Entrenamiento asistido por aparatos electromecánicos para caminar después de un accidente cerebrovascular
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT | |
| Participants | Country: Switzerland | |
| Interventions | 2 arms:
| |
| Outcomes | Outcomes were recorded at baseline and after 3 weeks and 6 months | |
| Notes | Unpublished data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Methods | RCT Method of randomisation: opaque, closed envelopes | |
| Participants | Country: Korea Inclusion criteria: > 6 months after stroke, ability to walk over 10 meters, gait speed > 0.4 m/s, MMSE ≥ 24 Exclusion criteria: uncontrolled health condition, comorbidity or disability other than stroke precluding gait training | |
| Interventions | 2 arms:
| |
| Outcomes | Outcomes were recorded at baseline and after 2 weeks. Outcome measures: gait speed, cadence, step length, double support period (GAITRite system), balance, level of balance confidence (ABC scale) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was performed by selection of an opaque, closed envelope in which the group assignment was written, which was given to the physical therapist. |
| Allocation concealment (selection bias) | Low risk | By sealed envelopes |
| Blinding of outcome assessment (detection bias) | Low risk | Described as blinded by an assessor not participating in study |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts |
| Methods | Randomised cross‐over trial | |
| Participants | Country: Denmark | |
| Interventions | 2 arms:
| |
| Outcomes | Outcomes were recorded at baseline, after 3 and 6 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Shuffling envelopes |
| Allocation concealment (selection bias) | Low risk | Using sealed, shuffled envelopes |
| Blinding of outcome assessment (detection bias) | High risk | No blinding was done. |
| Incomplete outcome data (attrition bias) | Low risk | ITT |
| Methods | RCT Method of randomisation: random number generator | |
| Participants | Country: USA Inclusion criteria: > 12 months after stroke, medically stable, initial gait speed between 0.4 and 0.8 m/s, > 17 MMSE, sit unsupported for 30 s, walk ≥ 10 m with maximum 1 person, follow a 3‐step command | |
| Interventions | 2 arms:
| |
| Outcomes | Outcomes were recorded at baseline and after visit 10 and 18, 3‐month follow‐up | |
| Notes | NCT01994395 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomisation described as "random number generator". |
| Allocation concealment (selection bias) | High risk | Allocation concealment not described. |
| Blinding of outcome assessment (detection bias) | Low risk | Evaluated by a research physical therapist, who was blinded to the participant’s training group |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts |
| Methods | RCT | |
| Participants | Country: Republic of Korea | |
| Interventions | 2 arms:
| |
| Outcomes | Outcomes were recorded at baseline and after training:
| |
| Notes | This study describes the same study protocol and participants as described in the study Kim 2008, but provides further explanation of participant characteristics; the ID Chang 2012 therefore replaces the formerly review used ID Kim 2008. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation is unclear. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is unclear. |
| Blinding of outcome assessment (detection bias) | High risk | No |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Methods | RCT, cross‐over ITT: not stated | |
| Participants | Country: Korea Inclusion criteria: onset period of > 6 months, FAC < 2, independent ambulation before stroke, ability to understand and execute RAGT, no orthopaedic or neurosurgical problems in the lower extremities Exclusion criteria: weight > 120 kg; femoral length < 35 cm or > 47 cm; history of low‐extremity fracture after stroke, instability or subluxation of the hip joint, or pressure ulcers on the hips or lower extremities; any underlying disease preventing execution of RAGT | |
| Interventions | 2 arms:
| |
| Outcomes | Outcomes were recorded at baseline and after 4 and 8 weeks:
| |
| Notes | Higher dose of intervention in experimental group compared to control group; group differences at baseline (modified forward reach) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described. |
| Allocation concealment (selection bias) | High risk | Allocation not described. |
| Blinding of outcome assessment (detection bias) | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Methods | RCT ITT: yes | |
| Participants | Country: Singapore Inclusion criteria: unilateral haemorrhagic/ischaemic stroke, age between 18 and 80 years, independent ambulation pre‐stroke Exclusion criteria: > 8 weeks poststroke, FAC ≥ 4, cardiovascular instability, MMSE < 16, communication deficits, lower limb joint contractures | |
| Interventions | 2 arms:
| |
| Outcomes | Outcomes were recorded at baseline and 4, 8, 12, 24, and 48 weeks after baseline Outcome measures: walking ability (FAC), Barthel Index, gait velocity (10‐metre walk test), gait endurance (6‐minute walk test), health status (Stroke Impact Scale) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors state: "Randomization was performed using a computer‐generated sequence of random numbers." |
| Allocation concealment (selection bias) | Low risk | Authors state: "An independent department generated the random group allocation sequence and transferred the sequence to a series of serially numbered opaque envelopes, which were not opened and revealed until after acceptance into the study and the baseline tests, therefore ensuring allocation concealment." |
| Blinding of outcome assessment (detection bias) | Low risk | Authors state: "The data assessors were blinded to group allocation, but it was not possible to blind participants or the health care professionals providing interventions." |
| Incomplete outcome data (attrition bias) | Low risk | All data for all participants provided and analysed. Authors state: "An intention‐to‐treat approach was used. Data from subjects were analysed according to the group to which they were randomised, regardless of whether they completed the intervention. Participants failing to complete either intervention were asked to return for follow‐up." |
| Methods | RCT | |
| Participants | Country: Portugal | |
| Interventions | 2 arms:
| |
| Outcomes | Outcomes were recorded at baseline and after 4 weeks and 3 months:
After study end and at follow‐up, participants rated satisfaction with and efficiency of treatment in a self questionnaire (Likert scale). | |
| Notes | Published and unpublished data provided by the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of outcome assessment (detection bias) | High risk | Not done |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Methods | RCT | |
| Participants | Country: USA | |
| Interventions | 2 arms:
| |
| Outcomes | Outcomes were recorded at baseline and after 24 sessions:
| |
| Notes | This trial is described as ongoing; the results of the first 20 participants were reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Stated as concealed, but method not described. |
| Blinding of outcome assessment (detection bias) | Low risk | Yes |
| Incomplete outcome data (attrition bias) | Low risk | ITT stated. |
| Methods | RCT ITT: no | |
| Participants | Country: USA Inclusion criteria: first stroke; residual lower extremity hemiparesis involving the ankle (1/5 to 4/5 MRC); capable of generating at least trace muscle activation in PF‐DF; adequate language and neurocognitive function; sit in the chair for 30 to 60 minutes per session of ankle training Exclusion criteria: total plegia (0/5) at paretic ankle; fixed or painful contractures; dementia; orthopaedic, arthritic, or inflammatory condition limiting ankle movement; deep venous thrombosis or pulmonary thromboembolism; vision impairment; severe receptive or global aphasia | |
| Interventions | 2 arms:
| |
| Outcomes | Outcomes were recorded at baseline and at discharge. Outcome measures: walking ability (Functional Independence Measure walking), balance (Berg Balance Scale), walking velocity, active range of motion, muscle strength, spatiotemporal gait parameters (step time, step length, step symmetry), motor control variables (angular velocity, co‐ordination) | |
| Notes | Unclear amount of therapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not clearly described; authors only state "blocked randomisation" |
| Allocation concealment (selection bias) | High risk | Not described |
| Blinding of outcome assessment (detection bias) | High risk | Authors state "not blinded". |
| Incomplete outcome data (attrition bias) | High risk | 5 participants were excluded from analysis after randomisation. |
| Methods | RCT | |
| Participants | Country: Italy | |
| Interventions | 3 arms:
All participants received 10 x 50‐minute treatment sessions, 5 days a week, for 2 consecutive weeks | |
| Outcomes | Outcomes were recorded at baseline and after 2 weeks:
| |
| Notes | We combined the results of both robotic‐assisted groups (arms 1 and 2) into a single group, which we compared with the results of the control group (arm 3). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Software‐generated list |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of outcome assessment (detection bias) | High risk | Not done |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Methods | RCT ITT: no | |
