Antibioticoterapia de corta duración versus ciclo prolongado para la neumonía adquirida en el hospital en adultos graves
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | The PRORATA study. A multi‐centre study based in France. Unblinded, randomised controlled trial comparing strategy utilising serum procalcitonin measurement to guide initiation and cessation of antibiotics for ICU patients with suspected bacterial infection, with antibiotic administration according to an agreed guideline | |
| Participants | 630 adult ICU patients (8 medical, surgical or medical‐surgical ICUs; 6 hospitals). Mean age was 62; 66% male; SAPS II 47; SOFA score 7.8) with suspected bacterial infection (either at admission or during ICU stay). 89% patients were medical.16% enrolled patients were defined as being "Immunocompromised" (including patients with AIDS, solid organ transplant, haematological malignancy, prior chemotherapy or radiotherapy, and long‐term corticosteroid or other immunosuppressant therapy); identifying and excluding data from these patients for the purpose of the systematic review was not possible. There appear not to have been significant differences in baseline characteristics between treatment groups. Of 621 patients entered into the analysis, 214 (34%) had VAP or "HAP": 141 (23%) patients were diagnosed with VAP, and 73 (12%) patients had HAP not requiring mechanical ventilation. For all patients with microbiologically‐confirmed infection, initial antibiotic therapy was appropriate in 92% cases. Diagnostic criteria for VAP and HAP were not provided in the full‐text article Exclusions: pregnancy, expected ICU stay < 3 days, bone marrow transplant or chemotherapy‐induced neutropenia, infections requiring long‐term course antibiotic therapy (e.g. infective endocarditis), poor chance of survival (SAPS II > 65), DNR order | |
| Interventions | Patients randomised to intervention group (311 randomised; 4 withdrew consent; 307 included in analysis; 75 patients with VAP; 29 with HAP not requiring mechanical ventilation) had serum procalcitonin level measured at inclusion, at each infectious episode until day 28, and for every morning that antibiotics were administered. The guidance for withholding or stopping antibiotic therapy was: i. antibiotics to be withheld when procalcitonin was < 0.5 µg/L on day of study entry; ii. antibiotics to be discontinued when procalcitonin level had fallen to < 0.5 µg/L or to less than 20% of the peak procalcitonin concentration For patients randomised to control group (319 randomised; 4 withdrew; 1 randomised twice; 314 included in analysis; 66 patients with VAP; 44 patients with HAP not requiring mechanical ventilation), physicians were encouraged to administer antibiotics according to agreed recommendations (including duration of therapy) | |
| Outcomes | Most outcome data were presented for all patients, without specific reference to patients with VAP or HAP (non‐ventilated) For all patients, the following outcomes were presented:
For patients specifically with VAP, the following outcomes were presented:
| |
| Notes | Procalcitonin measurements were used to guide whether to initiate antibiotic therapy as well as when to discontinue therapy. A very low proportion of recruited patients were surgical, which may limit generalisability of results. There was a high incidence of protocol non‐adherence; the algorithm for antimicrobial therapy administration was not followed (overall) in 162 patients (53%) in the intervention group, nor for 141 patients in the control group (45%). For all patients, a non‐significant increase in 60‐day mortality was observed in the procalcitonin group (92/307 (30%) versus 82/ 314 (26.1%)] in the control group). Furthermore, the study may have been under‐powered to determine non‐inferiority of PCT‐guided therapy in terms of death, since it was based upon a 35% absolute mortality in the control group and a 10% between‐group mortality difference. For all patients, in the procalcitonin group there were non‐significantly greater relapse rates (20/ 307 (6.5%) versus 16/ 314 (5.1%) in control group; P = 0.45) and superinfection rates (106/ 307 (34.5%) versus 97/ 314 (30.9%) in the control group; P = 0.29) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Independent, centralised, computer‐generated randomisation sequence... was used to randomly assign patients." |
| Allocation concealment (selection bias) | Low risk | "Investigators were masked to assignment before... randomisation." |
| Blinding (performance bias and detection bias) | High risk | Unblinded study |
| Incomplete outcome data (attrition bias) | Low risk | 2 patients lost to follow‐up, 1 from intervention and 1 from control group. This is unlikely to have a clinically relevant impact |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | High risk | The algorithm for antimicrobial therapy administration was not followed (overall) in 162 patients (53%) for the intervention group, nor for 141 patients in the control group (45%) |
| Methods | The PNEUMA study, a multi‐centre study based in France. Unblinded, randomised controlled trial comparing fixed durations (8‐day versus 15‐day) of antibiotic therapy for VAP. Randomisation occurred 3 days after bronchoscopy confirming diagnosis of VAP | |
| Participants | 401 adult patients. 51 French ICUs. 72% male; mean age 61; episodes due to NF‐GNB 32.5%, MRSA 11.2%; mean SAPS II score 45; mean SOFA score 7.4 at admission. VAP was diagnosed according to the following criteria: new and persistent radiographic infiltrate, plus 1 of: purulent tracheal secretions, temperature of 38.4 °C or higher, or leukocyte count > 10,000/µL; and positive quantitative culture of 104 cfu/mL from BAL or 103 cfu/mL from PSB. Duration of mechanical ventilation prior to VAP: 13.6 days. No significant differences between groups at baseline, with the exception of significantly higher proportion of men (76.6%) in 8‐day regimen versus men in 15‐day regimen (67.6%; P = 0.046). All patients received appropriate initial antibiotics. Exclusions included early onset pneumonia (within 5 days of commencing mechanical ventilation) in patients who had received no antimicrobial therapy in the 15 days prior to diagnosis of pneumonia, and immunocompromised state, characterised by: neutropenia, AIDS, long‐term corticosteroids or other immunosuppressant therapy | |
| Interventions | 197 patients received fixed 8‐day course of antibiotics (chosen by treating physician); 204 patients received a 15‐day course | |
| Outcomes | The following outcome measures were reported:
| |
| Notes | Repeat bronchoscopy was performed on the basis of fever, purulent secretions, new or progressive pulmonary infiltrates, or deterioration in respiratory or haemodynamic parameters. It was not performed routinely, e.g. on completion of 8‐day course of therapy, and consequently data regarding persistent colonisation with NF‐GNB is not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was performed...and stratified... according to a computer‐generated random‐number table." |
| Allocation concealment (selection bias) | Low risk | "Randomisation performed centrally, using an interactive voice system... randomisation was not communicated to the investigators until day 8... On that day, investigators had to telephone the randomisation centre to receive the treatment assignment by fax." |
| Blinding (performance bias and detection bias) | High risk | "...Patients, medical and nursing staffs, and pharmacists remained blinded until [day 8]." However, importantly, no attempt was made to blind from day 8, i.e. the point from which thereafter allocation might make a significant difference |
| Incomplete outcome data (attrition bias) | Low risk | Following randomisation, 0 patients were lost to follow‐up. 1 patient excluded from analysis following withdrawal of consent |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available for examination. However, outcome measures are those expected and appropriately presented within the report |
| Other bias | Low risk | There was a significantly higher proportion of men in the 8‐day group (76.6%) compared with the 15‐day group (67.6%; P = 0.046). However, there were not significant differences in illness severity scores, prior duration of mechanical ventilation, prior antibiotic administration, micro‐organisms responsible for VAP, and antibiotic regimes to treat VAP Data regarding proportions of patients who contrary to protocol did not receive a full 8‐day or 15‐day course of antibiotics are not provided. However, absolute antibiotic‐treatment days and 28‐day antibiotic‐free day data indicates significantly less antibiotic exposure as a consequence of allocation to short or prolonged‐course therapy |
| Methods | Single‐centre study based in Tunisia. RCT comparing fixed durations (7‐day versus 10‐day) of antibiotic therapy for VAP | |
| Participants | Medical ICU. 30 adult patients (63% male; mean age 63 years; NF‐GNB 72%; SAPS II 42.4). VAP (onset more than 48 hours after mechanical ventilation in ICU) diagnosed was suspected on the basis of: new and persistent radiographic infiltrate, purulent secretions, fever or deteriorating gas exchange or white cell count and confirmed on quantitative analysis of culture of endotracheal aspirate (> 104 cfu/ml) or protected distal respiratory specimens (>103 cfu/ml). Mean onset of VAP after institution of mechanical ventilation: 10 days. Initial antibiotics were appropriate in 94% of cases. No significant differences in baseline characteristics between the 2 groups Exclusions include: second episode of pneumonia during single hospitalisation, terminal illness, failure to isolate bacterial growth 30 patients randomised from 39 patients with clinical features of VAP: 9 not enrolled because of terminal illness or failure to isolate bacteria | |
