Capsaicina tópica (alta concentración) para el dolor neuropático crónico en adultos
Appendices
Appendix 1. MEDLINE search strategy (via Ovid)
-
Capsaicin.sh
-
(capsaicin OR capsaicine OR capsici OR axsain OR capsidol OR capsig OR capsin OR capsina OR capsiplast OR capzasin‐P OR dolorac OR gelcen OR katrum OR "No pain‐HP" OR priltam OR "R‐gel" OR zacin OR zostrix OR capsicum).ti,ab,kw
-
1 OR 2
-
exp Administration, topical.sh
-
(topical$ OR cutaneous OR dermal OR transcutaneous OR transdermal OR percutaneous OR skin OR massage OR embrocation OR gel OR ointment OR aerosol OR cream OR crème OR lotion OR foam OR liniment OR spray OR rub OR balm OR salve OR emulsion OR oil OR patch OR plaster).ti,ab,kw
-
4 OR 5
-
Diabetic neuropathies.sh OR Peripheral neuropathies.sh OR Polyneuropathies.sh OR Neuralgia.sh
-
(neuropath$ OR diabet$ post‐herpetic OR neuralgia).ti,ab,kw
-
7 OR 8
-
(pain OR painful OR analgesi$).ti,ab,kw
-
randomized controlled trial.pt
-
controlled clinical trial.pt
-
randomized.ab
-
placebo.ab
-
drug therapy.fs
-
randomly.ab
-
trial.ab
-
groups.ab
-
OR/11‐18
-
3 AND 6 AND 9 AND 19
Appendix 2. EMBASE search strategy (via Ovid)
-
Capsaicin.sh
-
(capsaicin OR capsaicine OR capsici OR axsain OR capsidol OR capsig OR capsin OR capsina OR capsiplast OR capzasin‐P OR dolorac OR gelcen OR katrum OR "No pain‐HP" OR priltam OR "R‐gel" OR zacin OR zostrix OR capsicum).ti,ab,kw
-
1 OR 2
-
exp Topical Drug Administration.sh
-
(topical$ OR cutaneous OR dermal OR transcutaneous OR transdermal OR percutaneous OR skin OR massage OR embrocation OR gel OR ointment OR aerosol OR cream OR crème OR lotion OR foam OR liniment OR spray OR rub OR balm OR salve OR emulsion OR oil OR patch OR plaster).ti,ab,kw
-
4 OR 5
-
Diabetic Neuropathy.sh OR Peripheral neuropathy.sh OR Polyneuropathy.sh OR Neuralgia.sh
-
(neuropath$ OR diabet$ post‐herpetic OR neuralgia).ti,ab,kw
-
7 OR 8
-
(pain OR painful OR analgesi$).ti,ab,kw
-
clinical trials.sh
-
controlled clinical trials.sh
-
randomized controlled trial.sh
-
double‐blind procedure.sh
-
(clin$ adj25 trial$).ab
-
((doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ab
-
placebo$.ab
-
random$.ab
-
OR/11‐18
-
3 AND 6 AND 9 AND 10 AND 19
Appendix 3. CENTRAL search strategy
-
MeSH descriptor Capsaicin
-
(capsaicin OR capsaicine OR capsici OR axsain OR capsidol OR capsig OR capsin OR capsina OR capsiplast OR capzasin‐P OR dolorac OR gelcen OR katrum OR "No pain‐HP" OR priltam OR "R‐gel" OR zacin OR zostrix OR capsicum):ti,ab,kw
-
1 OR 2
-
exp MeSH descriptor Administration, topical
-