| Participants | Country: Republic of Korea 60 participants (30 in treatment group, 30 in control group) Inclusion criteria: clinical diagnosis of stroke < 3 months after stroke onset, first‐ever stroke, dependent ambulation with severe gait impairment (FAC < 2), and sufficient cognition to understand procedures and provide informed consent Exclusion criteria: contraindications for RAGT therapy; cerebellar or brainstem lesions that could affect autonomic or balance function; musculoskeletal disease involving the lower limbs, such as severe painful arthritis, osteoporosis, amputation, or joint contracture; and other concurrent neurological diseases (e.g. Parkinson's disease, multiple sclerosis, etc.) | |
| Interventions | 2 arms:
| |
| Outcomes | Outcomes were recorded at baseline and after the 4‐week intervention; all outcome parameters were measured within 3 days before and after 20 sessions of training:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Blinding of outcome assessment (detection bias) | Low risk | A physiatrist (rehabilitation doctor) who remained blinded to participant group and treatment throughout the entire study analysed outcome measures. |
| Incomplete outcome data (attrition bias) | High risk | No ITT |
| Methods | RCT | |
| Participants | Country: USA | |
| Interventions | 2 arms:
| |
| Outcomes | Outcomes were recorded at baseline and after 12 and 24 sessions, and at 3‐month follow‐up:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation table |
| Allocation concealment (selection bias) | Unclear risk | Not described in sufficient detail |
| Blinding of outcome assessment (detection bias) | High risk | Not done |
| Incomplete outcome data (attrition bias) | High risk | No ITT, analysis per protocol |
| Methods | RCT | |
| Participants | Country: USA | |
| Interventions | 2 arms:
| |
| Outcomes | Outcomes were recorded at baseline and after 12 sessions and at 6‐month follow‐up:
| |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | (Probably) shuffling envelopes |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed envelopes |
| Blinding of outcome assessment (detection bias) | High risk | No blinding |
| Incomplete outcome data (attrition bias) | High risk | 'As‐treated' analysis done |
| Methods | RCT | |
| Participants | Country: Germany | |
| Interventions | 2 arms:
Both groups received additional 30 minutes of physiotherapy daily. | |
| Outcomes | Outcomes were recorded at baseline and after 4 weeks:
| |
| Notes | Published and unpublished data provided by the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers generated by a computer program, block randomisation. |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding of outcome assessment (detection bias) | Low risk | Evaluating therapist blinded for group allocation. |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement of ‘low risk’ or ‘high risk’ |
| Methods | RCT ITT: no | |
| Participants | Country: Korea Inclusion criteria: first stroke < 1 year, plateau in recovery of the locomotor functions after a 30‐day conventional neurorehabilitation Exclusion criteria: severe spasticity (Modified Ashworth Scale 2), tremor, severe visual and cognitive impairments, musculoskeletal diseases, cardiopulmonary diseases, body weight of 135 kg; height of 150 cm | |
| Interventions | 2 arms:
| |
| Outcomes | Outcomes were recorded at baseline and after 4 and 8 weeks. Outcome measures: walking ability (FAC), balance (Berg Balance Scale), modified Barthel Index, spasticity (Modified Ashworth Scale), quality of life (EuroQol‐5 dimension) | |
| Notes | NCT02053233 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described. |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) | High risk | Not described |
| Incomplete outcome data (attrition bias) | High risk | No ITT |
| Methods | RCT | |
| Participants | Country: Republic of Korea | |
| Interventions | 2 arms:
| |
| Outcomes | Outcomes were recorded at baseline and after training
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Method neither described nor stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Methods | RCT | |
| Participants | Country: Austria | |
| Interventions | 2 arms:
| |
| Outcomes | Outcomes were recorded at baseline and after training phase:
| |
| Notes | Published as conference abstract and unpublished data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Software‐generated list |
| Allocation concealment (selection bias) | Low risk | Described concealed allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Described as blinded evaluator |
| Incomplete outcome data (attrition bias) | High risk | No ITT |
| Methods | RCT | |
| Participants | Country: Italy Exclusion criteria: presence of subarachnoid haemorrhages, sequelae of prior cerebrovascular accidents or other chronic disabling pathologies, orthopaedic injuries that could impair locomotion, spasticity that limited lower extremity range of motion to less than 80%, sacral skin lesions, MMSE score < 24, and hemispatial neglect, as evaluated by a neuropsychologist | |
| Interventions | 2 arms (including strata for motor function):
The standard physiotherapy, shared by both groups, was focused on facilitation of movement on the paretic side and upper limb exercises, as well as improving balance, standing, sitting, and transferring. | |
| Outcomes | Outcomes were recorded by a physician who was blinded to the treatment at baseline, after 4 weeks of the intervention, and at hospital discharge:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated electronically by www.random.org |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Yes |
| Incomplete outcome data (attrition bias) | Low risk | ITT done; missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. |
| Methods | RCT | |
| Participants | Country: USA | |
| Interventions | 2 arms:
| |
| Outcomes | Outcomes were recorded at baseline and at postintervention, 3 months' postintervention
| |
| Notes | NCT00975156; same study as Kelley 2013 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) | Low risk | Authors state: "Blinded assessors tested the participants at baseline, post‐intervention, and 3‐month follow‐up." |
| Incomplete outcome data (attrition bias) | Unclear risk | 1 participant in the control group was not analysed. |
| Methods | RCT Method of randomisation: random number table Blinding of outcome assessors: stated as 'yes' | |
| Participants | Country: Japan Inclusion criteria: first‐ever stroke < 5 weeks; age between 40 and 85 years; lower extremities Brunnstrom's recovery stage ≤ grade III; FAC ≤ 2; independence in walking before stroke Exclusion criteria: height between 145 and 180 cm; body weight over 100 kg; limitation in range of motion in the lower extremity; severe cardiovascular, respiratory, renal, or musculoskeletal disease; difficulty in communicating | |
| Interventions | 2 arms:
| |
| Outcomes | Outcomes were recorded at baseline and after 4 weeks. Outcome measures: Fugl‐Meyer Assessment lower extremity, muscle torque, walking ability (FAC), 10‐metre walk test, Functional Independence Measure | |
| Notes | Clinical group differences in FAC at baseline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment described. |
| Blinding of outcome assessment (detection bias) | Low risk | Described as blinded assessors |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts, no participants excluded from analysis. |
| Methods | RCT | |
| Participants | Country: Finland | |
| Interventions | 3 arms:
All participants practised gait for 15 sessions over 3 weeks (each session lasting 20 minutes) and received an additional 55 minutes daily physiotherapy. | |
| Outcomes | Outcomes were recorded at baseline and after 2 and 3 weeks and 6 months:
| |
| Notes | Published and unpublished data provided by the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An investigator not involved in the study randomly assigned participants to groups using concealed envelopes. |
| Allocation concealment (selection bias) | Low risk | Concealed envelopes |
| Blinding of outcome assessment (detection bias) | High risk | Not done |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear if reasons for missing outcome data are unlikely to be related to true outcome |
| Methods | RCT | |
| Participants | Country: Finland | |
| Interventions | Between June 2003 and December 2004, random allocation to 2 arms took place (2 walking exercise groups)
All participants received 55 minutes daily gait‐oriented physiotherapy and additional gait training for 15 sessions over 3 weeks (each session lasting maximum of 20 minutes of walking). Between January 2005 and February 2007, random allocation to 3 arms took place (3 walking exercise groups)
All participants received 55 minutes daily gait‐oriented physiotherapy and additional gait training for 15 sessions over 3 weeks (each session lasting maximum of 20 minutes of walking). However, CT‐Group received 1 or 2 physiotherapy sessions daily but not at the same intensity as in the other groups. | |
| Outcomes | Outcomes were recorded at baseline and after 3 weeks and 6 months:
| |
| Notes | Because we were interested in the effects of automated electromechanical‐ and robotic‐assisted gait‐training devices for improving walking after stroke, we combined the results of the CT‐Group and the WALK‐Group as one group, which we compared with the results from the GT‐Group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An investigator not involved in the study randomly assigned participants to groups using concealed envelopes. |
