Ginseng para la cognición
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by year of study]
| Methods | Randomized, double‐blind, placebo controlled trial. | |
| Participants | Country: Italy Setting: Single center, University of Pavia Number of participants randomized: 32 (male) Number of participants completed: 32 (male) Age (years): 21.9 ± 1.6 (treatment), 21.7 ± 1.6 (control) Weight (kg): 69.5 ± 8.4 (treatment), 69.6 ± 10 (control) Inclusion criteria: All participants were students at a local University College and were in good physical condition as assessed by a medical examination and conventional laboratory tests. Exclusion criteria: Not stated. Baseline performance were similar in the two groups as tested by psychometric tests except the choice reaction time. Choice reaction time was longer in the G115 group. | |
| Interventions | Comparison: G115 versus placebo 1. G115® (GINSANA, Pharmaton S.A., Switzerland): One capsule twice a day, taken at 8:00 and 13:00, corresponding to a total daily dose of 200mg. 2. Placebo: Identical lactose‐containing capsules 3. Duration of treatment: 12 weeks | |
| Outcomes | 1. Tapping test 2. Simple reaction time 3. Choice reaction time 4. Cancellation test 5. Digit symbol substitution test 6. Mental arithmetic 7. Logical deduction 8. Tolerability outcomes | |
| Notes | 1. This study was the first randomized, double‐blind placebo‐controlled study 2. Changes in psychometric test score from baseline to the final assessment were not suggested in the report. 3. The trial lacked an adequate description of methods of randomization and blinding. 4. We contacted Dr Emilio Perucca on 28 May 2009 for additional information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random number table |
| Allocation concealment? | Unclear risk | Allocation concealment was not mentioned and whether the sequence was concealed remained unclear. |
| Blinding? | Low risk | Both experimenters doing the tests and participants were blinded. |
| Incomplete outcome data addressed? | Low risk | All participants completed the study. |
| Methods | Randomized, double‐blind, placebo‐controlled trial. | |
| Participants | Country: Denmark Setting: Single center, Department of internal medicine, Gentofte University Hospital. Number of participants randomized: 127 Number of participants completed: 112 (38 male) Age (years): 51.4 ± 7.9 (treatment), 51.5 ± 9.1 (control) Schooling (years): 10.5 ±1.9 (treatment), 10.2 ± 1.9 (control) Advanced education: 78% (treatment), 82% (control) Inclusion criteria: Healthy volunteers older than 40 years. Exclusion criteria: Serious illness, diseases of the central nervous system, and abuse of alcohol or drugs. Participants receiving psychoactive medication that might interact with ginseng. For all of the tests except the Selective Reminding Test, the baseline values for the two groups were similar and corresponded to the high end of expected values for normally functioning participants. | |
| Interventions | Comparison: Gerimax Ginseng Extract versus placebo 1. Gerimax Ginseng Extract (Dansk Droge A/S, Ishøj, Denmark): 400 mg per day. 2. Placebo: Inactive, heavily soluble calcium preparation identical with the Gerimax. 3. Duration of treatment: 8 to 9 weeks | |
| Outcomes | 1. Simple Auditive Reaction Times Test 2. Simple Visual Reaction Times Test 3. Finger‐Tapping Test 4. D2 Test 5. Fluency Test 6. Selective Reminding Test 7. Logical Memory and Reproduction Test 8. Rey‐Oestrich Complex Figure Test 9. Wisconsin Card Sorting Test 10. Tolerability outcomes | |
| Notes | 1. The study was supported by a grant from Dansk Droge A/S (Ishøj, Denmark). 2. A comprehensive battery of cognitive tests was used to investigate cognitive function in healthy, middle‐aged participants. 3. The trial lacked an adequate description of methods of randomization. 4. Allocation concealment was not mentioned in the report. 5. We contacted Dr Jesper Sonne on 28 May 2009 for additional information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | A randomization code was made by a pharmacist in the company and the method of randomization code generation was unclear. |
| Allocation concealment? | Low risk | The randomization code was kept under lock until all results had been analysed. Not till then was the treatment allocation revealed. Members of the company were in no way involved in the conduct of the trial and had absolutely no access to participants or test procedures etc. |
| Blinding? | Low risk | Both participants and study managers were blinded. |
| Incomplete outcome data addressed? | Low risk | 15 (12%) dropped‐out, 6 due to illness and 9 for unknown reasons. Compliance was assessed by querying the participants and by counting the tablets returned. In no case did the returned tablets exceed 5% of the total number distributed to the participants. |
| Methods | Randomised, double‐blind, placebo‐controlled, two period cross‐over design | |
| Participants | Country: UK Setting: Single University center Number of participants randomized: 30 (15 male) Number of participants completed: not given Age (years): 18‐25 (Mean: 20) Inclusion criteria: Healthy undergraduate young volunteers taking no medication or herbal supplements. Of the 30 participants two were light smokers (<5 cigarettes per day and <2 per week, respectively). Participants agreed to refrain from smoking, and caffeine and alcohol consumption throughout each study day. No other dietary restrictions were implemented. Exclusion criteria: Not stated | |
| Interventions | Comparison: G115 versus placebo 1. G115® (Pharmaton S.A., Switzerland): 400 mg 2. Placebo 3. Subjects received two capsules of identical appearance, each containing either 200mg G115 or an inert placebo, in a counterbalanced order, with a seven‐day washout period between treatments. 4. Duration of treatment: 2 days | |
| Outcomes | 1. Primary outcome measures (a) Quality of memory factor (b) Speed of memory factor (c) Speed of attention factor (d) Accuracy of attention 2. Secondary outcome measures (a) Working memory sub‐factor (b) Secondary memory sub‐factor (c) CDR factor scores | |
| Notes | 1. The fourth author Petrini O was employed by Pharmaton SA, the producer of the standardised ginseng extract G115 used in the trial. 2. The trial aimed to evaluate the effect of ginseng (400 mg) administration on cognitive performance and mood in healthy young volunteers. 3. Methods of blinding and adverse effects of G115 were not stated in the report. 4. We contacted Professor Keith A. Wesnes on 21 July 2009 for additional information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | A person not involved in the trial carried out randomisation manually using a randomisation table. |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? | Low risk | Subjects and test administrators were blinded. |
| Incomplete outcome data addressed? | Unclear risk | Exact number of participants completed the study was not stated. However, from the degree of freedom in the paired T‐test we concluded that authors used number of participants randomized to calculate the results. |
| Methods | Randomized, double‐blind, placebo‐controlled, two period cross‐over design | |
| Participants | Country: UK Setting: Single University center Number of participants randomized: 18 (5 male) Number of participants completed: 16 Age (years): 38.31 ± 10.3 Inclusion criteria: Healthy undergraduate young volunteers taking no illicit social drugs, and were free from "over the‐counter" or prescribed medications, with the exception, for some female volunteers, of the contraceptive pill. Participants fasted overnight and were alcohol and caffeine free for 12 hours prior to all assessment sessions, and also abstained from psychoactive products during the testing day. Exclusion criteria: Heavy smokers (>5 cigarettes/day). No significant differences between all baseline assessments on all measures within the study. | |
| Interventions | Comparison: Korean Panax ginseng extract versus placebo 1. Korean Panax ginseng extract (Cheong Kwan Jang, Korea Ginseng Corporation, Seoul, Republic of Korea): 200 mg 2. Placebo: Apparently identical with the intervention drug 3. Each participant took either ginseng or placebo for 8 weeks, with a 4 week placebo washout period between treatment arms. 4. Duration of treatment: 8 weeks | |
| Outcomes | 1. Cognitive Drug Research (CDR) computerised assessment battery 2. Working Memory Tasks (a) Corsi Block Tapping task (b) 3‐back task (c) Alphabetic working memory 3. Subjective mood and 'quality of life' measures (a) Bond‐Lader Mood scales (b) World Health Organisation Quality of Life questionnaire‐BREF: (WHOQOL‐BREF) 4. Blood glucose parameters | |
| Notes | 1. The effects of Korean ginseng extract (200 mg) on cognitive performance, gluco‐regulatory parameters and ratings of subjective mood and 'quality of life' were investigated. 2. Detailed information of the Korean ginseng extract were not given in the report. 3. The report lacked an adequate description of randomization, blinding, reason of drop‐outs and adverse effects of Korean ginseng extract. 4. Authors did not analyse results of the first treatment period after randomization as it would have too little statistical power to be interpretable. 5. We contacted Professor David Kennedy on 27 May 2009 and Dr Jonathon Reay on 8 July 2009 for additional information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random number generator allocating participants to two groups. |
| Allocation concealment? | Low risk | All treatments were packaged and coded by a disinterested third party, who retained the emergency code break for use in the event of any serious adverse events. |
| Blinding? | Low risk | Participants and all experimenters were blinded. |
| Incomplete outcome data addressed? | Low risk | Two participants failed to complete the trial (16 evaluable sets of data). Reasons unrelated to treatment i.e. simply dropped out of the study, and as it was an extended treatment period they did not have time to replace them. |
| Methods | A randomized, double‐blind, fixed‐dose, placebo‐controlled, parallel group trial | |
| Participants | Country: South Korea Setting: Single University center, Kyung Hee University Medical Center Number of participants randomized: 118 (42 male) Number of participants completed: 99 Age (years): 59.4 ± 5.1 (treatment), 59 ± 5 (control) Schooling (years): 12.2 ± 3.4 (treatment), 11.3 ± 2.9 (control) Inclusion criteria: Cognitively intact adults were required to have completed six or more years of education and have no difficulty reading or writing. A score≥borderline scores of 16.9 at ages 65 to 84 or 18.9 at ages 55 to 64 on the memory subscale of the Korean‐Dementia Rating Scale (K‐DRS) and a score of >24 on the Korean Version of the Mini Mental State Examination (MMSE‐K). Exclusion criteria: Individuals who had histories of neurological disorders, including stroke, head injury, psychiatric disorders (mental retardation, schizophrenia, depression with ≥21 on the Beck's Depression Inventory (BDI) scores), drug abuse, alcohol dependence/abuse, or a disease or surgery that could influence drug absorption, were excluded from this study before the K‐DRS test or MMSE‐K test. Individuals who were being treated with hormones, antidepressants or other psychoactive medications, who had internal medical problems on blood test (except stable hypertension or diabetes mellitus with medication), who had an unstable medical state, were pregnant or would become pregnant, were undernourished, or who drank more than eight cups of coffee per day also were excluded. Participants who had participated in other clinical trials in the last month were also excluded. Participants were excluded from the study if they did not take more than 8 packs of study medicines in any 2‐week period. No significant differences between all baseline assessments on all measures within the study. | |
| Interventions | Comparison: HT008‐1 versus placebo 1. HT008‐1 (Lot. No.001) (NeuMed Inc., Korea): two pouches daily with a daily dose of 5200mg (an average of 100 mg/ kg). In this clinical study, HT008‐1 was prepared as a liquid containing 2600 mg of standardized extracts in a 30 ml pouch of solution. 2. Placebo: two pouches daily that did not differ in appearance (e.g., color, size, smell, or taste) from HT008‐1. 3. Duration of treatment: 8 weeks | |
| Outcomes | 1. Wechsler Memory Scale‐III (WMS‐III) (a) Logical memory I (b) Logical memory II (c) Verbal paired associates I (d) Verbal paired associates II (e) Letter–Number Sequencing (f) Spatial span (g) Auditory recognition delayed 2. World Health Organization Quality of Life Assessment Instruments‐BREF (WHOQoL‐Bref) (a) Overall quality of life (b) General health (c) Physical health (d) Psychological health (e) Social relationships (f) Environment 3. Tolerability outcomes | |
| Notes | This work was supported by a grant (PF 0320201‐00) of Plant Diversity Research Center of 21st Century Frontier Research Program (Ministry of Science and Technology, Korea), and by grants from the Seoul R&D Program (10524) and the Second Stage of Brain Korea 21 Project (Ministry of Education, Korea). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Through the online service of www.randomizer.org. |
| Allocation concealment? | Low risk | Through the online randomization service. |
| Blinding? | Low risk | To analyze the blinding efficacy, participants were asked to which group they belonged. It could be concluded that blinding was not broken from the testing result. |
| Incomplete outcome data addressed? | Low risk | Number (or %) of followed‐up from each group: HT008‐1 (50/59, 85%), Placebo (49/59, 83%); Reasons for loss: HT008‐1 (4‐lost to follow up, 1‐adverse events, 4‐protocol violation), Placebo (5‐lost to follow up, 2‐adverse events, 3‐protocol violation). |
Characteristics of excluded studies [ordered by year of study]
| Study | Reason for exclusion |
| Randomized, double‐blind, placebo controlled study to assess the effects of a combination of active substances including ginseng extract Gl15 on quality of life (QOL) in healthy, employed volunteers older than 25 years. Results: After 12 weeks of treatment, both the combination and placebo groups improved their general well being, but the improvement from baseline was more pronounced in those participants taking the combination of active substances (Gericomplex soft gelatin capsules containing 40 mg ginseng extract Gl15 and vitamins, minerals, and trace elements) twice daily. Conclusions: In healthy participants the combination of vital substances including Gl15 offered significant advantages over placebo treatment in terms of improvement in self‐assessed feelings of vitality, alertness, less time urgency (that is, feeling more relaxed), and appetite. The beneficial effects appeared to be more pronounced in those participants who were at a disadvantage and worse off before the study started. Reasons for exclusion: Cognitive function was not tested. | |
| Randomized, double‐blind, placebo controlled study to compares psychological well‐being and perceived quality of life in participants with age‐associated memory impairment (AAMI), who were administered Gegorvit® (Pharmaton S.A., Switzerland) or placebo for 9 months. Results: Drug‐treated AAMI subjects differ from controls in part by improved scores on objective cognitive tests but even more so by modifications of the correlations among indexes of psychological well‐being and quality of life. Conclusions: Ginseng might act on some of the mechanisms underlying AAMI and met the prerequisites as a candidate for drug therapy. Reasons for exclusion: Number (or %) of follow‐up from each group was not stated. Therefore, relevant data could not be extracted. | |
