Inmunoterapia con alérgenos específicos para el tratamiento del eccema atópico
Información
- DOI:
- https://doi.org/10.1002/14651858.CD008774.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 12 febrero 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Piel
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
MC was the contact person with the editorial base at the protocol stage; and RB, at the review stage. MC and RB designed the study and co‐wrote the protocol. HN, HW, and SD reviewed earlier drafts of the protocol and provided comments. RB co‐ordinated contributions from the co‐authors. HT, MC, LM, and RB screened papers against eligibility criteria, appraised the quality of papers, extracted data, and sought additional information form original authors. HT, RB, and HN assessed the risk of bias. HT, LM, and RB entered data into Review Manager (RevMan) and analysed and interpreted data. HT and RB wrote the final draft of the review with contributions from all authors.
Disclaimer
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Skin Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Sources of support
Internal sources
-
Imperial College, London, UK.
-
The University of Nottingham, UK.
-
The University of Malaga, Spain.
External sources
-
The National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
Declarations of interest
Herman Tam: nothing to declare.
Moises A Calderon: nothing to declare.
Logan Manikam: nothing to declare.
Helen Nankervis: nothing to declare.
Ignacio García Núñez: nothing to declare.
Hywel C Williams: nothing to declare.
Stephen Durham: "I have received research funding for immunotherapy trials in hay fever (but not eczema) via Imperial College from ALK‐Abelló, Denmark; Merck, USA; and BioTech Tools, Belgium; all are manufacturers of allergy vaccines (research in relation to vaccines for hay fever, not for eczema). I have acted as a paid advisor for Merck, USA, a manufacturer of allergy vaccines (in relation to allergy vaccines for hay fever, not for eczema). I have received consultancy fees via Imperial College from Circassia, UK; Stallergenes, France; and Biomay, Austria (in relation to vaccines for hay fever, not for eczema)."
Robert J Boyle: nothing to declare.
Acknowledgements
We are grateful to Finola Delamere and Laura Prescott from the Cochrane Skin Group for their valuable comments and assistance with writing the study protocol.
The Cochrane Skin Group editorial base wishes to thank Sue Jessop who was the Dermatology Editor for this review; Ben Carter and Esther van Zuuren who were the Statistical and Methods Editors, respectively; the clinical referees, Eric Simpson and another who wishes to remain anonymous; and the consumer referee, Anjna Rani.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Feb 12 | Specific allergen immunotherapy for the treatment of atopic eczema | Review | Herman Tam, Moises A Calderon, Logan Manikam, Helen Nankervis, Ignacio García Núñez, Hywel C Williams, Stephen Durham, Robert J Boyle | |
| 2010 Oct 06 | Specific allergen immunotherapy for the treatment of atopic eczema | Protocol | Moises A Calderon, Robert J Boyle, Helen Nankervis, Ignacio García Núñez, Hywel C Williams, Stephen Durham | |
Differences between protocol and review
HT and LM joined as a co‐authors.
Types of interventions: we specified allergen formulations as standardised allergen extracts for single allergen or mixed allergens and included intradermal and oral routes of immunotherapy because of recent evidence that these routes may be effective for allergen immunotherapy in general (Anagnostou 2014; Rotiroti 2012).
Types of outcome measures: we clarified the primary outcome 'Participant‐ or parent‐reported specific symptoms of eczema' by subjective measures such as itch and sleep disturbance (SCORing Atopic Dermatitis (SCORAD) part C).
Types of outcome measures: although not one of our prespecified outcomes, we analysed 'Participant‐ or parent‐rated eczema severity assessed using a non‐published scale' because we thought it was important to include it as a subcategory. Six studies reported this outcome in the form of Visual Analogue Scales.
Types of outcome measures: for consistency, we added 'physician‐rated' to the third secondary outcome.
Measures of treatment effect: we amended the measure of treatment effect in continuous data to be expressed as mean differences where possible. We planned to express dichotomous outcomes as number needed to treat (NNT), where appropriate, with a 95% confidence interval (CI) and the baseline risk to which it applies but did not because we identified no suitable findings to which a NNT might be applied, since the review findings were either negative or inconclusive.
Unit of analysis issues: we planned to use techniques appropriate for paired designs and data from parallel trials and cross‐over trials as separate subgroups to analyse cross‐over trials, since cross‐over studies may not be appropriate for immunotherapy studies. Our search did not identify any cross‐over trials.
We did not list non‐randomised controlled studies because we did not identify significant studies or data from non‐randomised controlled studies.
Where studies reported more than one active intervention, we planned to combine the two active interventions and analyse them together, but we included no trials with more than one eligible active intervention. Where studies reported non‐parametric statistics, we planned to include these in meta‐analyses where possible, following the guidance of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). However, there were no relevant studies.
Assessment of reporting biases: we planned to use funnel plots to assess publication bias graphically (if there were sufficient included studies) and to use Begg and Egger tests (Begg 1994; Egger 1997) to assess it statistically; however, we did not have a sufficient number of included studies.
Sensitivity analysis: we planned to undertake sensitivity analysis for the allocation of missing data by best and worst case analysis. If we had found significant heterogeneity between studies, we planned to explore possible reasons for this, which would have included risk of bias in the included studies. However, we did not perform posthoc sensitivity analyses because of the small number of studies that contributed to meta‐analyses.
Appendices: we updated the search strategy for ongoing trial databases to identify relevant trials.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Animals; Child; Humans;
PICO

