Short‐course versus prolonged‐course antibiotic therapy for hospital‐acquired pneumonia in critically ill adults
Abstract
Background
Pneumonia is the most common hospital‐acquired infection affecting patients in the intensive care unit (ICU). However, current national guidelines for the treatment of hospital‐acquired pneumonia (HAP) are several years old and the diagnosis of pneumonia in mechanically ventilated patients (VAP) has been subject to considerable recent attention. The optimal duration of antibiotic therapy for HAP in the critically ill is uncertain.
Objectives
To assess the effectiveness of short versus prolonged‐course antibiotics for HAP in critically ill adults, including patients with VAP.
Search methods
We searched CENTRAL (2015, Issue 5), MEDLINE (1946 to June 2015), MEDLINE in‐process and other non‐indexed citations (5 June 2015), EMBASE (2010 to June 2015), LILACS (1982 to June 2015) and Web of Science (1955 to June 2015).
Selection criteria
We considered all randomised controlled trials (RCTs) comparing a fixed 'short' duration of antibiotic therapy with a 'prolonged' course for HAP (including patients with VAP) in critically ill adults.
Data collection and analysis
Two review authors conducted data extraction and assessment of risk of bias. We contacted trial authors for additional information.
Main results
We identified six relevant studies involving 1088 participants. This included two new studies published after the date of our previous review (2011). There was substantial variation in participants, in the diagnostic criteria used to define an episode of pneumonia, in the interventions and in the reported outcomes. We found no evidence relating to patients with a high probability of HAP who were not mechanically ventilated. For patients with VAP, overall a short seven‐ or eight‐day course of antibiotics compared with a prolonged 10‐ to 15‐day course increased 28‐day antibiotic‐free days (two studies; N = 431; mean difference (MD) 4.02 days; 95% confidence interval (CI) 2.26 to 5.78) and reduced recurrence of VAP due to multi‐resistant organisms (one study; N = 110; odds ratio (OR) 0.44; 95% CI 0.21 to 0.95), without adversely affecting mortality and other recurrence outcomes. However, for cases of VAP specifically due to non‐fermenting Gram‐negative bacilli (NF‐GNB), recurrence was greater after short‐course therapy (two studies, N = 176; OR 2.18; 95% CI 1.14 to 4.16), though mortality outcomes were not significantly different. One study found that a three‐day course of antibiotic therapy for patients with suspected HAP but a low Clinical Pulmonary Infection Score (CPIS) was associated with a significantly lower risk of superinfection or emergence of antimicrobial resistance, compared with standard (prolonged) course therapy.
Authors' conclusions
On the basis of a small number of studies and appreciating the lack of uniform definition of pneumonia, we conclude that for patients with VAP not due to NF‐GNB a short, fixed course (seven or eight days) of antibiotic therapy appears not to increase the risk of adverse clinical outcomes, and may reduce the emergence of resistant organisms, compared with a prolonged course (10 to 15 days). However, for patients with VAP due to NF‐GNB, there appears to be a higher risk of recurrence following short‐course therapy. These findings do not differ from those of our previous review and are broadly consistent with current guidelines. There are few data from RCTs comparing durations of therapy in non‐ventilated patients with HAP, but on the basis of a single study, short‐course (three‐day) therapy for HAP appears not to be associated with worse clinical outcome, and may reduce the risk of subsequent infection or the emergence of resistant organisms when there is low probability of pneumonia according to the CPIS.
PICO
Plain language summary
Short‐course versus long‐course antibiotic treatment for hospital‐acquired pneumonia in adult intensive care patients
Review question
We reviewed the evidence from randomised controlled trials (RCTs) that compared the effects of a short course of antibiotics with a long course for intensive care patients with hospital‐acquired pneumonia (HAP).
Background
Hospital‐acquired pneumonia is the major cause of hospital‐acquired infection in the intensive care unit (ICU). There are a number of factors that make the critically ill more likely to develop pneumonia, among which the most important is tracheal intubation performed in conjunction with mechanical ventilation; thus, the majority of ICU patients with HAP have what is termed ventilator‐associated pneumonia (VAP).
There is concern that an unnecessarily long course of antibiotic therapy may lead to patients acquiring antibiotic‐resistant organisms, which may be more difficult to recognise and treat when they cause infection and may increase drug costs. On the other hand, too short a course risks the treatment failing.
Study characteristics
The evidence was current as of June 2015. We identified six RCTs, which had enrolled 1088 patients. The studies took quite different approaches to their investigations, and we found only one study that had explored the duration of antibiotic therapy for ICU patients who had HAP, but were not mechanically ventilated.
Key results
For patients with VAP, our main finding was that a course of seven or eight days of antibiotics was associated with an overall decrease in antibiotic administration and reduced the recurrence of pneumonia due to resistant organisms when compared with a 10‐ to 15‐day course. Furthermore, this was achieved without any significant effect on mortality. Nevertheless, in cases when VAP was due to a particular type of organism ('non‐fermenting Gram‐negative bacillus'), which can be difficult to eradicate with antibiotics, the risk of pneumonia recurring appeared higher after a short course of treatment.
One study found that for patients with possible (but low probability) HAP a short (three‐day) course of therapy seemed to be associated with a lower chance of acquiring resistant organisms or of subsequent infections being due to a resistant organism.
Quality of evidence
The quality of evidence for the main outcome measures was low to moderate. The main reasons that the quality was not high were that only a small number of studies were identified, and that there were differences in patient populations, in the nature of the interventions between studies and in the reported outcomes.
Authors' conclusions
Summary of findings
| Should short‐course antibiotic therapy versus prolonged‐course antibiotic therapy be used in critically ill patients with hospital‐acquired pneumonia? | ||||||
| Patient or population: hospital‐acquired pneumonia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Prolonged‐course antibiotic therapy | Short‐course antibiotic therapy | |||||
| Mortality | 175 per 1000 | 201 per 1000 | OR 1.18 | 598 | ⊕⊕⊕⊝ | — |
| Mortality NF‐GNB | 265 per 1000 | 255 per 1000 | OR 0.95 | 179 | ⊕⊕⊝⊝ | — |
| Mortality MRSA | 238 per 1000 | 286 per 1000 | OR 1.28 | 42 | ⊕⊕⊕⊝ | — |
| Recurrence of pneumonia | 180 per 1000 | 237 per 1000 | OR 1.41 | 733 | ⊕⊕⊝⊝ | — |
| Recurrence of pneumonia NF‐GNB | 247 per 1000 | 417 per 1000 | OR 2.18 | 176 | ⊕⊕⊕⊝ | — |
| Recurrence of pneumonia MRSA | 370 per 1000 | 479 per 1000 | OR 1.56 | 49 | ⊕⊕⊕⊝ | — |
| 28‐day antibiotic‐free days | The mean 28‐day antibiotic free days in the intervention groups was | 431 | ⊕⊕⊝⊝ | — | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Low total number of events. | ||||||
Background
Description of the condition
Hospital‐acquired pneumonia (HAP) is usually defined as pneumonia occurring 48 hours or more after hospital admission and not incubating at time of admission (ATS/IDSA 2005). It remains an important cause of healthcare‐associated morbidity and mortality, occurring among the general hospitalised population with a rate of 5 to 10 per 1000 hospital admissions (ATS/IDSA 2005). Patients in the intensive care unit (ICU, also referred to as intensive therapy unit, ITU) appear to be at particular risk and in this setting pneumonia is the most common cause of hospital‐acquired infection in Europe and the United States (Burgmann 2010; Richards 2000; Vincent 1995).
Over 90% of pneumonia episodes developing in ICUs occur in patients who are intubated and mechanically ventilated (ATS/IDSA 2005); ventilator‐associated pneumonia (VAP) therefore represents a very significant sub‐set of HAP occurring in ICUs. VAP has been defined as pneumonia that occurs more than 48 to 72 hours after tracheal intubation (ATS/IDSA 2005) and is thought to affect 10% to 20% patients receiving mechanical ventilation for more than 48 hours (Safdar 2005). Endotracheal intubation probably increases the risk of developing pneumonia by facilitating the growth of potential bacterial pathogens within the respiratory tract (Chastre 2002). Patients are more likely to develop VAP if they are sicker, older, have undergone prior surgery, or are admitted with neurological and/or cardiovascular failure (Blot 2013; Chastre 2002). Recently it has been suggested that 'ventilator‐associated tracheobronchitis' (VAT), i.e. ventilator‐associated respiratory tract infection in the absence of chest x‐ray changes, may be a precursor of VAP (Dallas 2011), and that antibiotic therapy may prevent an episode from developing into pneumonia (Agrafiotis 2010).
An episode of HAP has adverse consequences for the critically ill. Mortality attributable to an episode of VAP (as opposed to the severe underlying illness) has been estimated to be 4% to 13% (Bekaert 2011; Melsen 2011; Melsen 2013). Furthermore, lengths of ICU and hospital stay are significantly prolonged when VAP develops and associated costs have been estimated to range from USD 10,000 to 40,000 per patient (Rello 2002; Safdar 2005; Warren 2003).