| Randomized, double‐blind, placebo controlled study to investigate the effect of ginseng on newly diagnosed non‐insulin dependent diabetes mellitus (NIDDM) participants. Results: Ginseng 100 mg or 200 mg (Dansk Droge, Copenhagen) daily for 8 weeks elevated mood, improved mood, vigor, well‐being, and psychomotor performance, but not memory, and reduced fasting blood glucose (FBG) and body weight. The 200 mg dose of ginseng improved glycated hemoglobin, serum aminoterminalpropeptide (PIIINP), and physical activity. The participants completed the study without any side effects. Conclusions: Ginseng might be a useful therapeutic adjunct in the management of NIDDM. Reasons for exclusion: Neither baseline results nor change from baseline values could be extracted. | |
| A double‐blind, placebo controlled study to examine the effect of ginseng (Gericomplex, Pharmaton S.A., Switzerland) as an adjuvant to treatment and rehabilitation of geriatric participants. Results: After treated with two capsules of ginseng or placebo for 8 weeks, length of stay in hospital did not differ in the two groups. Both groups also improved to the same degree on the various functional outcome measures, except for the Kendrick Object Learning test, where the placebo group improved more markedly. Conclusions: No identifiable effects of ginseng as an adjuvant to treatment and rehabilitation of geriatric participants were observed. Reasons for exclusion: A clinical controlled study. "Randomization" was never mentioned in the paper. No additional information of sequence generation could be obtained from the first author. | |
| Randomized, double‐blind, placebo‐controlled, Latin square cross‐over study to observe whether acute administration of Panax ginseng extract (G115®, Pharmaton SA) might have effects on mood and cognitive performance. Results: "Quality of Memory" and the associated "Secondary Memory" factor at all time points following 400mg of ginseng improved significantly. Both the 200 and 600mg doses were associated with a significant decrement of the "Speed of Attention" factor at later testing times only. Subjective ratings of alertness were also reduced 6h following the two lowest doses. Conclusions: Single doses of ginseng were capable of a dose dependent modulation of cognitive performance in healthy young adults. Reasons for exclusion: Data could not be extracted from the complicated Latin square cross‐over study. We contacted Professor David Kennedy on 27 May 2009 for additional information. | |
| Randomized, double‐blind, placebo controlled study to assess the time‐dependent effects of Panax ginseng on health‐related quality of life (HRQOL) in healthy young volunteers. Results: After 4 weeks of therapy, higher scores in social functioning, mental health, and the mental component summary scales were observed in participants randomized to Panax ginseng (Ginsana), these differences did not persist to the 8‐week time point. The incidence of adverse effects was 33% in the Panax ginseng group compared with 17% in the placebo group. Participants given Panax ginseng (58%) were more likely to state that they received active therapy than participants given placebo. Conclusions: Panax ginseng (Ginsana) improves aspects of mental health and social functioning after 4 weeks of therapy, although these differences attenuate with continued use. Reason for exclusion: Cognitive function was not tested. | |
| Randomized, single‐blind, placebo controlled study to evaluate the effect of King's Brain pill (Compound Chinese ginseng extract from herbs) on the treatment and the delaying of memory decline in the elderly with mild cognitive impairment (MCI) in a community by a year follow‐up of neuropsychology. Results: Mean MMSE score increased after treated with 4 pills of King's Brain pill (1.5 g) thrice a day for 3 months, while decreased at one year follow‐up point, but still higher than that in placebo group (P<0.05). Verbal Learning Test score was significantly increased at follow‐up point in the King's Brain pill group , which was significantly higher than that in the placebo group (P<0.01). The total score of memory items on BNPT battery (Bristol Memory Disorders Clinic‐Revised) was significantly increased at follow‐up point in the King's Brain pill group, higher than that in Piracetam group (P<0.05) and the placebo group ( P<0.01) . Conclusions: King's Brain spill had protective effect on cognitive and memory decline in elderly with MCI. Reasons for exclusion: King's Brain pill was a compound containing ginseng as a major component. Sales manager from Henan Wanxi Pharmaceutical Company said that King's Brain pill (Jin Si Wei) was not a compound on the market. Clinical information of King's Brain pill besides this study could not be found from academic database and the company. | |
| This was a poster presentation at the American Stroke Association's 28th International Stroke Conference. A randomized, double‐blind, pilot study to evaluate the effectiveness of a compound of Chinese ginseng tablet (King's Brain pill) in the treatment of memory impairment in participants with dementia after stroke. Results: After given one tablet of ginseng compound thrice a day for 12 weeks, a significant increase in mean scores on the HVLT and increase in total memory scores have been observed. There were greater improvements in episodic memeory function assessing story recall, delayed word recall, verbal learning and verbal recognition and visual recognition in the compound Chinese ginseng group than in the duxil‐controlled group. Conclusions: Treatment with the compound of Chinese ginseng extract might improve memory function in participants with mild and moderate dementia after stroke and might warrant further research treatment strategies for VaD. Reasons for exclusion: King's Brain pill was a compound containing ginseng as a major component. Not placebo controlled trial, since the comparison was Duxil. | |
| This was an abstract for the Asia Pacific scientific forum new discoveries in cardiovascular disease and stroke: Bench to bedside to community. It was the same as the abstract of Tian 2003a. | |
| Randomized, double‐blind, counterbalanced, placebo‐controlled study not only provided an examination of the cognitive and mood effects of guarana in healthy young volunteers, but also assessed the potential for additive or synergistic effects following the common, commercially available, combination of guarana with Panax ginseng. Results: In comparison to placebo, 75 mg of a dried ethanolic extract of guarana (approx 12% caffeine), 200 mg of Panax ginseng extract (G115®, Pharmaton SA), and their combination (75 mg/200 mg) resulted in improved task performance throughout the day. In the case of guarana, improvements were seen across 'attention' tasks (but with some evidence of reduced accuracy), and on a sentence verification task. While also increasing the speed of attention task performance, both ginseng and the ginseng/guarana combination also enhanced the speed of memory task performance, with little evidence of modulated accuracy. Guarana and the combination, and to a lesser extent ginseng, also led to significant improvements in serial subtraction task performance. Conclusions: These results provided the first demonstration in humans of the psychoactive effects of guarana, and confirmation of the psychoactive properties of ginseng. Reasons for exclusion: The Latin‐square design cross‐over trial compared other types of intervention besides ginseng was excluded since it was very difficult to evaluate the 'carry‐over' effect due to other intervention. Data could not be extracted from the complicated Latin square cross‐over study. | |
| Randomized, double‐blind, placebo‐controlled, Latin square cross‐over study to investigate the effects of two separate single doses (200 mg and 400 mg) of Panax ginseng extract (G115®, Pharmaton SA) on changes in fasted blood glucose levels and performance during sustained mentally demanding tasks. Results: 200 mg ginseng could significantly improved Serial Sevens subtraction task performance and significantly reduced subjective mental fatigue throughout all (with the exception of one time point in each case) of the post‐dose completions of the 10min battery. Conclusions: Panax ginseng could improve performance and subjective feelings of mental fatigue during sustained mental activity. Reasons for exclusion: Data could not be extracted from the complicated Latin square cross‐over study. We contacted Professor David Kennedy on 27 May 2009 and Dr Jonathon Reay on 8 July 2009 for additional information. | |
| Randomized, double‐blind, counterbalanced, placebo‐controlled study to investigate the effects of single doses of Panax ginseng, glucose, and a combination of Panax ginseng and glucose on blood glucose levels and cognitive performance during sustained 'mentally demanding' tasks. Results: Both Panax ginseng and glucose enhanced performance of a mental arithmetic task and ameliorated the increase in subjective feelings of mental fatigue experienced by participants during the later stages of the sustained, cognitively demanding task performance. Accuracy of performing the Rapid Visual Information Processing task (RVIP) was also improved following the glucose load. There was no evidence of a synergistic relationship between Panax ginseng and exogenous glucose ingestion on any cognitive outcome measure. Panax ginseng caused a reduction in blood glucose levels 1 hour following consumption when ingested without glucose. Conclusions: These results confirmed that Panax ginseng might possess glucoregulatory properties and could enhance cognitive performance. Reasons for exclusion: The Latin‐square design cross‐over trial compared other types of intervention besides ginseng was excluded since it was very difficult to evaluate the 'carry‐over' effect due to other intervention. Data could not be extracted from the complicated Latin square cross‐over study. | |
| Randomized, double‐blind, placebo controlled, two‐period cross‐over study to assess the behavioural and mood effects of chronic ingestion of Panax ginseng (G115). Results: Results revealed improvements in working memory following a single acute dose of Panax ginseng have been observed, whereas, following chronic dosing results revealed both improvements and decrements in aspects of cognition and mood. Conclusions: An observed effect on a performance measure following chronic ingestion can be further modulated by that day's acute dose. Reasons of exclusion: Only the abstract has been published and data were not obtainable from the author. | |
| Randomized, open‐label pilot study to evaluate the adjunctive effect of Korean red ginseng (KRG) in AD. Results: Participants in the high‐dose (9 g/day) KRG group showed significant improvement on the ADAS and CDR after 12 weeks of KRG therapy when compared with those in the control group. Both low‐dose (4.5 g/day) and high‐dose (9 g/day) KRG groups showed improvement from baseline MMSE when compared with the control group, but this improvement was not statistically significant. Two participants in the low‐dose KRG group complained of feeling feverish and two participants in the high‐dose KRS group complained of nausea. Conclusions: KRG showed good efficacy for the treatment of AD; however, further studies with larger samples of participants and a longer efficacy trial should be conducted to confirm the efficacy of KRG. Reasons for exclusion: This was not a blinded study. The trial compared KRG as an adjuvant therapy to conventional anti‐dementia medications, not placebo controlled trial. | |
| Randomized, open‐label prospective study to evaluate clinical efficacy of Panax ginseng in the cognitive performance of AD participants. Results: After 12 weeks of the Panax ginseng powder (4.5 g/d) treatment, the cognitive subscale of ADAS and the MMSE score began to show improvements and continued up to 12 weeks. At 12 weeks after the ginseng discontinuation, the improved ADAS and MMSE scores declined to the levels of the control group. The adverse events (e.g. heat‐sense, dizziness, nausea, anorexia, diarrhea and headache) were mild and transient. Conclusions: Panax ginseng was clinically effective in the cognitive performance of AD participants. Reasons for exclusion: This was an open‐label trial with no blinding performed. Both Ginseng group and control group continued the conventional therapy, not placebo controlled trial. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Cognitive, emotional, physical, and psychosocial effects of three weeks' prospective double‐blind placebo controlled cross‐over exposure to Panax Quinquefolius L (REMEMBER‐fX), with optional six months' open label follow‐up |
| Methods | Randomized, double‐blind, placebo controlled, cross‐over assignment trial |
| Participants | Inclusion Criteria:
Exclusion Criteria:
|
| Interventions | HT1001 (Panax Quinquefolius L, REMEMBER‐fX) |
| Outcomes | Primary outcome measures: Use of HT1001 will improve objective measures of psychomotor speed, sustained attention, working memory, declarative memory, and or executive skills. |
| Starting date | July 2007 |
| Contact information | Scot E Purdon, PhD, Department of Psychiatry, University of Alberta |
| Notes | Last visit took place on April 25, 2009. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Attention and concentration, cancellation (No. of digits/2 min) (Change from baseline: week 12) Show forest plot | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐1.76, 5.76] |
| Analysis 1.1 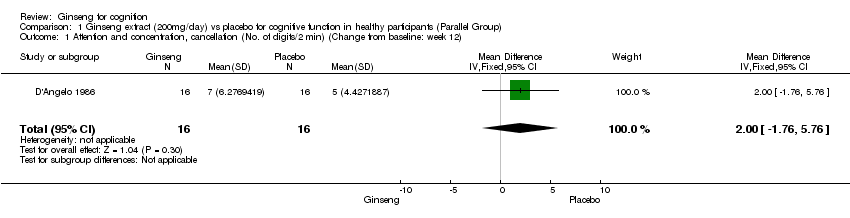 Comparison 1 Ginseng extract (200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 1 Attention and concentration, cancellation (No. of digits/2 min) (Change from baseline: week 12). | ||||
| 2 Speed of processing, Mental arithmetic (correct responses/s) (Change from baseline: week 12) Show forest plot | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [0.03, 0.21] |
| Analysis 1.2  Comparison 1 Ginseng extract (200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 2 Speed of processing, Mental arithmetic (correct responses/s) (Change from baseline: week 12). | ||||
| 3 Speed of processing, Logical deduction (correct responses/s) (Change from baseline: week 12) Show forest plot | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.02, 0.06] |
| Analysis 1.3  Comparison 1 Ginseng extract (200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 3 Speed of processing, Logical deduction (correct responses/s) (Change from baseline: week 12). | ||||
| 4 Speed of processing, Digit symbol substitution (No. of symbols/90s) (Change from baseline: week 12) Show forest plot | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐5.32, 3.32] |
| Analysis 1.4 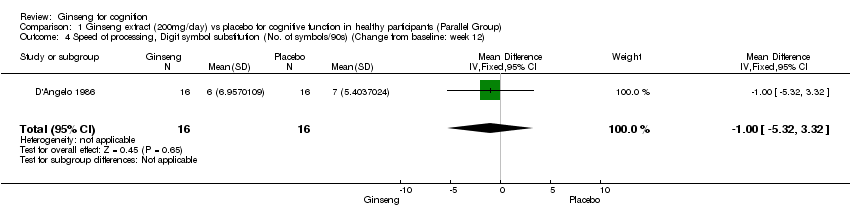 Comparison 1 Ginseng extract (200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 4 Speed of processing, Digit symbol substitution (No. of symbols/90s) (Change from baseline: week 12). | ||||
| 5 Sensorimotor function, choice reaction time (s/10‐no. of errors) (Change from baseline: week 12) Show forest plot | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.02, ‐0.01] |
| Analysis 1.5  Comparison 1 Ginseng extract (200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 5 Sensorimotor function, choice reaction time (s/10‐no. of errors) (Change from baseline: week 12). | ||||
| 6 Sensorimotor function, simple visual reaction time (s) (Change from baseline: week 12) Show forest plot | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.01, 0.01] |
| Analysis 1.6  Comparison 1 Ginseng extract (200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 6 Sensorimotor function, simple visual reaction time (s) (Change from baseline: week 12). | ||||
| 7 Sensorimotor function, simple auditory reaction time (s) (Change from baseline: week 12) Show forest plot | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.01, 0.01] |
| Analysis 1.7 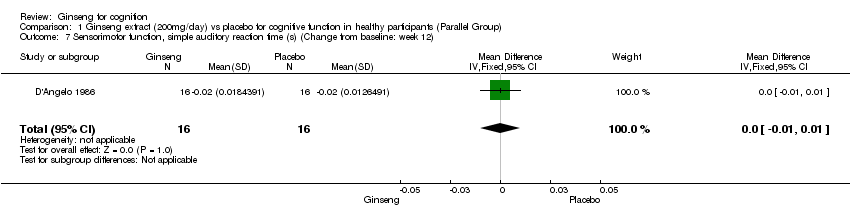 Comparison 1 Ginseng extract (200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 7 Sensorimotor function, simple auditory reaction time (s) (Change from baseline: week 12). | ||||
