Intervenciones psicosociales para mujeres embarazadas en programas ambulatorios de tratamiento de drogas ilegales en comparación con otras intervenciones
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | RCT. | |
| Participants | 20 pregnant women enrolled in methadone maintenance. Mean age 27.6; 78.6% non‐minority (11/14); 78.6% single; 100% unemployed; 8(± 6) weeks gestational age upon entry into MMT; 2.7 mean days cocaine use in past 30 days. Exclusion: > 28 weeks pregnant. | |
| Interventions | Daily MMT, weekly group counselling, three times/week urine toxicology screening for all participants.
No difference between groups in terms of MMT dose (mean 50 mg). | |
| Outcomes | Attendance was measured in terms of % number of groups attended. Infant outcomes measured as mean gestational age at delivery, mean weight, and mean number of days in the hospital. Urine toxicology was measure as % positive for cocaine, opiates, or other drugs. | |
| Notes | Unable to measure retention as not reported. We attempted to contact trial authors but data was unavailable. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "a total of 20 women provided informed consent and were randomly enrolled…" No details were provided related to randomization methods. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Incomplete outcome data (attrition bias) | High risk | "A total of 20 women provided informed consent and were randomly enrolled. Of these, one delivered within 1 week of providing consent, one was hospitalized for sedative detoxification, and four, all of whom had been on the methadone programme for several months or years when they became pregnant, did not participate in any groups or study assessments and were considered dropouts." 20 patients randomized and only 14 analysed. 6 dropouts (unclear from which randomized groups). Exclusions in analysis were lost to follow‐up because they did not attend meetings or because of early labour. These are all possible outcomes of the review and their exclusion biases results. Also analysis was per protocol not ITT. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No references to outcome assessor blinding made. |
| Methods | RCT. | |
| Participants | 12 cocaine‐dependent pregnant women: 58% African American, 8% Hispanic, 33% White; 92% never married or separated/widowed/divorced; 75% high school; 83% unemployed; 67% gravida 5 or more, 25% gravida 3 to 4; 75% in second trimester; DSM‐III‐R: 92% (10/11) cocaine primary drug of abuse, 8% (1/11) both heroin and cocaine dependent. Exclusion: Cessation of cocaine use greater than 30 days prior to enrolment. | |
| Interventions | All received PNC, behaviourally‐based drug counselling, nutritional education, and HIV counselling.
| |
| Outcomes | Retention in treatment as number remaining in treatment. Abstinence as average of individual %. Attendance in PNC as number of visits. Perinatal outcomes as number of preterm labour, or preterm delivery, or both. ASI composite scores presented as a bar graph. All outcomes reported as chi square with P value only when significant. | |
| Notes | Treatment facility provided free transportation and child care. We attempted to contact trial authors but received no response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Following stratification on referral source (self vs. court or probation or parole), subjects were randomly assigned to one of two treatment groups…" No details provided related to randomization methods. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Incomplete outcome data (attrition bias) | Low risk | Attrition details outlined for the 12 women, all 12 carried throughout the analysis. Results have detailed participant numbers for each outcome. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No references to outcome assessor blinding made. |
| Methods | RCT. Groups significantly different for race. No difference in drug use and psychiatric comorbidity. | |
| Participants | 77 pregnant opioid‐dependent women randomized with 14 disqualified post‐randomization, ≤ 26 weeks gestational age, receiving MMT and ≥5 cigarettes/day. Mean age 29.7; 84% African American; 79% single or never married; 97% unemployed; 94% less than high school education. DSM‐III‐R: all heroin dependent (100%), 41 (35%) cocaine dependent, 10 (16%) marijuana dependent, 17 (27%) alcohol dependent, all (100%) nicotine dependent. Exclusion criteria: not stated. | |
| Interventions | For all MMT, no information on urine monitoring, or counselling/therapy. All received USD 10 voucher after initial battery and USD20 when 10‐week interview completed. Mean MMT dose 65.2 mg. All received PNC and substance abuse counselling ‐ no details described.
Duration 10 weeks. | |
| Outcomes | Retention in treatment as % attrition. Stage of change and stage movement. Urine toxicology done at the 10‐week follow‐up. | |
| Notes | Randomization occurred during residential treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Seven women refused study participation and 77 patients completed baseline assessment and were randomized". No specific methods for randomization outlined. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Incomplete outcome data (attrition bias) | High risk | "At the 10‐week follow‐up, participant attrition was 14% (n = 9), with missing participants evenly distributed between the MET (n = 4) and SC (n = 5) groups. The only difference between completers and those lost at follow‐up was average methadone dose during treatment (M=50.8mg vs. 36.3 mg, respectively), t(61)=‐6.34,p<.0001." Some information was provided related to attrition (total number of individuals who dropped out). Outcome measures did not explicitly state the number of participants analyses were based on. Furthermore, details were not provided related to which groups the initially disqualified participants came from, making attrition data more unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No mention of blinding of outcome assessors. |
| Methods | RCT. | |
| Participants | 93 pregnant women enrolled in substance use treatment, 18 years of age or older, meeting DSM‐III‐R criteria. Average age 28.9; 88% African American; 75% single; 50% less than HS education; 97% unemployed; DSM‐III‐R: 75% cocaine dependence, 74% opiate dependence, 50% both; 25 women (27%) received MMT. Exclusion criteria: not stated. | |
| Interventions | For all participants, treatment services included a 7‐day residential stay followed by a 30‐day intensive treatment (7 days/week, 6.5 hours/day). For individuals receiving MMT, no information on doses.
