Intervenciones educativas, de apoyo y conductuales para mejorar el uso de aparatos de presión positiva continua de las vías respiratorias en adultos con apnea obstructiva del sueño
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised parallel‐group trial. All randomly assigned participants accounted for | |
| Participants | N = 12 Mean age: 65.5, AHI: 43.5, Desaturation: 77.05 ± 9.47 Inclusion criteria: > 55 years of age, RDI (AHI): > 10, Mini Mental Status Examination: > 25 Exclusion criteria: other ICSD, other treatment for apnoea, claustrophobia Participants had received prior treatment with CPAP | |
| Interventions | Intervention Two sessions. Session 1: review of participants' sleep data; symptoms; review of performance of cognitive tests; review of importance of treatment; review of PSG and CPAP; discussion of advantages and disadvantages of treatment; development of goals for therapy. Session 2: examination of compliance data for week one; discussion of noticeable changes with treatment; discussion of changes not apparent (hypertension/cardiac problems); troubleshooting discomfort; discussion of realistic aims of treatment; review of treatment goals Control Two sessions: general discussion of sleep architecture and opinions on sleep clinic Study duration: 12 weeks | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by 'urns', stratification by age, RDI, nadir O2 pretreatment, vigilance |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) | High risk | Not done for treatment group assignment 'None of the subjects were told that their CPAP machines were measuring their compliance via internal microprocessors' |
| Incomplete outcome data (attrition bias) | Unclear risk | Information not available |
| Methods | Randomised parallel‐group study | |
| Participants | N = 154 Intervention group: Age: 47.0, Male sex: 48%, AHI: 46.1, ESS: 12.6, BMI: 35 Control group: Age: 52, Male sex: 57%, AHI: 48.2, ESS: 11.9, BMI: 35.8 Inclusion criteria: new diagnosis of moderate to severe OSA by full in‐lab polysomnography, naive to CPAP Exclusion criteria: diagnosis by split night polysomnography, severe neurological or unstable psychiatric illness, congestive heart failure, end‐stage renal disease | |
| Interventions | Intervention Two 45‐minute face‐to‐face education sessions delivered by a trained nurse one and two weeks after initiation of PAP treatment. One additional booster phone call at week three Education comprised pathophysiology, medical and behavioural consequences of OSA and benefits of treatment Control Standard care consisting of physician discussing the benefits of treatment before and after diagnosis. Regular follow‐up visits with physicians, usually eight to 10 weeks after PAP initiation Study duration: 52 weeks | |
| Outcomes |
| |
| Notes | The study comprised three treatment arms. We consider the effects of the two treatment arms and the one control arms as separate studies. Interventions were initiated one week after initiation of CPAP Unpublished study. Currently under review for publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were urn randomly assigned in a 1:1 ratio |
| Allocation concealment (selection bias) | Unclear risk | No sufficient information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Participants, physicians and other healthcare providers were blinded to whether participants were enrolled in the study. Research staff who downloaded adherence data were blinded to group membership. Participants were informed that machine would be accessed periodically to determine 'how the device was working at night'. Given the nature of the intervention, it is unlikely that true blinding of participants was achieved. Furthermore, the same nurse delivered two different interventions in the study arms |
| Incomplete outcome data (attrition bias) | High risk | 27 of 80 participants in intervention group and 25 of 74 participants in control group dropped out from the study. Non‐completers were not included in outcome analysis |
| Methods | Randomised parallel‐group study | |
| Participants | N = 147 Intervention group: Age: 52, Male sex: 45%, AHI: 45.7, ESS: 11.6, BMI: 35 Control group: Age: 52, Male sex: 57%, AHI: 48.2, ESS: 11.9, BMI: 35.8 Inclusion criteria: new diagnosis of moderate to severe OSA by full in‐lab polysomnography, naive to CPAP Exclusion criteria: diagnosis by split night polysomnography, severe neurological or unstable psychiatric illness, congestive heart failure, end‐stage renal disease | |
| Interventions | Intervention Two 45‐minute face‐to‐face Motivational Enhancement Therapy (MET) sessions delivered by a trained nurse one and two weeks after initiation of PAP treatment. One additional booster phone call at week three. MET consisted of individually tailored counselling focused on addressing ambivalence regarding consistent use of PAP, participant‐specific information on OSA, symptom change, treatment expectations, goal development and refinement and enhancing participant's motivation Control Standard care involved the physician discussing the benefits of treatment before and after diagnosis. Regular follow‐up visits with physicians, usually eight to 10 weeks after PAP initiation Study duration: 52 weeks | |
| Outcomes |
| |
| Notes | The study comprised three treatment arms. We consider the effects of the two treatment arms and the one control arm as separate studies. Interventions were initiated one week after initiation of CPAP Unpublished study. Currently under review for publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were urn randomly assigned in a 1:1 ratio |
| Allocation concealment (selection bias) | Unclear risk | No sufficient information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Participants, physicians and other healthcare providers were blinded to whether participants were enrolled in the study. Research staff who downloaded adherence data were blinded to group membership. Participants were informed that machine would be accessed periodically to determine 'how the device was working at night'. Given the nature of the intervention, it is unlikely that blinding of participants was achieved. Furthermore, the same nurse delivered two different interventions in the study arms |
| Incomplete outcome data (attrition bias) | High risk | 26 of 73 participants in intervention group and 25 of 74 participants in control group dropped out from the study. Non‐completers were not included in outcome analysis |
| Methods | Randomised, parallel‐group study | |
| Participants | N = 133 Intervention group: Age: 53.7, Male sex: 82%, AHI 61, ESS: 10.3, BMI: 33.2 Control group: Age: 54, Male sex: 70%, AHI: 57.4, ESS: 12.4, BMI: 33 Inclusion criteria: newly diagnosed, moderate to severe OSA, CPAP naive Exclusion criteria: use of sedatives, drug abuse, cardiac co‐morbidities, COPD, other sleep disorders | |
| Interventions | Intervention 10‐Minute videotape on OSA, its consequences and CPAP therapy. In addition, routine information on diagnosis and treatment of OSA given by physician Control Standard information on OSA and CPAP therapy given by the same physician Study duration: 24 weeks | |
| Outcomes |
| |
| Notes | Unpublished information on study design and outcomes obtained from study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by a set of numbers prepared and randomly assigned by a clinician not involved in the study |
| Allocation concealment (selection bias) | Low risk | Randomisation by a third party |
| Blinding (performance bias and detection bias) | Unclear risk | The primary investigator and the statistician were blinded to the study group assignment. Participants were aware of machine usage monitoring. Given the nature of the intervention, it is unlikely that participant blinding was achieved |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed the study, and no data were missing |
| Methods | Randomised parallel‐group trial | |
| Participants | N = 40 Mean age: 51.7, Mean AHI: 49.4, ESS: 10.9 ± 5.1, Lowest 02, Sat: 75.6% ± 14.4, MSLT: 6 ± 3.9 Recruited from clinic | |
| Interventions | Intervention I Telephone call each week during trial (max trial time of two months) Intervention II Two printed documents Control No additional support Study duration: eight weeks | |
| Outcomes |
| |
| Notes | Two of 33 used Bi‐PAP. Both CPAP‐naive users and those who had been on CPAP before trial were studied. Reading done at enrolment and at between 1 to 2 months after enrolment Difference in AHI between active and control groups at baseline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) | High risk | Not done for treatment group assignment Participants' readout of CPAP machine usage data during telephone call to clinic |
| Incomplete outcome data (attrition bias) | High risk | Non‐completers excluded from analysis |
| Methods | Randomised parallel‐group study. Methods of randomisation not reported | |
| Participants | N = 30 Mean age: 46, BMI: 38, AHI: 40, Functional Outcomes of Sleep Questionnaire: TLC: 15.3, Control: 13.8 Inclusion criteria: participants starting nasal CPAP therapy; > 18 years; English‐speaking; > 15 episodes of apnoea or hypopnoea/h Exclusion criteria: not described | |
| Interventions | Telephone‐linked communications technology (TLC) versus usual care. TLC consisted of a computerised digitised human speech programme. TLC asks questions designed to elicit information from participant regarding adherence, education and reinforcement Study duration: eight weeks | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; other information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) | High risk | Participants aware of treatment group assignment Intervention involved communication regarding participant's CPAP machine usage |
| Incomplete outcome data (attrition bias) | Low risk | All completed |
| Methods | Randomised, parallel‐group study | |
| Participants | N = 50 No baseline characteristics were reported. Participants recruited after diagnosis of OSA confirmed with polysomnography and before initiation with CPAP treatment. No information on withdrawals were reported Inclusion criteria: not specified | |
| Interventions | Education course aim at desensitisation versus standard physician follow‐up Study duration: 24 weeks | |
| Outcomes |
| |
| Notes | Unpublished abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) | High risk | No blinding undertaken Not enough information available to ascertain awareness of CPAP machine usage |
| Incomplete outcome data (attrition bias) | High risk | Non‐completers excluded |
| Methods | Randomised parallel‐group study | |
| Participants | N = 75 Mean age: 53.5, Mean AHI: 41.6, ESS: Control group: 9.7, Intervention group: 9.9 Inclusion criteria: adult (≥ 19 years), moderate to severe OSA (AHI ≥ 15) Exclusion criteria: active cardiopulmonary or psychiatric disease, previously treated for OSA, no access to telephone line in bedroom, not able to return for follow‐up | |
| Interventions | Intervention Physiological data (PAP adherence, applied PAP, mask leak, residual respiratory events) were downloaded using modem attached to the PAP device and sent across the telephone line each morning. Downloaded information was reviewed every weekday except holidays by the research coordinator, who contacted the participant if poor compliance or other problems with treatment (e.g. mask leak) were detected. Participants were advised over the phone or visited the PAP coordinator. Standard care identical to control group Control 20‐Minute orientation to PAP session and mask fitting. Participants contacted after two days to check adherence and to troubleshoot problems, followed up at four to six weeks and at three months; each time, physiological data downloaded from machines and any problems with treatment addressed. In addition, data downloaded at eight weeks Study duration: 12 weeks | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | '...sequential numbered envelopes' |
| Allocation concealment (selection bias) | Unclear risk | envelopes were prepared by one of the study investigators |
| Blinding (performance bias and detection bias) | High risk | No blinding undertaken Intervention involved communication regarding participant's CPAP machine usage |
| Incomplete outcome data (attrition bias) | Unclear risk | 'intention to treat approach', high discontinuation rate (control group: 10/36, telemedicine group: 11/39) |
| Methods | Randomised, parallel study. Method of randomisation not reported. ITT | |
| Participants | N = 80 78:2 (M:F), Mean age: 51, Mean AHI: 58, ESS: 13 Inclusion criteria: AHI ≥ 15, plus daytime sleepiness or two other major symptoms of the syndrome; resident within 50 miles of Edinburgh Exclusion criteria: prior use of CPAP; coexisting COPD, asthma or neurological problems | |
| Interventions | Intervention Full explanation of need for and benefits of CPAP by sleep physician, 20‐minute video education programme, given mask to try for 20 minutes, titration of CPAP pressure overnight with following day discharge, nurses telephoned on days two and 21, reviewed in hospital at one, three and six months. Initial education at home with partner, two extra nights in hospital, sleep nurses' home visits to participant and partner at seven, 14 and 28 days and four months after starting CPAP Control Full explanation of need for and benefits of CPAP by sleep physician, 20‐minute video education programme, given mask to try for 20 minutes, titration of CPAP pressure overnight with following day discharge, nurses telephoned on days two and 21, reviewed in hospital at one, three and six months Duration: 24 weeks | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Each participant was randomly assigned with predetermined |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) | Unclear risk | Single‐blind: 'Patients were blinded to the group to which they were allocated' Not enough information available to ascertain awareness of CPAP machine usage |
| Incomplete outcome data (attrition bias) | Unclear risk | 'Data were analysed on an intention‐to‐treat basis' |
| Methods | Randomised, parallel‐group study | |
| Participants | N = 108 Mean age: 45, Mean AHI: 48, All participants newly diagnosed with OSA Inclusion criteria: diagnosis of OSA (AHI > 10 and subjective daytime sleepiness) | |
| Interventions | Intervention 10‐Minute CPAP education programme by respiratory nurse, brochure on OSA and CPAP treatment in Chinese, short trial CPAP therapy with comfortable mask for 30 minutes, CPAP titration on second night of study by AutoSet, nursing support following day, follow‐up by nursing staff and physician at 1 and 3 months. Locally produced 15‐minute videotape, additional nurse led 15‐minute educational session, review by physicians at weeks one and two, respiratory nurse telephone call on days one and two, weeks one, two, four, eight and 12 Control 10‐Minute CPAP education programme by respiratory nurse, brochure on OSA and CPAP treatment in Chinese, short trial CPAP therapy with comfortable mask for 30 minutes, CPAP titration on second night of study by AutoSet, nursing support following day, follow‐up by nursing staff and physician at 1 and 3 months. Study duration: 12 weeks | |
| Outcomes |
| |
| Notes | 91 participants had to purchase or rent their machines. 17 participants (10 in AS group and seven in BS group) qualified for state support | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; other information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) | Unclear risk | Not specified. Participants provided subjective CPAP machine usage data |
| Incomplete outcome data (attrition bias) | Unclear risk | Data were analysed on an intention‐to‐treat basis |
| Methods | Prospective, single‐blinded interventional study | |