| 8 Pure motor function, tapping (taps/30s) (Change from baseline: week 12) Show forest plot | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐8.0 [‐14.66, ‐1.34] |
| Analysis 1.8  Comparison 1 Ginseng extract (200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 8 Pure motor function, tapping (taps/30s) (Change from baseline: week 12). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Attention and concentration, D2 (total score) (Change from baseline: week 8 to 9) Show forest plot | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [‐11.15, 19.15] |
| Analysis 2.1  Comparison 2 Ginseng extract (400mg/day) vs placebo for cognitive function healthy participants (Parallel Group), Outcome 1 Attention and concentration, D2 (total score) (Change from baseline: week 8 to 9). | ||||
| 2 Learning and Memory, selective reminding (error index) (Change from baseline: week 8 to 9) Show forest plot | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | 4.4 [0.82, 7.98] |
| Analysis 2.2  Comparison 2 Ginseng extract (400mg/day) vs placebo for cognitive function healthy participants (Parallel Group), Outcome 2 Learning and Memory, selective reminding (error index) (Change from baseline: week 8 to 9). | ||||
| 3 Learning and Memory, logical memory and reproduction (units lost) (Change from baseline: week 8 to 9) Show forest plot | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.74, 0.74] |
| Analysis 2.3 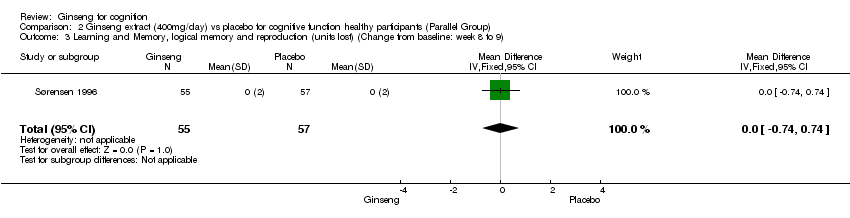 Comparison 2 Ginseng extract (400mg/day) vs placebo for cognitive function healthy participants (Parallel Group), Outcome 3 Learning and Memory, logical memory and reproduction (units lost) (Change from baseline: week 8 to 9). | ||||
| 4 Learning and Memory, Rey‐Oestrich complex figure (units lost) (Change from baseline: week 8 to 9) Show forest plot | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐1.46, 1.86] |
| Analysis 2.4 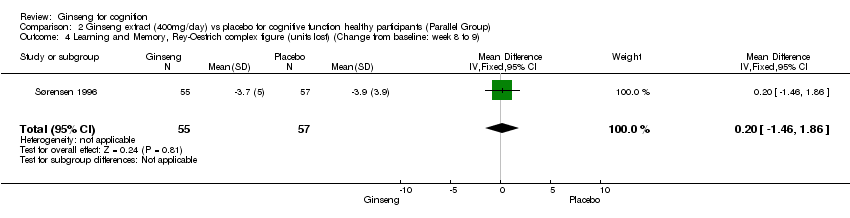 Comparison 2 Ginseng extract (400mg/day) vs placebo for cognitive function healthy participants (Parallel Group), Outcome 4 Learning and Memory, Rey‐Oestrich complex figure (units lost) (Change from baseline: week 8 to 9). | ||||
| 5 Sensorimotor function, simple auditory reaction times (10 Percentile) (Change from baseline: week 8 to 9) Show forest plot | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐6.60 [‐15.72, 2.52] |
| Analysis 2.5  Comparison 2 Ginseng extract (400mg/day) vs placebo for cognitive function healthy participants (Parallel Group), Outcome 5 Sensorimotor function, simple auditory reaction times (10 Percentile) (Change from baseline: week 8 to 9). | ||||
| 6 Sensorimotor function simple auditory reaction times (50 Percentile) (Change from baseline: week 8 to 9) Show forest plot | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐9.8 [‐22.69, 3.09] |
| Analysis 2.6 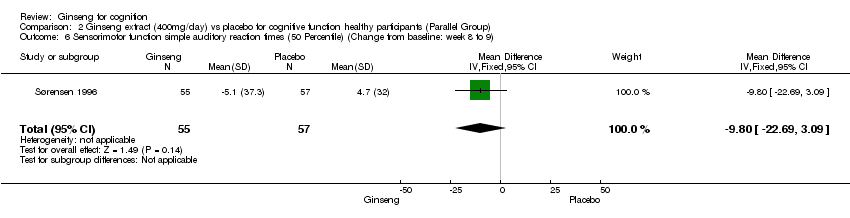 Comparison 2 Ginseng extract (400mg/day) vs placebo for cognitive function healthy participants (Parallel Group), Outcome 6 Sensorimotor function simple auditory reaction times (50 Percentile) (Change from baseline: week 8 to 9). | ||||
| 7 Sensorimotor function, simple visual reaction times (10 Percentile) (Change from baseline: week 8 to 9) Show forest plot | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐11.26, 3.86] |
| Analysis 2.7 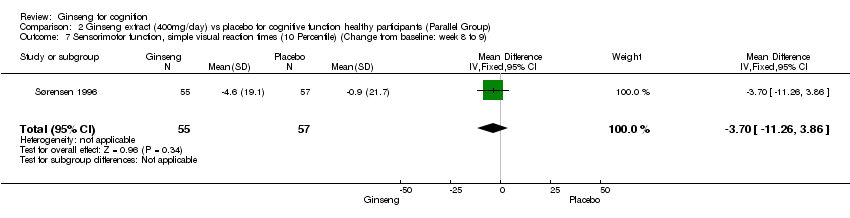 Comparison 2 Ginseng extract (400mg/day) vs placebo for cognitive function healthy participants (Parallel Group), Outcome 7 Sensorimotor function, simple visual reaction times (10 Percentile) (Change from baseline: week 8 to 9). | ||||
| 8 Sensorimotor function, simple visual reaction times (50 Percentile) (Change from baseline: week 8 to 9) Show forest plot | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐2.2 [‐11.63, 7.23] |
| Analysis 2.8 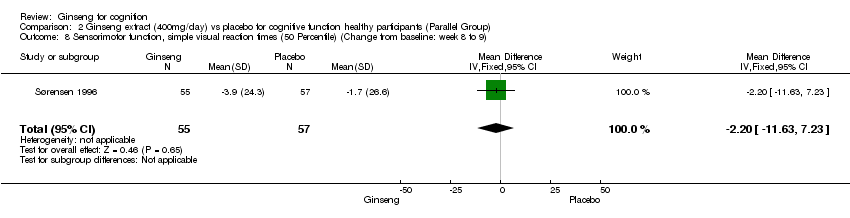 Comparison 2 Ginseng extract (400mg/day) vs placebo for cognitive function healthy participants (Parallel Group), Outcome 8 Sensorimotor function, simple visual reaction times (50 Percentile) (Change from baseline: week 8 to 9). | ||||
| 9 Pure motor function, finger‐tapping (maximum) (Change from baseline: week 8 to 9) Show forest plot | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐1.6 [‐3.67, 0.47] |
| Analysis 2.9 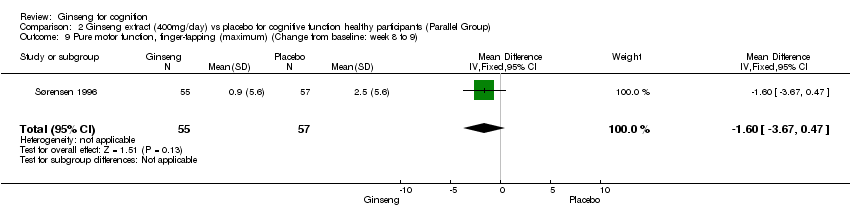 Comparison 2 Ginseng extract (400mg/day) vs placebo for cognitive function healthy participants (Parallel Group), Outcome 9 Pure motor function, finger‐tapping (maximum) (Change from baseline: week 8 to 9). | ||||
| 10 Pure motor function, finger‐tapping (50 Percentile) (Change from baseline: week 8 to 9) Show forest plot | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐5.07, 0.07] |
| Analysis 2.10  Comparison 2 Ginseng extract (400mg/day) vs placebo for cognitive function healthy participants (Parallel Group), Outcome 10 Pure motor function, finger‐tapping (50 Percentile) (Change from baseline: week 8 to 9). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Wechsler Memory Scale‐III (WMS‐III), Logical memory I (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.62, 1.02] |
| Analysis 3.1  Comparison 3 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 1 Wechsler Memory Scale‐III (WMS‐III), Logical memory I (Change from baseline: week 8). | ||||
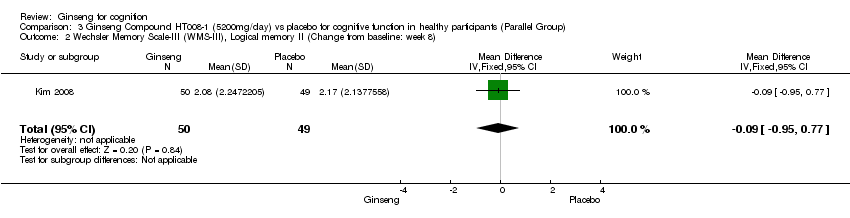
| 2 Wechsler Memory Scale‐III (WMS‐III), Logical memory II (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.95, 0.77] |
| Analysis 3.2 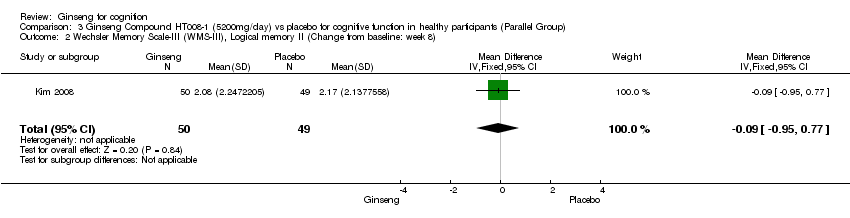 Comparison 3 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 2 Wechsler Memory Scale‐III (WMS‐III), Logical memory II (Change from baseline: week 8). | ||||
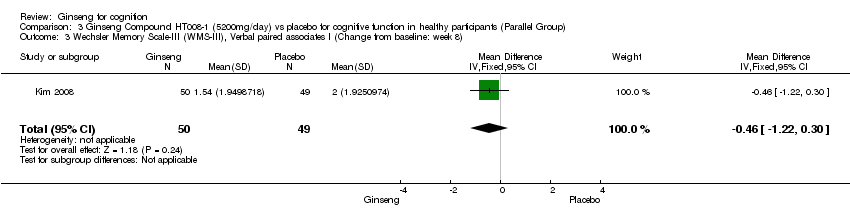
| 3 Wechsler Memory Scale‐III (WMS‐III), Verbal paired associates I (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐1.22, 0.30] |
| Analysis 3.3 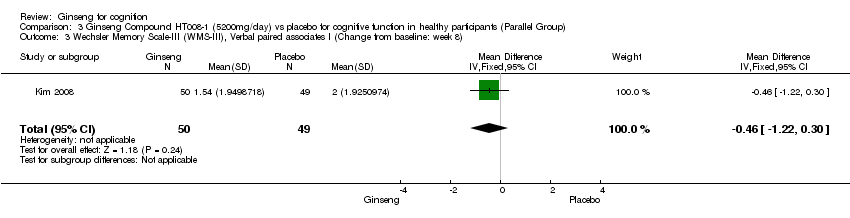 Comparison 3 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 3 Wechsler Memory Scale‐III (WMS‐III), Verbal paired associates I (Change from baseline: week 8). | ||||
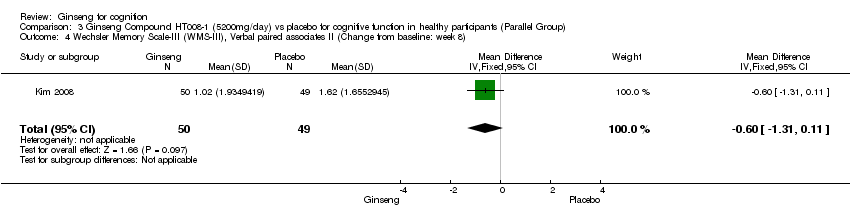
| 4 Wechsler Memory Scale‐III (WMS‐III), Verbal paired associates II (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.31, 0.11] |
| Analysis 3.4 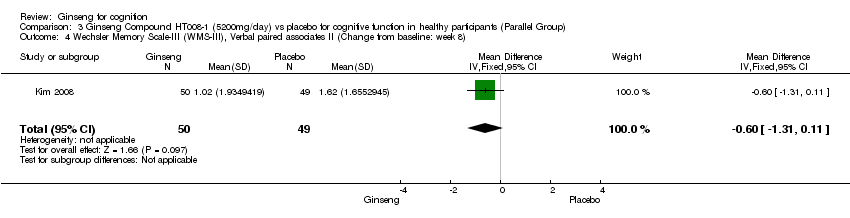 Comparison 3 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 4 Wechsler Memory Scale‐III (WMS‐III), Verbal paired associates II (Change from baseline: week 8). | ||||
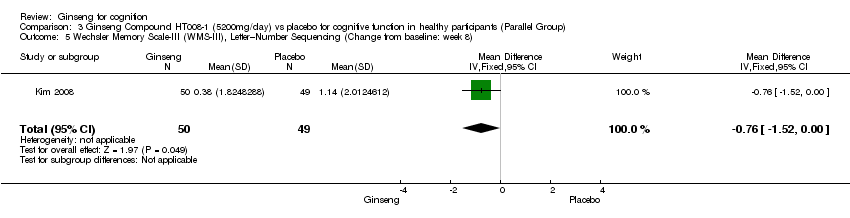
| 5 Wechsler Memory Scale‐III (WMS‐III), Letter–Number Sequencing (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐0.76 [‐1.52, ‐0.00] |
| Analysis 3.5 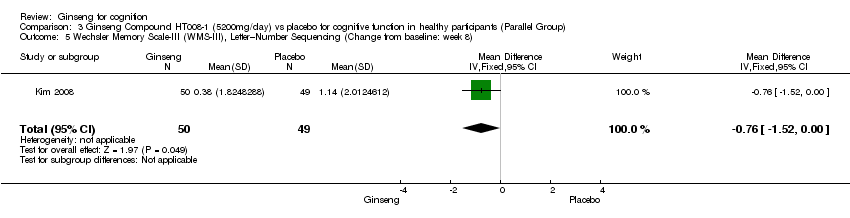 Comparison 3 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 5 Wechsler Memory Scale‐III (WMS‐III), Letter–Number Sequencing (Change from baseline: week 8). | ||||
| 6 Wechsler Memory Scale‐III (WMS‐III), Spatial span (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐1.16, 0.70] |
| Analysis 3.6  Comparison 3 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 6 Wechsler Memory Scale‐III (WMS‐III), Spatial span (Change from baseline: week 8). | ||||
| 7 Wechsler Memory Scale‐III (WMS‐III), Auditory recognition delayed (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 0.38 [‐0.43, 1.19] |
| Analysis 3.7  Comparison 3 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 7 Wechsler Memory Scale‐III (WMS‐III), Auditory recognition delayed (Change from baseline: week 8). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Working Memory, Speed of carrying out 3‐Back task (Change from baseline: week 4) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐0.06 [‐0.10, ‐0.02] | |
| Analysis 4.1 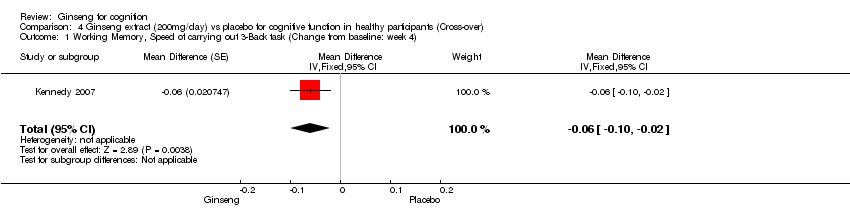 Comparison 4 Ginseng extract (200mg/day) vs placebo for cognitive function in healthy participants (Cross‐over), Outcome 1 Working Memory, Speed of carrying out 3‐Back task (Change from baseline: week 4). | ||||
| 2 Working Memory, Speed of carrying out 3‐Back task (Change from baseline: week 8) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐0.06 [‐0.11, ‐0.01] | |
| Analysis 4.2 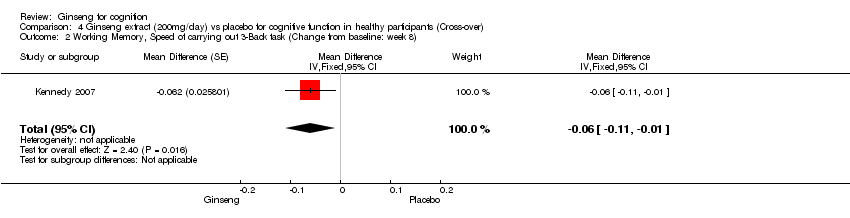 Comparison 4 Ginseng extract (200mg/day) vs placebo for cognitive function in healthy participants (Cross‐over), Outcome 2 Working Memory, Speed of carrying out 3‐Back task (Change from baseline: week 8). | ||||
| 3 Working Memory, Corsi Block Digit span (Change from baseline: week 4) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | 0.28 [‐0.02, 0.57] | |
| Analysis 4.3  Comparison 4 Ginseng extract (200mg/day) vs placebo for cognitive function in healthy participants (Cross‐over), Outcome 3 Working Memory, Corsi Block Digit span (Change from baseline: week 4). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Speed of attention (Change from baseline: day 2) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐25.61 [‐46.79, ‐4.43] | |
| Analysis 5.1  Comparison 5 Ginseng extract (400mg/day) vs placebo for cognitive function in healthy participants (Cross‐over), Outcome 1 Speed of attention (Change from baseline: day 2). | ||||
| 2 Continuity of attention (Change from baseline: day 2) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐0.32 [‐1.65, 1.01] | |
| Analysis 5.2  Comparison 5 Ginseng extract (400mg/day) vs placebo for cognitive function in healthy participants (Cross‐over), Outcome 2 Continuity of attention (Change from baseline: day 2). | ||||
| 3 Quality of memory (Change from baseline: day 2) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐2.28 [‐28.57, 24.01] | |
| Analysis 5.3 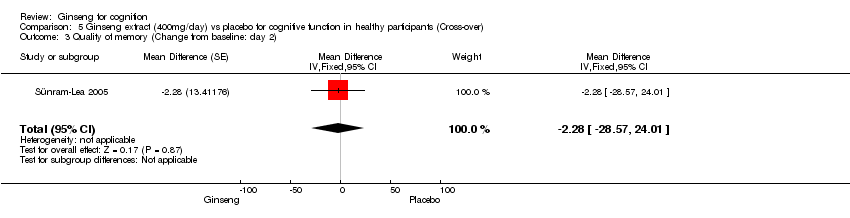 Comparison 5 Ginseng extract (400mg/day) vs placebo for cognitive function in healthy participants (Cross‐over), Outcome 3 Quality of memory (Change from baseline: day 2). | ||||
| 4 Speed of memory (Change from baseline: day 2) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | 28.17 [‐82.25, 138.59] | |
| Analysis 5.4 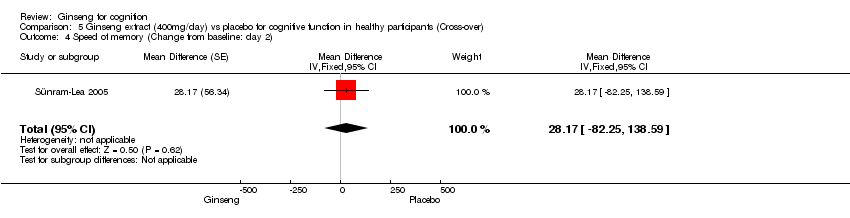 Comparison 5 Ginseng extract (400mg/day) vs placebo for cognitive function in healthy participants (Cross‐over), Outcome 4 Speed of memory (Change from baseline: day 2). | ||||
| 5 Working memory (Change from baseline: day 2) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | 0.05 [‐0.02, 0.12] | |
| Analysis 5.5 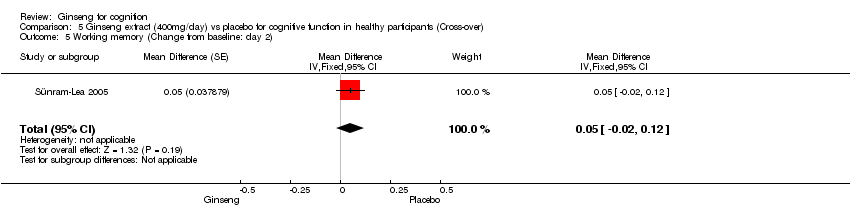 Comparison 5 Ginseng extract (400mg/day) vs placebo for cognitive function in healthy participants (Cross‐over), Outcome 5 Working memory (Change from baseline: day 2). | ||||
| 6 Secondary memory (Change from baseline: day 2) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐2.33 [‐27.70, 23.04] | |
| Analysis 5.6  Comparison 5 Ginseng extract (400mg/day) vs placebo for cognitive function in healthy participants (Cross‐over), Outcome 6 Secondary memory (Change from baseline: day 2). | ||||
| 7 CDR battery, Choice Reaction Time (Change from baseline: day 2) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐22.09 [‐36.92, ‐7.26] | |
| Analysis 5.7 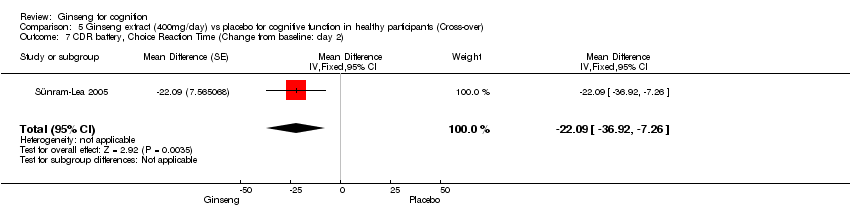 Comparison 5 Ginseng extract (400mg/day) vs placebo for cognitive function in healthy participants (Cross‐over), Outcome 7 CDR battery, Choice Reaction Time (Change from baseline: day 2). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mood, Calmness ( Bond‐Lader Visual Analogue Scales) (Change from baseline: week 4) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐9.31 [‐14.29, ‐4.34] | |
| Analysis 6.1 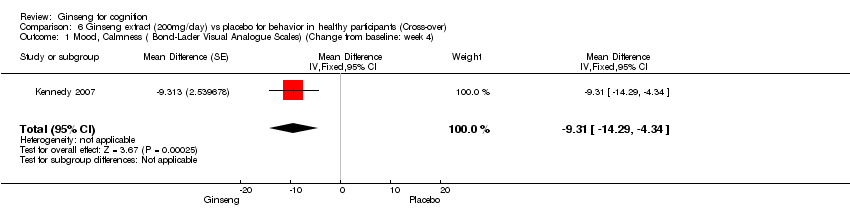 Comparison 6 Ginseng extract (200mg/day) vs placebo for behavior in healthy participants (Cross‐over), Outcome 1 Mood, Calmness ( Bond‐Lader Visual Analogue Scales) (Change from baseline: week 4). | ||||
| 2 Mood, Calmness ( Bond‐Lader Visual Analogue Scales) (Change from baseline: week 8) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐5.91 [‐7.96, ‐3.85] | |
| Analysis 6.2  Comparison 6 Ginseng extract (200mg/day) vs placebo for behavior in healthy participants (Cross‐over), Outcome 2 Mood, Calmness ( Bond‐Lader Visual Analogue Scales) (Change from baseline: week 8). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mood, Alertness ( Bond‐Lader Visual Analogue Scales) (Change from baseline: day 2) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | 1.2 [‐4.68, 7.08] | |
| Analysis 7.1  Comparison 7 Ginseng extract (400mg/day) vs placebo for behavior in healthy participants (Cross‐over), Outcome 1 Mood, Alertness ( Bond‐Lader Visual Analogue Scales) (Change from baseline: day 2). | ||||
| 2 Mood, Contentedness ( Bond‐Lader Visual Analogue Scales) (Change from baseline: day 2) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐0.63 [‐4.00, 4.74] | |
| Analysis 7.2  Comparison 7 Ginseng extract (400mg/day) vs placebo for behavior in healthy participants (Cross‐over), Outcome 2 Mood, Contentedness ( Bond‐Lader Visual Analogue Scales) (Change from baseline: day 2). | ||||
| 3 Mood, Calmness ( Bond‐Lader Visual Analogue Scales) (Change from baseline: day 2) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐1.88 [‐9.56, 5.80] | |
| Analysis 7.3 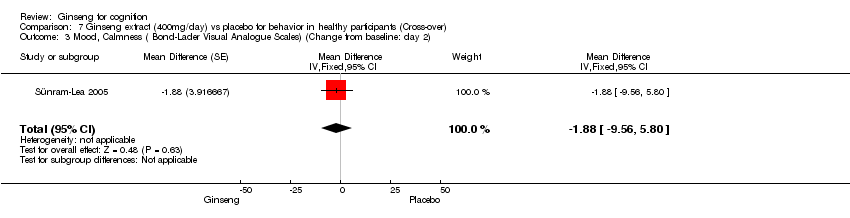 Comparison 7 Ginseng extract (400mg/day) vs placebo for behavior in healthy participants (Cross‐over), Outcome 3 Mood, Calmness ( Bond‐Lader Visual Analogue Scales) (Change from baseline: day 2). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 WHOQOL‐BREF, Overall quality of life (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [0.01, 0.35] |
| Analysis 8.1 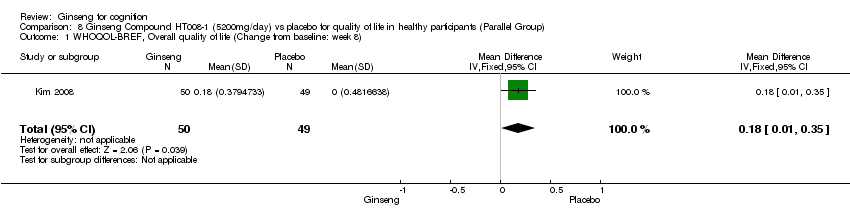 Comparison 8 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for quality of life in healthy participants (Parallel Group), Outcome 1 WHOQOL‐BREF, Overall quality of life (Change from baseline: week 8). | ||||
| 2 WHOQOL‐BREF, General health (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 0.34 [0.16, 0.52] |
| Analysis 8.2 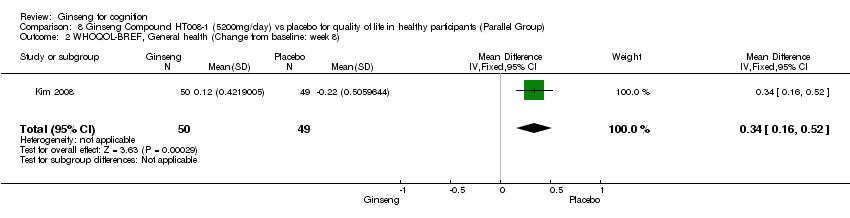 Comparison 8 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for quality of life in healthy participants (Parallel Group), Outcome 2 WHOQOL‐BREF, General health (Change from baseline: week 8). | ||||
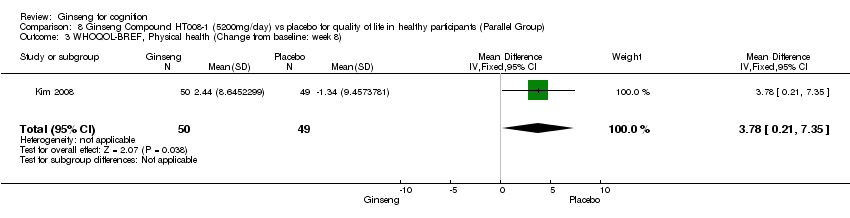
| 3 WHOQOL‐BREF, Physical health (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 3.78 [0.21, 7.35] |
| Analysis 8.3  Comparison 8 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for quality of life in healthy participants (Parallel Group), Outcome 3 WHOQOL‐BREF, Physical health (Change from baseline: week 8). | ||||
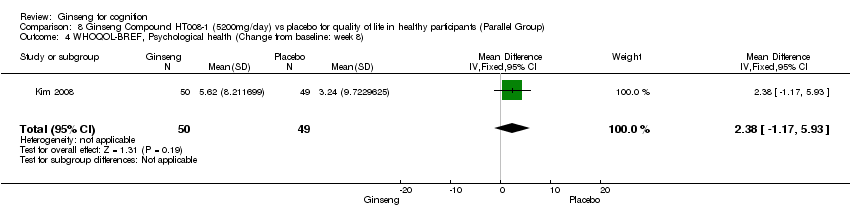
| 4 WHOQOL‐BREF, Psychological health (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 2.38 [‐1.17, 5.93] |
| Analysis 8.4  Comparison 8 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for quality of life in healthy participants (Parallel Group), Outcome 4 WHOQOL‐BREF, Psychological health (Change from baseline: week 8). | ||||
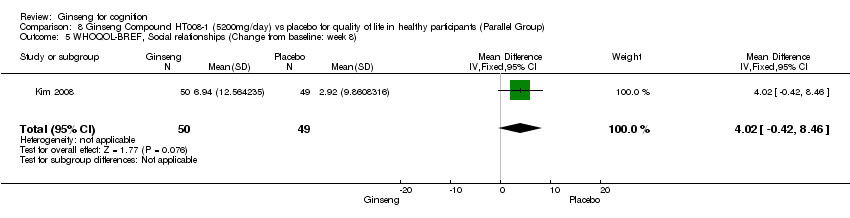
| 5 WHOQOL‐BREF, Social relationships (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 4.02 [‐0.42, 8.46] |
| Analysis 8.5  Comparison 8 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for quality of life in healthy participants (Parallel Group), Outcome 5 WHOQOL‐BREF, Social relationships (Change from baseline: week 8). | ||||
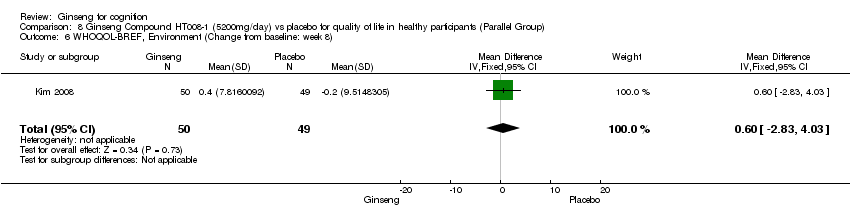
| 6 WHOQOL‐BREF, Environment (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐2.83, 4.03] |
| Analysis 8.6 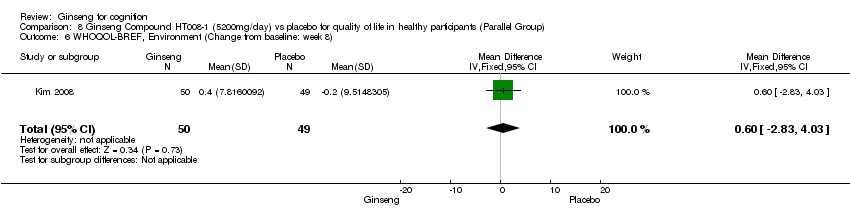 Comparison 8 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for quality of life in healthy participants (Parallel Group), Outcome 6 WHOQOL‐BREF, Environment (Change from baseline: week 8). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 WHOQOL‐BREF, Social relationships (Change from baseline: week 8) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | 1.33 [0.43, 2.24] | |
| Analysis 9.1  Comparison 9 Ginseng extract (200mg/day) vs placebo for quality of life in healthy participants (Cross‐over), Outcome 1 WHOQOL‐BREF, Social relationships (Change from baseline: week 8). | ||||

