Insuflaciones sostenidas versus estándar durante la reanimación neonatal para prevenir la mortalidad y mejorar los resultados respiratorios
Información
- DOI:
- https://doi.org/10.1002/14651858.CD004953.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 14 julio 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Neonatología
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Dr. Bruschettini and Dr. O'Donnell performed the literature search, extracted and analysed data, and wrote the manuscript. Prof. Davis performed the literature search, extracted data, checked the analysis, and reviewed the manuscript. Prof. Morley and Dr. Moja reviewed the manuscript. Dr. Zappettini performed the literature search, extracted data, and reviewed the manuscript. Dr. Calevo analysed data, checked the analysis, and reviewed the manuscript.
Sources of support
Internal sources
-
Institute for Clinical Sciences, Lund University; Research & Development, Section for HTA Analysis, Skåne University Hospital, Lund, Sweden.
-
Royal Women's Hospital, Melbourne, Australia.
-
University of Melbourne, Australia.
-
Istituto Giannina Gaslini, Genoa, Italy.
External sources
-
Murdoch Childrens Research Insitute, Australia.
-
National Health and Medical Research Council, Australia.
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA.
-
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C
-
Declarations of interest
MB, COD, PD, CM, LM, SZ, and MGC have no known conflicts of interest to declare.
Acknowledgements
We thank Drs. Lindner, te Pas, Harling, El‐Farghali (for El‐Chimi 2017), Mercadante, Nuntnarumit (Jiravisitkul 2017), Schmolzer (for Ngan 2017 and Schmölzer 2015), and Schwaberger for their gracious assistance in providing extra data.
We acknowledge the help of Ms. Jennifer Spano and Ms. Colleen Ovelman in conducting literature searches for this update of the review.
Version history
| Published | Title | Stage | Authors | Version |
| 2020 Mar 18 | Sustained versus standard inflations during neonatal resuscitation to prevent mortality and improve respiratory outcomes | Review | Matteo Bruschettini, Colm PF O'Donnell, Peter G Davis, Colin J Morley, Lorenzo Moja, Maria Grazia Calevo | |
| 2017 Jul 14 | Sustained versus standard inflations during neonatal resuscitation to prevent mortality and improve respiratory outcomes | Review | Matteo Bruschettini, Colm PF O'Donnell, Peter G Davis, Colin J Morley, Lorenzo Moja, Simona Zappettini, Maria Grazia Calevo | |
| 2015 Jul 01 | Sustained versus standard inflations during neonatal resuscitation to prevent mortality and improve respiratory outcomes | Review | Colm PF O'Donnell, Matteo Bruschettini, Peter G Davis, Colin J Morley, Lorenzo Moja, Maria Grazia Calevo, Simona Zappettini | |
| 2004 Oct 18 | Sustained inflations for neonatal resuscitation | Protocol | Colm PF O'Donnell, Peter G Davis, Colin J Morley | |
Differences between protocol and review
We added clinically relevant outcomes (surfactant administration, need for mechanical ventilation, retinopathy of prematurity, and PDA).
We planned subgroup analyses according to gestational age (< 37 weeks, ≥ 37 weeks), ventilation device used (self‐inflating bag, flow‐inflating bag, T‐piece, mechanical ventilator), patient interface used (face mask, ETT, nasopharyngeal tube), and duration of sustained inflation (> 1 second to 5 seconds, > 5 seconds). We were unable to conduct any subgroup analyses as few trials met the inclusion criteria.
For this update, we made the post hoc decision to add a comparison based on use of chest compression during resuscitation. Moreover, we specified Unit of analysis issues and Sensitivity analysis.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Ductus Arteriosus, Patent [epidemiology];
- Hospital Mortality;
- Intubation, Intratracheal [methods, mortality];
- Positive‐Pressure Respiration [*methods, mortality];
- Pulmonary Surfactants [administration & dosage];
- Randomized Controlled Trials as Topic;
- Respiration, Artificial [statistics & numerical data];
- Resuscitation [*methods];
- Time Factors;
Medical Subject Headings Check Words
Humans; Infant, Newborn;
PICO
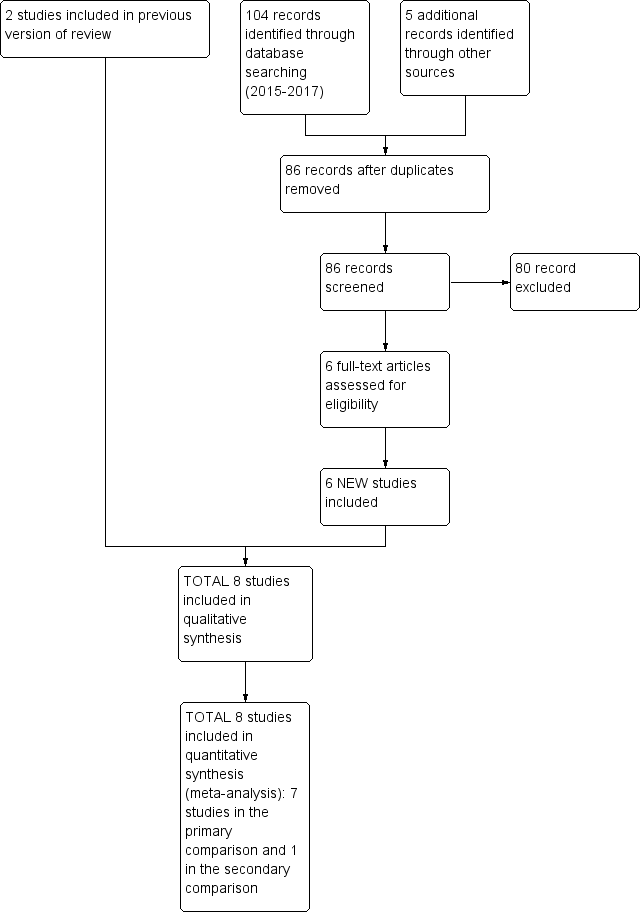
Study flow diagram: review update.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials.