| Participants | N = 72 M/F: 62/10, Mean age: 51.4, Mean AHI control group: 42, All participants newly diagnosed with OSA Inclusion criteria: diagnosis of OSA (based on home sleep study) and subjective daytime sleepiness | |
| Interventions | Intervention 20‐Minute educational video about SAHS. Telephone interview by research assistant between days two and five after CPAP issued to identify early problems and advise. Extra appointment to see sleep physician within seven to 14 days after being issued CPAP. Further appointment with sleep physician at one, six and 12 months Control Participants provided telephone number for support within office hours. Sleep physician reviewed participants at one, six and 12 months Study duration: 52 weeks | |
| Outcomes |
| |
| Notes | Only 20/36 participants in the intervention group watched the educational video tape Eight of the 17 defaulters returned machines at different times of the year and had negligible hours of use | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned using block tables |
| Allocation concealment (selection bias) | Low risk | 'The sequence of group assignment was indeed concealed from the investigators undergoing the screening and assessments, especially those recording/analysing machine hours' |
| Blinding (performance bias and detection bias) | Unclear risk | Single‐blinded: participant unaware of what 'intensive' or standard support comprised 'The CPAP clock‐timers were hidden with a plastic strip. Patients were not informed about the timers, and all covers were intact at each review; both patients and those recording |
| Incomplete outcome data (attrition bias) | High risk | Non‐completers not included in analysis of usage data |
| Methods | Randomised parallel‐group trial | |
| Participants | N = 57 Mean age: 58, Mean AHI: 58 Inclusion criteria: AHI > 30, no prior treatment for OSA | |
| Interventions | Intervention Reinforced education by the homecare team: home visit by technician at installation and further visits for explanation at one week, one month and two and three months of treatment for repetition of education and problem solving Reinforced education by prescriber: written material on CPAP use; explanation of OSA and CPAP with side effects; emphasis on importance of compliance with CPAP and detailed demonstration Control Standard education by the homecare network. Homecare visit to supply the CPAP machine, fit the mask and explain the technique of using the apparatus. CPAP mechanism and method of using the machine and mask were explained. Participant was encouraged to ask questions and could phone at any time to resolve problems Reinforced education by prescriber: written material on CPAP use; explanation of OSA and CPAP with side effects; emphasis on importance of compliance with CPAP and detailed demonstration Study duration: follow‐up to 52 weeks (intervention administered at outset of study). Data extracted at three months: 'During the remaining 9 months following the initial study design, there was no specific follow‐up protocol and patients benefited from the standard homecare surveillance recommended in the ANTADIR network, with a review every 3 months' | |
| Outcomes |
| |
| Notes | The study comprised four arms. We created four intervention/control comparisons and considered the effects of each as a separate study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; other information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) | High risk | Not done |
| Incomplete outcome data (attrition bias) | High risk | ‘One hundred thirty‐three patients were initially scheduled. However, complete initial data were obtained in only 112 patients who were definitively included in the study’ |
| Methods | Randomised parallel‐group trial | |
| Participants | N = 55 Mean age: 58, Mean AHI: 58 Inclusion criteria: AHI > 30, no prior treatment for OSA | |
| Interventions | Intervention Reinforced education by the homecare team: home visit by technician at installation and further visits for explanation at one week, one month and two and three months of treatment for repetition of education and problem solving Standard education by the prescriber Control Standard education by the homecare network. Homecare visit to supply the CPAP machine, fit the mask and explain the technique of using the apparatus. CPAP mechanism and method of using the machine and mask were explained. Participant was encouraged to ask questions and could phone at any time to resolve problems Standard education by the prescriber Study duration: follow‐up to 52 weeks (intervention administered at outset of study). Data extracted at three months: 'During the remaining 9 months following the initial study design, there was no specific follow‐up protocol and patients benefited from the standard homecare surveillance recommended in the ANTADIR network, with a review every 3 months' | |
| Outcomes |
| |
| Notes | The study comprised four arms. We created four intervention/control comparisons and considered the effects of each as a separate study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; other information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) | High risk | Not done |
| Incomplete outcome data (attrition bias) | High risk | ‘One hundred thirty‐three patients were initially scheduled. However, complete initial data were obtained in only 112 patients who were definitively included in the study’ |
| Methods | Randomised parallel‐group trial | |
| Participants | N = 55 Mean age: 58, Mean AHI: 58 Inclusion criteria: AHI > 30, no prior treatment for OSA | |
| Interventions | Intervention Reinforced education by the homecare team: home visit by technician at installation and further visits for explanation at one week, one month and two and three months of treatment for repetition of education and problem solving Reinforced education by prescriber: written material on CPAP use; explanation of OSA and CPAP with side effects; emphasis on importance of compliance with CPAP and detailed demonstration Control Reinforced education by the homecare team: home visit by technician at installation and further visits for explanation at one week, one month and two and three months of treatment for repetition of education and problem solving Standard education by the prescriber Study duration: follow‐up to 12 months (intervention administered at outset of study). Data extracted at three months: 'During the remaining 9 months following the initial study design, there was no specific follow‐up protocol and patients benefited from the standard homecare surveillance recommended in the ANTADIR network, with a review every 3 months' | |
| Outcomes |
| |
| Notes | The study comprised four arms. We created four intervention/control comparisons and considered the effects of each as a separate study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; other information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) | Unclear risk | Not done |
| Incomplete outcome data (attrition bias) | High risk | ‘One hundred thirty‐three patients were initially scheduled. However, complete initial data were obtained in only 112 patients who were definitively included in the study’ |
| Methods | Randomised parallel‐group trial | |
| Participants | N = 57 Mean age: 58, Mean AHI: 58 Inclusion criteria: AHI > 30, no prior treatment for OSA | |
| Interventions | Intervention Standard education by the homecare network. Homecare visit to supply the CPAP machine, fit the mask and explain the technique of using the apparatus. CPAP mechanism and method of using the machine and mask were explained. Participant was encouraged to ask questions and could phone at any time to resolve problems Reinforced education by prescriber: written material on CPAP use; explanation of OSA and CPAP with side effects; emphasis on importance of compliance with CPAP and detailed demonstration Control Standard education by the homecare network. Homecare visit to supply the CPAP machine, fit the mask and explain the technique of using the apparatus. CPAP mechanism and method of using the machine and mask were explained. Participant was encouraged to ask questions and could phone at any time to resolve problems Standard education by the prescriber Study duration: follow‐up to 12 months (intervention administered at outset of study). Data extracted at three months: 'During the remaining 9 months following the initial study design, there was no specific follow‐up protocol and patients benefited from the standard homecare surveillance recommended in the ANTADIR network, with a review every 3 months' | |
| Outcomes |
| |
| Notes | The study comprised four arms. We created four intervention/control comparisons and considered the effects of each as a separate study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; other information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) | High risk | Not done |
| Incomplete outcome data (attrition bias) | High risk | ‘One hundred thirty‐three patients were initially scheduled. However, complete initial data were obtained in only 112 patients who were definitively included in the study’ |
| Methods | Randomised parallel‐group study | |
| Participants | N = 100 Intervention group: Age: 55.1, Male: 58.5%, ESS: 10.8, RDI: 36.2 Control group: Age: 57.8, Male: 71.7%, ESS: 11.1, RDI: 32.4 Inclusion criteria: OSA confirmed by polysomnography, age ≥ 18, naive to CPAP Exclusion criteria: need for bi‐level ventilation, failed to complete CPAP titration, severe depression | |
| Interventions | Intervention Three sessions of CPAP‐specific Motivational Interview Nurse Therapy (MINT) one month apart. Each session lasted approximately 30 minutes. In addition, all participants received standard one‐on‐one 45‐minute education session conducted on the day of CPAP titration. Participants were followed up at two to four weeks by physician and at two months by a nurse. A questionnaire and a machine meter data on adherence were obtained at one, three and 12 months Control Standard one‐on‐one 45‐minute education session conducted on the day of CPAP titration. Participants were followed up at two to four weeks by physician and at two months by a nurse Study duration: 52 weeks | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned using envelopes with group allocation; no blocking or stratification used |
| Allocation concealment (selection bias) | Low risk | '...opaque, unlabelled envelopes...shuffled by a research assistant...placed into an allocation box held in a secured clinic area.' Administrative officers not otherwise involved in the study withdrew an envelope and booked the participant's future appointments accordingly |
| Blinding (performance bias and detection bias) | High risk | Participants and intervention nurses were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | 'The adherence analyses were by intent‐to‐treat...The multiple imputation method for substitution missing data was used...All univariate and bivariate statistical assumptions were met' |
| Methods | Randomised parallel‐group open‐label | |
| Participants | N = 39 All veterans Intervention group: N = 22, Age: 53 ± 14, Men: 100%, BMI: 35, AHI: 36.7 Control group: N = 17, Age: 50 ± 14, BMI: 33, Men: 100%, AHI: 37.5 Inclusion criteria: new diagnosis of OSA, AHI > five, full night or split night polysomnography, Age: 21 to 85, no sedative medications used Exclusion criteria: central or complex sleep apnoea, requirement of oxygen or Bi‐PAP, unstable medical co‐morbidities, irregular lifestyle pattern, excess alcohol use | |
| Interventions | Intervention Peer‐driven system (PBS); trained peers with OSA and good CPAP adherence record were paired with newly diagnosed participants over three months. During two face‐to‐face sessions and eight telephone‐based conversations, trained peers shared their experiences on coping strategies with CPAP, knowledge of perceived vulnerabilities of untreated OSA, motivated participants and promoted methods for improving efficacy of CPAP Control group Usual care: CPAP initiation and education class, participants were asked to send CPAP adherence 'smart cards' and were followed up at one and three months Study duration: 12 weeks | |
| Outcomes |
| |
| Notes | Additional information on study methods and mean CPAP adherence obtained from the study author These data were available from a pilot study. Larger trial is currently being undertaken | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was accomplished by computer‐generated assignment placed in sealed envelopes that were opened in a predetermined sequence of numbered and sealed envelopes |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding (performance bias and detection bias) | Unclear risk | Observers who evaluated outcomes and care providers were blinded to group allocation. Participants were not blinded to the intervention and were aware of CPAP adherence monitoring |
| Incomplete outcome data (attrition bias) | Unclear risk | Two of 17 participants in the control group lost to follow‐up versus zero in the intervention group No information on how this attrition was dealt with |
| Methods | Randomised, parallel‐group trial | |
| Participants | N = 100 M/F: 86/15, Mean age: 56, RDI: 26, ESS: 10.5 Inclusion criteria: newly diagnosed with OSA All participants referred for CPAP treatment. 109 screened and nine refused to participate | |
| Interventions | Intervention Cognitive‐behavioural therapy. Two one‐hour group sessions; slide presentation on sleep, OSA and treatment. CPAP machine on display and relaxation techniques in the event of anxiety caused by wearing CPAP mask Participants also benefited from video presentation with emphasis on perseverance with treatment and educational pamphlet made available Control Treatment as usual: one standardised group education session; explanation of CPAP titration process; familiarisation with equipment used and procedure to be followed on the titration night. Explanation of side effects, all participants strongly encouraged to contact staff to obtain relevant help and support. Participants assessed and fitted with comfortable mask to be worn during titration Study duration: CBT over course of one week before home treatment with CPAP. Assessment of CPAP made after four weeks | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | '...a sequence generated with a blocking factor of 4' |
| Allocation concealment (selection bias) | Low risk | 'An investigator not involved with recruitment or provision of treatment independently randomised participants using a sequence generated with a blocking factor of 4. Allocation concealment was achieved with sequentially numbered, opaque, sealed envelopes' |
| Blinding (performance bias and detection bias) | Unclear risk | Not possible/attempted for participants; assessors and technicians not informed of treatment groups 'Staff members were blinded to which group participants had been allocated and the 3 usual CPAP therapists strictly adhered to a script' Participants not informed that machine usage would be monitored |
| Incomplete outcome data (attrition bias) | Unclear risk | High attrition rate in control group (17/48 refused to take CPAP home) 'Analysis was by intention to treat, and we measured hours |
| Methods | Randomised parallel‐group study | |
| Participants | N = 30 Age: 46, Male sex: 30%, African Americans: 66.7%, AHI: 44, RDI: 56, ESS: 11.6, BMI: 42 Inclusion criteria: age 18 to 65, CPAP naive, reported intent to use CPAP; other sleep, psychiatric or health problems were not exclusion criteria | |
| Interventions | Intervention Written personalised feedback report, including detailed information on severity of the disease, self‐reported daytime sleepiness, individually estimated risk of adverse health outcome and risk of motor vehicle accident, all compared with normative data. Feedback addressed barriers to using CPAP, ambivalence about treatment and difficulties of behaviour change and promoted self‐efficacy and personal responsibility for choosing to use CPAP Control Written information from the American Academy of Sleep Medicine on OSA, Snoring and PAP therapy for OSA Study duration: three months | |