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Ginseng extract (200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 1 Attention and concentration, cancellation (No. of digits/2 min) (Change from baseline: week 12).

Comparison 1 Ginseng extract (200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 2 Speed of processing, Mental arithmetic (correct responses/s) (Change from baseline: week 12).
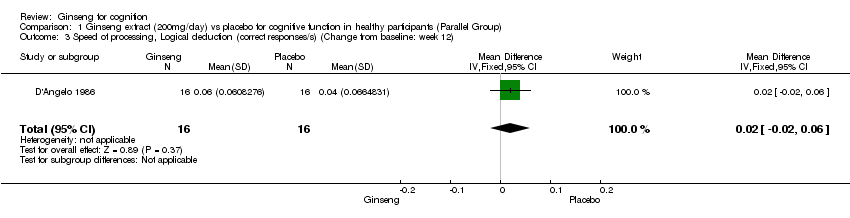
Comparison 1 Ginseng extract (200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 3 Speed of processing, Logical deduction (correct responses/s) (Change from baseline: week 12).
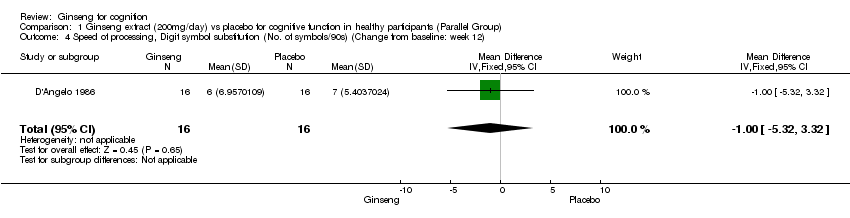
Comparison 1 Ginseng extract (200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 4 Speed of processing, Digit symbol substitution (No. of symbols/90s) (Change from baseline: week 12).
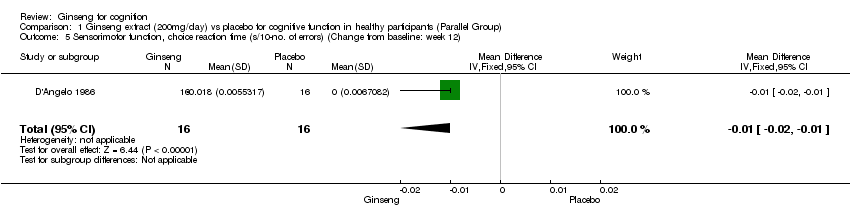
Comparison 1 Ginseng extract (200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 5 Sensorimotor function, choice reaction time (s/10‐no. of errors) (Change from baseline: week 12).
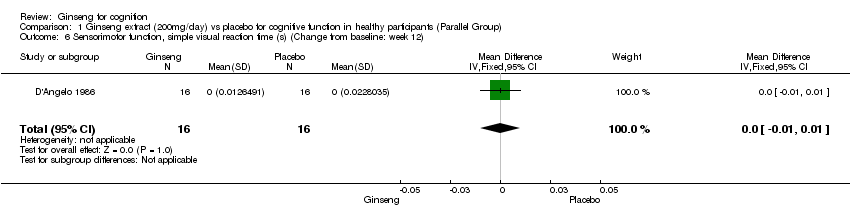
Comparison 1 Ginseng extract (200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 6 Sensorimotor function, simple visual reaction time (s) (Change from baseline: week 12).