| Allocation concealment (selection bias) | Low risk | Allocation was performed by an independent person not otherwise involved with the participants. |
| Blinding of outcome assessment (detection bias) | High risk | No |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear whether reasons for missing outcome data are unlikely to be related to true outcome |
| Methods | RCT | |
| Participants | Country: Italy | |
| Interventions | 2 arms:
| |
| Outcomes | Outcomes were recorded at baseline, 1 month Primary outcome:
Secondary outcomes:
| |
| Notes | 1 group received additional gait training (performance bias). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Software‐generated random order |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded evaluator |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts |
| Methods | RCT | |
| Participants | Country: Germany | |
| Interventions | 2 arms:
| |
| Outcomes | Outcomes were recorded at baseline and after 4 weeks and 6 months. Primary outcomes:
Participants who were ambulatory (FAC 4 or 5) or reaching a Barthel Index > 75 were defined as responders to therapy. Secondary outcomes:
| |
| Notes | Published and unpublished data provided by the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Lots indicating A or B were prepared in sealed envelopes; a person not involved in the study allocated participants to groups using the concealed envelopes. |
| Allocation concealment (selection bias) | Low risk | Concealed envelopes |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded primary outcomes (a person not involved in the study rated videotapes of participants) |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis done; missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. |
| Methods | Cross‐over RCT | |
| Participants | Country: Austria | |
| Interventions | 2 arms (A: Lokomat, B: physiotherapy):
| |
| Outcomes | Outcomes were recorded at baseline and after 3 weeks (they were additionally recorded after 6 and 9 weeks, but only outcomes of the first phase were included in this review). | |
| Notes | Unpublished data provided by the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By random numbers |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Methods | RCT | |
| Participants | Country: Israel | |
| Interventions | 2 arms:
| |
| Outcomes | Outcomes were recorded at baseline and after 3, 6, and 9 weeks:
| |
| Notes | Published and unpublished data provided by the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block sampling |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) | High risk | Not done |
| Incomplete outcome data (attrition bias) | High risk | For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk was enough to induce clinically relevant bias into intervention effect estimate. |
| Methods | RCT ITT: no | |
| Participants | Country: USA Inclusion criteria: single stroke, significant leg weakness and gait alterations < 6 months before study entry, independent in household ambulation Exclusion criteria: ongoing physical therapy for the leg and/or gait and mobility, botulinum toxin injections < 3 months before study entry, no further planned injections, other neurologic disorders, excessive spasticity of lower limb (Ashworth Scale > 3), uncontrolled hypertension, unstable coronary artery disease, contractures of lower limb, impaired cognition (MMSE score < 24) | |
| Interventions | 2 arms:
| |
| Outcomes | Outcomes were recorded at baseline, after 6, 10, and 19 weeks Outcome measures: Timed Up and Go Test, 10‐metre walk test, 6‐minute walk test, Five‐Times‐Sit‐to‐Stand Test, Berg Balance Scale, California Functional Evaluation, Emory Functional Ambulation Profile | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear, not described |
| Blinding of outcome assessment (detection bias) | Low risk | Performed by 1 physical therapist blinded to group assignment |
| Incomplete outcome data (attrition bias) | High risk | No ITT |
| Methods | Cross‐over RCT | |
| Participants | Country: Japan | |
| Interventions | 2 arms (only the first 12 weeks before cross‐over are described here; A: no training, B: gait training with Gait Master 4, 1 session: 20 minutes):
| |
| Outcomes | Outcomes were recorded weekly over a 24‐week period:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) | High risk | No |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Methods | RCT | |
| Participants | Country: Hong Kong, China | |
| Interventions | 3 arms:
The study consisted of 1 training session per weekday for 4 weeks. | |
| Outcomes |
| |
| Notes | Published and unpublished data provided by the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Concealed |
| Blinding of outcome assessment (detection bias) | Low risk | Yes for primary outcome |
| Incomplete outcome data (attrition bias) | Unclear risk | ITT unclear |
| Methods | RCT Blinding of outcome assessors: yes | |
| Participants | Country: Turkey Inclusion criteria: adult male (> 18 years), ability to ambulate 10 metres without personal assistance, and not receiving any other physical therapy Exclusion criteria: body weight more than 300 pounds (135 kg), FAC score < 3 and not able to walk consistently or independently within the community, cognitive deficits, cardiac disease, spasticity of the lower limbs preventing robotic walking, traumatic stroke, intracranial space occupying lesion‐induced strokes, and seizures | |
| Interventions | 2 arms:
| |
| Outcomes | Outcomes were assessed at baseline and after 2 and 8 weeks:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number list |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) | Low risk | Stated as blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Methods | RCT | |
| Participants | Country: Hong Kong, China Mean age: 53 years | |
| Interventions | 2 arms
| |
| Outcomes | Outcomes were assessed at baseline and after 8 weeks:
| |
| Notes | Published and unpublished data provided by the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Low risk | A person not involved in the study was asked to draw 1 of the opaque envelopes inside which group assignment was established each time a new participant entered the study. |
| Blinding of outcome assessment (detection bias) | High risk | No |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Methods | RCT | |
| Participants | Country: USA Mean age: 51 years | |
| Interventions | 2 arms
| |
| Outcomes | Outcomes were assessed at baseline and after 6 and 12 weeks :
| |
| Notes | Published and unpublished data provided by the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described. |
| Allocation concealment (selection bias) | High risk | Not described |
| Blinding of outcome assessment (detection bias) | High risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | No ITT |
| Methods | RCT ITT: no | |
| Participants | Country: Japan Inclusion criteria: stroke < 6 months Exclusion criteria: not ambulating prior to stroke, FAC 4 or 5, severe cardiac disease, NYHA III or IV, severe disturbance of consciousness (Japan Coma Scale II or III), size limitations for the robotic orthosis, skin disease, pacemaker | |
| Interventions | 2 arms:
| |
| Outcomes | Outcomes were recorded at baseline, after 12 training sessions Primary outcome:
Secondary outcomes:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Unclear risk | Odd‐numbered participants underwent gait training using the Hybrid Assistive Limb, and even‐numbered participants underwent conventional gait training (maybe even high risk of bias). |
| Blinding of outcome assessment (detection bias) | High risk | Not done |
| Incomplete outcome data (attrition bias) | High risk | No ITT |
| Methods | Cross‐over RCT | |
| Participants | Country: Germany | |
| Interventions | 2 arms:
Treated as inpatients for five 15‐ to 20‐minute sessions per week for 2 weeks | |
| Outcomes | Outcomes were recorded at baseline and after 2 weeks (additionally after 4 and 6 weeks, but only the first phase was included in this review):
| |
| Notes | We used the first treatment phase only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By envelopes |
| Allocation concealment (selection bias) | Low risk | Concealed envelopes that were drawn by an independent person |
| Blinding of outcome assessment (detection bias) | Low risk | Yes |
| Incomplete outcome data (attrition bias) | Low risk | No missing data |
| Methods | RCT | |
| Participants | Country: USA | |
| Interventions | 2 arms:
| |
| Outcomes | Outcomes were recorded at baseline and after 4 weeks
| |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Low risk | Randomisation list was overseen by 1 of the investigators who had no contact with participants until group assignment was revealed. |
| Blinding of outcome assessment (detection bias) | High risk | No |
| Incomplete outcome data (attrition bias) | Low risk | No missing data |
ABC: Activities‐specific Balance Confidence
ADL: activities of daily living
CT: computed tomography
FAC: Functional Ambulation Category
ITT: intention‐to‐treat
MMSE: Mini–Mental State Examination
MRC: Medical Research Council
MRI: magnetic resonance imaging
NIHSS: National Institutes of Health Stroke Scale
NYHA: New York Heart Association