PRISMA flow diagram

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
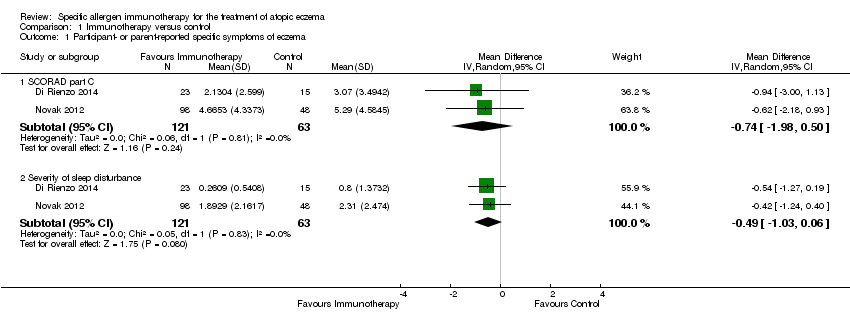
Comparison 1 Immunotherapy versus control, Outcome 1 Participant‐ or parent‐reported specific symptoms of eczema.

Comparison 1 Immunotherapy versus control, Outcome 2 Adverse events.

Comparison 1 Immunotherapy versus control, Outcome 3 Investigator‐ or physician‐rated global disease severity.
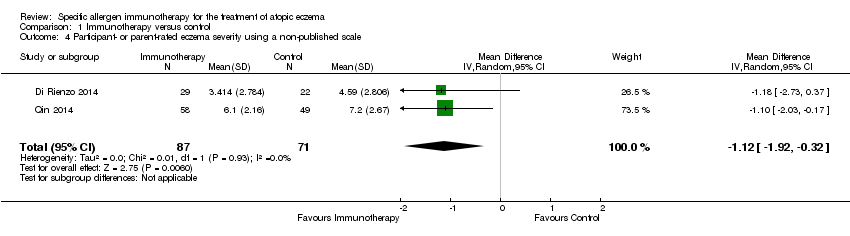
Comparison 1 Immunotherapy versus control, Outcome 4 Participant‐ or parent‐rated eczema severity using a non‐published scale.

Comparison 1 Immunotherapy versus control, Outcome 5 Investigator‐rated eczema severity assessed using a published scale.

Comparison 1 Immunotherapy versus control, Outcome 6 Use of other medications for eczema.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 1 Participant‐ or parent‐reported specific symptoms of eczema ‐ SCORAD part C by route of immunotherapy.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 2 Participant‐ or parent‐reported specific symptoms of eczema ‐ severity of sleep disturbance by route of immunotherapy.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 3 Participant‐ or parent‐reported specific symptoms of eczema ‐ SCORAD part C by allergen type.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 4 Participant‐ or parent‐reported specific symptoms of eczema ‐ severity of sleep disturbance by allergen type.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 5 Participant‐ or parent‐reported specific symptoms of eczema ‐ SCORAD part C by participant age.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 6 Participant‐ or parent‐reported specific symptoms of eczema ‐ itch severity by participant age.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 7 Participant‐ or parent‐reported specific symptoms of eczema ‐ severity of sleep disturbance by participant age.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 8 Participant‐ or parent‐reported specific symptoms of eczema ‐ itch severity by severity at randomisation.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 9 Participant‐ or parent‐reported specific symptoms of eczema ‐ severity of sleep disturbance by severity at randomisation.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 10 Adverse events: any local reaction by route of immunotherapy.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 11 Adverse events: any systemic reaction by route of immunotherapy.
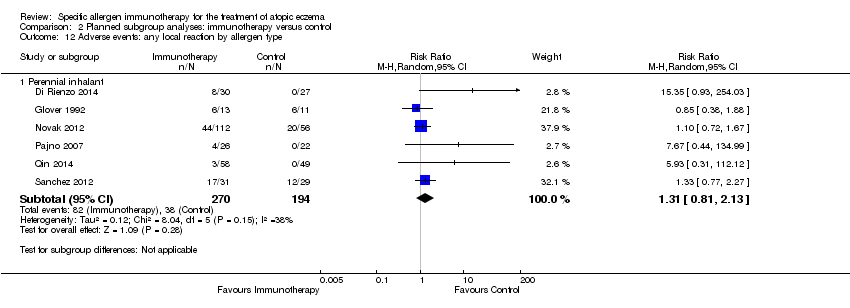
Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 12 Adverse events: any local reaction by allergen type.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 13 Adverse events: any systemic reaction by allergen type.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 14 Adverse events: any local reaction by participant age.

