Intervenciones psicosociales para mujeres embarazadas en programas ambulatorios de tratamiento de drogas ilegales en comparación con otras intervenciones
Appendices
Appendix 1. Search strategy
For the original search executed in May 2006, we searched the Cochrane Drugs and Alcohol Group Register (May 2006); CENTEAL, (The Cochrane Library, Issue 3, 2005); MEDLINE (1996 to August 2006); EMBASE (January 1996 to August 2006); and CINAHL (January 1982 to August 2006). We followed the 'optimal' MEDLINE and EMBASE sensitive search strategies devised by the Cochrane Collaboration for RCTs as published in Section 6.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) in order to identify studies relevant to this Cochrane review.
Search strategy to locate drug abuse
1. SUBSTANCE ADJ RELATED ADJ DISORDERS
2. SUBSTANCE‐RELATED‐DISORDERS#.DE.
3. ADDICT$4.TI,AB.
4. (OVERDOS$2 OR OVER‐DOS$2).TI,AB.
5. INTOXICAT$3.TI,AB.
6. (ABSTINEN$2 OR ABSTAIN$2).TI,AB.
7. WITHDRAW$2.TI,AB.
8. (ABUSE$2 OR USE).TI,AB.
9. (EXCESSIVE$2 ADJ USE$1).TI,AB.
10. (USE$2 ADJ DISORDER$2).TI,AB.
11. PSYCHOSES‐SUBSTANCE‐INDUCED
12. PSYCHOSES‐SUBSTANCE‐INDUCED#.DE.
13. 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12
Search strategy to identify drugs
14.HEROIN or HEROIN#.W..DE. or HEROIN.TI,AB.
15.NARCOTICS or NARCOTICS#.W..DE.
16.OPIOID.TI,AB. Or OPIATE.TI,AB. Or OPIATE.RN.
17.ANTI‐ANXIETY‐AGENTS#.DE.
18.BENZODIAZEPINE or BENZODIAZEPINES#.W..DE.
19.BARBITURATES or BARBITURATES#.W..DE. or BARBITURATES.TI,AB.
20.AMPHETAMINES or AMPHETAMINE#.W..DE. or AMPHETAMINE.TI,AB.
21.DESIGNER ADJ DRUGS or DESIGNER‐DRUGS#.DE. or (DESIGNER ADJ DRUGS).TI,AB. or (DESIGNER ADJ DRUGS).RN.
22.HALLUCINOGENS or HALLUCINOGENS#.W..DE. or HALLUCINOGENS.TI,AB. or HALLUCINOGENS.RN.
23.STREET ADJ DRUGS or STREET‐DRUGS#.DE. or(STREET ADJ DRUGS).TI,AB. or STREET‐DRUGS.TI,AB.
24.COCAINE or COCAINE#.W..DE. or COCAINE.TI,AB. or COCAINE.RN.
25.ALOCHOLS or ALCOHOLS#.W..DE. or ALCOHOL.TI,AB.
26.LYSERGIC ADJ ACID or LYSERGIC‐ACID#.DE. or (LYSERGIC ADJ ACID).TI,AB. or (LYSERGIC ADJ ACID).RN. or LSD.TI,AB. or LSD.RN. or LSD or LYSERGIC‐ACID‐DIETHYLAMIDE#.DE.
27.KETAMINE or KETAMINE#.W..DE. or KETAMINE.TI,AB. or KETAMINE.RN.
28.CANNABIS or CANNABIS#.W..DE. or CANNABIS.TI,AB. or CANNABIS.RN.
29.MARIHUANA.TI,AB. or MARIHUANA.RN. or MARIJUANA or MARIJUANA‐SMOKING#.DE. or MARIJUANA‐ABUSE#.DE. or MARIJUANA.TI,AB.
30.HASHISH or HASHISH.TI,AB.
31.OPIUM or OPIUM#.W..DE. or OPIUM.TI,AB.
32.INHALANT$2.TI,AB. or (INHALANT$2 ADJ ABUSE$2).TI,AB.
33.SOLVENT or SOLVENTS#.W..DE. or SOLVENT$2.TI,AB. or SOLVENT$2.RN.
34.(STEROID$2 ADJ ABUSE).TI,AB. or ANABOLIC ADJ STEROIDS or ANABOLIC‐AGENTS#.DE.
