imunoterapija alergenima je liječenje koje uključuje davanje injekcija ili kapi ispod jezika koje sadrže tvari na koje je osoba alergična. Takvo liječenje može smanjiti ozbiljnost alergije te time ublažiti simptome atopijskog ekcema. U ovom Cochrane sustavnom pregledu ispitano je da li je specifična terapija alergenima bolja ili lošija od standardnog liječenja ili placeba u smanjenju ozbiljnosti i simptoma, prema procjeni sudionika istraživanja, roditelja ili istraživača.
Istraživačko pitanje
Je li specifična imunoterapija alergenima djelotvoran način liječenja za ljude s atopijskim ekcemom?
Značajke istraživanja
Dokazi se temelje na literaturi objavljenoj do srpnja 2015. Pronašli smo 12 studija, sa 733 sudionika, u kojima su sudjelovala i djeca i odrasli. Studije su provedene u specijalističkim alergološkim centrima u devet zemalja. Trajanje istraživanja odvijalo se u rasponu od četiri mjeseca do tri godine. Imunoterapija je davana sudionicima na četiri različita načina. Proizvođači alergena su financirali sedam od dvanaest studija.
Ključni rezultati
Nismo pronašli nikakve dokaze u studijama u našem pregledu koji bi pokazali da bi specifična terapija alergenima mogla biti učinkovit lijek za atopijski ekcem za ublažavanje ozbiljnosti i simptoma bolesti, temeljem procjene sudionika istraživanja ili roditelja. Pronašli smo ograničene dokaze prema kojima bi takva terapija mogla ublažiti ozbiljnost bolesti, prema ocjeni istražitelja. Imunoterapija nije uzrokovala ni više štete od standardnog lijeka ili placeba.
Kvaliteta dokaza
Sveukupno, kvaliteta dokaza je bila niska. Autori su kvalitetu dokaza ocijenili niskom uglavnom zbog različitih rezultata u različitim studijama, manjka neutralnosti u nekim studijama i zato što je relativno malo studija opisalo rezultate koji bi bili važni za pacijenta. Buduće bi studije trebale koristiti visokokvalitetne alergenske smjese s dokazanim najboljim rezultatima za druga alergijska stanja te bi trebala uključiti rezultate koje bi procjenjivali sudionici istraživanja.
Authors' conclusions
Summary of findings
| Specific immunotherapy compared with no immunotherapy for atopic eczema | ||||||
| Patient or population: adults and children with atopic eczema and inhalant allergen sensitisation Settings: specialist allergy centres in the UK (2 trials), Italy (3 trials), USA, Germany, Belgium, Poland, Columbia, and China Intervention: specific allergen immunotherapy Comparison: no immunotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No immunotherapy | Specific allergen immunotherapy | |||||
| Participant‐ or parent‐reported global assessment of disease severity Follow‐up: 6 to 12 months | See comments | See comments | Not estimable | 44a | ⊕⊕⊝⊝ | Improvement in 7/9 participants (78%) in the immunotherapy group and 3/11 participants (27%) in the placebo group (RR 2.85, 95% CI 1.02 to 7.96; P = 0.04 (Warner 1978)) 8/13 participants (62%) in the immunotherapy group and 9/11 participants (81%) in the placebo group (RR 0.75, 95% CI 0.45 to 1.26; P = 0.38 (Glover 1992)) Due to unexplained statistical heterogeneity, we did not pool the data |
| Participant‐ or parent‐reported specific symptoms of eczema Follow‐up: 12 to 18 months SCORAD part C measured as a combination of 2 Visual Analogue Scales (1 for itch, 1 for sleep disturbance), each on a scale from 0, no specific symptoms, to 10, maximum specific symptoms | The mean SCORAD part C score ranged across control groups from 3.07 to 5.29 The mean SCORAD part C sleep severity score ranged across control groups from 0.8 to 2.31 | The mean SCORAD part C score in the immunotherapy group was on average 0.74 lower (95% CI ‐1.98 to 0.50) The mean SCORAD part C sleep severity score in the immunotherapy group was on average 0.49 lower (95% CI ‐1.03 to 0.06) | ‐ | 339a (6) | ⊕⊝⊝⊝ | Itch: SCORAD part C itch severity at the end of treatment: MD ‐0.24, 95% CI ‐1.00 to 0.52; I² = 0% for Di Rienzo 2014 and Novak 2012 Itch severity score: MD ‐4.20, 95% CI ‐3.69 to ‐4.71 for Sanchez 2012 Due to unexplained statistical heterogeneity, we did not pool the data |
| Adverse events ‐ any systemic reaction Follow‐up: 6 to 18 months | Low‐risk population | RR 0.78 (0.41 to 1.49) | 492a | ⊕⊕⊕⊝ | ‐ | |
| 0 per 1000 | 0 per 1000 | |||||
| Medium‐risk population | ||||||
| 71 per 1000 | 55 per 1000 | |||||
| High‐risk population | ||||||
| 163 per 1000 | 127 per 1000 | |||||
| Investigator‐ or physician‐rated global assessment of disease severity Follow‐up: 1 to 3 years | Low‐risk population | RR 1.48 (1.16 to 1.88) | 286a (7) | ⊕⊝⊝⊝ | ‐ | |
| 0 per 1000 | 0 per 1000 (0 to 10) | |||||
| Medium‐risk population | ||||||
| 471 per 1000 | 697 per 1000 (546 to 885) | |||||
| High‐risk population | ||||||
| 778 per 1000 | 1151 per 1000 (903 to 1462) | |||||
| Investigator‐ or physician‐rated eczema severity using a published scale Follow‐up: 12 to 18 months | The mean SCORAD score ranged across control groups from 26.7 to 32.6 | The mean SCORAD score in the immunotherapy group was on average 5.79 lower (95% CI ‐7.92 to ‐3.66) | ‐ | 435a (6) | ⊕⊝⊝⊝ | ‐ |
| Participant or parent‐rated eczema severity using a published scale Follow‐up: 12 to 18 months | See comment | See comment | Not estimable | 184a | ⊕⊕⊝⊝ | SCORAD part C used as the specific eczema symptom score (Di Rienzo 2014; Novak 2012) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Assumed risks are based on the total control group risk across all included studies (medium risk population) and the included studies with the lowest (low risk population) and highest (high risk population) control group risks. | ||||||
Background
We have listed unfamiliar terms in the glossary of terms in Table 1.
| Term | Definition |
| Anaphylaxis | A serious, life‐threatening allergic reaction |
| Fissuration | Formation of tears in the skin |
| Intradermally | Into the skin (dermis), below the epidermis |
| Lichenification | Thickening and hardening of the skin |
| Monovalent | 1 kind of antibody |
| Perennial | Long‐lasting continually |
| Photopheresis | A form of apheresis and photodynamic therapy |
| Sublingual | Under the tongue |
| Vesicles | Fluid‐filled cavities |
Description of the condition
Atopic eczema (AE) is a chronic inflammatory skin condition that affects 15% to 30% of children and 2% to 10% of adults world wide (Odhiambo 2009; Williams 2006). The terms 'atopic eczema' and 'atopic dermatitis' are synonymous. Severe itching and patches of dry inflamed skin in varying locations depending on the age of the person characterise this condition (Akdis 2006). In infants, AE is usually found on the cheeks, forehead, or scalp. In childhood, AE usually involves the hands, feet, wrists, ankles, and the creases of the elbows and backs of the knees (Akdis 2006). In adults, AE causes dry scaly patches and large plaques of thickened (lichenified) skin in the flexural folds; the face and neck; the upper arms and back; and the backs of the hands, feet, fingers, and toes (Akdis 2006). Strictly speaking, the term 'atopic eczema' "should only refer to individuals who have the physical features of eczema plus evidence of specific immunoglobulin E (IgE) antibodies to common environmental allergens such as house dust mite" (Johansson 2004). We have used this strict definition throughout this review unless we have specified otherwise.
Several observations suggest that allergens may be important causes of atopic eczema. Firstly, direct exposure of the skin to environmental allergens, including perennial allergens like house dust mite, and seasonal allergens like pollen has been shown to increase the severity of atopic eczema (Capristo 2004; Purvis 2005; Schäfer 1999). Secondly, other diseases triggered by allergens are common in those with atopic eczema. For example, of those children who develop the condition during the first two years of life, an estimated 50% may develop asthma during subsequent years (Warner 2001). Finally, those with more severe AE have an increased risk of asthma and allergic rhinitis (Gustafsson 2000; Illi 2004).
Despite the current available topical treatment with emollients; corticosteroids; calcineurin inhibitors; and other treatments, such as antibiotics, people with atopic eczema often cannot keep their condition completely under control. In some cases, the medications used can cause more harm than benefit (Akdis 2006). Therefore, considering the atopic background of the disease and its possible correlation with allergen‐triggering factors, some other types of treatment have been proposed, which include specific allergen immunotherapy (SIT) (Darsow 2012).