Comparison 1 Ginseng extract (200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 7 Sensorimotor function, simple auditory reaction time (s) (Change from baseline: week 12).
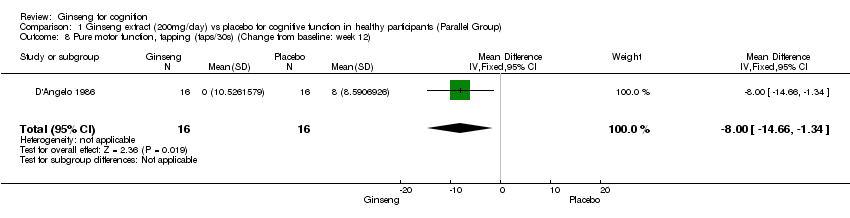
Comparison 1 Ginseng extract (200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 8 Pure motor function, tapping (taps/30s) (Change from baseline: week 12).
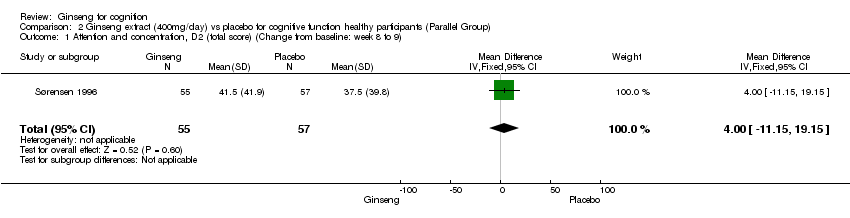
Comparison 2 Ginseng extract (400mg/day) vs placebo for cognitive function healthy participants (Parallel Group), Outcome 1 Attention and concentration, D2 (total score) (Change from baseline: week 8 to 9).