(topical* OR cutaneous OR dermal OR transcutaneous OR transdermal OR percutaneous OR skin OR massage OR embrocation OR gel OR ointment OR aerosol OR cream OR crème OR lotion OR foam OR liniment OR spray OR rub OR balm OR salve OR emulsion OR oil OR patch OR plaster):ti,ab,kw
-
4 OR 5
-
MeSH descriptor (Diabetic neuropathies OR Peripheral neuropathies OR Polyneuropathies OR Neuralgia)
-
(neuropath* OR diabet* post‐herpetic OR neuralgia):ti,ab,kw
-
7 OR 8
-
(pain OR painful OR analgesi*):ti,ab,kw
-
Randomized Controlled Trial:pt.
-
MeSH descriptor Double‐Blind Method
-
(double or treble or triple) NEXT (blind* or mask*)):ti,ab,kw.
-
random*:ti,ab,kw.
-
OR/11‐14
-
3 AND 6 AND 9 AND 10 AND 15
-
Limit 16 to Clinical Trials (CENTRAL)
Appendix 4. Summary of outcomes in individual studies: efficacy
| Study ID | Treatment | Clinical improvement |
| Backonja 2008 | (1) capsaicin patch 8%, n = 206 | At 12 weeks ≥ 30% pain reduction from baseline: (1) 91/206, (2) 69/196 ≥ 2 points reduction in pain from baseline: At 8 weeks ≥ 30% pain reduction from baseline: (1) 87/206, (2) 63/196 ≥ 2 points reduction in pain from baseline: PGIC (slightly/much/very much improved): |
| Clifford 2012 | (1) capsaicin patch 8% 30 min, n = 167 | At 12 weeks ≥ 30% pain reduction from baseline: |
| Irving 2011 | (1) capsaicin patch 8%, n = 212 | At 12 weeks ≥ 50% pain reduction from baseline: (1) 64/212, (2) 43/204 ≥ 30% pain reduction from baseline: (1) 100/212, (2) 71/204 PGIC (much and very much improved): (1) 83/212, (2) 50/204 ≥ 2 points reduction in pain from baseline: (1) 91/212, (2) 59/204 At 8 weeks ≥ 50% pain reduction from baseline: (1) 61/212, (2) 41/204 ≥ 30% pain reduction from baseline: (1) 98/212, (2) 69/204 PGIC (much and very much improved): (1) 71/212, (2) 49/204 ≥ 2 points reduction in pain from baseline: (1) 89/212, (2) 53/204 |
| Simpson 2008 | (1) capsaicin patch 8% 30 min, n = 72 | At 12 weeks ≥ 30% pain reduction from baseline: [capsaicin combined 76/225] PGIC (much and very much improved): (1) 23/72, (2) 18/78, (3) 20/75, (4) 9/82 [capsaicin combined 61/225] |
| Webster 2010a | (1) capsaicin patch 8% 30 min, n = 72 | At 8 weeks ≥ 50% pain reduction from baseline: (1) 17/72, (2) 21/77, (3) 17/73, (4) 8/77 ≥ 30% pain reduction from baseline: (1) 27/72, (2) 27/77, (3) 29/73, (4) 22/77 At 12 weeks PGIC (slight, much and very much improved): (1) 122/222 [capsaicin combined], (2) 32/77 |
| Webster 2010b | (1) capsaicin patch 8%, n = 102 | At 12 weeks ≥ 50% pain reduction from baseline: (1) 40/102, (2) 19/53 ≥ 30% pain reduction from baseline: (1) 50/102, (2) 26/53 PGIC (much and very much improved): (1) 41/102, (2) 15/53 At 8 weeks ≥ 50% pain reduction from baseline: (1) 37/102, (2) 19/53 ≥ 30% pain reduction from baseline: (1) 50/102, (2) 24/53 PGIC (much and very much improved): (1) 43/102, (2) 14/53 |