| Interventions | 14 patients randomised to receive 7‐day course of antibiotics; 16 patients to receive 16‐day course. Choice of antibiotic: on microbiology advice, taking into account whether early (up to including 5 days after commencing mechanical ventilation) or late‐onset VAP, and whether risk factors for multi‐resistant bacteria present, and modified according to culture/sensitivity results 94% patients received appropriate initial antibiotic therapy | |
| Outcomes | The following outcome measures were presented:
| |
| Notes | Data regarding protocol violations and patients lost to follow‐up not available. Unable to make contact trial with authors to request supplementary information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of random number table |
| Allocation concealment (selection bias) | Unclear risk | Inadequately reported |
| Blinding (performance bias and detection bias) | Unclear risk | Inadequately reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Inadequately reported |
| Selective reporting (reporting bias) | Unclear risk | Inadequately reported |
| Other bias | Low risk | No evidence of other source of bias |
| Methods | 2 centre study, based in Uruguay, conducted May 2003 to December 2006 (during the period December 2005 to April 2006 recruitment was interrupted while eligible patients were enrolled in the Pontet 2007 study). Randomised controlled study comparing fixed short (8‐day) and long (12‐day) courses of antibiotic therapy for VAP. | |
| Participants | 77 patients (medical, surgical and neurosurgical; mean age 59 years, 53% male, 63.6% NF‐GNB, 9.1% MRSA; median APACHE II score 21, MODS score 5, SOFA score 6) with VAP. VAP was diagnosed on the basis of: new and persistent radiographic infiltrates, 2 of temperature ≥38.5 ºC or <36 ºC, leukocytes ≥12,000/ mm3 or ≤ 4 x103/ mm3, and BAL culture ≥ 104 cfu/ml, or positive ETA plus CPIS > 6, or micro‐organism in ≥ 2 blood cultures with identical sensitivity to tracheal secretions and in absence of other possible infection, or positive culture of pleural fluid. Mean time after commencing mechanical ventilation before onset of VAP: 9.3 days. 68% patients had received a prior course of antibiotics. In 97% cases, initial antibiotic therapy for VAP was appropriate. There were no significant differences in baseline characteristics between treatment groups | |
| Interventions | 77 patients randomised to 8‐day treatment or 12‐day course on Day 8 of antibiotic therapy. Antibiotic choice was that of the attending physician; in 75/77 (97%) cases, initial antibiotic therapy was appropriate. The most commonly used antibiotics were: cefoperazone‐sulbactam, carbapenem and other third‐generation cephalosporins. In 51% cases, antibiotic combinations were used | |
| Outcomes | The following outcomes were studied:
| |
| Notes | 0 patients were lost to follow‐up. No patient received a shorter duration of antibiotic therapy than allocated; data incomplete for patients who had antibiotics continued beyond the allocated duration | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation using random number table |
| Allocation concealment (selection bias) | Low risk | Sealed, numbered envelopes opened sequentially |
| Blinding (performance bias and detection bias) | High risk | Unblinded study |
| Incomplete outcome data (attrition bias) | Low risk | No patients lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Unclear. Data incomplete for patients who had antibiotics continued beyond the allocated duration |
| Other bias | Low risk | Study appears to be free of other sources of bias |
| Methods | Single‐centre, medical ICU from USA. Randomised controlled study comparing strategy involving discontinuation policy to decrease antibiotic administration for VAP with standard‐therapy | |
| Participants | 302 patients receiving antibiotic treatment for VAP were randomised. VAP was diagnosed on the basis of: new persistent radiographic infiltrates together with one of: positive pleural culture (same organism as in sputum/tracheal aspirate), radiographic cavitation, histopathologic evidence of pneumonia, or 2 of: fever, leukocytosis and purulent tracheal aspirate or sputum. Mean age 60 years; 50% male; 29% COPD; APACHE II score 23; mean CPIS 7.1; Pseudomonas or Acinetobacter infection 11%; MRSA 20%. 25% patients had received prior antibiotics. Proportion of patients receiving appropriate initial antibiotic therapy was 94% overall. 18.6% patients were defined as "Immunosuppressed". No significant differences in baseline characteristics between groups Exclusions: transfers to host institution because of lack of capacity at external institutions | |
| Interventions | 154 patients randomised to discontinuation group; one of investigators recommended discontinuation of antibiotics to treating physician on weekdays if: non‐infectious cause for radiographic infiltrates identified, signs and symptoms of active infection had resolved (in terms of temperature, white cell count, radiographic appearance, sputum characteristics and PaO2/ FiO2 ratio). 148 patients were randomised to the control group. Recommendations regarding choice of antibiotic therapy were made to both groups | |
| Outcomes | Reported outcome measures:
| |
| Notes | Outcome data was missing for 4 patients in the intervention group, and for 8 patients in the control group. Among intervention group, recommendations to discontinue antibiotics were given to the physicians of 142 patients (94.7%); among 88.7% of these patients antibiotics were discontinued within 48 hours of the recommendation. Trial author contacted, but no further information available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding (performance bias and detection bias) | High risk | Intervention involved investigators contacting the physician team |
| Incomplete outcome data (attrition bias) | High risk | Outcome data missing in 12/302 (4%) cases; no information given regarding missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Low risk | There do not appear to be other major potential sources of bias |
| Methods | 4‐centre Uruguayan study, conducted December 2005 to April 2006; 2 of these centres also recruited to the study Medina 2007, but outside this time period. Unblinded, randomised controlled study comparing PCT‐guided strategy for antibiotic cessation versus standard therapy for VAP | |
| Participants | 81 adult patients (medical, surgical, cardiothoracic and neurosurgical; mean age 54 years, 63% male; NF‐GNB 23.4%; MRSA 6.1%; Day 1 MODS 6.2; Day 1 SOFA score 4.8) with suspected VAP. VAP was diagnosed on the basis of: new and persistent radiographic infiltrates, 2 of temperature ≥ 38.5 ºC or < 36 ºC, leukocytes ≥12,000/ mm3 or ≤ 4 x103/ mm3, and BAL culture ≥ 104 cfu/ml, or positive ETA plus CPIS > 6, or micro‐organism in ≥ 2 blood cultures with identical sensitivity to tracheal secretions and in absence of other possible infection, or positive culture of pleural fluid. Patients were excluded on the basis of: AIDS, leukaemia or immunosuppression. A further 14 patients were excluded after randomisation; from control group, 5 patients were excluded because of short duration of treatment (3 patients) or negative BAL (3 patients); from the PCT group, 9 patients were excluded post‐randomisation because Day 1 or 2 PCT was < 0.5 ng/mL. There were no significant baseline differences between groups in terms of demographics, underlying disease or prior antibiotic administration | |
| Interventions | Intervention: for the group receiving PCT‐guided therapy; if at day 7 PCT was < 0.5 ng/ml, antibiotic discontinuation was encouraged. Duration of therapy in control group was according to pre‐existing guidelines in place at each ICU. Choice of antibiotic therapy was that of the treating physician. Most commonly used antibiotics were: cephalosporins, ampicillin‐sulbactam and amikacin; antibiotic combinations were used in 54.5% cases | |
| Outcomes | Data are presented as a per‐protocol analysis. The following outcome measures were reported:
| |
| Notes | All patients in the procalcitonin group completed at least 7 days of antibiotic therapy. At Day 7, antibiotics were discontinued for all patients with procalcitonin < 0.5 ng/ml | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation using random number table |
| Allocation concealment (selection bias) | Low risk | Sealed, numbered envelopes opened in sequence |
| Blinding (performance bias and detection bias) | High risk | Treating team were made aware of assignment on Day 7, when PCT measured and communicated to PCT group |
| Incomplete outcome data (attrition bias) | Low risk | No patients lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | High risk | Potential source of bias as this is a per‐protocol analysis; exclusion of 9 patients with low PCT measurements in the PCT group may exclude a higher proportion of relatively well patients compared with the control group |
| Methods | Single‐centre study from the USA. Randomised controlled trial comparing strategy of 3 days' ciprofloxacin monotherapy versus standard therapy (duration and antibiotic choice at the discretion of physician) for patients with pulmonary infiltrates but low‐probability pneumonia | |
