Capsaicina tópica (alta concentración) para el dolor neuropático crónico en adultos
Resumen
Antecedentes
Esta revisión es una actualización de"Capsaicina tópica (alta concentración) para el dolor neuropático crónico en adultos" actualizada por última vez en el número 2, 2013. Las cremas tópicas con capsaicina se utilizan para tratar el dolor neuropático periférico. Después de la aplicación en la piel, la capsaicina mejoró la sensibilidad, seguida de un período con reducción de la sensibilidad y, después de aplicaciones repetidas, una desensibilización persistente. Se desarrollaron parches de capsaicina de alta concentración (8%) para aumentar la cantidad de capsaicina administrada; se pensó que la administración rápida mejoraba la tolerabilidad porque los nociceptores cutáneos perdían su función rápidamente. La aplicación única evita la falta de cumplimiento. Solamente está disponible la formulación de parches de capsaicina al 8%, con una concentración de capsaicina cerca de 100 veces mayor que las cremas convencionales. La capsaicina tópica a alta concentración se administra como una aplicación de un parche único en la parte afectada. Se debe aplicar bajo condiciones muy controladas, normalmente con un anestésico local, debido a la intensa sensación inicial de ardor que provoca. Se espera que los efectos beneficiosos perduren cerca de 12 semanas, cuando se podría hacer otra aplicación.
Objetivos
Revisar la evidencia de los ensayos controlados sobre la eficacia y la tolerabilidad de la capsaicina a alta concentración (8%) aplicada de forma tópica para el dolor neuropático crónico en adultos.
Métodos de búsqueda
Para esta actualización, se realizaron búsquedas en CENTRAL, MEDLINE, Embase, en dos registros de ensayos clínicos y en el sitio web de una compañía farmacéutica hasta el 10 de junio de 2016.
Criterios de selección
Estudios aleatorizados, doble ciego, controlados con placebo de al menos seis semanas de duración, que utilizaron capsaicina tópica de alta concentración (5% o más) para tratar el dolor neuropático.
Obtención y análisis de los datos
Dos autores de la revisión, de forma independiente, buscaron los estudios, extrajeron los datos sobre la eficacia y los eventos adversos y examinaron la calidad de los estudios y el potencial sesgo. Cuando fue posible el análisis combinado, se utilizaron datos dicotómicos para calcular los riesgos relativos y los números necesarios a tratar para un evento adicional, mediante métodos estándar.
Los resultados de eficacia que reflejan el alivio del dolor de larga duración después de una sola aplicación del fármaco fueron de la Impresión Global del Cambio del Paciente (IGCP) en puntos específicos, generalmente ocho y 12 semanas. También se evaluaron las puntuaciones medias de dolor durante las semanas 2 a 8 y 2 a 12 y el número de participantes con una reducción de la intensidad del dolor de al menos el 30% o al menos el 50% con respecto al valor inicial, y la información sobre los eventos adversos y los retiros.
Se evaluó la calidad de la evidencia mediante GRADE y se creó una tabla de "Resumen de los hallazgos".
Resultados principales
Se incluyeron ocho estudios, con 2488 participantes, dos estudios más y 415 participantes más que la versión anterior de esta revisión. En general, los estudios fueron de buena calidad metodológica; sólo se juzgó un estudio con alto riesgo de sesgo, debido a su pequeño tamaño. Dos estudios utilizaron un control con placebo y seis utilizaron capsaicina tópica al 0,04% como placebo "activo" para ayudar a mantener el cegamiento. Los resultados de eficacia se informaron de forma inconsistente, lo que dio lugar a que los análisis de la mayoría de los resultados se basaran en datos incompletos.
Para la neuralgia postherpética, se encontraron cuatro estudios (1272 participantes). Tanto a las ocho como a las 12 semanas, aproximadamente un 10% más de los participantes informaron haber mejorado más o mucho más con la capsaicina de alta concentración que con el placebo "activo"; las estimaciones puntuales del número necesario a tratar para un resultado beneficioso adicional (NNT) fueron 8,8 (intervalo de confianza [IC] del 95%: 5,3 a 26) a las ocho semanas y 7,0 (IC del 95%: 4,6 a 15) a las 12 semanas (dos estudios, 571 participantes; evidencia de calidad moderada). Más participantes (alrededor del 10%) tuvieron reducciones promedio de la intensidad del dolor de dos a ocho semanas y de dos a 12 semanas sobre el valor inicial de al menos el 30% y al menos el 50% con capsaicina que con el control, con valores NNT entre 10 y 12 (dos a cuatro estudios, 571 a 1272 participantes; evidencia de muy baja calidad).
Para la neuropatía dolorosa por VIH, se encontraron dos estudios (801 participantes). Un estudio informó la proporción de participantes que mejoraron más o mucho más a las 12 semanas (27% con capsaicina de alta concentración y 10% con placebo "activo"). Para ambos estudios, más participantes (alrededor del 10%) tuvieron reducciones promedio de la intensidad del dolor de dos a 12 semanas sobre el valor inicial de al menos el 30% con capsaicina que con control, con un NNT de 11 (evidencia de muy baja calidad).
Para la neuropatía diabética periférica, se encontró un estudio (369 participantes). Informó acerca de un 10% más de participantes que mejoraron más o mucho más a las ocho y 12 semanas. Un estudio pequeño de 46 participantes con dolor persistente después de la herniorrafia inguinal no mostró una diferencia entre la capsaicina y el placebo para la reducción del dolor (evidencia de muy baja calidad).
La calidad de la evidencia para los resultados de eficacia se redujo de uno a tres niveles debido a la escasez de datos, la imprecisión, los posibles efectos de los métodos de imputación y la susceptibilidad al sesgo de publicación.
Los eventos adversos locales fueron frecuentes, pero no se informaron de manera consistente. Los eventos adversos graves no fueron más frecuentes con el tratamiento activo (3,5%) que con el control (3,2%). Los retiros por eventos adversos no difirieron entre los grupos, pero los retiros por falta de eficacia fueron algo más frecuentes con el control que con el tratamiento activo, en base a un pequeño número de eventos (seis a ocho estudios, 21 a 67 eventos; evidencia de calidad moderada, disminuida debido a pocos eventos). No hubo muertes que se consideraran relacionadas con la medicación de estudio.
Conclusiones de los autores
La capsaicina tópica de alta concentración utilizada para tratar la neuralgia posherpética, la neuropatía por VIH y la neuropatía diabética dolorosa generó más participantes con niveles moderados o sustanciales de alivio del dolor que el tratamiento de control con una concentración mucho menor de capsaicina. Estos resultados deben interpretarse con cautela, ya que la calidad de la evidencia fue moderada o muy baja. La proporción adicional que se benefició del control no fue grande, pero para los que sí obtuvieron altos niveles de alivio del dolor, por lo general hubo mejorías adicionales en el sueño, la fatiga, la depresión y la calidad de vida. La capsaicina tópica de alta concentración es similar en sus efectos a otras terapias para el dolor crónico.
PICO
Resumen en términos sencillos
Capsaicina aplicada a la piel para el dolor neuropático crónico en adultos
Conclusión
Existe evidencia de calidad moderada de que los parches de capsaicina de alta concentración (8%) pueden proporcionar un alivio moderado del dolor, o mejor, a una minoría de pacientes con neuralgia posherpética, y evidencia de muy baja calidad de que benefician a los pacientes con neurosis por VIH y neuropatía diabética periférica.
Antecedentes
El dolor neuropático es provocado por el daño a los nervios, ya sea por lesión o enfermedad. El dolor se describe como crónico si se ha experimentado en la mayoría de los días durante al menos tres meses. La capsaicina se obtiene de los pimientos chile picantes. Se piensa que alivia el dolor neuropático crónico al hacer que los nervios sean insensibles a los mensajes de dolor. Esta revisión es una actualización de la última publicada en 2013, y se trata de una preparación altamente concentrada de capsaicina (8%) que debe administrarse en condiciones cuidadosamente controladas en una clínica u hospital, a menudo con anestesia local, ya que sin precauciones especiales puede causar inicialmente dolor y una sensación de ardor en la piel. Se utiliza sólo para tratar áreas localizadas de dolor. La aplicación única está diseñada para producir alivio del dolor durante hasta tres meses.
Características de los estudios
Se realizaron búsquedas en las bases de datos científicas para obtener estudios que consideraran los efectos de la capsaicina de alta concentración en adultos con dolor neuropático moderado o intenso. El tratamiento debía tener efectos medidos durante al menos ocho semanas. La evidencia está actualizada hasta junio de 2016.
Ocho estudios cumplieron los criterios de inclusión, incluidos dos nuevos estudios para esta actualización. Los estudios fueron bien realizados.
Resultados clave
En siete estudios, con 2442 participantes, se encontró que el tratamiento proporcionó buenos niveles de alivio del dolor a un pequeño número de participantes con algunos tipos de dolor neuropático (dolor después del herpes zóster y dolor por lesión nerviosa asociado con la infección por VIH), y probablemente también en otro tipo (pies dolorosos debido a los nervios dañados causados por la diabetes). Alrededor de cuatro de cada 10 pacientes tuvieron al menos un alivio moderado del dolor con capsaicina en comparación con tres de cada 10 con control. El control fue un tratamiento que se veía igual pero que no contenía altos niveles de capsaicina, sin nada agregado o muy pequeñas cantidades de capsaicina agregada. En un estudio pequeño (46 participantes) en pacientes con dolor persistente después de la cirugía de hernia, no pareció ser mejor que el control.
En todos los pacientes que se someten a este tratamiento, puede haber problemas cutáneos localizados de corta duración, como enrojecimiento, quemazón o dolor. Los problemas graves parecen ser poco frecuentes y no fueron más frecuentes en estos ensayos con capsaicina de alta concentración que con el control con capsaicina de muy baja concentración o placebo.
Un número ligeramente mayor de pacientes tratados con control en lugar de capsaicina abandonaron los estudios debido a la falta de beneficios, pero no hubo diferencias entre los grupos para los abandonos debido a los efectos secundarios.
Calidad de la evidencia
Se juzgó la calidad de la evidencia como moderada o muy baja para los resultados de alivio del dolor, principalmente porque sólo un número pequeño de estudios y un número moderado de participantes proporcionaron información para cada resultado. Se consideró la calidad de la evidencia como moderada para los efectos perjudiciales. La calidad moderada significa que la investigación adicional puede cambiar el resultado. Una calidad muy baja significa que existe una gran incertidumbre acerca de los resultados.
Authors' conclusions
Summary of findings
| High‐concentration (8%) capsaicin patch compared with control patch (0.4%) for postherpetic neuralgia | ||||||
| Patient or population: adults with postherpetic neuralgia Settings: community Intervention: high‐concentration (8%) capsaicin patch, single application Comparison: control patch (0.4% capsaicin), single application | ||||||
| Outcomes | Outcome with intervention | Outcome with comparator | RR, NNT, NNH, NNTp (95% CI) | Number of | Quality of the evidence | Comments |
|---|---|---|---|---|---|---|
| Substantial benefit | ||||||
| PGICvery much improved, week 8 and week 12 | No data | No data | ‐ | ‐ | Very low | No data |
| Moderate benefit | ||||||
| PGICmuch or very much improved, week 8 | 360 in 1000 | 250 in 1000 | RR 1.4 (1.1 to 1.8) NNT 8.8 (5.3 to 26) | 2 studies, 571 participants, 178 events | Moderate | Downgraded 1 level due to susceptibility to publication bias |
| PGIC much or very much improved, week 12 | 390 in 1000 | 250 in 1000 | RR 1.6 (1.2 to 2.0) NNT 7.0 (4.6 to 15) | 2 studies, 571 participants, 189 events | Moderate | Downgraded 1 level due to susceptibility to publication bias |
| Harm ‐ all conditions combined | ||||||
| Withdrawals due to lack of efficacy | 15 in 1000 | 31 in 1000 | RR 0.58 (0.32 to 1.04) NNTp 64 (34 to 610) | 6 studies, 2073 participants, 44 events | Moderate | Downgraded 1 level due to imprecision (few events, wide CI) |