Forest plot of comparison: 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, outcome: 1.1 Death.

Forest plot of comparison: 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, outcome: 1.4 Endotracheal intubation.

Forest plot of comparison: 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, outcome: 1.5 Surfactant administration.
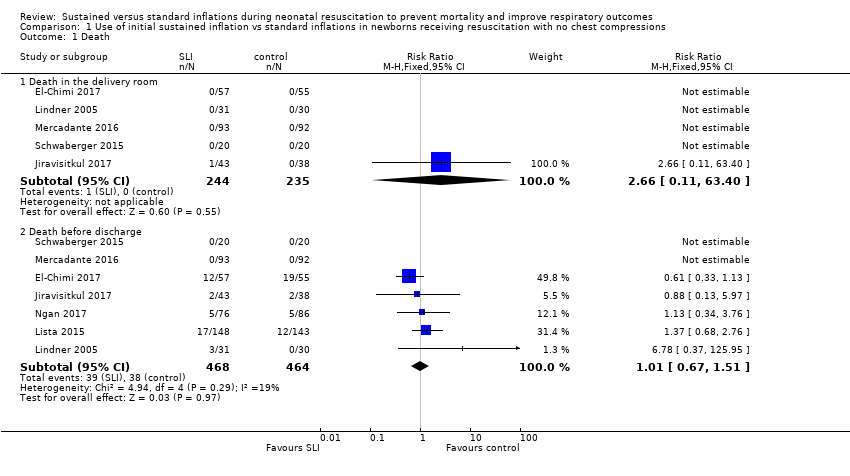
Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 1 Death.

Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 2 Apgar at 1 minute.

Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 3 Apgar at 5 minutes.

Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 4 Endotracheal intubation.
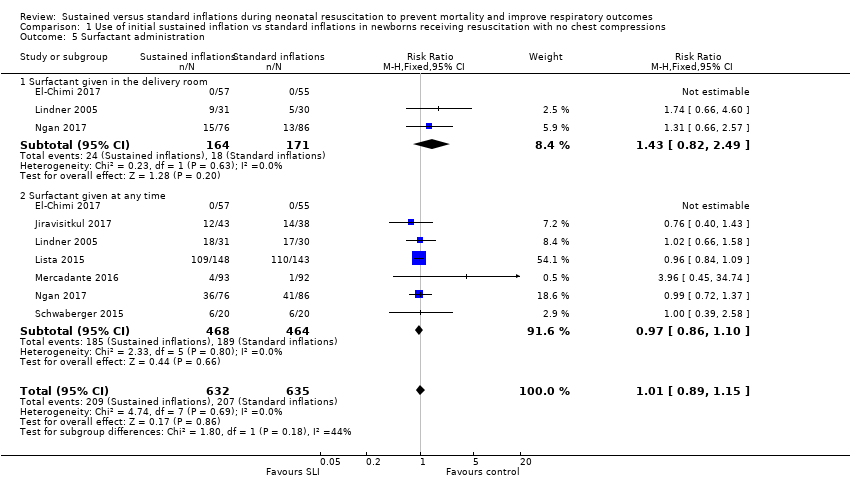
Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 5 Surfactant administration.
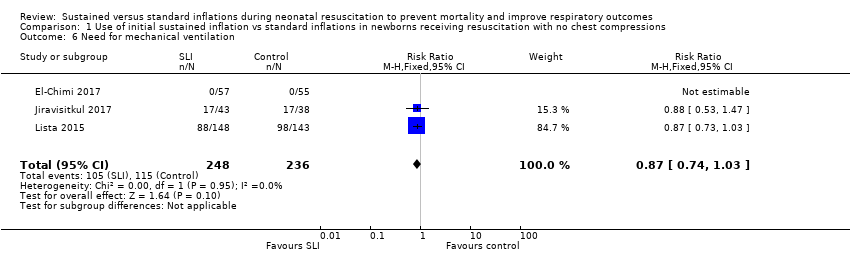
Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 6 Need for mechanical ventilation.
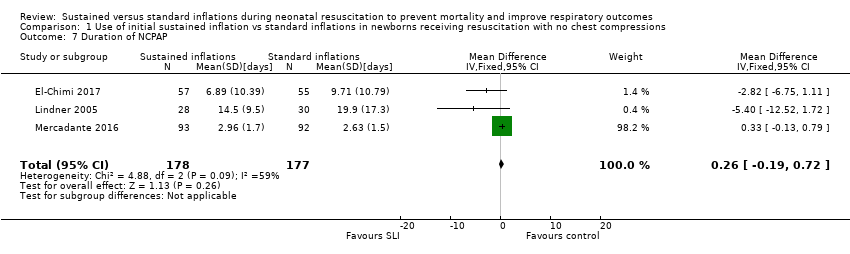
Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 7 Duration of NCPAP.

Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 8 Duration of mechanical ventilation.

Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 9 Duration of respiratory support (NCPAP + MV).

Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 10 Duration of supplemental oxygen requirement.

Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 11 Chronic lung disease.

Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 12 Pneumothorax.

Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 13 Cranial ultrasound abnormalities.

Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 14 Retinopathy of prematurity (ROP) stage ≥ 3.

Comparison 1 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with no chest compressions, Outcome 15 Patent ductus arteriosus (PDA).

Comparison 2 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with chest compressions, Outcome 1 Death.

Comparison 2 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with chest compressions, Outcome 2 Endotracheal intubation.

Comparison 2 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with chest compressions, Outcome 3 Surfactant administration.

Comparison 2 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with chest compressions, Outcome 4 Chronic lung disease.

Comparison 2 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with chest compressions, Outcome 5 Pneumothorax.

