Monoterapia con ribavirina para la hepatitis C crónica
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Study design: randomised clinical trial. | |
| Participants | Inclusion criteria: Exclusion criteria: Characteristics of included patients: | |
| Interventions | Ribavirin 1200 mg daily for 36 weeks versus placebo. | |
| Outcomes | Outcomes assessed 16 weeks after the end of treatment. | |
| Notes | The trial included naive, relapsers, and non‐responders. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described. |
| Allocation concealment? | Low risk | "Allocation concealed at independent pharmacy." |
| Blinding? | Low risk | Patients were randomised "in equal numbers to receive 200 mg ribavirin capsules or three identically appearing placebo capsules twice daily. The placebo was composed of capsules whose primary ingredients were lactose and cellulose." |
| Incomplete outcome data addressed? | Unclear risk | Report that "two patients refused follow‐up biopsy." |
| Free of selective reporting? | Unclear risk | Protocol is not available. The authors reported virological response at end of treatment only, but they reported on clinical outcomes and adverse reactions. |
| Early stopping? | Unclear risk | Not described. |
| Baseline characteristics | Low risk | There was no baseline imbalance in important characteristics. |
| Free of source of funding bias? | High risk | Grant from Valeant Pharmaceuticals International. |
| Methods | Study design: randomised clinical trial. | |
| Participants | Inclusion criteria: Exclusion criteria: Characteristics of included patients: | |
| Interventions | Ribavirin 1200 mg daily for eight weeks versus placebo. | |
| Outcomes | Outcomes assessed at end of treatment. | |
| Notes | Previous antiviral treatment unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described. |
| Allocation concealment? | Unclear risk | Not described. |
| Blinding? | Low risk | placebo‐controlled trial. |
| Incomplete outcome data addressed? | Unclear risk | Not described. |
| Free of selective reporting? | Unclear risk | Protocol is not available. The authors reported in the abstract only end of treatment biochemical response. |
| Early stopping? | Unclear risk | Not described. |
| Baseline characteristics | Unclear risk | Not described. |
| Free of source of funding bias? | Unclear risk | Not described. |
| Methods | Study design: randomised clinical trial. | |
| Participants | Inclusion criteria: Exclusion criteria: Characteristics of included patients: | |
| Interventions | Ribavirin 15 mg/kg daily versus interferon 3 million units thrice a week for 26 weeks. | |
| Outcomes | Outcomes assessed 52 weeks after end of treatment. | |
| Notes | This trial also includes a treatment group receiving ribavirin plus interferon. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Table of random numbers." |
| Allocation concealment? | Low risk | "Allocation concealed by sealed envelopes." |
| Blinding? | High risk | No blinding. |
| Incomplete outcome data addressed? | Unclear risk | Not described. |
| Free of selective reporting? | Unclear risk | Protocol is not available. Reported on the primary outcomes in the review. |
| Early stopping? | Unclear risk | Not described. |
| Baseline characteristics | Low risk | No relevant significant difference. |
| Free of source of funding bias? | Unclear risk | Not described. |
| Methods | Study design: randomised clinical trial. | |
| Participants | Inclusion criteria: Exclusion criteria: Characteristics of included patients: | |
| Interventions | Ribavirin 1200 mg daily for 12 months versus placebo. | |
| Outcomes | Outcomes assessed 26 weeks after end of treatment. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Table of random numbers. |
| Allocation concealment? | Unclear risk | Not described. |
| Blinding? | Low risk | Use of placebo identical in appearance and given in the same manner as ribavirin. |
| Incomplete outcome data addressed? | Low risk | "All patients were followed for the full 48 weeks and all had liver biopsy." |
| Free of selective reporting? | Unclear risk | Protocol is not available. Reported on the primary outcomes in the review. |
| Early stopping? | Unclear risk | Not described. |
| Baseline characteristics | Low risk | No significant difference. |
| Free of source of funding bias? | High risk | Support from Valeant Pharmaceuticals International. |
| Methods | Study design: randomised clinical trial. | |
| Participants | Inclusion criteria: Exclusion criteria: Characteristics of included patients: | |
| Interventions | Ribavirin 1000 to 1200 mg daily versus placebo for 24 weeks. | |
| Outcomes | Outcomes assessed 24 weeks after end of treatment. | |
| Notes | The trial included naive, relapsers, and non‐responders. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Table of random numbers. |
| Allocation concealment? | Low risk | Allocation concealed by sealed envelopes. |
| Blinding? | Low risk | "Use identical placebo capsules." |
| Incomplete outcome data addressed? | Low risk | Analysed with intention to treat: "93 had HCV‐RNA measured and 77 had liver biopsy at end of follow‐up." |
| Free of selective reporting? | Unclear risk | Protocol is not available. The authors reported on the primary outcomes in the review. |
| Early stopping? | Low risk | Sample size estimation of 120 patients; 118 patients were randomised. |
| Baseline characteristics | Low risk | No significant difference. |