Description of the intervention
Specific allergen immunotherapy (SIT) is a treatment for allergic disease that involves the administration of an allergen in high doses in order to induce immune tolerance to that allergen and relieve symptoms (Calderon 2007). For example, in people with hay fever who are allergic to grass pollen, SIT may involve treatment with injections, drops, or tablets of grass pollen over a period of months in order to relieve symptoms (Calderon 2007; Wilson 2005). Specific allergen immunotherapy is the only treatment shown to provide longer‐term benefit in allergic diseases after treatment has stopped (Durham 1999). It has been shown to be an effective treatment for allergic rhinitis and allergic asthma, although the treatment carries a risk of severe allergic reaction (Calderon 2007; CSM report 1986; Wilson 2005).
How the intervention might work
Specific allergen immunotherapy works by inducing changes in the immune response to the relevant allergen, so that in diseases caused by an abnormal response to that allergen, there may be an improvement in symptoms (Allam 2006). The specific immune changes caused by SIT include an increase in activity of suppressive components of the immune system (regulatory T cells) and an increase in antibodies (immunoglobulin G (IgG) antibodies) to the allergen (Bussmann 2007; Bussmann 2009; Maintz 2007). The presence of allergic sensitisation in those with AE and the relationship between AE and other allergic diseases suggest that allergic immune responses are an important part of the disease process in AE (Gustafsson 2000; Illi 2004; Warner 2001). It is therefore plausible that SIT might be able to reduce symptoms in people with AE by inhibiting abnormal immune responses to allergens.
Why it is important to do this review
Specific allergen immunotherapy is a disease‐modifying treatment that reduces symptoms in people with other allergic conditions: allergic rhinitis, allergic conjunctivitis, and asthma (Abramson 2003; Calderon 2007; Dahl 2006; Didier 2007; Penagos 2008). Hence, SIT might be potentially effective in reducing AE. An evaluation of its effects on skin manifestations in the context of randomised controlled trials could provide an alternative treatment for people with AE.
The plans for this review were published as a protocol 'Specific allergen immunotherapy for the treatment of atopic eczema' (Calderon 2010).
Objectives
To assess the effects of specific allergen immunotherapy (SIT), including subcutaneous, sublingual, intradermal, and oral routes, compared with placebo or a standard treatment in people with atopic eczema.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Adults and children with atopic eczema (AE) and allergic sensitisation to an inhalant or food allergen. "Allergy needed to be proven using an objective test such as a positive skin prick test or high circulating levels of allergen‐specific IgE antibody detected by a specific blood test for allergy called the radioallergosorbent test. Trials focusing on allergic rhinitis or asthma without eczema were excluded" (Calderon 2011). Where trials included participants with and without AE, we only included the trial if the results for the participants with AE were separately reported.
Types of interventions
High‐dose immunotherapy with standardised allergen extracts for single allergen or mixed allergens administered by the sublingual (under the tongue), subcutaneous (under the skin), intradermal (into the skin), or oral route compared with placebo or a standard treatment, such as emollients, topical corticosteroids, or topical calcineurin inhibitors. We considered all appropriate allergens at all doses and all durations of treatment.
Types of outcome measures
Primary outcomes
-
Participant‐ or parent‐reported global assessment of disease severity at the end of treatment, i.e. the proportion with good or excellent improvement at this time as reported in the trials (whether treatment was given for one, two, or three years, or other duration).
-
Participant‐ or parent‐reported specific symptoms of eczema, by subjective measures such as itch or sleep disturbance (SCORing Atopic Dermatitis (SCORAD) part C).
-
Adverse events, such as acute episodes of asthma or anaphylaxis.
Secondary outcomes
-
Investigator‐ or physician‐rated global assessment of disease severity at the end of treatment, i.e. the proportion with good or excellent improvement at this time as reported in the trials (whether treatment was given for one, two, or three years, or other duration).
-
Parent‐ or participant‐rated eczema severity assessed using a published scale (e.g. Patient Oriented Eczema Measure (POEM)).
-
Investigator‐ or physician‐rated eczema severity assessed using a published scale (e.g. SCORAD).
-
Use of other medication for treatment of eczema during the intervention period (e.g. topical/systemic corticosteroids, calcineurin inhibitors, or oral antihistamines).
-
Validated eczema‐related quality of life scores (e.g. Dermatitis Family Impact Questionnaire, Children's Dermatology Life Quality Index) (Lewis‐Jones 1995).
Search methods for identification of studies
We aimed to identify all relevant randomised controlled trials (RCTs) regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
We searched the following databases up to 21 July 2015:
-
the Cochrane Skin Group Specialised Register using the terms '(dermatitis or eczema) and (immuno* or allerg*)';
-
the Cochrane Central Register of Controlled Trials (CENTRAL) 2015, Issue 7, in the Cochrane Library using the search strategy in Appendix 1;
-
MEDLINE via Ovid (from 1946) using the strategy in Appendix 2;
-
EMBASE via Ovid (from 1974) using the strategy in Appendix 3;
-
LILACS (Latin American and Caribbean Health Science Information database, from 1982) using the strategy in Appendix 4;
-
the Global Resource of EczemA Trials. Centre of Evidence Based Dermatology, accessed at www.greatdatabase.org.uk, using the terms 'immuno* or allerg*' in the title or keywords of records and restricting to included studies only; and
-
Web of Science™ (from 2005) using the strategy in Appendix 5.
Trials registers
We searched the following trials registers up to 3 August 2015 using the terms 'immunotherapy and (eczema or dermatitis)'.
-
The International Standard Randomised Controlled Trial Number (ISRCTN) registry (www.isrctn.com).
-
The US National Institutes of Health Ongoing Trials Register (www.clinicaltrials.gov).
-
The Australian New Zealand Clinical Trials Registry (www.anzctr.org.au).
-
The World Health Organization International Clinical Trials Registry platform (apps.who.int/trialsearch/).
-
The Ongoing Skin Trials Register (www.nottingham.ac.uk/ongoingskintrials).
Searching other resources
We created a database of first and last names of authors of potentially eligible studies and searched the Science Citation Index Expanded (SCI‐EXPANDED, 1945 to the present) using these names in order to identify further relevant studies.
Reference lists
We checked the bibliographies of each included study and of published reviews for further reports of relevant trials.
Correspondence
We contacted the primary author of each included study to identify additional published and unpublished studies. We contacted allergen immunotherapy product manufacturers to request details of published or unpublished studies of allergen immunotherapy that included eczema as an outcome measure.
Conference proceedings
We searched the abstracts of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology meetings from 2010 to 2015.
Data collection and analysis
Some parts of the methods section of this review uses text that was originally published in other Cochrane Reviews co‐authored by RB and MC (predominantly Boyle 2012 and Calderon 2011). We included a 'Summary of findings' table where we used the Grading of Recommendations Assessment, Development and Education (GRADE) approach to assess the quality of the evidence for the primary and secondary outcomes.
Selection of studies
Two authors, RB and MC or HT, independently checked titles and abstracts identified from the searches, looked at the full text of all studies of possible relevance for assessment, and decided which trials met the inclusion criteria. The authors resolved any disagreements by discussing issues with each other, and the planned recourse to a third author (HN) for arbitration did not prove necessary. We sought further information from trial authors when needed to confirm eligibility.
Data extraction and management
Two authors, RB and HT or LM, independently extracted data from included trials and entered data into a specially designed data extraction sheet, and the authors met to compare results. MC, RB, and HT wrote to all authors to request additional information as required. Two authors, RB and HT or LM, entered the data into Review Manager (RevMan).
Assessment of risk of bias in included studies
We assessed and documented the risk of bias in the included studies by concentrating on the following six parameters to assess quality: random sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other potential sources of bias as specified in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Three authors, RB, HT, and HN, independently assessed risk of bias: we were not masked to study details. We met to resolve any disagreements, and the planned recourse to a fourth author, MC, for arbitration did not prove necessary.
The 'Risk of bias' tables, which are part of the 'Characteristics of included studies' tables, addressed each domain for each study.
Measures of treatment effect
For continuous data, we calculated individual and pooled statistics as mean differences (MD) where studies used the same outcome measure and reported them with a 95% confidence interval (CI) where possible. For dichotomous outcomes, we expressed results as a risk ratio (RR) with 95% CI, where possible. We were unable to express the result for dichotomous outcomes as number needed to treat (NNT) as we had originally planned.
Unit of analysis issues
We planned to analyse cross‐over trials through the use of techniques appropriate for paired designs and data from parallel trials and cross‐over trials as separate subgroups, since cross‐over studies may not be appropriate for immunotherapy studies. Our search did not identify any cross‐over trials.
We planned to list non‐randomised controlled studies but did not discuss them further because we did not identify significant studies or data from non‐randomised controlled studies.
Where studies reported more than one active intervention, we planned to combine the two active interventions and analyse them together, but we included no trials with more than one eligible active intervention. Where studies reported non‐parametric statistics, we planned to include these in meta‐analyses where possible, following the guidance of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
We contacted authors when a paper did not present details about study design or descriptive statistics for outcomes (mean, standard deviation (SD)). If the authors did not respond within a reasonable time (six to eight weeks) to at least two separate written requests for information, we conducted the review based on available information.