Considerable effort has been made in recent years to refine VAP definitions, yet doubt remains as to the optimal means of identifying an episode of VAP for clinical and for surveillance purposes. Traditionally, radiological evidence of pneumonia, systemic criteria (e.g. pyrexia or abnormal white cell count) and/or pulmonary criteria (e.g. presence of bronchial breathing or worsening cough) have been required to make a diagnosis of VAP (ATS/IDSA 2005; HELICS 2004; Horan 2008). However, practically it can be very difficult to make a distinction between VAP and other conditions occurring in mechanically ventilated critically ill patients, including non‐infective processes such as atelectasis and pulmonary oedema. Quantitative culture of invasive bronchoscopic respiratory specimen (e.g. broncho‐alveolar lavage, BAL) may have particular appeal for diagnosing VAP in patient groups with greater likelihood of a non‐pulmonary cause for systemic inflammation (e.g. trauma or surgical patients, Sharpe 2015), and may reduce antibiotic prescription (Conway Morris 2011; Fagon 2000), but is not often performed in European units (Koulenti 2009). Indeed, there may be variable tendency to perform chest radiography before starting a course of antibiotics for suspected ICU‐acquired respiratory tract infection (Szakmany 2013), and disagreement on the presence or absence of radiographic signs (Wunderink 2000), or clinical pulmonary features.
The Centers for Disease Control and Prevention's National Healthcare Safety Network (CDC/NHSN) have lately attempted to improve the reliability of surveillance for ventilator‐associated morbidity in the United States, concentrating on objective criteria (i.e. changes in oxygenation, inflammatory markers, the initiation of antibiotics and positive microbiology) and introducing a classification of "ventilator‐associated events" (VAEs) (Magill 2013), of which "possible VAP [PVAP]" now represents one example. Some concern has been raised at this relatively early stage that although developing a "ventilator‐associated complication" (VAC) on the basis of worse oxygenation may predict poorer clinical outcome (Boyer 2015; Hayashi 2013; Klein Klouwenberg 2014; Muscedere 2013), the overlap between an episode of VAC (defined according to surveillance needs) and an episode of historically defined VAP (according to the radiographic, clinical and microbiological criteria) appears to be limited (Boyer 2015; Hayashi 2013; Klein Klouwenberg 2014; Muscedere 2013; Stoeppel 2014). The potential utility of these newly defined conditions with respect to antibiotic prescription at the bedside is uncertain (Kipnis 2014).
Description of the intervention
A number of strategies to guide duration of antibiotic therapy in the treatment of HAP for the critically ill have been described. Antibiotics administered according to a fixed duration is the focus of this review (Chastre 2003a; Ibrahim 2001). National guidelines published within the last decade have recommended a course of antibiotic therapy for HAP of up to eight days when there has been good response to therapy (ATS/IDSA 2005; BSAC 2008), but a prolonged course of two to three weeks if response has been poor, or when infection is due to a non‐fermenting Gram‐negative bacillus (NF‐GNB), such as Pseudomonas aeruginosa (P. aeruginosa) (ATS/IDSA 2005), an organism particularly difficult to eradicate from the respiratory tract despite in vitro sensitivity to antibiotic therapy (Dennesen 2001; Visscher 2008), and an independent risk factor for VAP recurrence and mortality (Combes 2007; Kollef 1995).
Other strategies to limit duration of antibiotic therapy were explored in a previous version of this review (Pugh 2011). Such strategies may individualise and discontinue therapy according to resolution of clinical features (Micek 2004), to microbiological criteria (e.g. quantitative reduction in culture of respiratory specimens; Mueller 2007) or to a relevant biomarker, such as procalcitonin (Bouadma 2010; Stolz 2009a). Studies of procalcitonin‐guided antibiotic therapy in critically ill patients (e.g. Bouadma 2010; Jensen 2011; Nobre 2008; Stolz 2009a) have been explored in recent dedicated systematic reviews of patients with sepsis (Kopterides 2010) and acute respiratory tract infection (Schuetz 2012a; Schuetz 2012b). Of further interest is the recently reported use of extended infusion administration (e.g. Chant 2012) and novel methods of administration, e.g. nebulisation (e.g. Niederman 2012).
How the intervention might work
Resolution of clinical abnormalities associated with VAP is often observed within the first week of antibiotic therapy (Chastre 2003a; Dennesen 2001; Kollef 2012), particularly among ICU survivors (Luna 2003). For those that have responded to treatment, further prolongation of antibiotic therapy could lead to emergence of resistant organisms (Chastre 2003a; Dennesen 2001), may increase the risk of developing Clostridium difficile (C. difficile)‐associated disease (Bignardi 1998; Dubberke 2014) and antibiotic‐related toxicity, and will increase pharmacy costs.
Why it is important to do this review
Globally, nearly three‐quarters of ICU patients receive antibiotic therapy (Vincent 2009), and when prescribed to treat infection (as opposed to prophylaxis) the focus is directed overwhelmingly at the respiratory tract (Vincent 2009). Appropriate duration of antibiotic therapy is a key consideration in the optimal antimicrobial management (or antibiotic stewardship) of critically ill patients with HAP (Kollef 2012b); too short a course of therapy risks treatment failure, whereas too long a course of therapy carries unnecessary costs and poses potential risks for the individual patient and to other patients through the emergence of resistant organisms.
The optimal duration of antibiotic therapy for HAP (including VAP) in the critically ill is uncertain. Furthermore, when HAP is due to NF‐GNB, such as P. aeruginosa, the potential risk of treatment failure following short‐course therapy may be greater.
Objectives
To assess the effectiveness of short versus prolonged‐course antibiotics for HAP in critically ill adults, including patients with ventilator‐associated pneumonia (VAP).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing a 'short' versus 'prolonged' course of antibiotic therapy for clinical and microbiological outcomes in critically ill patients with established HAP (including VAP).
Types of participants
ICU patients (16 years and older) with HAP (including VAP) diagnosed by clinical and/or radiological features and/or quantitative culture of respiratory specimens. We excluded data regarding patients with haematological malignancy, chemically induced immune suppression or HIV/AIDS where possible.
Types of interventions
RCTs comparing a fixed 'short' (eight days or less) duration of antibiotic therapy with a 'prolonged' (including standard care) course of antibiotic therapy.
Types of outcome measures
Primary outcomes
-
28‐day mortality.
-
Recurrence of pneumonia (diagnosed on the basis of clinical and/or microbiological criteria).
-
28‐day antibiotic‐free days.
Secondary outcomes
-
ICU mortality.
-
In‐hospital mortality.
-
Clinical resolution of pneumonia (according to clinical and/or microbiological criteria).
-
Relapse of pneumonia.
-
Subsequent infection due to 'resistant organisms' (for example, methicillin‐resistant Staphylococcus aureus (S. aureus) (MRSA)).
-
Duration of ICU stay.
-
Duration of hospital stay.
-
Duration of mechanical ventilation (where appropriate).
-
Mechanical ventilation‐free days (where appropriate).
-
Mortality attributable to HAP.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL 2015, Issue 5), MEDLINE (1946 to 2015), MEDLINE in‐process and other non‐indexed citations (5 June 2015), EMBASE (to June 2015), LILACS (1982 to June 2015) and Web of Science (1955 to June 2015).
We used the search strategy described in Appendix 1 to search MEDLINE and CENTRAL. We combined the MEDLINE search strategy with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We modified these terms to search EMBASE (Appendix 2), LILACS (Appendix 3) and Web of Science (Appendix 4). We imposed no publication or language restrictions.
Searching other resources
We also searched the World Health Organization (WHO) International Clinical Trials Platform registers of controlled clinical trials, clinicaltrials.gov and the UK Clinical Trials Gateway (5 June 2015). We searched the Database of Abstracts of Reviews of Effects (DARE) for additional reviews. We searched reference lists of identified studies and review papers, and the OpenGrey database of grey literature. We also searched abstracts of recent conferences: International Symposium for Intensive Care and Emergency Medicine, European Society for Intensive Care Medicine, Society of Critical Care Medicine, American Thoracic Society, British Thoracic Society, European Respiratory Society, Chest, Infectious Disease Society of America and Interscience Conference on Antimicrobial Agents, and Chemotherapy.
Data collection and analysis
Selection of studies
Two review authors (RP, CG) independently analysed and reviewed the titles and abstracts of retrieved records for possible inclusion in this review. We used the following inclusion criteria: RCT, participants included ICU patients with HAP, comparison of fixed 'short' (eight days or less) duration of antibiotic therapy with 'prolonged' course antibiotic therapy, and relevant outcome data. We attempted to contact trial authors to clarify methods where indicated. We resolved any disagreements about study inclusion through consensus and, where needed, we involved a third review author (GD).
Data extraction and management
We used a standardised data extraction form. Two review authors (RP, CG) extracted data and entered these data into a Review Manager (RevMan) document (RevMan 2014). We contacted trial authors for any missing data.