35.(ANABOLIC ADJ AGENT$2).TI,AB. AND PERFORM$6.TI,AB.
36.METHADONE or METHADONE#.W..DE. or METHADONE.TI,AB. METHADONE.RN.
37.14 OR 15 OR 16 OR 17 OR 18 OR 19 OR 20 OR 21 OR 22 OR 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29 OR 30 OR 31 OR 32 OR 33 OR 34 OR 35 OR 36
Search strategy to locate interventions
38.PATIENT ADJ COMPLIANCE or PATIENT‐COMPLIANCE#.DE.
39.(ATTENDANCE ADJ INCENTIVE$2).TI,AB.
40.INCENTIVE$2.TI,AB. or VOUCHER$2.TI,AB.
41.PSYCHOTHERAPY or PSYCHOTHERAPY#.W..DE.
42.BEHAVIOR‐THERAPY#.DE. or BEHAVIOR ADJ THERAPY
43.REINFORCEMENT ADJ PSYCHOLOGY or REINFORCEMENT‐PSYCHOLOGY#.DE. or REINFORCEMENT.TI,AB.
44.MOTIVATION or MOTIVATION#.W..DE. or MOTIVATION ADJ INTERVIEWING
45.(CONTINGENCY ADJ MANAGEMENT).TI,AB.
46.38 OR 39 OR 40 OR 41 OR 42 OR 43 OR 44 OR 45
Search strategy to identify pregnancy
47.PREGNANCY or PREGNANCY#.W..DE. or PREGNAN$4.TI,AB.
Search strategy to locate RCTs and different types of studies
48.PT=RANDOMIZED‐CONTROLLED‐TRIAL or RANDOMIZED ADJ CONTROLLED ADJ TRIAL or RANDOMIZED‐CONTROLLED‐TRIALS#.DE.
49.PT=CONTROLLED‐CLINICAL‐TRIAL or RANDOM ADJ ALLOCATION or RANDOM‐ALLOCATION.DE.
50.DOUBLE ADJ BLIND ADJ METHOD or DOUBLE‐BLIND‐METHOD.DE.
51.SINGLE‐BLIND‐METHOD.DE.
52.PT=CLINICAL‐TRIAL$ or CLINICAL ADJ TRIALS or CLINICAL‐TRIALS#.DE. or (CLINIC$2 ADJ TRIAL$2).TI,AB.
53.((SINGL$2 OR DOUBL$2 OR TREBL$2 OR TRIPL$2) ADJ (BLIND$2 OR MASK$2)).TI,AB.
54.PLACEBOS or PLACEBOS#.W..DE. or PLACEBO$2.TI,AB.
55.RANDOM$4.TI,AB.
56.RESEARCH ADJ DESIGN or RESEARCH‐DESIGN#.DE.
57.COMPARATIVE ADJ STUDY or COMPARATIVE‐STUDY.DE.
58.EVALUATION‐STUDIES#.DE.
59.PROSPECTIVE‐STUDIES#.DE.
60.48 OR 49 OR 51 OR 52 OR 53 OR 54 OR 55 OR 57 OR 59
Search strategy to locate studies for this review
61.13 and 37 and 46 and 47 and 60
Appendix 2. CENTRAL search strategy
-
MeSH descriptor Substance‐Related Disorders explode all trees
-
((stimulant* or polydrug* or drug* or substance) near (abstain* or abstinen* or abus* or addict* or dependen* or disorder* or intoxicat* or misuse* or over dos* or overdos* or withdraw*)):ab,ti
-
(#1 OR #2)
-
(abstain* or abstinen* or abus* or addict* or drug user* or dependen* or inject* drug* or intoxicat* or misus* or overdos* or illicit use* or withdraw*):ti,ab,kw
-
MeSH descriptor Heroin explode all trees
-
(heroin or morphine* or diamorphine or diacetylmorphine or morfin* or narcotic* or methadone):ti,ab,kw
-
MeSH descriptor Methadone explode all trees
-
(opioid* or opiate* or opium):ti,ab,kw
-
MeSH descriptor Narcotics explode all trees
-
MeSH descriptor Anti‐Anxiety Agents explode all trees
-
MeSH descriptor Benzodiazepines explode all trees
-
(benzodiazepine):ti,ab,kw
-
MeSH descriptor Barbiturates explode all trees
-
(barbiturates):ti,ab,kw
-
MeSH descriptor Amphetamine explode all trees