Assessment of heterogeneity
We used the I² statistic to test for heterogeneity and assumed substantial statistical heterogeneity if the I² was greater than 50% (Higgins 2002). We used sensitivity or subgroup analysis to explore any statistical or clinical heterogeneity (see below). Quantitative analyses of outcomes were, wherever possible, on an intention‐to‐treat basis, i.e. participants were evaluated in the groups to which they were randomised, rather than according to the actual treatment that they received.
We gave consideration to the appropriateness of meta‐analysis in the presence of significant clinical or statistical heterogeneity and used a random‐effects model.
Assessment of reporting biases
We planned to use funnel plots to assess publication bias graphically (if there were sufficient included studies) and Begg and Egger tests to assess it statistically (Begg 1994; Egger 1997); however, we did not have a sufficient number of included studies.
Data synthesis
We planned to combine appropriate data from individual studies in a meta‐analysis only if heterogeneity measured by I² was less than 75% with the use of a random‐effects model. Where meta‐analyses were not applicable, we used a narrative synthesis of outcomes from relevant studies.
Subgroup analysis and investigation of heterogeneity
We planned five a priori subgroup analyses.
-
Immunotherapy type: sublingual and subcutaneous.
-
Allergen type: seasonal inhalant, perennial inhalant, food, and microbial.
-
Age of participants: up to four years, five to 11, 12 to 17, and 18 or over.
-
Immunotherapy regimens to be subdivided empirically into low, intermediate, and high dose therapy according to content of major allergen per dose (e.g. Phleum p5 for grass, Bet v1 for birch pollen, Fel d1 for cat, etc.):
-
for subcutaneous immunotherapy, content of major allergen 1 mcg to 5 mcg, 6 mcg to 10 mcg, and greater than 11 mcg per four‐ to six‐weekly maintenance injection doses; and
-
for sublingual immunotherapy, content of major allergen 1 mcg to 5 mcg, 6 mcg to 10 mcg, and greater than 11 mcg per daily maintenance sublingual dose (or equivalent if taken less frequently).
-
-
Severity of AE at randomisation: mild (SCORAD mean objective score of 0 to 15), moderate (SCORAD mean objective score of 16 to 40), and severe (SCORAD mean objective score of greater than 40).
Sensitivity analysis
We planned to undertake sensitivity analysis for the allocation of missing data by best and worst case analysis. If we had found significant heterogeneity between studies, we planned to explore possible reasons for this, which would have included risk of bias in the included studies. However, we did not perform posthoc sensitivity analyses because of the small number of studies that contributed to meta‐analyses.
Results
Description of studies
See the 'Characteristics of included studies', 'Characteristics of excluded studies', 'Characteristics of studies awaiting classification', and 'Characteristics of ongoing studies' tables.
Results of the search
The search identified 1550 references from electronic databases and six additional reports from other sources (three from screening references of review articles and three from ongoing trials registries), which gave a total of 1556 records (see the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram in Figure 1). We excluded 1465 references based on titles and abstracts. MC or HT and RB selected 91 records for which they screened the full text. We excluded 64 records and listed one as an ongoing study. Overall, 26 reports of 12 separate studies met the inclusion criteria (Di Rienzo 2014; Galli 1994; Glover 1992; Kaufman 1974; Leroy 1993; Luna‐Pech 2013; Novak 2012; Pajno 2007; Qin 2014; Sanchez 2012; Silny 2006; Warner 1978). We contacted the authors of all of the 12 included trials for original data and clarification of methods; we received further details from the authors or their collaborators for four trials (Di Rienzo 2014; Novak 2012; Sanchez 2012; Warner 1978).
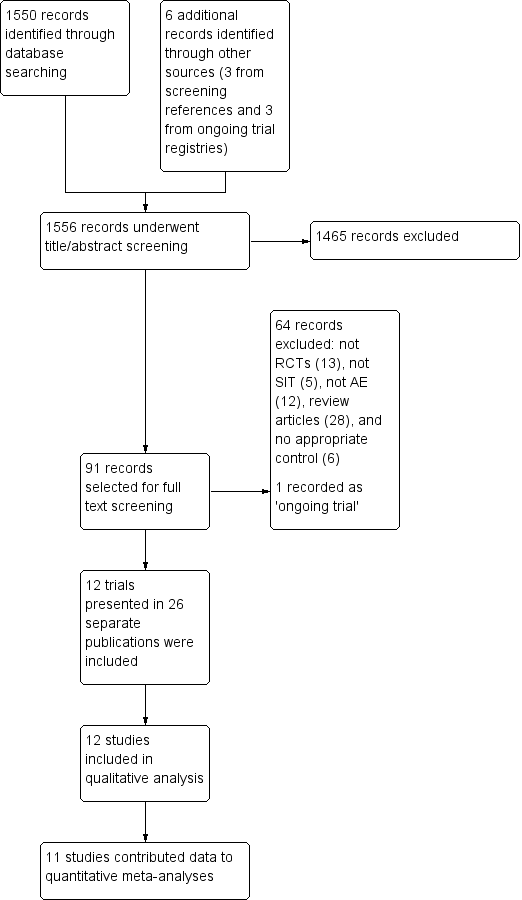
PRISMA flow diagram
Included studies
We included 12 studies, with a total of 733 participants.
Setting
Studies were conducted in specialist allergy centres in the UK (Glover 1992; Warner 1978), Italy (Di Rienzo 2014; Galli 1994; Pajno 2007), the USA (Kaufman 1974), Germany (Novak 2012), Belgium (Leroy 1993), Poland (Silny 2006), Columbia (Sanchez 2012), Mexico (Luna‐Pech 2013), and China (Qin 2014).
Participants
Two trials studied adults (Novak 2012; Qin 2014), six studied children (Di Rienzo 2014; Galli 1994; Glover 1992; Luna‐Pech 2013; Pajno 2007; Warner 1978), and four studied both children and adults (Kaufman 1974; Leroy 1993; Sanchez 2012; Silny 2006). Ten studies were restricted to people allergic to Dermatophagoides pteronyssinus or Dermatophagoides farinae (house dust mites) or both (Di Rienzo 2014; Galli 1994; Glover 1992; Leroy 1993; Luna‐Pech 2013; Novak 2012; Pajno 2007; Qin 2014; Sanchez 2012; Warner 1978), one study was restricted to people allergic to house dust mites or grass pollen (Silny 2006), and one study was restricted to people allergic to a group of unspecified inhalant antigens (Kaufman 1974).
Interventions
The 12 included studies were all of specific allergen immunotherapy (SIT). Of these, six trials studied subcutaneous immunotherapy (SCIT) (Glover 1992; Kaufman 1974; Novak 2012; Sanchez 2012; Silny 2006; Warner 1978), four studied sublingual immunotherapy (SLIT) (Di Rienzo 2014; Luna‐Pech 2013; Pajno 2007; Qin 2014), one studied intradermal immunotherapy (Leroy 1993), and one studied oral immunotherapy (Galli 1994).
Eight trials compared the intervention with a placebo (Glover 1992; Kaufman 1974; Leroy 1993; Luna‐Pech 2013; Novak 2012; Pajno 2007; Silny 2006; Warner 1978), and four compared the intervention with a standard treatment (Di Rienzo 2014; Galli 1994; Qin 2014; Sanchez 2012). The duration of treatment was less than a year in one trial, Leroy 1993, and at least a year in Di Rienzo 2014, Galli 1994, Glover 1992, Kaufman 1974, Luna‐Pech 2013, Novak 2012, Pajno 2007, Qin 2014, Sanchez 2012, Silny 2006, and Warner 1978.
Outcomes
With regard to our prespecified primary outcomes, two studies reported 'Participant‐ or parent‐reported global assessment of disease severity at the end of treatment' (Glover 1992; Warner 1978), six studies reported 'Participant‐ or parent‐reported specific symptoms of eczema, by subjective measures' (Di Rienzo 2014; Glover 1992; Leroy 1993; Novak 2012; Pajno 2007; Sanchez 2012), and seven studies reported 'Adverse events' (Di Rienzo 2014; Glover 1992; Novak 2012; Pajno 2007; Qin 2014; Sanchez 2012; Silny 2006).
With regard to our prespecified secondary outcomes, seven studies reported 'Investigator‐ or physician‐rated global assessment of disease severity at the end of treatment' (Di Rienzo 2014; Galli 1994; Kaufman 1974; Leroy 1993; Qin 2014; Sanchez 2012; Silny 2006), two studies reported 'Parent‐ or participant‐rated eczema severity assessed using a published scale' in the form of SCORing Atopic Dermatitis (SCORAD) part C (Di Rienzo 2014; Novak 2012), six studies reported 'Investigator‐ or physician‐rated eczema severity assessed using a published scale' (Di Rienzo 2014; Luna‐Pech 2013; Novak 2012; Qin 2014; Pajno 2007; Sanchez 2012), eight studies reported 'Use of other medication for treatment of eczema during the intervention period' (Glover 1992; Kaufman 1974; Luna‐Pech 2013; Novak 2012; Pajno 2007; Qin 2014; Sanchez 2012; Silny 2006), and one study reported 'Validated eczema‐related quality of life scores' (Novak 2012).