| Free of source of funding bias? | High risk | Grant from Valeant Pharmaceuticals International. |
| Methods | Study design: randomised clinical trial. | |
| Participants | Inclusion criteria: Exclusion criteria: Characteristics of included patients: | |
| Interventions | Ribavirin 600 mg daily for 8 weeks, then 1000 mg daily for 8 weeks, then 1200 mg daily for 8 weeks versus placebo. | |
| Outcomes | Outcomes assessed 12 weeks after end of treatment. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described. |
| Allocation concealment? | Unclear risk | Not described. |
| Blinding? | Low risk | Use of placebo. |
| Incomplete outcome data addressed? | Unclear risk | Not described. |
| Free of selective reporting? | Unclear risk | Protocol is not available. The authors reported only on the end of treatment virological response. |
| Early stopping? | Unclear risk | Not described. |
| Baseline characteristics | Low risk | No significant difference. |
| Free of source of funding bias? | High risk | Support from ICI‐Frama Ind. Farmac. Ltda. Grants from CNPq and FAPESP |
| Methods | Study design: randomised clinical trial. | |
| Participants | Inclusion criteria: Exclusion criteria: Characteristics of included patients: | |
| Interventions | Ribavirin 1200 mg daily versus placebo for 36 weeks. | |
| Outcomes | Outcomes assessed 24 weeks after end of treatment. | |
| Notes | Previous antiviral treatment unclear. A comparison group with 15 patients were receiving interferon, but they were not randomised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described. |
| Allocation concealment? | Unclear risk | Not described. |
| Blinding? | Low risk | Placebo‐controlled trial. |
| Incomplete outcome data addressed? | Unclear risk | Not described. |
| Free of selective reporting? | Unclear risk | Protocol is not available. The authors reported only on the end of treatment virological response. |
| Early stopping? | Unclear risk | Not described. |
| Baseline characteristics | Low risk | No significant difference. |
| Free of source of funding bias? | Low risk | Grant from Children's Miracle Network Reasearch Fund. |
| Methods | Study design: randomised clinical trial. | |
| Participants | Inclusion criteria: Exclusion criteria: Characteristics of included patients: | |
| Interventions | Ribavirin 1000 to 1200 mg daily versus placebo for 48 weeks. | |
| Outcomes | Outcomes assessed 24 weeks after end of treatment. | |
| Notes | All patients were treated with interferon plus ribavirin for 24 weeks. Those who did not clear hepatitis C virus were subsequently randomised to ribavirin versus placebo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described. |
| Allocation concealment? | Unclear risk | Not described. |
| Blinding? | Low risk | "Identical appearing placebo tablets." |
| Incomplete outcome data addressed? | Low risk | "All had HCV‐RNA measured and all but two had liver biopsy." |
| Free of selective reporting? | Unclear risk | Protocol is not available. The authors reported on adverse events and clinical outcomes but not on sustained virological response. |
| Early stopping? | Unclear risk | Not described. |
| Baseline characteristics | Low risk | No significant difference. |
| Free of source of funding bias? | High risk | Support from Schering‐Ploigh research Institute (placebo tablets). |
| Methods | Study design: randomised clinical trial. | |
| Participants | Inclusion criteria: Exclusion criteria: Characteristics of included patients: | |
| Interventions | Ribavirin 800 to 1000 mg daily versus no intervention versus interferon beta 3 million units thrice a week for 24 weeks versus no intervention. | |
| Outcomes | Outcomes assessed 24 weeks after end of treatment. | |
| Notes | Previous antiviral treatment unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described. |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? | High risk | No blinding. |
| Incomplete outcome data addressed? | Unclear risk | Not described. |
| Free of selective reporting? | Unclear risk | Protocol is not available. The authors reported on the primary outcomes in the review. |
| Early stopping? | Unclear risk | Not described. |
| Baseline characteristics | Low risk | No significant difference. |
| Free of source of funding bias? | Unclear risk | Not described. |
| Methods | Study design: randomised clinical trial. | |
| Participants | Inclusion criteria: Exclusion criteria: Characteristics of included patients: | |
| Interventions | Ribavirin 1200 mg daily versus interferon alfa‐2b 3 million units thrice weekly with or without ribavirin 1200 mg daily for six weeks | |
| Outcomes | Outcomes assessed four weeks after end of treatment. | |
| Notes | Patients who completed the full 10 weeks were offered ribavirin plus interferon for 24 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Sequence generation by computer. |
| Allocation concealment? | Low risk | Allocation concealed by sealed envelopes. |
| Blinding? | High risk | No blinding. |
| Incomplete outcome data addressed? | Unclear risk | Not described. |
| Free of selective reporting? | Unclear risk | Protocol is not available. The authors did not report on virological response. |
| Early stopping? | Unclear risk | Not described. |
| Baseline characteristics | Unclear risk | Not described. |
| Free of source of funding bias? | Unclear risk | Not described. |
| Methods | Study design: randomised clinical trial. | |
| Participants | Inclusion criteria: Exclusion criteria: Characteristics of included patients: | |