| Withdrawals due to adverse events | 8.0 in 1000 | 9.2 in 1000 | RR 0.80 (0.36 to 1.8) NNTp not calculated | 8 studies, 2487 participants, 21 events | Moderate | Downgraded 1 level due to sparse data (few events) |
| Serious adverse events | 35 in 1000 | 32 in 1000 | RR 1.1 (0.70 to 1.8) NNH not calculated | 7 studies, 1993 participants, 67 events | Moderate | Downgraded 1 level due to sparse data (few events) |
| Death | 4 events | 2 events | Not calculated | 8 studies, 2487 participants | Very low | Downgraded 3 levels as only six events, so no better grading possibleNo death was judged related to study medication by study authors |
| CI: confidence interval; NNH: number needed to treat for one additional harmful outcome; NNT: number needed to treat for one additional beneficial outcome; NNTp: number needed to treat to prevent one withdrawal event; PGIC: Patient Global Impression of Change; RR: risk ratio. | ||||||
| Descriptors for levels of evidence (EPOC 2015): † Substantially different: a large enough difference that it might affect a decision. | ||||||
Background
This is an update of a review of high‐concentration (8%) capsaicin for relief of neuropathic pain, published in 2013 (Derry 2013). Low‐concentration capsaicin, usually as a cream or spray that requires regular application, is considered in a separate review (Derry 2012).
Description of the condition
The 2011 International Association for the Study of Pain definition of neuropathic pain is "pain caused by a lesion or disease of the somatosensory system" (Jensen 2011), based on an earlier consensus meeting (Treede 2008). Neuropathic pain is a consequence of a pathological maladaptive response of the nervous system to 'damage' from a wide variety of potential causes. It is characterised by pain in the absence of a noxious stimulus and may be spontaneous (continuous or paroxysmal) in its temporal characteristics or be evoked by sensory stimuli (dynamic mechanical allodynia where pain is evoked by light touch of the skin). Neuropathic pain is associated with a variety of sensory loss (numbness) and sensory gain (allodynia) clinical phenomena, the exact pattern of which vary between person and disease, perhaps reflecting different pain mechanisms operating in an individual person and therefore potentially predictive of response to treatment (Demant 2014; Helfert 2015; von Hehn 2012). Preclinical research hypothesises a bewildering array of possible pain mechanisms that may operate in people with neuropathic pain, which largely reflect pathophysiological responses in both the central and peripheral nervous systems, including neuronal interactions with immune cells (Baron 2012; Calvo 2012; von Hehn 2012). Overall, even the most effective of available drugs provide only modest benefit in treating neuropathic pain (Finnerup 2015; Moore 2013a), and a robust classification of neuropathic pain is not yet available (Finnerup 2013).
Neuropathic pain is usually divided according to the cause of nerve injury. There may be many causes, but common causes of neuropathic pain include diabetes (painful diabetic neuropathy (PDN)), shingles (postherpetic neuralgia (PHN)), amputation (phantom limb pain), neuropathic pain after surgery or trauma, stroke or spinal cord injury, trigeminal neuralgia, and HIV infection. Sometimes the cause is not known.
Many people with neuropathic pain conditions are significantly disabled with moderate or severe pain for many years. Chronic pain conditions comprised five of the 11 top‐ranking conditions for years lived with disability in 2010 (Vos 2012), and are responsible for considerable loss of quality of life, employment, and increased healthcare costs (Bouhassira 2012; Moore 2014a).
In systematic reviews, the overall prevalence of neuropathic pain in the general population is reported to be between 7% and 10% (van Hecke 2014), and about 7% in one systematic review of studies published since 2000 (Moore 2014a). In individual countries, prevalence rates have been reported as 3.3% in Austria (Gustorff 2008), 6.9% in France (Bouhassira 2008), and up to 8% in the UK (Torrance 2006). Some forms of neuropathic pain are increasing, particularly PDN and postsurgical chronic pain (which is often neuropathic in origin)(Hall 2008). The prevalence of PHN is likely to fall if vaccination against the herpes virus becomes widespread.
Estimates of incidence vary between individual studies for neuropathic pain associated with particular conditions, often because of small numbers of cases. In primary care in the UK between 2002 and 2005, the incidences (per 100,000 person‐years' observation) were 28 (95% confidence interval (CI) 27 to 30) for PHN, 27 (95% CI 26 to 29) for trigeminal neuralgia, 0.8 (95% CI 0.6 to 1.1) for phantom limb pain, and 21 (95% CI 20 to 22) for PDN (Hall 2008). Other research groups have estimated an incidence of 4 in 100,000 per year for trigeminal neuralgia (Katusic 1991; Rappaport 1994), and of 12.6 per 100,000 person‐years for trigeminal neuralgia and 3.9 per 100,000 person‐years for PHN in one study of facial pain in the Netherlands (Koopman 2009).
Neuropathic pain is difficult to treat effectively, with only a minority of people experiencing a clinically relevant benefit from any one intervention. A multidisciplinary approach is now advocated, with pharmacological interventions being combined with physical or cognitive interventions, or both. Conventional analgesics are usually thought to be ineffective, but without evidence to support or refute that view. Some people with neuropathic pain may derive some benefit from a topical lidocaine patch or low‐concentration topical capsaicin, though evidence about benefits is uncertain (Derry 2012; Derry 2014). The earlier review of high‐concentration topical capsaicin indicated benefit in some people with PHN (Derry 2013). Treatment for neuropathic pain is more usually with so‐called unconventional analgesics (pain modulators), for example, with antidepressants such as duloxetine and amitriptyline (Lunn 2014; Moore 2012a; Sultan 2008), or antiepileptic drugs such as gabapentin or pregabalin (Moore 2009; Moore 2014b; Wiffen 2013).
The proportion of people who achieve worthwhile pain relief (typically at least 50% pain intensity reduction (PIR); Moore 2013b) with any one intervention is small, generally only 10% to 25% more than with placebo, with numbers needed to treat for an additional beneficial outcome (NNT) usually between 4 and 10 (Kalso 2013; Moore 2013a; Moore 2014c). Neuropathic pain is not particularly different from other chronic pain conditions in that only a small proportion of trial participants have a good response to treatment (Moore 2013a).
The current National Institute for Health and Care Excellence (NICE) guidance for the pharmacological management of neuropathic pain suggests offering a choice of amitriptyline, duloxetine, gabapentin, or pregabalin as initial treatment for neuropathic pain (with the exception of trigeminal neuralgia), with switching if first, second, or third drugs tried are not effective or not tolerated (NICE 2013). This concurs with other recent guidance (Finnerup 2015).
Topical agents are most likely to be used for localised, peripheral neuropathies.
Description of the intervention
Topical medications are applied externally and are taken up through the skin. They exert their effects close to the site of application, and there is no substantial systemic uptake or distribution. This compares with transdermal application, where the medication is applied externally and is taken up through the skin, but relies on systemic distribution for its effect.
Low‐concentration capsaicin creams have not convincingly been shown to be effective for neuropathic pain (Derry 2012). The initial burning sensation felt on application of capsaicin limits the amount of active substance that can be applied at one time, which necessitates frequent (four times per day) application, and reduces compliance with treatment. The high‐concentration (8%) patch was developed to increase the amount of capsaicin delivered to the skin, and improve tolerability. Rapid delivery is thought to improve tolerability because cutaneous nociceptors are 'defunctionalised' quickly, and the single application avoids both noncompliance and contamination of the home environment with particles of dried capsaicin cream (Anand 2011). At the time of this review, the 8% patch is the only high‐strength formulation of capsaicin commercially available, although different strengths and formulations have been investigated in clinical trials. For the purposes of this review, we considered 5% or greater to be a high concentration.
The treatment is usually applied as a single application dermal patch over the area where painful symptoms are felt. Each patch (280 cm2) contains capsaicin 640 μg/cm2, and can be cut to treat smaller areas and irregular shapes, or up to four patches can be used simultaneously to treat large areas, such as the back (eMC 2012). The skin to which patches are applied should not be broken or irritated. The skin is usually treated with a topical local anaesthetic (e.g. topical lidocaine 4% for 60 minutes) before application because the capsaicin may cause an intense burning sensation, and the anaesthetic is then washed off thoroughly, and the skin dried, before the patch is applied. Studies suggest that skin cooling is as effective as topical anaesthetic for relieving initial burning (Knolle 2013), or that any form of pretreatment in unnecessary (Kern 2014). The patch is left in place for 30 minutes when applied to the feet, or 60 minutes for other areas, before removal and careful cleansing of the skin with a specially formulated cleanser, to remove any residual capsaicin. Application must be carried out in a healthcare centre by trained personnel, and patients are usually monitored for up to two hours after treatment. Stringent conditions are required, and as well as using trained healthcare professionals, the treatment setting needs to be well ventilated and spacious due to the vapour of the capsaicin, and cough due to inhalation of capsaicin particles or dust is a hazard for both the healthcare professionals and the patients. Treatment can be repeated after 12 weeks if necessary.
High‐concentration capsaicin is available by prescription only; it was first licensed in Europe and the US in 2009. It was originally licensed in the European Union (EU) to treat neuropathic pain in patients without diabetes, but in 2015 the restriction on patients with diabetes was lifted. In the US, it is licensed only to treat PHN. The US Food and Drug Administration refused a license for neuropathic pain in HIV in 2012. We could not find information about marketed products outside Europe and the US.
How the intervention might work
Capsaicin is the active compound present in chilli peppers, responsible for making them hot when eaten. It binds to nociceptors (sensory receptors responsible for sending signals that cause the perception of pain) in the skin, and specifically to the TRVP1 receptor, which controls movement of sodium and calcium ions across the cell membrane. Initially, binding opens the ion channel (influx of sodium and calcium ions), causing depolarisation and the production of action potentials, which are usually perceived as itching, pricking, or burning sensations. Repeated applications or high concentrations give rise to a long‐lasting effect, which has been termed 'defunctionalisation', probably owing to a number of different effects that together overwhelm the cell's normal functions, and can lead to reversible degeneration of nerve terminals (Anand 2011).
Adverse events from capsaicin are mainly at the application site (burning, stinging, erythema), and systemic events are rare. Achieving double‐blind conditions in placebo‐controlled trials using capsaicin can therefore be difficult.
Why it is important to do this review
Since the review in 2013, there have been new studies involving high‐dose capsaicin in different neuropathic pain conditions and in different formulations. The licensed indications have also changed for Europe (and possibly other jurisdictions). It is important, therefore, to update the review to include the latest information to inform clinical practice.
Objectives