Comparison 2 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with chest compressions, Outcome 6 Cranial ultrasound abnormalities.

Comparison 2 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with chest compressions, Outcome 7 Retinopathy of prematurity (ROP) stage ≥ 3.

Comparison 2 Use of initial sustained inflation vs standard inflations in newborns receiving resuscitation with chest compressions, Outcome 8 Patent ductus arteriosus (PDA).
| Use of initial sustained inflation compared with standard inflations in newborns receiving resuscitation with no chest compressions during resuscitation | ||||||
| Patient or population: preterm infants resuscitated using PPV at birth Settings: delivery room in Europe (Austria, Germany, Italy), Canada, Egypt, Thailand | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard inflations in newborns receiving resuscitation with no chest compressions | Use of initial sustained inflation | |||||
| Death ‐ death in the delivery room | Study population | RR 2.66 | 479 | ⊕⊕⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Death ‐ death before discharge | Study population | RR 1.01 | 932 | ⊕⊕⊕⊝ | ||
| 82 per 1000 | 83 per 1000 | |||||
| Need for mechanical ventilation | Study population | RR 0.87 | 484 | ⊕⊕⊕⊝ | ||
| 487 per 1000 | 424 per 1000 | |||||
| Chronic lung disease ‐ BPD any grade | Study population | RR 0.9 | 220 | ⊕⊕⊕⊝ | ||
| 483 per 1000 | 435 per 1000 | |||||
| Chronic lung disease ‐ moderate to severe BPD | Study population | RR 0.95 | 683 | ⊕⊕⊕⊝ | ||
| 257 per 1000 | 244 per 1000 | |||||
| Pneumothorax ‐ any time | Study population | RR 1.44 | 851 | ⊕⊕⊝⊝ | ||
| 33 per 1000 | 48 per 1000 | |||||
| Cranial ultrasound abnormalities ‐ intraventricular haemorrhage grade 3 to 4 | Study population | RR 0.89 | 635 | ⊕⊕⊕⊝ | ||
| 120 per 1000 | 107 per 1000 | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| Assumed risk is the risk of the control arm. aLimitations in study design: all studies at high or unclear risk of bias in at least one domain | ||||||
| Use of initial sustained inflation compared with standard inflations in newborns receiving resuscitation with chest compressions during resuscitation | ||||||
| Patient or population: preterm infants resuscitated by PPV at birth Settings: delivery room in Europe (Austria, Germany, Italy), Canada, Egypt, Thailand | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard inflations in newborns receiving resuscitation with chest compressions | Use of initial sustained inflation | |||||
| Death ‐ death before discharge | See comment | See comment | Not estimable | 9 | ⊕⊝⊝⊝ | Only 1 trial included |
| Chronic lung disease ‐ moderate to severe BPD | See comment | See comment | Not estimable | 7 | ⊕⊝⊝⊝ | Only 1 trial included |
| Pneumothorax ‐ any time | See comment | See comment | Not estimable | 9 | ⊕⊝⊝⊝ | Only 1 trial included |
| Cranial ultrasound abnormalities ‐ intraventricular haemorrhage grade 3 to 4 | See comment | See comment | Not estimable | 9 | ⊕⊝⊝⊝ | Only 1 trial included |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| Assumed risk is the risk of the control arm. aLimitations in study design: included study at high or unclear risk of bias in four domains | ||||||
| Trial (no. infants) | Antenatal steroids | Gestational age, weeks | Birth weight, grams | Device/Interface | Interventions/Controls | ||||
| SLI | Control | SLI | Control | SLI | Control | SLI and control | SLI | Control | |
| El‐Chimi 2017 (112) | 39% | 34.5% | mean 31.1 (SD 1.7) | mean 31.3 (SD 1.7) | mean 1561 (SD 326) | mean 1510 (SD 319) | Mask and T‐piece in SLI group Mask and self‐inflating bag with an oxygen reservoir in control group | PIP of 20 cmH2O for 15 seconds, followed by PEEP of 5 cmH2O If needed: a second SLI of 15 seconds of 25 cmH2O for 15 seconds, followed by PEEP of 6 cmH2O; then a third SLI of 15 seconds of 30 cmH2O for 15 seconds, followed by PEEP of 7 cmH2O If still not satisfactory: intubated in delivery room | PIP maximum 40 cmH2O, rate of 40 to 60 breaths/min for 30 seconds |
