Effects and safety of preventive oral iron or iron+folic acid supplementation for women during pregnancy
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomisation: adequate by means of computer‐generated numbers. Allocation concealment: adequate. Blinding: adequate. Participant and care provider blinded. Loss to follow up: adequate. Less than 5%. | |
| Participants | 97 healthy women attending prenatal care at National Maternity Hospital, Dublin, Ireland with singleton pregnancy, during their first trimester of pregnancy, and with haemoglobin equal or higher than 140 g/L were assigned to the groups. Women were excluded if they had a recent blood transfusion, chronic respiratory disease, chronic hypertension, renal disease, diabetes mellitus, history of haematologic disorder and alcohol dependence. | |
| Interventions | Women were randomly assigned to one of two groups: group 1: received iron and folic acid tablets, one tablet to be taken by mouth twice daily (each tablet contained 0.5 mg of folic acid and 60 mg elemental iron); group 2: placebo tablets also to be taken by mouth twice daily. | |
| Outcomes | Maternal: haemoglobin, hematocrit, serum erythropoietin concentrations at baseline and at 24, 28, 32, 36 and 40 wk; serum ferritin at baseline and at 36 wk; number of hypertensive disorders, antepartum haemorrhage, cesarean delivery. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate by means of computer‐generated numbers. |
| Allocation concealment? | Low risk | Adequate. |
| Blinding? | Low risk | Adequate. |
| Incomplete outcome data addressed? | Low risk | Adequate ‐ less than 5% lost to follow up. |
| Methods | Randomisation: unclear ‐ method not stated. Allocation concealment: unclear. Blinding: unclear ‐ participant blinded. Provider/assessor not stated or clear. Loss to follow up: inadequate ‐ 37 women (28%) were excluded for analysis. | |
| Participants | 133 women referred to investigators from a population of women attending an antenatal clinic for the fist time in Yangoon (also known as Rangoon), Myanmar (Burma). Women with severe anaemia were excluded from the trial during the intervention for treatment. | |
| Interventions | Women were randomly assigned to one of four groups starting at 22‐25 wks: group 1: one ferrous sulphate tablet containing 60 mg of elemental iron, and one placebo tablets twice daily; group 2: one tablet containing 60 mg of elemental iron as ferrous sulphate, and one tablet containing 0.5 mg of folic acid twice daily; group 3: two placebo tablets twice daily; group 4: one placebo tablet and one tablet containing 0.5 mg of folic acid twice daily. Administration of the treatments was carefully supervised. Supplementation started at 22‐25 wks until term. | |
| Outcomes | Maternal: haemoglobin concentrations at baseline, at term (38‐40th wk) and 4‐7 wk postpartum, serum iron, serum and red cell folate activity and hypersegmented polimorph count at baseline, at 38‐40th wk and postpartum. | |
| Notes | Supervised. 32 women who had taken other supplements or whose Hb level at full term was not available were excluded from the analysis. Three women from group 3 and two from group 4 developed severe anaemia and were also withdrawn from analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear ‐ method not stated. |
| Allocation concealment? | Unclear risk | Unclear. |
| Blinding? | Unclear risk | Unclear ‐ participant blinded. Provider/assessor not stated or clear. |
| Incomplete outcome data addressed? | High risk | Inadequate ‐ 37 women (28%) were excluded for analysis. |
| Methods | Randomisation: adequate by means of a randomised list stratified by age, parity and initial haemoglobin level. Allocation concealment: adequate. Numbered bottles of tablets and code was broken after study completed for group 1 and 2. Blinding: inadequate. Participant and provider were blinded to treatment for groups 1 and 2. The control group did not get a placebo. Loss to follow up: inadequate ‐ more than 20% were lost to follow up to the postnatal visit. | |
| Participants | 200 women before 20th week of gestation and Hb above 100 g/L attending antenatal clinic at the Maternity Hospital in Glossop Terrace, Cardiff, England, United Kingdom were studied. Exclusion criteria included urinary infection and threatened miscarriage, confusion over therapy, intercurrent illness and difficult veins, intolerant to the iron form, premature labor. | |
| Interventions | Women were randomly allocated to three groups: group 1: received 122 mg of elemental iron as ferrous sulphate daily; group 2: received 122 mg of elemental iron as ferrous sulphate plus 3.4 mg of folic acid daily; group 3: no treatment. A group 4 was formed as some subjects (n = 38) from group 3 received iron supplements for treatment of anaemia in the course of the intervention. They are excluded for analysis. Women were supplemented from week 20 to week 40 of gestation. | |
| Outcomes | Maternal: haemoglobin concentrations, blood and plasma volume, haematocrit (not reported), red cell volume, albumin and globulin fractions, oedema, intrapartum haemorrhage. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate by means of a randomised list stratified by age, parity and initial haemoglobin level. |
| Allocation concealment? | Low risk | Adequate. |