To review the evidence from controlled trials on the efficacy and tolerability of topically applied, high‐concentration (8%) capsaicin for neuropathic pain in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised, double‐blind trials comparing high‐concentration (typically 8%) topical capsaicin with placebo or other active treatment for neuropathic pain, with at least 10 participants per treatment arm. We excluded studies published only as short abstracts (usually meeting reports) or studies of experimentally induced pain.
Types of participants
Adults (aged 16 years or more) with neuropathic pain of at least moderate intensity (Collins 1997) resulting from any cause, with a duration of at least 12 weeks and as defined in the study using accepted diagnostic criteria.
Types of interventions
Included studies had at least one treatment arm using a single application of high‐concentration (8%) topical capsaicin, and a comparator arm using placebo or other active treatment.
Types of outcome measures
We anticipated that studies would use a variety of outcome measures, with most studies using standard subjective scales (numerical rating scale or visual analogue scale) for pain intensity or pain relief, or both. We were particularly interested in Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) definitions for moderate and substantial benefit in chronic pain studies (Dworkin 2008). These are defined as:
-
at least 30% pain relief over baseline (moderate);
-
at least 50% pain relief over baseline (substantial);
-
much or very much improved on Patient Global Impression of Change scale (PGIC; moderate);
-
very much improved on PGIC (substantial).
These dichotomous outcomes are important where pain responses do not follow a normal (Gaussian) distribution. People with chronic pain desire high levels of pain relief, ideally more than 50% PIR, and ideally having no worse than mild pain (Moore 2013b; O'Brien 2010).
Primary outcomes
-
Participant‐reported pain intensity reduction (PIR) of 30% or greater.
-
Participant‐reported PIR of 50% or greater.
-
Patient Global Impression of Change (PGIC) much or very much improved.
-
PGIC very much improved.
Secondary outcomes
-
Any pain‐related outcome indicating some improvement.
-
Withdrawals due to lack of efficacy and adverse events.
-
Participants experiencing local adverse events (application site events) and systemic adverse events.
-
Participants experiencing any serious adverse event. Serious adverse events typically include any untoward clinical occurrence or effect that at any dose results in death, is life‐threatening, requires hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability or incapacity, is a congenital anomaly or birth defect, is an 'important medical event' that may jeopardise the person, or may require an intervention to prevent one of the above characteristics or consequences.
-
Specific adverse events, particularly local skin reactions.
We anticipated that outcomes would be reported after different durations of treatment, and extracted data reported around 8 to 12 weeks as this is the expected duration of a single‐dose administration of high‐concentration topical capsaicin, but not generally to examine outcomes at less than 6 weeks because that would be considered an inadequate duration of effect. Where longer‐duration outcomes were available, we also extracted these. We also anticipated that reporting of adverse events would vary between trials with regard to the terminology used, method of ascertainment, and categories that were reported (e.g. occurring in at least 5% of participants or where there was a statistically significant difference between treatment groups). Care was taken to identify these details.
Search methods for identification of studies
Electronic searches
For this update, we searched the following databases, without language restriction.
-
Cochrane Central Register of Controlled Trials (CENTRAL, via the Cochrane Register of Studies Online database) to 10 June 2016.
-
MEDLINE via Ovid (January 2012 to 10 June 2016).
-
Embase via Ovid (January 2012 to 10 June 2016).
See Appendix 1 for the CENTRAL search strategy, Appendix 2 for the MEDLINE search strategy, and Appendix 3 for the Embase search strategy.
Searching other resources
We also searched the reference lists of review articles and included studies, together with two clinical trials databases (ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform (ICTRP); www.who.int/ictrp/en/), and the Astellas clinical trials website. We did not search grey literature and short abstracts, or directly contact manufacturers and license holders for unpublished clinical trial data for this update.
Data collection and analysis
Two review authors independently selected the studies for inclusion, assessed risk of bias, and extracted data. One review author entered data for analyses, which was checked by another review author. We resolved disagreements through discussion.
Selection of studies
We reviewed the titles and abstracts of studies identified by the searches on‐screen to eliminate those that clearly did not satisfy the inclusion criteria. We obtained full reports of the remaining studies to determine inclusion in the review. We considered cross‐over studies only if data from the first treatment period were reported separately.
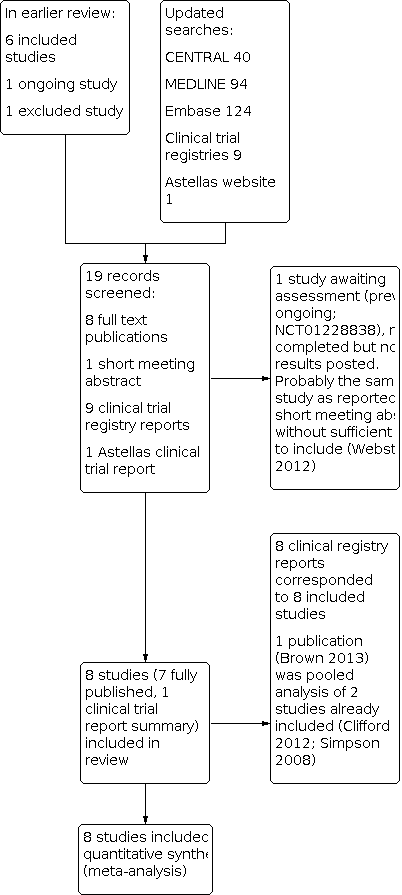
We did not anonymise the studies before assessment. We have included a Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow chart (Figure 1).
Study flow diagram.
Data extraction and management
We abstracted information on participants, interventions, and outcomes from the original reports into a standard data extraction form and checked for agreement before entry into Review Manager 5 (RevMan 2014) or any other analysis tool. We included information about the pain condition and number of participants treated, drug and dosing regimen, study design (placebo or active control), study duration and follow‐up, analgesic outcome measures and results, withdrawals, and adverse events. We did not contact authors for further information.
Assessment of risk of bias in included studies
We used the Oxford Quality Score (Jadad 1996) as the basis for inclusion, limiting inclusion to studies that, as a minimum, were randomised and double‐blind.
The review authors independently assessed risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 8, Higgins 2011), and adapted from those used by the Cochrane Pregnancy and Childbirth Group, with any disagreements resolved by discussion. We assessed the following for each study.
-
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process, such as random number table; computer random number generator); unclear risk of bias (method used to generate sequence not clearly stated). We excluded studies at a high risk of bias that used a nonrandom process (odd or even date of birth; hospital or clinic record number).
-
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment assessed whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment. We assessed the methods as: low risk of bias (telephone or central randomisation; consecutively numbered sealed opaque envelopes); unclear risk of bias (method not clearly stated). We excluded studies that did not conceal allocation and were therefore at a high risk of bias (open list).
-
Blinding of participants and personnel, and outcome assessment (checking for possible performance and detection bias). We assessed the methods used to blind study participants and personnel from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study stated that it was blinded and described the method used to achieve blinding, identical tablets; matched in appearance and smell); unclear risk of bias (study stated that it was blinded but did not provide an adequate description of how it was achieved). We excluded studies at a high risk of bias that were not double‐blind.
-
Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk (fewer than 10% of participants did not complete the study or used 'baseline observation carried forward' analysis, or both); unclear risk of bias (used 'last observation carried forward' (LOCF) analysis); high risk of bias (used 'completer' analysis).
-
Size (checking for possible biases confounded by small size). Small studies have been shown to overestimate treatment effects, probably due to methodological weaknesses (Dechartres 2013; Nüesch 2010). We assessed studies as: low risk of bias if they had at least 200 participants per treatment arm; unclear risk of bias if they had 50 to 200 participants per treatment arm; high risk of bias if they had fewer than 50 participants per treatment arm.
Measures of treatment effect
We used risk ratio (RR) to establish statistical difference. We used numbers needed to treat for an additional beneficial outcome (NNT) and pooled percentages as an absolute measure of benefit.
We used the following terms to describe adverse outcomes in terms of harm or prevention of harm:
-
When significantly fewer adverse outcomes occurred with capsaicin than with control (placebo or active) we used the term the number needed to treat to prevent one event (NNTp).
-
When significantly more adverse outcomes occurred with capsaicin compared with control (placebo or active) we used the term the number needed to treat for an additional harmful outcome or cause one event (NNH).
Unit of analysis issues
We accepted randomisation to the individual participant only. In the event of a study having more than one active treatment arm, in which data were not combined for analysis, we planned to split the control treatment arm between active treatment arms. For cross‐over studies, we planned to use only the first period data.
Dealing with missing data
The most likely source of missing data was expected to be from participants dropping out from the studies. We looked specifically for evidence of LOCF and used a dichotomous responder analysis, where a responder was defined as a participant who experienced the predefined outcome and remained in the study (e.g. did not withdraw due to adverse events). LOCF is a potential source of major bias in chronic pain studies (Moore 2012b).
For all outcomes, we carried out analyses, as far as possible, on a modified intention‐to‐treat (ITT) basis (we included all participants who were randomised and received an intervention). Where sufficient information was reported, we added back missing data in the analyses we undertook.
Assessment of heterogeneity
We planned to deal with clinical heterogeneity by combining studies that examined similar conditions, and to assess statistical heterogeneity visually (L'Abbé 1987), and with using the I2 statistic. When the I2 value was greater than 50%, we considered possible reasons for this.
Assessment of reporting biases
We planned to assess publication bias by examining the number of participants in trials with zero effect (RR 1.0) needed for the point estimate of the NNT to increase beyond a clinically useful level (Moore 2008a). In this case, we specified a clinically useful level as an NNT of 10 for clinical improvement at 8 or 12 weeks.
Data synthesis
We conducted analyses of all efficacy outcomes according to type of painful condition, because interventions are known to have different effects in different types of neuropathic pain (Moore 2009). For adverse events, we combined all conditions.
At least 200 participants had to be available for any outcome before we pooled studies (Moore 1998). Where appropriate, we calculated RR with 95% CI using a fixed‐effect model (Morris 1995). We calculated NNT and NNH with 95% CIs using the pooled number of events, using the method devised by Cook and Sackett (Cook 1995). We assumed a statistically significant difference from control when the 95% CI of the RR did not include the number one.
If we had found significant clinical heterogeneity and considered it appropriate to combine studies, we would have investigated it using a random‐effects model.
Quality of the evidence
We used the GRADE system to assess the quality of the evidence related to the key outcomes listed in Types of outcome measures, as appropriate (Appendix 4). Two review authors independently rated the quality of each outcome.
We paid particular attention to inconsistency, where point estimates vary widely across studies or CIs of studies show minimal or no overlap (Guyatt 2011), and potential for publication bias, based on the amount of unpublished data required to make the result clinically irrelevant (Moore 2008a).