For MMT subgroup: could receive USD5 for each negative urine and USD25 or USD50 bonus for 5 or 7 days drug‐free urine, and additional USD20 if completed all urine samplings and or attendance card monitoring. For non MMT: USD5 each day attended at least 4 hours treatment, USD25 or USD50 bonus for attending 5 to 6 or 7 days. (n = 40) | |
| Outcomes | Attendance was measured in days and hours. Urine toxicology was not reported. Retention or loss to follow‐up was not reported. | |
| Notes | Transportation and childcare provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "On admission, both MM and AT subjects were recruited and randomly assigned to one of two incentive conditions…" No explicit methods of randomization outlined. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | No details related to attrition provided. No detailed results included, unclear which participant numbers outcomes are based on. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No references to outcome assessor blinding made. |
| Methods | RCT. | |
| Participants | 80 pregnant women on MMT, greater than 18 years of age, meeting DSM‐II‐R criteria for opiate dependence with cocaine abuse, admitted for first time for substance abuse treatment. Mean age 28; mean gestational age 23.4; 96% unemployed; 85% single/never married; 76% African American; 20% chronic medical conditions (HTN, DM, HIV); DSM‐III‐R: 100% opiate dependent, 69% cocaine, 5% marijuana, 10% alcohol. | |
| Interventions | For all participants treatment consisted of a 7‐day residential followed by 7 days of intensive outpatient (7 days/week, 6.5 hours/day). Treatment consisted of group counselling and at least once a week individual psychotherapy. All received MMT, mean dose 42.
Duration 14 days. | |
| Outcomes | Attendance was measured as mean full day attendance as well as "perfect treatment attendance" defined as attendance on at least 13 or 14 full days of treatment. Retention was measured as the % drop out. Urine samples were collected daily from days 8 to 14 and reported as % positive. | |
| Notes | Transportation, child care, on site PNC and psychiatric consultation provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization procedure involved patients selecting one of two different color chips from a hat with replacement following each selection…" Specific process outlined that provides participants with equal chance of assignment. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Incomplete outcome data (attrition bias) | Low risk | Attrition data specified and descriptions of different analytic methods to account for missing data outlined. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No references to outcome assessor blinding made. |
| Methods | RCT. | |
| Participants | 89 women at least 18 years old, with a single fetus, self‐reported heroin or cocaine use in the past 30 days, completion of a 7‐night inpatient stay on an assisted living unit and willingness to live in recovery housing or other drug‐free housing. | |
| Interventions | Both groups received usual care involving group and individual counseling and psychoeducation, medically‐assisted withdrawal for patients either refusing methadone maintenance or not meeting current opioid dependence criteria, methadone maintenance for qualifying opioid‐dependent patients, care management, obstetrical care, psychiatric evaluation and treatment, general medical management.
| |
| Outcomes | One month post‐randomization treatment outcomes included days retained in CAP treatment before delivery. Measures at baseline and 1 month after: number of days in recovery housing; heroin and cocaine use; employment status; illegal activity. Maternal and neonatal outcomes at delivery: Maternal: enrolment at CAP at delivery; urine screening positive for any illegal drug at delivery. Neonatal outcomes: estimated gestational age at delivery; prematurity (< 37 weeks); birth weight, number of days of hospitalization after birth. | |
| Notes | On‐site child care and paediatric care also provided to all participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random assignment of participants to one of the two treatment conditions was performed by a staff member with no participant contact who generated a random condition assignment with a computer program…" Type of computer programme is unknown, but was likely sufficient for randomization. |
| Allocation concealment (selection bias) | Low risk | "random assignment of participants to one of the two treatment conditions was performed by a staff member with no participant contact who generated a random condition assignment with a computer program…" |
| Incomplete outcome data (attrition bias) | Low risk | From caption of Figure 1: "All analyses reported in this paper are based on the data from these 89 participants and their respective 89 neonates". Detailed attrition data with flowchart. 89 patients randomized and 89 patients data at follow‐up. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No mention of blinding of outcome assessors. |
| Methods | RCT. | |
| Participants | 71 pregnant women who used illicit drugs in pregnancy, aged 18 years or older. (1) 35 (2) 36. Average age 27.1; 73% single/never married; 88% unemployed; 82% receiving government income; 32% African American, 50% Caucasian; DSM‐III‐R: 49% cocaine dependent, 28% marijuana, 4% alcohol. Exclusion: no obvious impairment (acute psychosis or organic illness). | |
| Interventions | For all participants, substance treatment counselling provided. Nature of counselling not elaborated.
Study duration 2 months. | |
| Outcomes | Attendance reported as number and % attended. Urine was screened at intake and then randomly once per week and were reported as a mean proportion of negative screens. | |
| Notes | Gender‐specific treatment centre. Transportation and childcare included. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | We used a stratified random sample procedure based on ethnicity and drug of choice (cocaine/crack cocaine, marijuana, amphetamine/methamphetamine, alcohol, or PCP) to assign participants to conditions. Comment: No specific descriptions of randomization methods were made. |
| Allocation concealment (selection bias) | Unclear risk | No references to allocation concealment procedures made. |
| Incomplete outcome data (attrition bias) | Unclear risk | Specific attrition data was not included. Some information related to number of sessions attended and no‐show rates for specific sessions were detailed, but overall study attrition was not outlined clearly. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No mention of blinding of outcome assessors. |
| Methods | RCT. | |
| Participants | 92 pregnant women enrolled in MMT who injected drugs. Mean age 26.2; mean years education 10.2; 53% ever sex worker; mean gestational age 22 weeks; DSM‐III‐R: 85% opiate dependent, 15% cocaine, 59% marijuana, 32% alcohol, 98% nicotine. | |
| Interventions | All participants received MMT (mean methadone dose 49 mg) and counselling about HIV risk.