| Interventions | Ribavirin 1000 to 1200 mg daily versus interferon alfa‐2b 3 million units daily versus interferon 3 million units thrice weekly versus no intervention for 12 weeks. | |
| Outcomes | Outcomes assessed at end of treatment. | |
| Notes | This study also includes a treatment group receiving ribavirin plus interferon. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described. |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? | High risk | No blinding. |
| Incomplete outcome data addressed? | Unclear risk | Not described. |
| Free of selective reporting? | Unclear risk | Protocol is not available. DId not report on sustained virological response. |
| Early stopping? | Unclear risk | Not described. |
| Baseline characteristics | Unclear risk | Not described. |
| Free of source of funding bias? | Unclear risk | Not described. |
| Methods | Study design: randomised clinical trial. | |
| Participants | ‐ Non‐responders to previous interferon treatment. | |
| Interventions | Leukocyte interferon 3 million units thrice a week or ribavirin 1000 mg daily for 26 weeks. | |
| Outcomes | Outcomes assessed at the end of treatment. | |
| Notes | The trial also includes a third group receiving ribavirin plus interferon. Furthermore, the ribavirin group received interferon after ribavirin. Thus, only data at end of ribavirin treatment are included. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Use of computer. |
| Allocation concealment? | Unclear risk | Not described. |
| Blinding? | High risk | No double blinding. |
| Incomplete outcome data addressed? | Low risk | Report on patients dropping out from study. |
| Free of selective reporting? | Unclear risk | Protocol is not available. |
| Early stopping? | Unclear risk | Not described. |
| Baseline characteristics | Low risk | No significant difference. |
| Free of source of funding bias? | Unclear risk | Not described. |
| Methods | Study design: randomised clinical trial. | |
| Participants | Inclusion criteria: Exclusion criteria: Characteristics of included patients: | |
| Interventions | Ribavirin 1200 mg daily for 48 weeks or placebo. | |
| Outcomes | Outcomes assessed 16 weeks after end of treatment. | |
| Notes | Treatment naive. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described. |
| Allocation concealment? | Low risk | Allocation concealed at independent medical department. |
| Blinding? | Low risk | Use of placebo. Furthermore, monitor and nursing staff were blinded. |
| Incomplete outcome data addressed? | Low risk | Number and reasons for drop‐outs clearly described in 'Final report' page 38 to 40. |
| Free of selective reporting? | Low risk | Protocol available and reported on relevant and pre‐specified outcomes. |
| Early stopping? | Low risk | Sample size estimation fulfilled. |
| Baseline characteristics | Low risk | No significant difference. |
| Free of source of funding bias? | High risk | Grant from Valeant Pharmaceuticals International. |
| Methods | Study design: randomised clinical trial. | |
| Participants | Inclusion criteria: Exclusion criteria: Characteristics of included patients: | |
| Interventions | Ribavirin 1000 to 1200 mg daily versus ribavirin placebo versus interferon 3 million units thrice weekly for 24 weeks. | |
| Outcomes | Outcomes assessed 24 weeks after end of treatment. | |
| Notes | This trial also includes a treatment group receiving ribavirin plus interferon. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described. |
| Allocation concealment? | Low risk | Allocation concealment: sealed envelopes. |
| Blinding? | Low risk | "Use of matched placebo". |
| Incomplete outcome data addressed? | Low risk | Number and reasons for drop‐outs described (Figure 1). |
| Free of selective reporting? | Unclear risk | Protocol is not available. The authors reported on the primary outcomes in the review. |
| Early stopping? | Low risk | Sample size estimation fulfilled. |
| Baseline characteristics | Low risk | No significant difference. |
| Free of source of funding bias? | High risk | Financial support form Valeant Pharmaceuticals International and Schering Plough. |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing different ribavirin plus interferon regimens. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing different ribavirin plus interferon regimens. | |
| Randomised trial comparing different ribavirin plus interferon regimens. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing different ribavirin plus interferon regimens. | |
| Observational study. | |
| Randomised trial comparing different ribavirin plus interferon regimens. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Observational study. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial including patients who had undergone liver transplantation | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing different ribavirin plus interferon regimens. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Observational study. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Observational study. | |
| Observational study. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing ribavirin vs viramidine. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Observational study. | |
| Randomised trial comparing different ribavirin plus interferon regimens. | |
| Randomised trial comparing different ribavirin plus interferon regimens. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing ribavirin plus interferon with ribavirin plus interferon plus amantadine. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing ribavirin versus amantadine versus historical control group without antiviral treatment. | |