-
(amphetamine* or methamphetamine*):ti,ab,kw
-
(Designer near/2 Drug*):ti,ab,kw
-
MeSH descriptor Hallucinogens explode all trees
-
(ecstasy or MDMA or hallucinogen*):ti,ab,kw
-
MeSH descriptor Street Drugs explode all trees
-
MeSH descriptor Cocaine explode all trees
-
(crack or cocaine):ti,ab,kw
-
(alcohol*):ti,ab,kw
-
MeSH descriptor Lysergic Acid explode all trees
-
(Lysergic NEXT Acid):ti,ab,kw
-
(LSD):ti,ab,kw
-
(ketamine):ti,ab,kw
-
MeSH descriptor Ketamine explode all trees
-
MeSH descriptor Cannabis explode all trees
-
(cannabis or marijuana or marihuana or hashish):ti,ab,kw
-
(Inhalant NEXT abuse ):ti,ab,kw
-
(Solvent*):ti,ab,kw
-
MeSH descriptor Solvents explode all trees
-
(anabolic near steroid*):ti,ab,kw
-
MeSH descriptor Anabolic Agents explode all trees
-
(steroid* near/3 abuse):ti,ab
-
(anabolic near agent*):ti,ab
-
(#5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37)
-
(#4 AND #38)
-
(#3 OR #39)
-
MeSH descriptor Patient Compliance explode all trees
-
(patient NEXT compliance):ti,ab,kw
-
MeSH descriptor Psychotherapy explode all trees
-
MeSH descriptor Reinforcement (Psychology) explode all trees
-
MeSH descriptor Motivation explode all trees
-
(behavi* near/3 therap*):ti,ab
-
(psychotherap* or psychosocial or voucher* or incentive* or reinforcement or motivation*):ti,ab,kw
-
(contingency near management):ti,ab,kw
-
(#41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48)
-
MeSH descriptor Pregnancy explode all trees
-
(pregnan*):ti,ab,kw
-
(#50 OR #51)
-
(#40 AND #49 AND #52)
Appendix 3. PubMed search strategy
-
Substance‐related disorders[MeSH]
-
Psychoses, Substance‐Induced[MeSH]
-
(abstain*[tiab] OR abstinen*[tiab] OR abus*[tiab] OR addict*[tiab] OR dependen*[tiab] OR disorder*[tiab] OR intoxicat*[tiab] OR misuse*[tiab] OR use*[tiab] OR over‐dos*[tiab] OR overdos*[tiab] OR withdraw*[tiab])
-
#1 OR #2 OR #3
-
Heroin [MH]
-
Anti‐anxiety agents [MeSH]
-
Benzodiazepines [MeSH]
-
Barbiturates [MeSH]
-
Amphetamines [MeSH]
-
Designer drugs [MeSH]
-
"designer drug"[tiab]
-
"illicit drug"[tiab]
-
Antidepressive agents [MeSH]
-
Hallucinogens [MeSH]
-
Street drugs [MeSH] OR street‐drug* [tiab]
-
Cocaine [MeSH]
-
Lysergic acid [MeSH] or lysergic‐acid* [tiab] or LSD[tiab]
-
Ketamine [MeSH]
-
Cannabis [MeSH]
-
Opium [MeSH]
-
"steroid abuse"[tiab]
-
Anabolic steroids [MeSH]
-
drug*[tiab] OR substance[tiab] OR polidrug*[tiab] OR alcohol*[tiab] OR amphetamine*[tiab] OR cannabis[tiab] OR marihuana[tiab] or marijuana[tiab] OR "hash oil*"[tiab] OR hashish[tiab] OR cocaine[tiab] OR hallucinogen* [tiab] OR heroin[tiab] OR mdma[tiab] OR ecstasy[tiab] OR methamphetamine*[tiab] OR narcotic* [tiab] OR ketamine[tiab] OR opioid*[tiab] OR opiate* [tiab] OR opium[tiab] OR tranquilizer*[tiab] OR tranquiliser*[tiab] OR inhalant*[tiab] OR barbiturate*[tiab] OR solvent*[tiab] OR stimulant*[tiab]