Duration 9 months. | |
| Outcomes | Retention was measured as a proportion. Attendance was measured as the average number of missed appointments. | |
| Notes | Researchers attempted to contact patients lost to follow‐up through the Department of Social Security and Department of Health. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study states that subjects were randomly allocated to the intervention or control group, but does not outline specific randomization methods. |
| Allocation concealment (selection bias) | Unclear risk | No specific references to allocation concealment methods were made. |
| Incomplete outcome data (attrition bias) | Unclear risk | "Five subjects dropped out of the intervention condition; three before any sessions and four after two or more sessions. There were no differences in any of the variables (demographic, drug use or HIV‐risk taking behaviour) between those who remained in the study and those who dropped out. There were 40 subjects in each group at the post‐intervention assessment. Of these 80 subjects, 74 (92.5%) were contactable 9 months later. One refused to participate further, giving a 91% follow‐up rate of those who complete pre‐ and post‐intervention assessments." Attrition information provided, but differences between the intervention and control group not detailed. In addition, specific participant numbers for different outcomes not stated. |
| Blinding of outcome assessment (detection bias) | Low risk | "Assessments occurred at pre‐intervention, post‐intervention and at 9‐month follow‐up. Follow‐up assessments were conducted by an interviewer blind to the subject’s group membership." Specific references to the blinding of the interviewer conducting follow‐up assessments makes the risk of detection bias low. |
| Methods | RCT. | |
| Participants | 40 pregnant, unemployed, women 18 to 50 years old on MMT, and with positive urine toxicology for opiates within 6 weeks prior to enrolment. Mean age 32; 83% African America; 65% HS or greater education; 7.5% married; 100% unemployed; 100% used cocaine; 75% used cocaine. | |
| Interventions | For all participants, substance abuse counselling and MMT provided. Details of counselling not given. No mention of MMT doses or schedule.
Duration 24 weeks. | |
| Outcomes | Retention in treatment defined as remaining in the study through 24 weeks and reported as N and %. Urine toxicology reported as % negative over total study period for each group, and reported as overall positive and drug‐specific positive. Attendance in Therapeutic Workplace was calculated and presented in a bar graph for each woman. | |
| Notes | Transportation and childcare provided at no cost. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A modified dynamic balanced randomization was used to randomize patients sequentially to the treatment conditions." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Incomplete outcome data (attrition bias) | Low risk | Attrition details outlined, reasons for leaving also outlined. Different methods utilized to attempt to account for missing data. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No references to outcome assessor blinding made. |
| Methods | RCT. Unable to assess baseline difference between groups as results reported in terms of MMT vs. not MMT. | |
| Participants | 142 pregnant women in a comprehensive substance abuse treatment programme, > 18 years of age, and who met DSM‐III‐R criteria for opiate or cocaine dependence, or both. Mean age 28.4; mean years of education 11; estimated gestational age 22 weeks; 53(70%) unemployed; 81.4% single/never married; DSM‐III‐R: 79.6% used cocaine; 83.8% used cocaine. | |
| Interventions | For all participants, treatment consisted of 7 days residential care followed by 30 days of day treatment (7 days/week, 6.5 hours/day). Group counselling with once/week individual counselling. Obstetrics/Gynecology services on site. For individuals on MMT, no mention of doses.
Duration 30 days. | |
| Outcomes | Retention in treatment defined as remaining in treatment for 30 days and reported as number of days. Mean number of days also calculated. | |
| Notes | Results were not reported in terms of intervention vs. control group, rather in terms of MMT vs. not MMT. All individuals were randomized to USD0, USD1, USD5 or USD10 group, however USD0 and USD1 were grouped together as "control". Only USD5 and USD10 groups were analyzed as "intervention". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects were told that within 24 h prior to transfer from residential to IDT, they would be randomly assigned to one of the four incentive groups…" No description of specific randomization process used. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | Only breakdown of methadone vs. non‐methadone maintained provided, limited information related to incentive condition assigned. Presumably data is for all randomized individuals, given fact that retention and attendance are primary outcomes, unclear if this impacts outcomes. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No references to outcome assessor blinding made. |
| Methods | RCT. | |
| Participants | 91 pregnant drug‐dependent women enrolled in comprehensive drug use treatment programme ‐ 18 years of age or older who met the DSM‐III‐R criteria for opiate, or cocaine dependence, or both. Exclusion: Received methadone pharmacotherapy, delivered prematurely or aborted during study period, were administratively discharged from study, had extended or reduced residential stays, had acute psychiatric distress that prohibited study participation. Mostly in early 30s, 74% single/never married, 84% African American, and 95% unemployed, mean education of 11.2 years (SD = 1.9). 79.0% cocaine dependence, 37.1% opiate dependence, 17.7% alcohol dependence, and 17.7% cannabis dependence. | |
| Interventions | For all participants, treatment included 7 days of residential care followed by 30 days intensive outpatient care.