| Outcomes |
| |
| Notes | Participants were not provided machines but obtained them 'naturalistically', most commonly through insurance. Most participants were low‐income African Americans | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) | Unclear risk | 'Physicians were blind to study participation and participants were blind to their study condition.' Patients were aware that CPAP usage was monitored. Despite intended blinding, it is likely that participants would have been able to distinguish the two interventions |
| Incomplete outcome data (attrition bias) | Unclear risk | Only two incidents of missing data in each group. However, in addition, participants who took longer to obtain machines (n = 5 in control group and n = 2 in intervention group did not obtain devices by two weeks) were included from the start and had CPAP usage recorded as 0 hours per session. It is possible that financial burden prevented some participants from acquiring CPAP machines in a timely fashion |
| Methods | Randomised, parallel‐group trial. The study presented was a secondary analysis on a subset of participants from the parent study (N = 122) | |
| Participants | N = 51 (intervention: 32; placebo: 19) 26 M Inclusion criteria: identified as non‐adherent CPAP users from a parent study (N = 122); all used CPAP for < four hours per night | |
| Interventions | Intervention Internet‐based application aimed at encouraging problem solving and preparedness in application of CPAP Control Internet‐based application similar in format to intervention but directed activities in neutral health topics (vitamin intake) Study duration: 16 weeks | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule with gender as a stratification variable |
| Allocation concealment (selection bias) | Unclear risk | Information not provided |
| Blinding (performance bias and detection bias) | Unclear risk | Single‐blind: Participants in the control group were given different content delivered in similar way to intervention Outcome assessors not aware of assignment of treatment groups No information on whether participants were aware that CPAP usage was being monitored |
| Incomplete outcome data (attrition bias) | Low risk | Data for all participants presented |
| Methods | Randomised parallel‐group trial | |
| Participants | N = 19 Mean age: 63 Inclusion criteria: non‐adherent with CPAP for three months, after initial education on CPAP use and supplemental audiotaped/videotaped reinforcement at two and four weeks | |
| Interventions | Intervention Two‐way telehealth sessions mediated by video link‐up through phone line. Research nurse emphasised nightly, bedtime routine for CPAP. After standardised protocols, nurse visually assessed participant, guided correct CPAP routine and determined whether the CPAP mask fits properly. Nurse described consequences of non‐adherence and managing barriers to CPAP use. Benefits of nightly CPAP use for general health were emphasised Control Two‐way telehealth sessions mediated by video link‐up through phone line. Protocols drawn up to mimic content delivered to intervention group. Instead of CPAP‐related information, participants given content on vitamin intake Study duration: 12 weeks of scheduled telehealth sessions | |
| Outcomes |
| |
| Notes | Non‐adherence in the study defined as less than four hours of CPAP use per night for fewer than nine of 14 consecutive nights’ use TJL emailed for details of randomisation and outcome data 12/09/2008. Carol Smith responded 15/09/2008 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | '...randomised and done via computer software generated random assignment’ |
| Allocation concealment (selection bias) | Low risk | '...allocation sequence and treatment group assignment concealed from investigators conducting the screening and ongoing assessments' |
| Blinding (performance bias and detection bias) | Unclear risk | Single‐blind; nursing interventionist staff aware of different content delivered by video link‐up Machine usage was measured via smart card by blinded sleep lab personnel. Information on participants' awareness of CPAP machine usage was insufficient for us to determine how this might have affected the study |
| Incomplete outcome data (attrition bias) | Low risk | All participants finished follow‐up and contributed to data on adherence. Two satisfaction surveys were not submitted (one from each group) |
| Methods | Randomised parallel‐group trial | |
| Participants | N = 97 Mean age: 63.4, Male sex: 55%, Mean AHI: Intervention group: 52.3, Control group: 47.3 Inclusion criteria: new diagnosis of OSA, age ≥ 18, AHI ≥ 20 Exclusion criteria: positive screening for drug or alcohol abuse, depression requiring hospitalisation | |
| Interventions | All participants received usual education on OSA and demonstration of CPAP equipment Intervention Audiotaped music along with softly spoken directions on relaxation techniques and habit‐promoting instructions for using CPAP nightly. Participants received information packet, which included CPAP use reminder placard, handouts on benefits of CPAP adherence and health consequences of poor compliance, four‐week diary for recording experience with CPAP Control group Audiotaped music along with spoken information about vitamins. Information packet was the same in format and length as the intervention group, but content was on vitamins Study duration: 24 weeks | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomly assigned using computerised random assignment programme |
| Allocation concealment (selection bias) | Low risk | Participants recruited by 'nurses who had no knowledge of group assignment' |
| Blinding (performance bias and detection bias) | Unclear risk | Single‐blind; '...placebo intervention was used to mimic the daily activities in the experimental treatment...' CPAP usage was measured via smart cards by blinded personnel. Nurses administering experimental or placebo control interventions aware of different content of these interventions. Unclear whether participants were aware of machine usage monitoring. Personnel analysing data on compliance were blind to allocation of treatment |
| Incomplete outcome data (attrition bias) | Unclear risk | Intention‐to‐treat analysis but imbalanced N of dropouts: Intervention group: 11/55 (20%), Control group: 13/42 (31%) at six months. Unclear whether reasons for dropouts were balanced across groups |
| Methods | Randomised parallel‐group trial | |
| Participants | N = 250 Median age: 55.0 years, 82% Men, Median BMI: 35.1 Intervention group: AHI: 36, ESS: 10 Conrol group: AHI: 40.5, ESS: 11 Inclusion criteria: age 18 to 80 years, AHI > 10 Exclusion criteria: not reported | |
| Interventions | Intervention Automated telephone‐linked communication system adapted for CPAP (TLC‐CPAP), designed around the concepts of motivational interviewing. Digitised human speech was used, and participants were communicating with it via touch tone keypad of their telephones. The TLC‐CPAP content included assessment of the participant's experience with CPAP, self‐reported machine use, feedback and counselling to enhance adherence and side effect management. Participants were required to make weekly calls to TLC‐CPAP during the first month and monthly thereafter. Printed reports were sent to the participant's physician. Participants were encouraged to contact physician directly if any excessive symptoms or side effects of treatment encountered Control 'Attention placebo control' group received general education on a variety of health topics via a telephone‐linked communication (TLC) system. Participants were required to make calls on the same schedule as the intervention group Study duration: 52 weeks | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | '...randomisation stratified by sex, age and AHI using a randomised block design' |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding of participants attempted by developing an 'attention placebo control group'. However, given the nature of the intervention, participants may have been aware of group assignment. Participants in the intervention group self‐reported frequency and duration of CPAP usage. It is unclear whether participants in the control group were aware of CPAP usage monitoring '...all data were collected by research assistants blind to group assignment'. Unclear whether the same applied to outcome assessors |
| Incomplete outcome data (attrition bias) | Low risk | Data were analysed by intention to treat. Multiple‐imputation procedure was implemented to account for missing data in the outcome of CPAP use due to loss to follow‐up. 20/124 in the intervention group and 15/126 in the control group lost to follow‐up at 12 months |
| Methods | Randomised parallel‐group trial | |
| Participants | N = 45 (40 presented as baseline and completed) Mean age: 59, Male: 98%, AHI: 39, ESS: 12.6 Inclusion criteria: AHI ≥ 15, no prior CPAP treatment, stable sleep environment Exclusion criteria: allergies/sensitivity to mask or mask material, previous use of any other PAP device (e.g. bi‐level PAP, auto‐adjusting PAP), current use of prescribed supplemental oxygen or significant comorbid medical conditions that could interfere with daily use of CPAP | |
| Interventions | Intervention Review of compliance and efficacy data. Monitored information garnered as objective compliance data and subjective reports of usage. Follow‐up tailored to how CPAP used by participants. Details on how many total hours the PAP unit was used each night at therapeutic pressure. Efficacy data consisted of the amount of mask leakage (L/s) and the AHI (total number of apnoeas and hypopnoeas per hour of sleep) Control Telephone call from staff one week after CPAP initiation and office follow‐up visit at one month. Participants encouraged to call clinic any time with problems or concerns Study duration: eight weeks | |
| Outcomes |
| |
| Notes | TJL emailed for randomisation 12/09/2008. Carl Stepnowsky responded 15/09/2008 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | '...we used the uniform random number generator in R to select all sequences of 4 randomly with equal probability so that the occurrence of 3 in a row being assigned to the same group would be extremely rare' |
| Allocation concealment (selection bias) | Low risk | 'The randomisation scheme was concealed until the time at which the intervention was assigned. The randomisation scheme was generated by the project statistician and carried out by research staff immediately after the informed consent procedure and the completion of the baseline questionnaires' |
| Blinding (performance bias and detection bias) | Unclear risk | Participants in both groups received a monitoring unit All participants likely to be aware that CPAP usage was measured |
| Incomplete outcome data (attrition bias) | High risk | ‘There were five CPAP “rejectors,” or patients who decided within the first day or two that they did not want to pursue CPAP as the primary treatment for their OSA. Our study did not have a “run‐in” period, which could have helped identify these patients prior to the intervention’ |
| Methods | Randomised parallel‐group trial | |
| Participants | N = 133 Mean age: 45, ESS: 14, CPAP pressure: 8.9 All participants were service or ex‐service personnel in USA Inclusion criteria: 18 to 64 years; RDI ≥ five; English speaking Exclusion criteria: acute illness, hospitalised participants, significant nocturnal hypoxaemia, ESS < eight, disorder interfering with ability to use computer at home (i.e. blindness), major mental illness, physical disability that interfered with optimal use of computer, prior use of CPAP, undergoing concurrent therapy for OSA (MAD, surgery) | |
| Interventions | Intervention Education on first day; film on OSA and CPAP; instruction in use of CPAP; encouragement to attend sleep clinic. Computer programme (Health Buddy) delivering questions on a daily basis; responses were monitored by sleep medicine practitioners. If persistent high‐risk answers given, this prompted a sleep practitioner to contact the participant within 24 hours Control Education on first day; film on OSA and CPAP; instruction in use of CPAP; encouragement to attend sleep clinic. Follow‐up at one month with a scheduled clinic visit; telephone consultation Study duration: four weeks | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. Participants stratified according to age, gender and severity of symptoms |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) | High risk | Not undertaken 'Each day, the patient was greeted with three questions regarding reported hours of nasal CPAP use...' |
| Incomplete outcome data (attrition bias) | Unclear risk | Information not available |
| Methods | Randomised parallel‐group study | |
| Participants | N = 76 Intervention group: Male: 89.5%, AHI: 35.7, ESS: 13.4, Control group: Male: 81.6%, AHI: 38.5, ESS: 13.9 Incusion criteria: new diagnosis of OSA, AHI ≥ 10, above elementary school education, 'conscious mind and able to communicate clearly' Exclusion criteria: personal or family history of mental illness, drug or alcohol abuse, severe cognitive impairment, 'concurrent oncologic or psychiatric diseases' | |
| Interventions | Intervention Three nights of CPAP titration in the first week, four‐hour group education session on OSA and CPAP in the first week, participants were given a brochure describing benefits of CPAP and CD containing a 20‐minute video demonstrating how to optimise CPAP treatment, 24‐hour consultation telephone line to the sleep nurses was available Control One night of CPAP titration in the hospital in the first week Study duration: 12 weeks | |
| Outcomes |
| |
| Notes | The study comprised four treatment arms: three intervention groups and one control group. We consider the effects of the three intervention arms as separate studies | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'The patients were randomly assigned...by block randomisation' |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) | High risk | Not performed Information on participants' awareness of CPAP machine usage monitoring not available |
| Incomplete outcome data (attrition bias) | Unclear risk | 'The patients' CPAP adherence rates and dropout rates were analysed on an intention‐to‐treat basis' |
| Methods | Randomised parallel‐group study | |
| Participants | N = 76 Intervention group: Male: 76.3%, AHI: 41.2, ESS: 14.7 Control group: Male: 81.6%, AHI: 38.5, ESS: 13.9 Incusion criteria: new diagnosis of OSA, AHI ≥ 10, above elementary school education, 'conscious mind and able to communicate clearly' Exclusion criteria: personal or family history of mental illness, drug or alcohol abuse, severe cognitive impairment, 'concurrent oncologic or psychiatric diseases' | |
| Interventions | Intervention One night of CPAP titration in the hospital, 12 × 40 minute group Progressive Muscle Relaxation (PMR) practice sessions over 12 weeks, one per week. Self‐practice of PMR before each CPAP treatment. Brochure and CD with a guide for PMR practice at home Control One night of CPAP titration in the hospital in the first week Study duration: 12 weeks | |
| Outcomes |
| |
| Notes | The study comprised four treatment arms: three intervention groups and one control group. We consider the effects of the three intervention arms as separate studies | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'The patients were randomly assigned...by block randomisation' |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) | High risk | Not performed Information on participants' awareness of CPAP machine usage monitoring not available |