PF‐DF: plantar flexion and dorsiflexion
RAGT: robot‐assisted gait training
RCT: randomised controlled trial
SF‐36: 36‐Item Short Form Health Survey
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Intervention: both groups received robot‐assisted gait training (the experimental group also received functional electrical stimulation on the ankle dorsiflexor of the affected side). | |
| Uses a sliding rehabilitation machine, not robotic training as experimental condition | |
| Did not investigate electromechanical‐ and robotic‐assisted gait‐training devices as stated in the protocol of this review: bicycle training versus treadmill walking versus variable surface training were investigated | |
| Investigates brain stimulation, and both groups participated in identical locomotor training with a robotic gait orthosis | |
| Did not meet inclusion criteria of this review: not an RCT | |
| Compared different robotic applications | |
| Did not investigate electromechanical‐ and robotic‐assisted gait‐training devices as stated in the protocol of this review: no electromechanical‐assisted devices were compared | |
| Did not meet inclusion criteria of this review: not an RCT | |
| Did not meet inclusion criteria of this review: not an RCT | |
| Investigated upper limbs | |
| Did not meet inclusion criteria of this review: experimental and control groups received a kind of assisted stepping therapy in a seated position. This study investigated the effects of virtual reality as an adjunct to stepping training. After discussion, we reached consensus to exclude this study from our review. | |
| Investigated the i‐Walker, a rollator vehicle | |
| Compared different robotic approaches | |
| Did not meet inclusion criteria of this review: the experimental group received a kind of assisted stepping therapy in a seated position. This study investigated the effect of the NuStep apparatus. After discussion, we reached consensus to exclude this study from our review. | |
| Uses a treadmill training approach as experimental condition (Gait Trainer 2 analysis system, Biodex Medical Systems, Inc., Shirley, NY, USA) | |
| According to the information on ClinicalTrials.gov (NCT00125619), this is a 1‐arm, non‐randomised trial. | |
| Investigated upper limbs | |
| Both groups received the same robotic treatment. | |
| Did not meet inclusion criteria of this review: the study describes preliminary findings of an initial sample of 9 participants; the experimental group received treadmill training or gait training | |
| Did not meet inclusion criteria of this review: the experimental group received a specialised locomotor training including early intensive physiotherapy with tilt table, limb load monitor, resistance exercises, and treadmills to promote functional recovery. After discussion, we reached consensus to exclude this study. | |
| Did not meet inclusion criteria of this review: the experimental group received specialised locomotor training including early intensive physiotherapy with tilt table, limb load monitor, resistance exercises and treadmills to promote functional recovery. After discussion, we reached consensus to exclude this study. | |
| Not an RCT | |
| Not an RCT, control groups were age and sex matched | |
| Compared 2 treadmill exercise options | |
| Compared different modes (resistance versus assistance training) of the same robotic device |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Probably an RCT |
| Participants | Unclear |
| Interventions | Unclear |
| Outcomes | Unclear |
| Notes | — |
| Methods | Probably an RCT |
| Participants | People after stroke, number unclear |
| Interventions | 2 arms:
|
| Outcomes | Unclear |
| Notes | This study was presented at the 5th World Congress of Physical Medicine and Rehabilitation. |
| Methods | Probably an RCT |
| Participants | Unclear |
| Interventions | Unclear |
| Outcomes | Unclear |
| Notes | — |
| Methods | Probably an RCT |
| Participants | 34 non‐ambulatory stroke survivors |
| Interventions | 2 arms:
|
| Outcomes | Unclear |
| Notes | This study was presented at the 5th World Congress of Physical Medicine and Rehabilitation. |
| Methods | Probably an RCT |
| Participants | Unclear |
| Interventions | Unclear |
| Outcomes | Unclear |
| Notes | Study was found in the Proceedings of the 1st International Congress of International Society of Physical and Rehabilitation Medicine (ISPRM), 2001 July 7‐13. |
| Methods | Probably an RCT |
| Participants | Unclear |
| Interventions | Unclear |
| Outcomes | Unclear |
| Notes | — |
| Methods | Probably an RCT |
| Participants | Unclear |
| Interventions | Unclear |
| Outcomes | Unclear |
| Notes | — |
| Methods | Probably an RCT |
| Participants | Unclear |
| Interventions | Unclear |
| Outcomes | Unclear |
| Notes | — |
| Methods | Probably a randomised cross‐over trial |
| Participants | 23 participants with stroke (stroke onset < 6 months) Group 1 (N = 13; mean: 60.9 ± 9.6 years) Group 2 (N = 10; mean: 61.1 ± 14.6 years) |
| Interventions | Gait training with the Stride Management Assist device (Honda R&D Co., Ltd. Japan) (20 minutes/time, 5 times per week for 4 weeks) Conventional rehabilitation (40 minutes/time) 4 weeks |
| Outcomes |
|
| Notes | Published as abstract |
| Methods | Probably an RCT |
| Participants | Unclear |
| Interventions | Unclear |
| Outcomes | Unclear |
| Notes | No longer at ClinicalTrials.gov |
| Methods | Probably an RCT |
| Participants | Unclear |
| Interventions | Unclear |
| Outcomes | Unclear |
| Notes | ‐ |
| Methods | Probably an RCT |
| Participants | Unclear |
| Interventions | N = 10 in gait training with active‐assistive gait device group N = 10 in control group |
| Outcomes |
|
| Notes | Conference abstract |
| Methods | Probably an RCT |
| Participants | Unclear |
| Interventions | Unclear |
| Outcomes | Unclear |
| Notes | ‐ |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Use of a powered robotic exoskeleton to promote walking recovery after stroke: study protocol for a randomised controlled trial |
| Methods | Single‐blind randomised controlled trial to evaluate the efficacy of a powered mobile exoskeleton (Ekso) on improving walking ability in people early after stroke |
| Participants | 50 individuals admitted for stroke rehabilitation in Canada, within 4 weeks' poststroke and needing second person assist to walk, will be randomly assigned to either a usual care group or exoskeleton group for 5 days/week for 4 weeks. |
| Interventions | 2 arms:
|
| Outcomes | Outcomes will be measured at baseline, 4 weeks later at discharge, and at 6 months after program ends. Primary outcome:
Secondary outcomes:
|
| Starting date | Unknown |
| Contact information | Louie DR1,2 1University of British Columbia, Vancouver, Canada 2Rehabilitation Research Program, Vancouver Coastal Health Research Institute, Vancouver, Canada |
| Notes | Presented at the Canadian Stroke Conference, Toronto, 2015 September 17 |
| Trial name or title | Efficacy of a mechanical gait repetitive training technique compared with a usual rehabilitation program on gait recovery in hemiparetic stroke patients |
| Methods | RCT with 2 arms |
| Participants | Country: France |
| Interventions | 4‐week rehabilitation programme comparing physiotherapy and gait trainer therapy with physiotherapy alone |
| Outcomes | Primary outcomes: walking speed (time needed to walk 10 metres) after the 4‐week rehabilitation programme |
| Starting date | March 2006 |
| Contact information | Principal Investigator: |
| Notes | Expected total enrolment: 122 participants |
| Trial name or title | Effects of gait training with assistance of a robot‐driven gait orthosis in hemiparetic patients after stroke |
| Methods | Randomised 2 arms |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Robot‐assisted gait training |
| Outcomes | Not provided |
| Starting date | Unknown status |
| Contact information | Not provided |
| Notes | The recruitment status of this study is unknown. The completion date has passed, and the status has not been verified in more than 2 years. |
| Trial name or title | Comparative study of GangTrainer GT1, Lokomat and conventional physiotherapy (GALOP) |
| Methods | RCT with 3 arms |
| Participants | Inclusion criteria: first supratentorial stroke (ischaemic, haemorrhagic, or intracerebral haemorrhage) resulting in hemiparesis; interval from stroke 3 to 12 weeks; non‐ambulatory (FAC < 3); free sitting on bedside for 1 minute, with both feet on the floor and holding onto bedside by hands; Barthel Index 25 to 65 |
| Interventions | Group A: 30 minutes of treatment on the GangTrainer GT1 and 30 minutes of conventional physiotherapy every workday for 8 weeks |
| Outcomes | Primary outcomes: FAC, modified Emory Functional Ambulation Secondary outcomes: Barthel Index, 10‐metre walk test, 6‐minute walk test on the floor, Medical Research Council, Rivermead Visual Gait Assessment, EuroQol‐5 Dimensions (EQ‐5D) |
| Starting date | August 2010 |
| Contact information | Contact: Andreas Waldner, MD; +39 0471 471 471; [email protected] |
| Notes | Estimated enrolment: 120 |
| Trial name or title | A randomised controlled trial on hemiplegic gait rehabilitation: robotic locomotor training versus conventional training in subacute stroke |
| Methods | RCT with 2 arms |