| |
| Outcomes | Rate of premature residential treatment dropout against medical advice (AMA). Length of stay in treatment. Mean days prior to AMA. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Those providing informed consent were randomly assigned to either standard care or an escalating voucher schedule.." No specific description of randomization process used. |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment. |
| Incomplete outcome data (attrition bias) | Low risk | As attrition is a primary outcome, incomplete data does not seem to be an issue. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome assessors not mentioned. |
| Methods | RCT. | |
| Participants | 222 pregnant women provided written consent, but 143 were randomized. Pregnant women with an estimated gestational age of < 28 weeks who were opioid dependent and methadone stabilized. Average of 30.0 years old (SD = 5.2), 71.4% African American, 69.9% never married, 11.6 (SD = 1.5) mean years of education, 6.0% currently employed. Exclusion: not receiving methadone maintenance, non‐compliant with study or CAP procedures, had a miscarriage or terminated the pregnancy, transferred programmes, or had a negative pregnancy test. | |
| Interventions |
Study duration was 13 weeks or until delivery with 1 week of inpatient treatment (when participants could earn two vouchers and 12 outpatient weeks during which participants could earn three vouchers weekly). | |
| Outcomes | Drug abstinence (number of urine screening tests negative for both opiates and cocaine, number of negative urine tests prior to the first positive test and longest consecutive number of negative urine tests), opioid use (with similar parameters as drug abstinence), cocaine use (with similar parameters). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "participants were randomly assigned to one of the treatment conditions within 5 days of program admission…" No specific description of randomization used. |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment processes. |
| Incomplete outcome data (attrition bias) | Unclear risk | "Ten cases were missing data on one or more of the concomitant variables so were dropped from the sample, reducing the final sample to 133 cases." Some information about attrition given, but breakdown of assignment groups and attrition was not provided. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No mention of outcome assessor blinding. |
| Methods | RCT. | |
| Participants | 200 women participating in the pregnant women treatment programmes at the four participating community treatment programmes (CTPs). At least 18 years of age and pregnant. Identified as needing substance use treatment via the CTP's usual screening procedure. Participants mostly unmarried, unemployed, and had, on average, high school education. Sample was fairly diverse in terms of race and ethnicity. Significant baseline differences between the intervention and control participants. Intervention condition had significantly older participants, significantly more minority participants, with significantly more years of education, and with significantly more participants with cocaine as their primary drug of choice (marijuana was primary drug of choice in the control group). Exclusion: not pregnant, did not need to enter substance abuse treatment, under 18, not interested in participating, had unstable living arrangements, had plans to relocate within 4 months, pending legal charges, required inpatient treatment, suicidal/homicidal risks, more than 32 weeks pregnant. | |
| Interventions |
Active study phase was four weeks (three sessions of MET or TAU provided in the 28 day period). | |
| Outcomes | Treatment utilization, defined as ratio of number of outpatient treatment hours attended to the number of hours scheduled. Number of weeks in which at least one treatment session was attended. Number of weeks until treatment dropout, dropout defined as failure to attend any treatment provided by the CTP for three consecutive weeks. Substance use measured by self‐report and qualitative toxicology results. | |
| Notes | Participants in both conditions were encouraged to participate in other treatment services offered by the CTP (group treatment, case management). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Urn randomization to balance three dichotomous variables: pressure to attend treatment, self report of drug and alcohol use and need for methadone maintenance". Unclear what type of urn randomization was used, but method likely adequate. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Incomplete outcome data (attrition bias) | Low risk | Attrition information provided (e.g. 200 randomized, 162 completed the 1‐month active phase) and statistical analyses related to attrition showed no difference between intervention and control group (P > 0.5). |
| Blinding of outcome assessment (detection bias) | Unclear risk | No references to outcome assessor blinding made. |
| Methods | RCT. | |
| Participants | 183 women attending two hospital‐based reproductive health clinics in Connecticut between June 2006 and July 2010. Women met inclusion criteria if they: were aged > 16 years of age, were fluent in English or Spanish, had not yet completed 28 weeks of pregnancy at screening, were planning to deliver at a collaborating hospital, and using alcohol or an illicit drug other than opiates during the 28 days prior to screening or scored at least a "3" on the modified TWEAK survey. Women were excluded if they: were already engaged in substance use treatment, endorsed nicotine or opiates as their only substance, had plans to relocate, were not willing to provide consent, were an imminent danger to themselves or their fetus, or if they required inpatient general medical or psychiatric treatment. | |