| Observational study. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Observational study. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Observational study. | |
| Observational study. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Possibly randomised trial comparing different ribavirin plus interferon regimens. | |
| Observational study. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Observational study. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing different ribavirin plus interferon regimens. | |
| Observational study. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Observational study. | |
| Observational study. | |
| Randomised trial comparing different ribavirin plus interferon regimens. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Observational study. | |
| Randomised trial comparing different ribavirin plus interferon regimens. | |
| Randomised trial concerning recurrent HCV infection in liver transplant recipients. | |
| Observational study. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing different ribavirin plus interferon regimens. | |
| Randomised trial comparing ribavirin vs viramidine. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Observational study. | |
| Observational study. | |
| Randomised trial comparing different ribavirin plus interferon regimens. | |
| Observational study. | |
| Observational study. | |
| Randomised trial comparing different ribavirin plus interferon regimens. | |
| Observational study. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Observational study. | |
| Randomised trial comparing ribavirin vs viramidine. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing different ribavirin plus interferon regimens. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Observational study. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing different regimens of interferon plus amantadine plus ribavirin. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised patients that responded (cleared HCV‐RNA) to combination therapy to ribavirin versus placebo. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Observational study. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing ribavirin vs viramidine. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Observational study. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing different ribavirin plus interferon regimens. | |
| Randomised trial comparing different ribavirin plus interferon regimens. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial in liver transplant recipients. | |
| Observational study. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised patients that responded (cleared HCV‐RNA) to combination therapy to ribavirin versus placebo. | |
| Observational study. | |
| Randomised trial comparing different ribavirin plus interferon regimens. | |
| Observational study. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing ribavirin plus interferon versus ribavirin. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. | |
| Observational study. | |
| Randomised trial comparing ribavirin plus interferon versus interferon. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure of serum virological response (number of patients with detectable hepatitis C virus RNA) Show forest plot | 10 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1 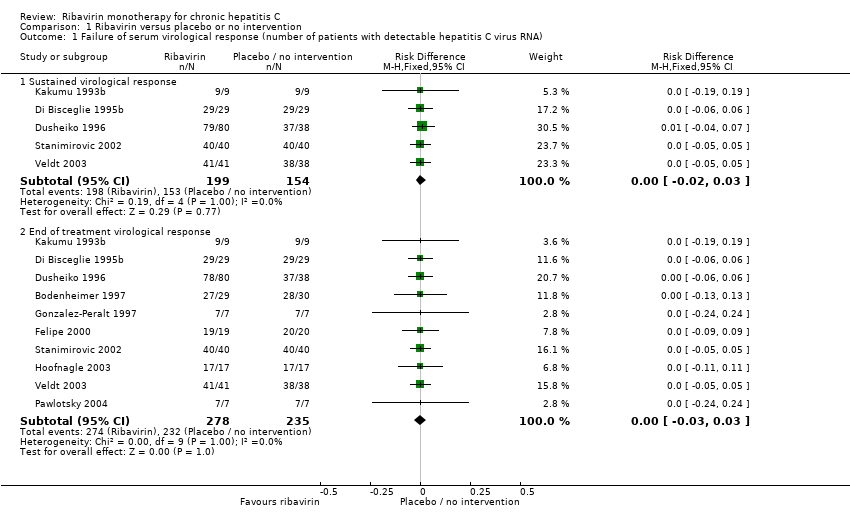 Comparison 1 Ribavirin versus placebo or no intervention, Outcome 1 Failure of serum virological response (number of patients with detectable hepatitis C virus RNA). | ||||
| 1.1 Sustained virological response | 5 | 353 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.02, 0.03] |
| 1.2 End of treatment virological response | 10 | 513 | Risk Difference (M‐H, Fixed, 95% CI) | ‐ [‐0.03, 0.03] |
| 2 Liver‐related morbidity and all‐cause mortality Show forest plot | 11 | 521 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.02, 0.03] |
| Analysis 1.2  Comparison 1 Ribavirin versus placebo or no intervention, Outcome 2 Liver‐related morbidity and all‐cause mortality. | ||||
| 3 Adverse events Show forest plot | 7 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3 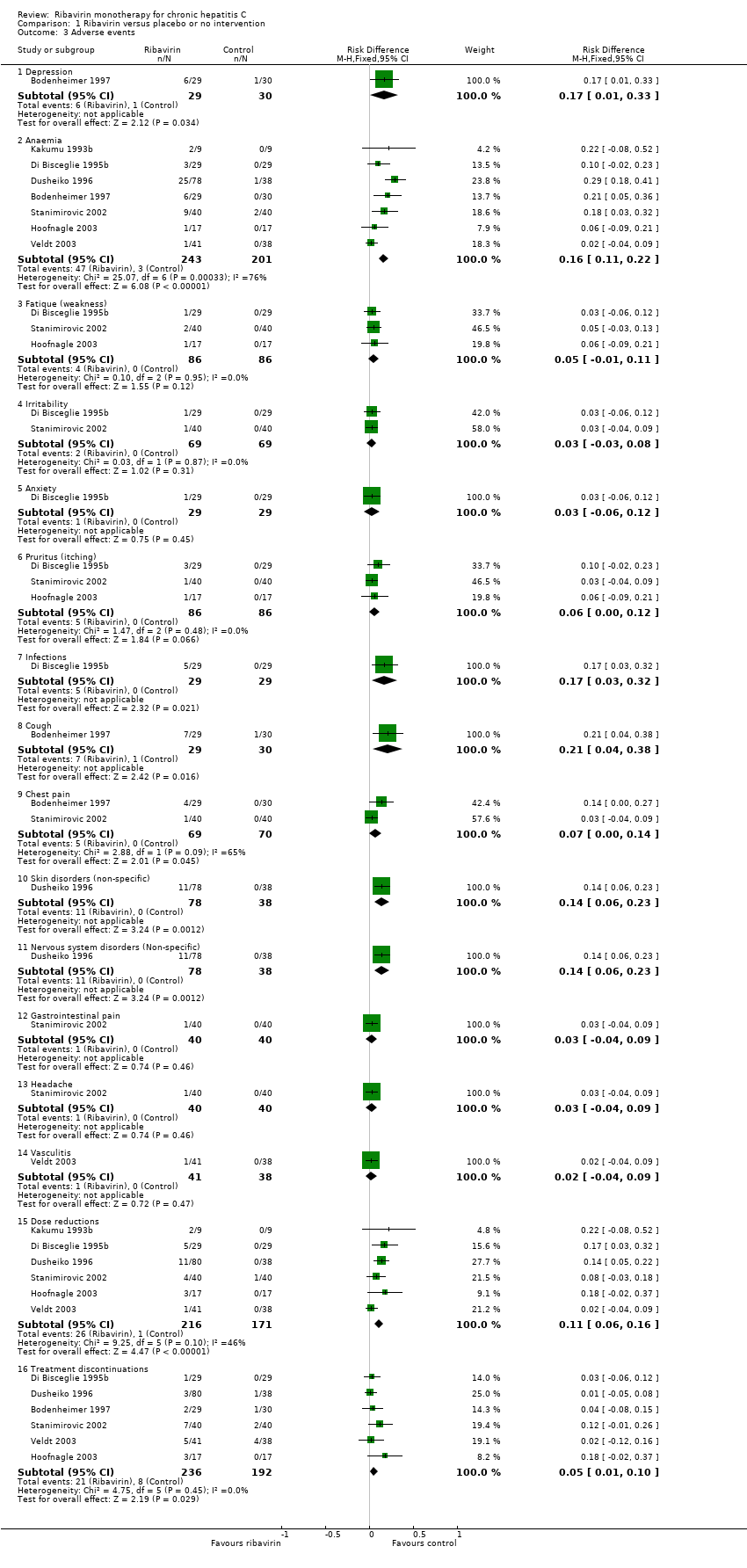 Comparison 1 Ribavirin versus placebo or no intervention, Outcome 3 Adverse events. | ||||
| 3.1 Depression | 1 | 59 | Risk Difference (M‐H, Fixed, 95% CI) | 0.17 [0.01, 0.33] |
| 3.2 Anaemia | 7 | 444 | Risk Difference (M‐H, Fixed, 95% CI) | 0.16 [0.11, 0.22] |
| 3.3 Fatique (weakness) | 3 | 172 | Risk Difference (M‐H, Fixed, 95% CI) | 0.05 [‐0.01, 0.11] |
| 3.4 Irritability | 2 | 138 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [‐0.03, 0.08] |
| 3.5 Anxiety | 1 | 58 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [‐0.06, 0.12] |
| 3.6 Pruritus (itching) | 3 | 172 | Risk Difference (M‐H, Fixed, 95% CI) | 0.06 [‐0.00, 0.12] |
| 3.7 Infections | 1 | 58 | Risk Difference (M‐H, Fixed, 95% CI) | 0.17 [0.03, 0.32] |
| 3.8 Cough | 1 | 59 | Risk Difference (M‐H, Fixed, 95% CI) | 0.21 [0.04, 0.38] |
| 3.9 Chest pain | 2 | 139 | Risk Difference (M‐H, Fixed, 95% CI) | 0.07 [0.00, 0.14] |
| 3.10 Skin disorders (non‐specific) | 1 | 116 | Risk Difference (M‐H, Fixed, 95% CI) | 0.14 [0.06, 0.23] |
| 3.11 Nervous system disorders (Non‐specific) | 1 | 116 | Risk Difference (M‐H, Fixed, 95% CI) | 0.14 [0.06, 0.23] |