Assessment of risk of bias in included studies
Two review authors (RP, GD) independently assessed the validity of studies using the Cochrane 'Risk of bias' tool (Sterne 2011). We resolved disagreements through consensus.
Measures of treatment effect
We used the following dichotomous data for measuring treatment effect:
-
mortality;
-
clinical resolution;
-
recurrence;
-
relapse;
-
infection with 'resistant organism'.
In addition, we collected the following continuous data:
-
durations of stay;
-
duration of mechanical ventilation;
-
ventilation‐ and antibiotic‐free days.
Unit of analysis issues
We did not identify significant unit of analysis issues (for example, randomisation of a group of individuals rather than an individual in a cluster‐randomised trial, or allocation of an individual to multiple interventions in a cross‐over trial).
Dealing with missing data
All studies presented data on the basis of the intention‐to‐treat (ITT) principle, i.e. all patients included in the study at the point of randomisation were analysed according to their assigned treatment group, regardless of whether or not treatment was completed.
Assessment of heterogeneity
We anticipated sources of heterogeneity relating to: participation factors (preceding durations of hospitalisation and mechanical ventilation, bacterial pathogen, prior antibiotic administration and illness severity) and intervention factors (class of antibiotic and method of administration). We anticipated that methodological diversity would be a significant source of heterogeneity. We assessed heterogeneity on the basis of the I2 statistic, interpreting a value below 40% as not suggestive of important heterogeneity (Higgins 2002).
Assessment of reporting biases
We attempted to minimise reporting bias by searching for unpublished trials and references to trial registries. Due to the small number of studies identified, we did not perform funnel plot tests for funnel plot asymmetry.
Data synthesis
We used the mean difference (MD) as a summary statistic for continuous measures and the odds ratio (OR) for dichotomous measures. We chose a random‐effects model for meta‐analysis given the clinical and methodological diversity of studies, and limited scope to explore heterogeneity through subgroup analysis.
Subgroup analysis and investigation of heterogeneity
A priori subgroups were:
-
duration of hospitalisation prior to development of pneumonia;
-
ventilator‐ and non‐ventilator‐associated HAP;
-
duration of mechanical ventilation prior to development of pneumonia;
-
administration of antibiotic therapy during hospital admission prior to development of pneumonia;
-
severity of illness;
-
chronic respiratory illness;
-
bacterial pathogen: NF‐GNB and MRSA;
-
class of antibiotic(s).
However, outcome data were insufficiently reported for individual subgroups to enable investigation of sources of heterogeneity with the exception of bacterial pathogen.
We did not perform meta‐regression owing to the low number of included studies (Deeks 2011).
Sensitivity analysis
We intended to perform sensitivity analysis if there were apparent differences in participants, interventions or methodology, irrespective of measures of study heterogeneity (I2 statistic).
The studies with which we had particular concerns in this respect were: Medina 2007 (since data has been published in abstract form only), Capellier 2012 (since participants were specifically selected for likelihood of "early‐onset" ventilator‐associated pneumonia) and Kollef 2012 (due to multiple differences between intervention measures), and on this basis, pooled results excluding data from these studies are presented in Appendix 5.
Results
Description of studies
Results of the search
The searches of CENTRAL (2015, Issue 5), MEDLINE (Ovid) from 1946 to June 2015, MEDLINE in‐process and other non‐indexed citations to June 2015, EMBASE to June 2015, LILACS to June 2015 and Web of Science to June 2015 produced 5878 results (after removal of duplicates). Further searching of DARE within The Cochrane Library 2015, Issue 5 identified 131 records. Searches of additional databases produced the following: Clinical.Trials.gov 113 results, WHO International Clinical Trials Registry Platform 83 results, UK Clinical Trials Gateway one result.
Of these, we undertook full‐text review of 12 potentially eligible studies (Capellier 2012; CCCTG 2006; Chastre 2003a; Chastre 2003b; Fagon 2000; Fekih Hassen 2009; Kim 2012; Kollef 2012; Micek 2004; Sanchez‐Nieto 1998; Singh 1998; Singh 2000). We identified five other potential studies from review articles and reference lists (Ibrahim 2001; Kollef 2005; Peery 2001; Rello 2004; Sole 2000). We found a further four studies on investigation of the 'grey literature', including relevant database searches and review of conference abstracts (Labelle 2012; Maldonado‐Ortiz 2004; Medina 2007; Wolff 2003). Review of trials registers identified three potentially relevant ongoing studies (NCT01994980; NCT00410527; NCT01554657).
Included studies
Six studies met the criteria for inclusion in this review, reporting data from a total of 1088 participants (Capellier 2012; Chastre 2003a; Fekih Hassen 2009; Kollef 2012; Medina 2007; Singh 2000). Summary data are presented in the Characteristics of included studies table. Of these, five were RCTs comparing fixed durations of antibiotic therapy in patients with VAP (Capellier 2012; Chastre 2003a; Fekih Hassen 2009; Kollef 2012; Medina 2007). The sixth study was a RCT comparing short (three‐day) course therapy with 'standard' duration therapy for patients with a low probability of HAP, of whom only 58% were mechanically ventilated. There are substantial differences between this study and the others, and data from this study are therefore not included in meta‐analysis (Singh 1998).
Of these six studies, only Kollef 2012 appears to have been blinded, though details about blinding were not available for Fekih Hassen 2009. However, as discussed below, there is a potential significant source of bias in Kollef 2012, since different antibiotic and antibiotic administration regimens were adopted for the seven‐day course (doripenem) and the 10‐day course (imipenem).
Participants
In terms of study size, the multi‐centre French Pneuma study dominates, having enrolled 401 participants (Chastre 2003a). A second multi‐centre French study (Capellier 2012) and an international multi‐centre study (Kollef 2012) recruited 225 and 274 participants, respectively, although in Kollef 2012 primary analysis is focused on the 167 participants fulfilling a "microbiological intention‐to‐treat (MITT)". A Uruguayan study, Medina 2007, recruited 77 participants and has not yet, to our knowledge, been published in a peer‐reviewed journal. A smaller single‐centre Tunisian study, which recruited 30 participants, has been published in a French language journal (Fekih Hassen 2009). The single‐centre US study by Singh 2000 enrolled 81 participants.
With respect to patient characteristics, the Pneuma study was specifically designed to compare durations of antibiotic therapy in a population likely to have a high proportion of more resistant bacteria, including NF‐GNB such as P. aeruginosa, by excluding participants with early VAP (within the first five days of mechanical ventilation) and no antimicrobial therapy within the preceding 15 days (Chastre 2003a); 33% cases of VAP were due to NF‐GNB, and 11% due to MRSA. Kollef 2012 intended to study participants with late‐onset VAP and for the "MITT" group to include only patients with Gram‐negative VAP; of this MITT group, 31% cases of VAP were due to mono‐microbial NF‐GNB; there were no cases of mono‐microbial MRSA VAP, but in 6.6% of cases MRSA was identified with another Gram‐negative organism. Higher proportions of VAP due to NF‐GNB were reported in Fekih Hassen 2009 (72%) and in Medina 2007 (64%). In contrast, Capellier 2012 intended to enrol participants with early‐onset VAP (mechanical ventilation for more than 24 hours, but less than eight days), and there were no cases of VAP due to NF‐GNB or MRSA. Lastly, Singh 2000 sought to recruit patients with a low probability of pneumonia, as determined by a Clinical Pulmonary Infection Score (CPIS, a composite score based upon high or low temperature, leukocyte count, quality of tracheal secretion, oxygenation and presence of radiographic infiltrates) of less than seven at day one and day three; pathogens associated with an episode of HAP were incompletely reported.
Antibiotics had been administered prior to the onset of VAP in 100% participants in Kollef 2012, 84% of participants in Chastre 2003a, and 68% of participants in Medina 2007. Data regarding previous antibiotic use were not provided in Fekih Hassen 2009 nor in Singh 2000, and prior antibiotic administration for pneumonia was an exclusion criterion in Capellier 2012.
Where reported, duration of mechanical ventilation prior to onset of VAP differed between studies: 14 days (Chastre 2003a), 10 days (Fekih Hassen 2009), nine days (Medina 2007), and three days (Capellier 2012). This duration was not reported in Kollef 2012, and in Singh 2000 42% participants were not mechanically ventilated at enrolment.
Presence of underlying respiratory disease was inadequately reported for comparison between studies, and for illness severity scores only limited comparison was possible; mean SAPS II (Simplified Acute Physiology Score II) scores were 45 for Chastre 2003a, 43 for Fekih Hassen 2009, and 39 for Capellier 2012; SOFA (Sequential Organ Failure Assessment) scores were mean 7.3 for Chastre 2003a and 5.8 for Kollef 2012, and median 6 for Medina 2007.