| Interventions | Women were randomized to one of two groups:
| |
| Outcomes | Outcomes were assessed as measured at intake, delivery, and 3 months post‐delivery. The primary outcome was the percentage of days of any alcohol or drug use in the prior 28 days. Secondary outcomes measured abstinence from substances (alcohol and drugs) according to self‐report, urine toxicology and combined self‐report and urine. Birth outcomes were also analyzed. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated block randomization used. |
| Allocation concealment (selection bias) | Low risk | A statistician or project member who had no direct contact with subjects maintained allocation. |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis not performed. Women were excluded from analysis if there were no follow‐up assessments and if there were early deliveries. |
| Blinding of outcome assessment (detection bias) | Low risk | Urine toxicology used. |
CBT = Cognitive behavioural therapy; MET = Motivational enhancement therapy; MI = Motivational interviewing; MMT = Methadone maintenance therapy; OB/Gyn = Obstetrical/gynecological;
BA = brief advice therapy.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Inappropriate study design: a systematic review. | |
| Inappropriate study design: review article. | |
| Inappropriate study design: review article. | |
| Inappropriate study design: matched case control, not randomized. | |
| Uses Winhusen 2008 data and looks at consecutive weeks attended (as proxy for retention). | |
| Inappropriate study design: not randomized. | |
| Excluded as the type of participants were not in the inclusion criteria: alcohol dependent, not illicit drug‐using. | |
| Inappropriate study design: retrospective cohort. | |
| Inappropriate study design: not randomized; and no intervention was analysed. | |
| Inappropriate study design: not a RCT; and because there was no intervention studies. This article concerned the development of a Quality of Life index. | |
| Inappropriate study design: retrospective case review. | |
| Excluded as the intervention was not within the scope of the review: comparison of methadone trough levels between symptomatic and asymptomatic women. | |
| Inappropriate study design: cohort study with non‐perinatal controls. | |
| Inappropriate study design: cohort study. | |
| Inappropriate study design: cohort study. | |
| Uses Winhusen 2008 data and looks at impact of therapist on abstinence. | |
| Inappropriate study design: review article. | |
| Excluded as the intervention was not in scope of the review: pharmacological intervention. | |
| Inappropriate study design: retrospective cohort. | |
| Excluded as the study design and participants were not in the scope of the review: cohort study with comparison group and the participants were infants with perinatal illicit drug exposure. | |
| Excluded as the study design and intervention were not in the scope of the review: review article. | |
| Excluded as the study design and intervention were not in the scope of the review: Cochrane review. | |
| Excluded as the study design and intervention were not in the scope of the review: case series evaluating a pharmacological intervention. | |
| Inappropriate study design: retrospective case series. | |
| Inappropriate study design: cohort study. | |
| Inappropriate study design: cohort study. | |
| Excluded as the study participants were not in the scope of the review: targets male partners of pregnant women. | |
| Inappropriate study design: cohort study. | |
| Uses Winhusen 2008 data with birth outcomes, but does not stratify. | |
| Inappropriate study design: case series. | |
| Excluded as the study participants and intervention were not in the scope of the review: postpartum patients and no psychosocial intervention. | |
| Excluded as the study participants were not in the scope of the review: postpartum. | |
| Excluded as the study participants were not in the scope of the review: targeted postpartum women. | |
| Uses Winhusen 2008 and looks at impact of baseline motivation on abstinence. | |
| Inappropriate study design: review article. | |
| Inappropriate study design. | |
| Excluded as no data related to pregnant women was specifically referenced and data could not be stratified. | |
| Inappropriate study design: cohort study. | |
| Inappropriate study design: cohort study. | |
| Inappropriate study design: cohort study. | |
| Inappropriate study design: cohort study. | |
| Excluded as citation was only for oral session. | |
| Analyzes cigarette smoking and treatment for cigarette use (no an illicit substance). | |
| Inappropriate study design: case series. | |
| Inappropriate study design: cohort study with historical control. | |
| Inappropriate study design: case series. | |
| Inappropriate study design: cohort study. | |
| Inappropriate study design: review article. | |
| Review article comparing a pilot study and a RCT. | |
| Inappropriate study design: review article. | |
| Inappropriate study design: cohort study. | |
| Inappropriate study design: cohort study. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Preterm birth (< 37 weeks gestation) Show forest plot | 3 | 264 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.34, 1.51] |
| Analysis 1.1  Comparison 1 Any psychosocial intervention vs. control: neonatal outcomes, Outcome 1 Preterm birth (< 37 weeks gestation). | ||||
| 2 Positive neonatal toxicology at delivery (any drug) Show forest plot | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [0.86, 4.24] |
| Analysis 1.2  Comparison 1 Any psychosocial intervention vs. control: neonatal outcomes, Outcome 2 Positive neonatal toxicology at delivery (any drug). | ||||
