Donepezilo para el deterioro cognitivo leve
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | 24‐week, randomized, double‐blind, parallel‐group, placebo‐controlled study | |
| Participants | Country: USA | |
| Interventions | 1. donepezil (10 mg/day) | |
| Outcomes | NYU paragraph test delayed recall | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | 6 month, randomized, double‐blind, parallel‐group, placebo‐controlled study | |
| Participants | Country: USA 15 participants, mean age 71 | |
| Interventions | 1. donepezil (10 mg/day) | |
| Outcomes | MMSE, Hopkins Verbal Learning Test revised | |
| Notes | The main objective was to study cerebral blood flow using MRI and PET scans. | |
| Methods | 3‐year, randomized, double‐blind, parallel‐group, placebo‐controlled study | |
| Participants | Country: USA | |
| Interventions | 1. donepezil (10 mg/day) | |
| Outcomes | time to development of possible or probable AD | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Treatment not blinded. | |
| Patients presented with depression and cognitive impairment. All patients receiving sertraline | |
| Retrospective comparison, treatment not blinded |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | NCT00182845 |
| Methods | |
| Participants | age >65 years |
| Interventions | 1. donepezil |
| Outcomes | post‐operative cognitive decline |
| Starting date | feb 2005 |
| Contact information | Stephanie Munger [email protected] |
| Notes | sponsored by Pfizer |
| Trial name or title | NCT00100022 |
| Methods | |
| Participants | age 45‐90 |
| Interventions | 1. donepezil |
| Outcomes | Cognitive function |
| Starting date | dec 2003 |
| Contact information | |
| Notes | sponsored by Pfizer and Eisai |
| Trial name or title | NCT00042172 |
| Methods | |
| Participants | MCI, but normal ADL |
| Interventions | first 6 months |
| Outcomes | PET scan to assess brain activity during memory tasks |
| Starting date | june 2002 |
| Contact information | University of Iowa Departatment of Psychiatry, Iowa City, Iowa 52242 |
| Notes | sponsored by NIMH |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cognitive function (change from baseline at 24 weeks) ITT‐LOCF Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 donepezil (10mg/day) vs placebo, Outcome 1 Cognitive function (change from baseline at 24 weeks) ITT‐LOCF. | ||||
| 1.1 ADAS‐Cog 13 item | 1 | 262 | Mean Difference (IV, Fixed, 95% CI) | 1.90 [0.51, 3.29] |
| 1.2 NYU Paragraph Test Delayed Recall | 1 | 262 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.41, 1.01] |
| 1.3 NYU Paragraph Test Immediate Recall | 1 | 262 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.11, 1.31] |
| 1.4 WMS (Wechsler) Digit Span Backwards | 1 | 262 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.05, 1.05] |
| 1.5 Symbol Digit Modalities | 1 | 262 | Mean Difference (IV, Fixed, 95% CI) | 1.7 [‐0.11, 3.51] |
| 2 Total number of withdrawals at 24 weeks Show forest plot | 1 | 270 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.37 [1.33, 4.22] |
| Analysis 1.2  Comparison 1 donepezil (10mg/day) vs placebo, Outcome 2 Total number of withdrawals at 24 weeks. | ||||
| 3 Total number of withdrawals due to adverse event at 24 weeks Show forest plot | 1 | 270 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.54 [1.65, 7.60] |
| Analysis 1.3  Comparison 1 donepezil (10mg/day) vs placebo, Outcome 3 Total number of withdrawals due to adverse event at 24 weeks. | ||||
| 4 Total number of patients who suffered at least one adverse event by 24 weeks Show forest plot | 1 | 270 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.52 [1.34, 4.76] |
| Analysis 1.4  Comparison 1 donepezil (10mg/day) vs placebo, Outcome 4 Total number of patients who suffered at least one adverse event by 24 weeks. | ||||
| 5 Total number of patients who suffered at least one adverse event of diarrhoea by 24 weeks Show forest plot | 1 | 270 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.71 [2.23, 9.97] |
| Analysis 1.5  Comparison 1 donepezil (10mg/day) vs placebo, Outcome 5 Total number of patients who suffered at least one adverse event of diarrhoea by 24 weeks. | ||||
| 6 Total number of patients who suffered at least one adverse event of nausea by 24 weeks Show forest plot | 1 | 270 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.52 [1.10, 5.75] |
| Analysis 1.6  Comparison 1 donepezil (10mg/day) vs placebo, Outcome 6 Total number of patients who suffered at least one adverse event of nausea by 24 weeks. | ||||
| 7 Total number of patients who suffered at least one adverse event of vomiting by 24 weeks Show forest plot | 1 | 270 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.30 [2.61, 26.37] |
| Analysis 1.7  Comparison 1 donepezil (10mg/day) vs placebo, Outcome 7 Total number of patients who suffered at least one adverse event of vomiting by 24 weeks. | ||||
| 8 Total number of patients who suffered at least one adverse event of leg cramps by 24 weeks Show forest plot | 1 | 270 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.63 [1.58, 13.55] |
| Analysis 1.8  Comparison 1 donepezil (10mg/day) vs placebo, Outcome 8 Total number of patients who suffered at least one adverse event of leg cramps by 24 weeks. | ||||
| 9 Total number of patients who suffered at least one adverse event of abnormal dreams by 24 weeks Show forest plot | 1 | 270 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.31 [2.61, 10.79] |
| Analysis 1.9  Comparison 1 donepezil (10mg/day) vs placebo, Outcome 9 Total number of patients who suffered at least one adverse event of abnormal dreams by 24 weeks. | ||||
| 10 Total number of patients who suffered at least one adverse event of depression by 24 weeks Show forest plot | 1 | 270 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.43 [0.76, 54.11] |
| Analysis 1.10  Comparison 1 donepezil (10mg/day) vs placebo, Outcome 10 Total number of patients who suffered at least one adverse event of depression by 24 weeks. | ||||
| 11 Total number of patients who suffered at least one adverse event of insomnia by 24 weeks Show forest plot | 1 | 270 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.18 [0.85, 5.60] |
| Analysis 1.11  Comparison 1 donepezil (10mg/day) vs placebo, Outcome 11 Total number of patients who suffered at least one adverse event of insomnia by 24 weeks. | ||||
| 12 Number who progress to AD or another dementia after 1 year of treatment Show forest plot | 1 | 512 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.21, 0.72] |
| Analysis 1.12  Comparison 1 donepezil (10mg/day) vs placebo, Outcome 12 Number who progress to AD or another dementia after 1 year of treatment. | ||||
| 13 Number who progress to AD or another dementia after 3 years of treatment Show forest plot | 1 | 512 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.57, 1.25] |
| Analysis 1.13  Comparison 1 donepezil (10mg/day) vs placebo, Outcome 13 Number who progress to AD or another dementia after 3 years of treatment. | ||||