Comparison 2 Ginseng extract (400mg/day) vs placebo for cognitive function healthy participants (Parallel Group), Outcome 2 Learning and Memory, selective reminding (error index) (Change from baseline: week 8 to 9).

Comparison 2 Ginseng extract (400mg/day) vs placebo for cognitive function healthy participants (Parallel Group), Outcome 3 Learning and Memory, logical memory and reproduction (units lost) (Change from baseline: week 8 to 9).
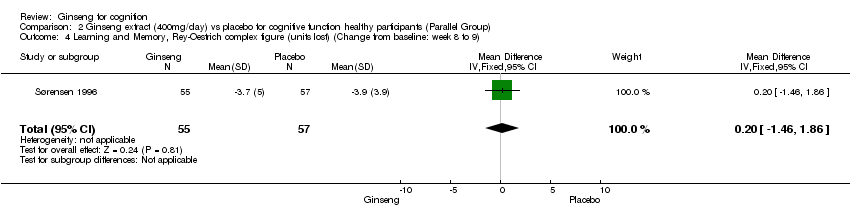
Comparison 2 Ginseng extract (400mg/day) vs placebo for cognitive function healthy participants (Parallel Group), Outcome 4 Learning and Memory, Rey‐Oestrich complex figure (units lost) (Change from baseline: week 8 to 9).
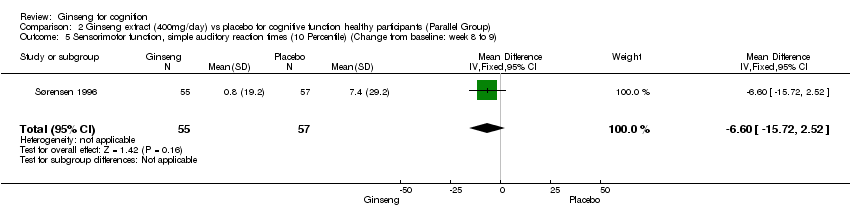
Comparison 2 Ginseng extract (400mg/day) vs placebo for cognitive function healthy participants (Parallel Group), Outcome 5 Sensorimotor function, simple auditory reaction times (10 Percentile) (Change from baseline: week 8 to 9).