| PGIC ‐ patient global impression of change; PR ‐ pain relief | ||
Appendix 5. Summary of outcomes in individual studies: adverse events and withdrawals
| Study ID | Treatment | Local AEs | Systemic AEs | Serious AEs | Withdrawals/exclusions |
| Backonja 2008 | (1) capsaicin patch 8%, n = 206 | Mostly transient, mild to moderate Erythema: (1) 193/205, (2) 128/197 Pain: (1) 114/205, (2) 43/197 Papules: (1) 20/205, (2) 6/197 Pruritus: (1) 10/205, (2) 6/197 Oedema: (1) 12/205, (2) 2/197 | Nausea, vomiting, nasopharyngitis, sinusitis, back pain, dizziness, headache, worsening of PHN, hypertension ‐ all reported at < 5% per group, with no clear difference between groups | (1) 10/205 | AE: (1) 1/205, (2) 0/197 LoE: (1) 10/205, (2) 9/197 Lost to follow‐up: (1) 3/205, (2) 2/197 Other: (1) 5/205, (2) 7/197 |
| Clifford 2012 | (1) capsaicin patch 8% 30 min, n = 167 | Generally mild or moderate. Groups combined Erythema: (1) 176/332, (2) 58/162 Pain: (1) 274/332, (2) 62/162 Papules: (1) 12/332, (2) 0/162 Pruritus: (1) 12/332, (2) 2/162 Oedema: (1) 4/332, (2) 5/162 | Diarrhoea, nausea, respiratory tract infection, pain, worsening neuropathy ‐ all reported, generally < 5% per group | Approximately 6% in all groups with "infections and infestations" 1 death in capsaicin 60 min group (judged unrelated) | AE: (1) 0/167, (2) 2/165 (1 death), (3) 0/72, (4) 1/90 Lost to follow‐up: (1) 3/167, (2) 2/165 (3) 2/73 (4) 0/89 LoE: (1) 0/167 (2) 1/165 (3) 0/73 (4) 1/89 Other: (1) 8/167 (2) 6/165 (3) 0/73 (4) 6/89 |
| Irving 2011 | (1) capsaicin patch 8%, n = 212 | Most mild or moderate. Erythema: (1) 194/212, (2) 141/204 Pain: (1) 134/212, (2) 57/204 Papules: (1) 15/212 (2) 5/204 Pruritus: (1) 6/212 (2) 3/204 Oedema: (1) 13/212 (2) 0/204 | Nausea, vomiting, sinusitis, respiratory tract infection, musculoskeletal disorders, dizziness, headache ‐ all reported, most < 5% per group | (1) 11/212 (1 death) | AE: (1) 4/212 (1 death), (2) 3/304 Lost to follow‐up: (1) 4/212, (2) 5/204 LoE: (1) 1/212, (2) 5/204 Other: (1) 11/212, (2) 5/204 |
| Simpson 2008 | (1) capsaicin patch 8% 30 min, n = 72 | Self limiting and mild to moderate Pain: (1) 47/225, (2) 7/82 Papules: (1) 11/225, (2) 1/82 Pruritus: (1) 39/225, (2) 5/82 Swelling: (1) 29/225, (2) 7/82 | Diarrhoea, nausea, vomiting, fatigue, infections, musculoskeletal disorders, dizziness, headache, psychiatric disorders ‐ all reported, < 5% per group | (1) 1/225 (2) 2/82 All deaths, judged unrelated to study medication | AE: (1) 3/225 (1 death), (2) 3/82 (2 deaths) Lost to follow‐up: (1) 13/225, (2) 4/82 LoE: (1) 1/225, (2) 2/82 Other: (1) 5/225, (2) 2/82 |