Comparison 2 Planned subgroup analyses: immunotherapy versus control, Outcome 15 Adverse events: any systemic reaction by participant age.
| Specific immunotherapy compared with no immunotherapy for atopic eczema | ||||||
| Patient or population: adults and children with atopic eczema and inhalant allergen sensitisation Settings: specialist allergy centres in the UK (2 trials), Italy (3 trials), USA, Germany, Belgium, Poland, Columbia, and China Intervention: specific allergen immunotherapy Comparison: no immunotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No immunotherapy | Specific allergen immunotherapy | |||||
| Participant‐ or parent‐reported global assessment of disease severity Follow‐up: 6 to 12 months | See comments | See comments | Not estimable | 44a | ⊕⊕⊝⊝ | Improvement in 7/9 participants (78%) in the immunotherapy group and 3/11 participants (27%) in the placebo group (RR 2.85, 95% CI 1.02 to 7.96; P = 0.04 (Warner 1978)) 8/13 participants (62%) in the immunotherapy group and 9/11 participants (81%) in the placebo group (RR 0.75, 95% CI 0.45 to 1.26; P = 0.38 (Glover 1992)) Due to unexplained statistical heterogeneity, we did not pool the data |
| Participant‐ or parent‐reported specific symptoms of eczema Follow‐up: 12 to 18 months SCORAD part C measured as a combination of 2 Visual Analogue Scales (1 for itch, 1 for sleep disturbance), each on a scale from 0, no specific symptoms, to 10, maximum specific symptoms | The mean SCORAD part C score ranged across control groups from 3.07 to 5.29 The mean SCORAD part C sleep severity score ranged across control groups from 0.8 to 2.31 | The mean SCORAD part C score in the immunotherapy group was on average 0.74 lower (95% CI ‐1.98 to 0.50) The mean SCORAD part C sleep severity score in the immunotherapy group was on average 0.49 lower (95% CI ‐1.03 to 0.06) | ‐ | 339a (6) | ⊕⊝⊝⊝ | Itch: SCORAD part C itch severity at the end of treatment: MD ‐0.24, 95% CI ‐1.00 to 0.52; I² = 0% for Di Rienzo 2014 and Novak 2012 Itch severity score: MD ‐4.20, 95% CI ‐3.69 to ‐4.71 for Sanchez 2012 Due to unexplained statistical heterogeneity, we did not pool the data |
| Adverse events ‐ any systemic reaction Follow‐up: 6 to 18 months | Low‐risk population | RR 0.78 (0.41 to 1.49) | 492a | ⊕⊕⊕⊝ | ‐ | |
| 0 per 1000 | 0 per 1000 | |||||
| Medium‐risk population | ||||||
| 71 per 1000 | 55 per 1000 | |||||
| High‐risk population | ||||||
| 163 per 1000 | 127 per 1000 | |||||
| Investigator‐ or physician‐rated global assessment of disease severity Follow‐up: 1 to 3 years | Low‐risk population | RR 1.48 (1.16 to 1.88) | 286a (7) | ⊕⊝⊝⊝ | ‐ | |
| 0 per 1000 | 0 per 1000 (0 to 10) | |||||
| Medium‐risk population | ||||||
| 471 per 1000 | 697 per 1000 (546 to 885) | |||||
| High‐risk population | ||||||
| 778 per 1000 | 1151 per 1000 (903 to 1462) | |||||
| Investigator‐ or physician‐rated eczema severity using a published scale Follow‐up: 12 to 18 months | The mean SCORAD score ranged across control groups from 26.7 to 32.6 | The mean SCORAD score in the immunotherapy group was on average 5.79 lower (95% CI ‐7.92 to ‐3.66) | ‐ | 435a (6) | ⊕⊝⊝⊝ | ‐ |
| Participant or parent‐rated eczema severity using a published scale Follow‐up: 12 to 18 months | See comment | See comment | Not estimable | 184a | ⊕⊕⊝⊝ | SCORAD part C used as the specific eczema symptom score (Di Rienzo 2014; Novak 2012) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Assumed risks are based on the total control group risk across all included studies (medium risk population) and the included studies with the lowest (low risk population) and highest (high risk population) control group risks. | ||||||
| Term | Definition |
| Anaphylaxis | A serious, life‐threatening allergic reaction |
| Fissuration | Formation of tears in the skin |
| Intradermally | Into the skin (dermis), below the epidermis |
| Lichenification | Thickening and hardening of the skin |
| Monovalent | 1 kind of antibody |
| Perennial | Long‐lasting continually |
| Photopheresis | A form of apheresis and photodynamic therapy |
| Sublingual | Under the tongue |
| Vesicles | Fluid‐filled cavities |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participant‐ or parent‐reported specific symptoms of eczema Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 SCORAD part C | 2 | 184 | Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐1.98, 0.50] |
| 1.2 Severity of sleep disturbance | 2 | 184 | Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐1.03, 0.06] |
| 2 Adverse events Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Any local reaction | 7 | 484 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.89, 1.81] |
| 2.2 Any systemic reaction | 7 | 492 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.41, 1.49] |