| Incomplete outcome data (attrition bias) | Unclear risk | 'The patients' CPAP adherence rates and dropout rates were analysed on an intention‐to‐treat basis' |
| Methods | Randomised parallel‐group study | |
| Participants | N = 76 Intervention group: Male: 81.6, AHI: 43.1, ESS: 14.5 Control group: Male: 89.5%, AHI‐35.7, ESS: 13.4 Incusion criteria: new diagnosis of OSA, AHI ≥ 10, above elementary school education, 'conscious mind and able to communicate clearly' Exclusion criteria: personal or family history of mental illness, drug or alcohol abuse, severe cognitive impairment, 'concurrent oncologic or psychiatric diseases' | |
| Interventions | Intervention Three nights of CPAP titration in the hospital. Combination of interventions as in Education and PMR group (see above) Control The control for this intervention was the intervention arm of Wang 2011a Study duration: 12 weeks | |
| Outcomes |
| |
| Notes | The study comprised four treatment arms: three intervention groups and one control group. We consider the effects of the three intervention arms as separate studies. In this study, for the combined intervention, Education + PMR, the control group was Education | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'The patients were randomly assigned...by block randomisation' |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) | High risk | Not performed Information on participants' awareness of CPAP machine usage monitoring not available |
| Incomplete outcome data (attrition bias) | Unclear risk | 'The patients' CPAP adherence rates and dropout rates were analysed on an intention‐to‐treat basis' |
| Methods | Randomised parallel‐group study | |
| Participants | N = 76 Intervention group: Male: 81.6, AHI: 43.1, ESS: 14.5 Control group: 76.3%, AHI: 41.2, ESS: 14.7 Inclusion criteria: new diagnosis of OSA, AHI ≥ 10, above elementary school education, 'conscious mind and able to communicate clearly' Exclusion criteria: personal or family history of mental illness, drug or alcohol abuse, severe cognitive impairment, 'concurrent oncologic or psychiatric diseases' | |
| Interventions | Intervention Three nights of CPAP titration in the hospital. Combination of interventions as in Education + PMR group (see above) Control The control for this intervention was the intervention arm of Wang 2011b Study duration: 12 weeks | |
| Outcomes |
| |
| Notes | The study comprised four treatment arms: three intervention groups and one control group. We consider the effects of the three intervention arms as separate studies. In this study, for the combined intervention, Education + PMR, the control group was PMR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'The patients were randomly assigned...by block randomisation' |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) | High risk | Not performed Information on participants' awareness of CPAP machine usage monitoring not available |
| Incomplete outcome data (attrition bias) | Unclear risk | 'The patients' CPAP adherence rates and dropout rates were analysed on an intention‐to‐treat basis' |
| Methods | Randomised, unblinded parallel‐group trial | |
| Participants | N = 93 Mean age: 48, BMI: 38, Mean duration of symptoms: 5.4 years, % smokers (treatment: 26%; control: 49%), Mean AHI: 9, ESS: 13 Inclusion criteria: > 20 years; RDI > four; newly diagnosed OSAHS Exclusion criteria: not reported | |
| Interventions | Intervention During initial visit, participants received explanations of OSA and CPAP by physician and respiratory therapist. Short instructional video (15‐minute tape of interview between two 'blue collar' workers discussing what CPAP felt like and how it helped them) Control During initial visit, participants received explanations of OSA and CPAP by physician and respiratory therapist. Control group members were interviewed Both groups received instruction at outset on using CPAP Study duration: four weeks | |
| Outcomes |
| |
| Notes | Not able to assess machine usage, as 13 of the 57 participants who returned for their one‐month clinic visit had | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; blocks of 10 to ensure balanced group design |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) | High risk | Not undertaken Information on participants' awareness of CPAP machine usage was insufficient for us to determine how this might have affected the study |
| Incomplete outcome data (attrition bias) | Unclear risk | Information not available |
AHI: Apnoea Hypopnoea Index; BiPAP: Bi‐level positive airway pressure; CPAP: Continuous positive airway pressure; ESS: Epworth Sleepiness Scale; FOSQ: Functional Outcomes of Sleep Questionnaire (quality of life instrument); OSA: Obstructive sleep apnoea; RDI: Respiratory Disturbance Index; SAQLI: Sleep Apnoea Quality of Life Index.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Allocation to the standard or intensive support group was based on proximity of participant's address to the sleep centre, and no randomisation occurred. Participants were randomly assigned only to APAP or CPAP treatment. This unpublished information was obtained from the study authors | |
| Cross‐over study | |
| Implemented intervention could not be classified as supportive, educational, psychological or behavioural | |
| Participants randomly assigned to CPAP treatment or no treatment | |
| CBT programme given before randomisation to CPAP | |
| Excluded for the same reasons as Damjanovic 2009 | |
| Based on description of the study, it is unlikely that randomisation took place. No contact details of study authors available; therefore not possible to obtain further clarification on the trial design | |
| Data for only 19 of 128 enrolled participants analysed and reported | |
| Clinical review conducted by different practitioners. No systematic intervention that is educational or behavioural in nature | |
| No control group. Participants randomly assigned to positively or negatively framed education | |
| Participants randomly assigned to immediate or delayed CPAP prescription |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised parallel‐group study |
| Participants | N = 206 Intervention group: Age: 49, Male sex: 70%, AHI: 30.4, ESS: 12.0, BM: 35.8 Control group: Age: 47, Male sex: 72%, AHI: 39.9, ESS: 12, BMI: 34.4 Exclusion criteria: poor fluency in English and previous use of CPAP |
| Interventions | Intervention 30‐Minute group education session on sleep, OSA, risks of untreated OSA and CPAP treatment. CBT session including slides on health/social benefits of using CPAP and video of real‐life successful CPAP users. CBT session was delivered to a group of three or four participants Control Same 30‐minute group education session on sleep, OSA and CPAP usage. Social reciprocity consisting of afternoon tea served while participants watched a video of a patient undergoing diagnostic and CPAP studies Study duration: six months |
| Outcomes |
|
| Notes | Inconsistency of preliminary data on CPAP adherence reported in an abstract form (e.g. SE of mean CPAP adherence difference derived from CI differs 100 times from that calculated from SD). Further characteristics of intervention and control groups required Information from the study authors that final withdrawal figures are different from those initially reported The study has been submitted for journal publication, and further information may be available |
| Methods | Randomised parallel‐group |
| Participants | N = 20 Obese, OSA patients Other baseline details not available Inclusion criteria not available |
| Interventions | Intervention Tele‐assisted rehabilitation programme consisting of individualised exercise programme to lose weight and monitor CPAP compliance. Regular phone call interview every two weeks to assess OSA symptoms, problems with CPAP, adherence to exercise programme and weight control Control Standard care, otherwise not specified Study duration: six months |
| Outcomes |
|
| Notes | DRW emailed for further study information 30/06/2012 |
| Methods | Randomised parallel‐group |
| Participants | N = not specified Baseline details not available Inclusion criteria: newly diagnosed OSA |
| Interventions | All participants underwent titration at baseline. Fixed‐pressure CPAP was used throughout the study Intervention: self‐monitoring group Control: non–self‐monitoring group Study duration: not enough information presented on duration of study |
| Outcomes |
|
| Notes | TJL emailed for study information 12/09/2008 |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Machine usage (hours/night) Show forest plot | 13 | 803 | Mean Difference (IV, Random, 95% CI) | 0.82 [0.36, 1.27] |
| Analysis 1.1 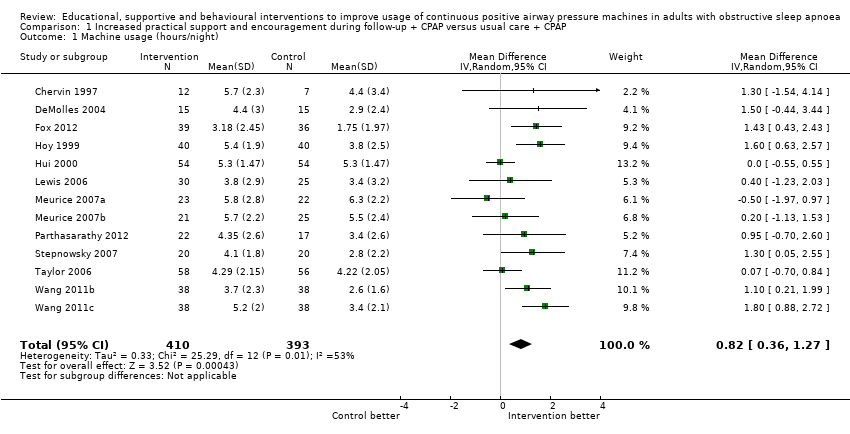 Comparison 1 Increased practical support and encouragement during follow‐up + CPAP versus usual care + CPAP, Outcome 1 Machine usage (hours/night). | ||||
| 2 Machine usage, sensitivity analysis: excluding participants aware of machine usage monitoring Show forest plot | 6 | 378 | Mean Difference (IV, Fixed, 95% CI) | 1.07 [0.61, 1.52] |
| Analysis 1.2 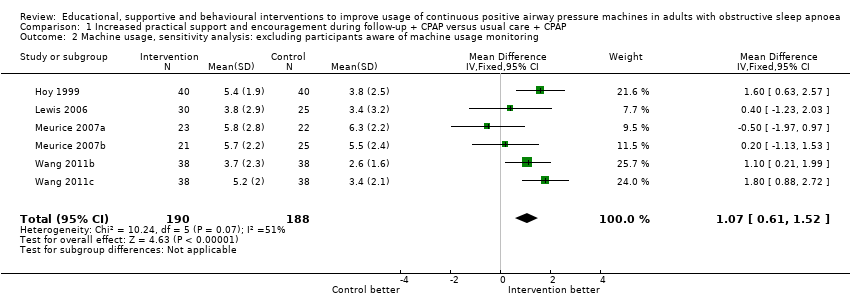 Comparison 1 Increased practical support and encouragement during follow‐up + CPAP versus usual care + CPAP, Outcome 2 Machine usage, sensitivity analysis: excluding participants aware of machine usage monitoring. | ||||
| 3 Machine usage, sensitivity analysis: adherence in control group < four hours/night Show forest plot | 8 | 471 | Mean Difference (IV, Fixed, 95% CI) | 1.36 [0.96, 1.76] |
| Analysis 1.3  Comparison 1 Increased practical support and encouragement during follow‐up + CPAP versus usual care + CPAP, Outcome 3 Machine usage, sensitivity analysis: adherence in control group < four hours/night. | ||||
| 4 N deemed adherent (≥ four hours/night) Show forest plot | 4 | 268 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.06 [1.22, 3.47] |
| Analysis 1.4  Comparison 1 Increased practical support and encouragement during follow‐up + CPAP versus usual care + CPAP, Outcome 4 N deemed adherent (≥ four hours/night). | ||||
| 5 Epworth Sleepiness Scale scores Show forest plot | 8 | 501 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.81, 0.62] |
| Analysis 1.5  Comparison 1 Increased practical support and encouragement during follow‐up + CPAP versus usual care + CPAP, Outcome 5 Epworth Sleepiness Scale scores. | ||||
| 6 Quality of life: Functional Outcomes of Sleep Questionnaire Show forest plot | 2 | 70 | Mean Difference (IV, Fixed, 95% CI) | 0.98 [‐0.84, 2.79] |
| Analysis 1.6  Comparison 1 Increased practical support and encouragement during follow‐up + CPAP versus usual care + CPAP, Outcome 6 Quality of life: Functional Outcomes of Sleep Questionnaire. | ||||
| 7 Quality of life: Sleep Apnoea Quality of Life Index (SAQLI) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.7  Comparison 1 Increased practical support and encouragement during follow‐up + CPAP versus usual care + CPAP, Outcome 7 Quality of life: Sleep Apnoea Quality of Life Index (SAQLI). | ||||
| 8 Mood Show forest plot | 3 | 312 | Mean Difference (IV, Fixed, 95% CI) | ‐0.94 [‐1.55, ‐0.33] |
| Analysis 1.8  Comparison 1 Increased practical support and encouragement during follow‐up + CPAP versus usual care + CPAP, Outcome 8 Mood. | ||||
| 8.1 HAD Scale for Anxiety | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐2.95, 0.75] |
| 8.2 HAD Scale for Depression | 3 | 232 | Mean Difference (IV, Fixed, 95% CI) | ‐0.93 [‐1.57, ‐0.28] |
| 9 Withdrawals Show forest plot | 12 | 903 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.44, 0.97] |
| Analysis 1.9 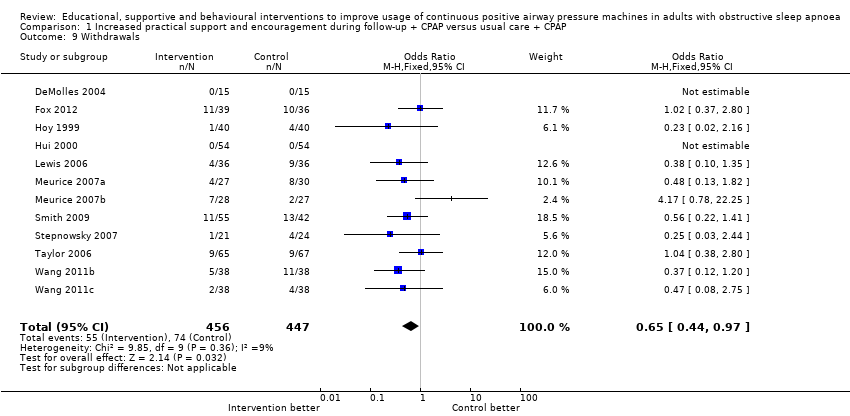 Comparison 1 Increased practical support and encouragement during follow‐up + CPAP versus usual care + CPAP, Outcome 9 Withdrawals. | ||||
| 10 AHI on treatment Show forest plot | 2 | 115 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐1.62, 1.48] |
| Analysis 1.10 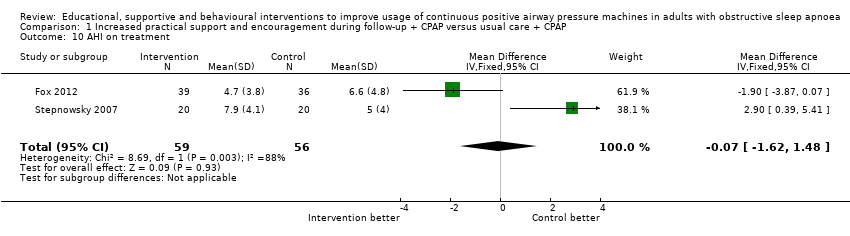 Comparison 1 Increased practical support and encouragement during follow‐up + CPAP versus usual care + CPAP, Outcome 10 AHI on treatment. | ||||
| 11 Maintenance of Wakefulness Test (MWT) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.11  Comparison 1 Increased practical support and encouragement during follow‐up + CPAP versus usual care + CPAP, Outcome 11 Maintenance of Wakefulness Test (MWT). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Machine usage (hours/night) Show forest plot | 7 | 508 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [0.27, 0.93] |
| Analysis 2.1 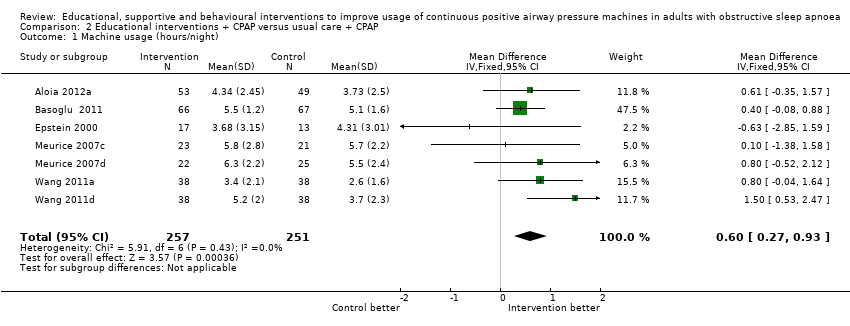 Comparison 2 Educational interventions + CPAP versus usual care + CPAP, Outcome 1 Machine usage (hours/night). | ||||
| 2 N deemed adherent (≥ four hours/night) Show forest plot | 3 | 285 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.09, 2.95] |
| Analysis 2.2  Comparison 2 Educational interventions + CPAP versus usual care + CPAP, Outcome 2 N deemed adherent (≥ four hours/night). | ||||
| 3 Epworth Sleepiness Scale scores Show forest plot | 5 | 336 | Mean Difference (IV, Fixed, 95% CI) | ‐1.17 [‐2.07, ‐0.26] |
| Analysis 2.3  Comparison 2 Educational interventions + CPAP versus usual care + CPAP, Outcome 3 Epworth Sleepiness Scale scores. | ||||
| 4 Quality of life: Sleep Apnoea Quality of Life Index (SAQLI) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.4  Comparison 2 Educational interventions + CPAP versus usual care + CPAP, Outcome 4 Quality of life: Sleep Apnoea Quality of Life Index (SAQLI). | ||||
| 5 HAD Scale for Depression Show forest plot | 2 | 152 | Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐1.25, 0.22] |
| Analysis 2.5 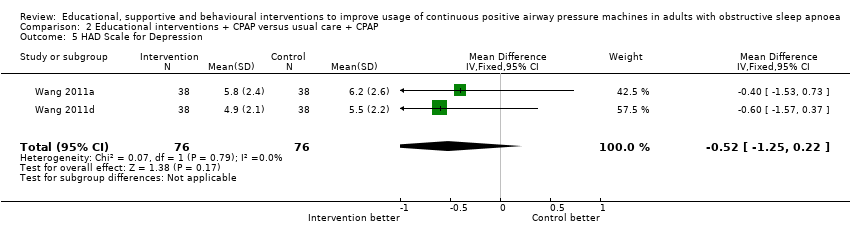 Comparison 2 Educational interventions + CPAP versus usual care + CPAP, Outcome 5 HAD Scale for Depression. | ||||
| 6 Withdrawal Show forest plot | 8 | 683 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.45, 0.98] |
| Analysis 2.6  Comparison 2 Educational interventions + CPAP versus usual care + CPAP, Outcome 6 Withdrawal. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Machine usage (hours/night) Show forest plot | 6 | 584 | Mean Difference (Random, 95% CI) | 1.44 [0.43, 2.45] |
| Analysis 3.1  Comparison 3 Behavioural therapy + CPAP versus control + CPAP, Outcome 1 Machine usage (hours/night). | ||||
| 2 Sensitivity analysis: excluding participants aware of machine usage monitoring Show forest plot | 5 | Mean Difference (Fixed, 95% CI) | 1.54 [0.99, 2.09] | |
| Analysis 3.2 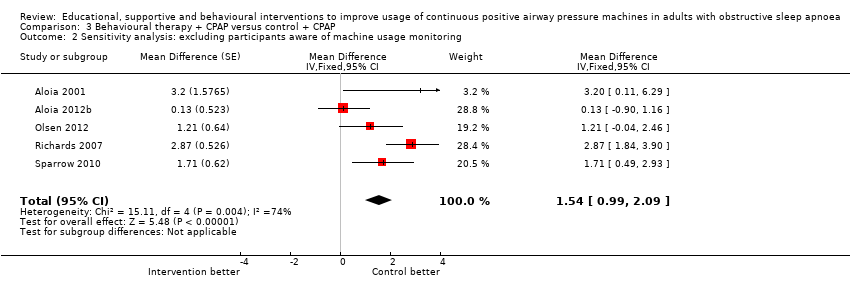 Comparison 3 Behavioural therapy + CPAP versus control + CPAP, Outcome 2 Sensitivity analysis: excluding participants aware of machine usage monitoring. | ||||
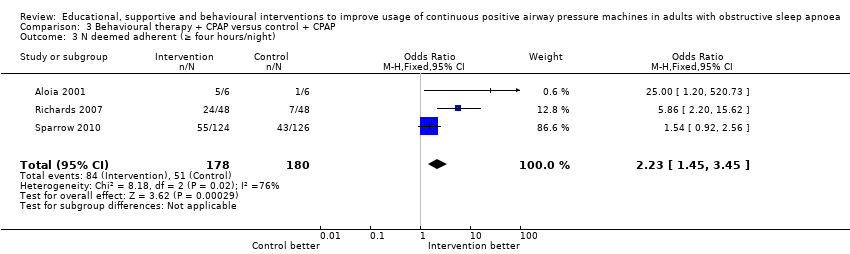
| 3 N deemed adherent (≥ four hours/night) Show forest plot | 3 | 358 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.23 [1.45, 3.45] |
| Analysis 3.3  Comparison 3 Behavioural therapy + CPAP versus control + CPAP, Outcome 3 N deemed adherent (≥ four hours/night). | ||||
| 4 Epworth Sleepiness Scale score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.4  Comparison 3 Behavioural therapy + CPAP versus control + CPAP, Outcome 4 Epworth Sleepiness Scale score. | ||||
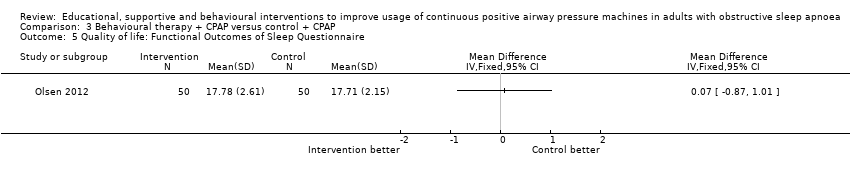
| 5 Quality of life: Functional Outcomes of Sleep Questionnaire Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.5 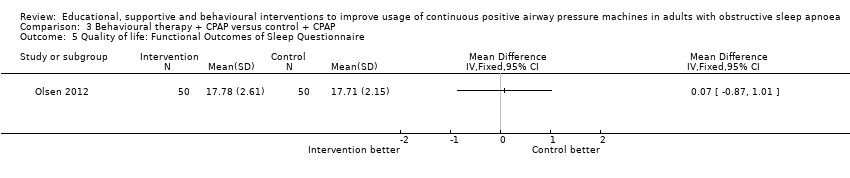 Comparison 3 Behavioural therapy + CPAP versus control + CPAP, Outcome 5 Quality of life: Functional Outcomes of Sleep Questionnaire. | ||||
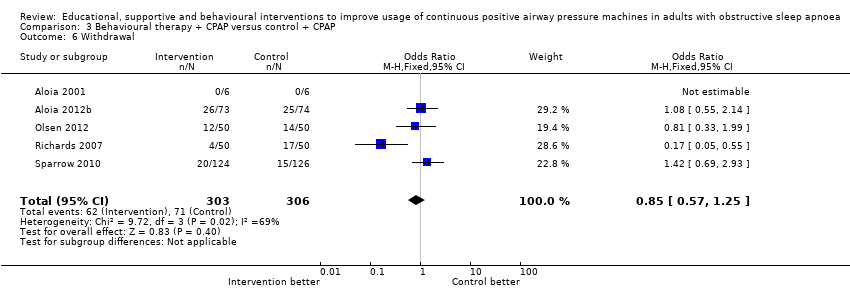
| 6 Withdrawal Show forest plot | 5 | 609 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.57, 1.25] |
| Analysis 3.6  Comparison 3 Behavioural therapy + CPAP versus control + CPAP, Outcome 6 Withdrawal. | ||||