Comparison 1 donepezil (10mg/day) vs placebo, Outcome 1 Cognitive function (change from baseline at 24 weeks) ITT‐LOCF.

Comparison 1 donepezil (10mg/day) vs placebo, Outcome 2 Total number of withdrawals at 24 weeks.
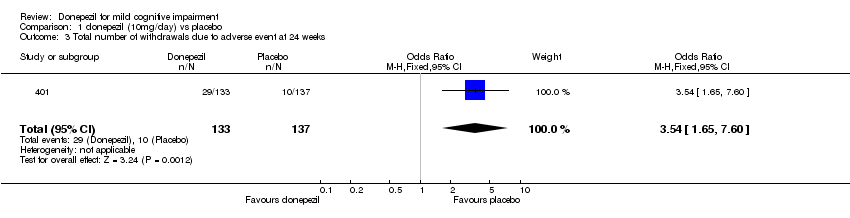
Comparison 1 donepezil (10mg/day) vs placebo, Outcome 3 Total number of withdrawals due to adverse event at 24 weeks.
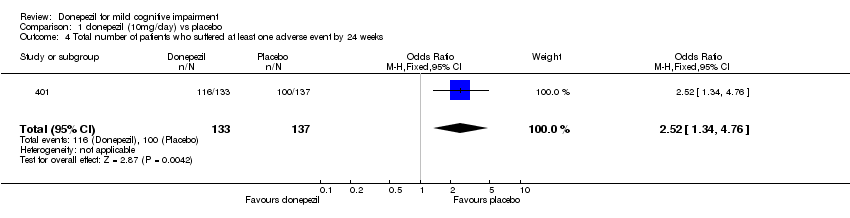
Comparison 1 donepezil (10mg/day) vs placebo, Outcome 4 Total number of patients who suffered at least one adverse event by 24 weeks.

Comparison 1 donepezil (10mg/day) vs placebo, Outcome 5 Total number of patients who suffered at least one adverse event of diarrhoea by 24 weeks.
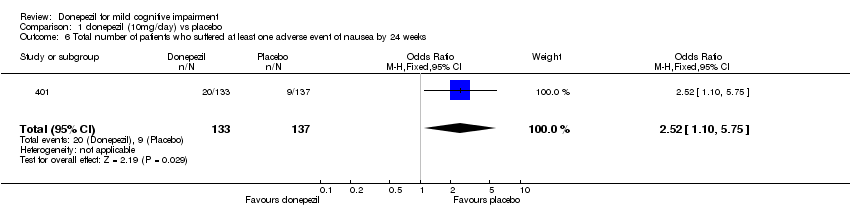
Comparison 1 donepezil (10mg/day) vs placebo, Outcome 6 Total number of patients who suffered at least one adverse event of nausea by 24 weeks.

Comparison 1 donepezil (10mg/day) vs placebo, Outcome 7 Total number of patients who suffered at least one adverse event of vomiting by 24 weeks.