| 2.3 Tiredness | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 5.08 [0.66, 39.02] |
| 2.4 Headache | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 2.56 [0.11, 59.75] |
| 3 Investigator‐ or physician‐rated global disease severity Show forest plot | 6 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [1.16, 1.88] |
| 4 Participant‐ or parent‐rated eczema severity using a non‐published scale Show forest plot | 2 | 158 | Mean Difference (IV, Random, 95% CI) | ‐1.12 [‐1.92, ‐0.32] |
| 5 Investigator‐rated eczema severity assessed using a published scale Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Total SCORAD | 3 | 244 | Mean Difference (IV, Random, 95% CI) | ‐5.79 [‐7.92, ‐3.66] |
| 6 Use of other medications for eczema Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participant‐ or parent‐reported specific symptoms of eczema ‐ SCORAD part C by route of immunotherapy Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Subcutaneous immunotherapy | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Sublingual immunotherapy | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Participant‐ or parent‐reported specific symptoms of eczema ‐ severity of sleep disturbance by route of immunotherapy Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Subcutaneous immunotherapy | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Sublingual immunotherapy | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Participant‐ or parent‐reported specific symptoms of eczema ‐ SCORAD part C by allergen type Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Perennial inhalant | 2 | 184 | Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐1.98, 0.50] |
| 4 Participant‐ or parent‐reported specific symptoms of eczema ‐ severity of sleep disturbance by allergen type Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Perennial inhalant | 2 | 184 | Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐1.03, 0.06] |
| 5 Participant‐ or parent‐reported specific symptoms of eczema ‐ SCORAD part C by participant age Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 18 years or over | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Participant‐ or parent‐reported specific symptoms of eczema ‐ itch severity by participant age Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 18 years or over | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Participant‐ or parent‐reported specific symptoms of eczema ‐ severity of sleep disturbance by participant age Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 18 years or over | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Participant‐ or parent‐reported specific symptoms of eczema ‐ itch severity by severity at randomisation Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Moderate (SCORAD mean objective score 16 to 40) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Severe (SCORAD mean objective score > 40) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Participant‐ or parent‐reported specific symptoms of eczema ‐ severity of sleep disturbance by severity at randomisation Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Moderate (SCORAD mean objective score 16 to 40) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Severe (SCORAD mean objective score > 40) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Adverse events: any local reaction by route of immunotherapy Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Subcutaneous | 5 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.90, 1.55] |
| 10.2 Sublingual | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 9.76 [1.28, 74.26] |
| 11 Adverse events: any systemic reaction by route of immunotherapy Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Subcutaneous | 5 | 328 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.34, 2.00] |
| 11.2 Sublingual | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.29, 1.89] |
| 12 Adverse events: any local reaction by allergen type Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 Perennial inhalant | 6 | 464 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.81, 2.13] |
| 13 Adverse events: any systemic reaction by allergen type Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 Perennial inhalant | 6 | 472 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.41, 1.49] |
| 14 Adverse events: any local reaction by participant age Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 18 years or over | 2 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.44, 4.23] |
| 15 Adverse events: any systemic reaction by participant age Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15.1 18 years or over | 2 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.38, 1.47] |