Three studies measured other outcomes: one measured total serum immunoglobulin E (IgE) levels, specific IgE levels, and skin prick test results (Glover 1992); another measured specific IgE levels and other serum inflammatory parameters associated with either allergic inflammation or its suppression, including eosinophilic cationic protein (ECP), soluble interleukin 2 receptor (sIL‐2R), interferon gamma (IFN‐gamma), or interleukins 4, 5, and 10 (Silny 2006); and a third measured specific serum IgG4 levels (Qin 2014).
Only two of the five publications that reported outcomes from the Pajno 2007 study contributed data to the review, because the other three publications did not report atopic eczema outcomes.
Excluded studies
We rejected the other 64 titles for the following reasons: not a randomised controlled trial (RCT) (13), not SIT (five), not atopic eczema (AE) (12), review articles (28), and no appropriate control (six). The reason we included these articles for the full text review stage is that from the title or abstract we could not exclude the possibility that they were RCTs of adults or children with AE and allergic sensitisation, but after assessment of the full text, we excluded them.
Studies awaiting classification
There were no studies awaiting classification.
Ongoing studies
There was one ongoing trial with no outcome data available at the time of review (see the 'Characteristics of ongoing studies' table). The contacts for the trial NCT00310492 did not respond to our request for further information.
Risk of bias in included studies
Full details are shown in the 'Characteristics of included studies' tables. Please see the 'Risk of bias' summary (review authors' judgements about each 'Risk of bias' item for each included study, Figure 2).
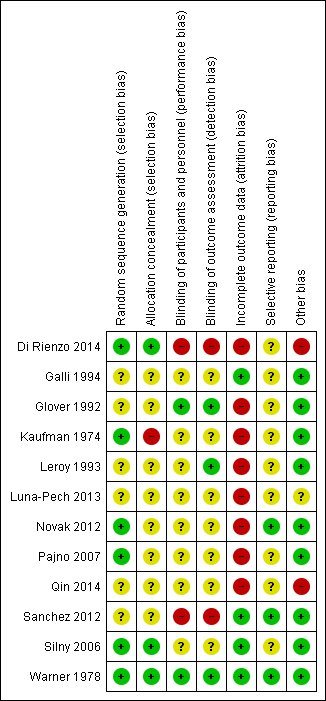
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
Random sequence generation
There was a low risk of bias related to generation of randomisation sequence concealment in six studies, Di Rienzo 2014, Kaufman 1974, Novak 2012, Pajno 2007, Silny 2006, Warner 1978, and unclear risk in the following six studies: Galli 1994, Glover 1992, Leroy 1993, Luna‐Pech 2013, Qin 2014, and Sanchez 2012.
Allocation
There was a low risk of bias related to allocation concealment in three studies (Di Rienzo 2014; Silny 2006; Warner 1978), high risk in one study (Kaufman 1974), and unclear risk in eight studies due to insufficient details provided (Galli 1994; Glover 1992; Leroy 1993; Luna‐Pech 2013; Novak 2012; Pajno 2007; Sanchez 2012; Qin 2014).
Blinding
There was a low risk of bias related to blinding of participants and personnel in two studies (Glover 1992; Warner 1978), which were either double blinded or triple blinded; high risk in two studies (Di Rienzo 2014; Sanchez 2012), which were open label; and unclear risk in eight studies due to insufficient details provided (Galli 1994; Kaufman 1974; Leroy 1993; Luna‐Pech 2013; Novak 2012; Pajno 2007; Qin 2014; Silny 2006).
There was a low risk of bias related to blinding of outcome assessors in three studies (Glover 1992; Leroy 1993; Warner 1978); high risk in two studies (Di Rienzo 2014; Sanchez 2012), which were open label; and unclear risk in seven studies (Galli 1994; Kaufman 1974; Luna‐Pech 2013; Novak 2012; Pajno 2007; Qin 2014; Silny 2006), four of which were unclear regarding whether they included outcome assessors in the double blinding (Kaufman 1974; Novak 2012; Pajno 2007; Silny 2006).
Incomplete outcome data
There was a low risk of bias related to incomplete outcome data in four studies, Galli 1994, Sanchez 2012, Silny 2006, and Warner 1978, where loss to follow‐up rates were low, and high risk in eight studies where loss to follow up rates were high (up to 51%) or postrandomisation exclusions were noted: Di Rienzo 2014, Glover 1992, Kaufman 1974, Leroy 1993, Luna‐Pech 2013, Qin 2014, Novak 2012, and Pajno 2007.
Selective reporting
There was a low risk of bias related to selective reporting in three studies where the specified outcomes in the methodology were reported in the results, Novak 2012, Sanchez 2012, Warner 1978, and unclear risk in nine studies: Di Rienzo 2014, Galli 1994, Glover 1992, Kaufman 1974, Leroy 1993, Luna‐Pech 2013, Pajno 2007, Qin 2014, and Silny 2006.
Other potential sources of bias
There was low risk of bias related to other sources in nine studies (Galli 1994; Glover 1992; Kaufman 1974; Leroy 1993; Novak 2012; Pajno 2007; Sanchez 2012; Silny 2006; Warner 1978), high risk in two studies where the manufacturer funded the study either partly or wholly and the authors were affiliated with the manufacturer (Di Rienzo 2014; Qin 2014), and unclear risk in one study where it was unclear whether the authors were affiliated with the manufacturer (Luna‐Pech 2013).
Effects of interventions
See: Summary of findings for the main comparison Specific allergen immunotherapy versus no immunotherapy
See summary of findings Table for the main comparison for the main comparison 'specific allergen immunotherapy versus no immunotherapy'.
Primary outcomes
1. Participant‐ or parent‐reported global assessment of disease severity at the end of treatment
One study, Warner 1978, measured this outcome as whether the eczema was improved, there was no change, or it was worse as rated by the participants or parents. These data were available for 20 participants at the end of the treatment (nine active, 11 placebo), with improvement in 7/9 (78%) of the immunotherapy group and 3/11 (27%) in the placebo group (risk ratio (RR) 2.85, 95% confidence interval (CI) 1.02 to 7.96). Another study, Glover 1992, measured this outcome as whether the eczema was better, the same, or worse as rated by parents. These data were available for 24 participants, with improvement in 8/13 (62%) of those in the active treatment group and 9/11 (81%) in the placebo group (RR 0.75, 95% CI 0.45 to 1.26). We did not perform meta‐analysis because of high heterogeneity between the two studies (I² = 83%). The high loss to follow‐up rate and as‐treated analysis in the study by Glover 1992 may have contributed to the significant heterogeneity. The quality of the evidence was low.
2. Participant‐ or parent‐reported specific symptoms of eczema, by subjective measures
We used original data shared by the authors of two studies, Di Rienzo 2014 and Novak 2012, to calculate SCORing Atopic Dermatitis (SCORAD) part C scores at the end of treatment, and the components of SCORAD part C, which are itch measured by Visual Analogue Scales (VAS) and sleep disturbance measured by VAS, each on a scale from 0 to 10. Meta‐analysis, with a total of 184 participants, showed no significant difference in SCORAD part C (mean difference (MD) ‐0.74, 95% CI ‐1.98 to 0.50; I² = 0%; Analysis 1.1) or severity of sleep disturbance (MD ‐0.49, 95% CI ‐1.03 to 0.06; I² = 0%; Analysis 1.1).
The authors of Sanchez 2012 provided original data that showed subjective symptom scores at the end of the treatment on a scale of 0 to 100, where higher scores meant more symptoms, and a component of the symptom score, which measured itching severity on a scale of 0 to 10, where higher scores also mean more symptoms. These data were available for 60 participants at the end of the treatment (31 active, 29 placebo), with a mean overall severity score of 37.3 (95% CI 32.4 to 42.1) in the immunotherapy group and 80.8 (95% CI 75.8 to 85.7) in the control group (P < 0.001) and a mean itch severity score of 3.2 (95% CI 2.3 to 4.0) in the immunotherapy group and 7.5 (95% CI 6.9 to 8.0) in the control group (P < 0.001). The difference between groups in change in itch severity score from baseline was also statistically significant (MD ‐4.20, 95% CI ‐3.69 to ‐4.71).
For itch severity, we did not meta‐analyse data from these three studies because of extreme heterogeneity (I² = 98%), which was attributable to the open label study of Sanchez 2012. When we excluded this study from meta‐analysis, combined data from Novak 2012 and Di Rienzo 2014 showed no significant difference in SCORAD part C itch severity (MD ‐0.24, 95% CI ‐1.00 to 0.52; I² = 0%).
One study, Glover 1992, reported symptoms in the form of itch score presented graphically that showed no significant difference between the active and placebo groups. One study, Leroy 1993, reported a mean itch score of 2.2 (or 33% reduction from baseline) after immunotherapy compared with 2.6 (or 19% reduction from baseline) in the control group. The authors did not comment on whether this difference was statistically significant and did not respond to our request for further data.
Other studies reported insufficient data, such as Pajno 2007, or did not measure this outcome, such as Galli 1994, Kaufman 1974, Luna‐Pech 2013, Qin 2014, Silny 2006, and Warner 1978.