Pneumonia definitions varied between studies. All included the need for radiological and clinical features; in several studies the diagnosis of primary episode of VAP necessitated a threshold quantitative culture of broncho‐alveolar lavage (BAL; Capellier 2012; Chastre 2003a, the "MITT" cohort in Kollef 2012) or protected specimen brush (PSB) samples (Chastre 2003a). In Fekih Hassen 2009, threshold quantitative culture was permissible from endotracheal aspirate (ETA) or PSB, and in Medina 2007, quantitative culture was not required if the CPIS was above six, or blood or pleural cultures were positive. In contrast, for Singh 2000 an episode of HAP was defined according to the development of new infiltrates on chest radiography together with clinical suspicion, and microbiological confirmation was not required.
CPIS was not reported by Chastre 2003a or by Capellier 2012. However, CPIS was six or above in 92% participants in Kollef 2012 and 67% in Fekih Hassen 2009. In Singh 2000, patients with CPIS above six were excluded from study, and mean CPIS was 4.9 on day of enrolment.
Interventions
For the majority of studies, a short course of seven or eight days was compared with a prolonged course of 10 to 15 days. Capellier 2012 and Chastre 2003a allocated patients to receive either eight days or 15 days of antibiotic therapy, Fekih Hassen 2009 and Kollef 2012 to seven days or 10 days of therapy, and Medina 2007 to eight days or 12 days of therapy. Singh 2000 allocated patients whose CPIS remained less than seven on the third day of antibiotic therapy to receive either three days' antibiotic therapy or non‐fixed "standard course" therapy (typically 10 to 21 days at the start of the recruitment), as determined by the treating physician.
The most common classes of antibiotics used in Chastre 2003a were an aminoglycoside or quinolone plus beta‐lactam (91%); in Medina 2007, beta‐lactams were used in 90% of cases and aminoglycosides in 27% overall. In Capellier 2012, all participants received a beta‐lactam for the allocated duration, combined with an aminoglycoside for the first five days of therapy. Carbapenems were used in 100% cases in Kollef 2012, with doripenem in the short‐course group and imipenem in the prolonged‐therapy group. Antibiotic class data are not presented in Fekih Hassen 2009. In Singh 2000, ciprofloxacin monotherapy was used in the short‐course group, but varied widely in the standard‐therapy group (including glycopeptide, beta‐lactam, aminoglycoside and macrolide therapy).
Initial antibiotic therapy was appropriate (according to respiratory specimen culture and sensitivity results) in all cases in Capellier 2012 and Chastre 2003a (from which any patients initially treated with inappropriate antibiotics were subsequently excluded) and in Kollef 2012 (in which the pathogen's imipenem minimum inhibitory concentration, MIC, was confirmed as less than 8 μg/mL). In one case among each of the short‐course and prolonged‐therapy groups the initial antibiotic therapy was inappropriate in both Fekih Hassen 2009 and Medina 2007. Appropriateness of initial therapy is not described in Singh 2000.
Uniquely among the included studies, Kollef 2012 allocated patients in the short‐course doripenem group to receive their antibiotic therapy as a theoretically favourable four‐hour infusion every eight hours (according to pharmacokinetic/pharmacodynamic models), whereas in the prolonged‐course group imipenem was administered as a one‐hour infusion eight‐hourly.
Outcomes
Although some unambiguous outcome measures were reported in the included studies (mortality, length of stay, duration of mechanical ventilation), discussion is required regarding other measures, including 'clinical resolution', 'recurrence' and 'relapse'.
The term 'clinical resolution' used in this review refers to the outcome measures described by Capellier 2012 ('clinical cure', evaluated at day 21 according to clinical and radiographic criteria), Kollef 2012 ('clinical cure', or resolution of clinical features and improvement or lack of progression of radiographic response to therapy, evaluated at day 10 of study) and Medina 2007 ('clinical resolution', described as lessening of symptoms and signs of infection such that additional therapy is not required, evaluation day unspecified).
'Recurrence' data were presented by Capellier 2012, Chastre 2003a, Fekih Hassen 2009, and Medina 2007. In Chastre 2003a, an episode of recurrence, whether relapse (i.e. due to same initial causative organism) or super‐infection (i.e. due to other organism), again required threshold quantitative culture of BAL or PSB; distal respiratory tract sampling could be prompted by changes in oxygenation or haemodynamic status, or development of fever, purulent secretions or new infiltrates on chest radiography. For Capellier 2012, a relapse was defined as culture of original organism in conjunction with clinical or radiological signs of pneumonia or worsening SOFA score. In Fekih Hassen 2009, episodes of recurrence ('réinfection') were described in terms of infection due to initial organism or other organism, presumably using the same clinical, radiographic and microbiological criteria as for a primary episode of VAP. Medina 2007 defined episodes of recurrence according to clinical and microbiological features occurring 72 hours or more after completing an initial course of antibiotic therapy.
Excluded studies
We excluded studies if they had been published as a full paper elsewhere (Chastre 2003b; Singh 1998; Wolff 2003), were not RCTs (Ibrahim 2001; Kollef 2005; Rello 2004), investigated a diagnostic strategy (CCCTG 2006; Fagon 2000; Peery 2001; Sanchez‐Nieto 1998; Sole 2000), or used a de‐escalation or discontinuation clinical protocol (Kim 2012; Micek 2004), or biomarker (Bouadma 2010; Micek 2004; Pontet 2007; Stolz 2009a), to guide duration of therapy rather than comparing fixed durations of therapy. We also excluded two studies published in abstract form: Maldonado‐Ortiz 2004 because of inadequate outcome data and perceived risk of bias, and Labelle 2012 because fixed courses of therapy appear not to have been utilised and duration of therapy was not significantly different between groups.
Please refer to the Characteristics of excluded studies table.
Risk of bias in included studies
Risk of bias is presented in detail in the Characteristics of included studies table, presented graphically in Figure 1 and summarised in Figure 2.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
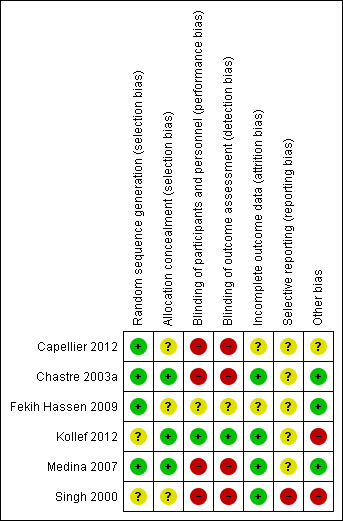
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation was adequate in four studies (Capellier 2012; Chastre 2003a; Fekih Hassen 2009; Medina 2007), and unclear in two studies (Kollef 2012; Singh 1998). Allocation concealment was adequate in three studies (Chastre 2003a; Kollef 2012; Medina 2007), and unclear in three studies (Capellier 2012; Fekih Hassen 2009; Singh 2000).
Blinding
In only one of the studies were there efforts to blind (Kollef 2012). In one study it was unclear whether blinding had been used and contact with the authors has been unsuccessful (Fekih Hassen 2009). Four studies were unblinded (Capellier 2012; Chastre 2003a; Medina 2007; Singh 2000).
Incomplete outcome data
Incomplete outcome data reporting was adequate in four studies (Chastre 2003a; Kollef 2012; Medina 2007; Singh 2000), and unclear in two (Capellier 2012; Fekih Hassen 2009).
Selective reporting
Selective reporting appears to have been a feature of one study (Singh 2000), and was unclear in five (Capellier 2012; Chastre 2003a; Fekih Hassen 2009; Kollef 2012; Medina 2007).
Other potential sources of bias
Interpretation of Kollef 2012 is made more difficult by there having been multiple interventions: the study compares a short course of one antibiotic administered according to theoretically favourable pharmacokinetic modelling versus a prolonged course of another antibiotic administered over a shorter, more conventional time course. Furthermore, the study was stopped early, having enrolled approximately half of the target (480) recruitment, on the basis of inferior efficacy and greater mortality in the short‐course therapy group. Lastly, data from five sites, which had enrolled 41 patients, were excluded from primary analysis because of poor compliance with agreed good clinical practice.
Singh 1998 was stopped early by an institutional review board. Results for patients managed in the 'experimental' group appear to have influenced management of patients subsequently allocated to the 'standard therapy group'.
Effects of interventions
Outcomes are presented in detail (including forest plots) in the Data and analyses section below. Outcomes are summarised in summary of findings Table for the main comparison below.
Primary outcomes
1. 28‐day mortality
Twenty‐eight‐day mortality was reported in three studies and did not differ significantly between short‐course and prolonged‐course therapy groups upon meta‐analysis (odds ratio (OR) 1.18; 95% confidence interval (CI) 0.77 to 1.80; Analysis 1.1); despite differences in study characteristics, heterogeneity appeared to be low (I2 statistic = 0%) (Chastre 2003a; Fekih Hassen 2009; Kollef 2012). Twenty‐eight‐day mortality was reported as significantly greater in the small subgroup of patients in Kollef 2012 with ventilator‐associated pneumonia (VAP) due toP. aeruginosa treated with short‐course therapy (6/17 (35.3%) versus 0/10 (0.0%); 95% CI 12.6% to 58.0%; Kollef 2012). However, including data for patients with other non‐fermenting Gram‐negative bacilli (NF‐GNB) VAP (i.e. Acinetobacter spp.) from this study together with patients with NF‐GNB VAP from Chastre 2003a in meta‐analysis, there was no significant difference in 28‐day mortality between short‐ and prolonged‐course therapy (Analysis 1.1). Similarly, we identified no differences in 28‐day mortality between intervention groups for patients with VAP due to MRSA, and between groups for patients with VAP not due to MRSA/NF‐GNB, or with an unspecified organism.