| 3.12 Gastrointestinal pain | 1 | 80 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [‐0.04, 0.09] |
| 3.13 Headache | 1 | 80 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [‐0.04, 0.09] |
| 3.14 Vasculitis | 1 | 79 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.04, 0.09] |
| 3.15 Dose reductions | 6 | 387 | Risk Difference (M‐H, Fixed, 95% CI) | 0.11 [0.06, 0.16] |
| 3.16 Treatment discontinuations | 6 | 428 | Risk Difference (M‐H, Fixed, 95% CI) | 0.05 [0.01, 0.10] |
| 4 Failure of biochemical response (number of patients with elevated transaminase) Show forest plot | 10 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Ribavirin versus placebo or no intervention, Outcome 4 Failure of biochemical response (number of patients with elevated transaminase). | ||||
| 4.1 Sustained biochemical response | 5 | 294 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.05, 0.06] |
| 4.2 End of treatment biochemical response | 10 | 509 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.23 [‐0.29, ‐0.17] |
| 5 Failure of histologic response (number of patients without improvement in liver histology) Show forest plot | 3 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Ribavirin versus placebo or no intervention, Outcome 5 Failure of histologic response (number of patients without improvement in liver histology). | ||||
| 5.1 Combined necro‐inflammatory and fibrosis score (Intention to treat) | 3 | 211 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.14 [‐0.25, ‐0.02] |
| 5.2 Combined necro‐inflammatory and fibrosis score (Per protocol) | 3 | 156 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.20 [‐0.35, ‐0.06] |
| 6 Quality of life Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Ribavirin versus placebo or no intervention, Outcome 6 Quality of life. | ||||
| 6.1 Improvement of fatigue at end of treatment | 1 | 59 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.11 [‐0.27, 0.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure of serum virological response (number of patients with detectable hepatitis C virus RNA) Show forest plot | 5 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Ribavirin versus interferon, Outcome 1 Failure of serum virological response (number of patients with detectable hepatitis C virus RNA). | ||||
| 1.1 Sustained virological response | 2 | 48 | Risk Difference (M‐H, Fixed, 95% CI) | 0.13 [‐0.04, 0.29] |
| 1.2 End of treatment virological response | 5 | 151 | Risk Difference (M‐H, Fixed, 95% CI) | 0.17 [0.07, 0.27] |
| 2 Liver‐related and all‐cause morbidity and mortality Show forest plot | 5 | 151 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.06, 0.06] |
| Analysis 2.2  Comparison 2 Ribavirin versus interferon, Outcome 2 Liver‐related and all‐cause morbidity and mortality. | ||||
| 3 Failure of biochemical response (number of patients with elevated alanine aminotransferase) Show forest plot | 4 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3 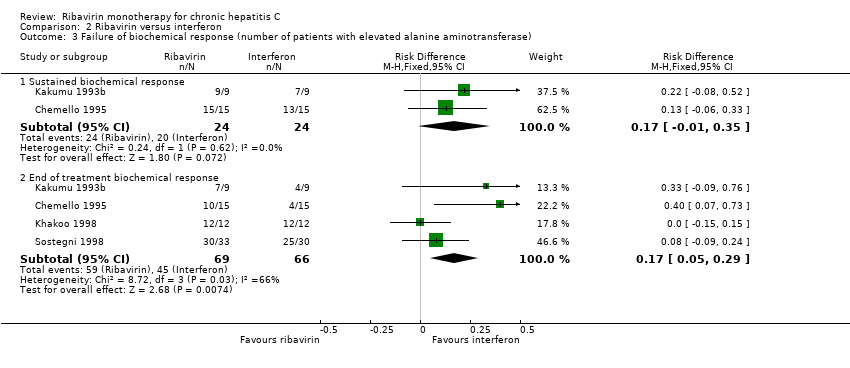 Comparison 2 Ribavirin versus interferon, Outcome 3 Failure of biochemical response (number of patients with elevated alanine aminotransferase). | ||||
| 3.1 Sustained biochemical response | 2 | 48 | Risk Difference (M‐H, Fixed, 95% CI) | 0.17 [‐0.01, 0.35] |
| 3.2 End of treatment biochemical response | 4 | 135 | Risk Difference (M‐H, Fixed, 95% CI) | 0.17 [0.05, 0.29] |
| 4 Adverse events Show forest plot | 4 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2 Ribavirin versus interferon, Outcome 4 Adverse events. | ||||
| 4.1 Flu‐like syndrome (non‐specific) | 1 | 30 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.2 [‐0.42, 0.02] |
| 4.2 Weight loss | 1 | 30 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.18, 0.18] |
| 4.3 Fatique/Weakness | 2 | 54 | Risk Difference (M‐H, Fixed, 95% CI) | 0.11 [‐0.09, 0.31] |
| 4.4 Irritability | 2 | 93 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.12, 0.11] |
| 4.5 Abdominal pain | 2 | 93 | Risk Difference (M‐H, Fixed, 95% CI) | 0.13 [0.03, 0.23] |
| 4.6 Diarrhea | 1 | 30 | Risk Difference (M‐H, Fixed, 95% CI) | 0.13 [‐0.06, 0.33] |
| 4.7 Pruritus | 2 | 93 | Risk Difference (M‐H, Fixed, 95% CI) | 0.06 [‐0.03, 0.15] |
| 4.8 Herpes labialis | 1 | 30 | Risk Difference (M‐H, Fixed, 95% CI) | 0.13 [‐0.11, 0.37] |
| 4.9 Anaemia | 2 | 93 | Risk Difference (M‐H, Fixed, 95% CI) | 0.09 [‐0.01, 0.18] |
| 4.10 Headache | 1 | 24 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.17 [‐0.51, 0.17] |
| 4.11 Pharyngitis | 1 | 24 | Risk Difference (M‐H, Fixed, 95% CI) | 0.08 [‐0.12, 0.29] |
| 4.12 Viral infections | 1 | 24 | Risk Difference (M‐H, Fixed, 95% CI) | 0.08 [‐0.12, 0.29] |
| 4.13 Myalgia | 1 | 24 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.08 [‐0.29, 0.12] |
| 4.14 Dose reductions | 3 | 72 | Risk Difference (M‐H, Fixed, 95% CI) | 0.08 [‐0.04, 0.21] |
| 4.15 Treatment discontinuations | 3 | 117 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.09, 0.11] |

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included trials.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Trial sequential analysis illustrating that the cumulative Z‐curve (blue) has not crossed the monitoring boundary (red) but have surpassed the information size (n = 232) needed to detect of reject an effect size corresponding to a number needed to treat of 10 patient (or fewer) for ribavirin. Thus we can rule out a number needed to treat of 10 patients (or fewer) for ribavirin regarding sustained virological response.