| Participants | Study entry criteria: new‐onset pulmonary infiltrate associated with possible nosocomial pneumonia. Modified Clinical Pulmonary Infection Score (CPIS) < 7 on Day 1 (suggesting low probability pneumonia) 81 adult medical and surgical ICU patients; 47 (58%) receiving mechanical ventilation; mean age 66.7 years; no data on sex. APACHE III score 41.8; mean CPIS 4.9. Prior mean duration of ICU stay 8.8 days and duration of mechanical ventilation 7.6 days. Chronic obstructive airways disease present in 27% cases. 5% patients were transplant recipients. With the exception of abnormal respiration (92% experimental group versus 71% standard‐therapy group, P = 0.016), there were no significant differences in baseline characteristics between the 2 groups Exclusions: HIV, chemotherapy‐induced neutropenia, concurrent antibiotic administration (other than surgical prophylaxis), flouroquinolone allergy | |
| Interventions | Randomised at Day 1 of episode of possible pneumonia. Intervention group (N = 39): 3 days' ciprofloxacin monotherapy. At Day 3 if CPIS < 7, antibiotics would be discontinued; if CPIS > 6, therapy would be continued, with choice of agent and duration of therapy at the discretion of treating physician, and incorporating microbiology results Standard therapy (N = 42): choice and duration of antibiotic therapy at choice of treating physician | |
| Outcomes | The following outcomes were reported:
| |
| Notes | Pathogens associated with possible HAP were incompletely presented For patients allocated to the short‐course therapy, 0 patients with CPIS < 7 at Day 3 had antibiotics continued beyond 72 hours A significant decrease in duration of therapy in "Standard therapy" group was observed with time (P = 0.0001), thought to be a result of unblinded nature of study. The study was terminated by institutional review board as it was deemed "unethical to continue study." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation process not described |
| Allocation concealment (selection bias) | Unclear risk | Randomisation/concealment not described |
| Blinding (performance bias and detection bias) | High risk | This was an unblinded study |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | High risk | The components of the composite measure of "emergence of antimicrobial resistance or superinfection" are not reported |
| Other bias | High risk | Stopped early by institutional review board. Results for patients managed in "experimental" group appear to have influenced management of patients subsequently allocated to "standard therapy group" |
| Methods | The ProVAP study, an international, multi‐centre study involving 7 ICUs in USA and Switzerland. Unblinded, randomised controlled trial comparing strategy utilising serum procalcitonin measurement to guide discontinuation of antibiotics for ICU patients with VAP, with antibiotic administration according to an agreed guideline | |
| Participants | 101 patients with VAP; 36% NF‐GNB; 10% MRSA; mean age 56; 75% male; 27% surgical patients; 19% with chronic obstructive airways disease. Mean SAPS II score 43; ODIN score 2.1; SOFA score 7.8. Mean duration of mechanical ventilation before onset of VAP 6 days. 75% received antibiotics during the 14 days before VAP. 89% patients had CPIS ≥ 6.ODIN score was slightly higher in the control group than the procalcitonin group (2.3 versus 1.9; P = 0.042); otherwise, there were no significant differences in baseline characteristics. VAP diagnosed according to clinical criteria: new or persistent infiltrates on chest radiography with at least 2 of purulent tracheal secretions, temperature > 38 ºC, leukocyte count > 11,000/ µL or < 3000/ µL; microbiological confirmation was not an essential criterion, and indeed was absent in 27% patients. Exclusions: treatment with immunosuppressants or long‐term corticosteroid treatment; or underlying immunosuppressant disorder; co‐existing extrapulmonary infection | |
| Interventions | 101 patients were randomised on day of diagnosis of VAP (Day 0). Serum procalcitonin levels were measured from Day 0 to Day 10 for all patients (including patients in the control group). For the 51 patients in the intervention group, discontinuation of antibiotic therapy was encouraged according to an algorithm 72 hours (Day 2) after randomisation if PCT was < 0.5 µg/L or had decreased to 20% or less of level on Day 0 For the 50 patients randomised to the control group, procalcitonin levels were withheld, and duration of therapy was determined by treating physician In both intervention and control groups, choice of antibiotic therapy was that of the attending physician | |
| Outcomes | The following outcome measures were reported:
| |
| Notes | It is unclear how many patients were treated with antibiotics beyond the point at which discontinuation was advocated by the protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Arbitrary allocation to... treatment assignments based on sealed, opaque envelopes." |
| Allocation concealment (selection bias) | Low risk | "Treating physicians were not aware of envelope contents before randomisation." |
| Blinding (performance bias and detection bias) | High risk | Not blinded |
| Incomplete outcome data (attrition bias) | Low risk | No patients lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Complete data on numbers of patients for whom physicians violate (PCT) protocol not presented |
| Other bias | Low risk | There appear not to be other major potential sources of bias |
APACHE II score: Acute Physiology and Chronic Health Evaluation II score
BAL: broncho‐alveolar lavage
cfu/ml: colony‐forming units per millilitre
COPD: chronic obstructive airways disease
CPIS: clinical pulmonary infection score
DNR order: do not resuscitate order
ETA: endo‐tracheal aspirate
FiO2: fraction of inspired oxygen (in a gas mixture)
HAP: hospital‐acquired pneumonia
ICU: intensive care unit
ITU: intensive therapy unit
MODS: multiple organ dysfunction score
MRSA: methicillin‐resistant Staphylococcus aureus
NF‐GNB: non‐fermenting Gram‐negative bacilli
ODIN: organ dysfunction and/or infection score
PaO2: partial pressure of oxygen
PCT: procalcitonin
PSB: protected specimen brush
SAPS II: Simplified Acute Physiology Score II
SOFA score: Sequential Organ Failure Assessment score
VAP: ventilator‐associated pneumonia
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| A randomised controlled study investigating diagnostic strategies (quantitative versus qualitative culture of respiratory tract specimens) on outcome from VAP | |
| This 2‐by‐2 factorial randomised controlled study aiming to compare: i. invasive and non‐invasive diagnostic strategies for diagnosis of VAP, and ii. initial empiric treatment with meropenem or meropenem plus ciprofloxacin for suspected VAP. There was no significant difference in number of antibiotic‐free days between groups of patients allocated to invasive or non‐invasive strategies | |
| Data subsequently published in full (Chastre 2003a) | |
| This was a randomised study designed to compare strategies for diagnosis and selection of initial antimicrobial therapy of VAP, not duration of therapy. For groups of patients randomised to both invasive and non‐invasive diagnostic strategies, recommended duration of therapy in the presence of positive respiratory culture (i.e. confirmed pneumonia) was 14 days | |
| This was a randomised controlled study comparing a strategy using serial procalcitonin (PCT) measurement to guide discontinuation of antibiotic therapy for treatment of sepsis in 110 surgical intensive care patients with standard therapy. Of these patients, 43 had "pneumonia"; it is unclear what proportion had nosocomial or community‐acquired pneumonia, and what proportion of patients was receiving mechanical ventilation at time of diagnosis. Duration of antibiotic therapy was significantly shorter in the intervention (PCT‐guided) group (5.9 +/‐ 1.7 days versus 7.9 +/‐ 0.5 days), but outcome data relevant to this systematic review are restricted to duration of ITU stay and hospital mortality | |
| Non‐randomised before‐and‐after study investigating a clinical guideline regarding initial treatment and subsequent discontinuation of antibiotic therapy | |
| This was an RCT investigating effectiveness of an antibiotic de‐escalation protocol for patients with low probability of HAP (according to CPIS and culture results), published in abstract form only. It has not been possible to contact study authors for further details. It is unclear whether it was a single or multi‐centre study. 109 patients (unclear whether exclusively adults or not) with HAP (diagnostic criteria not described) were randomised to the de‐escalation protocol (N = 54) or standard therapy (N = 55). The protocol is not described nor relevant outcome data presented adequately for inclusion in this review. In addition, the significantly higher rate of appropriate initial antibiotic therapy in the de‐escalation group represents a potential risk of bias | |
| Prospective observational study investigating application of previously described clinical guideline (Ibrahim 2001) for patients with suspected VAP, but negative quantitative BAL results | |