Study flow diagram.
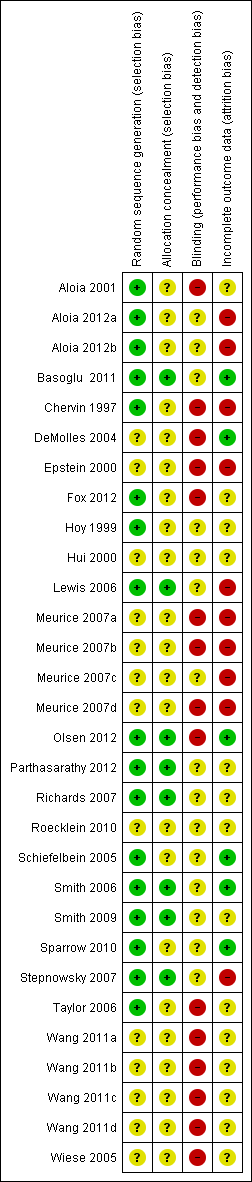
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
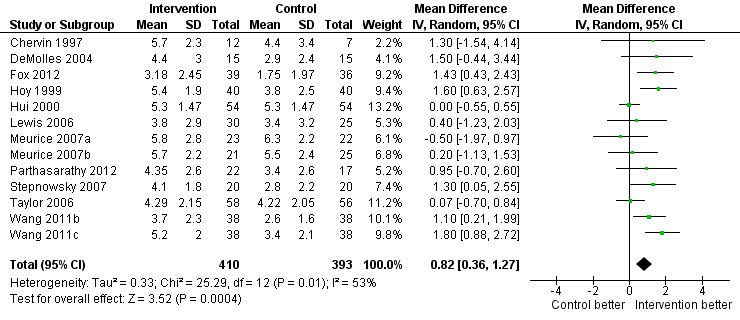
Forest plot of comparison: 1 Increased psychological and/or practical support during follow‐up + CPAP versus usual care + CPAP, outcome: 1.1 Machine usage (hours/night)—first arm/parallel studies.