In addition, there may be circumstances where the overall rating for a particular outcome needs to be adjusted as recommended by GRADE guidelines (Guyatt 2013a). For example, if there are so few data that the results are highly susceptible to the random play of chance, or if studies use LOCF imputation in circumstances where there are substantial differences in adverse event withdrawals, one would have no confidence in the result, and would need to downgrade the quality of the evidence by three levels, to very low quality. In circumstances where there were no data reported for an outcome, we would have reported the level of evidence as very low quality (Guyatt 2013b).
'Summary of findings' table
We included a 'Summary of findings' table, as set out in the author guide (PaPaS 2012), and recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 4, Higgins 2011; summary of findings Table 1). We included key information concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the outcomes of 'substantial benefit' (PGIC very much improved from weeks 2 to 8 and weeks 2 to 12), 'moderate benefit' (PGIC much or very much improved from weeks 2 to 8 and weeks 2 to 12), withdrawals due to adverse events, withdrawals due to lack of efficacy, serious adverse events, and death (a particular serious adverse event).
For the 'Summary of findings' table we used the following descriptors for levels of evidence (EPOC 2015).
-
High: This research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different† is low.
-
Moderate: This research provides a good indication of the likely effect. The likelihood that the effect will be substantially different† is moderate.
-
Low: This research provides some indication of the likely effect. However, the likelihood that it will be substantially different† is high.
-
Very low: This research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different† is very high.
† Substantially different: a large enough difference that it might affect a decision.
Subgroup analysis and investigation of heterogeneity
We planned all efficacy analyses to be according to individual painful conditions. We did not plan further subgroup analyses since experience of previous reviews indicated that there would be too few data for any meaningful subgroup analysis.
Sensitivity analysis
We did not plan any sensitivity analyses for this update.
Results
Description of studies
Results of the search
The earlier review included six studies, excluded one study, and identified one ongoing study. Updated searches identified 40 articles in CENTRAL, 94 in MEDLINE, and 124 in Embase. After screening titles and abstracts, we obtained full copies of two published reports. We also identified nine relevant clinical study reports in trial registries and one on the Astellas website. One study was reported in a short meeting abstract.
One of the published reports was a new study that satisfied our inclusion criteria (Bischoff 2014). The other published report was a pooled analysis of two studies already included in the review (Brown and colleagues, reporting on Clifford 2012 and Simpson 2008). The unpublished study on the Astellas website satisfied our inclusion criteria (STEP 2014); this has since been published, and checked against our data extraction (Simpson and colleagues, 2016, see under STEP 2014). Eight reports identified in clinical trial registries related to the six previously included studies, the new published study, and the study identified on the Astellas website. The remaining report was previously identified as an ongoing study, which has now completed, but no results have been posted (NCT01228838). It is likely that this study is the one reported in a short meeting abstract (Webster and colleagues, see under NCT01228838). There was insufficient information to include this study in the review, and it has been placed in 'Studies awaiting assessment'. See Figure 1.
Included studies
We included eight studies, with 2488 participants. Six were in the earlier review (Backonja 2008; Clifford 2012; Irving 2011; Simpson 2008; Webster 2010a; Webster 2010b) and two were new studies (Bischoff 2014; STEP 2014).
Participants had pain due to PHN (Backonja 2008; Backonja 2010; Irving 2011; Webster 2010a; Webster 2010b), HIV‐neuropathy (Clifford 2012; Simpson 2008), painful PDN (STEP 2014), and persistent pain after inguinal herniorrhaphy (Bischoff 2014). In all studies, pain was of at least moderate severity and was frequently unresponsive to, or poorly controlled by, conventional therapy. In studies of PHN, the mean age of participants was 70 to 71 years and men and women were enrolled in approximately equal numbers. In studies of HIV‐neuropathy, the mean age of participants was 48 and 50 years and about 90% were men. For PDN, the mean age of participants was 63 years and 58% were men, while for persistent pain after inguinal herniorrhaphy, the mean age was 54 years and over 90% were men.
The duration of application of high‐concentration topical capsaicin varied between 30 and 90 minutes, with most participants treated for 60 minutes. Clifford 2012; Simpson 2008 and Webster 2010a tested different durations in participants with HIV‐neuropathy and PHN, while STEP 2014 treated the feet of all participants with PDN for 30 minutes. Bischoff 2014 treated participants with persistent pain after inguinal herniorrhaphy for 60 minutes.
All the included studies used a 'placebo' comparator. Because application of capsaicin to the skin, particularly at this high concentration, initially causes erythema (redness) and a burning or stinging sensation in many people, maintaining the double‐blind status of studies is problematic. Most studies used a low dose (0.04%) of capsaicin in the control patch to produce some degree of skin irritation without effective analgesia, in an attempt to prevent participants from guessing their treatment allocation, but two studies did not (Bischoff 2014; STEP 2014). We refer to these control patches as 'low‐concentration capsaicin control' and 'placebo control', respectively.
Most studies permitted stable treatment with concomitant oral or transdermal drugs (opioids of morphine equivalent 60 mg/day or less) to be continued for neuropathic pain without change in dose or frequency, but all topical medications were discontinued at least seven days before the study. STEP 2014 did not allow any oral, transdermal, or parenteral opioids at any dose in the seven days preceding patch application.
Details of included studies are in the Characteristics of included studies table.
Excluded studies
We excluded one study after reading the full report, because study duration was only 4 weeks (Backonja 2010; see the Characteristics of excluded studies table).
Risk of bias in included studies
We scored each study for methodological quality using the Oxford Quality Score; all studies scored 4/5 except Bischoff 2014, which scored 5/5, and Simpson 2008, which scored 3/5.
We completed a 'Risk of bias' table for all studies for sequence generation, allocation concealment, blinding, incomplete outcome data, and size. We judged all the studies to be low or unclear risk of bias for all criteria except Bischoff 2014, which we judged at high risk of bias for size. In most cases where the risk was assessed as 'unknown' it was likely that the methods were rigorous, but the reporting inadequate (e.g. randomisation, allocation concealment). Where there was incomplete outcome data due to missing values, the most relevant outcomes were reported without LOCF imputation.
Full details can be found in the Characteristics of included studies table and Figure 2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Only Bischoff 2014 clearly reported the method of randomisation, but since these studies were carried out under rigorous conditions by the pharmaceutical company it is likely that the schedule was computer‐generated. Only Backonja 2008 and Irving 2011 adequately described the method used to conceal the sequence allocation.
Blinding
Studies were all described as double‐blind, and this was generally well described.
Incomplete outcome data
The nature of the studies was that all participants received the single application of topical high‐concentration capsaicin at the start of the study, but there were some withdrawals or losses to follow‐up thereafter, although these were generally small. Modified LOCF analysis was used for some efficacy outcomes, but no imputation was used for weekly pain scores or patient global assessment of treatment, where nonreporting was regarded as nonresponse. All participants were included for safety analyses.
Selective reporting
All relevant outcomes were reported according to the study methods, although there was inconsistency between studies in the exact outcomes reported.
Other potential sources of bias
Bischoff 2014 had small treatment groups, which can be associated with overestimation of effect. Otherwise, studies were generally large and apparently well conducted, so there were no other obvious sources of bias.
Effects of interventions
Types of efficacy outcomes reported
In these studies, participants with chronic pain were given a single 30‐ to 90‐minute intervention with high‐concentration topical capsaicin, and their pain was then measured over the following 8 to 12 weeks. Because the intervention itself could cause localised pain at the application site, no pain measurements were generally made in the first post‐treatment week. The outcomes then reported were of two distinct types.
-
We assessed the longevity of benefit from a PGIC made at specific points, usually 8 and 12 weeks after drug administration. Responses of much or very much improved equated to 'moderate benefit', and very much improved to 'substantial benefit'. We considered these outcomes to provide the most reliable evidence. The expected pattern would be early, but not later, differences between active and control interventions.
-
Most studies calculated average pain scores over weeks 2 to 8 and 2 to 12, and recorded the number of participants with PIR of at least 30% or at least 50% over baseline. We considered these outcomes to provide less reliable evidence because they used data averaged over the study duration, and these studies used LOCF imputation for most missing data. These outcomes might be regarded as assessing whether the intervention 'worked' in providing a larger proportion of participants with adequate pain relief with the intervention than with control. Because the largest difference between active treatment and control typically occurred in the first 4 to 6 weeks after treatment, these measures did not adequately address for how long the benefits lasted.
Not all studies reported all of these outcomes, so data were inconsistently available for pooling and analysis. We have used what we consider to be the most reliable evidence for the 'Summary of findings' table.
Details of study efficacy outcomes are in Appendix 5, adverse events and withdrawals in Appendix 6, and patch tolerability in Appendix 7. Preliminary analyses demonstrated that duration of administration of high‐concentration topical capsaicin of 30 and 90 minutes for PHN and HIV‐neuropathy resulted in no discernible difference in efficacy from 60 minutes (Analysis 1.1; Analysis 1.3), so in the following analyses we combined results for different durations of patch application.
Postherpetic neuralgia
PHN was the neuropathic pain condition in four studies involving 1272 participants (742 exposed to high‐concentration topical capsaicin, 530 to low‐concentration 0.04% capsaicin control) (Backonja 2008; Irving 2011; Webster 2010a; Webster 2010b). Not all outcomes were reported in all studies, with the exception of at least 30% PIR over 2 to 8 weeks compared with baseline pain, which all four studies reported.
Pain intensity reduction of 30% or greater, or 50% or greater
Results for the different levels of PIR are shown in the 'Summary of results A' table. The magnitude of the treatment effect was similar for at least 30% reduction (moderate benefit) and at least 50% reduction (substantial benefit) over baseline for the average weekly pain intensity over 2 to 8 (at least 30% PIR 2 to 8 weeks; at least 50% PIR 2 to 8 weeks) and 2 to 12 weeks (at least 30% PIR 2 to 12 weeks; at least 50% PIR 2 to 12 weeks), with NNT point estimates of between 10 and 12 in comparisons with low‐concentration capsaicin controls (Figure 3; Analysis 1.2; Analysis 1.3; Analysis 1.4).