3. Adverse events
Seven studies reported local or systemic reactions to treatment (Di Rienzo 2014; Glover 1992; Novak 2012; Pajno 2007; Qin 2014; Sanchez 2012; Silny 2006).
In addition to individual studies, meta‐analysis, with a total of 484 participants, showed no statistically significant increase in risk of local reactions (RR 1.27, 95% CI 0.89 to 1.81; I² = 25%; Analysis 1.2). Data from seven of the 12 studies contributed to this effect estimate (Di Rienzo 2014; Glover 1992; Novak 2012; Pajno 2007; Qin 2014; Sanchez 2012; Silny 2006).
In addition to individual studies, meta‐analysis with a total of 492 participants showed no statistically significant increase in risk of systemic reactions (RR 0.78, 95% CI 0.41 to 1.49; I² = 0%; Analysis 1.2), with 18 events observed in the immunotherapy group and 15 in the control group. Data from four of 12 studies contributed to this effect estimate (Glover 1992; Novak 2012; Pajno 2007; Qin 2014). However, there were no systemic reactions reported in three studies (Di Rienzo 2014; Sanchez 2012; Silny 2006).
One study, Pajno 2007, with 48 participants, measured other adverse reactions and showed no statistically significant increase in risk of tiredness (RR 5.08, 95% CI 0.66 to 39.02; Analysis 1.2) or headache (RR 2.56, 95% CI 0.11 to 59.75; Analysis 1.2).
Secondary outcomes
1. Investigator‐ or physician‐rated global assessment of disease severity at the end of treatment
Six studies reported investigator‐ or physician‐rated global assessment of disease severity (Di Rienzo 2014; Galli 1994; Kaufman 1974; Qin 2014; Sanchez 2012; Silny 2006). Meta‐analysis, with 262 participants, showed significant improvement in disease severity (RR 1.48, 95% CI 1.16 to 1.88; I² = 19%; Analysis 1.3).
One study, Leroy 1993, with 24 participants, reported improvement in 70% of all of the participants that used an investigator‐rated index of disease severity at a threshold of 50% improvement. This was significant between the treatment and the placebo group (P < 0.003), but there were no separate data for the treatment and placebo group, so we could not include them in a meta‐analysis.
Other studies did not measure this outcome (Glover 1992; Luna‐Pech 2013; Novak 2012; Pajno 2007; Warner 1978).
2. Parent‐ or participant‐rated eczema severity assessed using a published scale
None of the studies reported participant‐ or parent‐rated eczema severity using a published scale, except for two studies that we have mentioned above, Di Rienzo 2014 and Novak 2012, which recorded SCORAD part C, which we included in this systematic review as a parent‐ or participant‐rated specific eczema symptom (MD ‐0.74, 95% CI ‐1.98 to 0.50; I² = 0%; Analysis 1.1).
Participant‐ or parent‐rated eczema severity assessed using a non‐published scale
Although this was not a prespecified outcome, we felt it important to include. Four studies measured participant‐ or parent‐rated eczema severity assessed using non‐published Visual Analogue Scales (VAS) on a scale of 0 to 10 (0 = no symptoms, 10 = maximal symptoms). Meta‐analysis of two studies (Di Rienzo 2014; Qin 2014), with a total of 158 participants, showed statistically significant lower end‐of‐treatment VAS scores (MD ‐1.12, 95% CI ‐1.92 to ‐0.32; I² = 0%; Analysis 1.4). We used original data shared by the authors of one study, Di Rienzo 2014, to conduct this analysis.
The other two studies only provided original data listed as illustrative text: Pajno 2007 reported a VAS that measured overall eczema symptoms with 10.7% improvement in the treatment group and 13.1% worsening in the placebo group (P = 0.07), but the study did not report absolute values. Leroy 1993 reported a VAS that measured participant general well‐being with a significant improvement in the treatment group (P = 0.008) but not in the control group, but again, did not report absolute values. Authors of the latter two studies did not respond to our requests for original data for inclusion in a meta‐analysis.
3. Investigator‐ or physician‐rated eczema severity assessed using a published scale
Six studies reported 'Investigator‐ or physician‐rated eczema severity assessed using a published scale' in the form of SCORAD (Di Rienzo 2014; Luna‐Pech 2013; Novak 2012; Pajno 2007; Qin 2014; Sanchez 2012). Authors of two studies supplied original data for end‐of‐treatment SCORAD (Novak 2012; Sanchez 2012). Meta‐analysis of three trials (Di Rienzo 2014; Novak 2012; Sanchez 2012), with 244 participants, showed significant improvement in end of treatment SCORAD (MD ‐5.79, 95% CI ‐7.92 to ‐3.66; I² = 0%; Analysis 1.5).
One study, Qin 2014, reported reduction ratios in SCORAD and classified scores as cure (greater than 90%), marked effect (60% to 89%), improvement (20% to 59%), and ineffective (less than 19%). The total efficacy (defined as percentage of participants with change in SCORAD ≥ 60%) was significantly greater in the specific allergen immunotherapy (SIT) group (77.78%) than in the control group (53.85%) (P < 0.05) and was included as a dichotomous 'Investigator‐ or physician‐rated global assessment of disease severity at the end of treatment' outcome in a meta‐analysis in this review (RR 1.48, 95% CI 1.16 to 1.88; I² = 19%; Analysis 1.3). Another study, Luna‐Pech 2013, found a significant change in SCORAD between immunotherapy (‐18.4 ± 6.5) and control (‐6.6 ± 4.1) (P = 0.008). This effect was greater for participants with severe eczema at baseline. A further study, Pajno 2007, suggested greater SCORAD improvement with the SIT than in controls in graphical data (P < 0.001), but no numerical data were available. No data for end of treatment SCORAD scores from these three studies were available for inclusion in a meta‐analysis.
One study, Glover 1992, reported no significant difference in a non‐published scale that measured erythema, lichenification, and surface damage between the immunotherapy and the placebo groups. Another study, Galli 1994, reported no significant difference between treatment groups, using a non‐published scale that measured severity of erythema, vesicles, fissuration, lichenification, and itching.
4. Use of other medication for treatment of eczema during the intervention period
One study, Silny 2006, with 20 participants, reported no statistically significant difference between the treatment groups in the use of topical steroids for mild to moderate flares of AE (RR 1.33, 95% CI 0.74 to 2.41; Analysis 1.6). Another study, Glover 1992, reported no significant difference in the use of topical steroids between the treatment groups. (There were no numerical data for meta‐analysis.) One study, Sanchez 2012, reported a significant reduction in the use of topical steroids and tacrolimus during one year of immunotherapy (P = 0.02), but there was no such reduction in the control group.
Two studies reported the use of systemic steroids for AE. One study, Kaufman 1974, with 26 participants, required the use of systemic steroids in 8/16 participants (50%) in the immunotherapy group and 4/10 participants (40%) in the placebo group (P = 0.70). Another study, Sanchez 2012, with 60 participants, reported a significant increase in systemic steroid use in 12/29 participants (41%) in the control group compared with 4/31 participants (13%) in the immunotherapy group (P = 0.02). We did not perform meta‐analysis because of the high heterogeneity (I² = 76%). The reason for high heterogeneity between these two studies was unclear.
Another study, Novak 2012, with 168 participants, reported a non‐significant 32% difference in the median AUC (area under the curve) of medication score, a culmination of topical medication and overall consumption of systemic medication (19,330 in the immunotherapy group and 28,420 in the placebo group; P = 0.08). These data were not in a format suitable for incorporation into a meta‐analysis.
One study, Pajno 2007, reported a significant decrease in the use of rescue medications (oral hydroxyzine and topical steroids, respectively) in the immunotherapy group. There were 171 occasions where rescue medications were used in the immunotherapy group compared with 346 occasions in the placebo group (P = 0.03). The rescue medications were used on 93 days in the immunotherapy group and 158 days in the placebo groups (P = 0.01).
One study, Luna‐Pech 2013, reported significantly less use of rescue medications (not defined) in the treatment group compared with the control group, but no details were provided.
Another study, Qin 2014, reported an average daily drug score (one point for symptomatic use of levocetirizine hydrochloride tablet, mometasone furoate cream, or mupirocin ointment each day; and six points for every six‐day course of clarithromycin for superinfection). Average daily drug score was lower in the treatment group (mean 0.5, standard deviation (SD) 0.4) than in the control group (mean 1.3, SD 0.7) (P < 0.01).
Other studies did not report this outcome (Di Rienzo 2014; Galli 1994; Leroy 1993; Warner 1978). None of the studies reported the use of oral antihistamines or calcineurin inhibitors as separate outcomes.
5. Validated eczema‐related quality of life scores
One study, Novak 2012, reported a validated eczema‐related quality of life score, the Dermatology Life Quality Index (DLQI), at the end of treatment. We used original data kindly provided by the trial authors to calculate DLQI at the end of treatment, which showed no difference between the treatment groups ‐ a median of 3 (interquartile range (IQR) 1.0 to 8.0) for immunotherapy and a median of 3.5 (IQR 1.0 to 10.5) for placebo (P = 0.525).