| Jiravisitkul 2017 (81) | 63% | 74% | 25 to 28 weeks: 29 to 32 weeks: | 25 to 28 weeks: 29 to 32 weeks: | mean 1206 (SD 367) | mean 1160 (SD 411) | Mask and T‐piece | PIP of 25 cmH2O for 15 seconds If HR 60 to 100 beats/min and/or poor respiratory effort: a second SLI (25 cmH2O, 15 seconds) | PIP 15 to 20 cmH2O, PEEP 5 cmH2O for 30 seconds, followed by resuscitation according to AHA guidelines |
| Lindner 2005 (61) | 81% | 80% | median 27.0 (IQR 25.0 to 28.9) | median 26.7 (IQR 25.0 to 28.9) | median 870 (IQR 410 to 1320) | median 830 (IQR 370 to 1370) | Nasopharyngeal tube (fixed at 4 to 5 cm) and mechanical ventilator | PIP of 20 cmH2O for 15 seconds If response was not satisfactory: 2 further SLIs of 15 seconds (25 and 30 cmH2O). Then PEEP at 4 to 6 cmH2O | PIP 20 cmH2O, PEEP 4 to 6 cmH2O; inflation time 0.5 seconds; inflation rate 60 per min. Then, PEEP at 4 to 6 cmH2O |
| Lista 2015 (301) | 87% | 91% | mean 26.8 (SD 1.2); 25 to 26 weeks: 27 to 28 weeks: | mean 26.8 (SD 1.1); 25 to 26 weeks: 27 to 28 weeks: | mean 894 (SD 247) | mean 893 (SD 241) | Mask and T‐piece | PIP 25 cmH2O for 15 seconds. Then reduced to PEEP of 5 cmH2O | PEEP 5 cmH2O, followed by resuscitation according to AHA guidelines |
| Mercadante 2016 (185) | 40% | 32% | mean 35.2 (SD 0.8) | mean 35.2 (SD 0.8) | mean 2345 (SD 397) | mean 2346 (SD 359) | Mask and T‐piece | PIP 25 cmH2O for 15 seconds, followed by PEEP of 5 cmH2O. In case of persistent heart failure (HR < 100 bpm): SLI repeated | PEEP 5 cmH2O, followed by resuscitation according to AAP guidelines |
| Ngan 2017 (162) | 78% | 70% | mean 28 (SD 2.5) | mean 28 (SD 2.5) | mean 1154 (SD 426) | mean 1140 (SD 406) | Mask and T‐piece | Two PIPs of 24 cmH2O. Duration of first SLI was 20 seconds. Duration of second SLI was 20 or 10 seconds, guided by ECO2 values. After SLIs, CPAP if breathing spontaneously or, if found to have apnoea or laboured breathing, mask IPPV at a rate of 40 to 60 bpm | IPPV, rate of 40 to 60 inflations/min until spontaneous breathing, at which time CPAP will be provided |
| Schmölzer 2015 (9) | 80%a | 100%a | mean 24.6 (SD 1.3)a | mean 25.6 (SD 2.3)a | mean 707 (SD 208)a | mean 808 (SD 192)a | Mask and T‐piecea | PIP for 20 + 20 secondsa during chest compressions | 3:1 compression:ventilation ratio according to resuscitation guidelines |
| Schwaberger 2015 (40) | not reported | not reported | mean 32.1 (SD 1.4) | mean 32.1 (SD 1.6) | mean 1692 (SD 297) | mean 1722 (SD 604) | Mask and T‐piece | PIP 30 cmH2O for 15 seconds, to be repeated once or twice with HR remaining < 100 bpm. Infants with HR > 100 bpm: PPV at 30 cmH2O PIP or CPAP at PEEP level of 5 cmH2O depending on respiratory rate | Resuscitation according to AHA guidelines PEEP 5 cmH2O if respiratory rate > 30 and signs of respiratory distress PPV at 30 cmH2O PIP if insufficient breathing efforts |
| aInformation provided by study authors | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Death in the delivery room | 5 | 479 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.66 [0.11, 63.40] |
| 1.2 Death before discharge | 7 | 932 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.67, 1.51] |
| 2 Apgar at 1 minute Show forest plot | 5 | 529 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.26, 0.09] |
| 3 Apgar at 5 minutes Show forest plot | 6 | 641 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.13, 0.08] |
| 4 Endotracheal intubation Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Endotracheal intubation in the delivery room | 5 | 601 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.62, 1.19] |
| 4.2 Endotracheal intubation within 24 hours | 2 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.4 [0.53, 3.68] |
