| 1 Cognitive function (change from baseline at 24 weeks) ITT‐LOCF Show forest plot | 1 | | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
|
| 1.1 ADAS‐Cog 13 item | 1 | 262 | Mean Difference (IV, Fixed, 95% CI) | 1.90 [0.51, 3.29] |
| 1.2 NYU Paragraph Test Delayed Recall | 1 | 262 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.41, 1.01] |
| 1.3 NYU Paragraph Test Immediate Recall | 1 | 262 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.11, 1.31] |
| 1.4 WMS (Wechsler) Digit Span Backwards | 1 | 262 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.05, 1.05] |
| 1.5 Symbol Digit Modalities | 1 | 262 | Mean Difference (IV, Fixed, 95% CI) | 1.7 [‐0.11, 3.51] |
| 2 Total number of withdrawals at 24 weeks Show forest plot | 1 | 270 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.37 [1.33, 4.22] |
|
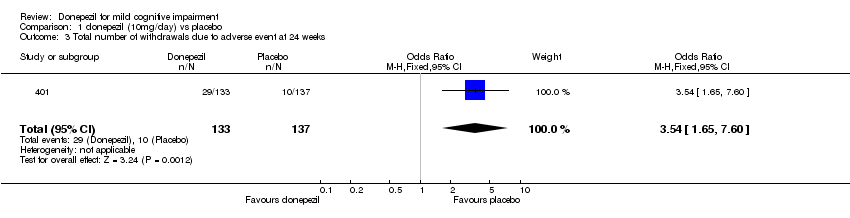
| 3 Total number of withdrawals due to adverse event at 24 weeks Show forest plot | 1 | 270 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.54 [1.65, 7.60] |
|
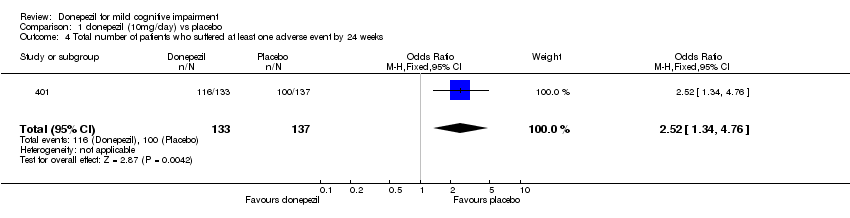
| 4 Total number of patients who suffered at least one adverse event by 24 weeks Show forest plot | 1 | 270 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.52 [1.34, 4.76] |
|
| 5 Total number of patients who suffered at least one adverse event of diarrhoea by 24 weeks Show forest plot | 1 | 270 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.71 [2.23, 9.97] |
|
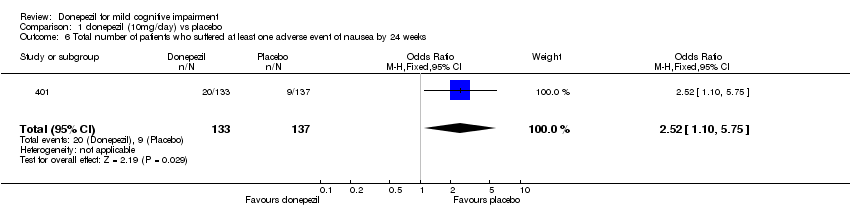
| 6 Total number of patients who suffered at least one adverse event of nausea by 24 weeks Show forest plot | 1 | 270 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.52 [1.10, 5.75] |
|
| 7 Total number of patients who suffered at least one adverse event of vomiting by 24 weeks Show forest plot | 1 | 270 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.30 [2.61, 26.37] |
|
| 8 Total number of patients who suffered at least one adverse event of leg cramps by 24 weeks Show forest plot | 1 | 270 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.63 [1.58, 13.55] |
|
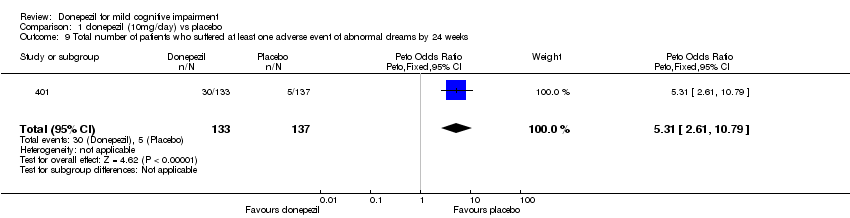
| 9 Total number of patients who suffered at least one adverse event of abnormal dreams by 24 weeks Show forest plot | 1 | 270 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.31 [2.61, 10.79] |
|
| 10 Total number of patients who suffered at least one adverse event of depression by 24 weeks Show forest plot | 1 | 270 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.43 [0.76, 54.11] |
|
| 11 Total number of patients who suffered at least one adverse event of insomnia by 24 weeks Show forest plot | 1 | 270 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.18 [0.85, 5.60] |
|
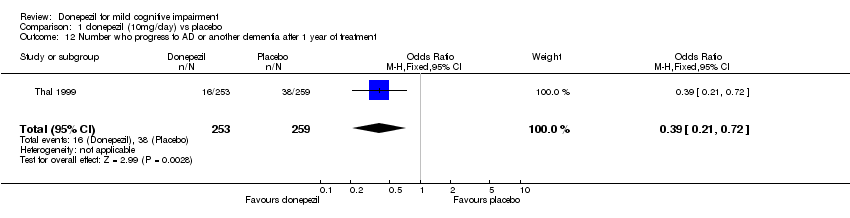
| 12 Number who progress to AD or another dementia after 1 year of treatment Show forest plot | 1 | 512 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.21, 0.72] |
|
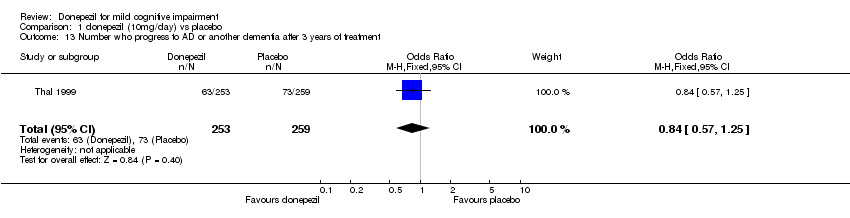
| 13 Number who progress to AD or another dementia after 3 years of treatment Show forest plot | 1 | 512 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.57, 1.25] |
|