We performed sensitivity analysis to exclude data from Kollef 2012, given concerns regarding multiple interventions, premature cessation and compliance of several centres with agreed good clinical practice, but this did not lead to significant differences in 28‐day mortality on meta‐analysis (Appendix 5).
2. Recurrence of pneumonia
Recurrence data were presented in four VAP studies (Capellier 2012; Chastre 2003a; Fekih Hassen 2009; Medina 2007). Overall, there was a trend to increased recurrence following a short course of therapy (OR 1.41; 95% CI 0.94 to 2.12; Analysis 1.2; I2 statistic = 5%, suggesting low heterogeneity) in treatment of VAP. However, meta‐analysis of the two studies reporting recurrence data specifically for patients with VAP due to NF‐GNB demonstrated significantly greater recurrence for VAP due to NF‐GNB in the short‐course group (OR 2.18; 95% CI 1.14 to 4.16; P value = 0.02; Analysis 1.2; I2 statistic = 0%), though recurrence rate due to multi‐resistant organism was lower in the short‐course group in one study (OR 0.44; 95% CI 0.21 to 0.95; data from Chastre 2003a only; Analysis 1.11).
We performed a sensitivity analysis to exclude data from Medina 2007 (data currently published in abstract form only) and from Capellier 2012 (early‐onset VAP). On exclusion of data from Medina 2007, increased recurrence following short‐course therapy for VAP due to NF‐GNB was no longer statistically significant (Appendix 5).
3. 28‐day antibiotic‐free days
Chastre 2003a and Fekih Hassen 2009 provided data on 28‐day antibiotic‐free days for patients with VAP (total number of antibiotic‐free days over the 28‐day period, which begins with onset of VAP and commencement of antibiotics). Antibiotic‐free days were significantly greater in the short‐course group (mean difference (MD) 4.02 days; 95% confidence interval (CI) 2.26 to 5.78; Analysis 1.3; I2 statistic = 68%, indicating a high degree of heterogeneity) among patients with VAP due to all organisms (two studies), and in subgroups of patients with VAP due to NF‐GNB or MRSA (one study).
With regards to other measures of antibiotic exposure, for HAP patients randomised to the short‐course therapy group in Singh 2000, 28% of patients received antibiotics for more than three days, compared with 97% in the standard‐therapy group (P value = 0.0001).
Secondary outcomes
1. Intensive care unit (ICU) mortality
We identified no significant differences between short‐ and prolonged‐course antibiotic therapy with respect to ICU mortality, reported in two studies (Fekih Hassen 2009; Medina 2007) (Analysis 1.4).
2. In‐hospital mortality
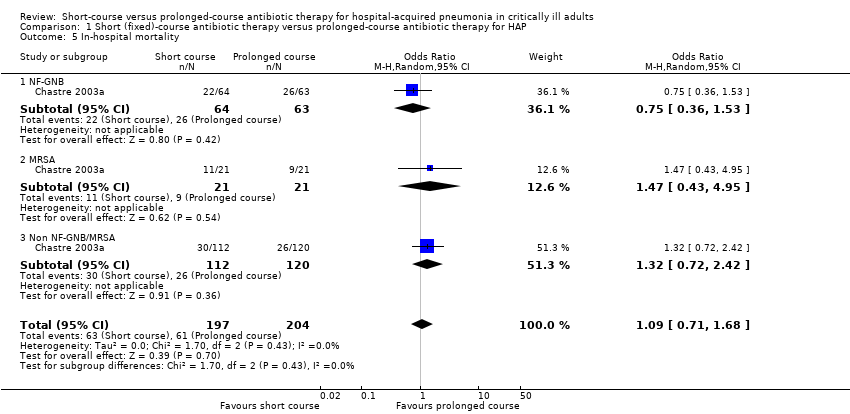
We identified no significant differences between short‐ and prolonged‐course antibiotic therapy with respect to hospital mortality, reported in one study (Chastre 2003a) (Analysis 1.5).
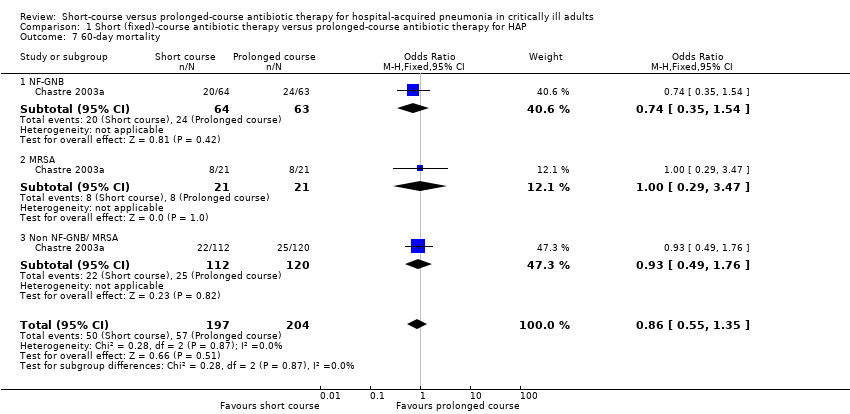
Other mortality outcomes were reported with respect to: 21 days (Capellier 2012; Analysis 1.6), 60 days (Chastre 2003a; Analysis 1.7), and 90 days (Capellier 2012; Analysis 1.8). Again, we identified no significant differences between short‐ and prolonged‐course antibiotic therapy.
We separately considered data from Singh 1998, given the high proportion of non‐ventilated patients and the prerequisite that all included patients were at low risk of having pneumonia, according to Clinical Pulmonary Infection Score (CPIS). A trend towards lower 30‐day mortality was identified by the study authors in the short‐course group (OR 0.33; 95% CI 0.10 to 1.03). Outcomes for patients who were and were not mechanically ventilated were not reported separately.
3. Clinical resolution of pneumonia
Clinical resolution outcomes were reported in three studies: Capellier 2012 (evaluated at day 21), Kollef 2012 (evaluated at day 10) and Medina 2007 (unclear evaluation date). We observed a non‐significantly lower clinical resolution between treatment groups (OR 0.75; 95% CI 0.49 to 1.15; Analysis 1.9; I2 statistic = 0%) for all patients, and for the small subgroup patients with VAP due to NF‐GNB reported in one study (Kollef 2012). The authors of this last study also noted a significantly lower CPIS on day 11 among the prolonged‐therapy group compared with the short‐course group.
4. Relapse of pneumonia
Relapse of pneumonia was non‐significantly higher following short‐course therapy for VAP among patients as a whole (OR 1.70; 95% CI 0.97 to 2.97; Analysis 1.10) and the subgroup with NF‐GNB (OR 2.08; 95% CI 0.92 to 4.70) in two studies (Capellier 2012; Chastre 2003a).
5. Subsequent infection due to 'resistant organisms'
Recurrence rate due to multi‐resistant organisms was lower in the short‐course group in one study (OR 0.44; 95% CI 0.21 to 0.95; data from Chastre 2003a only; Analysis 1.11).
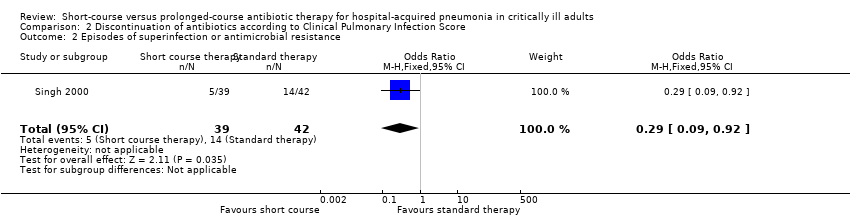
Singh 2000 reported a composite measure of antibiotic resistance and/or superinfection following treatment of HAP; in the short‐course group, this adverse outcome rate was found to be significantly lower at 13% versus 33% (P value = 0.03; Analysis 2.2).
