Contenido relacionado
Revisiones y protocolos relacionados
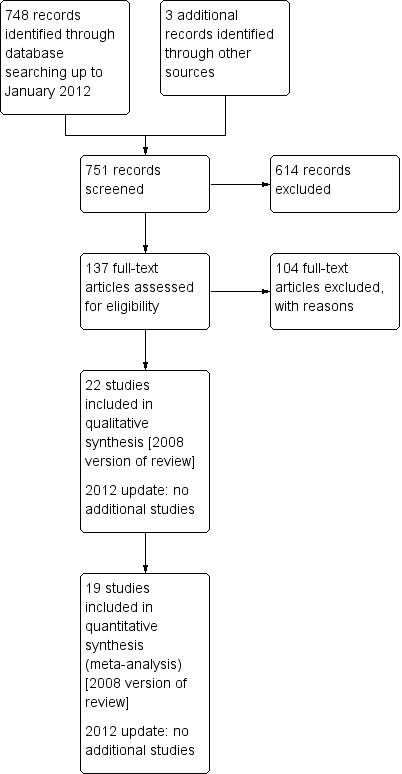
Christopher J Cates, Toby J Lasserson | 14 marzo 2012
Christopher J Cates, Matthew J Cates | 16 julio 2008
Christopher J Cates, L. Susan Wieland, Marta Oleszczuk, Kayleigh M Kew | 6 febrero 2014
E. Haydn Walters, Peter G Gibson, Toby J Lasserson, Julia AE Walters | 24 enero 2007
E. Haydn Walters, Julia AE Walters, Peter G Gibson | 22 julio 2002
Sadia Janjua, Stefanie Schmidt, Montse Ferrer, Christopher J Cates | 25 septiembre 2019
Emma J Welsh, Christopher J Cates | 8 septiembre 2010
Sarah Appleton, Terry Jones, Phillippa Poole, Toby J Lasserson, Robert Adams, Brian Smith, Julia Muhammed | 19 julio 2006
Orlagh O'Shea, Elizabeth Stovold, Christopher J Cates | 14 abril 2021
Agonistas beta2 de acción prolongada para la enfermedad pulmonar obstructiva crónica poco reversible
Sarah Appleton, Phillippa Poole, Brian J Smith, Antony Veale, Toby J Lasserson, Matthew Ming Ki Chan, Christopher J Cates | 19 julio 2006
Podcast relacionado
Respuestas clínicas Cochrane
Adarsh Gupta | 13 marzo 2017