Trial sequential analysis was performed with a type I error of 5% and type II error of 20% (80% power). We assumed a baseline event rate of 97% without sustained virological response in the placebo/no intervention group. We used an event rate of 87% (number needed to treat = 10 patients) without sustained virological response in the ribavirin group.
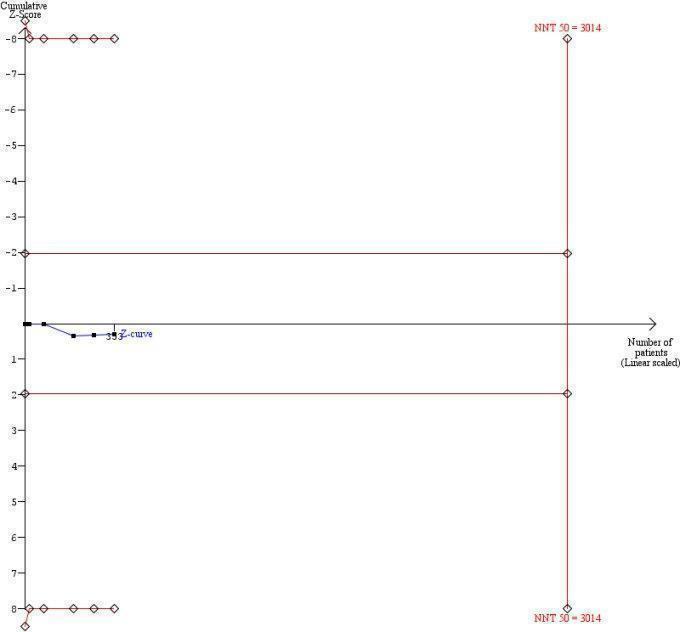
Trial sequential analysis illustrating that the cumulative Z‐curve (blue) has not crossed the monitoring boundary (red) nor reached the information size (n = 3014) needed to detect of reject an effect size corresponding to a number needed to treat of 50 (or fewer) for ribavirin. Thus we can not rule out a number needed to treat of 50 (or fewer) for ribavirin regarding sustained virological response. Additionally 2661 (3014 ‐ 353) patients are needed to detect or reject this effect size.
Trial sequential analysis was performed with a type I error of 5% and type II error of 20% (80% power). We assumed a baseline event rate of 97% for sustained virological response in the placebo/no intervention group. We used an event rate of 95% (number needed to treat = 50) without sustained virological response in the ribavirin group.
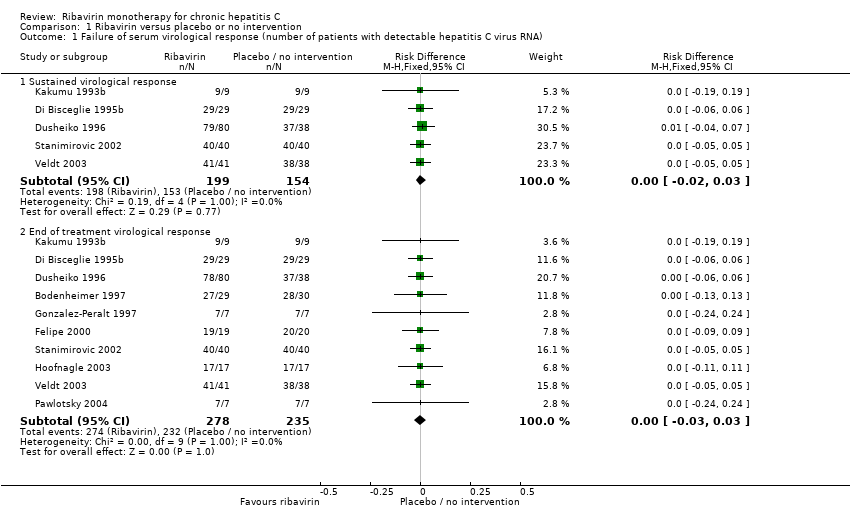
Comparison 1 Ribavirin versus placebo or no intervention, Outcome 1 Failure of serum virological response (number of patients with detectable hepatitis C virus RNA).

Comparison 1 Ribavirin versus placebo or no intervention, Outcome 2 Liver‐related morbidity and all‐cause mortality.