| 4.3 Endotracheal intubation by 72 hours of age | 5 | 811 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.79, 1.09] |
| 5 Surfactant administration Show forest plot | 7 | 1267 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.89, 1.15] |
| 5.1 Surfactant given in the delivery room | 3 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.82, 2.49] |
| 5.2 Surfactant given at any time | 7 | 932 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.86, 1.10] |
| 6 Need for mechanical ventilation Show forest plot | 3 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.74, 1.03] |
| 7 Duration of NCPAP Show forest plot | 3 | 355 | Mean Difference (IV, Fixed, 95% CI) | 0.26 [‐0.19, 0.72] |
| 8 Duration of mechanical ventilation Show forest plot | 5 | 524 | Mean Difference (IV, Fixed, 95% CI) | ‐5.37 [‐6.31, ‐4.43] |
| 9 Duration of respiratory support (NCPAP + MV) Show forest plot | 2 | 243 | Mean Difference (IV, Fixed, 95% CI) | 0.69 [0.23, 1.16] |
| 10 Duration of supplemental oxygen requirement Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 Chronic lung disease Show forest plot | 6 | 903 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.77, 1.13] |
| 11.1 BPD any grade | 2 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.69, 1.19] |
| 11.2 Moderate to severe BPD | 5 | 683 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.74, 1.22] |
| 12 Pneumothorax Show forest plot | 7 | 932 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.76, 2.61] |
| 12.1 During first 48 hours | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.06, 13.65] |
| 12.2 At any time | 6 | 851 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.76, 2.72] |
| 13 Cranial ultrasound abnormalities Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Intraventricular haemorrhage grade 3‐4 | 5 | 635 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.58, 1.37] |
| 13.2 IVH any grade | 2 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.40, 1.69] |
| 13.3 Cystic periventricular leukomalacia | 5 | 635 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.24, 1.44] |
| 14 Retinopathy of prematurity (ROP) stage ≥ 3 Show forest plot | 5 | 632 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.44, 1.10] |
| 15 Patent ductus arteriosus (PDA) Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 PDA ‐ pharmacological treatment | 6 | 745 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.90, 1.30] |
| 15.2 PDA ‐ surgical closure | 3 | 412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.27, 1.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Death before discharge | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Endotracheal intubation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Endotracheal intubation in the delivery room | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Surfactant administration Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Surfactant given in the delivery room | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Chronic lung disease Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Moderate to severe BPD | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Pneumothorax Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 At any time | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Cranial ultrasound abnormalities Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Intraventricular haemorrhage grade 3 to 4 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 IVH any grade | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Retinopathy of prematurity (ROP) stage ≥ 3 Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Patent ductus arteriosus (PDA) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 PDA ‐ pharmacological treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