-
#5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22
-
Patient Compliance[MH]
-
Psychotherapy[MH]
-
reinforcement psychology[MeSH Terms]
-
motivation[mh]
-
(behavi*[tiab] AND therap*[tiab])
-
(psychotherap*[tiab] OR psychosocial[tiab] OR voucher*[tiab] OR incentive*[tiab] OR reinforcement[tiab] OR motivation*[tiab])
-
(contingency[tiab] AND management[tiab])
-
#25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31
-
"pregnancy"[MeSH Terms]
-
pregnan*[tiab]
-
#33 OR #34
-
randomized controlled trial [pt]
-
controlled clinical trial [pt]
-
randomized [tiab]
-
placebo [tiab]
-
drug therapy [sh]
-
randomly [tiab]
-
trial [tiab]
-
groups [tiab]
-
#36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43
-
animals [mh] NOT humans [mh]
-
#44 NOT #45
-
#4 AND #24 AND #32 AND #35 AND #46
Appendix 4. EMBASE search strategy
-
'addiction'/exp
-
dependen*:ab,ti OR addict*:ab,ti OR overdos*:ab,ti OR intoxicat*:ab,ti OR abstin*:ab,ti OR abstain:ab,ti OR withdraw*:ab,ti OR abus*:ab,ti OR use*:ab,ti OR misus*:ab,ti OR disorder*:ab,ti
-
#1 OR #2
-
'diamorphine'/exp
-
diamorphine:ab,ti OR heroin:ab,ti OR narcotic*:ab,ti OR drug*:ab,ti OR polydrug:ab,ti OR substance:ab,ti OR opioid:ab,ti OR opiate:ab,ti OR opium:ab,ti OR hallucinogen*:ab,ti OR amphetamine*:ab,ti OR barbiturate:ab,ti OR inhalant*:ab,ti OR morphine:ab,ti OR ecstasy:ab,ti OR mdma:ab,ti
-
'street drug'/exp
-
'designer drug'/exp
-
'lysergic acid'/exp OR 'lysergic acid':ab,ti OR lsd:ab,ti
-
'cocaine'/exp OR cocaine:ab,ti
-
'alcohol'/exp OR alcohol:ab,ti
-
'ketamine'/exp OR ketamine:ab,ti
-
'cannabis'/exp OR cannabis:ab,ti OR hashish:ab,ti OR marihuana:ab,ti OR marijuana:ab,ti
-
'inhalant abuse'/exp OR inhalant:ab,ti
-
'solvent'/exp OR solvent:ab,ti
-
'methadone'/exp OR methadone:ab,ti
-
'anabolic agent'/exp
-
steroid*:ab,ti AND abuse:ab,ti
-
anabolic:ab,ti AND agent*:ab,ti
-
'benzodiazepine'/exp OR benzodiazepine:ab,ti
-
'amphetamine'/exp OR amphetamine:ab,ti
-
#4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20
-
'patient compliance'/exp
-
'psychotherapy'/exp
-
'reinforcement'/exp
-
'motivation'/exp
-
incentive*or:ab,ti OR voucher*:ab,ti OR psychotherap*:ab,ti OR psychosocial*:ab,ti OR reinforcement:ab,ti OR motivation*:ab,ti
-
#22 OR #23 OR #24 OR #25 OR #26
-
'pregnancy'/exp
-
pregnan*:ab,ti
-
#28 OR #29
-
'crossover procedure'/exp OR 'double blind procedure'/exp OR 'single blind procedure'/exp OR 'controlled clinical trial'/exp OR 'clinical trial'/exp OR placebo:ab,ti OR 'double blind':ab,ti OR 'single blind':ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti OR random*:ab,ti OR factorial*:ab,ti OR crossover:ab,ti OR (cross:ab,ti AND over:ab,ti) OR 'randomized controlled trial'/exp