Subgroup analyses
We undertook 16 planned subgroup analyses where data were available. We did not undertake further sensitivity analyses because of the small number of trials that contributed data to the analyses.
-
Immunotherapy type: sublingual and subcutaneous.
-
Allergen type: seasonal inhalant, perennial inhalant, food, and microbial.
-
Age of participants: up to four years, five to 11, 12 to 17, and 18 or over.
-
Immunotherapy regimens to be subdivided empirically into low, intermediate, and high dose therapy according to content of major allergen per dose (e.g. Phleum p5 for grass, Bet v1 for birch pollen, Fel d1 for cat, etc.):
-
for subcutaneous immunotherapy, content of major allergen 1 mcg to 5 mcg, 6 mcg to 10 mcg, and greater than 11 mcg per four‐ to six‐weekly maintenance injection doses; and
-
for sublingual immunotherapy, content of major allergen 1 mcg to 5 mcg, 6 mcg to 10 mcg, and greater than 11 mcg per daily maintenance sublingual dose (or equivalent if taken less frequently).
-
-
Severity of AE at randomisation: mild (SCORAD mean objective score of 0 to 15), moderate (SCORAD mean objective score of 16 to 40), and severe (SCORAD mean objective score of greater than 40).
First, we analysed our primary outcome measure 'Participant‐ or parent‐reported global assessment of disease severity at the end of treatment'. Two studies reported dichotomous outcomes that we did not combine in meta‐analyses because of significant heterogeneity (I² = 83%) (Glover 1992; Warner 1978). We did not perform subgroup analyses because both studies fell under the same subgroup categories (subcutaneous route, perennial allergen, and both children and adults). One study, Warner 1978, showed significant improvement in 7/9 participants (78%) in the immunotherapy group compared with 3/11 participants (27%) in the placebo group (P = 0.04). Another study, Glover 1992, showed significant improvement in 8/13 participants (62%) in the active group compared with 9/11 (81%) in the placebo group (P = 0.38).
Next, we analysed our primary outcome measure 'Participant‐ or parent‐reported specific symptoms of eczema, by subjective measures' in nine subgroup analyses. We found no evidence that this outcome differed according to the following.
-
Route of immunotherapy: SCORAD part C (subcutaneous: MD ‐0.62, 95% CI ‐2.18 to 0.93) (sublingual: MD ‐0.94, 95% CI ‐3.00 to 1.13) (test for subgroup differences: I² = 0%; Analysis 2.1). With regard to itch, meta‐analysis was not possible due to extreme heterogeneity (I² = 99%) attributable to the study of Sanchez 2012. Without this study in the analysis, the test for subgroup difference between sublingual and subcutaneous immunotherapies and their controls was not significant (I² = 0%) for sleep disturbance (subcutaneous: MD ‐0.42, 95% CI ‐1.24 to 0.40) (sublingual: MD ‐0.54, 95% CI ‐1.27 to 0.19) (test for subgroup differences: I² = 0%; Analysis 2.2).
-
Allergen type: SCORAD part C (seasonal inhalant: MD not estimable) (perennial inhalant: MD ‐0.74, 95% CI ‐1.98 to 0.50; Analysis 2.3) (food: MD not estimable) (microbial: MD not estimable). With regard to itch, meta‐analysis was not possible due to extreme heterogeneity (I² = 99%) attributable to the study of Sanchez 2012. Without this study in the analysis, the test for subgroup differences for seasonal inhalant and perennial inhalant immunotherapies was not significant (I² = 0%) for sleep disturbance (seasonal inhalant: MD not estimable) (perennial inhalant: MD ‐0.49, 95% CI ‐1.03 to 0.06; Analysis 2.4) (food: MD not estimable) (microbial: MD not estimable).
-
Participant age: SCORAD part C (up to four years: MD not estimable) (five to 11 years of age: MD not estimable) (12 to 17 years of age: MD not estimable) (18 years of age or over: (MD ‐0.62, 95% CI ‐2.18 to 0.93; Analysis 2.5); itch (up to four years of age: MD not estimable) (five to 11 years of age: MD not estimable) (12 to 17 years of age: MD not estimable) (18 years of age or over: MD ‐0.20, 95% CI ‐1.05 to 0.64; Analysis 2.6); or sleep disturbance (up to four years of age: MD not estimable) (five to 11 years of age: MD not estimable) (12 to 17 years of age: MD not estimable) (18 years of age or over: MD ‐0.42, 95% CI ‐1.24 to 0.40; Analysis 2.7).
-
Severity at randomisation using original data from one study for the outcomes itch and sleep disturbance (Novak 2012). In the moderate severity subgroup, data were available for 37 participants (23 in the immunotherapy group and 14 in the placebo group): itch did not differ significantly between groups ‐ with a median of 1.7 (IQR 0.3 to 3.5) for immunotherapy and 1.7 (IQR 0.5 to 3.7) for placebo (P = 0.96) ‐ nor did sleep disturbance ‐ with a median of 0.3 (IQR 0.1 to 2.8) for immunotherapy and 0.5 (IQR 0.3 to 1.5) for placebo (P = 0.53). In the severe subgroup, data were available for 109 participants (75 in the active group and 34 in the placebo group): itch did not differ significantly between groups ‐ with a median of 2.0 (IQR 0.7 to 4.1) for immunotherapy and 2.9 (IQR 1.3 to 5.4) for placebo (P = 0.22) ‐ nor did sleep disturbance ‐ with a median of 1.1 (IQR 0.4 to 3.3) for immunotherapy and 1.9 (IQR 0.6 to 5.1) for placebo (P = 0.14). During treatment, we also calculated the change in itch in the moderate (MD 1.01, 95% CI ‐1.31 to 3.33) and severe subgroups (MD 0.10, 95% CI ‐1.38 to 1.58; Analysis 2.8) and sleep disturbance in the moderate (MD 0.38, 95% CI ‐1.32 to 2.09) and severe subgroups (MD ‐0.31, 95% CI ‐1.66 to 1.04; Analysis 2.9). We found no significant difference between the immunotherapy and control groups.
Last, we analysed our primary outcome 'Adverse events' in six subgroup analyses. We found evidence that this outcome differed significantly according to the following:
-
route of immunotherapy: local reactions were greater in the immunotherapy group than the control group by the sublingual (RR 9.76, 95% CI 1.28 to 74.26) but not the subcutaneous route (RR 1.18, 95% CI 0.90 to 1.55) (test for subgroup differences: I² = 76%; Analysis 2.10).
We found no evidence that this outcome differed between the immunotherapy or control groups according to the following:
-
route of immunotherapy: systemic reactions (subcutaneous: RR 0.82, 95% CI 0.34 to 2.00) (sublingual: RR 0.74, 95% CI 0.29 to 1.89) (test for subgroup differences: I² = 0%; Analysis 2.11);
-
allergen type: local reactions (seasonal inhalant: RR not estimable) (perennial inhalant: RR 1.31, 95% CI 0.81 to 2.13; Analysis 2.12) (food: RR not estimable) (microbial: RR not estimable); systemic reactions (seasonal inhalant: RR not estimable) (perennial inhalant: RR 0.78, 95% CI 0.41 to 1.49; Analysis 2.13) (food: RR not estimable) (microbial: RR not estimable); and
-
participant age: local reactions (up to four years: RR not estimable) (five to 11: RR not estimable) (12 to 17: RR not estimable) (18 years or over: RR 1.37, 95% CI 0.44 to 4.23; Analysis 2.14); systemic reactions (up to four years: RR not estimable) (five to 11: RR not estimable) (12 to 17: RR not estimable) (18 years or over: RR 0.74, 95% CI 0.38 to 1.47; Analysis 2.15).
There were no data available for other subgroup analyses of our primary outcomes.
Discussion
Summary of main results
We identified 12 randomised controlled clinical trials of specific allergen immunotherapy (SIT) for the treatment of atopic eczema (AE), which included 733 participants with eczema and allergic sensitisation to an inhalant allergen. The studies were of children and adult participants allergic to house dust mite, grass pollen, and other inhalant allergens; and immunotherapy via subcutaneous, sublingual, oral, and intradermal routes. We judged nine studies to have a high risk of bias due to high rates of loss to follow up or postrandomisation exclusions, Di Rienzo 2014, Glover 1992, Kaufman 1974, Leroy 1993, Luna‐Pech 2013, Novak 2012, Pajno 2007, Qin 2014, or non‐blinded outcome assessment, Di Rienzo 2014, Sanchez 2012.
For our prespecified primary outcomes 'Participant‐ or parent‐reported global assessment of disease severity at the end of treatment' (two studies, 44 participants, low quality evidence) and 'Participant‐ or parent‐reported specific symptoms of eczema, by subjective measures' (six studies, 339 participants, very low quality evidence), SIT is not an effective treatment for AE (summary of findings Table for the main comparison). However, the results for our secondary outcomes 'Investigator‐ or physician‐rated global assessment of disease activity at the end of treatment' (seven studies, 286 participants) and 'Investigator‐ or physician‐rated eczema severity assessed using a published scale (e.g. SCORing Atopic Dermatitis (SCORAD))' (six studies, 435 participants) indicated SIT was effective, although the quality of the evidence was low and very low for these two outcomes, respectively. Our other secondary outcomes 'Parent‐ or participant‐rated eczema severity assessed using a published scale' (two studies, 184 participants) and 'Validated eczema‐related quality of life scores' (one study, 168 participants) showed no difference with SIT.