Forest plot of comparison: 1 Increased practical support and encouragement during follow‐up + CPAP versus usual care + CPAP, outcome: 1.3 Machine usage, sensitivity analysis: adherence in control group =< four hours/night.

Funnel plot of comparison: 1 Increased practical support and encouragement + CPAP versus usual care + CPAP, outcome: 1.1 Machine usage (hours/night).

Forest plot of comparison: 2 Educational interventions + CPAP versus usual care + CPAP, outcome: 2.1 Machine usage (hours/night).

Forest plot of comparison: 4 Behavioural therapy + CPAP versus control + CPAP, outcome: 4.1 Machine usage.
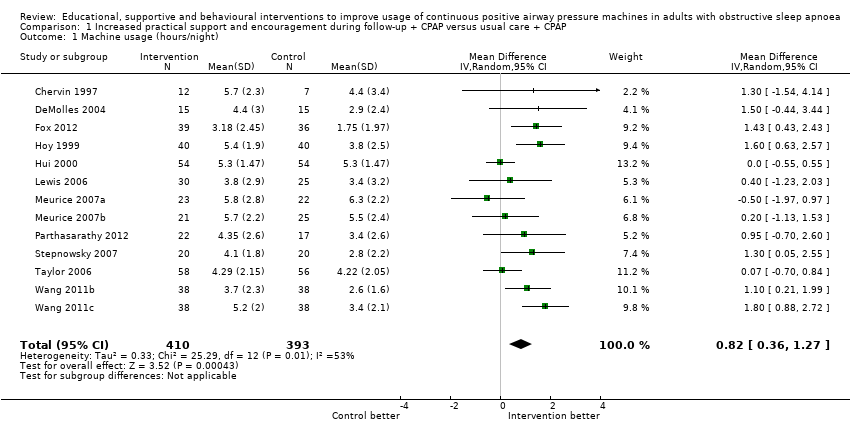
Comparison 1 Increased practical support and encouragement during follow‐up + CPAP versus usual care + CPAP, Outcome 1 Machine usage (hours/night).