| 3 Low birth weight (< 2500 g) Show forest plot | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.36, 1.43] |
| Analysis 1.3  Comparison 1 Any psychosocial intervention vs. control: neonatal outcomes, Outcome 3 Low birth weight (< 2500 g). | ||||
| 4 Days hospitalized after delivery Show forest plot | 2 | 103 | Mean Difference (IV, Random, 95% CI) | ‐1.27 [‐2.52, ‐0.03] |
| Analysis 1.4  Comparison 1 Any psychosocial intervention vs. control: neonatal outcomes, Outcome 4 Days hospitalized after delivery. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Positive urine drug test (end of treatment) Show forest plot | 3 | 367 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.75, 1.73] |
| Analysis 2.1  Comparison 2 Any psychosocial intervention vs. control: maternal outcomes, Outcome 1 Positive urine drug test (end of treatment). | ||||
| 2 Positive urine at 1 month+ Show forest plot | 1 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.55, 2.31] |
| Analysis 2.2  Comparison 2 Any psychosocial intervention vs. control: maternal outcomes, Outcome 2 Positive urine at 1 month+. | ||||
| 3 Positive urine at delivery Show forest plot | 2 | 217 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.52, 2.65] |
| Analysis 2.3  Comparison 2 Any psychosocial intervention vs. control: maternal outcomes, Outcome 3 Positive urine at delivery. | ||||
| 4 Retention at treatment completion Show forest plot | 9 | 743 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.93, 1.06] |
| Analysis 2.4  Comparison 2 Any psychosocial intervention vs. control: maternal outcomes, Outcome 4 Retention at treatment completion. | ||||
| 5 Short term treatment retention Show forest plot | 6 | 514 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.90, 1.10] |
| Analysis 2.5  Comparison 2 Any psychosocial intervention vs. control: maternal outcomes, Outcome 5 Short term treatment retention. | ||||
| 6 Retention in treatment at delivery Show forest plot | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.50, 1.88] |
| Analysis 2.6  Comparison 2 Any psychosocial intervention vs. control: maternal outcomes, Outcome 6 Retention in treatment at delivery. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Positive urine drug test (end of treatment) Show forest plot | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [0.86, 4.24] |
| Analysis 3.1  Comparison 3 CM vs. control: maternal outcomes, Outcome 1 Positive urine drug test (end of treatment). | ||||
| 2 Positive urine at delivery Show forest plot | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [0.86, 4.24] |
| Analysis 3.2  Comparison 3 CM vs. control: maternal outcomes, Outcome 2 Positive urine at delivery. | ||||
| 3 Retention at treatment completion Show forest plot | 6 | 388 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.92, 1.16] |
| Analysis 3.3  Comparison 3 CM vs. control: maternal outcomes, Outcome 3 Retention at treatment completion. | ||||
| 4 Short term treatment retention Show forest plot | 2 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.70, 1.73] |
| Analysis 3.4  Comparison 3 CM vs. control: maternal outcomes, Outcome 4 Short term treatment retention. | ||||
| 5 Retention at delivery Show forest plot | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.50, 1.88] |
| Analysis 3.5  Comparison 3 CM vs. control: maternal outcomes, Outcome 5 Retention at delivery. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Positive urine drug test (end of treatment) Show forest plot | 2 | 278 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.63, 1.48] |
| Analysis 4.1  Comparison 4 MIB vs. control: maternal outcomes, Outcome 1 Positive urine drug test (end of treatment). | ||||
| 2 Positive urine drug test at three months (follow‐up) Show forest plot | 1 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.55, 2.31] |
| Analysis 4.2  Comparison 4 MIB vs. control: maternal outcomes, Outcome 2 Positive urine drug test at three months (follow‐up). | ||||
| 3 Positive urine at delivery Show forest plot | 1 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.57, 1.24] |
| Analysis 4.3  Comparison 4 MIB vs. control: maternal outcomes, Outcome 3 Positive urine at delivery. | ||||
| 4 Retention at treatment completion Show forest plot | 3 | 355 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.89, 1.06] |
| Analysis 4.4  Comparison 4 MIB vs. control: maternal outcomes, Outcome 4 Retention at treatment completion. | ||||
| 5 Short term treatment retention Show forest plot | 3 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.88, 1.12] |
| Analysis 4.5  Comparison 4 MIB vs. control: maternal outcomes, Outcome 5 Short term treatment retention. | ||||
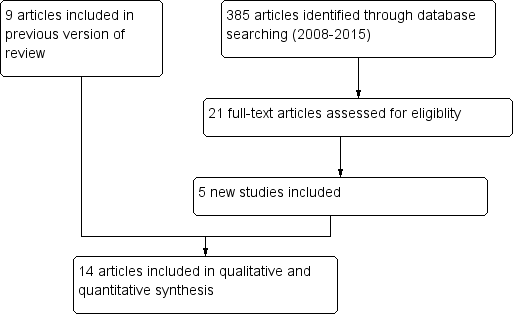
Study flow diagram.
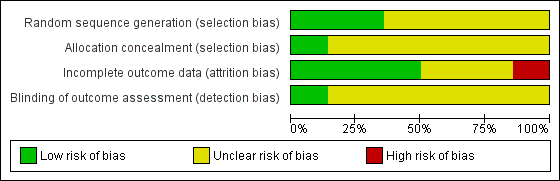
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
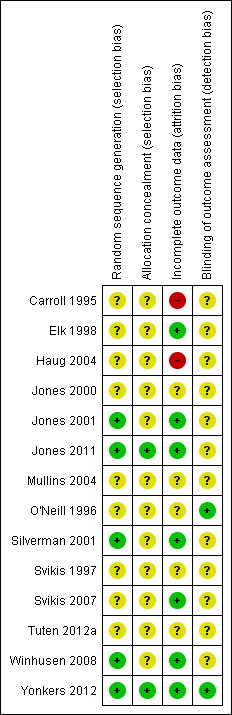
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
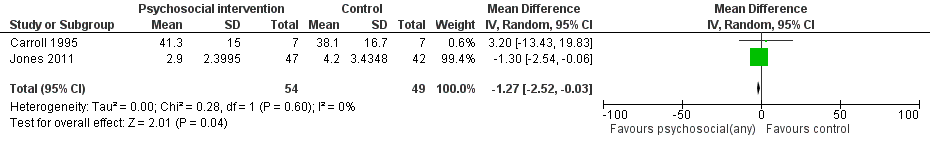
Forest plot of comparison: 1 Neonatal outcomes any psychosocial intervention vs. control, outcome: 1.5 Mean days hospitalized after delivery.
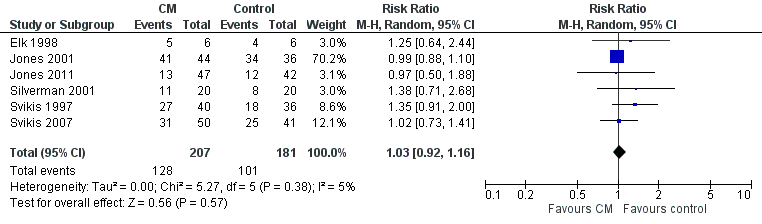
Forest plot of comparison: 2 CM vs. control, outcome: 3.1 Retention in treatment at the end of study.

Comparison 1 Any psychosocial intervention vs. control: neonatal outcomes, Outcome 1 Preterm birth (< 37 weeks gestation).

Comparison 1 Any psychosocial intervention vs. control: neonatal outcomes, Outcome 2 Positive neonatal toxicology at delivery (any drug).
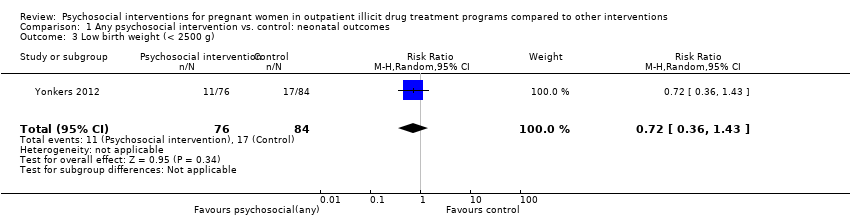
Comparison 1 Any psychosocial intervention vs. control: neonatal outcomes, Outcome 3 Low birth weight (< 2500 g).

