Contenido relacionado
Revisiones y protocolos relacionados
Ronald L Koretz, Maria Pleguezuelo, Vasiliki Arvaniti, Pilar Barrera Baena, Ruben Ciria, Kurinchi Selvan Gurusamy, Brian R Davidson, Andrew K Burroughs | 31 enero 2013
Jesper Brok, Lise Lotte Gluud, Christian Gluud | 20 enero 2010
Goran Hauser, Tahany Awad, Jesper Brok, Kristian Thorlund, Davor Štimac, Mahasen Mabrouk, Christian Gluud, Lise Lotte Gluud | 28 febrero 2014
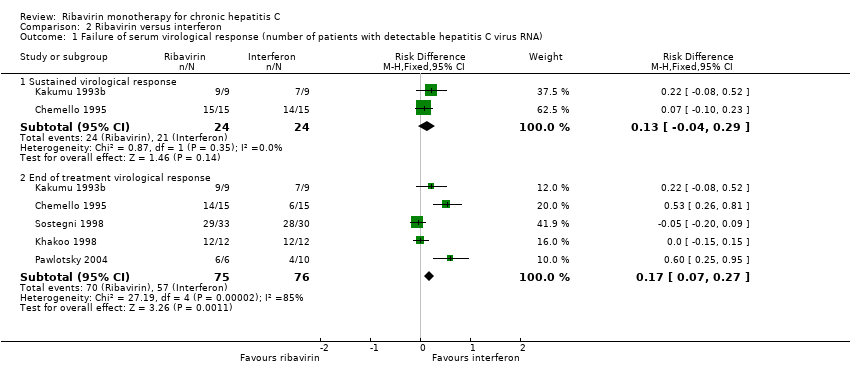
Jesper Brok, Martin Thyge Mellerup, Kim Krogsgaard, Christian Gluud | 19 abril 2004
Interferón para los pacientes con hepatitis C crónica que no han recibido tratamiento con interferón
Robert P Myers, Corinne Regimbeau, Thierry Thevenot, Vincent Leroy, Philippe Mathurin, Pierre Opolon, Jean Pierre Zarski, Thierry Poynard | 22 abril 2002
Kristiana Nikolova, Christian Gluud, Berit Grevstad, Janus C Jakobsen | 6 abril 2014
Lise Lotte Gluud, Kim Krogsgaard, Christian Gluud | 22 abril 2002
Mieke H Lamers, Mark Broekman, Joost PH Drenth, Christian Gluud | 3 mayo 2014
Maria Kalafateli, Elena Buzzetti, Douglas Thorburn, Brian R Davidson, Emmanuel Tsochatzis, Kurinchi Selvan Gurusamy | 3 diciembre 2018
Janus C Jakobsen, Emil Eik Nielsen, Joshua Feinberg, Kiran Kumar Katakam, Kristina Fobian, Goran Hauser, Goran Poropat, Snezana Djurisic, Karl Heinz Weiss, Milica Bjelakovic, Goran Bjelakovic, Sarah Louise Klingenberg, Jian Ping Liu, Dimitrinka Nikolova, Ronald L Koretz, Christian Gluud | 18 septiembre 2017