For our primary outcome 'Adverse events', SIT was not associated with increased risk of local (seven studies, 484 participants) or systemic (seven studies, 492 participants, moderate evidence) adverse reactions. Also, SIT was not associated with an increased need for topical (one study, 20 participants) or systemic (two studies, 86 participants) corticosteroid use during the studies.
Three studies had more positive findings than the others. One, Sanchez 2012, reported a marked improvement in participant‐ or parent‐reported symptoms and smaller but statistically significant improvements in investigator‐ or physician‐reported global eczema severity and total SCORAD (a 5.8‐point greater improvement) compared with untreated participants. Another, Qin 2014, reported a significantly greater investigator‐ or physician‐rated global disease severity, defined as change in SCORAD ≥ 60% in SIT (77.78%) compared with the control (53.85%) (P < 0.05). A further study, Luna‐Pech 2013, reported a significant change in investigator‐ or physician‐rated global disease severity through assessment of SCORAD in SIT (mean ‐18.4, SD 6.5) compared with the control (mean ‐6.6, SD 4.1) (P = 0.008), with a greater effect in those with severe eczema at baseline. No original data were available for inclusion in meta‐analyses.
Subgroup analyses identified a low confidence of effect that sublingual immunotherapy was associated with more local adverse reactions compared with subcutaneous immunotherapy. Other subgroup analyses did not identify a type of allergen, a participant age, or a severity of AE at randomisation with a different efficacy or safety profile, although these analyses were generally inconclusive due to the limited data available.
Overall completeness and applicability of evidence
Overall, we found low quality of evidence that specific allergen immunotherapy is effective in the treatment of atopic eczema. The varied disease severity scales and symptom scores used across the trials generally limited the meta‐analyses. In those with comparable data, some outcomes were significant. Wide confidence intervals for many outcome measures reflected relatively small studies and varied methodologies. Several outcomes were based on analysis from a single trial, Novak 2012, with a large number of participants but high loss to follow up. Three trials, Di Rienzo 2014, Qin 2014, Sanchez 2012, had more positive findings than the others and showed a clear beneficial effect on participant‐ or parent‐reported eczema symptoms and investigator‐ or physician‐reported global eczema severity in the form of SCORAD. It is not clear why the findings of these trials differed, but there was a risk of detection bias due to lack of blinding of participants or investigators in at least two trials (Di Rienzo 2014; Sanchez 2012). We found that adverse reaction rates were not significantly increased with immunotherapy in the included studies, but other evidence suggests that SIT carries a significantly increased risk of severe allergic reactions (Calderon 2007). While this might suggest that the allergic sensitisation present in the trial participants is of little clinical relevance or that the allergen extracts used were of low potency, it may equally reflect the small number of trials and participants that contributed to the adverse events analyses.
Quality of the evidence
Our overall judgement of the quality of the body of evidence that contributed to the results of the review, using the Grading of Recommendations Assessment, Development and Education (GRADE) approach (Higgins 2011), was low. The reasons we downgraded were relatively few trials and participants, lack of blinding in at least two trials, wide confidence intervals, moderate risk of bias with high loss to follow up as the main concern, and significant heterogeneity between the estimate of treatment effects for a primary outcome.
Potential biases in the review process
The strengths of this review were the adherence to our published protocol and the repeated efforts to acquire original data from study authors in order to maximise opportunities for meta‐analysis and clarify methodological uncertainties. The limited number of included studies did not allow formal assessment for publication bias. We analysed different outcome measures as separate analyses, which limited the opportunities to pool data from different studies that used different outcome assessment tools.
Agreements and disagreements with other studies or reviews
Three other systematic reviews of SIT for the treatment of AE have been undertaken. In one review (Bae 2013), the authors identified eight of the 12 trials included in this review but analysed the data in a different way, by pooling heterogeneous outcomes 'measured by any scoring systems', which may not be appropriate (Tam 2013). In contrast to our review, they found moderate evidence that SIT may be an effective treatment for AE both in all participants studied (odds ratio (OR) for improved eczema 5.35, 95% confidence interval (CI) 1.61 to 17.77) and in subgroup analyses of participants with severe eczema at randomisation (OR 3.13, 95% CI 1.31 to 7.47) and studies that used subcutaneous immunotherapy (OR 4.27, 95% CI 1.36 to 13.39). The different outcomes in their review are likely due to the unconventional approaches for extracting and combining data from the included trials. There was no registered protocol for their review, so we cannot confirm that the inclusion criteria and outcome measures were determined a priori.
In a systematic review that used the GRADE recommendations (Gendelman 2013), the authors identified five of the nine trials included in our review, and an additional two that we excluded (Ring 1982; Werfel 2006). The review did not perform meta‐analyses. Similar to our review, they found only weak strength of recommendations for the use of SIT to treat AE. They also reported similar methodological shortcomings, including high losses to follow up.
In a similar systematic review on sublingual immunotherapy only that used the GRADE recommendations (Gendelman 2015), the authors identified three of the 12 trials included in our review and an additional two that we excluded (Cadario 2007; Mastrandrea 2000). The review did not perform meta‐analyses. Similar to our study, they found only weak strength of recommendations for the use of sublingual immunotherapy to treat AE with a large placebo effect in two studies. They also reported similar methodological shortcomings, which included lack of blinding, lack of control, and lack of randomisation.

PRISMA flow diagram

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
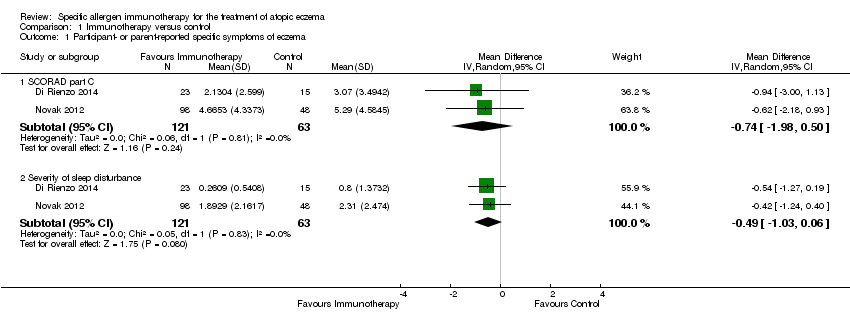
Comparison 1 Immunotherapy versus control, Outcome 1 Participant‐ or parent‐reported specific symptoms of eczema.

Comparison 1 Immunotherapy versus control, Outcome 2 Adverse events.

Comparison 1 Immunotherapy versus control, Outcome 3 Investigator‐ or physician‐rated global disease severity.
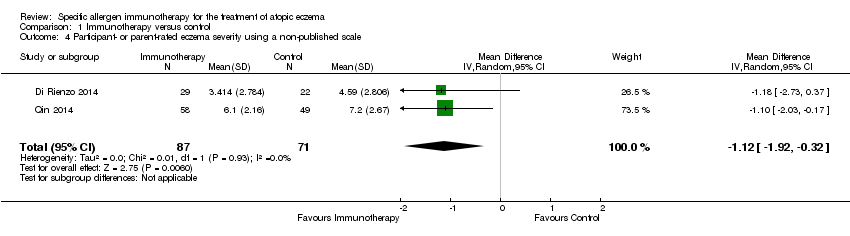
Comparison 1 Immunotherapy versus control, Outcome 4 Participant‐ or parent‐rated eczema severity using a non‐published scale.

Comparison 1 Immunotherapy versus control, Outcome 5 Investigator‐rated eczema severity assessed using a published scale.

Comparison 1 Immunotherapy versus control, Outcome 6 Use of other medications for eczema.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 1 Participant‐ or parent‐reported specific symptoms of eczema ‐ SCORAD part C by route of immunotherapy.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 2 Participant‐ or parent‐reported specific symptoms of eczema ‐ severity of sleep disturbance by route of immunotherapy.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 3 Participant‐ or parent‐reported specific symptoms of eczema ‐ SCORAD part C by allergen type.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 4 Participant‐ or parent‐reported specific symptoms of eczema ‐ severity of sleep disturbance by allergen type.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 5 Participant‐ or parent‐reported specific symptoms of eczema ‐ SCORAD part C by participant age.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 6 Participant‐ or parent‐reported specific symptoms of eczema ‐ itch severity by participant age.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 7 Participant‐ or parent‐reported specific symptoms of eczema ‐ severity of sleep disturbance by participant age.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 8 Participant‐ or parent‐reported specific symptoms of eczema ‐ itch severity by severity at randomisation.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 9 Participant‐ or parent‐reported specific symptoms of eczema ‐ severity of sleep disturbance by severity at randomisation.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 10 Adverse events: any local reaction by route of immunotherapy.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 11 Adverse events: any systemic reaction by route of immunotherapy.