| Participants | Country: Thailand Exclusion criteria: unstable general medical condition, severe malposition or fixed contracture of joint with an extension deficit > 30°, any functional impairment before stroke, cannot adequately co‐operate in training, severe communication problems, severe cognitive‐perceptual deficits |
| Interventions | Group A: conventional therapy: 50 minutes individual physiotherapy and 60 minutes individual occupational therapy per workday (5 times per week) for 4 consecutive weeks |
| Outcomes | Primary outcomes: FAC 0 to 5 and Barthel Index 0 to 100 |
| Starting date | January 2011 |
| Contact information | Principal Investigator: Ratanapat Chanubol, MD, Rehabilitation Department, Prasat Neurological Institute, Mahidol University |
| Notes | Study Completion Date: July 2012 Enrolment: 60 |
| Trial name or title | Robot walking rehabilitation in stroke patients |
| Methods | RCT with 3 arms |
| Participants | Inclusion criteria: between the ages of 18 and 95 years, able to walk 25 feet unassisted or with assistance, first acute event of cerebrovascular stroke, unilateral paresis, ability to understand and follow simple instructions, ability to walk without assistance before stroke, endurance sufficient to stand at least 20 minutes unassisted per participant report |
| Interventions | Experimental group: Robot G‐EO: each participant will be asked to perform 15 sessions (3 to 5 days a week for 4 up to 5 weeks) consisting of a treatment cycle using the G‐EO system device, according to individually tailored exercise scheduling |
| Outcomes | |
| Starting date | September 2012 |
| Contact information | Contact: Patrizio Sale, MD; [email protected] |
| Notes | Estimated enrolment: 90 |
| Trial name or title | Effects of locomotion training with assistance of a robot‐driven gait orthosis in hemiparetic patients after subacute stroke |
| Methods | Randomised trial 2 arms |
| Participants | Inclusion criteria
Exclusion criteria
72 first‐ever stroke patients who could not walk independently (FAC < 2), and suffered stroke within 6 months were enrolled and randomly assigned into 2 groups. People with congestive heart failure, malignancies, cardiopulmonary dysfunctions, and who could not walk independently before their stroke were excluded. |
| Interventions | 2 groups received 30 minutes of conventional gait training including neurodevelopmental treatment:
|
| Outcomes | Independent walking ability (FAC ≥ 3), FAC, Motricity index, Fugl‐Meyer Assessment, Modified Barthel Index, Medical Research Council were assessed for lower extremity muscles before, during (2 weeks), and after training. Independent walking ability was followed until 3 months. |
| Starting date | March 2012 |
| Contact information | Yonsei University |
| Notes |
| Trial name or title | Human‐machine system for the H2 lower limb exoskeleton (H2‐NeuroExo) |
| Methods | RCT with 2 arms |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: robot‐assisted rehabilitation participants will receive robot‐assisted training with the H2 lower limb powered exoskeleton. They will perform walking and other lower limb exercises (as applicable) while wearing the H2 lower limb powered exoskeleton. Training will involve 3 sessions per week for 4 weeks, each lasting about 1.5 hours. Control: supervised motor practice participants will perform walking and other lower limb exercises (as applicable) under the supervision of a research physical therapist. Training will involve 3 sessions per week for 4 weeks, each lasting about 1.5 hours. |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | March 2014 |
| Contact information | clinicaltrials.gov/ct2/show/study/NCT02114450#contacts |
| Notes |
| Trial name or title | Interactive exoskeleton robot for walking ‐ ankle joint |
| Methods | Randomised 2 arms |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | June 2015 |
| Contact information | Raymond KY Tong, Professor, Chinese University of Hong Kong |
| Notes |
| Trial name or title | Adaptive ankle robot control system to reduce foot‐drop in chronic stroke |
| Methods | Randomised 2 arms |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | September 2015 |
| Contact information | clinicaltrials.gov/ct2/show/study/NCT02483676?term=NCT02483676&rank=1#contacts |
| Notes |
| Trial name or title | New technology for individualised, intensive training of gait after stroke ‐ phase III trials, study II |
| Methods | Randomised 3 arms |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | October 2015 |
| Contact information | clinicaltrials.gov/ct2/show/study/NCT02545088?term=NCT02545088&rank=1#contacts |
| Notes |
| Trial name or title | Robot assisted gait training in patients with infratentorial stroke |
| Methods | Randomised 2 arms |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | April 2015 |
| Contact information | clinicaltrials.gov/ct2/show/study/NCT02680691?term=NCT02680691&rank=1#contacts |
| Notes |
| Trial name or title | Clinical trial of robot‐assisted‐gait‐training (RAGT) in stroke patients (Walkbot) |
| Methods | Randomised 2 arms |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Primary outcome measure
Secondary outcome measures
|
| Starting date | March 2014 |
| Contact information | P&S Mechanics Co., Ltd. |
| Notes |
| Trial name or title | Clinical applicability of robot‐assisted gait training system in acute stroke patients |
| Methods | Randomised 2 arms |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Device: HIWIN Robotic Gait Training System˜: the HIWIN Robotic Gait Training System is an automatic training system that combines weight‐bearing standing, repetitive stepping, and gait training |
| Outcomes |
|
| Starting date | May 2016 |
| Contact information | China Medical University Hospital |
| Notes | Other study ID: CMUH105‐REC1‐037 |
| Trial name or title | Robot‐assisted gait training for patients with stroke |
| Methods | Randomised 2 arms |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Standard hospital‐based rehabilitation for people with stroke |
| Outcomes |
|
| Starting date | May 2016 |
| Contact information | Principal Investigator: Nai‐Hsin Meng, MD, China Medical University Hospital |
| Notes | HIWIN‐CMU‐C‐105‐1 |
| Trial name or title | Gait pattern analysis and feasibility of gait training with a walking assist robot in stroke patients and elderly adults |
| Methods | Randomised 2 treatment groups |
| Participants | Number of participants: 54 (n = 27 per group) Inclusion criteria
Exclusion criteria
|
| Interventions | Group 1 : gait rehabilitation with hip assist robot (Samsung Hip Assist v1): 10 sessions (5 sessions: treadmill gait training/5 sessions: overground gait training), 30 minutes per session Group 2 : gait rehabilitation without hip assist robot: 10 sessions (5 sessions: treadmill gait training/5 sessions: overground gait training), 30 minutes per session |
| Outcomes |
|
| Starting date | September 2015 |
| Contact information | Yun‐Hee Kim, MD, PhD; 82‐2‐3410‐2824 [email protected] |
| Notes |
FAC: Functional Ambulation Category
NYHA: New York Heart Association
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
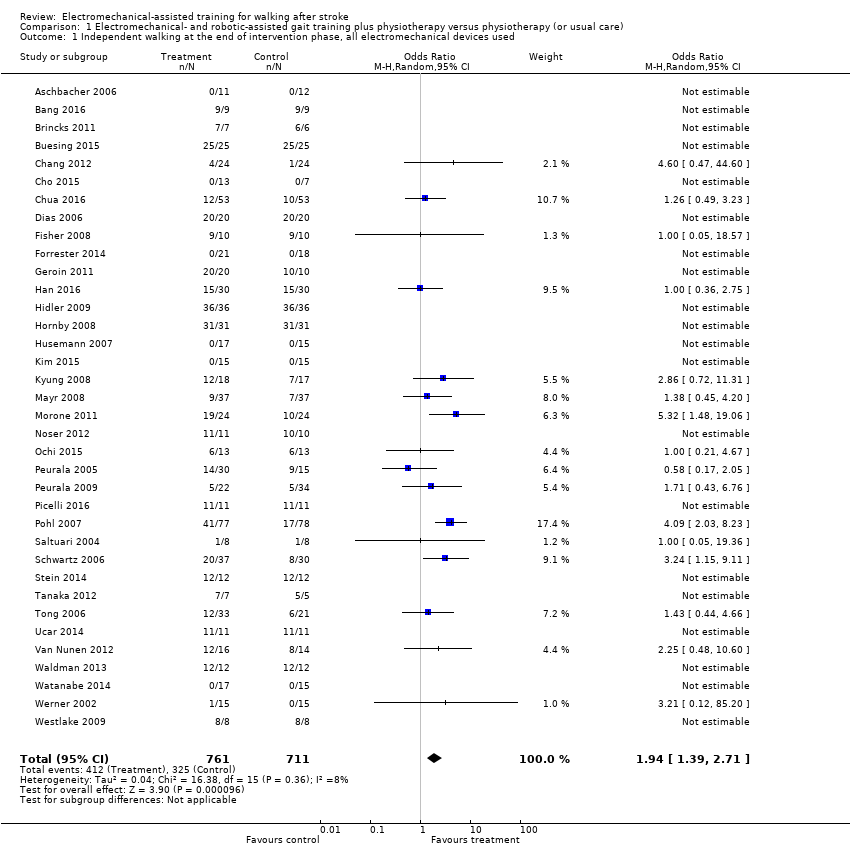
| 1 Independent walking at the end of intervention phase, all electromechanical devices used Show forest plot | 36 | 1472 | Odds Ratio (M‐H, Random, 95% CI) | 1.94 [1.39, 2.71] |
| Analysis 1.1  Comparison 1 Electromechanical‐ and robotic‐assisted gait training plus physiotherapy versus physiotherapy (or usual care), Outcome 1 Independent walking at the end of intervention phase, all electromechanical devices used. | ||||
| 2 Recovery of independent walking at follow‐up after study end Show forest plot | 6 | 496 | Odds Ratio (M‐H, Random, 95% CI) | 1.93 [0.72, 5.13] |
| Analysis 1.2  Comparison 1 Electromechanical‐ and robotic‐assisted gait training plus physiotherapy versus physiotherapy (or usual care), Outcome 2 Recovery of independent walking at follow‐up after study end. | ||||
| 3 Walking velocity (metres per second) at the end of intervention phase Show forest plot | 24 | 985 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.00, 0.09] |
| Analysis 1.3 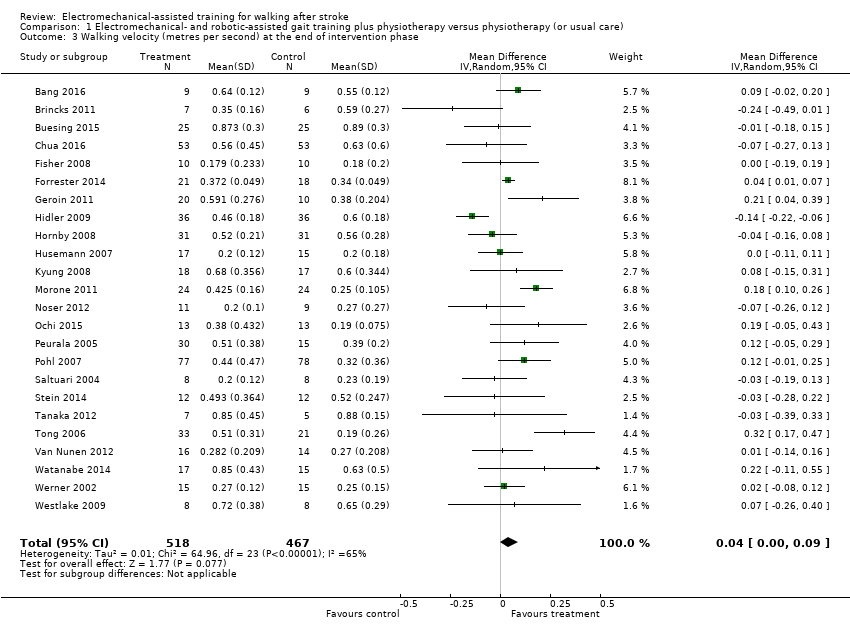 Comparison 1 Electromechanical‐ and robotic‐assisted gait training plus physiotherapy versus physiotherapy (or usual care), Outcome 3 Walking velocity (metres per second) at the end of intervention phase. | ||||
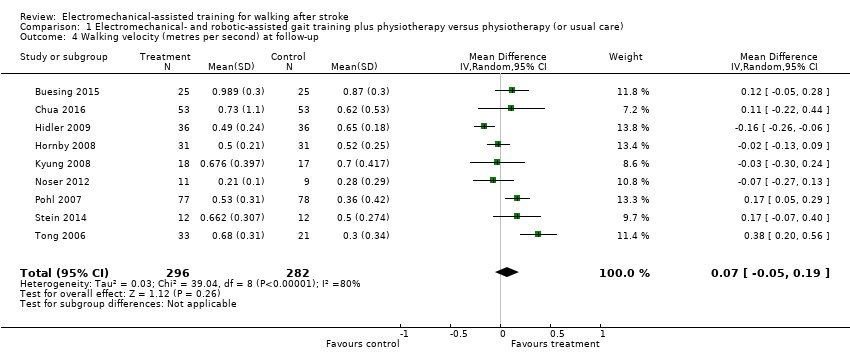
| 4 Walking velocity (metres per second) at follow‐up Show forest plot | 9 | 578 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.05, 0.19] |
| Analysis 1.4  Comparison 1 Electromechanical‐ and robotic‐assisted gait training plus physiotherapy versus physiotherapy (or usual care), Outcome 4 Walking velocity (metres per second) at follow‐up. | ||||
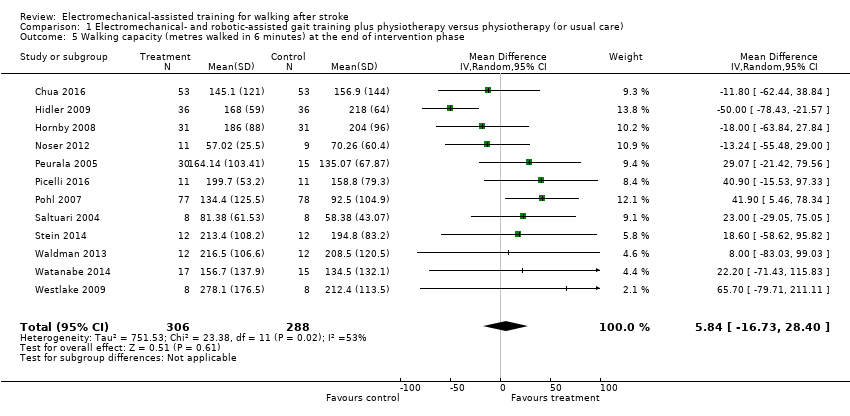
| 5 Walking capacity (metres walked in 6 minutes) at the end of intervention phase Show forest plot | 12 | 594 | Mean Difference (IV, Random, 95% CI) | 5.84 [‐16.73, 28.40] |
| Analysis 1.5  Comparison 1 Electromechanical‐ and robotic‐assisted gait training plus physiotherapy versus physiotherapy (or usual care), Outcome 5 Walking capacity (metres walked in 6 minutes) at the end of intervention phase. | ||||
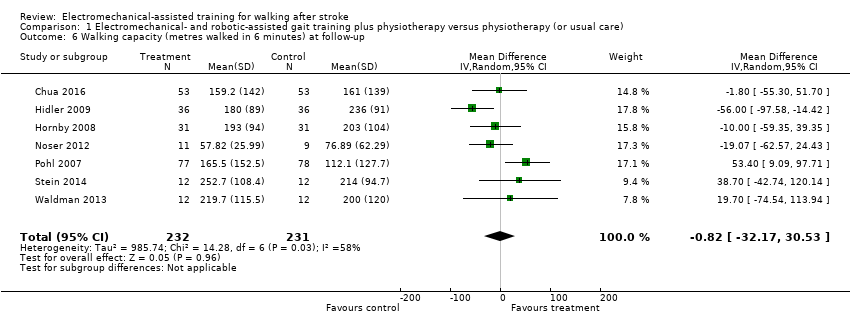
| 6 Walking capacity (metres walked in 6 minutes) at follow‐up Show forest plot | 7 | 463 | Mean Difference (IV, Random, 95% CI) | ‐0.82 [‐32.17, 30.53] |