-
#3 AND #21 AND #27 AND #30 AND #31
Appendix 5. CINAHL search strategy
-
(MH "Substance Use Disorders+")
-
(MH "Psychoses, Substance‐Induced+")
-
TX(drug N3 addict*) or TX(drug N3 dependen*) or TX(drug N3 abuse*) or TX(drug N3 misus*)
-
TX(substance N3 addict*) or TX(substance N3 dependen*) or TX(substance N3 abuse*) or TX(substance N3 misus*)
-
TX(addict* OR overdos* OR intoxicat* OR abstin* OR abstain OR withdraw* OR abus* OR misus* OR disorder* OR dependen*)
-
TX(use* N2 drug) or TX(use* N2 disorder) or TX(use* N2 illicit)
-
TX(use* N2 drug) or TX(use* N2 disorder) or TX(use* N2 illicit)
-
S1 or S2 or S3 or S4
-
S5 or S6 or S7
-
TX(polydrug or alcohol or opioid or opiate or opium or hallucinogen or cocaine or benzodiazepine* or amphetamine*or “anti‐anxiety‐agents” or barbiturate* or “lysergic acid” or ketamine or cannabis or marihuana or marijuana or hashish or inhalant* or solvent or steroid* or methadone or morphine)
-
MH "Narcotics"
-
MH "Designer Drugs"
-
(MH "Hallucinogens+")
-
(MH "Methadone")
-
(MH "Amphetamines+")
-
(MH "Ketamine")
-
S10 or S11 or S12 or S13 or S14 or S15 or S16
-
S9 and S17
-
S8 or S18
-
(MH "Patient Compliance+")
-
(MH "Motivational Interviewing") OR (MM "Counseling")
-
(MH "Psychotherapy+")
-
TI incentive* OR voucher OR psychotherap* OR psychosocial* OR reinforcement OR motivation* OR contingent* OR advice
-
AB incentive* OR voucher OR psychotherap* OR psychosocial* OR reinforcement OR motivation* OR contingent* OR advice
-
TI (contingency N1 management) OR AB (contingency N1 management)
-
TI (behaviour* N2 therapy) OR AB (behaviour* N2 therapy)
-
(MH "Reinforcement (Psychology)+")
-
S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27
-
(MH "Pregnancy")
-
TX pregnan*
-
S29 or S30
-
MH "Clinical Trials+"
-
PT Clinical trial
-
TI clinic* N1 trial* or AB clinic* N1 trial*
-
TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* )
-
AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* )
-
TI randomi?ed control* trial* or AB randomi?ed control* trial*
-
MH "Random Assignment"
-
TI random* allocat* or AB random* allocat*
-
MH "Placebos"
-
TI placebo* or AB placebo*
-
MH "Quantitative Studies"
-
S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42
-
S19 and S28 and S31 and S43
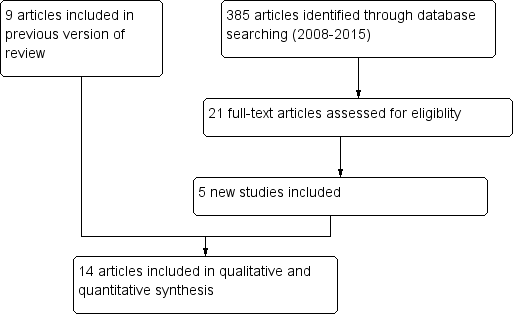
Study flow diagram.
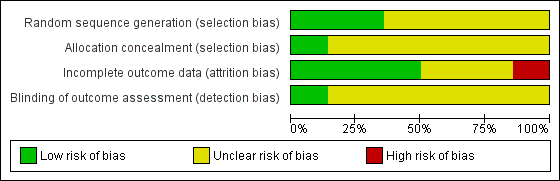
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
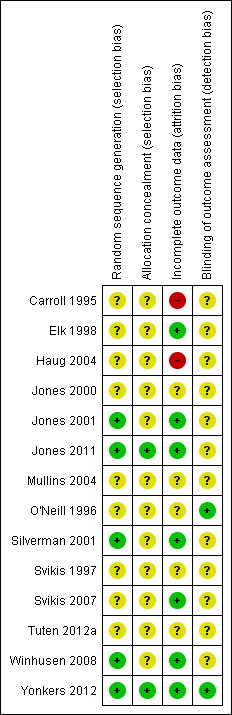
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
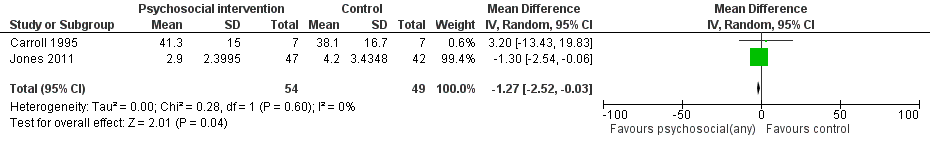
Forest plot of comparison: 1 Neonatal outcomes any psychosocial intervention vs. control, outcome: 1.5 Mean days hospitalized after delivery.