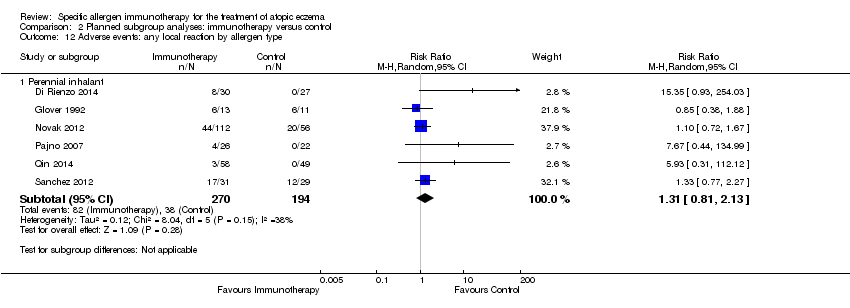
Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 12 Adverse events: any local reaction by allergen type.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 13 Adverse events: any systemic reaction by allergen type.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 14 Adverse events: any local reaction by participant age.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 15 Adverse events: any systemic reaction by participant age.
| Specific immunotherapy compared with no immunotherapy for atopic eczema | ||||||
| Patient or population: adults and children with atopic eczema and inhalant allergen sensitisation Settings: specialist allergy centres in the UK (2 trials), Italy (3 trials), USA, Germany, Belgium, Poland, Columbia, and China Intervention: specific allergen immunotherapy Comparison: no immunotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No immunotherapy | Specific allergen immunotherapy | |||||
| Participant‐ or parent‐reported global assessment of disease severity Follow‐up: 6 to 12 months | See comments | See comments | Not estimable | 44a | ⊕⊕⊝⊝ | Improvement in 7/9 participants (78%) in the immunotherapy group and 3/11 participants (27%) in the placebo group (RR 2.85, 95% CI 1.02 to 7.96; P = 0.04 (Warner 1978)) 8/13 participants (62%) in the immunotherapy group and 9/11 participants (81%) in the placebo group (RR 0.75, 95% CI 0.45 to 1.26; P = 0.38 (Glover 1992)) Due to unexplained statistical heterogeneity, we did not pool the data |
| Participant‐ or parent‐reported specific symptoms of eczema Follow‐up: 12 to 18 months SCORAD part C measured as a combination of 2 Visual Analogue Scales (1 for itch, 1 for sleep disturbance), each on a scale from 0, no specific symptoms, to 10, maximum specific symptoms | The mean SCORAD part C score ranged across control groups from 3.07 to 5.29 The mean SCORAD part C sleep severity score ranged across control groups from 0.8 to 2.31 | The mean SCORAD part C score in the immunotherapy group was on average 0.74 lower (95% CI ‐1.98 to 0.50) The mean SCORAD part C sleep severity score in the immunotherapy group was on average 0.49 lower (95% CI ‐1.03 to 0.06) | ‐ | 339a (6) | ⊕⊝⊝⊝ | Itch: SCORAD part C itch severity at the end of treatment: MD ‐0.24, 95% CI ‐1.00 to 0.52; I² = 0% for Di Rienzo 2014 and Novak 2012 Itch severity score: MD ‐4.20, 95% CI ‐3.69 to ‐4.71 for Sanchez 2012 Due to unexplained statistical heterogeneity, we did not pool the data |
| Adverse events ‐ any systemic reaction Follow‐up: 6 to 18 months | Low‐risk population | RR 0.78 (0.41 to 1.49) | 492a | ⊕⊕⊕⊝ | ‐ | |
| 0 per 1000 | 0 per 1000 | |||||
| Medium‐risk population | ||||||
| 71 per 1000 | 55 per 1000 | |||||
| High‐risk population | ||||||
| 163 per 1000 | 127 per 1000 | |||||
| Investigator‐ or physician‐rated global assessment of disease severity Follow‐up: 1 to 3 years | Low‐risk population | RR 1.48 (1.16 to 1.88) | 286a (7) | ⊕⊝⊝⊝ | ‐ | |
| 0 per 1000 | 0 per 1000 (0 to 10) | |||||
| Medium‐risk population | ||||||
| 471 per 1000 | 697 per 1000 (546 to 885) | |||||
| High‐risk population | ||||||
| 778 per 1000 | 1151 per 1000 (903 to 1462) | |||||
| Investigator‐ or physician‐rated eczema severity using a published scale Follow‐up: 12 to 18 months | The mean SCORAD score ranged across control groups from 26.7 to 32.6 | The mean SCORAD score in the immunotherapy group was on average 5.79 lower (95% CI ‐7.92 to ‐3.66) | ‐ | 435a (6) | ⊕⊝⊝⊝ | ‐ |
| Participant or parent‐rated eczema severity using a published scale Follow‐up: 12 to 18 months | See comment | See comment | Not estimable | 184a | ⊕⊕⊝⊝ | SCORAD part C used as the specific eczema symptom score (Di Rienzo 2014; Novak 2012) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Assumed risks are based on the total control group risk across all included studies (medium risk population) and the included studies with the lowest (low risk population) and highest (high risk population) control group risks. | ||||||
| Term | Definition |
| Anaphylaxis | A serious, life‐threatening allergic reaction |
| Fissuration | Formation of tears in the skin |
| Intradermally | Into the skin (dermis), below the epidermis |
| Lichenification | Thickening and hardening of the skin |
| Monovalent | 1 kind of antibody |
| Perennial | Long‐lasting continually |
| Photopheresis | A form of apheresis and photodynamic therapy |
| Sublingual | Under the tongue |
| Vesicles | Fluid‐filled cavities |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participant‐ or parent‐reported specific symptoms of eczema Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 SCORAD part C | 2 | 184 | Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐1.98, 0.50] |
| 1.2 Severity of sleep disturbance | 2 | 184 | Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐1.03, 0.06] |
| 2 Adverse events Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Any local reaction | 7 | 484 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.89, 1.81] |
| 2.2 Any systemic reaction | 7 | 492 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.41, 1.49] |
| 2.3 Tiredness | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 5.08 [0.66, 39.02] |
| 2.4 Headache | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 2.56 [0.11, 59.75] |
| 3 Investigator‐ or physician‐rated global disease severity Show forest plot | 6 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [1.16, 1.88] |
| 4 Participant‐ or parent‐rated eczema severity using a non‐published scale Show forest plot | 2 | 158 | Mean Difference (IV, Random, 95% CI) | ‐1.12 [‐1.92, ‐0.32] |
| 5 Investigator‐rated eczema severity assessed using a published scale Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Total SCORAD | 3 | 244 | Mean Difference (IV, Random, 95% CI) | ‐5.79 [‐7.92, ‐3.66] |
| 6 Use of other medications for eczema Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participant‐ or parent‐reported specific symptoms of eczema ‐ SCORAD part C by route of immunotherapy Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Subcutaneous immunotherapy | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Sublingual immunotherapy | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Participant‐ or parent‐reported specific symptoms of eczema ‐ severity of sleep disturbance by route of immunotherapy Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Subcutaneous immunotherapy | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Sublingual immunotherapy | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Participant‐ or parent‐reported specific symptoms of eczema ‐ SCORAD part C by allergen type Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Perennial inhalant | 2 | 184 | Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐1.98, 0.50] |
| 4 Participant‐ or parent‐reported specific symptoms of eczema ‐ severity of sleep disturbance by allergen type Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Perennial inhalant | 2 | 184 | Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐1.03, 0.06] |
| 5 Participant‐ or parent‐reported specific symptoms of eczema ‐ SCORAD part C by participant age Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 18 years or over | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Participant‐ or parent‐reported specific symptoms of eczema ‐ itch severity by participant age Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 18 years or over | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Participant‐ or parent‐reported specific symptoms of eczema ‐ severity of sleep disturbance by participant age Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 18 years or over | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Participant‐ or parent‐reported specific symptoms of eczema ‐ itch severity by severity at randomisation Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Moderate (SCORAD mean objective score 16 to 40) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Severe (SCORAD mean objective score > 40) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Participant‐ or parent‐reported specific symptoms of eczema ‐ severity of sleep disturbance by severity at randomisation Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Moderate (SCORAD mean objective score 16 to 40) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Severe (SCORAD mean objective score > 40) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Adverse events: any local reaction by route of immunotherapy Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Subcutaneous | 5 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.90, 1.55] |
| 10.2 Sublingual | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 9.76 [1.28, 74.26] |
| 11 Adverse events: any systemic reaction by route of immunotherapy Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Subcutaneous | 5 | 328 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.34, 2.00] |
| 11.2 Sublingual | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.29, 1.89] |
| 12 Adverse events: any local reaction by allergen type Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 Perennial inhalant | 6 | 464 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.81, 2.13] |
| 13 Adverse events: any systemic reaction by allergen type Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 Perennial inhalant | 6 | 472 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.41, 1.49] |
| 14 Adverse events: any local reaction by participant age Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 18 years or over | 2 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.44, 4.23] |
| 15 Adverse events: any systemic reaction by participant age Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15.1 18 years or over | 2 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.38, 1.47] |