| Analysis 1.6 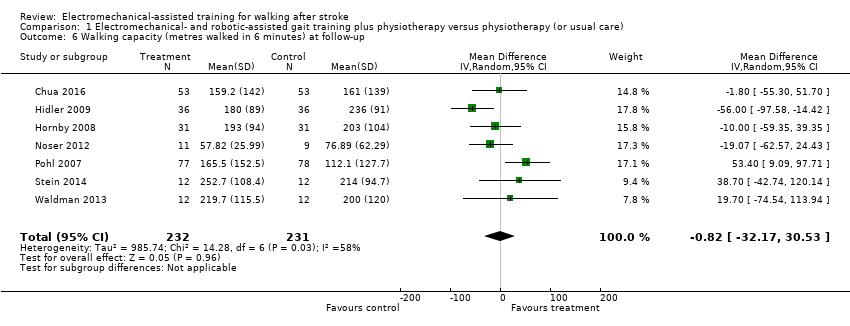 Comparison 1 Electromechanical‐ and robotic‐assisted gait training plus physiotherapy versus physiotherapy (or usual care), Outcome 6 Walking capacity (metres walked in 6 minutes) at follow‐up. | ||||
| 7 Acceptability of electromechanical‐assisted gait training devices during intervention phase: dropouts Show forest plot | 36 | 1472 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.43, 1.05] |
| Analysis 1.7  Comparison 1 Electromechanical‐ and robotic‐assisted gait training plus physiotherapy versus physiotherapy (or usual care), Outcome 7 Acceptability of electromechanical‐assisted gait training devices during intervention phase: dropouts. | ||||
| 8 Death from all causes until the end of intervention phase Show forest plot | 36 | 1472 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.02] |
| Analysis 1.8 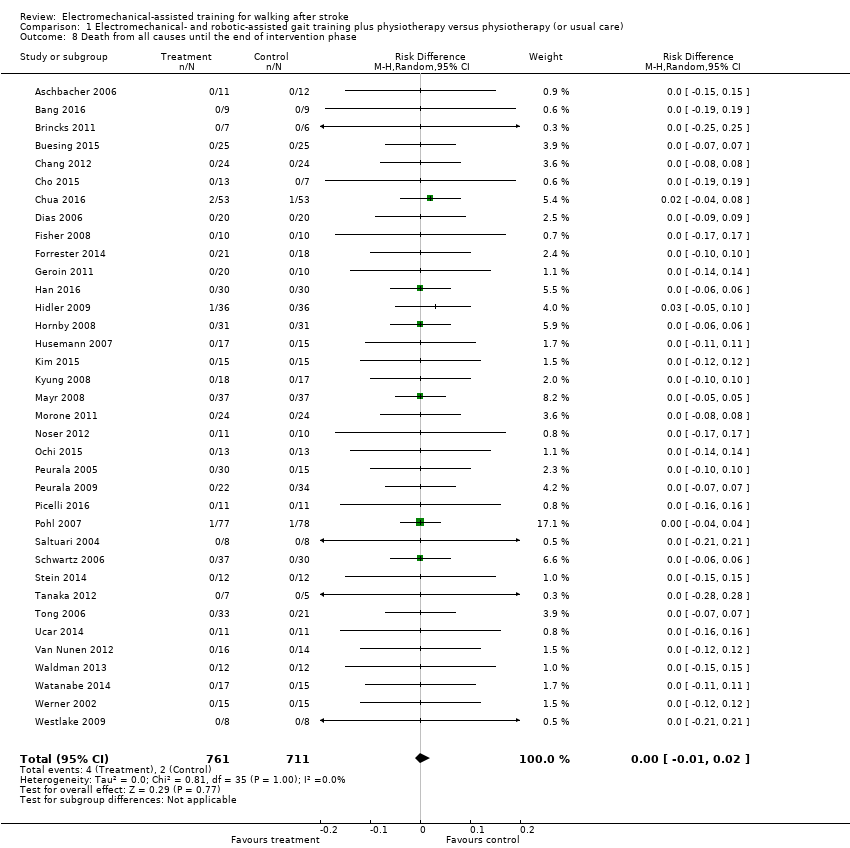 Comparison 1 Electromechanical‐ and robotic‐assisted gait training plus physiotherapy versus physiotherapy (or usual care), Outcome 8 Death from all causes until the end of intervention phase. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Regaining independent walking ability Show forest plot | 36 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Planned sensitivity analysis by trial methodology, Outcome 1 Regaining independent walking ability. | ||||
| 1.1 All studies with adequate sequence generation process | 20 | 949 | Odds Ratio (M‐H, Random, 95% CI) | 1.80 [1.06, 3.08] |
| 1.2 All studies with adequate concealed allocation | 17 | 831 | Odds Ratio (M‐H, Random, 95% CI) | 1.87 [1.12, 3.12] |
| 1.3 All studies with blinded assessors for primary outcome | 16 | 762 | Odds Ratio (M‐H, Random, 95% CI) | 1.81 [1.10, 2.98] |
| 1.4 All studies without incomplete outcome data | 14 | 590 | Odds Ratio (M‐H, Random, 95% CI) | 2.23 [1.16, 4.29] |
| 1.5 All studies excluding the largest study Pohl 2007 | 35 | 1317 | Odds Ratio (M‐H, Random, 95% CI) | 1.65 [1.17, 2.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Independent walking at the end of intervention phase, all electromechanical devices used Show forest plot | 36 | Odds Ratio (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.1 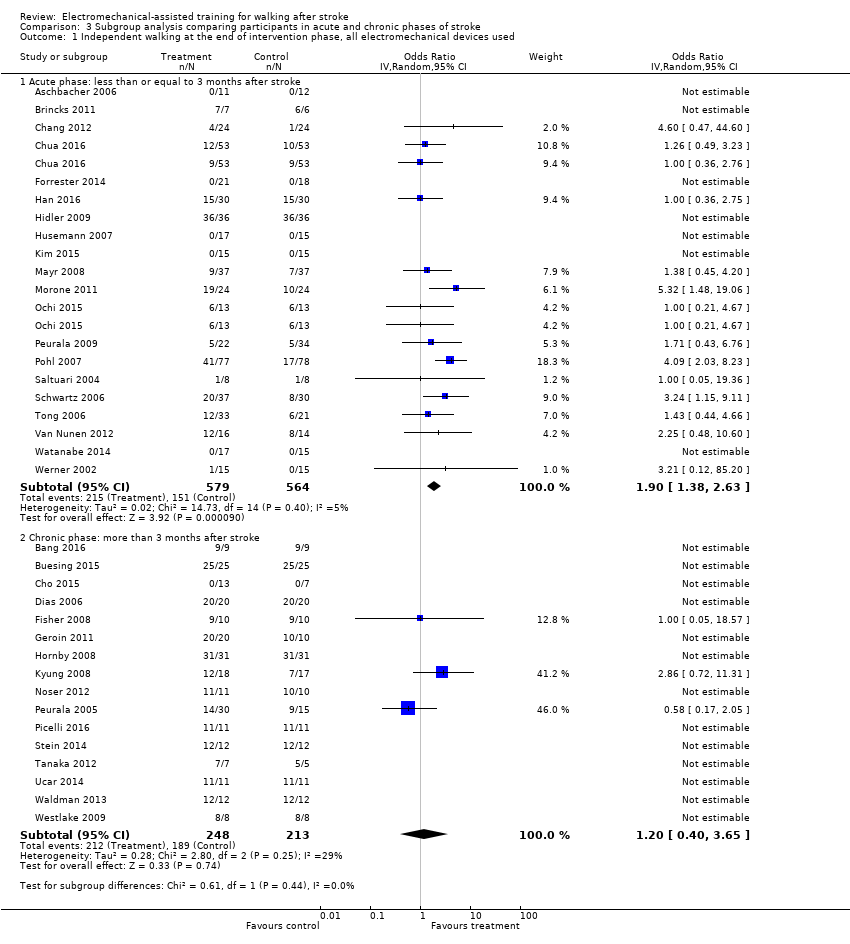 Comparison 3 Subgroup analysis comparing participants in acute and chronic phases of stroke, Outcome 1 Independent walking at the end of intervention phase, all electromechanical devices used. | ||||
| 1.1 Acute phase: less than or equal to 3 months after stroke | 20 | 1143 | Odds Ratio (IV, Random, 95% CI) | 1.90 [1.38, 2.63] |
| 1.2 Chronic phase: more than 3 months after stroke | 16 | 461 | Odds Ratio (IV, Random, 95% CI) | 1.20 [0.40, 3.65] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recovery of independent walking: ambulatory status at study onset Show forest plot | 36 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.1  Comparison 4 Post hoc sensitivity analysis: ambulatory status at study onset, Outcome 1 Recovery of independent walking: ambulatory status at study onset. | ||||
| 1.1 Studies that included independent walkers | 15 | 500 | Odds Ratio (M‐H, Random, 95% CI) | 1.38 [0.45, 4.20] |
| 1.2 Studies that included dependent and independent walkers | 9 | 340 | Odds Ratio (M‐H, Random, 95% CI) | 1.90 [1.11, 3.25] |
| 1.3 Studies that included dependent walkers | 12 | 632 | Odds Ratio (M‐H, Random, 95% CI) | 1.90 [1.04, 3.48] |
| 2 Walking velocity: ambulatory status at study onset Show forest plot | 24 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
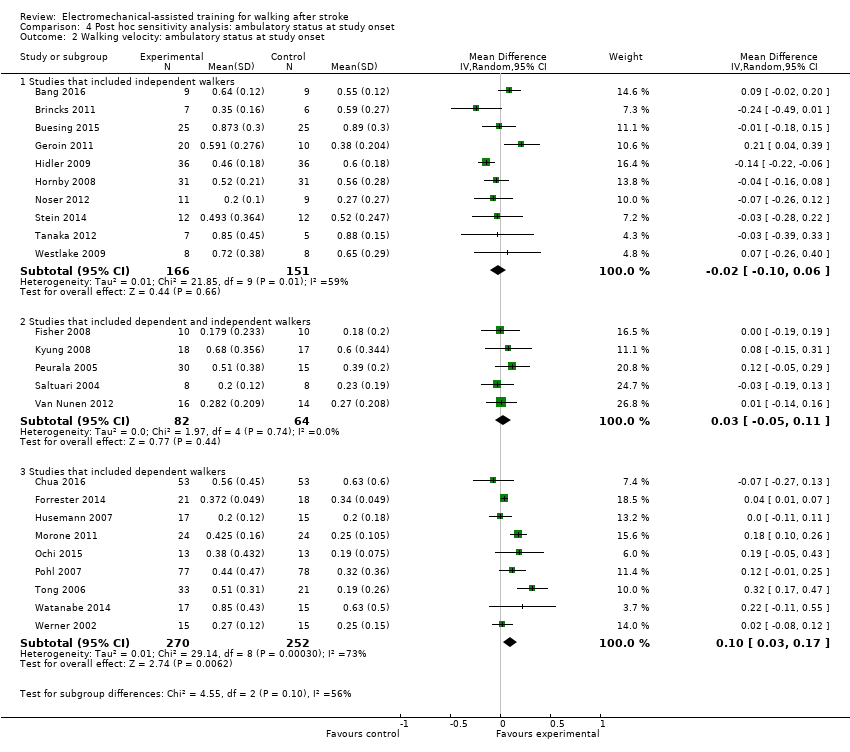
| Analysis 4.2 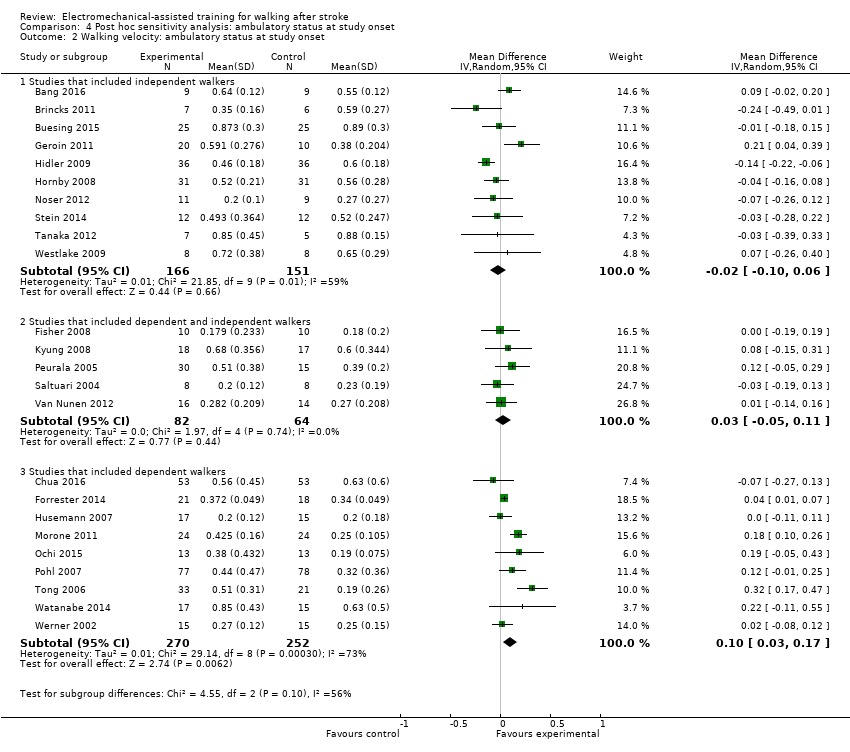 Comparison 4 Post hoc sensitivity analysis: ambulatory status at study onset, Outcome 2 Walking velocity: ambulatory status at study onset. | ||||
| 2.1 Studies that included independent walkers | 10 | 317 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.10, 0.06] |
| 2.2 Studies that included dependent and independent walkers | 5 | 146 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.05, 0.11] |
| 2.3 Studies that included dependent walkers | 9 | 522 | Mean Difference (IV, Random, 95% CI) | 0.10 [0.03, 0.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
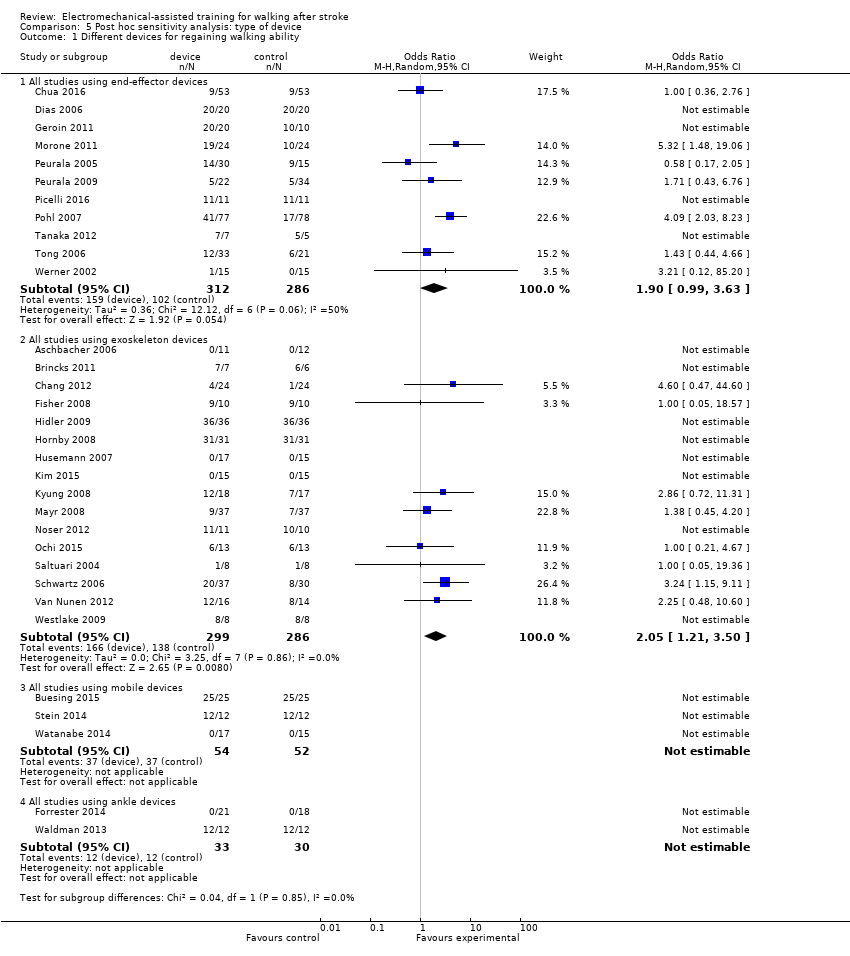
| 1 Different devices for regaining walking ability Show forest plot | 32 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.1  Comparison 5 Post hoc sensitivity analysis: type of device, Outcome 1 Different devices for regaining walking ability. | ||||
| 1.1 All studies using end‐effector devices | 11 | 598 | Odds Ratio (M‐H, Random, 95% CI) | 1.90 [0.99, 3.63] |
| 1.2 All studies using exoskeleton devices | 16 | 585 | Odds Ratio (M‐H, Random, 95% CI) | 2.05 [1.21, 3.50] |
| 1.3 All studies using mobile devices | 3 | 106 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 All studies using ankle devices | 2 | 63 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Different devices for regaining walking speed Show forest plot | 24 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 5.2 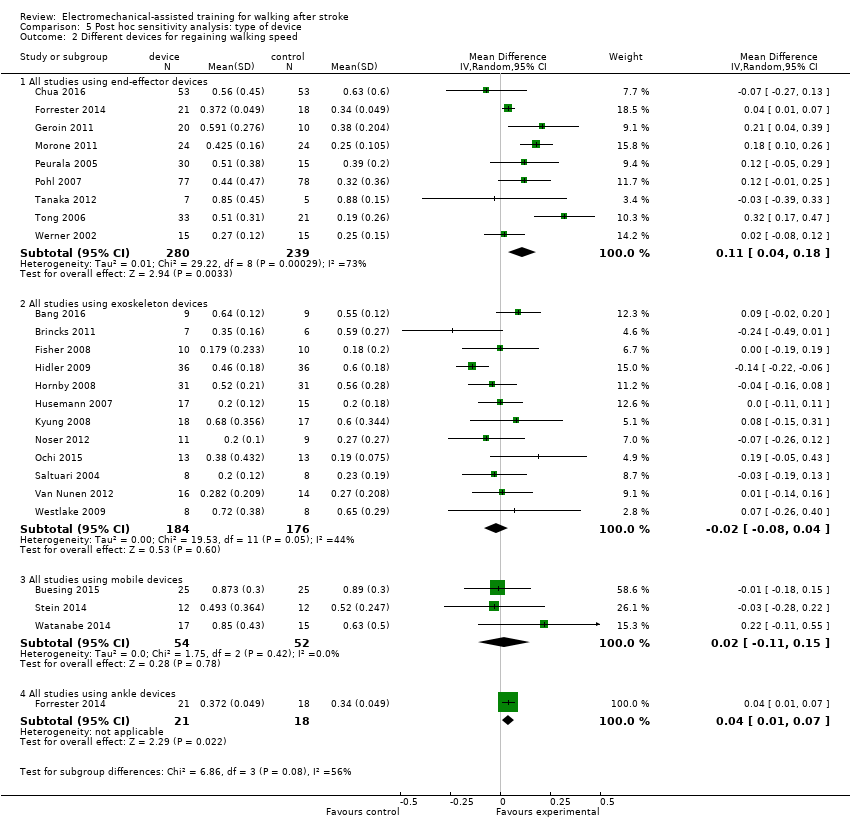 Comparison 5 Post hoc sensitivity analysis: type of device, Outcome 2 Different devices for regaining walking speed. | ||||
| 2.1 All studies using end‐effector devices | 9 | 519 | Mean Difference (IV, Random, 95% CI) | 0.11 [0.04, 0.18] |
| 2.2 All studies using exoskeleton devices | 12 | 360 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.08, 0.04] |
| 2.3 All studies using mobile devices | 3 | 106 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.11, 0.15] |
| 2.4 All studies using ankle devices | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 0.04 [0.01, 0.07] |
| 3 Different devices for regaining walking capacity Show forest plot | 12 | 594 | Mean Difference (IV, Random, 95% CI) | 5.84 [‐16.73, 28.40] |
| Analysis 5.3  Comparison 5 Post hoc sensitivity analysis: type of device, Outcome 3 Different devices for regaining walking capacity. | ||||
| 3.1 All studies using end‐effector devices | 4 | 328 | Mean Difference (IV, Random, 95% CI) | 27.50 [3.64, 51.36] |
| 3.2 All studies using exoskeleton devices | 5 | 186 | Mean Difference (IV, Random, 95% CI) | ‐15.64 [‐46.34, 15.05] |
| 3.3 All studies using mobile devices | 2 | 56 | Mean Difference (IV, Random, 95% CI) | 20.06 [‐39.52, 79.63] |
| 3.4 All studies using ankle devices | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 8.0 [‐83.03, 99.03] |

Study flow diagram.
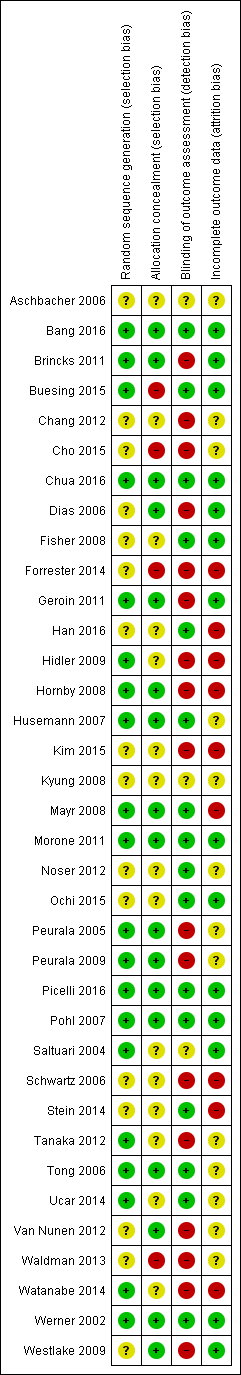
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
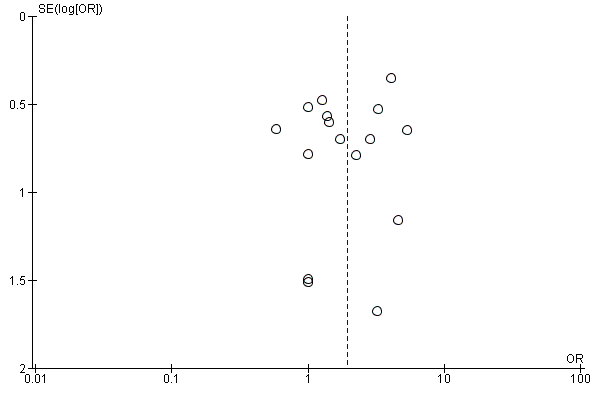
Funnel plot of comparison: 1 Electromechanical‐ and robotic‐assisted gait training plus physiotherapy versus physiotherapy (or usual care), outcome: 1.1 Independent walking at the end of intervention phase, all electromechanical devices used.

Funnel plot of comparison: 1 Electromechanical‐ and robotic‐assisted gait training plus physiotherapy versus physiotherapy (or usual care), outcome: 1.3 Walking velocity (metres per second) at the end of intervention phase.
Comparison 1 Electromechanical‐ and robotic‐assisted gait training plus physiotherapy versus physiotherapy (or usual care), Outcome 1 Independent walking at the end of intervention phase, all electromechanical devices used.

Comparison 1 Electromechanical‐ and robotic‐assisted gait training plus physiotherapy versus physiotherapy (or usual care), Outcome 2 Recovery of independent walking at follow‐up after study end.

Comparison 1 Electromechanical‐ and robotic‐assisted gait training plus physiotherapy versus physiotherapy (or usual care), Outcome 3 Walking velocity (metres per second) at the end of intervention phase.
Comparison 1 Electromechanical‐ and robotic‐assisted gait training plus physiotherapy versus physiotherapy (or usual care), Outcome 4 Walking velocity (metres per second) at follow‐up.
Comparison 1 Electromechanical‐ and robotic‐assisted gait training plus physiotherapy versus physiotherapy (or usual care), Outcome 5 Walking capacity (metres walked in 6 minutes) at the end of intervention phase.
Comparison 1 Electromechanical‐ and robotic‐assisted gait training plus physiotherapy versus physiotherapy (or usual care), Outcome 6 Walking capacity (metres walked in 6 minutes) at follow‐up.

Comparison 1 Electromechanical‐ and robotic‐assisted gait training plus physiotherapy versus physiotherapy (or usual care), Outcome 7 Acceptability of electromechanical‐assisted gait training devices during intervention phase: dropouts.

Comparison 1 Electromechanical‐ and robotic‐assisted gait training plus physiotherapy versus physiotherapy (or usual care), Outcome 8 Death from all causes until the end of intervention phase.

Comparison 2 Planned sensitivity analysis by trial methodology, Outcome 1 Regaining independent walking ability.

Comparison 3 Subgroup analysis comparing participants in acute and chronic phases of stroke, Outcome 1 Independent walking at the end of intervention phase, all electromechanical devices used.

Comparison 4 Post hoc sensitivity analysis: ambulatory status at study onset, Outcome 1 Recovery of independent walking: ambulatory status at study onset.
Comparison 4 Post hoc sensitivity analysis: ambulatory status at study onset, Outcome 2 Walking velocity: ambulatory status at study onset.
Comparison 5 Post hoc sensitivity analysis: type of device, Outcome 1 Different devices for regaining walking ability.

Comparison 5 Post hoc sensitivity analysis: type of device, Outcome 2 Different devices for regaining walking speed.