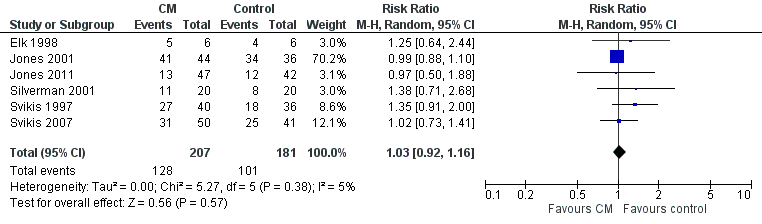
Forest plot of comparison: 2 CM vs. control, outcome: 3.1 Retention in treatment at the end of study.

Comparison 1 Any psychosocial intervention vs. control: neonatal outcomes, Outcome 1 Preterm birth (< 37 weeks gestation).

Comparison 1 Any psychosocial intervention vs. control: neonatal outcomes, Outcome 2 Positive neonatal toxicology at delivery (any drug).
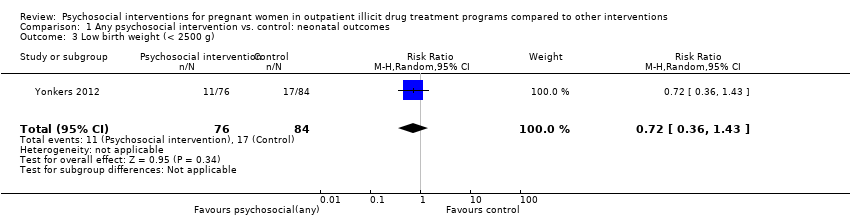
Comparison 1 Any psychosocial intervention vs. control: neonatal outcomes, Outcome 3 Low birth weight (< 2500 g).

Comparison 1 Any psychosocial intervention vs. control: neonatal outcomes, Outcome 4 Days hospitalized after delivery.

Comparison 2 Any psychosocial intervention vs. control: maternal outcomes, Outcome 1 Positive urine drug test (end of treatment).
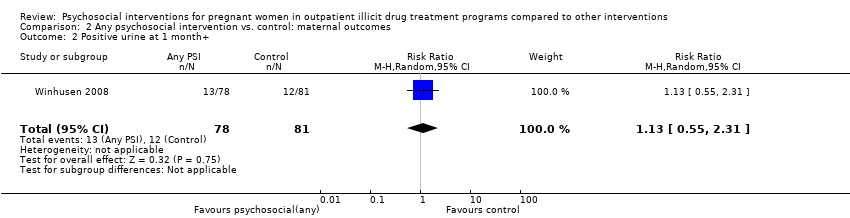
Comparison 2 Any psychosocial intervention vs. control: maternal outcomes, Outcome 2 Positive urine at 1 month+.

Comparison 2 Any psychosocial intervention vs. control: maternal outcomes, Outcome 3 Positive urine at delivery.

Comparison 2 Any psychosocial intervention vs. control: maternal outcomes, Outcome 4 Retention at treatment completion.

Comparison 2 Any psychosocial intervention vs. control: maternal outcomes, Outcome 5 Short term treatment retention.

Comparison 2 Any psychosocial intervention vs. control: maternal outcomes, Outcome 6 Retention in treatment at delivery.
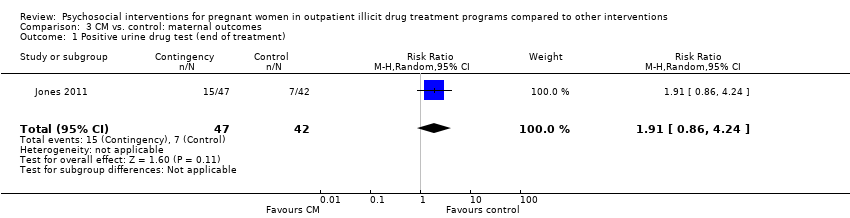
Comparison 3 CM vs. control: maternal outcomes, Outcome 1 Positive urine drug test (end of treatment).

Comparison 3 CM vs. control: maternal outcomes, Outcome 2 Positive urine at delivery.