Comparison 2 Ginseng extract (400mg/day) vs placebo for cognitive function healthy participants (Parallel Group), Outcome 6 Sensorimotor function simple auditory reaction times (50 Percentile) (Change from baseline: week 8 to 9).
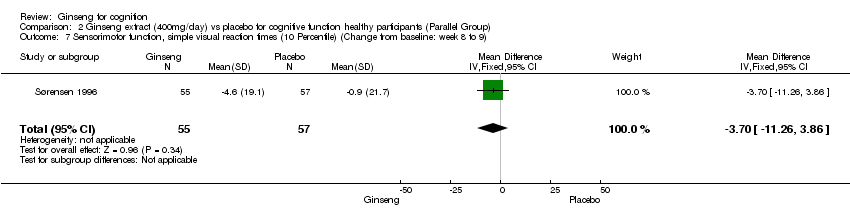
Comparison 2 Ginseng extract (400mg/day) vs placebo for cognitive function healthy participants (Parallel Group), Outcome 7 Sensorimotor function, simple visual reaction times (10 Percentile) (Change from baseline: week 8 to 9).
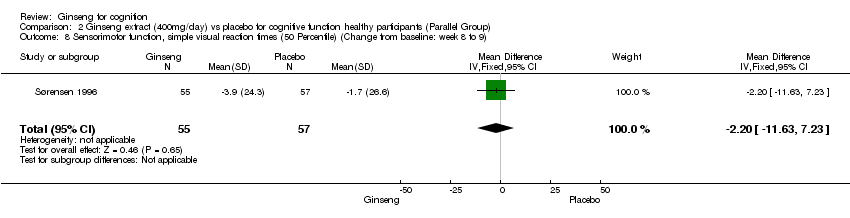
Comparison 2 Ginseng extract (400mg/day) vs placebo for cognitive function healthy participants (Parallel Group), Outcome 8 Sensorimotor function, simple visual reaction times (50 Percentile) (Change from baseline: week 8 to 9).
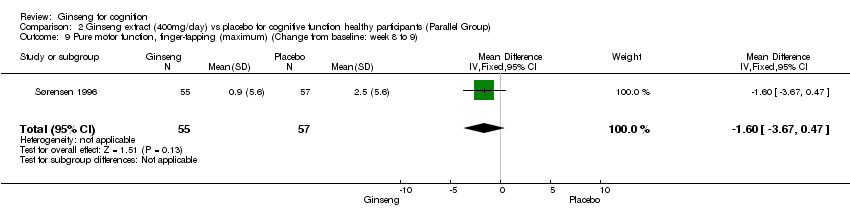
Comparison 2 Ginseng extract (400mg/day) vs placebo for cognitive function healthy participants (Parallel Group), Outcome 9 Pure motor function, finger‐tapping (maximum) (Change from baseline: week 8 to 9).
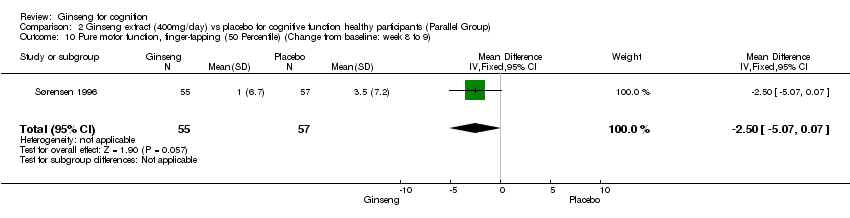
Comparison 2 Ginseng extract (400mg/day) vs placebo for cognitive function healthy participants (Parallel Group), Outcome 10 Pure motor function, finger‐tapping (50 Percentile) (Change from baseline: week 8 to 9).

Comparison 3 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 1 Wechsler Memory Scale‐III (WMS‐III), Logical memory I (Change from baseline: week 8).
Comparison 3 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 2 Wechsler Memory Scale‐III (WMS‐III), Logical memory II (Change from baseline: week 8).
Comparison 3 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 3 Wechsler Memory Scale‐III (WMS‐III), Verbal paired associates I (Change from baseline: week 8).
Comparison 3 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 4 Wechsler Memory Scale‐III (WMS‐III), Verbal paired associates II (Change from baseline: week 8).
Comparison 3 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 5 Wechsler Memory Scale‐III (WMS‐III), Letter–Number Sequencing (Change from baseline: week 8).
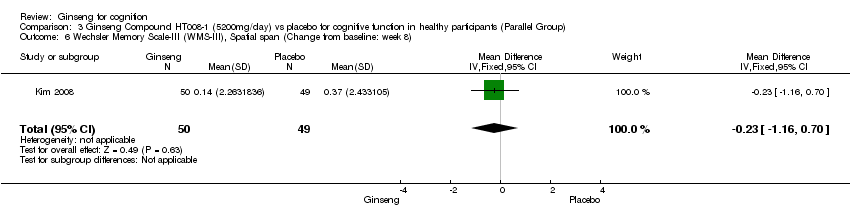
Comparison 3 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 6 Wechsler Memory Scale‐III (WMS‐III), Spatial span (Change from baseline: week 8).

Comparison 3 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for cognitive function in healthy participants (Parallel Group), Outcome 7 Wechsler Memory Scale‐III (WMS‐III), Auditory recognition delayed (Change from baseline: week 8).
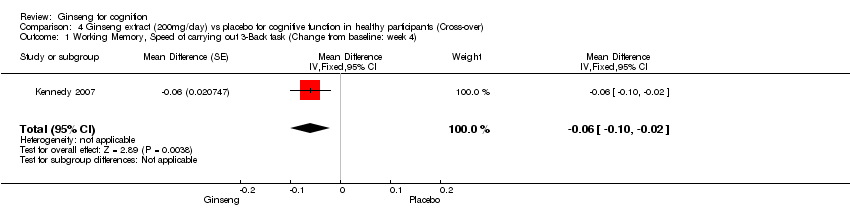
Comparison 4 Ginseng extract (200mg/day) vs placebo for cognitive function in healthy participants (Cross‐over), Outcome 1 Working Memory, Speed of carrying out 3‐Back task (Change from baseline: week 4).
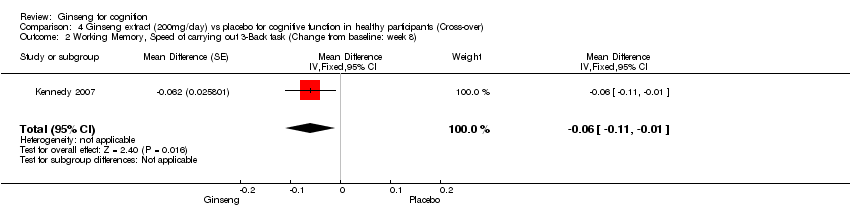
Comparison 4 Ginseng extract (200mg/day) vs placebo for cognitive function in healthy participants (Cross‐over), Outcome 2 Working Memory, Speed of carrying out 3‐Back task (Change from baseline: week 8).

Comparison 4 Ginseng extract (200mg/day) vs placebo for cognitive function in healthy participants (Cross‐over), Outcome 3 Working Memory, Corsi Block Digit span (Change from baseline: week 4).

Comparison 5 Ginseng extract (400mg/day) vs placebo for cognitive function in healthy participants (Cross‐over), Outcome 1 Speed of attention (Change from baseline: day 2).

Comparison 5 Ginseng extract (400mg/day) vs placebo for cognitive function in healthy participants (Cross‐over), Outcome 2 Continuity of attention (Change from baseline: day 2).

Comparison 5 Ginseng extract (400mg/day) vs placebo for cognitive function in healthy participants (Cross‐over), Outcome 3 Quality of memory (Change from baseline: day 2).
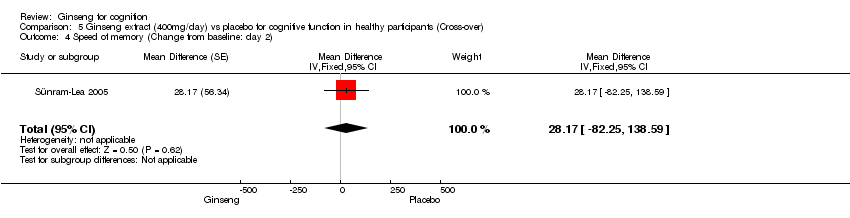
Comparison 5 Ginseng extract (400mg/day) vs placebo for cognitive function in healthy participants (Cross‐over), Outcome 4 Speed of memory (Change from baseline: day 2).

Comparison 5 Ginseng extract (400mg/day) vs placebo for cognitive function in healthy participants (Cross‐over), Outcome 5 Working memory (Change from baseline: day 2).

Comparison 5 Ginseng extract (400mg/day) vs placebo for cognitive function in healthy participants (Cross‐over), Outcome 6 Secondary memory (Change from baseline: day 2).
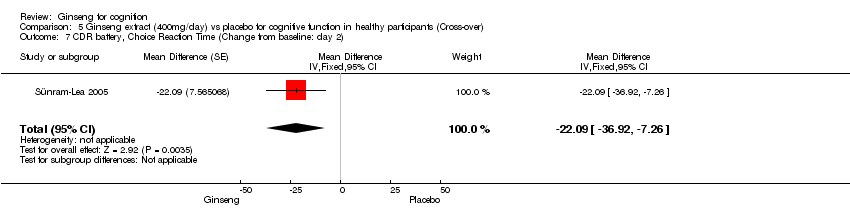
Comparison 5 Ginseng extract (400mg/day) vs placebo for cognitive function in healthy participants (Cross‐over), Outcome 7 CDR battery, Choice Reaction Time (Change from baseline: day 2).
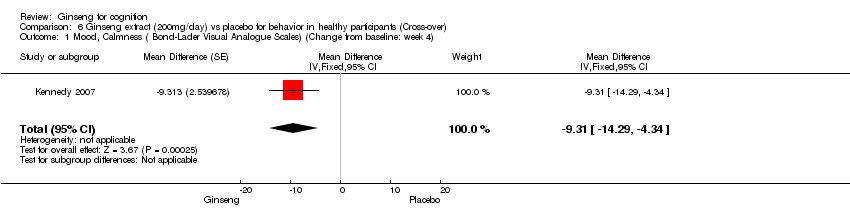
Comparison 6 Ginseng extract (200mg/day) vs placebo for behavior in healthy participants (Cross‐over), Outcome 1 Mood, Calmness ( Bond‐Lader Visual Analogue Scales) (Change from baseline: week 4).

Comparison 6 Ginseng extract (200mg/day) vs placebo for behavior in healthy participants (Cross‐over), Outcome 2 Mood, Calmness ( Bond‐Lader Visual Analogue Scales) (Change from baseline: week 8).