Forest plot of comparison: 1 High‐concentration (8%) capsaicin versus control (single dose), outcome: 1.1 Postherpetic neuralgia ‐ at least 50% pain intensity reduction over weeks 2 to 8.
We downgraded the quality of the evidence to very low because of imprecision (wide CIs), because of the uncertain effects of the use of LOCF imputation, and because of susceptibility to publication bias with point estimates for NNT of 10 or above.
Patient Global Impression of Change much or very much improved
Results for PGIC are shown in the 'Summary of results A' table.
There were no data reported for PGIC very much improved (substantial benefit).
Only two of the four studies reported PGIC outcomes of much or very much improved (moderate benefit) at 8 and 12 weeks (Irving 2011; Webster 2010b). At both 8 and 12 weeks, there was a significant benefit for high‐concentration over low‐concentration topical capsaicin, with point estimates of the NNTs of 8.8 for high‐concentration and 7.0 for low‐concentration (Figure 4).

Forest plot of comparison: 1 High‐concentration (8%) capsaicin versus control (single dose), outcome: 1.5 Postherpetic neuralgia ‐ Patient Global Impression of Change much or very much improved at 8 and 12 weeks.
We downgraded the quality of the evidence to moderate due to susceptibility to publication bias as null effect data from only about 250 participants would be needed to raise the NNT above 10 (Moore 2008a). See summary of findings Table 1.
Summary of results A
| Outcome | Number | Per cent with outcome | RR | NNT | ||
|---|---|---|---|---|---|---|
| Trials | Participants | 8% Capsaicin | Control | |||
| Postherpetic neuralgia | ||||||
| ≥ 30% PIR 2 to 8 weeks | 4 | 1272 | 43 | 34 | 1.3 (1.1 to 1.5) | 11 (6.8 to 26) |
| ≥ 30% PIR 2 to 12 weeks | 3 | 973 | 46 | 37 | 1.3 (1.1 to 1.5) | 10 (6.3 to 28) |
| ≥ 50% PIR 2 to 8 weeks | 3 | 870 | 29 | 20 | 1.4 (1.1 to 1.9) | 12 (7.2 to 41) |
| ≥ 50% PIR 2 to 12 weeks | 2 | 571 | 33 | 24 | 1.3 (1.0 to 1.7) | 11 (6.1 to 62) |
| PGIC much/very much improved at 8 weeks | 2 | 571 | 36 | 25 | 1.4 (1.1 to 1.8) | 8.8 (5.3 to 26) |
| PGIC much/very much improved at 12 weeks | 2 | 571 | 39 | 25 | 1.6 (1.2 to 2.0) | 7.0 (4.6 to 15) |
| HIV‐neuropathy | ||||||
| ≥30% PIR 2 to 12 weeks | 2 | 801 | 39 | 30 | 1.4 (1.1 to 1.7) | 11 (6.2 to 47) |
| PGIC much/very much 12 weeks* | 1 | 307 | 27 | 10 | 2.8 (1.4 to 5.6) | 5.8 (3.8 to 12) |
| Peripheral diabetic neuropathy | ||||||
| ≥ 30% PIR 2 to 8 weeks* | 1 | 369 | 40 | 33 | 1.2 (0.92 to 1.6) | Not calculated |
| ≥ 30% PIR 2 to 12 weeks* | 1 | 369 | 41 | 32 | 1.3 (0.98 to 1.7) | Not calculated |
| ≥ 50% PIR 2 to 8 weeks* | 1 | 369 | 21 | 18 | 1.2 (0.77 to 1.8) | Not calculated |
| ≥ 50% PIR 2 to 12 weeks* | 1 | 369 | 22 | 19 | 1.2 (0.77 to 1.7) | Not calculated |
| PGIC much/very much 8 weeks* | 1 | 369 | 38 | 28 | 1.3 (1.0 to 1.8) | 10 (5.2 to 520) |
| PGIC much/very much 12 weeks* | 1 | 369 | 36 | 28 | 1.2 (0.92 to 1.7) | Not calculated |
| * Note that these results are from > 200 participants, but from a single study and so should be treated with caution. They are reported for comparative purposes only. | ||||||
| CI: confidence interval; NNT: number needed to treat for an additional beneficial outcome; PGIC: Patient Global Impression of Change; PIR: pain intensity reduction; RR: risk ratio | ||||||
HIV‐neuropathy
Painful HIV‐neuropathy was the neuropathic pain condition in two studies involving 801 participants (557 exposed to high‐concentration topical capsaicin, 244 to low‐concentration 0.04% capsaicin control) (Clifford 2012; Simpson 2008). All outcomes were not reported in both studies, with the exception of at least 30% PIR over 2 to 12 weeks compared with baseline pain.
Pain intensity reduction of 30% or greater, or 50% or greater
Neither study reported at least 50% PIR over baseline (substantial benefit).
Both studies reported at least 30% PIR (moderate benefit) over 2 to 12 weeks compared with baseline ('Summary of results A' table) (Figure 5). The point estimate of the NNT was 11.