Comparison 1 Any psychosocial intervention vs. control: neonatal outcomes, Outcome 4 Days hospitalized after delivery.

Comparison 2 Any psychosocial intervention vs. control: maternal outcomes, Outcome 1 Positive urine drug test (end of treatment).
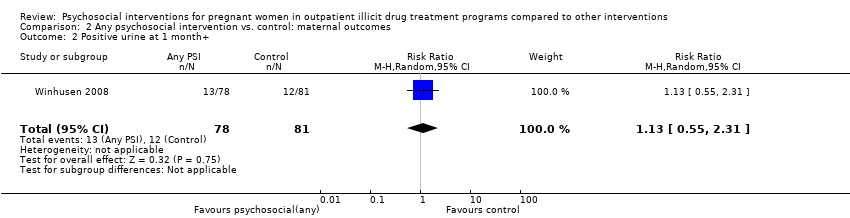
Comparison 2 Any psychosocial intervention vs. control: maternal outcomes, Outcome 2 Positive urine at 1 month+.

Comparison 2 Any psychosocial intervention vs. control: maternal outcomes, Outcome 3 Positive urine at delivery.

Comparison 2 Any psychosocial intervention vs. control: maternal outcomes, Outcome 4 Retention at treatment completion.

Comparison 2 Any psychosocial intervention vs. control: maternal outcomes, Outcome 5 Short term treatment retention.

Comparison 2 Any psychosocial intervention vs. control: maternal outcomes, Outcome 6 Retention in treatment at delivery.
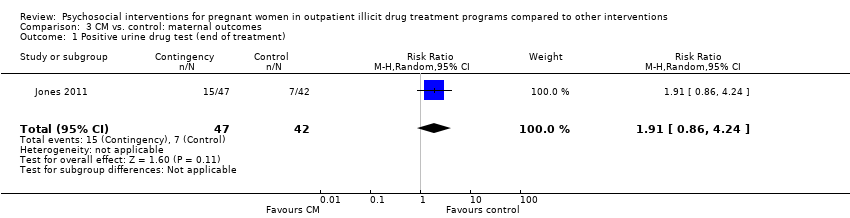
Comparison 3 CM vs. control: maternal outcomes, Outcome 1 Positive urine drug test (end of treatment).

Comparison 3 CM vs. control: maternal outcomes, Outcome 2 Positive urine at delivery.
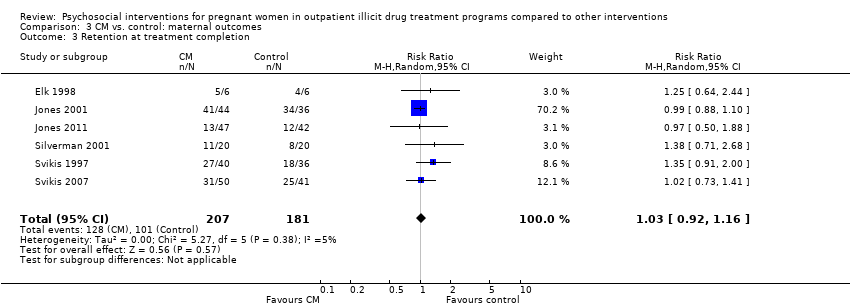
Comparison 3 CM vs. control: maternal outcomes, Outcome 3 Retention at treatment completion.

Comparison 3 CM vs. control: maternal outcomes, Outcome 4 Short term treatment retention.

Comparison 3 CM vs. control: maternal outcomes, Outcome 5 Retention at delivery.

Comparison 4 MIB vs. control: maternal outcomes, Outcome 1 Positive urine drug test (end of treatment).

Comparison 4 MIB vs. control: maternal outcomes, Outcome 2 Positive urine drug test at three months (follow‐up).
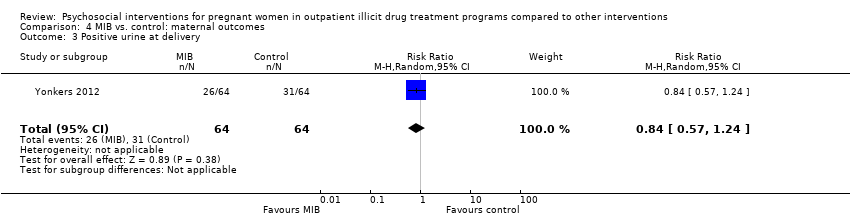
Comparison 4 MIB vs. control: maternal outcomes, Outcome 3 Positive urine at delivery.
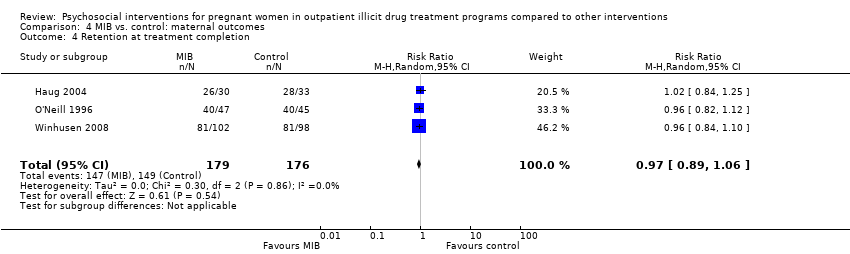
Comparison 4 MIB vs. control: maternal outcomes, Outcome 4 Retention at treatment completion.