Comparison 7 Ginseng extract (400mg/day) vs placebo for behavior in healthy participants (Cross‐over), Outcome 1 Mood, Alertness ( Bond‐Lader Visual Analogue Scales) (Change from baseline: day 2).
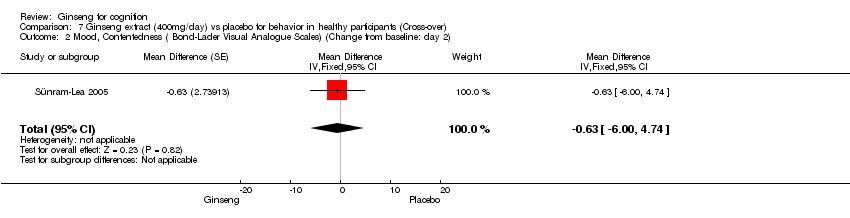
Comparison 7 Ginseng extract (400mg/day) vs placebo for behavior in healthy participants (Cross‐over), Outcome 2 Mood, Contentedness ( Bond‐Lader Visual Analogue Scales) (Change from baseline: day 2).

Comparison 7 Ginseng extract (400mg/day) vs placebo for behavior in healthy participants (Cross‐over), Outcome 3 Mood, Calmness ( Bond‐Lader Visual Analogue Scales) (Change from baseline: day 2).

Comparison 8 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for quality of life in healthy participants (Parallel Group), Outcome 1 WHOQOL‐BREF, Overall quality of life (Change from baseline: week 8).
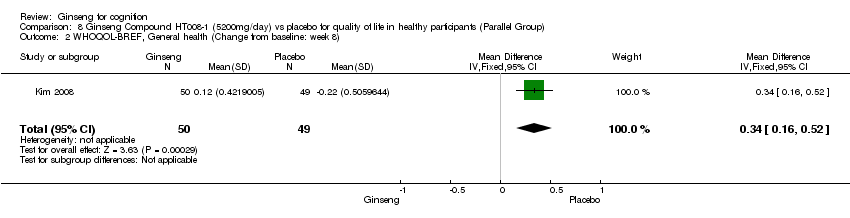
Comparison 8 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for quality of life in healthy participants (Parallel Group), Outcome 2 WHOQOL‐BREF, General health (Change from baseline: week 8).
Comparison 8 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for quality of life in healthy participants (Parallel Group), Outcome 3 WHOQOL‐BREF, Physical health (Change from baseline: week 8).
Comparison 8 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for quality of life in healthy participants (Parallel Group), Outcome 4 WHOQOL‐BREF, Psychological health (Change from baseline: week 8).
Comparison 8 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for quality of life in healthy participants (Parallel Group), Outcome 5 WHOQOL‐BREF, Social relationships (Change from baseline: week 8).
Comparison 8 Ginseng Compound HT008‐1 (5200mg/day) vs placebo for quality of life in healthy participants (Parallel Group), Outcome 6 WHOQOL‐BREF, Environment (Change from baseline: week 8).
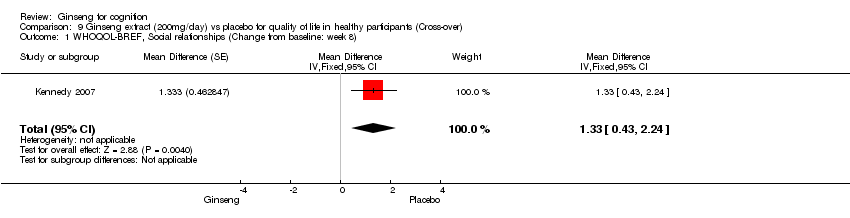
Comparison 9 Ginseng extract (200mg/day) vs placebo for quality of life in healthy participants (Cross‐over), Outcome 1 WHOQOL‐BREF, Social relationships (Change from baseline: week 8).
| Pinyin Name | English Name | Latin Name |
| Ren shen | Asian ginseng Asiatic ginseng Chinese ginseng Ginseng Korean ginseng Manchurian ginseng Oriental ginseng Red ginseng (steamed & dried peeled roots) White ginseng (sun‐dried roots) | Panax ginseng |
| Xi yang shen | American ginseng Ginseng (USA) Wild American ginseng Occidental ginseng | Panax quinquefolius |
| Da ye san qi Ri ben ren shen Zhu jie shen | Japanese ginseng | Panax japonicus |
| Xia ye zhu jie shen Xia ye jia ren shen | Narrow‐leaved Japanese ginseng | Panax japonicus |
| San qi | Notoginseng Sanchi ginseng (USA) San‐qi ginseng (USA) South China ginseng Tien‐qi ginseng Yunnan ginseng | Panax notoginseng |
| Jia ren shen | False ginseng Nepal ginseng Himalayan ginseng Pseudoginseng | Panax pseudoginseng |
| Xiu li jia ren shen | Elegant pseudoginseng Pearl ginseng | Panax pseudoginseng |
| Zhu zi shen | Pearl ginseng | Panax pseudoginseng |
| Bai san qi Ping bian san qi Tu san qi Ye san qi Zhu jie qi | Pingpien ginseng | Panax stipuleanatus |
| San ye ren shen | Dwarf ginseng Groundnut (USA) | Panax trifolius |
| Yue nan ren shen Ou mei san qi | Bamboo ginseng Vietnamese ginseng | Panax vietnamensis |
| Xia ye jia ren shen | Narrow‐leaved pseudoginseng | Panax wangianus |
| Jiang zhuang san qi | Ginger ginseng Ginger‐like pseudo‐ginseng | Panax zingiberensis |
| Reference: Multilingual multiscript plant name database http://www.plantnames.unimelb.edu.au/Sorting/Panax.html#bipinnatifidus | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Attention and concentration, cancellation (No. of digits/2 min) (Change from baseline: week 12) Show forest plot | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐1.76, 5.76] |
| 2 Speed of processing, Mental arithmetic (correct responses/s) (Change from baseline: week 12) Show forest plot | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [0.03, 0.21] |
| 3 Speed of processing, Logical deduction (correct responses/s) (Change from baseline: week 12) Show forest plot | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.02, 0.06] |
| 4 Speed of processing, Digit symbol substitution (No. of symbols/90s) (Change from baseline: week 12) Show forest plot | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐5.32, 3.32] |
| 5 Sensorimotor function, choice reaction time (s/10‐no. of errors) (Change from baseline: week 12) Show forest plot | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.02, ‐0.01] |
| 6 Sensorimotor function, simple visual reaction time (s) (Change from baseline: week 12) Show forest plot | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.01, 0.01] |
| 7 Sensorimotor function, simple auditory reaction time (s) (Change from baseline: week 12) Show forest plot | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.01, 0.01] |
| 8 Pure motor function, tapping (taps/30s) (Change from baseline: week 12) Show forest plot | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐8.0 [‐14.66, ‐1.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Attention and concentration, D2 (total score) (Change from baseline: week 8 to 9) Show forest plot | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [‐11.15, 19.15] |
| 2 Learning and Memory, selective reminding (error index) (Change from baseline: week 8 to 9) Show forest plot | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | 4.4 [0.82, 7.98] |
| 3 Learning and Memory, logical memory and reproduction (units lost) (Change from baseline: week 8 to 9) Show forest plot | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.74, 0.74] |
| 4 Learning and Memory, Rey‐Oestrich complex figure (units lost) (Change from baseline: week 8 to 9) Show forest plot | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐1.46, 1.86] |
| 5 Sensorimotor function, simple auditory reaction times (10 Percentile) (Change from baseline: week 8 to 9) Show forest plot | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐6.60 [‐15.72, 2.52] |
| 6 Sensorimotor function simple auditory reaction times (50 Percentile) (Change from baseline: week 8 to 9) Show forest plot | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐9.8 [‐22.69, 3.09] |
| 7 Sensorimotor function, simple visual reaction times (10 Percentile) (Change from baseline: week 8 to 9) Show forest plot | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐11.26, 3.86] |
| 8 Sensorimotor function, simple visual reaction times (50 Percentile) (Change from baseline: week 8 to 9) Show forest plot | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐2.2 [‐11.63, 7.23] |
| 9 Pure motor function, finger‐tapping (maximum) (Change from baseline: week 8 to 9) Show forest plot | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐1.6 [‐3.67, 0.47] |
| 10 Pure motor function, finger‐tapping (50 Percentile) (Change from baseline: week 8 to 9) Show forest plot | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐5.07, 0.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Wechsler Memory Scale‐III (WMS‐III), Logical memory I (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.62, 1.02] |
| 2 Wechsler Memory Scale‐III (WMS‐III), Logical memory II (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.95, 0.77] |
| 3 Wechsler Memory Scale‐III (WMS‐III), Verbal paired associates I (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐1.22, 0.30] |
| 4 Wechsler Memory Scale‐III (WMS‐III), Verbal paired associates II (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.31, 0.11] |
| 5 Wechsler Memory Scale‐III (WMS‐III), Letter–Number Sequencing (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐0.76 [‐1.52, ‐0.00] |
| 6 Wechsler Memory Scale‐III (WMS‐III), Spatial span (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐1.16, 0.70] |
| 7 Wechsler Memory Scale‐III (WMS‐III), Auditory recognition delayed (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 0.38 [‐0.43, 1.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Working Memory, Speed of carrying out 3‐Back task (Change from baseline: week 4) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐0.06 [‐0.10, ‐0.02] | |
| 2 Working Memory, Speed of carrying out 3‐Back task (Change from baseline: week 8) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐0.06 [‐0.11, ‐0.01] | |
| 3 Working Memory, Corsi Block Digit span (Change from baseline: week 4) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | 0.28 [‐0.02, 0.57] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Speed of attention (Change from baseline: day 2) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐25.61 [‐46.79, ‐4.43] | |
| 2 Continuity of attention (Change from baseline: day 2) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐0.32 [‐1.65, 1.01] | |
| 3 Quality of memory (Change from baseline: day 2) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐2.28 [‐28.57, 24.01] | |
| 4 Speed of memory (Change from baseline: day 2) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | 28.17 [‐82.25, 138.59] | |
| 5 Working memory (Change from baseline: day 2) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | 0.05 [‐0.02, 0.12] | |
| 6 Secondary memory (Change from baseline: day 2) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐2.33 [‐27.70, 23.04] | |
| 7 CDR battery, Choice Reaction Time (Change from baseline: day 2) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐22.09 [‐36.92, ‐7.26] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mood, Calmness ( Bond‐Lader Visual Analogue Scales) (Change from baseline: week 4) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐9.31 [‐14.29, ‐4.34] | |
| 2 Mood, Calmness ( Bond‐Lader Visual Analogue Scales) (Change from baseline: week 8) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐5.91 [‐7.96, ‐3.85] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mood, Alertness ( Bond‐Lader Visual Analogue Scales) (Change from baseline: day 2) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | 1.2 [‐4.68, 7.08] | |
| 2 Mood, Contentedness ( Bond‐Lader Visual Analogue Scales) (Change from baseline: day 2) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐0.63 [‐4.00, 4.74] | |
| 3 Mood, Calmness ( Bond‐Lader Visual Analogue Scales) (Change from baseline: day 2) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | ‐1.88 [‐9.56, 5.80] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 WHOQOL‐BREF, Overall quality of life (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [0.01, 0.35] |
| 2 WHOQOL‐BREF, General health (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 0.34 [0.16, 0.52] |
| 3 WHOQOL‐BREF, Physical health (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 3.78 [0.21, 7.35] |
| 4 WHOQOL‐BREF, Psychological health (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 2.38 [‐1.17, 5.93] |
| 5 WHOQOL‐BREF, Social relationships (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 4.02 [‐0.42, 8.46] |
| 6 WHOQOL‐BREF, Environment (Change from baseline: week 8) Show forest plot | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐2.83, 4.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 WHOQOL‐BREF, Social relationships (Change from baseline: week 8) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | 1.33 [0.43, 2.24] | |