Forest plot of comparison: 1 High‐concentration (8%) capsaicin versus control (single dose), outcome: 1.6 HIV‐neuropathy ‐ at least 30% pain intensity reduction over weeks 2 to 12.
We downgraded the quality of the evidence to very low because of imprecision (wide CIs), because of the uncertain effects of the use of LOCF imputation, and because of susceptibility to publication bias with a point estimate for NNT above 10.
Patient Global Impression of Change much or very much improved
We found no data for PGIC very much improved (substantial benefit).
One study reported PGIC of much or very much improved (moderate benefit) at 12 weeks (Simpson 2008). Results are shown in the 'Summary of results A' table for comparison, but they derive from a single study and should be interpreted with caution. There was a significant benefit for high‐concentration topical capsaicin over low‐concentration control, with a point estimate for the NNT of 5.8.
We downgraded the quality of the evidence to very low due to sparse data, imprecision, and susceptibility to publication bias.
Peripheral diabetic neuropathy
STEP 2014 treated 369 participants with painful PDN (186 exposed to high‐concentration topical capsaicin, 183 to placebo), and reported outcomes over 8 and 12 weeks.
Pain intensity reduction of 30% or greater, or 50% or greater
The study reported both 30% and 50% PIR over 2 to 8 and 2 to 12 weeks compared with baseline. About 10% more participants had at least a 30% reduction with high‐concentration capsaicin than with placebo at both time points. The response rate was lower for at least 50% PIRs, and only 3% higher with capsaicin than with placebo ('Summary of results A' table).
We downgraded the quality of the evidence to very low because of imprecision (wide CIs), uncertain effects of the use of LOCF imputation, and susceptibility to publication bias.
Patient Global Impression of Change much or very much improved
We found no data for PGIC very much improved (substantial benefit).
About 10% more participants reported PGIC outcomes of much or very much improved (moderate benefit) at 8 and 12 weeks with capsaicin than with placebo ('Summary of results A' table).
We downgraded the quality of the evidence to very low due to sparse data, imprecision, and susceptibility to publication bias.
Persistent pain after inguinal herniorrhaphy
Bischoff 2014 treated 45 participants with persistent pain after inguinal herniorrhaphy (23 exposed to high‐concentration capsaicin, 22 to placebo).
The study did not report any responder analyses, but did report no significant difference in the summed pain intensity difference (from baseline) between groups at 4, 8, or 12 weeks after treatment. We downgraded the quality of the evidence to very low due to sparse data.
Subgroup analysis
Dose and condition
Analysis by pain condition has been carried out in the primary analysis above.
Adverse events
Reporting of adverse events was inconsistent and incomplete (Appendix 6). Most studies did not report the precise methods used to collect adverse event data, such as use of direct or indirect questioning or participant diaries, or the timing of data collection, but they did consistently classify adverse events and serious adverse events according to the Medical Dictionary for Regulatory Activities. Most adverse events were transient and mild to moderate in intensity. Five studies reported adverse events occurring in at least 3% (Backonja 2008; Clifford 2012; Irving 2011; Webster 2010a; Webster 2010b), and two in at least 2% (Simpson 2008; STEP 2014), of participants in any treatment arm, together with any serious adverse events. Bischoff 2014 reported all adverse events. The most common events were application site (skin) reactions.
Local skin reactions
All included studies reported on local skin reactions. Two studies used placebo control patches (Bischoff 2014; STEP 2014), but in the other studies the control patches contained a low concentration (0.04%) of capsaicin to mimic the burning sensation of capsaicin without providing effective pain relief. It was not possible to determine the number of participants experiencing any type of local skin reaction since more than one symptom may appear in an individual participant. We chose to analyse 'erythema, pain, papules, and pruritus' as these were fairly consistently reported in individual studies. For analysis, we combined studies in the different pain conditions and all durations of application since there were no obvious differences or trends and the number of events was small.
Some studies captured all adverse events following application. We defined these as Group 1 studies, which comprised Backonja 2008; Bischoff 2014; Clifford 2012; and Irving 2011 (Analysis 1.7). The other studies reported adverse events differently; these Group 2 studies comprised Simpson 2008, STEP 2014, Webster 2010a, and Webster 2010b (Analysis 1.8; 'Summary of results B' table). Two Group 2 studies specifically stated that "treatment associated erythema, discomfort and pain on the day of treatment were not captured as adverse events but reported as dermal assessment scores or 'Pain Now' scores" (Webster 2010a; Webster 2010b). They reported very much lower rates of skin adverse events, presumably because events in the first day were not included. The other Group 2 studies did not specify whether they included skin reactions on the first day as adverse events, but they also had a very much lower rate (Simpson 2008; STEP 2014), and are analysed with the Webster studies (Webster 2010a; Webster 2010b).
We downgraded the quality of the evidence to moderate due to inconsistent reporting and assumptions made in pooling studies for analysis.
Summary of results B
| Outcome | Number | Per cent with outcome | RR (95% CI) | NNH (95% CI) | ||
|---|---|---|---|---|---|---|
| Studies | Participants | 8% Capsaicin | Control | |||
| Group 1 | ||||||
| Erythema | 4 | 1355 | 75 | 57 | 1.4 (1.3 to 1.5) | 5.5 (4.3 to 7.7) |
| Pain | 4 | 1355 | 69 | 29 | 2.3 (2.0 to 2.6) | 2.5 (2.2 to 2.8) |
| Papules | 3 | 1312 | 6.3 | 2.0 | 3.6 (1.9 to 6.9) | 23 (16 to 46) |
| Pruritus | 3 | 1312 | 3.7 | 2.0 | 2.0 (0.98 to 4.0) | Not calculated |
| Oedema | 3 | 1312 | 3.9 | 1.2 | 3.0 (1.4 to 6.2) | 38 (23 to 110) |
| Group 2 | ||||||
| Erythema | 1 | 129 | 5.3 | 0 | Not calculated | Not calculated |
| Pain | 4 | 1005 | 9.9 | 3.8 | 2.4 (1.4 to 4.1) | 16 (11 to 31) |
| Papules | 3 | 735 | 3.4 | 2.4 | 1.6 (0.59 to 4.2) | Not calculated |
| Pruritus | 3 | 735 | 14 | 9.4 | 1.6 (0.98 to 2.5) | Not calculated |
| Oedema | 3 | 735 | 8.0 | 6.1 | 1.3 (0.75 to 2.4) | Not calculated |
| CI: confidence interval; NNH: number needed to treat for an additional harmful outcome; RR: risk ratio | ||||||
Patch tolerability
Use of local anaesthetic before application, local cooling, and availability of short‐acting opioids for pain relief in the first few days following treatment all help to increase tolerability of the treatment. Most of the studies assessed tolerability by the number of participants able to complete at least 90% of the intended application time, the degree of dermal irritation two hours after application (FDA 1999), and the numbers of participants using medication for treatment‐related discomfort on days zero to five ('Summary of results C' table). For analysis, we have combined studies in different neuropathic pain conditions and for all durations of application since there were no obvious differences or trends and the number of events was small for most outcomes (Analysis 1.9). Bischoff 2014 reported only that one participant experienced severe pain at the application site, which necessitated patch removal, and was withdrawn from the study. STEP 2014 reported the number of participants with dermal irritation scores of 4 or greater (scale 0 to 7) at 15 and 60 minutes after patch removal (0/186 with capsaicin and 2/183 with placebo at both time points).
We downgraded the quality of the evidence to moderate due to inconsistent reporting and assumptions made in pooling studies for analysis.
Summary of results C
| Outcome | Number | Per cent with outcome | RR (95% CI) | NNH (95% CI) | ||
|---|---|---|---|---|---|---|
| Studies | Participants | 8% Capsaicin | Control | |||
| < 90% application time | 6 | 2074 | 1.7 | 0.3 | 3.3 (1.2 to 9.2) | 77 (45 to 260) |
| DIS > 2 at 2 hours | 3 | 1065 | 11 | 0.7 | 12 (4.0 to 34) | 9.6 (7.7 to 13) |
| DIS > 0 at 2 hours | 2 | 606 | 40 | 18 | 2.3 (1.6 to 3.2) | 4.5 (3.3 to 6.7) |
| Pain medication 0 to 5 days | 7 | 2442 | 43 | 17 | 2.5 (2.2 to 2.9) | 3.8 (3.4 to 4.4) |
| CI: confidence interval; DIS: dermal irritation score; NNH: number needed to treat for an additional harmful outcome; RR: risk ratio | ||||||
The estimate for NNH for achieving less than 90% of the scheduled patch application time should be interpreted with caution since the numbers of participants with this outcome were very small (22/1300 (1.9%) with capsaicin and 2/774 (0.58%) with control).
Systemic adverse events
Systemic adverse events included diarrhoea, nausea, vomiting, fatigue, infections, musculoskeletal disorders, hypertension, dizziness, and headache. Individual events generally occurred in fewer than 5% of participants in each treatment arm, with no obvious differences between different doses and control arms (Appendix 6). Three studies specifically reported on cough, which occurred in 2% to 3% of participants treated with high‐concentration capsaicin and 0% to 4% of participants treated with control (Simpson 2008; Webster 2010a; Webster 2010b). No further analysis of systemic adverse events was carried out.
Serious adverse events
Serious adverse events were uncommon. Seven studies provided data for analysis (Backonja 2008; Bischoff 2014; Irving 2011; Simpson 2008; STEP 2014; Webster 2010a; Webster 2010b); 41/1175 (3.5%) of participants treated with high‐concentration capsaicin and 26/818 (3.2%) of participants treated with control experienced serious adverse events, giving an RR of 1.1 (95% CI 0.70 to 1.8) (Analysis 1.10; Figure 6). The NNH was not calculated. The remaining study reported that serious adverse events occurred with similar frequency in both treatment groups (6%), and judged none to be treatment‐related (Clifford 2012).