| This multi‐centre randomised controlled study from Mexico enrolled 65 patients in a study intended to evaluate a strategy of early discontinuation of empirical antibiotic therapy: it has been published in abstract form only. 31 patients were allocated to early (< 8 days) discontinuation of empirical therapy versus 34 patients to late discontinuation (> 9 days). Patient characteristics and diagnostic criteria were not described. Outcome data presented in the abstract was inadequate for the study's inclusion in this review, and contact with a trial author did not yield any further information. Furthermore, a highly significant risk of bias was identified: antibiotic discontinuation at Day 8 was higher (70.6%) among patients allocated to late discontinuation than among patients allocated to early discontinuation (67.7%) | |
| This was a randomised controlled study comparing a strategy using serial measurements of procalcitonin (PCT) to guide cessation of antibiotic therapy with standard therapy in critically ill patients with sepsis or septic shock. 79 patients were randomised, of whom a total of 47 patients had sepsis of pulmonary origin. A high proportion of patients (68%) had community‐acquired sepsis. A significant proportion of patients in the PCT group (19%) had the protocol overridden to receive a longer course of antibiotics than advised by the algorithm. On intention‐to‐treat analysis, the difference in antibiotic days between control and intervention groups for all patients was not significant. A decision was made to exclude this study on the basis of small numbers of the subgroup of patients with suspected HAP or VAP and the lack of significant difference between groups in terms of duration of therapy | |
| This was a randomised study comparing diagnostic strategies for suspected VAP. It was not designed to investigate duration of therapy; there were no protocols to guide duration of antibiotic therapy | |
| This was an observational rather than a randomised controlled study. It investigated outcomes following introduction of a "De‐escalation" strategy which incorporated the initial administration of broad spectrum antibiotics and subsequent simplification of antibiotic treatment with culture results: 1. changing to monotherapy in absence of Pseudomonas sp; 2. shortening therapy to < 5 days if culture negative and > 48 hours of defervescence; 3. changing from broad to narrow spectrum agent on basis of culture results | |
| A randomised clinical trial comparing the effects of an invasive quantitative diagnostic strategy versus a non‐invasive strategy on management of and outcome from suspected VAP | |
| This was a RCT comparing a procalcitonin‐guided antibiotic discontinuation strategy with standard treatment for surgical intensive care patients with severe sepsis. The reasons for exclusion of this study are: 1. its very small size (of the 27 patients enrolled in this study, only 8 were diagnosed with pneumonia); 2. it is unclear what proportion of patients had nosocomial versus community‐acquired infection; 3. of the patients with "pneumonia" it is unclear what proportion were receiving mechanical ventilation at time of diagnosis; 4. limited outcome data relevant to this systematic review are published (in‐hospital mortality and duration of ICU stay) | |
| Data subsequently published in full (Singh 2000) | |
| A RCT to evaluate invasive versus non‐invasive diagnostic methods on outcome from VAP | |
| Data subsequently published in full (Stolz 2009a) | |
| RCT evaluating PCT‐guided strategies in the management of septic illness after multiple trauma or major surgery. The study makes no reference to patients with HAP | |
| Data subsequently published in full (Chastre 2003a) |
BAL: broncho‐alveolar lavage
CPIS: clinical pulmonary infection score
HAP: hospital‐acquired pneumonia
ICU: intensive care unit
ITU: intensive therapy unit
PCT: procalcitonin
RCT: randomised controlled trial
VAP: ventilator‐associated pneumonia
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Comparative study of C‐reactive protein versus procalcitonin to guide antibiotic therapy in patients with severe sepsis and septic shock admitted to the Intensive Care Unit |
| Methods | Randomised controlled trial |
| Participants | Adult ICU patients with severe sepsis or septic shock |
| Interventions | CRP‐guided antibiotic therapy versus procalcitonin‐guided antibiotic therapy |
| Outcomes | Primary: duration of antibiotic therapy for first episode infection, total antibiotic days, 28‐day antibiotic‐free days |
| Starting date | September 2009 |
| Contact information | http://clinicaltrials.gov/ct2/show/NCT00934011 |
| Notes | NCT00934011 |
| Trial name or title | Reduction of antibiotic use in the ICU: procalcitonin guided versus conventional antibiotic therapy in patients with sepsis in the ICU |
| Methods | Randomised controlled study |
| Participants | Adult ICU patients receiving antibiotic therapy for sepsis of suspected or proven focus of infection |
| Interventions | Procalcitonin‐guided antibiotic therapy versus standard antibiotic therapy |
| Outcomes | Primary outcome: duration of antibiotic therapy; secondary outcome: 28‐day mortality |
| Starting date | October 2009 |
| Contact information | http://clinicaltrials.gov/ct2/show/NCT00987818 |
| Notes | NCT00987818 |
| Trial name or title | The procalcitonin and survival study (PASS) |
| Methods | A randomised multi‐centre investigator‐initiated trial to investigate whether procalcitonin‐guided diagnostic and therapeutic strategies can improve survival in intensive care unit patients |
| Participants | 1000 critically ill patients |
| Interventions | "Standard of care" versus "standard of care and procalcitonin‐guided diagnostics and treatment of infection" |
| Outcomes | Primary outcome: 28‐day mortality |
| Starting date | In progress |
| Contact information | http://clinicaltrials.gov/ct2/show/NCT00271752 |
| Notes | NCT00271752 |
| Trial name or title | Randomised multicenter prospective study of procalcitonin‐guided treatment on antibiotic use and outcome in severe sepsis ICU patients without obvious infection |
| Methods | Randomised controlled trial |
| Participants | Adult patients hospitalised in resuscitation ward, severe sepsis symptomatology, 2 or more SIRS criteria, at least one organ deficiency, no infectious aetiology |
| Interventions | Duration of antibiotic therapy guided by procalcitonin level versus standard care |
| Outcomes | Primary outcome: antibiotic treatment at day 5 |
| Starting date | December 2007 |
| Contact information | http://clinicaltrials.gov/ct2/show/NCT01025180 |
| Notes | NCT01025180 |
| Trial name or title | Safety and efficacy of procalcitonin guided antibiotic therapy in adult intensive care units (ICU's) (SAPS) |
| Methods | Randomised controlled trial |
| Participants | Adult ICU patients with suspected or proven infection |
| Interventions | Procalcitonin‐guided antibiotic therapy versus standard therapy |
| Outcomes | Primary: 28‐day mortality; consumption of antibiotics expressed as the defined daily dosage and duration of antibiotic therapy expressed in days of therapy |
| Starting date | November 2009 |
| Contact information | http://clinicaltrials.gov/ct2/show/NCT01139489 |
| Notes | NCT01139489 |
| Trial name or title | Placebo‐controlled trial of sodium selenite and procalcitonin guided antimicrobial therapy in severe sepsis (SISPCT) |
| Methods | Prospective, randomised multi‐centre 2 x 2 trial |
| Participants | Adult patients with severe sepsis or septic shock, with onset of < 24 hours |
| Interventions | 2 x 2 trial: 1. Intravenous sodium‐selenite versus placebo 2. Procalcitonin‐guided antibiotic therapy versus alternative (non‐PCT guided) antibiotic protocol |
| Outcomes | Primary outcome: 28‐day mortality |
| Starting date | November 2009 |
| Contact information | www.clinicaltrials.gov/ct2/show/NCT00832039 |
| Notes | NCT00832039 |
CRP: C‐reactive protein
ICU: intensive care unit
PCT: procalcitonin
SIRS: Systemic Inflammatory Response Syndrome
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
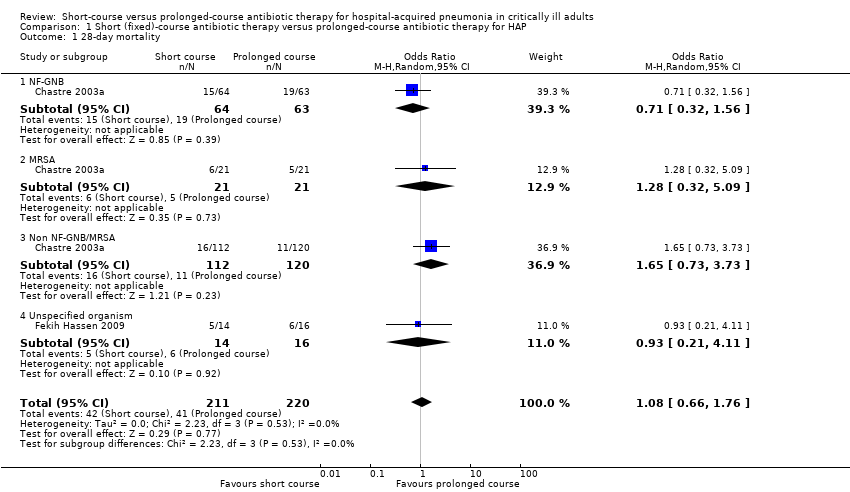
| 1 28‐day mortality Show forest plot | 2 | 431 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.66, 1.76] |
| Analysis 1.1  Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 1 28‐day mortality. | ||||
| 1.1 NF‐GNB | 1 | 127 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.32, 1.56] |
| 1.2 MRSA | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 1.28 [0.32, 5.09] |
| 1.3 Non NF‐GNB/MRSA | 1 | 232 | Odds Ratio (M‐H, Random, 95% CI) | 1.65 [0.73, 3.73] |
| 1.4 Unspecified organism | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.21, 4.11] |
| 2 Recurrence of pneumonia Show forest plot | 3 | 508 | Odds Ratio (M‐H, Random, 95% CI) | 1.37 [0.87, 2.17] |
| Analysis 1.2  Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 2 Recurrence of pneumonia. | ||||
| 2.1 NF‐GNB | 2 | 176 | Odds Ratio (M‐H, Random, 95% CI) | 2.18 [1.14, 4.16] |
| 2.2 MRSA | 2 | 49 | Odds Ratio (M‐H, Random, 95% CI) | 1.56 [0.12, 19.61] |
| 2.3 Non NF‐GNB/MRSA | 2 | 253 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.55, 1.78] |
| 2.4 Unspecified organism | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 1.17 [0.14, 9.59] |
| 3 28‐day antibiotic‐free days Show forest plot | 2 | 431 | Mean Difference (IV, Random, 95% CI) | 4.02 [2.26, 5.78] |
| Analysis 1.3  Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 3 28‐day antibiotic‐free days. | ||||
| 3.1 NF‐GNB | 1 | 127 | Mean Difference (IV, Random, 95% CI) | 4.5 [2.25, 6.75] |
| 3.2 MRSA | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 8.0 [4.14, 11.86] |
| 3.3 Non NF‐GNB/ MRSA | 1 | 232 | Mean Difference (IV, Random, 95% CI) | 3.70 [2.09, 5.31] |
| 3.4 Unspecified organism | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 2.3 [1.03, 3.57] |