Comparison 1 Increased practical support and encouragement during follow‐up + CPAP versus usual care + CPAP, Outcome 2 Machine usage, sensitivity analysis: excluding participants aware of machine usage monitoring.
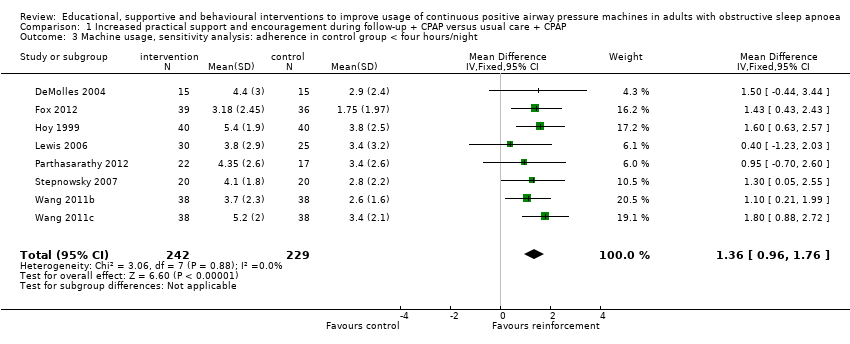
Comparison 1 Increased practical support and encouragement during follow‐up + CPAP versus usual care + CPAP, Outcome 3 Machine usage, sensitivity analysis: adherence in control group < four hours/night.

Comparison 1 Increased practical support and encouragement during follow‐up + CPAP versus usual care + CPAP, Outcome 4 N deemed adherent (≥ four hours/night).

Comparison 1 Increased practical support and encouragement during follow‐up + CPAP versus usual care + CPAP, Outcome 5 Epworth Sleepiness Scale scores.

Comparison 1 Increased practical support and encouragement during follow‐up + CPAP versus usual care + CPAP, Outcome 6 Quality of life: Functional Outcomes of Sleep Questionnaire.

Comparison 1 Increased practical support and encouragement during follow‐up + CPAP versus usual care + CPAP, Outcome 7 Quality of life: Sleep Apnoea Quality of Life Index (SAQLI).

Comparison 1 Increased practical support and encouragement during follow‐up + CPAP versus usual care + CPAP, Outcome 8 Mood.

Comparison 1 Increased practical support and encouragement during follow‐up + CPAP versus usual care + CPAP, Outcome 9 Withdrawals.

Comparison 1 Increased practical support and encouragement during follow‐up + CPAP versus usual care + CPAP, Outcome 10 AHI on treatment.

Comparison 1 Increased practical support and encouragement during follow‐up + CPAP versus usual care + CPAP, Outcome 11 Maintenance of Wakefulness Test (MWT).

Comparison 2 Educational interventions + CPAP versus usual care + CPAP, Outcome 1 Machine usage (hours/night).

Comparison 2 Educational interventions + CPAP versus usual care + CPAP, Outcome 2 N deemed adherent (≥ four hours/night).

Comparison 2 Educational interventions + CPAP versus usual care + CPAP, Outcome 3 Epworth Sleepiness Scale scores.

Comparison 2 Educational interventions + CPAP versus usual care + CPAP, Outcome 4 Quality of life: Sleep Apnoea Quality of Life Index (SAQLI).

Comparison 2 Educational interventions + CPAP versus usual care + CPAP, Outcome 5 HAD Scale for Depression.

Comparison 2 Educational interventions + CPAP versus usual care + CPAP, Outcome 6 Withdrawal.

Comparison 3 Behavioural therapy + CPAP versus control + CPAP, Outcome 1 Machine usage (hours/night).

Comparison 3 Behavioural therapy + CPAP versus control + CPAP, Outcome 2 Sensitivity analysis: excluding participants aware of machine usage monitoring.
Comparison 3 Behavioural therapy + CPAP versus control + CPAP, Outcome 3 N deemed adherent (≥ four hours/night).