Forest plot of comparison: 1 High‐concentration (8%) capsaicin versus control (single dose), outcome: 1.10 Serious adverse events.
One event was judged possibly related to study medication. This participant experienced increased blood pressure on the day of treatment following treatment with high‐concentration capsaicin (Backonja 2008).
We downgraded the quality of the evidence to moderate due to few events.
There were six deaths, four following treatment with high‐concentration capsaicin (one each in Clifford 2012; Irving 2011; Simpson 2008; Webster 2010a), and two following low‐concentration capsaicin control (both in Simpson 2008). None were judged related to study medication.
We downgraded the quality of the evidence to very low due to the very small number of events (six in total).
Withdrawals
Adverse events
There were 12 withdrawals due to adverse events in 1507 participants (0.80%) treated with high‐concentration capsaicin and nine withdrawals in 980 participants (0.92%) treated with control, giving an RR of 0.80 (95% CI 0.36 to 1.8); the NNH was not calculated (Analysis 1.11).
We downgraded the quality of the evidence to moderate (few events, wide CIs).
Lack of efficacy
There were 20 withdrawals due to lack of efficacy in 1298 participants (1.5%) treated with high‐concentration capsaicin and 24 withdrawals in 775 participants (3.1%) treated with control, giving an RR of 0.58 (95% CI 0.32 to 1.04); the NNTp was 64 (95% CI 34 to 610) (Analysis 1.11).
Withdrawals for other reasons (such as lost to follow‐up) were generally below 10% and evenly distributed between treatment arms (Appendix 6). No further statistical analysis of withdrawals was carried out.
We downgraded the quality of the evidence to moderate (few events, wide CIs).
Discussion
Summary of main results
A single application of a high‐concentration (8%) capsaicin patch for 30 to 90 minutes provides significant pain relief for up to 12 weeks in some people with chronic pain arising from PHN or HIV‐neuropathy. The evidence we considered most reliable and trustworthy generated NNTs between about 6 and 9 measured at 8 or 12 weeks; for every seven to nine people treated, one will experience improvement in pain over 12 weeks who would not have done with control. These results for an outcome at a specific point in time were supported by positive benefits with a similar order of magnitude for outcomes that we considered less reliable, of people with average PIR of at least 50% or at least 30% measured over periods of time between 2 and 12 weeks.
There were insufficient data to draw any conclusions about treatment of PDN or persistent pain after inguinal herniorrhaphy. For PDN, there was a similar difference in response rate (about 10%) between capsaicin and placebo as was seen in studies in PHN and HIV‐neuropathy for the outcomes of much or very much improved and at least 30% PIR, but the difference was only 3% for at least 50% PIR. For persistent pain after inguinal herniorrhaphy, there was no difference between capsaicin and placebo for summed pain intensity difference from baseline at any time point.
These results might be compared with an NNT of 5.4 (95% CI 3.9 to 9.2) over 12 weeks for 600 mg pregabalin compared with placebo in 702 participants with PHN (Moore 2009). The NNT for much or very much improved in 1121 participants treated with gabapentin (any dose) compared with placebo yielded an NNT of 5.5 (95% CI 4.3 to 7.7) (Moore 2011a). No other drug therapies have comparable data sets for estimation of efficacy in PHN.
Painful HIV‐neuropathy is a condition in which there are no large comparable data sets, but where few therapies appear to demonstrate any benefit (Phillips 2010); topical high‐concentration capsaicin is therefore notable for providing some evidence of effective pain relief.
The annual incidence of PDN appears to be increasing, at least in the UK, and its incidence is similar to that of PHN; topical capsaicin is not a common initial treatment (Hall 2013). A number of oral therapies have NNTs of 5 or 6 for at least 50% PIR after 12 weeks (duloxetine 60 mg or 120 mg, gabapentin at doses of 1200 mg or above, and pregabalin 600 mg daily; Kalso 2013).
Treatments for chronic pain are characterised by the small proportion of people who obtain high degrees of treatment‐specific pain relief. However, the benefits of good pain relief go far beyond pain itself, with associated benefits in terms of improved sleep, reduced fatigue and depression, an overall improvement in quality of life, and even the ability to spend more time in employment or looking after the family (Azevedo 2016; Gülfe 2010; Hoffman 2010; Ikenberg 2012; Moore 2009; Straube 2011).
Use of capsaicin at the high concentration of 8% is associated with increased local skin reactions, primarily burning, stinging, and erythema, that affects many people, whether or not they obtain good pain relief, but these effects can be managed and resolve quickly after the single application.
Overall completeness and applicability of evidence
The earlier review identified studies in only two neuropathic pain conditions (PHN and HIV‐neuropathy), and, while this update found additional studies in PDN and postsurgical (inguinal herniorrhaphy) pain, there were too few data to draw any sensible conclusions in these conditions. This leaves a gap in our knowledge of the utility of high‐concentration capsaicin in a considerable proportion of people with localised neuropathic pain.
We found no double‐blind studies using an active comparator, so no direct comparisons with other treatments can be made.
The decision to exclude studies of less than 6 weeks' duration reduced the amount of evidence available to us; we excluded a single study with only 38 participants amounting to only about 3% of the total number of participants (Backonja 2010). We feel this was justified because benefits extending to only 4 weeks are unlikely to outweigh the considerable efforts associated with high‐concentration capsaicin use, at least at the moment.
The largest deficiencies resulted from inconsistent reporting, especially of efficacy outcomes. For example, for five of the six efficacy outcomes reported from the four PHN studies, complete data were available for analysis for only one outcome, and for the other five the amount available varied between 45% and 76% of the total participants. Importantly, both of the most reliable outcomes for benefit at 8 or 12 weeks after application were calculated using only 45% of participants. For HIV‐neuropathy, only two of the six outcomes were reported, and only one less‐reliable outcome reported in both studies. For PDN, all six efficacy outcomes were reported, but for inguinal herniorrhaphy pain none of our desired outcomes were reported.
This represents a considerable loss of evidence from otherwise high‐quality, well‐conducted, and mainly large studies. It is a deficiency that could affect the applicability of the results we have, and probably reflects the difficulties in reporting large, detailed, and complex clinical trials within the severe constraints of the allowable size of papers for publication. The deficiency should be rectified. Rectification would require no more studies, but rather better access to trial data, perhaps in the form of clinical trial reports, as has been done before (Moore 2005; Moore 2008b; Moore 2011b; Straube 2010).
Adverse event reporting was also limited by different ways of reporting data. This is a problem that has been commented upon previously in pain studies, and more generally (Edwards 1999; Loke 2001). The review did not specifically look for, or find, safety data relating to quantitative sensory testing or intra‐epidermal nerve fibre density.
The studies reviewed provided no information about long‐term efficacy and safety with repeated applications, but this has been investigated in a number of longer‐duration (52 weeks) studies, in a variety of neuropathic pain conditions (PACE 2014; Simpson 2014; STRIDE 2014). Generally, these studies have not demonstrated any additional safety issues with up to seven patch applications, and in some participants the therapeutic efficacy is maintained, or even improved, over time.
Quality of the evidence
The included studies were of generally high methodological quality, although with deficiencies in describing the process of randomisation and allocation concealment. Data handling of missing data did not adversely affect quality or involve any possible biases in studies that contributed efficacy data for meta‐analyses, but imputation methods were unclear in the two studies added to this update.
Because topical capsaicin is associated with erythema, burning, and local pain, a 'true' placebo was thought likely to lead to immediate unblinding. Six studies used 0.04% topical capsaicin as an 'active' placebo control, one that would mimic the local adverse effects of capsaicin without longer‐term pain relief. Responses to various outcomes with control were of the order of 25% of participants benefiting for the outcome of much or very much improved using PGIC in PHN, compared with 15% to 20% for the same outcome with placebo in trials of pregabalin (Moore 2009) and gabapentin (Moore 2011a). That might suggest that the low‐concentration control had some very small longer‐term benefit that would work to diminish the apparent efficacy of high‐concentration topical capsaicin, but this should not be considered more than speculation with the evidence available, especially relating to benefits from low‐concentration capsaicin creams (Derry 2012). Moreover, in the single study of PDN that used a 'true' placebo patch and reported PGIC, the placebo response (28%) was remarkably similar to the 'active' placebo response.
The use of stable concomitant medication throughout the studies may also have reduced their sensitivity to demonstrate an effect of high‐concentration capsaicin over placebo.
We downgraded the quality of the evidence for efficacy outcomes to moderate or very low because of the small number of studies and events, because use of LOCF imputation meant possible bias for some outcomes, imprecision, and susceptibility to publication bias. For harms, we downgraded the evidence to moderate because, despite adequate numbers of studies and participants, there were few events.
Potential biases in the review process
We used an extensive search strategy, and examined bibliographies, reference lists, clinical trial registries, and a pharmaceutical company database. High‐concentration capsaicin is a relatively recent therapy and it is unlikely that the search overlooked relevant high‐quality large studies. However, the relatively high NNTs for high‐concentration topical capsaicin combined with incomplete reporting of PGIC outcomes means that, for substantial benefit in PHN, null effect data from only about 250 participants would be needed to raise the NNT above 10 (Moore 2008a), at which point the efficacy of the therapy might be regarded as very limited. For moderate benefit, null effect data from only about 80 participants would be required.
Agreements and disagreements with other studies or reviews
Several reviews of high‐concentration capsaicin have been published since our earlier review. Burness 2016 and Űçeyler 2014 were narrative reviews, including different study types and all conditions; neither carried out any pooled analyses. Mou 2013 included the four‐week study that we excluded, but not the two new studies in this update; pooled analysis for percentage change and responder outcomes was performed, generating odds ratios and RRs, but not NNTs. An abstract of a network meta‐analysis in PDN showed no difference in efficacy between capsaicin 8% and pregabalin, gabapentin, and duloxetine, but reduced systemic adverse events; there were too few details to assess the methods used (van Nooten 2015). There was general agreement between these reviews that high‐concentration capsaicin can provide moderate or substantial benefit to a minority of people with localised, peripheral neuropathic pain, and that some people continue to derive benefit with repeated applications.
One open‐label study compared a single application of high‐concentration capsaicin with oral gabapentin titrated to a maximum of 600 mg daily, and did not demonstrate superiority of capsaicin over 8 weeks (ELEVATE 2014). Slightly more participants achieved 'optimal therapeutic effect' with capsaicin (147/282, 52%) than with pregabalin (124/277, 45%). 'Optimal therapeutic effect' was defined as at least 30% reduction in average pain for the past 24 hours from baseline to week eight, no discontinuation due to lack of efficacy or tolerability, no change in background chronic medication, and no moderate or severe adverse events during the stable treatment period). Withdrawals due to adverse events, lack of efficacy, and participant choice were all higher with pregabalin than with capsaicin.