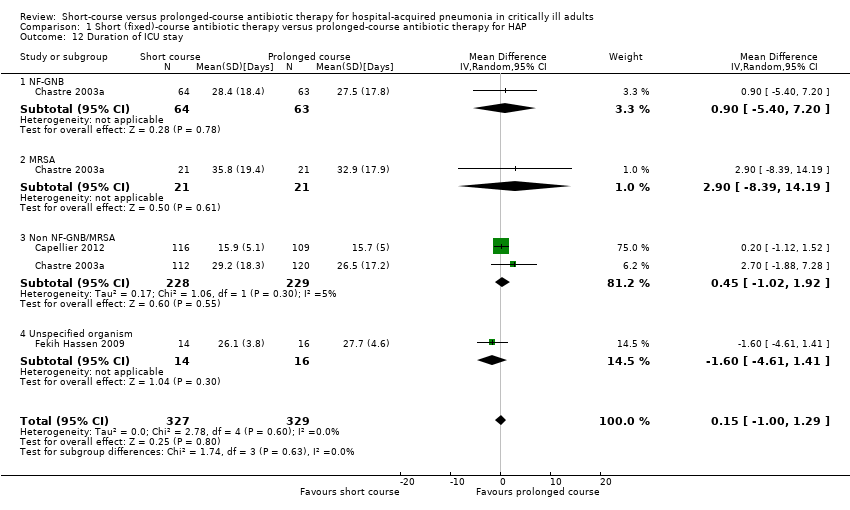
6. Duration of ICU stay
Duration of ICU stay did not differ significantly between patients with VAP treated according to short‐course and prolonged‐course therapy (Analysis 1.12).
Singh 2000 reported a significantly reduced mean length of ICU stay in the short‐course therapy group (9.4 versus 14.7 days; standard deviation (SD) data not presented; P value = 0.04).
7. Duration of hospital stay
Duration of hospital stay did not differ significantly between patients with VAP treated according to short‐course and prolonged‐course therapy (Analysis 1.13).
8. Duration of mechanical ventilation
Duration of mechanical ventilation did not differ significantly between patients with VAP treated according to short‐course and prolonged‐course therapy (Analysis 1.14).
9. Mechanical ventilation‐free days
28‐day mechanical ventilation‐free days were reported in two studies, and did not differ significantly between study groups: mean 8.7 days (SD 9.1) in the short‐course and 9.1 days (SD 9.4) in the prolonged‐therapy group (Chastre 2003a), and 3.4 (SD 1.9) days in the short‐course versus 2.1 (SD 1.8) days in the prolonged‐therapy group (Fekih Hassen 2009).
10. Mortality attributable to HAP
The included trials did not report this outcome.
Discussion
Summary of main results
We identified six studies, which had enrolled a total of 1088 participants with pneumonia (92% of whom had ventilator‐associated pneumonia (VAP)) defined according to diverse radiological, clinical and microbiological criteria. There were notable differences in patient characteristics and interventions between studies, though for the most part this was not reflected in statistical heterogeneity.
Considering the five studies that compared fixed durations of antibiotic therapy for VAP (seven to eight days versus 10 to 15 days), a shorter course of therapy was associated with significantly reduced antibiotic exposure in terms of 28‐day antibiotic‐free days in two studies (mean difference (MD) 4.02 days; 95% confidence interval (CI) 2.26 to 5.78), without any significant increase in mortality, duration of mechanical ventilation or duration of hospital stay, and irrespective of pathogen (Chastre 2003a; Fekih Hassen 2009). One study reported that a shorter course of antibiotic therapy was associated with a significant reduction in VAP recurrence due to multi‐resistant organisms (odds ratio (OR) 0.44; 95% CI 0.21 to 0.95) (Chastre 2003a).
Clinical resolution of VAP was non‐significantly lower (Capellier 2012; Kollef 2012; Medina 2007) (OR 0.75; 95% CI 0.49 to 1.15) and recurrence was non‐significantly greater (Capellier 2012; Chastre 2003a; Fekih Hassen 2009; Medina 2007) (OR 1.41; 95% CI 0.94 to 2.12) for patients treated with a short course of therapy. A significant increase in recurrence was observed in the subgroup of patients with VAP due to non‐fermenting Gram‐negative bacilli (NF‐GNB) (Chastre 2003a; Medina 2007) (OR 2.18; 95% CI 1.14 to 4.16), though not for those with VAP due to methicillin‐resistant Staphylococcus aureus (MRSA). Despite this, short‐course therapy for VAP due to NF‐GNB was not associated with other significantly poorer outcomes, in terms of mortality, mechanical ventilation‐free days and length of stay (summary of findings Table for the main comparison).
For critically ill patients with suspected HAP (whether mechanically ventilated or not) but a low probability of pneumonia (according to a day one and day three Clinical Pulmonary Infection Score (CPIS) below seven; Singh 2000), discontinuing antibiotic therapy at day three was associated with significantly shorter duration of antibiotic therapy (mean three days versus 9.8 days; P value = 0.0001 reported), a significantly reduced composite rate of superinfection and antimicrobial resistance (OR 0.29; 95% CI 0.09 to 0.92) and a significantly reduced duration of intensive care unit (ICU) stay.
Overall completeness and applicability of evidence
In this review, we have included only those studies using fixed durations of antibiotic therapy for hospital‐acquired pneumonia (HAP) in the critically ill; thus (unlike the previous version of our review), we have excluded studies that evaluated clinical response (e.g. Micek 2004) or a biomarker (procalcitonin; e.g. Stolz 2009a) as a guide to variable courses of antibiotic therapy. The included studies enrolled 1088 medical and surgical (including cardio‐thoracic and neuro‐surgical) critically ill adults with HAP from ICUs in Africa, Asia, Europe, and North, Central and South America. However, studies were few in number, and participants, interventions and reported outcomes varied considerably. Furthermore, we identified only one study presenting outcome data for patients with HAP who were not receiving mechanical ventilation; separate outcome data are not presented for those subgroups of patients who were and were not receiving mechanical ventilation, and indeed all of the enrolled patients were required to have 'low probability' of pneumonia according to CPIS (Singh 2000). All other included studies focused entirely on patients with VAP. This limitation is important since there may be differences in patient characteristics and in bacterial aetiology between ICU cases of nosocomial pneumonia acquired in the presence or absence of assisted mechanical ventilation (Esperatti 2010; Kohlenberg 2010); therefore, guidance that attempts to extrapolate from studies of patients with VAP to non‐ventilated patients with HAP may potentially be flawed.
For critically ill patients (not necessarily requiring mechanical ventilation) with new radiographic infiltrates and a suspicion but low probability of pneumonia (for example, according to CPIS on day of diagnosis and 72 hours later), a short course (three days) of appropriate antibiotic therapy appears to be more suitable than a prolonged (10‐ to 21‐day) course (Singh 2000), though the generalisability of empiric ciprofloxacin monotherapy may be debated. Current American (ATS/IDSA 2005), British (BSAC 2008), and Canadian (Rotstein 2008) guidelines do not make any specific recommendations for this particular scenario.
A fixed seven‐ to eight‐day course (rather than a 10‐ to 15‐day course) of antibiotic therapy appears to reduce overall antibiotic exposure and, on the basis of one study, to reduce the risk of further infection associated with resistant organisms. However, pooled data from two studies enrolling a total of 176 patients with VAP due to NF‐GNB, indicated that a fixed, short course of antibiotic therapy (seven or eight days) was associated with significantly increased risk of recurrence compared with a more prolonged course (12 or 15 days) (Chastre 2003a; Medina 2007). Outcome data for a subgroup of patients with VAP due to NF‐GNB were presented in only one other study; a trend to lower clinical resolution following short‐course therapy was seen among patients with and without VAP due to NF‐GNB, and a higher CPIS was observed at day 10 after short‐course therapy (Kollef 2012).
Given the (non‐significantly) lower clinical resolution among patients treated with short‐course therapy and significantly higher recurrence among patients with VAP due to NF‐GNB, data regarding the continued presence of bacterial pathogens (e.g. Pseudomonas sp.) in respiratory specimens during and after fixed‐duration therapy may also have been of interest but were not routinely reported in the included studies.
Diagnostic criteria for defining an episode of pneumonia were not uniform, and no study reported using the new Centers for Disease Control and Prevention (CDC) classification of ventilator‐associated events, including possible VAP (PVAP).
Unfortunately, insufficient outcome data were available to allow exploration of other subgroups of interest, for example: presence of chronic respiratory illness, illness severity, prior administration of antibiotic therapy, prior duration of mechanical ventilation (i.e. early‐ or late‐onset VAP) and class of antibiotic (including combination therapy). Durations of therapy incorporating more novel modes of administration of potential interest were confounded by multiple interventions (e.g. extended infusion versus standard infusion, doripenem versus imipenem, short course versus prolonged course) or did not meet the inclusion criteria for this review (e.g. use of nebulised antibiotics, such as Niederman 2012).
Subsequent infection due to resistant organisms was reported in only two studies (one of which reported as a composite measure only). Emergence of extra‐pulmonary infection, in particular, C. difficile‐associated disease, was surprisingly not reported at all.
Both American (ATS/IDSA 2005) and British (BSAC 2008) guidelines recommend that efforts should be made to shorten antibiotic therapy for HAP (including patients with VAP) to a duration of seven to eight days, where there has been clinical response and, in the case of American Thoracic Society/Infectious Diseases Society of America (ATS/IDSA) guidelines, where the causative organism is not P. aeruginosa. Overall, our review was broadly consistent with these guidelines; nevertheless, in the context of VAP due to NF‐GNB, short‐course therapy is unlikely to be appropriate for all patients and the optimal duration remains uncertain.