| 4 ITU mortality Show forest plot | 2 | 107 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.37, 1.91] |
| Analysis 1.4  Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 4 ITU mortality. | ||||
| 5 Non‐resolution of pneumonia Show forest plot | 1 | 77 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.80 [0.65, 5.02] |
| Analysis 1.5  Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 5 Non‐resolution of pneumonia. | ||||
| 5.1 NF‐GNB | 1 | 49 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.49, 7.40] |
| 5.2 MRSA | 1 | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | 11.0 [0.28, 433.80] |
| 5.3 Non NF‐GNB/MRSA | 1 | 21 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.16, 6.25] |
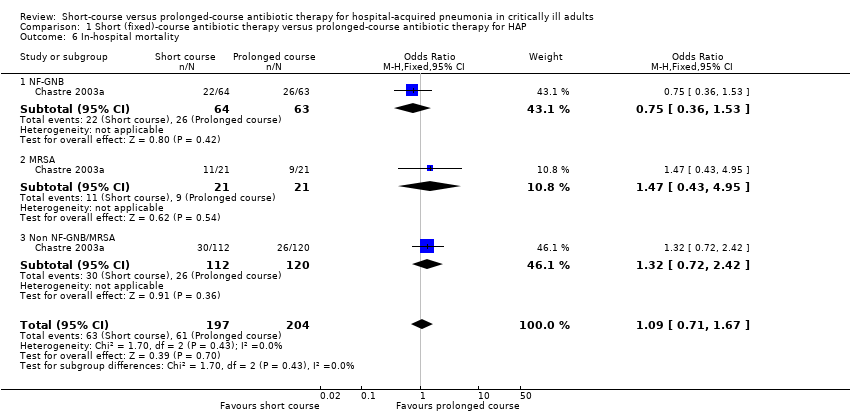
| 6 In‐hospital mortality Show forest plot | 1 | 401 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.71, 1.67] |
| Analysis 1.6  Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 6 In‐hospital mortality. | ||||
| 6.1 NF‐GNB | 1 | 127 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.36, 1.53] |
| 6.2 MRSA | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.43, 4.95] |
| 6.3 Non NF‐GNB/MRSA | 1 | 232 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.72, 2.42] |
| 7 Recurrence due to multi‐resistant organism Show forest plot | 1 | 110 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.21, 0.95] |
| Analysis 1.7  Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 7 Recurrence due to multi‐resistant organism. | ||||
| 8 Duration of ITU stay Show forest plot | 2 | 431 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐2.30, 2.27] |
| Analysis 1.8  Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 8 Duration of ITU stay. | ||||
| 8.1 NF‐GNB | 1 | 127 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐5.40, 7.20] |
| 8.2 MRSA | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 2.90 [‐8.39, 14.19] |
| 8.3 Non NF‐GNB/MRSA | 1 | 232 | Mean Difference (IV, Random, 95% CI) | 2.70 [‐1.88, 7.28] |
| 8.4 Unspecified organism | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐4.61, 1.41] |
| 9 Duration of hospital stay Show forest plot | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐4.11, 2.11] |
| Analysis 1.9  Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 9 Duration of hospital stay. | ||||
| 10 Duration of mechanical ventilation Show forest plot | 2 | 107 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.57, 0.55] |
| Analysis 1.10  Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 10 Duration of mechanical ventilation. | ||||
| 11 28‐day mechanical ventilation‐free days Show forest plot | 2 | 431 | Mean Difference (IV, Random, 95% CI) | 0.47 [‐0.97, 1.92] |
| Analysis 1.11  Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 11 28‐day mechanical ventilation‐free days. | ||||
| 11.1 NF‐GNB | 1 | 127 | Mean Difference (IV, Random, 95% CI) | 1.50 [‐1.77, 4.77] |
| 11.2 MRSA | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐6.37, 3.77] |
| 11.3 Non NF‐GNB/MRSA | 1 | 232 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐3.54, 1.14] |
| 11.4 Unspecified organism | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 1.30 [‐0.03, 2.63] |
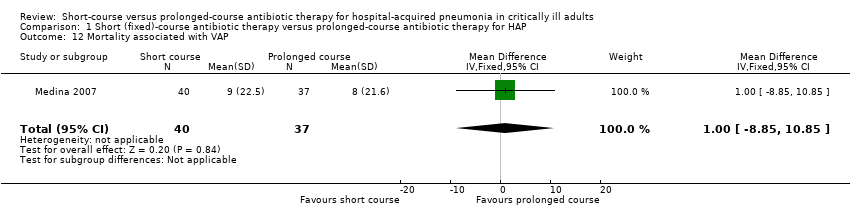
| 12 Mortality associated with VAP Show forest plot | 1 | 77 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐8.85, 10.85] |
| Analysis 1.12  Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 12 Mortality associated with VAP. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 30‐day mortality Show forest plot | 1 | 81 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.10, 1.03] |
| Analysis 2.1  Comparison 2 Discontinuation of antibiotics according to Clinical Pulmonary Infection Score, Outcome 1 30‐day mortality. | ||||
| 2 Episodes of superinfection or antimicrobial resistance Show forest plot | 1 | 81 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.09, 0.92] |
| Analysis 2.2  Comparison 2 Discontinuation of antibiotics according to Clinical Pulmonary Infection Score, Outcome 2 Episodes of superinfection or antimicrobial resistance. | ||||
| 3 Duration of antibiotic therapy Show forest plot | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 2.3  Comparison 2 Discontinuation of antibiotics according to Clinical Pulmonary Infection Score, Outcome 3 Duration of antibiotic therapy. | ||||
| 4 Duration of ITU stay Show forest plot | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 2.4  Comparison 2 Discontinuation of antibiotics according to Clinical Pulmonary Infection Score, Outcome 4 Duration of ITU stay. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of pneumonia Show forest plot | 1 | 290 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.48, 1.59] |
| Analysis 3.1  Comparison 3 Discontinuation of antibiotics according clinical guideline, Outcome 1 Recurrence of pneumonia. | ||||
| 2 Duration of antibiotic therapy Show forest plot | 1 | 290 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐3.21, ‐0.79] |
| Analysis 3.2  Comparison 3 Discontinuation of antibiotics according clinical guideline, Outcome 2 Duration of antibiotic therapy. | ||||
| 3 In‐hospital mortality Show forest plot | 1 | 290 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.49, 1.29] |
| Analysis 3.3  Comparison 3 Discontinuation of antibiotics according clinical guideline, Outcome 3 In‐hospital mortality. | ||||
| 4 Duration of ITU stay Show forest plot | 1 | 290 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.75, 1.35] |
| Analysis 3.4  Comparison 3 Discontinuation of antibiotics according clinical guideline, Outcome 4 Duration of ITU stay. | ||||
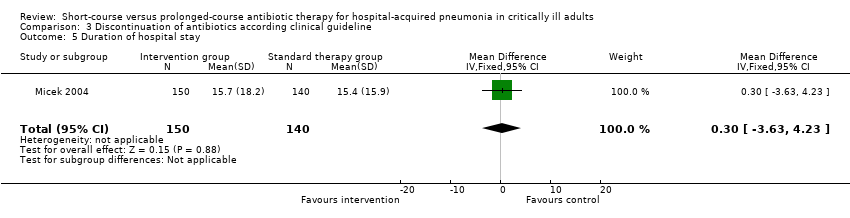
| 5 Duration of hospital stay Show forest plot | 1 | 290 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐3.63, 4.23] |
| Analysis 3.5  Comparison 3 Discontinuation of antibiotics according clinical guideline, Outcome 5 Duration of hospital stay. | ||||
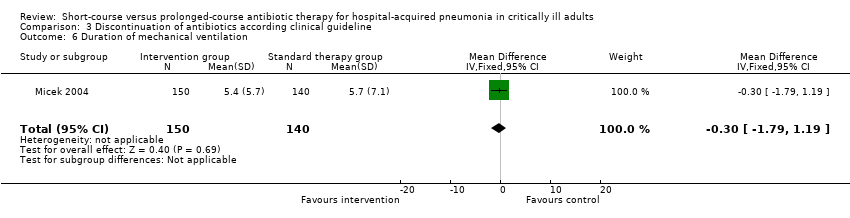
| 6 Duration of mechanical ventilation Show forest plot | 1 | 290 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.79, 1.19] |
| Analysis 3.6  Comparison 3 Discontinuation of antibiotics according clinical guideline, Outcome 6 Duration of mechanical ventilation. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 28‐day mortality Show forest plot | 3 | 308 | Odds Ratio (M‐H, Random, 95% CI) | 0.66 [0.39, 1.14] |
| Analysis 4.1  Comparison 4 Discontinuation of antibiotic therapy according to serum procalcitonin level, Outcome 1 28‐day mortality. | ||||
| 2 Recurrence of pneumonia Show forest plot | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.74, 5.70] |
| Analysis 4.2  Comparison 4 Discontinuation of antibiotic therapy according to serum procalcitonin level, Outcome 2 Recurrence of pneumonia. | ||||
| 3 28‐day antibiotic‐free days Show forest plot | 3 | 308 | Mean Difference (IV, Random, 95% CI) | 2.80 [1.39, 4.21] |
| Analysis 4.3  Comparison 4 Discontinuation of antibiotic therapy according to serum procalcitonin level, Outcome 3 28‐day antibiotic‐free days. | ||||
| 4 Duration of antibiotic therapy Show forest plot | 3 | 308 | Mean Difference (IV, Random, 95% CI) | ‐3.20 [‐4.45, ‐1.95] |
| Analysis 4.4  Comparison 4 Discontinuation of antibiotic therapy according to serum procalcitonin level, Outcome 4 Duration of antibiotic therapy. | ||||
| 5 In‐hospital mortality Show forest plot | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.25, 1.58] |
| Analysis 4.5  Comparison 4 Discontinuation of antibiotic therapy according to serum procalcitonin level, Outcome 5 In‐hospital mortality. | ||||
| 6 ITU mortality Show forest plot | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.26, 2.22] |
| Analysis 4.6  Comparison 4 Discontinuation of antibiotic therapy according to serum procalcitonin level, Outcome 6 ITU mortality. | ||||
| 7 Non‐resolution of pneumonia Show forest plot | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.38, 3.62] |
| Analysis 4.7  Comparison 4 Discontinuation of antibiotic therapy according to serum procalcitonin level, Outcome 7 Non‐resolution of pneumonia. | ||||
| 8 Recurrence due to resistant organism Show forest plot | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.49, 6.21] |
| Analysis 4.8  Comparison 4 Discontinuation of antibiotic therapy according to serum procalcitonin level, Outcome 8 Recurrence due to resistant organism. | ||||
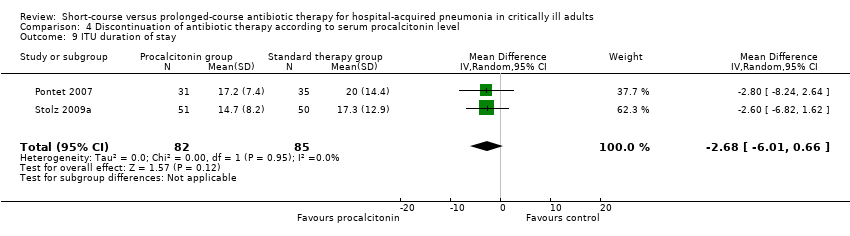
| 9 ITU duration of stay Show forest plot | 2 | 167 | Mean Difference (IV, Random, 95% CI) | ‐2.68 [‐6.01, 0.66] |
| Analysis 4.9  Comparison 4 Discontinuation of antibiotic therapy according to serum procalcitonin level, Outcome 9 ITU duration of stay. | ||||
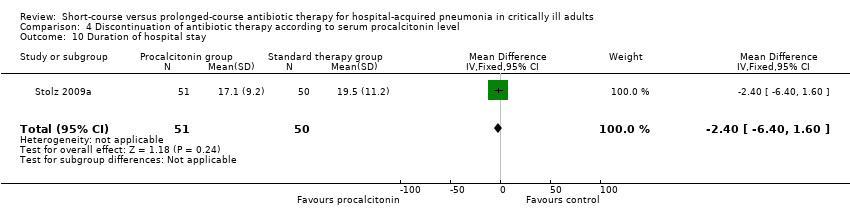
| 10 Duration of hospital stay Show forest plot | 1 | 101 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐6.40, 1.60] |
| Analysis 4.10  Comparison 4 Discontinuation of antibiotic therapy according to serum procalcitonin level, Outcome 10 Duration of hospital stay. | ||||
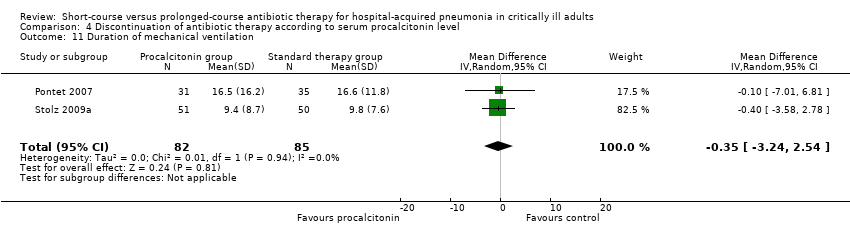
| 11 Duration of mechanical ventilation Show forest plot | 2 | 167 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐3.24, 2.54] |
| Analysis 4.11  Comparison 4 Discontinuation of antibiotic therapy according to serum procalcitonin level, Outcome 11 Duration of mechanical ventilation. | ||||
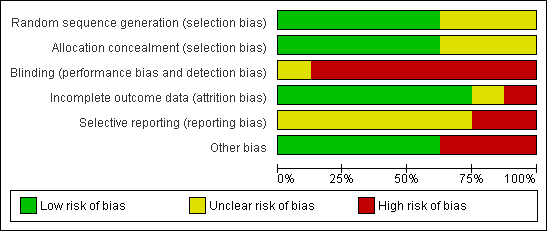
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
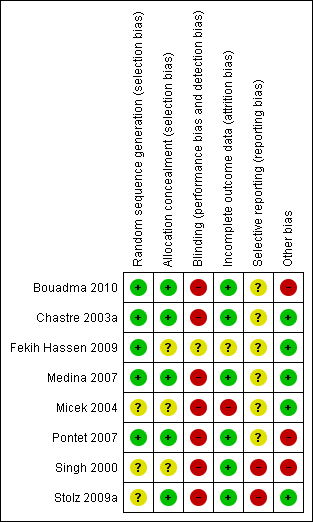
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 1 28‐day mortality.

Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 2 Recurrence of pneumonia.

Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 3 28‐day antibiotic‐free days.

Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 4 ITU mortality.
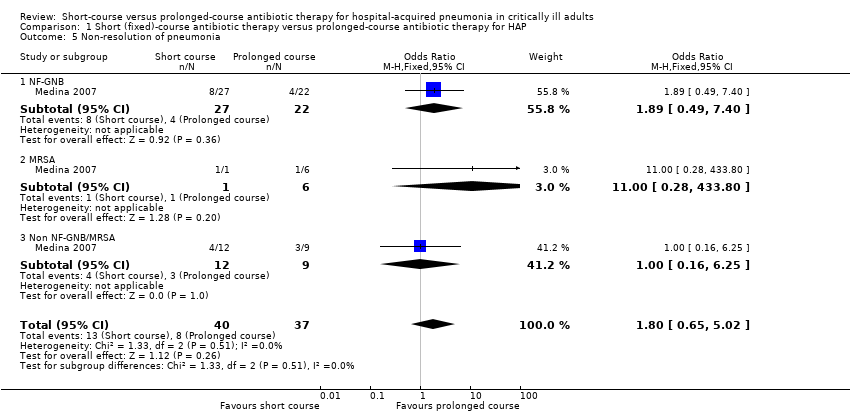
Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 5 Non‐resolution of pneumonia.
Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 6 In‐hospital mortality.

Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 7 Recurrence due to multi‐resistant organism.

Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 8 Duration of ITU stay.

Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 9 Duration of hospital stay.

Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 10 Duration of mechanical ventilation.

Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 11 28‐day mechanical ventilation‐free days.
Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 12 Mortality associated with VAP.

Comparison 2 Discontinuation of antibiotics according to Clinical Pulmonary Infection Score, Outcome 1 30‐day mortality.

Comparison 2 Discontinuation of antibiotics according to Clinical Pulmonary Infection Score, Outcome 2 Episodes of superinfection or antimicrobial resistance.

Comparison 2 Discontinuation of antibiotics according to Clinical Pulmonary Infection Score, Outcome 3 Duration of antibiotic therapy.

Comparison 2 Discontinuation of antibiotics according to Clinical Pulmonary Infection Score, Outcome 4 Duration of ITU stay.

Comparison 3 Discontinuation of antibiotics according clinical guideline, Outcome 1 Recurrence of pneumonia.

Comparison 3 Discontinuation of antibiotics according clinical guideline, Outcome 2 Duration of antibiotic therapy.

Comparison 3 Discontinuation of antibiotics according clinical guideline, Outcome 3 In‐hospital mortality.

Comparison 3 Discontinuation of antibiotics according clinical guideline, Outcome 4 Duration of ITU stay.
Comparison 3 Discontinuation of antibiotics according clinical guideline, Outcome 5 Duration of hospital stay.
Comparison 3 Discontinuation of antibiotics according clinical guideline, Outcome 6 Duration of mechanical ventilation.
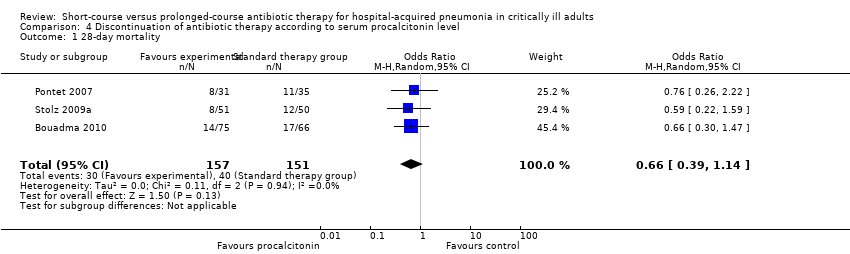
Comparison 4 Discontinuation of antibiotic therapy according to serum procalcitonin level, Outcome 1 28‐day mortality.

Comparison 4 Discontinuation of antibiotic therapy according to serum procalcitonin level, Outcome 2 Recurrence of pneumonia.

Comparison 4 Discontinuation of antibiotic therapy according to serum procalcitonin level, Outcome 3 28‐day antibiotic‐free days.
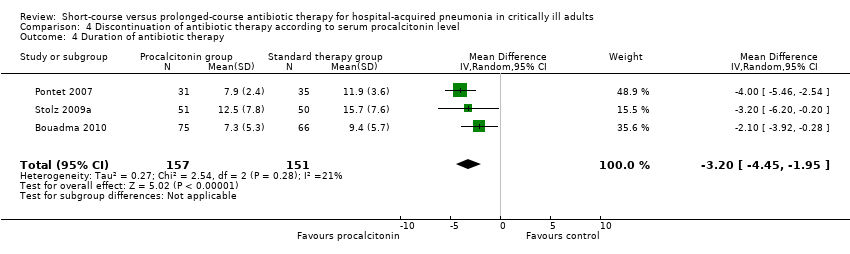
Comparison 4 Discontinuation of antibiotic therapy according to serum procalcitonin level, Outcome 4 Duration of antibiotic therapy.
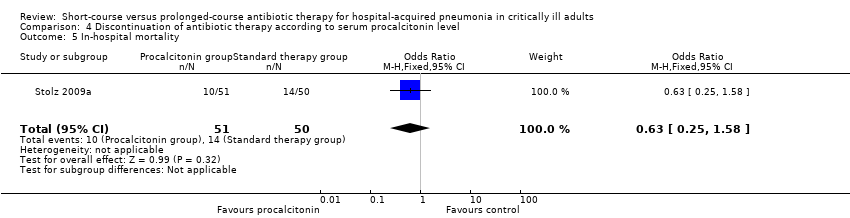
Comparison 4 Discontinuation of antibiotic therapy according to serum procalcitonin level, Outcome 5 In‐hospital mortality.

Comparison 4 Discontinuation of antibiotic therapy according to serum procalcitonin level, Outcome 6 ITU mortality.