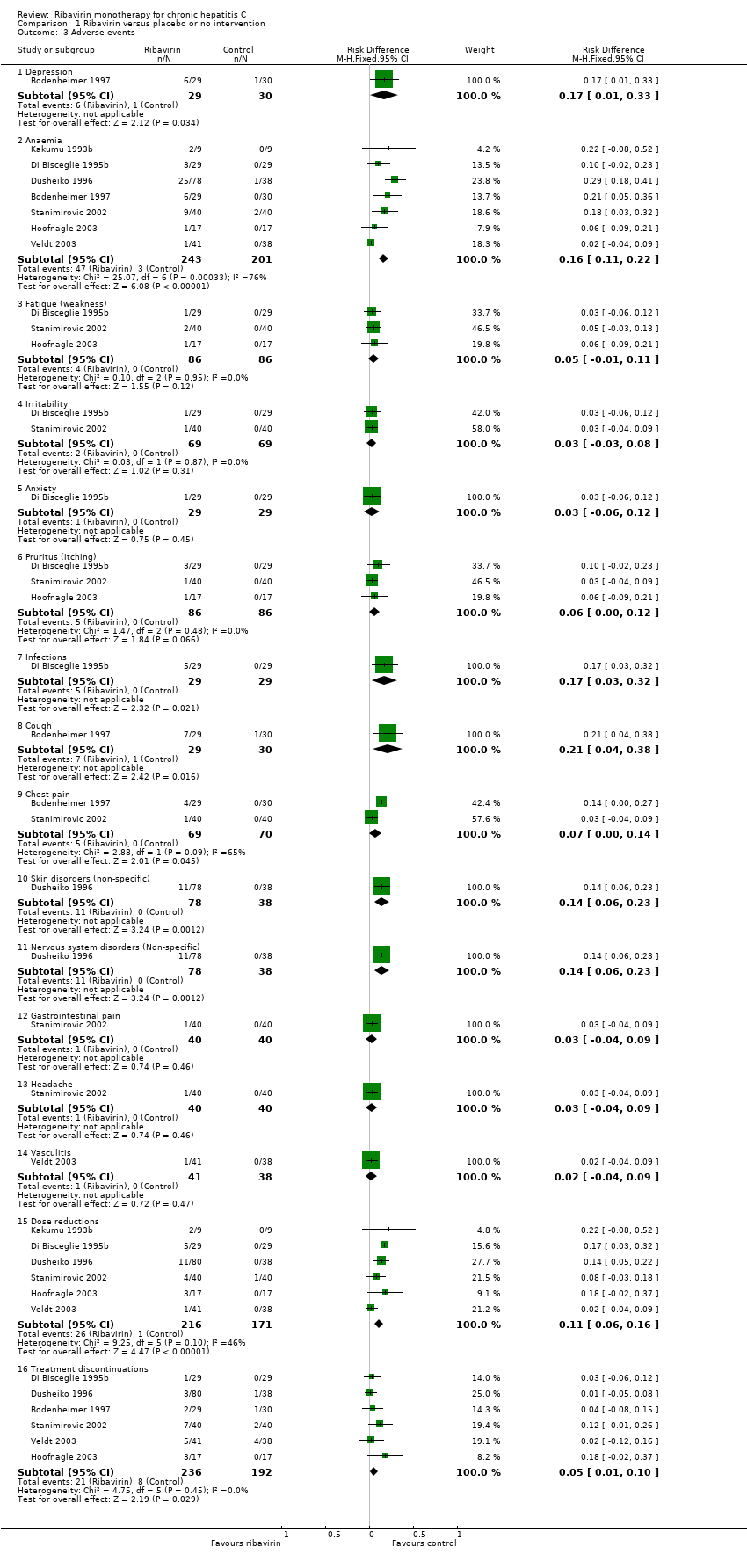
Comparison 1 Ribavirin versus placebo or no intervention, Outcome 3 Adverse events.

Comparison 1 Ribavirin versus placebo or no intervention, Outcome 4 Failure of biochemical response (number of patients with elevated transaminase).

Comparison 1 Ribavirin versus placebo or no intervention, Outcome 5 Failure of histologic response (number of patients without improvement in liver histology).
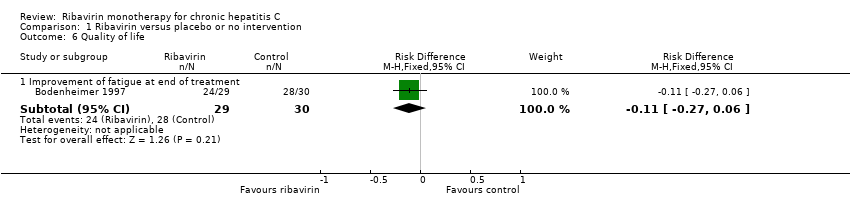
Comparison 1 Ribavirin versus placebo or no intervention, Outcome 6 Quality of life.
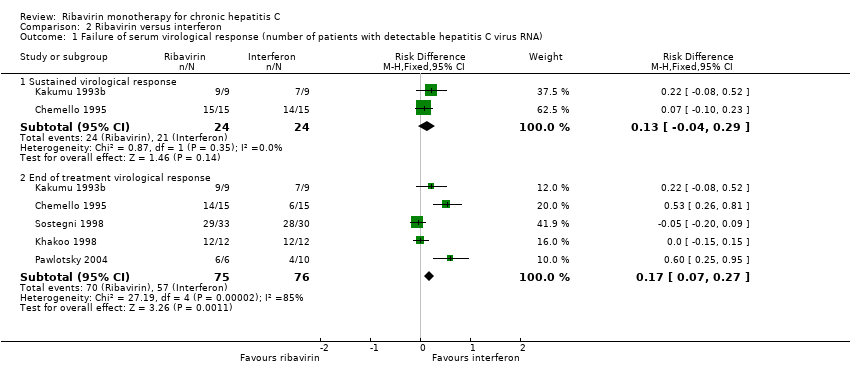
Comparison 2 Ribavirin versus interferon, Outcome 1 Failure of serum virological response (number of patients with detectable hepatitis C virus RNA).

Comparison 2 Ribavirin versus interferon, Outcome 2 Liver‐related and all‐cause morbidity and mortality.

Comparison 2 Ribavirin versus interferon, Outcome 3 Failure of biochemical response (number of patients with elevated alanine aminotransferase).