Comparison 5 Post hoc sensitivity analysis: type of device, Outcome 3 Different devices for regaining walking capacity.
| Electromechanical‐ and robotic‐assisted gait training plus physiotherapy compared to physiotherapy (or usual care) for walking after stroke | ||||||
| Patient or population: walking after stroke | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with physiotherapy (or usual care) | Risk with electromechanical‐ and robotic‐assisted gait training plus physiotherapy | |||||
| Independent walking at the end of intervention phase, all electromechanical devices used | Study population | OR 1.94 | 1472 | ⊕⊕⊕⊝ | ||
| 457 per 1000 | 615 per 1000 | |||||
| Recovery of independent walking at follow‐up after study end | Study population | OR 1.93 | 496 | ⊕⊕⊕⊝ | ||
| 551 per 1000 | 703 per 1000 | |||||
| Walking velocity (metres per second) at the end of intervention phase | The mean walking velocity (metres per second) at the end of intervention phase was 0. | MD 0.04 higher | ‐ | 985 | ⊕⊕⊝⊝ | |
| Walking velocity (metres per second) at follow‐up | The mean walking velocity (metres per second) at follow‐up was 0. | MD 0.07 higher | ‐ | 578 | ⊕⊕⊕⊝ | |
| Walking capacity (metres walked in 6 minutes) at the end of intervention phase | The mean walking capacity (metres walked in 6 minutes) at the end of intervention phase was 0. | MD 5.84 higher | ‐ | 594 | ⊕⊝⊝⊝ | |
| Walking capacity (metres walked in 6 minutes) at follow‐up | The mean walking capacity (metres walked in 6 minutes) at follow‐up was 0. | MD 0.82 lower | ‐ | 463 | ⊕⊝⊝⊝ | |
| Acceptability of electromechanical‐assisted gait‐training devices during intervention phase | Study population | OR 0.67 | 1472 | ⊕⊕⊝⊝ | ||
| 131 per 1000 | 92 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded due to several ratings of 'unclear' and 'high' risk of bias. | ||||||
| Study ID | Experimental: age, mean (SD) | Control: age, mean (SD) | Experimental: time poststroke | Control: time poststroke | Experimental: sex | Control: sex | Experimental: side paresis | Control: side paresis |
| 57 years | 65 years | ≤ 3 months | ≤ 3 months | 2 female | 4 female | Not stated | Not stated | |
| 54 years | 54 years | 12 months | 13 months | 5 male, 4 female | 4 male, 5 female | 4 right, 5 left | 4 right, 5 left | |
| 61 (median) years | 59 (median) years | 56 (median) days | 21 (median) days | 5 male, 2 female | 4 male, 2 female | 5 right, 2 left | 1 right, 5 left | |
| 60 years | 62 years | 7 years | 5 years | 17 male, 8 female | 16 male, 9 female | 13 right, 12 left | 12 right, 13 left | |
| 56 (12) years | 60 (12) years | 16 (5) days | 18 (5) days | 13 male, 7 female | 10 male, 7 female | 6 right, 14 left | 6 right, 11 left | |
| 55 (12) years | 55 (15) years | 15 months | 13 months | Not stated | Not stated | 6 right, 4 left (4 both) | 3 right, 1 left (3 both) | |
| 62 (10) years | 61 (11) years | 27 (11) days | 30 (14) days | 35 male, 18 female | 40 male, 13 female | 24 right, 29 left | 21 right, 32 left | |
| 70 (7) years | 68 (11) years | 47 (64) months | 48 (30) months | 16 male, 4 female | 14 male, 6 female | Not stated | Not stated | |
| Not stated | Not stated | Less than 12 months | Less than 12 months | Not stated | Not stated | Not stated | Not stated | |
| 63 years | 60 years | 12 days | 11 days | Not stated | Not stated | 9 right, 9 left | 7 right, 9 left | |
| 63 (7) years | 61 (6) years | 26 (6) months | 27 (6) months | 14 male, 6 female | 9 male, 1 female | Not stated | Not stated | |
| 68 (15) years | 63 (11) years | 22 (8) days | 18 (10) days | Not stated | Not stated | 20 right, 10 left | 14 right, 12 left | |
| 60 (11) years | 55 (9) years | 111 (63) days | 139 (61) days | 21 male, 12 female | 18 male, 12 female | 22 right, 11 left | 13 right, 17 left | |
| 57 (10) years | 57 (11) years | 50 (51) months | 73 (87) months | 15 male, 9 female | 15 male, 9 female | 16 right, 8 left | 16 right, 8 left | |
| 60 (13) years | 57 (11) years | 79 (56) days | 89 (61) days | 11 male, 5 female | 10 male, 4 female | 12 right, 4 left | 11 right, 3 left | |
| 54 (13) years | 50 (16) years | 80 (60) days | 120 (84) days | 9 male, 4 female | 10 male, 3 female | 8 right, 5 left | 10 right, 3 left | |
| 48 (8) years | 55 (16) years | 22 (23) months | 29 (12) months | 9 male, 8 female | 4 male, 4 female | 9 right, 8 left | 4 right, 4 left | |
| Not stated | Not stated | Between 10 days and 6 months | Between 10 days and 6 months | Not stated | Not stated | Not stated | Not stated | |
| 62 (11) years | 62 (14) years | 19 (11) days | 20 (14) days | 15 male, 9 female | 13 male, 11 female | 13 right, 11 left | 15 right, 9 left | |
| 67 (9) years | 64 (11) years | 1354 days | 525 days | 7 male, 4 female | 6 male, 4 female | Not stated | Not stated | |
| 62 (8) years | 66 (12) years | 23 (7) days | 26 (8) days | 11 male, 2 female | 9 male, 4 female | 6 right, 7 left | 5 right, 8 left | |
| 52 (8) years | 52 (7) years | 2.5 (2.5) years | 4.0 (5.8) years | 26 male, 4 female | 11 male, 4 female | 13 right, 17 left | 10 right, 5 left | |
| 67 (9) years | 68 (10) years | 8 (3) days | 8 (3) days | 11 male, 11 female | 18 male, 16 female | 11 right, 11 left | 14 right, 20 left | |
| 62 (10) years | 65 (3) years | 6 (4) years | 6 (4) years | 7 male, 4 female | 9 male, 2 female | Not stated | Not stated | |
| 62 (12) years | 64 (11) years | 4.2 (1.8) weeks | 4.5 (1.9) weeks | 50 male, 27 female | 54 male, 24 female | 36 right, 41 left | 33 right, 45 left | |
| 62 (13) years | 60 (19) years | 3.6 (4.6) months | 1.9 (0.8) months | 4 male, 4 female | 2 male, 6 female | Not stated | Not stated | |
| 62 (9) years | 65 (8) years | 22 (9) days | 24 (10) days | 21 male, 16 female | 20 male, 10 female | 17 right, 20 left | 8 right, 22 left | |
| 58 (11) years | 57 (15) years | 49 (39) months | 89 (153) months | Not stated | Not stated | Not stated | Not stated | |
| 63 (10) years | 60 (9) years | 55 (37) months | 65 (67) months | 10 male, 2 female | 9 right, 3 left | |||
| 71 (14) years | 64 (10) years | 2.5 (1.2) weeks | 2.7 (1.2) weeks | 19 male, 11 female | 12 male, 8 female | 13 right, 17 left | 7 right, 13 left | |
| 56 years | 62 years | Not stated | Not stated | Not stated | Not stated | Not stated | Not stated | |
| 53 (10) years | 2.1 (1.3) months | 16 male, 14 female | Not stated | Not stated | ||||
| 51 (8) years | 53 (7) years | 41 (20) months | 30 (22) months | Not stated | Not stated | Not stated | Not stated | |
| 67 (17) years | 76 (14) years | 59 (47) days | 51 (34) days | 7 male, 4 female | 4 male, 7 female | 6 right, 5 left | 5 right, 6 left | |
| 60 (9) years | 60 (9) years | 7.4 (2.0) weeks | 6.9 (2.1) weeks | 8 male, 7 female | 5 male, 10 female | 8 right, 7 left | 8 right, 7 left | |
| 59 (17) years | 55 (14) years | 44 (27) months | 37 (20) months | 6 male, 2 female | 7 male, 1 female | 4 right, 4 left | 3 right, 5 left | |
| SD: standard deviation | ||||||||
| Criteria | Stroke severity | Electromechanical device used | Duration of study intervention | Aetiology (ischaemic/haemorrhage) | Intensity of treatment per day | Description of the control intervention | Dropouts | Reasons for dropout and adverse events in the experimental group | Reasons for dropout and adverse events in the control group | Source of information |
| Not stated | Lokomat | 3 weeks | Not stated | 30 minutes, 5 times a week | Described as task‐oriented physiotherapy, 5 times a week for 3 weeks (2.5 hours a week) | 4 of 23 | Not stated | Not stated | Unpublished information in the form of a conference presentation | |
| Unclear | Lokomat | 4 weeks | 13/5 | 60 minutes, 5 times a week (20 sessions) | Described as treadmill training without body weight support | 0 of 18 | ‐ | ‐ | Published information | |
| Mean FIM, 92 of 126 points | Lokomat | 3 weeks | Not stated | Not stated | Physiotherapy | 0 of 13 | ‐ | ‐ | Unpublished and published information provided by the authors. | |
| Unclear | Wearable exoskeleton Stride Management Assist system (SMA) | 6 to 8 weeks | Unclear | 3 times per week for a maximum of 18 sessions | Functional task‐specific training (intensive overground training and mobility training) | 0 of 50 | ‐ | ‐ | Published information | |
| Not stated | Lokomat | 10 days | Not stated | 30 minutes daily for 10 days | Conventional gait training by physical therapists (with equal therapy time and same amount of sessions as experimental group) | 3 of 40 | Not described by group (3 participants dropped out: 1 due to aspiration pneumonia, and 2 were unable to co‐operate fully with the experimental procedure) | Unpublished and published information provided by the authors. | ||
| Mean Modified Barthel Index, 36 points | Lokomat | 8 weeks (2 phases, cross‐over after 4 weeks) | 4/14 (2 both) | 30 minutes, 3 times a week for 4 weeks | Bobath (neurophysiological exercises, inhibition of spasticity and synergy pattern) | 0 of 20 | ‐ | ‐ | Published information | |
| Mean Barthel Index, 49 points | Gait Trainer | 8 weeks | Not stated | Not stated | Physiotherapy including 25 minutes of stance/gait, 10 minutes cycling, 10 minutes tilt table standing | 20 of 106 | 2 death, 3 refusal, 1 medical problem, 1 transport problem (1 pain as adverse event) | 1 death, 6 refusal, 3 medical problem, 1 administrative problem (no adverse events) | Published information | |
| Mean Barthel Index, 75 points | Gait Trainer | 4 weeks | Not stated | 40 minutes, 5 times a week | Bobath method, 5 times a week for 5 weeks | 0 of 40 | ‐ | ‐ | Unpublished and published information provided by the authors. | |
| Not stated | AutoAmbulator | 24 sessions | Not stated | Minimum of 3 sessions a week up to 5 sessions; number of minutes in each session unclear | "Standard" physical therapy, 3 to 5 times a week for 24 consecutive sessions | 0 of 20 | 14 adverse events, no details provided | 11 adverse events, no details provided | Unpublished and published information provided by the authors. | |
| Mean FIM walk 1 point | Anklebot | 8 to 10 sessions (with ca. 200 repetitions) | Not stated | 60 minutes, 8 to 10 sessions | Stretching of the paretic ankle | 5 of 34 | Total of 5 dropouts in both groups (1 medical complication, 1 discharge prior study end, 2 time poststroke > 49 days, 1 non‐compliance) | Published information provided by the authors. | ||
| Mean European Stroke Scale, 80 points | Gait Trainer | 2 weeks | Not stated | 50 minutes, 5 times a week | Walking exercises according to the Bobath approach | 0 of 30 | ‐ | ‐ | Unpublished and published information provided by the authors. | |
| Not stated | Lokomat | 4 weeks | 33/23 | 30 minutes, 5 times a week | Neurodevelopmental techniques for balance and mobility | 4 0f 60 | ‐ | 4 unclear reasons | Published information provided by the authors. | |
| Not stated | Lokomat | 8 to 10 weeks (24 sessions) | 47/16 | 45 minutes, 3 days a week | Conventional gait training, 3 times a week for 8 to 10 weeks (24 sessions), each session lasted 1.5 hours | 9 of 72 | Not described by group (9 withdrew or were removed because of poor attendance or a decline in health, including 1 death, which according to the authors was unrelated to study) | Unpublished and published information provided by the authors. | ||
| Not stated | Lokomat | 12 sessions | 22/26 | 30 minutes, 12 sessions | Therapist‐assisted gait training, 12 sessions, each session lasted 30 minutes | 14 of 62 | 4 participants dropped out (2 discontinued secondary to leg pain during training, 1 experienced pitting oedema, and 1 had travel limitations) | 10 participants dropped out (4 discontinued secondary to leg pain, 1 experienced an injury outside therapy, 1 reported fear of falling during training, 1 presented with significant hypertension, 1 had travel limitations, and 2 experienced subjective exercise intolerance) | Published information provided by the authors. | |
| Median Barthel Index, 35 points | Lokomat | 4 weeks | 22/8 | 30 minutes, 5 times a week | Conventional physiotherapy, 30 minutes per day for 4 weeks | 2 of 32 | 1 participant enteritis | 1 participant pulmonary embolism | Information as provided by the authors | |