Comparison 4 Discontinuation of antibiotic therapy according to serum procalcitonin level, Outcome 7 Non‐resolution of pneumonia.
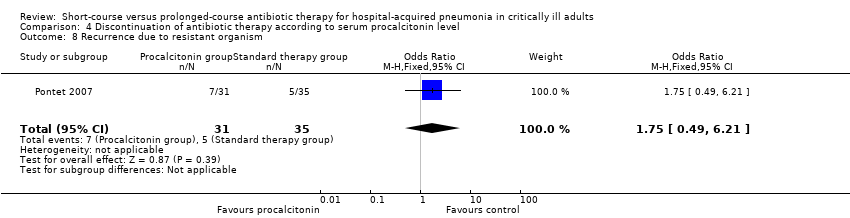
Comparison 4 Discontinuation of antibiotic therapy according to serum procalcitonin level, Outcome 8 Recurrence due to resistant organism.
Comparison 4 Discontinuation of antibiotic therapy according to serum procalcitonin level, Outcome 9 ITU duration of stay.
Comparison 4 Discontinuation of antibiotic therapy according to serum procalcitonin level, Outcome 10 Duration of hospital stay.
Comparison 4 Discontinuation of antibiotic therapy according to serum procalcitonin level, Outcome 11 Duration of mechanical ventilation.
| Should short (fixed duration)‐course antibiotic therapy versus prolonged‐course antibiotic therapy be used for critically ill patients with hospital‐acquired pneumonia? | ||||||
| Patient or population: critically ill patients with hospital‐acquired pneumonia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Prolonged‐course antibiotic therapy | Short (fixed duration)‐course antibiotic therapy | |||||
| 28‐day mortality | Study population | OR 1.08 | 431 | |||
| 186 per 1000 | 198 per 1000 | |||||
| Medium‐risk population | ||||||
| 270 per 1000 | 285 per 1000 | |||||
| 28‐day mortality ‐ NF‐GNB | Study population | OR 0.71 | 127 | See comment | ||
| 302 per 1000 | 235 per 1000 | |||||
| Medium‐risk population | ||||||
| 302 per 1000 | 235 per 1000 | |||||
| 28‐day mortality ‐ MRSA | Study population | OR 1.28 | 42 | See comment | ||
| 238 per 1000 | 286 per 1000 | |||||
| Medium‐risk population | ||||||
| 238 per 1000 | 286 per 1000 | |||||
| Recurrence of pneumonia | Study population | OR 1.37 | 508 | |||
| 245 per 1000 | 308 per 1000 | |||||
| Medium‐risk population | ||||||
| 227 per 1000 | 287 per 1000 | |||||
| Recurrence of pneumonia ‐ NF‐GNB | Study population | OR 2.18 | 176 | |||
| 247 per 1000 | 417 per 1000 | |||||
| Medium‐risk population | ||||||
| 241 per 1000 | 409 per 1000 | |||||
| Recurrence of pneumonia ‐ MRSA | Study population | OR 1.56 | 49 | |||
| 370 per 1000 | 478 per 1000 | |||||
| Medium‐risk population | ||||||
| 298 per 1000 | 398 per 1000 | |||||
| 28‐day antibiotic‐free days | The mean 28‐day antibiotic‐free days in the intervention groups was | 431 | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Discontinuation of antibiotics according to Clinical Pulmonary Infection Score for critically ill adults with hospital‐acquired pneumonia | ||||||
| Patient or population: critically ill adults with hospital‐acquired pneumonia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Discontinuation of antibiotics according to Clinical Pulmonary Infection Score | |||||
| 30‐day mortality | Study population | OR 0.33 | 81 | See comment | ||
| 310 per 1000 | 129 per 1000 | |||||
| Medium‐risk population | ||||||
| 310 per 1000 | 129 per 1000 | |||||
| Episodes of superinfection or antimicrobial resistance | Study population | OR 0.29 | 81 | See comment | ||
| 333 per 1000 | 126 per 1000 | |||||
| Medium‐risk population | ||||||
| 333 per 1000 | 126 per 1000 | |||||
| Duration of antibiotic therapy | See comment | See comment | Not estimable | 81 | See comment | |
| Duration of ITU stay | See comment | See comment | Not estimable | 81 | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Discontinuation of antibiotics according to clinical guideline for hospital‐acquired pneumonia in critically ill adults | ||||||
| Patient or population: patients with hospital‐acquired pneumonia in critically ill adults | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Discontinuation of antibiotics according to clinical guideline | |||||
| Recurrence of pneumonia | Study population | OR 0.88 | 290 | See comment | ||
| 193 per 1000 | 174 per 1000 | |||||
| Medium‐risk population | ||||||
| 193 per 1000 | 174 per 1000 | |||||
| Duration of antibiotic therapy | The mean duration of antibiotic therapy in the intervention groups was | 290 | See comment | |||
| In‐hospital mortality | Study population | OR 0.8 | 290 | See comment | ||
| 371 per 1000 | 321 per 1000 | |||||
| Medium‐risk population | ||||||
| 371 per 1000 | 321 per 1000 | |||||
| Duration of ICU stay | The mean duration of ICU stay in the intervention groups was | 290 | See comment | |||
| Duration of hospital stay | The mean duration of hospital stay in the intervention groups was | 290 | See comment | |||
| Duration of mechanical ventilation | The mean duration of mechanical ventilation in the intervention groups was | 290 | See comment | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Discontinuation of antibiotic therapy according to serum procalcitonin level for hospital‐acquired pneumonia in critically ill adults | ||||||
| Patient or population: hospital‐acquired pneumonia in critically ill adults | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Discontinuation of antibiotic therapy according to serum procalcitonin level | |||||
| 28‐day mortality | Study population | OR 0.66 | 308 | |||
| 265 per 1000 | 192 per 1000 | |||||
| Medium‐risk population | ||||||
| 258 per 1000 | 187 per 1000 | |||||
| Recurrence of pneumonia | Study population | OR 2.06 | 66 | See comment | ||
| 286 per 1000 | 452 per 1000 | |||||
| Medium‐risk population | ||||||
| 286 per 1000 | 452 per 1000 | |||||
| 28‐day antibiotic‐free days | The mean 28‐day antibiotic‐free days in the intervention groups was | 167 | ||||
| Duration of antibiotic therapy | The mean duration of antibiotic therapy in the intervention groups was | 308 | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 28‐day mortality Show forest plot | 2 | 431 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.66, 1.76] |
| 1.1 NF‐GNB | 1 | 127 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.32, 1.56] |
| 1.2 MRSA | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 1.28 [0.32, 5.09] |
| 1.3 Non NF‐GNB/MRSA | 1 | 232 | Odds Ratio (M‐H, Random, 95% CI) | 1.65 [0.73, 3.73] |
| 1.4 Unspecified organism | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.21, 4.11] |
| 2 Recurrence of pneumonia Show forest plot | 3 | 508 | Odds Ratio (M‐H, Random, 95% CI) | 1.37 [0.87, 2.17] |
| 2.1 NF‐GNB | 2 | 176 | Odds Ratio (M‐H, Random, 95% CI) | 2.18 [1.14, 4.16] |
| 2.2 MRSA | 2 | 49 | Odds Ratio (M‐H, Random, 95% CI) | 1.56 [0.12, 19.61] |
| 2.3 Non NF‐GNB/MRSA | 2 | 253 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.55, 1.78] |
| 2.4 Unspecified organism | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 1.17 [0.14, 9.59] |
| 3 28‐day antibiotic‐free days Show forest plot | 2 | 431 | Mean Difference (IV, Random, 95% CI) | 4.02 [2.26, 5.78] |
| 3.1 NF‐GNB | 1 | 127 | Mean Difference (IV, Random, 95% CI) | 4.5 [2.25, 6.75] |
| 3.2 MRSA | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 8.0 [4.14, 11.86] |
| 3.3 Non NF‐GNB/ MRSA | 1 | 232 | Mean Difference (IV, Random, 95% CI) | 3.70 [2.09, 5.31] |
| 3.4 Unspecified organism | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 2.3 [1.03, 3.57] |
| 4 ITU mortality Show forest plot | 2 | 107 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.37, 1.91] |
| 5 Non‐resolution of pneumonia Show forest plot | 1 | 77 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.80 [0.65, 5.02] |
| 5.1 NF‐GNB | 1 | 49 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.49, 7.40] |
| 5.2 MRSA | 1 | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | 11.0 [0.28, 433.80] |
| 5.3 Non NF‐GNB/MRSA | 1 | 21 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.16, 6.25] |
| 6 In‐hospital mortality Show forest plot | 1 | 401 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.71, 1.67] |
| 6.1 NF‐GNB | 1 | 127 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.36, 1.53] |
| 6.2 MRSA | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.43, 4.95] |
| 6.3 Non NF‐GNB/MRSA | 1 | 232 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.72, 2.42] |
| 7 Recurrence due to multi‐resistant organism Show forest plot | 1 | 110 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.21, 0.95] |
| 8 Duration of ITU stay Show forest plot | 2 | 431 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐2.30, 2.27] |
| 8.1 NF‐GNB | 1 | 127 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐5.40, 7.20] |
| 8.2 MRSA | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 2.90 [‐8.39, 14.19] |
| 8.3 Non NF‐GNB/MRSA | 1 | 232 | Mean Difference (IV, Random, 95% CI) | 2.70 [‐1.88, 7.28] |
| 8.4 Unspecified organism | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐4.61, 1.41] |
| 9 Duration of hospital stay Show forest plot | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐4.11, 2.11] |
| 10 Duration of mechanical ventilation Show forest plot | 2 | 107 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.57, 0.55] |
| 11 28‐day mechanical ventilation‐free days Show forest plot | 2 | 431 | Mean Difference (IV, Random, 95% CI) | 0.47 [‐0.97, 1.92] |
| 11.1 NF‐GNB | 1 | 127 | Mean Difference (IV, Random, 95% CI) | 1.50 [‐1.77, 4.77] |
| 11.2 MRSA | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐6.37, 3.77] |
| 11.3 Non NF‐GNB/MRSA | 1 | 232 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐3.54, 1.14] |
| 11.4 Unspecified organism | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 1.30 [‐0.03, 2.63] |
| 12 Mortality associated with VAP Show forest plot | 1 | 77 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐8.85, 10.85] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 30‐day mortality Show forest plot | 1 | 81 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.10, 1.03] |
| 2 Episodes of superinfection or antimicrobial resistance Show forest plot | 1 | 81 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.09, 0.92] |
| 3 Duration of antibiotic therapy Show forest plot | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Duration of ITU stay Show forest plot | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of pneumonia Show forest plot | 1 | 290 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.48, 1.59] |
| 2 Duration of antibiotic therapy Show forest plot | 1 | 290 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐3.21, ‐0.79] |
| 3 In‐hospital mortality Show forest plot | 1 | 290 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.49, 1.29] |
| 4 Duration of ITU stay Show forest plot | 1 | 290 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.75, 1.35] |
| 5 Duration of hospital stay Show forest plot | 1 | 290 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐3.63, 4.23] |
| 6 Duration of mechanical ventilation Show forest plot | 1 | 290 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.79, 1.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 28‐day mortality Show forest plot | 3 | 308 | Odds Ratio (M‐H, Random, 95% CI) | 0.66 [0.39, 1.14] |
| 2 Recurrence of pneumonia Show forest plot | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.74, 5.70] |
| 3 28‐day antibiotic‐free days Show forest plot | 3 | 308 | Mean Difference (IV, Random, 95% CI) | 2.80 [1.39, 4.21] |
| 4 Duration of antibiotic therapy Show forest plot | 3 | 308 | Mean Difference (IV, Random, 95% CI) | ‐3.20 [‐4.45, ‐1.95] |
| 5 In‐hospital mortality Show forest plot | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.25, 1.58] |
| 6 ITU mortality Show forest plot | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.26, 2.22] |
| 7 Non‐resolution of pneumonia Show forest plot | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.38, 3.62] |
| 8 Recurrence due to resistant organism Show forest plot | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.49, 6.21] |
| 9 ITU duration of stay Show forest plot | 2 | 167 | Mean Difference (IV, Random, 95% CI) | ‐2.68 [‐6.01, 0.66] |
| 10 Duration of hospital stay Show forest plot | 1 | 101 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐6.40, 1.60] |
| 11 Duration of mechanical ventilation Show forest plot | 2 | 167 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐3.24, 2.54] |