Comparison 2 Ribavirin versus interferon, Outcome 4 Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure of serum virological response (number of patients with detectable hepatitis C virus RNA) Show forest plot | 10 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Sustained virological response | 5 | 353 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.02, 0.03] |
| 1.2 End of treatment virological response | 10 | 513 | Risk Difference (M‐H, Fixed, 95% CI) | ‐ [‐0.03, 0.03] |
| 2 Liver‐related morbidity and all‐cause mortality Show forest plot | 11 | 521 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.02, 0.03] |
| 3 Adverse events Show forest plot | 7 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Depression | 1 | 59 | Risk Difference (M‐H, Fixed, 95% CI) | 0.17 [0.01, 0.33] |
| 3.2 Anaemia | 7 | 444 | Risk Difference (M‐H, Fixed, 95% CI) | 0.16 [0.11, 0.22] |
| 3.3 Fatique (weakness) | 3 | 172 | Risk Difference (M‐H, Fixed, 95% CI) | 0.05 [‐0.01, 0.11] |
| 3.4 Irritability | 2 | 138 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [‐0.03, 0.08] |
| 3.5 Anxiety | 1 | 58 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [‐0.06, 0.12] |
| 3.6 Pruritus (itching) | 3 | 172 | Risk Difference (M‐H, Fixed, 95% CI) | 0.06 [‐0.00, 0.12] |
| 3.7 Infections | 1 | 58 | Risk Difference (M‐H, Fixed, 95% CI) | 0.17 [0.03, 0.32] |
| 3.8 Cough | 1 | 59 | Risk Difference (M‐H, Fixed, 95% CI) | 0.21 [0.04, 0.38] |
| 3.9 Chest pain | 2 | 139 | Risk Difference (M‐H, Fixed, 95% CI) | 0.07 [0.00, 0.14] |
| 3.10 Skin disorders (non‐specific) | 1 | 116 | Risk Difference (M‐H, Fixed, 95% CI) | 0.14 [0.06, 0.23] |
| 3.11 Nervous system disorders (Non‐specific) | 1 | 116 | Risk Difference (M‐H, Fixed, 95% CI) | 0.14 [0.06, 0.23] |
| 3.12 Gastrointestinal pain | 1 | 80 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [‐0.04, 0.09] |
| 3.13 Headache | 1 | 80 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [‐0.04, 0.09] |
| 3.14 Vasculitis | 1 | 79 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.04, 0.09] |
| 3.15 Dose reductions | 6 | 387 | Risk Difference (M‐H, Fixed, 95% CI) | 0.11 [0.06, 0.16] |
| 3.16 Treatment discontinuations | 6 | 428 | Risk Difference (M‐H, Fixed, 95% CI) | 0.05 [0.01, 0.10] |
| 4 Failure of biochemical response (number of patients with elevated transaminase) Show forest plot | 10 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Sustained biochemical response | 5 | 294 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.05, 0.06] |
| 4.2 End of treatment biochemical response | 10 | 509 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.23 [‐0.29, ‐0.17] |
| 5 Failure of histologic response (number of patients without improvement in liver histology) Show forest plot | 3 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Combined necro‐inflammatory and fibrosis score (Intention to treat) | 3 | 211 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.14 [‐0.25, ‐0.02] |
| 5.2 Combined necro‐inflammatory and fibrosis score (Per protocol) | 3 | 156 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.20 [‐0.35, ‐0.06] |
| 6 Quality of life Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Improvement of fatigue at end of treatment | 1 | 59 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.11 [‐0.27, 0.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure of serum virological response (number of patients with detectable hepatitis C virus RNA) Show forest plot | 5 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Sustained virological response | 2 | 48 | Risk Difference (M‐H, Fixed, 95% CI) | 0.13 [‐0.04, 0.29] |
| 1.2 End of treatment virological response | 5 | 151 | Risk Difference (M‐H, Fixed, 95% CI) | 0.17 [0.07, 0.27] |
| 2 Liver‐related and all‐cause morbidity and mortality Show forest plot | 5 | 151 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.06, 0.06] |
| 3 Failure of biochemical response (number of patients with elevated alanine aminotransferase) Show forest plot | 4 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Sustained biochemical response | 2 | 48 | Risk Difference (M‐H, Fixed, 95% CI) | 0.17 [‐0.01, 0.35] |
| 3.2 End of treatment biochemical response | 4 | 135 | Risk Difference (M‐H, Fixed, 95% CI) | 0.17 [0.05, 0.29] |
| 4 Adverse events Show forest plot | 4 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Flu‐like syndrome (non‐specific) | 1 | 30 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.2 [‐0.42, 0.02] |
| 4.2 Weight loss | 1 | 30 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.18, 0.18] |
| 4.3 Fatique/Weakness | 2 | 54 | Risk Difference (M‐H, Fixed, 95% CI) | 0.11 [‐0.09, 0.31] |
| 4.4 Irritability | 2 | 93 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.12, 0.11] |
| 4.5 Abdominal pain | 2 | 93 | Risk Difference (M‐H, Fixed, 95% CI) | 0.13 [0.03, 0.23] |
| 4.6 Diarrhea | 1 | 30 | Risk Difference (M‐H, Fixed, 95% CI) | 0.13 [‐0.06, 0.33] |
| 4.7 Pruritus | 2 | 93 | Risk Difference (M‐H, Fixed, 95% CI) | 0.06 [‐0.03, 0.15] |
| 4.8 Herpes labialis | 1 | 30 | Risk Difference (M‐H, Fixed, 95% CI) | 0.13 [‐0.11, 0.37] |
| 4.9 Anaemia | 2 | 93 | Risk Difference (M‐H, Fixed, 95% CI) | 0.09 [‐0.01, 0.18] |
| 4.10 Headache | 1 | 24 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.17 [‐0.51, 0.17] |
| 4.11 Pharyngitis | 1 | 24 | Risk Difference (M‐H, Fixed, 95% CI) | 0.08 [‐0.12, 0.29] |
| 4.12 Viral infections | 1 | 24 | Risk Difference (M‐H, Fixed, 95% CI) | 0.08 [‐0.12, 0.29] |
| 4.13 Myalgia | 1 | 24 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.08 [‐0.29, 0.12] |
| 4.14 Dose reductions | 3 | 72 | Risk Difference (M‐H, Fixed, 95% CI) | 0.08 [‐0.04, 0.21] |
| 4.15 Treatment discontinuations | 3 | 117 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.09, 0.11] |