| Webster 2010a | (1) capsaicin patch 8% 30 min, n = 72 | Transient and mild to moderate Pain: 1/222, (2) 2/77 Papules: (1) 3/222, (2) 2/77 Pruritus: (1) 17/222, (2) 9/77 Swelling: (1) 3/222, (2) 5/77 | Diarrhoea, nausea, vomiting, infections, musculoskeletal disorders, dizziness, headache, cough ‐ all reported, mostly < 5% per group | (1 to 3) 10/222 (1 death) (4) 3/77 None considered related to study medication | AE: (1 to 3) 2/222, (4) 1/77 (death) Lost to follow‐up (1 to 3) 7/222, (4) 1/77 LoE: (1 to 3) 4/222, (4) 0/77 Other: (1 to 3) 9/222, (4) 2/77 |
| Webster 2010b | (1) capsaicin patch 8%, n = 102 | Transient and mild to moderate Erythema: (1) 4/102, (2) 0/53 Pain: (1) 4/102, (2) 2/53 Papules: (1) 4/102, (2) 2/53 Pruritus: (1) 17/102, (2) 6/53 Swelling: (1) 10/102, (2) 1/53 | Nausea, infections, musculoskeletal disorders, dizziness, cough, nasopharyngitis, hypertension ‐ all reported, mostly < 5% per group | (1) 7/102 | AE: None in either group Lost to follow‐up: (1) 5/102, (2) 0/53 LoE: (1) 3/102, (2) 7/53 Other: (1) 3/102, (2) 3/53 |
| AE ‐ adverse event; SAE ‐ serious adverse event | |||||
Appendix 6. Patch tolerability
| Study ID | Treatment | Completed < 90% application time | Dermal irritation score > 2 at 2 h | Rescue medication days 0 to 5 |
| Backonja 2008 | (1) capsaicin patch 8%, n = 206 | (1) 1/206 (2) 2/196 | "common but mild, transient and self‐limited" | (1) 99/206 (2) 32/196 |
| Clifford 2012 | (1) capsaicin patch 8% 30 min, n = 167 | (1 to 2) 10/332 (3 to 4) 0/162 | (1 to 2) 13/332 (3 to 4) 0/162 | (1 to 2) 246/332 (3 to 4) 53/162 |
| Irving 2011 | (1) capsaicin patch 8%, n = 212 | (1) 4/212 (2) 0/204 | (1) 6/212 (2) 1/204 | (1) 112/212 (2) 43/204 |
| Simpson 2008 | (1) capsaicin patch 8% 30 min, n = 72 | (1) 0/72 (2) 0/78 (3) 2/75 (4) 0/82 | > 0 at 2 h (1 to 3) 92/225 (4) 23/82 | (1 to 3) 124/225 (4) 19/82 |
| Webster 2010a | (1) capsaicin patch 8% 30 min, n = 72 | (1) 0/73 (2) 1/77 (3) 0/73 (4) 0/77 | > 0 at 2 h (1 to 3) 87/222 (4) 5/77 | (1 to 3) 12/222 (4) 3/77 |
| Webster 2010b | (1) capsaicin patch 8%, n = 102 | (1) 4/102 (2) 0/53 | (1) 53/102 | (1) 12/102 |
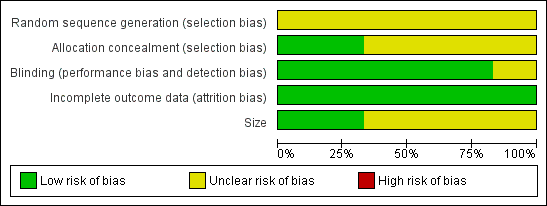
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Forest plot of comparison: 1 8% capsaicin versus control (single dose), outcome: 1.1 PGIC much or very much improved at 8 and 12 weeks.
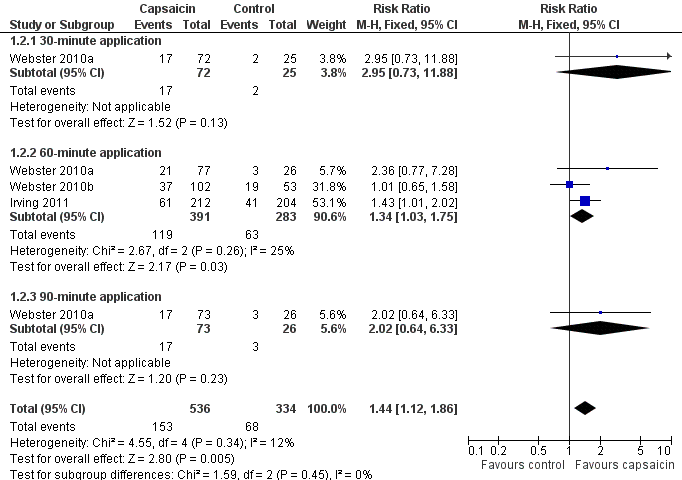
Forest plot of comparison: 1 8% capsaicin versus control (single dose), outcome: 1.2 PHN ‐ at least 50% pain intensity reduction over weeks 2 to 8.