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 High‐concentration (8%) capsaicin versus control (single dose), outcome: 1.1 Postherpetic neuralgia ‐ at least 50% pain intensity reduction over weeks 2 to 8.

Forest plot of comparison: 1 High‐concentration (8%) capsaicin versus control (single dose), outcome: 1.5 Postherpetic neuralgia ‐ Patient Global Impression of Change much or very much improved at 8 and 12 weeks.

Forest plot of comparison: 1 High‐concentration (8%) capsaicin versus control (single dose), outcome: 1.6 HIV‐neuropathy ‐ at least 30% pain intensity reduction over weeks 2 to 12.

Forest plot of comparison: 1 High‐concentration (8%) capsaicin versus control (single dose), outcome: 1.10 Serious adverse events.

Comparison 1: High‐concentration (8%) capsaicin versus control (single dose), Outcome 1: Postherpetic neuralgia (PHN) ‐ at least 50% pain intensity reduction over weeks 2 to 8

Comparison 1: High‐concentration (8%) capsaicin versus control (single dose), Outcome 2: PHN ‐ at least 50% pain intensity reduction over 2 to 12 weeks

Comparison 1: High‐concentration (8%) capsaicin versus control (single dose), Outcome 3: PHN ‐ at least 30% pain intensity reduction over weeks 2 to 8

Comparison 1: High‐concentration (8%) capsaicin versus control (single dose), Outcome 4: PHN ‐ at least 30% pain intensity reduction over weeks 2 to 12

Comparison 1: High‐concentration (8%) capsaicin versus control (single dose), Outcome 5: PHN ‐ Patient Global Impression of ChangePGIC much or very much improved at 8 and 12 weeks

Comparison 1: High‐concentration (8%) capsaicin versus control (single dose), Outcome 6: HIV‐neuropathy ‐ at least 30% pain intensity reduction over weeks 2 to 12

Comparison 1: High‐concentration (8%) capsaicin versus control (single dose), Outcome 7: Local skin reactions ‐ group 1

Comparison 1: High‐concentration (8%) capsaicin versus control (single dose), Outcome 8: Local skin reactions ‐ group 2

Comparison 1: High‐concentration (8%) capsaicin versus control (single dose), Outcome 9: Patch tolerability

Comparison 1: High‐concentration (8%) capsaicin versus control (single dose), Outcome 10: Serious adverse events

Comparison 1: High‐concentration (8%) capsaicin versus control (single dose), Outcome 11: Withdrawals
| High‐concentration (8%) capsaicin patch compared with control patch (0.4%) for postherpetic neuralgia | ||||||
| Patient or population: adults with postherpetic neuralgia Settings: community Intervention: high‐concentration (8%) capsaicin patch, single application Comparison: control patch (0.4% capsaicin), single application | ||||||
| Outcomes | Outcome with intervention | Outcome with comparator | RR, NNT, NNH, NNTp (95% CI) | Number of | Quality of the evidence | Comments |
|---|---|---|---|---|---|---|
| Substantial benefit | ||||||
| PGICvery much improved, week 8 and week 12 | No data | No data | ‐ | ‐ | Very low | No data |
| Moderate benefit | ||||||
| PGICmuch or very much improved, week 8 | 360 in 1000 | 250 in 1000 | RR 1.4 (1.1 to 1.8) NNT 8.8 (5.3 to 26) | 2 studies, 571 participants, 178 events | Moderate | Downgraded 1 level due to susceptibility to publication bias |
| PGIC much or very much improved, week 12 | 390 in 1000 | 250 in 1000 | RR 1.6 (1.2 to 2.0) NNT 7.0 (4.6 to 15) | 2 studies, 571 participants, 189 events | Moderate | Downgraded 1 level due to susceptibility to publication bias |
| Harm ‐ all conditions combined | ||||||
| Withdrawals due to lack of efficacy | 15 in 1000 | 31 in 1000 | RR 0.58 (0.32 to 1.04) NNTp 64 (34 to 610) | 6 studies, 2073 participants, 44 events | Moderate | Downgraded 1 level due to imprecision (few events, wide CI) |
| Withdrawals due to adverse events | 8.0 in 1000 | 9.2 in 1000 | RR 0.80 (0.36 to 1.8) NNTp not calculated | 8 studies, 2487 participants, 21 events | Moderate | Downgraded 1 level due to sparse data (few events) |
| Serious adverse events | 35 in 1000 | 32 in 1000 | RR 1.1 (0.70 to 1.8) NNH not calculated | 7 studies, 1993 participants, 67 events | Moderate | Downgraded 1 level due to sparse data (few events) |
| Death | 4 events | 2 events | Not calculated | 8 studies, 2487 participants | Very low | Downgraded 3 levels as only six events, so no better grading possibleNo death was judged related to study medication by study authors |
| CI: confidence interval; NNH: number needed to treat for one additional harmful outcome; NNT: number needed to treat for one additional beneficial outcome; NNTp: number needed to treat to prevent one withdrawal event; PGIC: Patient Global Impression of Change; RR: risk ratio. | ||||||
| Descriptors for levels of evidence (EPOC 2015): † Substantially different: a large enough difference that it might affect a decision. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Postherpetic neuralgia (PHN) ‐ at least 50% pain intensity reduction over weeks 2 to 8 Show forest plot | 3 | 870 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.12, 1.86] |
| 1.1.1 Using 30‐minute application | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.95 [0.73, 11.88] |
| 1.1.2 Using 60‐minute application | 3 | 674 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.03, 1.75] |
| 1.1.3 Using 90‐minute application | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.64, 6.33] |
| 1.2 PHN ‐ at least 50% pain intensity reduction over 2 to 12 weeks Show forest plot | 2 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.00, 1.71] |
| 1.3 PHN ‐ at least 30% pain intensity reduction over weeks 2 to 8 Show forest plot | 4 | 1268 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.13, 1.52] |
| 1.3.1 Using 30‐minute application | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.67, 2.69] |
| 1.3.2 Using 60‐minute application | 4 | 1072 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.12, 1.52] |
| 1.3.3 Using 90‐minute application | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.74, 2.95] |
| 1.4 PHN ‐ at least 30% pain intensity reduction over weeks 2 to 12 Show forest plot | 3 | 973 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.07, 1.45] |
| 1.5 PHN ‐ Patient Global Impression of ChangePGIC much or very much improved at 8 and 12 weeks Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.5.1 At 8 weeks | 2 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.10, 1.84] |
| 1.5.2 At 12 weeks | 2 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.20, 1.99] |
| 1.6 HIV‐neuropathy ‐ at least 30% pain intensity reduction over weeks 2 to 12 Show forest plot | 2 | 801 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.09, 1.68] |
| 1.6.1 Using 30‐minute application | 2 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.14, 2.46] |
| 1.6.2 Using 60‐minute application | 2 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.84, 1.44] |
| 1.6.3 Using 90‐minute application | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [0.83, 4.53] |
| 1.7 Local skin reactions ‐ group 1 Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.7.1 Erythema | 4 | 1355 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.32, 1.54] |
| 1.7.2 Pain | 4 | 1355 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.26 [1.98, 2.59] |
| 1.7.3 Papules | 3 | 1312 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.58 [1.87, 6.85] |
| 1.7.4 Pruritus | 3 | 1312 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.98, 4.03] |
| 1.7.5 Oedema | 3 | 1312 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.98 [1.44, 6.18] |
| 1.8 Local skin reactions ‐ group 2 Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.8.1 Erythema | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.31 [0.35, 114.82] |
| 1.8.2 Pain | 4 | 1105 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.39 [1.41, 4.05] |
| 1.8.3 Papules | 3 | 735 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.59, 4.24] |
| 1.8.4 Pruritus | 3 | 735 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.98, 2.50] |
| 1.8.5 Oedema | 3 | 735 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.75, 2.39] |
| 1.9 Patch tolerability Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.9.1 < 90% of application time | 6 | 2074 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.27 [1.17, 9.15] |
| 1.9.2 Dermal irritation score > 2 at 2 hours | 3 | 1065 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.80 [4.04, 34.48] |
| 1.9.3 Dermal irritation score > 0 at 2 hours | 2 | 606 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.28 [1.60, 3.26] |
| 1.9.4 Pain medication 0 to 5 days | 7 | 2442 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.52 [2.18, 2.92] |
| 1.10 Serious adverse events Show forest plot | 7 | 1993 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.70, 1.86] |
| 1.11 Withdrawals Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.11.1 Adverse events | 8 | 2487 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.36, 1.78] |
| 1.11.2 Lack of efficacy | 6 | 2073 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.32, 1.02] |