Quality of the evidence
Differences in participants, interventions and reported outcomes between studies meant that the number of studies contributing to a particular summary statistic was very small. We considered selection bias to be at low risk in three of the six included studies. Blinding occurred in only one of the six studies. We felt that there was significant other bias in two of the studies, because of multiple differences between intervention groups (Kollef 2012), and because of contamination of the control group with early termination of the study (Singh 2000). We therefore assessed the quality of evidence according to the GRADE classification as low to moderate for the main outcome measures (summary of findings Table for the main comparison).
Potential biases in the review process
We attempted to minimise bias in a number of ways. Our search strategy utilised the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE. In addition, we searched review articles and the 'grey literature' to minimise reporting bias. We established inclusion criteria prior to conduct of searches and, where necessary, we contacted trial authors to clarify study eligibility. We collected data using a standardised data collection form; when not available, we contacted trial authors to provide additional relevant data. We systematically assessed internal validity using the Cochrane 'Risk of bias' tool.
The potential for study heterogeneity was evident at the planning stage of this review, for example, in terms of methodology, diagnostic criteria, illness severity and antibiotic class. We identified relevant subgroups a priori. However, outcome data were typically not presented in sufficient detail to enable adequate subgroup analysis (e.g. according to prior administration of antibiotic therapy, prior duration of mechanical ventilation, classes of antibiotic including combination therapy) and given methodological differences we used a random‐effects model for meta‐analysis.
With a very small number of included studies, we were able to meta‐analyse data from a maximum of four (and more frequently only two or three) studies per outcome. We included studies that focused on early‐onset VAP (Capellier 2012), and those that primarily recruited patients with later‐onset VAP (e.g. Chastre 2003a; Kollef 2012), with the intention of analysing according causative organism (e.g. NF‐GNB). We felt that this approach was appropriate, particularly given that the relationship between early‐ versus late‐onset VAP and the likelihood of pneumonia being due to a resistant organism has been questioned in recent reports (Gastmeier 2009; Martin‐Loeches 2015; Restrepo 2013).
Due to marked differences in participants, interventions and reported outcomes between Singh 2000 and other studies, we considered outcomes for that study separately.
We performed sensitivity analysis to exclude the results of Kollef 2012 from 28‐day mortality data, which did not produce different findings. We also performed sensitivity analysis to exclude Capellier 2012 (given its focus on early onset VAP) and Medina 2007 (since it has been published only in abstract form) from the recurrence results, which led to recurrence among the subgroup of patients with VAP due to NF‐GNB no longer being statistically significant.
Agreements and disagreements with other studies or reviews
Three systematic reviews specifically focusing on duration of antibiotic therapy for VAP have been published (Dimopoulos 2013; Dugan 2003; Grammatikos 2008). The two earlier of these reviews included observational studies and studies focused on diagnostic strategies (for example, invasive versus noninvasive diagnosis of VAP, Fagon 2000). Notably, the Dugan 2003 review also appears to have been written before publication of the Pneuma study, the largest randomised controlled trial (RCT) to date to investigate duration of therapy for VAP (Chastre 2003a). The most recently published systematic review, Dimopoulos 2013, restricted itself to RCTs as did we, but excluded Singh 2000 (presumably on the basis that it included non‐ventilated patients and all patients had a low probability of pneumonia) and Medina 2007 (since it is currently published in abstract form only). In their review, relapse was chosen as a primary outcome measure rather than recurrence.
The conclusions of Grammatikos 2008, namely that short‐course antibiotic therapy for VAP not caused by NF‐GNB does not appear to adversely affect mortality, recurrence or length of stay (Grammatikos 2008), are consistent with the findings from our review and those of Dimopoulos 2013. Dugan 2003 concluded that shorter‐course therapy may be associated with reduced antimicrobial resistance, which is consistent with Chastre 2003a. However, studies published subsequent to their review have been less supportive of the benefits of short‐course therapy in reducing ICU and hospital length of stay. Having excluded Medina 2007, the systematic review published by Dimopoulos 2013 reported a non‐significantly greater relapse in patients with VAP due to NF‐GNB treated with short‐course therapy, rather than the significantly greater recurrence that we found upon meta‐analysis.
Our finding that short‐course antibiotic therapy for VAP in the absence of NF‐GNB may be adequate, without increased risk of recurrence or mortality, is consistent with data from observational studies. Dennesen 2001 demonstrated in 27 cases of VAP treated with appropriate initial antimicrobial therapy that clinical resolution occurred within the first six days of treatment and that S. pneumoniae, H. influenzae and S. aureus were eradicated within this period. In contrast, in Dennesen's study P. aeruginosa was isolated from endotracheal aspirates in all patients 15 days after the initiation of therapy, despite a mean 12.7 days of antibiotic treatment. Similarly, after seven days' appropriate antibiotic therapy for VAP, El Solh 2007 observed a disparity between clinical response and persistent respiratory colonisation, with P. aeruginosa continuing to be cultured from broncho‐alveolar lavage (BAL) despite improvements in CPIS. This persistence may be clinically significant. Zhuo 2008 demonstrated that the bacterial load in mechanically ventilated patients colonised with P. aeruginosa but without meeting the clinical criteria for VAP may predict outcome; in their study, high P. aeruginosa load was associated with a significantly greater 28‐day mortality.
A reliable measure of clinical or microbiological response to treatment has appeal as a means of identifying those for whom a shorter course of therapy may be appropriate, particularly among those with VAP due to NF‐GNB. Kollef observed 10 days after initiation of antibiotic therapy for VAP due to Gram‐negative bacteria, that CPIS was lower among patients treated for 10 days rather than seven days (Kollef 2012). In a non‐randomised study, use of repeat BAL to guide duration of therapy for VAP in surgical/trauma patients led to a reduction in duration of therapy among patients with VAP due to NF‐GNB compared with a control group (mean 10.7 versus 14.4 days respectively) without there being any overall increase in ventilator‐free ICU days, in‐hospital mortality and VAP relapse (Mueller 2007). Bouadma 2010, Pontet 2007, and Stolz 2009a reported reductions in antibiotic exposure in patients with VAP treated according to a procalcitonin‐guided algorithm compared with standard treatment in RCTs, without observing an increase in mortality. Recurrence rates did not differ between groups in one of these studies (Pontet 2007), though outcomes for patients specifically with NF‐GNB VAP were not reported at all.
To summarise, there are very few data for non‐ventilated patients with HAP. For patients with VAP, although short‐course (seven to eight days) therapy for VAP generally appears to be safe, the increased rate of recurrence of VAP due to NF‐GNB among patients administered a fixed, short course of antibiotic therapy demonstrated in our review may reflect inadequate microbiological and/or clinical response to treatment. It would therefore appear that further work is warranted to establish the optimal duration of therapy particularly for these patients, perhaps involving strategies that enable individualisation of treatment.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
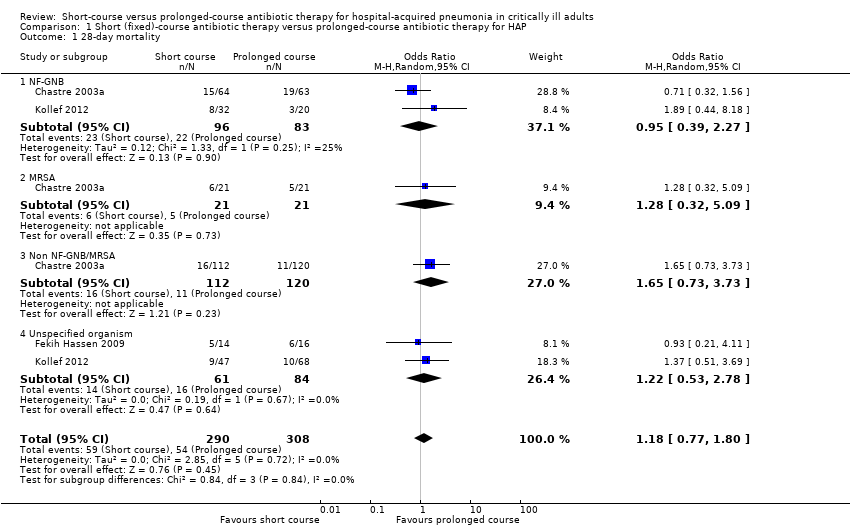
Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 1 28‐day mortality.