| Mean Barthel Index, 20 points | Walkbot | 4 weeks | 13/13 | 30 minutes, 5 times a week | Conventional physiotherapy (bed mobility, stretching, balance training, strengthening, symmetry training, treadmill training) | 4 of 30 | 1 rib fracture, 3 decline in health condition | Information as provided by the authors | ||
| Not stated | Lokomat | 4 weeks | 18/7 | 45 minutes, 3 days a week | Conventional physiotherapy, received equal time and sessions of conventional gait training | 10 of 35 | 1 participant dropped out for private reasons (travelling); adverse events not described | 9 participants refused after randomisation (reasons not provided); adverse events not described | Unpublished and published information provided by the authors. | |
| Not stated | Lokomat | 8 weeks | Not stated | Not stated | Add‐on conventional physiotherapy, received equal time and sessions of conventional gait training | 13 of 74 | 4 participants dropped out (reasons not provided); adverse events not described | 9 participants dropped out (reasons not provided) | Unpublished and published information provided by the authors. | |
| Canadian Neurological Scale, 6 points | Gait Trainer | 4 weeks | 41/7 | 40 minutes, 5 times a week | Focused on trunk stabilisation, weight transfer to the paretic leg, and walking between parallel | 21 of 48 | 12 (hypotension, referred weakness, knee pain, urinary infection, uncontrolled blood pressure, fever, absence of physiotherapist) | 9 (hypotension, referred weakness, knee pain, ankle pain, uncontrolled blood pressure, fever, absence of physiotherapist) | Information as provided by the authors | |
| Not stated | Lokomat | Unclear | Not stated | Not stated | Not stated | 1 of 21 | No dropouts; 2 serious adverse events (1 skin breakdown as a result of therapy, 1 second stroke during the post‐treatment phase) | 1 dropout due to protocol violation; 2 serious adverse events (1 sudden drop in blood pressure at participant's home leading to brief hospitalisation, 1 sudden chest pain before therapy leading to brief hospitalisation) | Information as provided by the authors | |
| Not stated | Gait‐assistance robot (consisting of 4 robotic arms for the thighs and legs, thigh cuffs, leg apparatuses, and a treadmill) | 4 weeks | 10/16 | 20 minutes, 5 times a week for 4 weeks, in addition to rehabilitation treatment | Range‐of‐motion exercises, muscle strengthening, rolling over and sit‐to‐stand and activity and gait exercises | 0 of 26 | ‐ | ‐ | Published information | |
| Scandinavian Stroke Scale, 42 points | Gait Trainer | 3 weeks | 25/20 | 20 minutes, 5 times a week for 3 weeks, in addition to rehabilitation treatment | Walking overground; all participants practised gait for 15 sessions over 3 weeks (each session lasting 20 minutes) | 0 of 45 | ‐ | ‐ | Published information | |
| Not stated | Gait Trainer | 3 weeks | 42/14 | 20 minutes, 5 times a week for 3 weeks, in addition to rehabilitation treatment | Overground walking training; in the other control group, 1 or 2 physiotherapy sessions daily but not at the same intensity as in the other groups | 9 of 56 | 5 dropouts (2 situation worsened after 1 to 2 treatment days; 1 had 2 unsuccessful attempts in device; 1 had scheduling problems; 1 felt protocol too demanding) | 4 dropouts (1 felt protocol too demanding; 2 situation worsened after 1 to 2 treatment days; 1 death) | Published information | |
| Not stated | G‐EO System Evolution | Experimental group (G‐EO) 30 minutes a day for 5 consecutive days | Not stated | 5 days in addition to botulinum toxin injection of calf muscles | None | 0 of 22 | ‐ | ‐ | Published information | |
| Mean Barthel Index, 37 points | Gait Trainer | 4 weeks | 124/31 | 20 minutes, 5 times a week | Physiotherapy every weekday for 4 weeks | 11 of 155 | 2 participants refused therapy, 1 increased cranial pressure, 1 relapsing pancreas tumour, 1 cardiovascular unstable | 4 participants refused therapy, 1 participant died, 1 myocardial infarction | Published information | |
| Not stated | Lokomat | 2 weeks | 13/3 | A‐B‐A study: in phase A, 30 minutes, 5 days a week | Physiotherapy every weekday for 3 weeks (phase B) | 0 of 16 | None | None | Unpublished and published information provided by the authors. | |
| Mean NIHSS, 11 points | Lokomat | 6 weeks | 49/67 | 30 minutes, 3 times a week | Physiotherapy with additional gait training 3 times a week for 6 weeks | 6 of 46 | 2 participants with leg wounds, 1 participant with recurrent stroke, 1 refused therapy | 1 participant with recurrent stroke, 1 with pulmonary embolism | Unpublished and published information provided by the authors. | |
| Not stated | Bionic leg device (AlterG) | 6 weeks | Not stated | 1 hour, 3 times a week for 6 weeks | Group exercises | 0 of 24 | ‐ | ‐ | Published information | |
| Mean FIM, 79 points | Gait Master4 | 4 weeks | Not stated | 20 minutes, 2 or 3 times a week (12 sessions) | Non‐intervention (non‐training) | 0 of 12 | ‐ | ‐ | Published information | |
| Mean Barthel Index, 51 points | Gait Trainer | 4 weeks | 39/11 | 20 minutes, 5 times a week | Conventional physiotherapy alone, based on Bobath concept | 4 of 50 | None | 2 participants discharged before study end, 1 participant readmitted to an acute ward, 1 participant deteriorating condition | Published information | |
| Not stated | Lokomat | 2 weeks | Not stated | 30 minutes, 5 times a week | Conventional physiotherapy at home (focused on gait) | 0 of 22 | ‐ | ‐ | Published information | |
| Not stated | Lokomat | 8 weeks | Not stated | 30 minutes, twice a week | Overground therapy | 0 of 30 | ‐ | ‐ | Unpublished and published information provided by the author. | |
| Not stated | Portable rehab robot (ankle device) | 6 weeks | Not stated | 3 times a week, 18 sessions | Stretching the plantar flexors and active exercises for ankle mobility and strength | 0 of 24 | ‐ | ‐ | Published information | |
| Not stated | Single‐leg version of the Hybrid Assistive Limb (HAL) | 4 weeks | 11/11 | 20 minutes, 12 sessions | Aimed to improve walking speed, endurance, balance, postural stability, and symmetry | 10 of 32 | 4 withdrew, 1 epilepsy, 1 technical reasons | 2 pneumonia, 2 discharged | Published information | |
| Mean Barthel Index, 38 points | Gait Trainer | 2 weeks | 13/12 | 20 minutes, 5 times a week | Gait therapy including treadmill training with body weight support | 0 of 30 | None | None | Published information | |
| Not stated | Lokomat | 4 weeks (12 sessions) | 8/8 | 30 minutes, 3 times a week | 12 physiotherapy sessions including manually guided gait training (3 times a week over 4 weeks) | 0 of 16 | None | None | Published information | |
| FIM: Functional Independence Measure | ||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Independent walking at the end of intervention phase, all electromechanical devices used Show forest plot | 36 | 1472 | Odds Ratio (M‐H, Random, 95% CI) | 1.94 [1.39, 2.71] |
| 2 Recovery of independent walking at follow‐up after study end Show forest plot | 6 | 496 | Odds Ratio (M‐H, Random, 95% CI) | 1.93 [0.72, 5.13] |
| 3 Walking velocity (metres per second) at the end of intervention phase Show forest plot | 24 | 985 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.00, 0.09] |
| 4 Walking velocity (metres per second) at follow‐up Show forest plot | 9 | 578 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.05, 0.19] |
| 5 Walking capacity (metres walked in 6 minutes) at the end of intervention phase Show forest plot | 12 | 594 | Mean Difference (IV, Random, 95% CI) | 5.84 [‐16.73, 28.40] |
| 6 Walking capacity (metres walked in 6 minutes) at follow‐up Show forest plot | 7 | 463 | Mean Difference (IV, Random, 95% CI) | ‐0.82 [‐32.17, 30.53] |
| 7 Acceptability of electromechanical‐assisted gait training devices during intervention phase: dropouts Show forest plot | 36 | 1472 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.43, 1.05] |
| 8 Death from all causes until the end of intervention phase Show forest plot | 36 | 1472 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Regaining independent walking ability Show forest plot | 36 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 All studies with adequate sequence generation process | 20 | 949 | Odds Ratio (M‐H, Random, 95% CI) | 1.80 [1.06, 3.08] |
| 1.2 All studies with adequate concealed allocation | 17 | 831 | Odds Ratio (M‐H, Random, 95% CI) | 1.87 [1.12, 3.12] |
| 1.3 All studies with blinded assessors for primary outcome | 16 | 762 | Odds Ratio (M‐H, Random, 95% CI) | 1.81 [1.10, 2.98] |
| 1.4 All studies without incomplete outcome data | 14 | 590 | Odds Ratio (M‐H, Random, 95% CI) | 2.23 [1.16, 4.29] |
| 1.5 All studies excluding the largest study Pohl 2007 | 35 | 1317 | Odds Ratio (M‐H, Random, 95% CI) | 1.65 [1.17, 2.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Independent walking at the end of intervention phase, all electromechanical devices used Show forest plot | 36 | Odds Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Acute phase: less than or equal to 3 months after stroke | 20 | 1143 | Odds Ratio (IV, Random, 95% CI) | 1.90 [1.38, 2.63] |
| 1.2 Chronic phase: more than 3 months after stroke | 16 | 461 | Odds Ratio (IV, Random, 95% CI) | 1.20 [0.40, 3.65] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recovery of independent walking: ambulatory status at study onset Show forest plot | 36 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Studies that included independent walkers | 15 | 500 | Odds Ratio (M‐H, Random, 95% CI) | 1.38 [0.45, 4.20] |
| 1.2 Studies that included dependent and independent walkers | 9 | 340 | Odds Ratio (M‐H, Random, 95% CI) | 1.90 [1.11, 3.25] |
| 1.3 Studies that included dependent walkers | 12 | 632 | Odds Ratio (M‐H, Random, 95% CI) | 1.90 [1.04, 3.48] |
| 2 Walking velocity: ambulatory status at study onset Show forest plot | 24 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Studies that included independent walkers | 10 | 317 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.10, 0.06] |
| 2.2 Studies that included dependent and independent walkers | 5 | 146 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.05, 0.11] |
| 2.3 Studies that included dependent walkers | 9 | 522 | Mean Difference (IV, Random, 95% CI) | 0.10 [0.03, 0.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Different devices for regaining walking ability Show forest plot | 32 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 All studies using end‐effector devices | 11 | 598 | Odds Ratio (M‐H, Random, 95% CI) | 1.90 [0.99, 3.63] |
| 1.2 All studies using exoskeleton devices | 16 | 585 | Odds Ratio (M‐H, Random, 95% CI) | 2.05 [1.21, 3.50] |
| 1.3 All studies using mobile devices | 3 | 106 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 All studies using ankle devices | 2 | 63 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Different devices for regaining walking speed Show forest plot | 24 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 All studies using end‐effector devices | 9 | 519 | Mean Difference (IV, Random, 95% CI) | 0.11 [0.04, 0.18] |
| 2.2 All studies using exoskeleton devices | 12 | 360 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.08, 0.04] |
| 2.3 All studies using mobile devices | 3 | 106 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.11, 0.15] |
| 2.4 All studies using ankle devices | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 0.04 [0.01, 0.07] |
| 3 Different devices for regaining walking capacity Show forest plot | 12 | 594 | Mean Difference (IV, Random, 95% CI) | 5.84 [‐16.73, 28.40] |
| 3.1 All studies using end‐effector devices | 4 | 328 | Mean Difference (IV, Random, 95% CI) | 27.50 [3.64, 51.36] |
| 3.2 All studies using exoskeleton devices | 5 | 186 | Mean Difference (IV, Random, 95% CI) | ‐15.64 [‐46.34, 15.05] |
| 3.3 All studies using mobile devices | 2 | 56 | Mean Difference (IV, Random, 95% CI) | 20.06 [‐39.52, 79.63] |
| 3.4 All studies using ankle devices | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 8.0 [‐83.03, 99.03] |