Forest plot of comparison: 1 8% capsaicin versus control (single dose), outcome: 1.6 HIVN ‐ at least 30% pain intensity reduction over weeks 2 to 12.

Skin adverse event rates with capsaicin and control. Yellow symbols are studies recording all events (Group 1). Pink symbols are studies specifying that events are not recorded on the first day after treatment (Group 2). The blue symbol did not specify the period over which events were recorded (Group 2). Size of symbol is proportional to the size of the study

Forest plot of comparison: 1 8% capsaicin versus control (single dose), outcome: 1.10 Serious adverse events.

Comparison 1 8% capsaicin versus control (single dose), Outcome 1 PGIC much or very much improved at 8 and 12 weeks.

Comparison 1 8% capsaicin versus control (single dose), Outcome 2 PHN ‐ at least 50% pain intensity reduction over weeks 2 to 8.

Comparison 1 8% capsaicin versus control (single dose), Outcome 3 PHN ‐ at least 50% pain intensity reduction over 2 to 12 weeks.

Comparison 1 8% capsaicin versus control (single dose), Outcome 4 PHN ‐ at least 30% pain intensity reduction over weeks 2 to 8.

Comparison 1 8% capsaicin versus control (single dose), Outcome 5 PHN ‐ at least 30% pain intensity reduction over weeks 2 to 12.
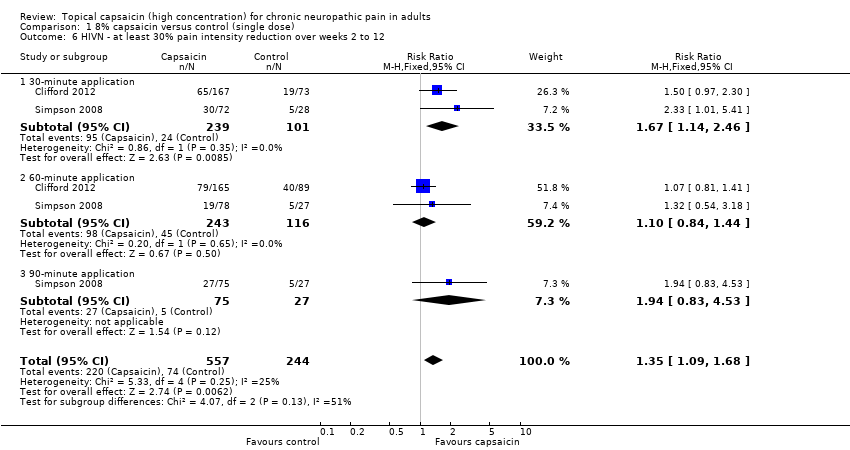
Comparison 1 8% capsaicin versus control (single dose), Outcome 6 HIVN ‐ at least 30% pain intensity reduction over weeks 2 to 12.

Comparison 1 8% capsaicin versus control (single dose), Outcome 7 Local skin reactions ‐ group 1.
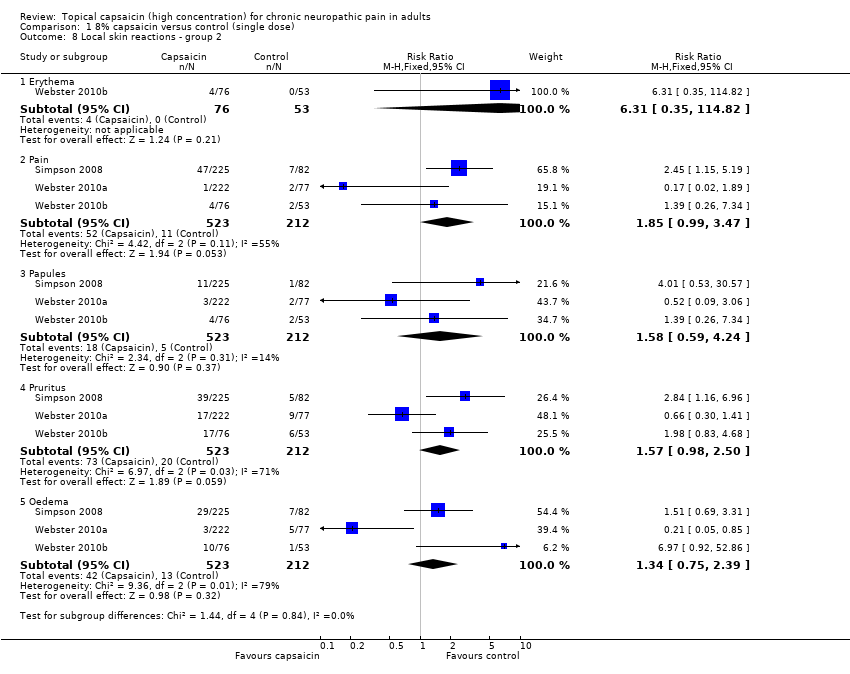
Comparison 1 8% capsaicin versus control (single dose), Outcome 8 Local skin reactions ‐ group 2.

Comparison 1 8% capsaicin versus control (single dose), Outcome 9 Patch tolerability.