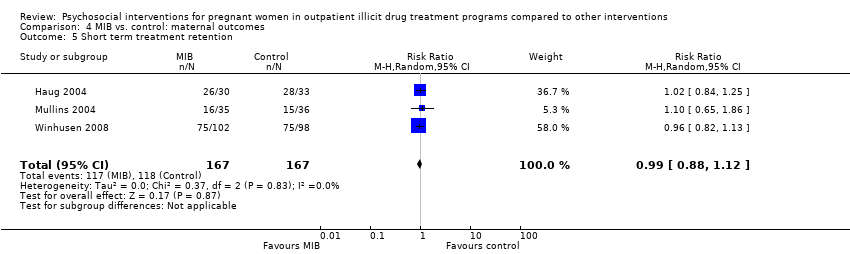
Comparison 4 MIB vs. control: maternal outcomes, Outcome 5 Short term treatment retention.
| Outcomes | Relative effect | No of participants | Quality of the evidence |
| Patients: Pregnant women enrolled in illicit drug treatment programs for any treatment of substance abuse or dependence of any drug Settings: Outpatient treatment facilities Intervention: Psychosocial interventions of any kind (including Contingency Management methods and Motivational Interviewing based techniques) alone or given in addition to usual care Comparison: Comprehensive usual care such as methadone maintenance, counselling, prenatal care (PNC), STD counselling and testing, transportation, and/or childcare | |||
| Preterm birth (< 37 weeks gestation) (Any psychosocial intervention vs. control) | RR 0.71 (95% CI 0.34 to 1.51) | 264 (3 studies) | ⊕⊕⊕⊝ |
| Low birth weight (< 2500 g) (Any psychosocial intervention vs. control) | RR 0.72 (95% CI 0.36 to 1.43) | 160 (1 study) | ⊕⊕⊕⊕ |
| Days hospitalized after delivery (Any psychosocial intervention vs. control) | MD ‐1.27 (95% CI ‐2.52 to ‐0.03) | 103 (2 studies) | ⊕⊕⊕⊝ |
| Retention at treatment completion (Any psychosocial intervention vs. control) | RR 0.99 (95% CI 0.93 to 1.06) | 743 (9 studies) | ⊕⊕⊝⊝ |
| Short term treatment retention (Any psychosocial intervention vs. control) | RR 1.00 (95% CI 0.90 to 1.10) | 514 (6 studies) | ⊕⊕⊝⊝ |
| Positive urine at delivery (Any psychosocial intervention vs. control) | RR 1.18 (95% CI 0.52 to 2.65) | 217 (2 studies) | ⊕⊕⊕⊕ high |
| Positive urine drug test (end of treatment) (Any psychosocial intervention vs. control) | RR 1.14 (95% CI 0.75 to 1.73) | 367 (3 studies) | ⊕⊕⊕⊝ |
| Retention at treatment completion (CM vs. control) | RR 1.03 (95% CI 0.92 to 1.16) | 388 (6 studies) | ⊕⊕⊝⊝ |
| Retention at treatment completion (MIB interventions vs. control) | RR 0.97 (95% CI 0.89 to 1.06) | 355 (3 studies) | ⊕⊕⊝⊝ |
| CI: Confidence interval; RR: Risk ratio; MD: Mean difference; CM: contingency management; MIB: motivational interviewing based. | |||
| GRADE Working Group grades of evidence | |||
| 1 Downgraded by one due to possible selection bias in one of the three included studies. 2 Downgraded by one due to possible attrition bias associated with one of the two studies. 3 Downgraded by two due to possible selection bias, attrition bias, and detection bias in majority of the included studies (all but two). 4 Downgraded by two due to possible selection bias, attrition bias, and detection bias in majority of the included studies (all but two). 5 Downgraded by one due to possible selection bias associated with one of the three studies. 6 Downgraded by two due to possible selection bias associated with four of the included studies. 7 Downgraded by two due to possible selection bias associated with two of the included studies. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Preterm birth (< 37 weeks gestation) Show forest plot | 3 | 264 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.34, 1.51] |
| 2 Positive neonatal toxicology at delivery (any drug) Show forest plot | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [0.86, 4.24] |
| 3 Low birth weight (< 2500 g) Show forest plot | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.36, 1.43] |
| 4 Days hospitalized after delivery Show forest plot | 2 | 103 | Mean Difference (IV, Random, 95% CI) | ‐1.27 [‐2.52, ‐0.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Positive urine drug test (end of treatment) Show forest plot | 3 | 367 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.75, 1.73] |
| 2 Positive urine at 1 month+ Show forest plot | 1 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.55, 2.31] |
| 3 Positive urine at delivery Show forest plot | 2 | 217 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.52, 2.65] |
| 4 Retention at treatment completion Show forest plot | 9 | 743 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.93, 1.06] |
| 5 Short term treatment retention Show forest plot | 6 | 514 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.90, 1.10] |
| 6 Retention in treatment at delivery Show forest plot | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.50, 1.88] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Positive urine drug test (end of treatment) Show forest plot | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [0.86, 4.24] |
| 2 Positive urine at delivery Show forest plot | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [0.86, 4.24] |
| 3 Retention at treatment completion Show forest plot | 6 | 388 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.92, 1.16] |
| 4 Short term treatment retention Show forest plot | 2 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.70, 1.73] |
| 5 Retention at delivery Show forest plot | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.50, 1.88] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Positive urine drug test (end of treatment) Show forest plot | 2 | 278 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.63, 1.48] |
| 2 Positive urine drug test at three months (follow‐up) Show forest plot | 1 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.55, 2.31] |
| 3 Positive urine at delivery Show forest plot | 1 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.57, 1.24] |
| 4 Retention at treatment completion Show forest plot | 3 | 355 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.89, 1.06] |
| 5 Short term treatment retention Show forest plot | 3 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.88, 1.12] |