Comparison 3 Behavioural therapy + CPAP versus control + CPAP, Outcome 4 Epworth Sleepiness Scale score.
Comparison 3 Behavioural therapy + CPAP versus control + CPAP, Outcome 5 Quality of life: Functional Outcomes of Sleep Questionnaire.
Comparison 3 Behavioural therapy + CPAP versus control + CPAP, Outcome 6 Withdrawal.
| Increased practical support and encouragement for adults with sleep apnoea | ||||||
| Patient or population: adults with sleep apnoea Comparison: CPAP Settings: community | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Increased practical support and encouragement | |||||
| Machine usage | Average CPAP machine usage ranged across control groups from | Mean machine usage in the intervention groups was | 803 | ⊕⊕⊝⊝ | ||
| N deemed adherent (≥ four hours/night) | 59 per 100 | 75 per 100 | OR 2.06 | 268 | ⊕⊕⊝⊝ | |
| Symptoms of sleepiness | Average Epworth symptom scores in control groups ranged from 4.5 to 13 | Mean symptoms of sleepiness in the intervention groups was | 501 | ⊕⊝⊝⊝ | ||
| Quality of life | Mean quality of life in the intervention groups was | 70 | ⊕⊕⊝⊝ | |||
| Quality of life | See comment | See comment | 108 | ⊕⊕⊝⊝ | Single study estimate | |
| Withdrawals | 17 per 100 | 11 per 100 | OR 0.65 | 903 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| 1Risk of bias (‐1): In the absence of blinding across studies, the study effect estimates are at risk of performance bias. | ||||||
| Educational interventions for adults with sleep apnoea | ||||||
| Patient or population: adults with sleep apnoea Comparison: CPAP Settings: community | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Educational interventions | |||||
| Machine usage | Average CPAP machine usage ranged across control groups from 2.6 to 5.7 hours per night | Mean machine usage in the intervention groups was | 508 | ⊕⊕⊕⊝ | ||
| N deemed adherent (≥4 hours/night) | 57 per 100 | 71 per 100 | OR 1.8 | 285 | ⊕⊕⊝⊝ | |
| Symptoms of sleepiness | Mean Epworth Sleepiness Scale scores across control groups ranged from 5.4 to 10.8 | Mean Epworth Sleepiness Scale scores in the intervention groups was | 336 | ⊕⊕⊕⊝ | ||
| Quality of life: Sleep Apnoea Quality of Life Index (SAQLI) | See comment | See comment | Not estimable | 89 | ⊕⊝⊝⊝ | Single study estimate |
| Withdrawal | 24 per 100 | 18 per 100 | OR 0.67 | 683 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| 1Risk of bias (‐1): In the absence of blinding across studies, effect estimates may be biased because of performance bias. | ||||||
| Behavioural therapy for adults with sleep apnoea who are using CPAP | ||||||
| Patient or population: adults with sleep apnoea Comparison: CPAP Settings: community | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Behavioural therapy | |||||
| Machine usage | See comment | Average machine usage in the intervention groups was | 584 | ⊕⊕⊝⊝ | Data analysed as generic inverse variance | |
| N deemed adherent (≥4 hours/night) | 28 per 100 | 47 per 100 | OR 2.23 | 358 | ⊕⊝⊝⊝ | |
| Symptoms | See comment | See comment | 100 | ⊕⊕⊝⊝ | Single study estimate | |
| Quality of life | See comment | See comment | 100 | ⊕⊕⊝⊝ | Single study estimate | |
| Withdrawal | 23 per 100 | 20 per 100 | OR 0.85 | 609 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| 1Risk of bias (‐1): In the absence of blinding across studies, effect estimates may be biased because of performance bias. | ||||||
| Study | N Screened | Entered | Completed | % Screened | % Entered |
| NA | 12 | 12 | NA | 100 | |
| 339 | 227 | 149 | 44 | 66 | |
| 246 | 133 | 133 | 54 | 100 | |
| NA (75% of those approached agreed to participate) | 40 | 33 | NA | 82.5 | |
| NA | 30 | 30 | NA | 100 | |
| NA | 50 | 43 | NA | 86 | |
| NA | 75 | 54 | NA | 72 | |
| NA | 80 | 75 | NA | 94 | |
| NA | 108 | 108 | NA | 100 | |
| 74 | 72 | 55 | 74 | 76 | |
| 133 | 112 | 91 | 68 | 81 | |
| 132 | 100 | 73 | 55 | 73 | |
| 49 | 39 | 37 | 76 | 95 | |
| 109 | 100 | 79 | 72 | 79 | |
| NA | 30 | 28 | NA | 93 | |
| NA | 51 | 51 | NA | 100 | |
| NA | 19 | 19 | NA | 100 | |
| NA | 97 | 73 | NA | 75 | |
| 423 | 250 | 115 | 27 | 46 | |
| 91 | 45 | 40 | 44 | 88 | |
| 160 | 132 | 114 | 71 | 86 | |
| NA | 152 | 130 | NA | 86 | |
| NA | 93 | 56 | NA | 60 |
| Intervention group | Study | Intervention | Control | Study duration (weeks) | ||
| Increased support and reinforcement components | Increased educational components | Behavioural therapy | ||||
| Increased support and reinforcement | Weekly telephone calls to monitor progress and troubleshoot | Written information on OSA and CPAP | Usual care | Eight | ||
| Computer‐based telecommunication system allowing for monitoring and reinforcing compliance | Education provided by the computer‐based telecommunication system | Usual care | Eight | |||
| Telecomunication system allowing for daily monitoring of CPAP usage, timely detection and troubleshooting of problems | Usual care | 12 | ||||
| 2 additional titration nights in hospital, 4 additional visits at home by sleep nurses | Initial education at home with partner | Usual care | 24 | |||
| 2 additional early reviews by sleep physician and frequent telephone calls by sleep nurses | Videotape and additional education session | Usual care | 12 | |||
| 1 additional early review by sleep physician and 1 early telephone interview with sleep nurse | Educational video | Usual care | 52 | |||
| 4 additional home visits in the first 3 months by sleep practitioner for problem solving | Written information and detailed explanation by the prescriber, additional education during home visits | Written information and detailed explanation by the prescriber + usual care | 52 | |||
| 4 additional home visits in the first 3 months by sleep practitioner for problem solving | Additional education during home visits | Usual care | 52 | |||
| 2 individual sessions and 8 telephone conversations with trained peer CPAP users providing support and sharing their positive experience with CPAP | Peers shared their knowledge on CPAP and OSA | Interventions delivered by peer contained elements of promoting self‐efficacy, risk perception, participant activation and motivation | Usual care | 12 | ||
| Internet‐based application aimed at encouraging CPAP use and problem solving | Internet‐based application similar in format to intervention but directed activities in neutral health topics (vitamin intake) | 16 | ||||
| Home video‐link sessions delivered by nurse, who guided correct CPAP use and provided problem solving | Nurse provided education on CPAP and OSA | Home video‐link sessions similar in form to intervention but directed activities in neutral health topics (vitamin intake) | 12 | |||
| Audiotaped music along with softly spoken directions on relaxation techniques and habit‐promoting instructions for using CPAP, user reminder placard | Handouts on benefits of CPAP adherence and health consequences of poor compliance | Audiotaped music along with spoken information about vitamins. Information packet similar in format to intervention, but content was on vitamins | 24 | |||
| Wireless telemonitoring of compliance and treatment efficacy on daily basis and acting on the data via prespecified clinical pathways | Usual care | Eight | ||||
| Internet‐based application aimed at monitoring self‐reported compliance, acting on the information in timely fashion | Usual care | Four | ||||
| Progressive muscle relaxation | Usual care | 12 | ||||
| Progressive muscle relaxation + 2 additional nights of CPAP titration | 4hour group education session, written information, video CD | Two additional nights of CPAP titration + four‐hour group education session, written information, video CD + usual care | 12 | |||
| Increased education | Two 45‐minute individual didactic sessions and one booster phone call by sleep nurse | Usual care | 52 | |||
| 10‐Minute educational video session on OSA and CPAP | Usual care | 24 | ||||
| Educational and desensitisation course | Usual care | 24 | ||||
| 4 additional home visits in the first 3 months by sleep practitioner for problem solving | Written information and detailed explanation by the prescriber, additional education during home visits | Four additional home visits in the first three months by sleep practitioner for problem solving and additional education + usual care | 52 | |||
| Written information and detailed explanation by the prescriber | Usual care | 52 | ||||
| 2 additional nights of CPAP titration | Four‐hour group education session, written information, video CD | Usual care | 12 | |||
| Progressive muscle relaxation + 2 additional nights of CPAP titration | Four‐hour group education session, written information, video CD | Progressive muscle relaxation + usual care | 12 | |||
| 15‐Minute educational video addressing misconception about OSA and barriers to effective CPAP treatment | Usual care | Four | ||||
| Behavioural therapy | Elements of education on consequences of OSA and efficacy of CPAP | Two 45‐minute sessions of cognitive‐behavioural therapy interventions | Two 45‐minute sessions involving discussion on sleep architecture and sleep clinic | 12 | ||
| Two 45‐minute sessions of Motivational Enhancement Therapy, one booster phone call | Usual care | 52 | ||||
| 45‐Minute individual education session | Three 30‐minute sessions of Motivational Interviewing Therapy | 45‐Minute educational session + usual care | 52 | |||
| Slide presentation and written information on OSA and CPAP | Two one‐hour group sessions of cognitive‐behavioural therapy | Usual care | Four | |||
| Written personalised feedback report framed according to Motivational Enhancement Theory | Written information from the American Academy of Sleep Medicine | 12 | ||||
| Side effects management module incorporated in the automated telephone‐linked communication system | Information exchange on OSA and CPAP incorporated in the automated telephone‐linked communication system | Automated telephone‐linked communication system designed around the concept of Motivational Interviewing, which allowed one to assess and enhance CPAP compliance | General education on unrelated health topics via automated telephone‐linked communication system | 52 | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Machine usage (hours/night) Show forest plot | 13 | 803 | Mean Difference (IV, Random, 95% CI) | 0.82 [0.36, 1.27] |
| 2 Machine usage, sensitivity analysis: excluding participants aware of machine usage monitoring Show forest plot | 6 | 378 | Mean Difference (IV, Fixed, 95% CI) | 1.07 [0.61, 1.52] |
| 3 Machine usage, sensitivity analysis: adherence in control group < four hours/night Show forest plot | 8 | 471 | Mean Difference (IV, Fixed, 95% CI) | 1.36 [0.96, 1.76] |
| 4 N deemed adherent (≥ four hours/night) Show forest plot | 4 | 268 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.06 [1.22, 3.47] |
| 5 Epworth Sleepiness Scale scores Show forest plot | 8 | 501 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.81, 0.62] |
| 6 Quality of life: Functional Outcomes of Sleep Questionnaire Show forest plot | 2 | 70 | Mean Difference (IV, Fixed, 95% CI) | 0.98 [‐0.84, 2.79] |
| 7 Quality of life: Sleep Apnoea Quality of Life Index (SAQLI) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Mood Show forest plot | 3 | 312 | Mean Difference (IV, Fixed, 95% CI) | ‐0.94 [‐1.55, ‐0.33] |
| 8.1 HAD Scale for Anxiety | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐2.95, 0.75] |
| 8.2 HAD Scale for Depression | 3 | 232 | Mean Difference (IV, Fixed, 95% CI) | ‐0.93 [‐1.57, ‐0.28] |
| 9 Withdrawals Show forest plot | 12 | 903 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.44, 0.97] |
| 10 AHI on treatment Show forest plot | 2 | 115 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐1.62, 1.48] |
| 11 Maintenance of Wakefulness Test (MWT) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Machine usage (hours/night) Show forest plot | 7 | 508 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [0.27, 0.93] |
| 2 N deemed adherent (≥ four hours/night) Show forest plot | 3 | 285 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.09, 2.95] |
| 3 Epworth Sleepiness Scale scores Show forest plot | 5 | 336 | Mean Difference (IV, Fixed, 95% CI) | ‐1.17 [‐2.07, ‐0.26] |
| 4 Quality of life: Sleep Apnoea Quality of Life Index (SAQLI) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 HAD Scale for Depression Show forest plot | 2 | 152 | Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐1.25, 0.22] |
| 6 Withdrawal Show forest plot | 8 | 683 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.45, 0.98] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Machine usage (hours/night) Show forest plot | 6 | 584 | Mean Difference (Random, 95% CI) | 1.44 [0.43, 2.45] |
| 2 Sensitivity analysis: excluding participants aware of machine usage monitoring Show forest plot | 5 | Mean Difference (Fixed, 95% CI) | 1.54 [0.99, 2.09] | |
| 3 N deemed adherent (≥ four hours/night) Show forest plot | 3 | 358 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.23 [1.45, 3.45] |
| 4 Epworth Sleepiness Scale score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Quality of life: Functional Outcomes of Sleep Questionnaire Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Withdrawal Show forest plot | 5 | 609 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.57, 1.25] |