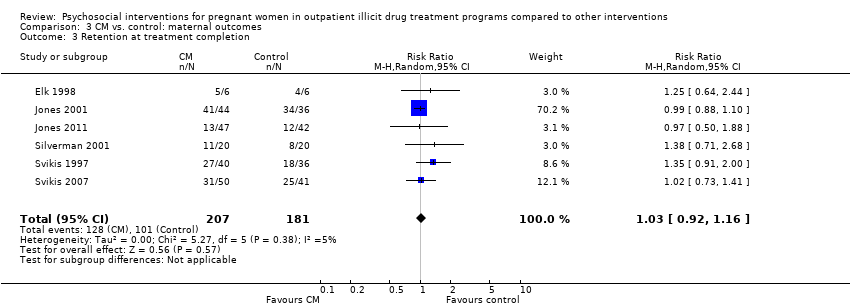
Comparison 3 CM vs. control: maternal outcomes, Outcome 3 Retention at treatment completion.

Comparison 3 CM vs. control: maternal outcomes, Outcome 4 Short term treatment retention.

Comparison 3 CM vs. control: maternal outcomes, Outcome 5 Retention at delivery.

Comparison 4 MIB vs. control: maternal outcomes, Outcome 1 Positive urine drug test (end of treatment).

Comparison 4 MIB vs. control: maternal outcomes, Outcome 2 Positive urine drug test at three months (follow‐up).
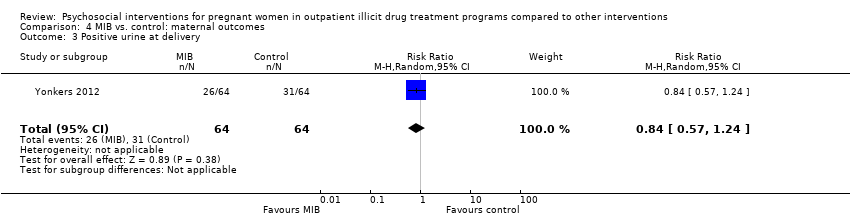
Comparison 4 MIB vs. control: maternal outcomes, Outcome 3 Positive urine at delivery.
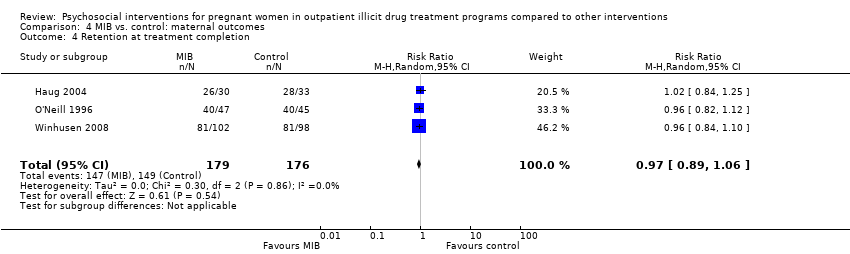
Comparison 4 MIB vs. control: maternal outcomes, Outcome 4 Retention at treatment completion.
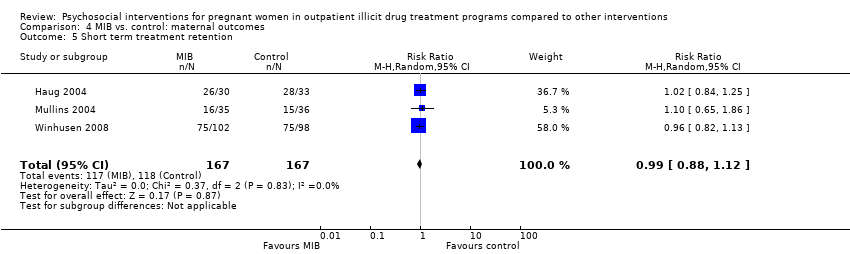
Comparison 4 MIB vs. control: maternal outcomes, Outcome 5 Short term treatment retention.