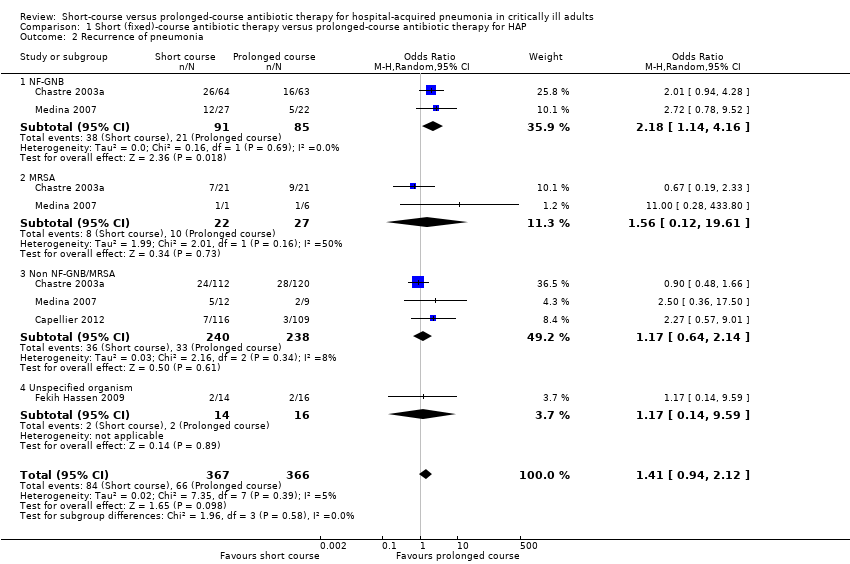
Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 2 Recurrence of pneumonia.

Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 3 28‐day antibiotic‐free days.
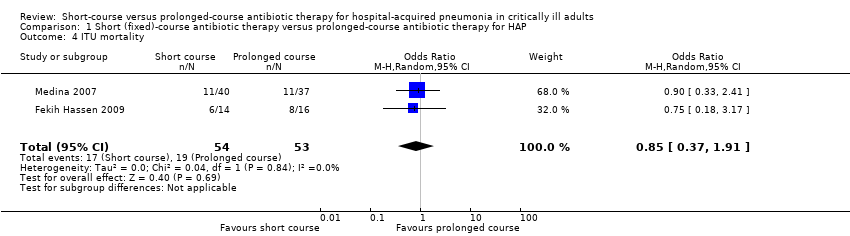
Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 4 ITU mortality.
Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 5 In‐hospital mortality.

Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 6 21‐day mortality.
Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 7 60‐day mortality.

Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 8 90‐day mortality.

Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 9 Clinical resolution.

Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 10 Relapse of pneumonia.

Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 11 Subsequent infection due to resistant organism.
Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 12 Duration of ICU stay.

Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 13 Duration of hospital stay.

Comparison 1 Short (fixed)‐course antibiotic therapy versus prolonged‐course antibiotic therapy for HAP, Outcome 14 Duration of mechanical ventilation.

Comparison 2 Discontinuation of antibiotics according to Clinical Pulmonary Infection Score, Outcome 1 30‐day mortality.
Comparison 2 Discontinuation of antibiotics according to Clinical Pulmonary Infection Score, Outcome 2 Episodes of superinfection or antimicrobial resistance.
| Should short‐course antibiotic therapy versus prolonged‐course antibiotic therapy be used in critically ill patients with hospital‐acquired pneumonia? | ||||||
| Patient or population: hospital‐acquired pneumonia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Prolonged‐course antibiotic therapy | Short‐course antibiotic therapy | |||||
| Mortality | 175 per 1000 | 201 per 1000 | OR 1.18 | 598 | ⊕⊕⊕⊝ | — |
| Mortality NF‐GNB | 265 per 1000 | 255 per 1000 | OR 0.95 | 179 | ⊕⊕⊝⊝ | — |
| Mortality MRSA | 238 per 1000 | 286 per 1000 | OR 1.28 | 42 | ⊕⊕⊕⊝ | — |
| Recurrence of pneumonia | 180 per 1000 | 237 per 1000 | OR 1.41 | 733 | ⊕⊕⊝⊝ | — |
| Recurrence of pneumonia NF‐GNB | 247 per 1000 | 417 per 1000 | OR 2.18 | 176 | ⊕⊕⊕⊝ | — |
| Recurrence of pneumonia MRSA | 370 per 1000 | 479 per 1000 | OR 1.56 | 49 | ⊕⊕⊕⊝ | — |
| 28‐day antibiotic‐free days | The mean 28‐day antibiotic free days in the intervention groups was | 431 | ⊕⊕⊝⊝ | — | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Low total number of events. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 28‐day mortality Show forest plot | 3 | 598 | Odds Ratio (M‐H, Random, 95% CI) | 1.18 [0.77, 1.80] |
| 1.1 NF‐GNB | 2 | 179 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.39, 2.27] |
| 1.2 MRSA | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 1.28 [0.32, 5.09] |
| 1.3 Non NF‐GNB/MRSA | 1 | 232 | Odds Ratio (M‐H, Random, 95% CI) | 1.65 [0.73, 3.73] |
| 1.4 Unspecified organism | 2 | 145 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.53, 2.78] |
| 2 Recurrence of pneumonia Show forest plot | 4 | 733 | Odds Ratio (M‐H, Random, 95% CI) | 1.41 [0.94, 2.12] |
| 2.1 NF‐GNB | 2 | 176 | Odds Ratio (M‐H, Random, 95% CI) | 2.18 [1.14, 4.16] |
| 2.2 MRSA | 2 | 49 | Odds Ratio (M‐H, Random, 95% CI) | 1.56 [0.12, 19.61] |
| 2.3 Non NF‐GNB/MRSA | 3 | 478 | Odds Ratio (M‐H, Random, 95% CI) | 1.17 [0.64, 2.14] |
| 2.4 Unspecified organism | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 1.17 [0.14, 9.59] |
| 3 28‐day antibiotic‐free days Show forest plot | 2 | 431 | Mean Difference (IV, Random, 95% CI) | 4.02 [2.26, 5.78] |
| 3.1 NF‐GNB | 1 | 127 | Mean Difference (IV, Random, 95% CI) | 4.5 [2.25, 6.75] |
| 3.2 MRSA | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 8.0 [4.14, 11.86] |
| 3.3 Non NF‐GNB/ MRSA | 1 | 232 | Mean Difference (IV, Random, 95% CI) | 3.70 [2.09, 5.31] |
| 3.4 Unspecified organism | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 2.3 [1.03, 3.57] |
| 4 ITU mortality Show forest plot | 2 | 107 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.37, 1.91] |
| 5 In‐hospital mortality Show forest plot | 1 | 401 | Odds Ratio (M‐H, Random, 95% CI) | 1.09 [0.71, 1.68] |
| 5.1 NF‐GNB | 1 | 127 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.36, 1.53] |
| 5.2 MRSA | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 1.47 [0.43, 4.95] |
| 5.3 Non NF‐GNB/MRSA | 1 | 232 | Odds Ratio (M‐H, Random, 95% CI) | 1.32 [0.72, 2.42] |
| 6 21‐day mortality Show forest plot | 1 | 225 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.41, 2.69] |
| 7 60‐day mortality Show forest plot | 1 | 401 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.55, 1.35] |
| 7.1 NF‐GNB | 1 | 127 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.35, 1.54] |
| 7.2 MRSA | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.29, 3.47] |
| 7.3 Non NF‐GNB/ MRSA | 1 | 232 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.49, 1.76] |
| 8 90‐day mortality Show forest plot | 1 | 198 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.49, 1.99] |
| 9 Clinical resolution Show forest plot | 3 | 472 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.49, 1.15] |
| 9.1 NF‐GNB | 1 | 55 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.16, 1.48] |
| 9.2 Unspecified organism | 3 | 417 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.51, 1.28] |
| 10 Relapse of pneumonia Show forest plot | 2 | 626 | Odds Ratio (M‐H, Random, 95% CI) | 1.70 [0.97, 2.97] |
| 10.1 NF‐GNB | 1 | 127 | Odds Ratio (M‐H, Random, 95% CI) | 2.08 [0.92, 4.70] |
| 10.2 MRSA | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.14, 3.64] |
| 10.3 Non NF‐GNB/MRSA | 1 | 232 | Odds Ratio (M‐H, Random, 95% CI) | 1.41 [0.51, 3.92] |
| 10.4 Unspecified organism | 1 | 225 | Odds Ratio (M‐H, Random, 95% CI) | 2.92 [0.58, 14.78] |
| 11 Subsequent infection due to resistant organism Show forest plot | 1 | 110 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.21, 0.95] |
| 12 Duration of ICU stay Show forest plot | 3 | 656 | Mean Difference (IV, Random, 95% CI) | 0.15 [1.00, 1.29] |
| 12.1 NF‐GNB | 1 | 127 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐5.40, 7.20] |
| 12.2 MRSA | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 2.90 [‐8.39, 14.19] |
| 12.3 Non NF‐GNB/MRSA | 2 | 457 | Mean Difference (IV, Random, 95% CI) | 0.45 [‐1.02, 1.92] |
| 12.4 Unspecified organism | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐4.61, 1.41] |
| 13 Duration of hospital stay Show forest plot | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐4.11, 2.11] |
| 14 Duration of mechanical ventilation Show forest plot | 3 | 332 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.51, 0.54] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 30‐day mortality Show forest plot | 1 | 81 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.10, 1.03] |
| 2 Episodes of superinfection or antimicrobial resistance Show forest plot | 1 | 81 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.09, 0.92] |