Comparison 1 donepezil (10mg/day) vs placebo, Outcome 8 Total number of patients who suffered at least one adverse event of leg cramps by 24 weeks.
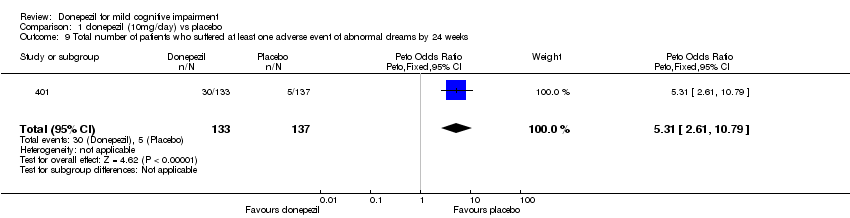
Comparison 1 donepezil (10mg/day) vs placebo, Outcome 9 Total number of patients who suffered at least one adverse event of abnormal dreams by 24 weeks.

Comparison 1 donepezil (10mg/day) vs placebo, Outcome 10 Total number of patients who suffered at least one adverse event of depression by 24 weeks.

Comparison 1 donepezil (10mg/day) vs placebo, Outcome 11 Total number of patients who suffered at least one adverse event of insomnia by 24 weeks.
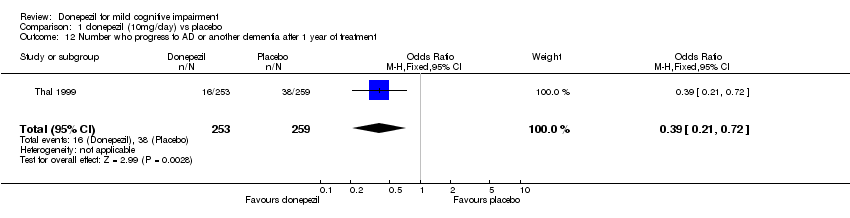
Comparison 1 donepezil (10mg/day) vs placebo, Outcome 12 Number who progress to AD or another dementia after 1 year of treatment.
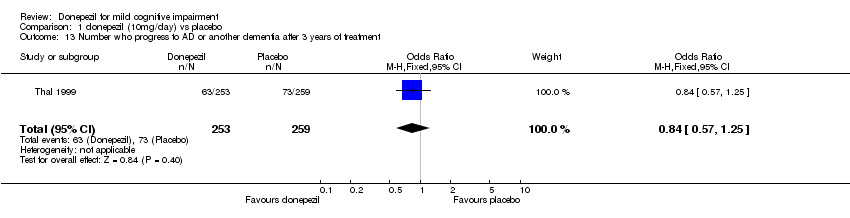
Comparison 1 donepezil (10mg/day) vs placebo, Outcome 13 Number who progress to AD or another dementia after 3 years of treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cognitive function (change from baseline at 24 weeks) ITT‐LOCF Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 ADAS‐Cog 13 item | 1 | 262 | Mean Difference (IV, Fixed, 95% CI) | 1.90 [0.51, 3.29] |
| 1.2 NYU Paragraph Test Delayed Recall | 1 | 262 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.41, 1.01] |
| 1.3 NYU Paragraph Test Immediate Recall | 1 | 262 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.11, 1.31] |
| 1.4 WMS (Wechsler) Digit Span Backwards | 1 | 262 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.05, 1.05] |
| 1.5 Symbol Digit Modalities | 1 | 262 | Mean Difference (IV, Fixed, 95% CI) | 1.7 [‐0.11, 3.51] |
| 2 Total number of withdrawals at 24 weeks Show forest plot | 1 | 270 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.37 [1.33, 4.22] |
| 3 Total number of withdrawals due to adverse event at 24 weeks Show forest plot | 1 | 270 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.54 [1.65, 7.60] |
| 4 Total number of patients who suffered at least one adverse event by 24 weeks Show forest plot | 1 | 270 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.52 [1.34, 4.76] |
| 5 Total number of patients who suffered at least one adverse event of diarrhoea by 24 weeks Show forest plot | 1 | 270 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.71 [2.23, 9.97] |
| 6 Total number of patients who suffered at least one adverse event of nausea by 24 weeks Show forest plot | 1 | 270 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.52 [1.10, 5.75] |
| 7 Total number of patients who suffered at least one adverse event of vomiting by 24 weeks Show forest plot | 1 | 270 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.30 [2.61, 26.37] |
| 8 Total number of patients who suffered at least one adverse event of leg cramps by 24 weeks Show forest plot | 1 | 270 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.63 [1.58, 13.55] |
| 9 Total number of patients who suffered at least one adverse event of abnormal dreams by 24 weeks Show forest plot | 1 | 270 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.31 [2.61, 10.79] |
| 10 Total number of patients who suffered at least one adverse event of depression by 24 weeks Show forest plot | 1 | 270 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.43 [0.76, 54.11] |
| 11 Total number of patients who suffered at least one adverse event of insomnia by 24 weeks Show forest plot | 1 | 270 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.18 [0.85, 5.60] |
| 12 Number who progress to AD or another dementia after 1 year of treatment Show forest plot | 1 | 512 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.21, 0.72] |
| 13 Number who progress to AD or another dementia after 3 years of treatment Show forest plot | 1 | 512 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.57, 1.25] |