| Outcomes | Relative effect | No of participants | Quality of the evidence |
| Patients: Pregnant women enrolled in illicit drug treatment programs for any treatment of substance abuse or dependence of any drug Settings: Outpatient treatment facilities Intervention: Psychosocial interventions of any kind (including Contingency Management methods and Motivational Interviewing based techniques) alone or given in addition to usual care Comparison: Comprehensive usual care such as methadone maintenance, counselling, prenatal care (PNC), STD counselling and testing, transportation, and/or childcare | |||
| Preterm birth (< 37 weeks gestation) (Any psychosocial intervention vs. control) | RR 0.71 (95% CI 0.34 to 1.51) | 264 (3 studies) | ⊕⊕⊕⊝ |
| Low birth weight (< 2500 g) (Any psychosocial intervention vs. control) | RR 0.72 (95% CI 0.36 to 1.43) | 160 (1 study) | ⊕⊕⊕⊕ |
| Days hospitalized after delivery (Any psychosocial intervention vs. control) | MD ‐1.27 (95% CI ‐2.52 to ‐0.03) | 103 (2 studies) | ⊕⊕⊕⊝ |
| Retention at treatment completion (Any psychosocial intervention vs. control) | RR 0.99 (95% CI 0.93 to 1.06) | 743 (9 studies) | ⊕⊕⊝⊝ |
| Short term treatment retention (Any psychosocial intervention vs. control) | RR 1.00 (95% CI 0.90 to 1.10) | 514 (6 studies) | ⊕⊕⊝⊝ |
| Positive urine at delivery (Any psychosocial intervention vs. control) | RR 1.18 (95% CI 0.52 to 2.65) | 217 (2 studies) | ⊕⊕⊕⊕ high |
| Positive urine drug test (end of treatment) (Any psychosocial intervention vs. control) | RR 1.14 (95% CI 0.75 to 1.73) | 367 (3 studies) | ⊕⊕⊕⊝ |
| Retention at treatment completion (CM vs. control) | RR 1.03 (95% CI 0.92 to 1.16) | 388 (6 studies) | ⊕⊕⊝⊝ |
| Retention at treatment completion (MIB interventions vs. control) | RR 0.97 (95% CI 0.89 to 1.06) | 355 (3 studies) | ⊕⊕⊝⊝ |
| CI: Confidence interval; RR: Risk ratio; MD: Mean difference; CM: contingency management; MIB: motivational interviewing based. | |||
| GRADE Working Group grades of evidence | |||
| 1 Downgraded by one due to possible selection bias in one of the three included studies. 2 Downgraded by one due to possible attrition bias associated with one of the two studies. 3 Downgraded by two due to possible selection bias, attrition bias, and detection bias in majority of the included studies (all but two). 4 Downgraded by two due to possible selection bias, attrition bias, and detection bias in majority of the included studies (all but two). 5 Downgraded by one due to possible selection bias associated with one of the three studies. 6 Downgraded by two due to possible selection bias associated with four of the included studies. 7 Downgraded by two due to possible selection bias associated with two of the included studies. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Preterm birth (< 37 weeks gestation) Show forest plot | 3 | 264 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.34, 1.51] |
| 2 Positive neonatal toxicology at delivery (any drug) Show forest plot | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [0.86, 4.24] |
| 3 Low birth weight (< 2500 g) Show forest plot | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.36, 1.43] |
| 4 Days hospitalized after delivery Show forest plot | 2 | 103 | Mean Difference (IV, Random, 95% CI) | ‐1.27 [‐2.52, ‐0.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Positive urine drug test (end of treatment) Show forest plot | 3 | 367 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.75, 1.73] |
| 2 Positive urine at 1 month+ Show forest plot | 1 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.55, 2.31] |
| 3 Positive urine at delivery Show forest plot | 2 | 217 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.52, 2.65] |
| 4 Retention at treatment completion Show forest plot | 9 | 743 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.93, 1.06] |
| 5 Short term treatment retention Show forest plot | 6 | 514 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.90, 1.10] |
| 6 Retention in treatment at delivery Show forest plot | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.50, 1.88] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Positive urine drug test (end of treatment) Show forest plot | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [0.86, 4.24] |
| 2 Positive urine at delivery Show forest plot | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [0.86, 4.24] |
| 3 Retention at treatment completion Show forest plot | 6 | 388 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.92, 1.16] |
| 4 Short term treatment retention Show forest plot | 2 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.70, 1.73] |
| 5 Retention at delivery Show forest plot | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.50, 1.88] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Positive urine drug test (end of treatment) Show forest plot | 2 | 278 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.63, 1.48] |
| 2 Positive urine drug test at three months (follow‐up) Show forest plot | 1 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.55, 2.31] |
| 3 Positive urine at delivery Show forest plot | 1 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.57, 1.24] |
| 4 Retention at treatment completion Show forest plot | 3 | 355 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.89, 1.06] |
| 5 Short term treatment retention Show forest plot | 3 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.88, 1.12] |