Comparison 1 8% capsaicin versus control (single dose), Outcome 10 Serious adverse events.
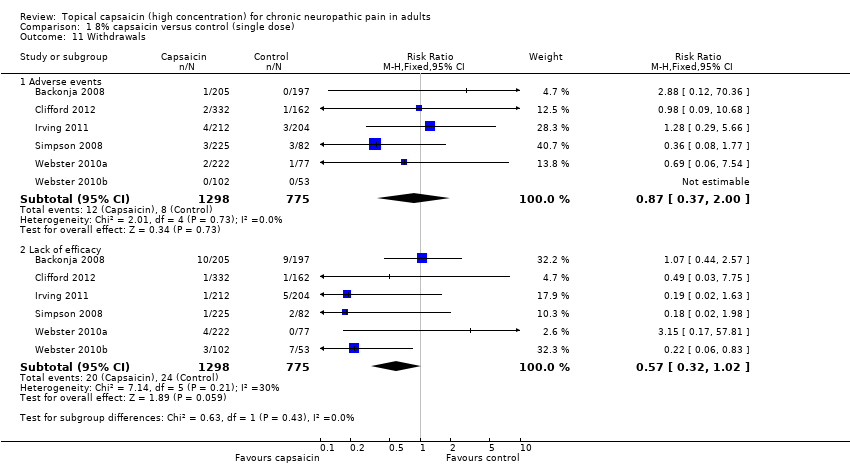
Comparison 1 8% capsaicin versus control (single dose), Outcome 11 Withdrawals.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PGIC much or very much improved at 8 and 12 weeks Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 8 weeks | 2 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.10, 1.84] |
| 1.2 12 weeks | 2 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.20, 1.99] |
| 2 PHN ‐ at least 50% pain intensity reduction over weeks 2 to 8 Show forest plot | 3 | 870 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.12, 1.86] |
| 2.1 30‐minute application | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.95 [0.73, 11.88] |
| 2.2 60‐minute application | 3 | 674 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.03, 1.75] |
| 2.3 90‐minute application | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.64, 6.33] |
| 3 PHN ‐ at least 50% pain intensity reduction over 2 to 12 weeks Show forest plot | 2 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.00, 1.71] |
| 4 PHN ‐ at least 30% pain intensity reduction over weeks 2 to 8 Show forest plot | 4 | 1268 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.13, 1.52] |
| 4.1 30‐minute application | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.67, 2.69] |
| 4.2 60‐minute application | 4 | 1072 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.12, 1.52] |
| 4.3 90‐minute application | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.74, 2.95] |
| 5 PHN ‐ at least 30% pain intensity reduction over weeks 2 to 12 Show forest plot | 3 | 973 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.07, 1.45] |
| 6 HIVN ‐ at least 30% pain intensity reduction over weeks 2 to 12 Show forest plot | 2 | 801 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.09, 1.68] |
| 6.1 30‐minute application | 2 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.14, 2.46] |
| 6.2 60‐minute application | 2 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.84, 1.44] |
| 6.3 90‐minute application | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [0.83, 4.53] |
| 7 Local skin reactions ‐ group 1 Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Erythema | 3 | 1312 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.30, 1.52] |
| 7.2 Pain | 3 | 1312 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.28 [1.99, 2.62] |
| 7.3 Papules | 3 | 1312 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.58 [1.87, 6.85] |
| 7.4 Pruritus | 3 | 1312 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.98, 4.03] |
| 7.5 Oedema | 3 | 1312 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.98 [1.44, 6.18] |
| 8 Local skin reactions ‐ group 2 Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Erythema | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.31 [0.35, 114.82] |
| 8.2 Pain | 3 | 735 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [0.99, 3.47] |
| 8.3 Papules | 3 | 735 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.59, 4.24] |
| 8.4 Pruritus | 3 | 735 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.98, 2.50] |
| 8.5 Oedema | 3 | 735 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.75, 2.39] |
| 9 Patch tolerability Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 < 90% of application time | 6 | 2074 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.27 [1.17, 9.15] |
| 9.2 Dermal irritation score > 2 at 2 h | 3 | 1065 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.80 [4.04, 34.48] |
| 9.3 Dermal irritation score > 0 at 2 h | 2 | 606 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.28 [1.60, 3.26] |
| 9.4 Rescue medication 0 to 5 d | 6 | 2073 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.47 [2.13, 2.87] |
| 10 Serious adverse events Show forest plot | 5 | 1579 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.82, 2.41] |
| 11 Withdrawals Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Adverse events | 6 | 2073 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.37, 2.00] |
| 11.2 Lack of efficacy | 6 | 2073 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.32, 1.02] |