| Blinding? | High risk | Inadequate. Participant and provider were blinded to treatment for groups 1 and 2. The control group did not get a placebo. |
| Incomplete outcome data addressed? | High risk | Inadequate ‐ more than 20% were lost to follow up to the postnatal visit. |
| Methods | Randomisation: adequate by random table numbers. Allocation concealment: adequate by means of sealed envelopes. Blinding: inadequate. Participant nor provider blinded. No placebo used. Loss to follow‐up: adequate ‐ less than 20% lost to follow up. | |
| Participants | 45 non‐anaemic women with singleton pregnancy and no major illnesses attending the University Hospital Obstetric and Gynaecologic Clinic in Antwerp, Belgium. | |
| Interventions | Women were randomly assigned to one of two groups: | |
| Outcomes | Maternal: haemoglobin, serum iron, serum transferrin and serum ferritin concentrations at 16, 28, 36 wks, delivery and 6 wks postpartum. | |
| Notes | Unsupervised. The randomisation was made for each clinic in Antwerp, and the results are presented separately by clinic. Compliance not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate ‐ by random table numbers. |
| Allocation concealment? | Low risk | Adequate, by means of sealed envelopes. |
| Blinding? | High risk | Inadequate. Participant nor provider blinded. No placebo used. |
| Incomplete outcome data addressed? | Low risk | Adequate ‐ less than 20% lost to follow up |
| Methods | Randomisation: unclear. method not stated. Allocation concealment: unclear. Blinding: unclear. Not specified. Loss to follow up: unclear. Not reported. | |
| Participants | 27 apparently healthy non‐anaemic pregnant women 17‐35 years of age from 4 participating obstetricians' private practice clinics from Montreal, Canada in their 1‐5th month of pregnancy with Hb 12 g/dL or higher in first trimester and 11 g/dL or higher in second trimester. Women with history of pathological blood loss or gross dietary imbalance were excluded. | |
| Interventions | Women were randomly assigned to two groups: group 1 received 39 mg elemental iron to be taken twice daily with meals (total daily 78 mg elemental iron) or group 2 who received no iron tablets. Both groups received one tablet of multivitamin supplement daily containing: copper citrate 2 mg, magnesium stearate 6 mg, manganese carbonate 0.3 mg, vitamin A 1000 IU, vitamin D 500 IU, bone flour 130 mg, vitamin B1 1 mg, vitamin B2 1 mg, brewer yeast concentrate 50 mg, niacinamide 5 mg, vitamin C 25 mg, sodium iodide 0.2 mg and folate 0.049 ug (naturally occurring). Duration of supplementation unclear. | |
| Outcomes | Maternal: Hb concentration, packed cell volume, reticulocyte count, sedimentation rate, total white blood cell and differential counts, serum iron, unsaturated and total iron binding capacity, serum B12, serum and RBC folate at baseline and at 32, 36, 39th wks and 7 days postpartum. | |
| Notes | Supervision unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear ‐ method not stated. |
| Allocation concealment? | Unclear risk | Unclear. |
| Blinding? | Unclear risk | Unclear. |
| Incomplete outcome data addressed? | High risk | Inadequate ‐ not reported. |
| Methods | Randomisation: inadequate ‐ quasi‐randomised study, assignment by sequence. Allocation concealment: inadequate. Blinding: adequate. Participant and provider blinded. Loss to follow up: adequate ‐ less than 20%. | |
| Participants | 251 women attending antenatal clinic at St Mary's Hospital, London, England, United Kingdom before 20th week of gestation. | |
| Interventions | Women were allocated by sequence to one of five groups: group 1: oral dose of 30 mg of elemental iron daily; group 2: oral dose of 60 mg of elemental iron daily; group 3: oral dose of 120 mg of elemental iron daily; group 4: placebo; group 5: 1 g of iron (Imferon, 4 x 250 mg) intravenously before week 20, and thereafter oral 60 mg of elemental iron as ferrous fumarate daily (not included in this review). Oral elemental iron provided as ferrous fumarate. | |
| Outcomes | Maternal: full blood count, serum iron at 20, 25, 30 and 37th week. Sternal marrow aspiration at 37 wks; antepartum haemorrhage, threatened abortion, urinary tract infection, fetal abnormalities, pregnancy hypertension, premature delivery and puerperal infection measured but not reported by groups. | |
| Notes | Compliance not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Inadequate ‐ quasi‐randomised study, assignment by sequence. |
| Allocation concealment? | High risk | Inadequate. |
| Blinding? | Low risk | Adequate. Participant and provider blinded. |
| Incomplete outcome data addressed? | Low risk | Adequate ‐ less than 20%. |
| Methods | Randomisation: adequate, using a set of random tables. Allocation concealment: unclear. Blinding: unclear ‐ participant and outcome assessor blinded. Provider blinding unclear. Loss to follow up: adequate. Ranged from 10%‐15%. | |
| Participants | 325 pregnant women with Hb (AA) and 232 pregnant women with Hb (AE) attending midwife centres in 80 villages from the Varin Chamrab district of Ubon Province, Thailand. Chronic illness, complicated pregnancy, severe anaemia (Hb < 80 g/L), haemoglobinopathies Hb (EE) and (EF), and unwillingness to cooperate were reason for exclusion. Individuals with Hb (AA) have normal hemoglobin genes. Individuals with Hb (AE) have a heterozygous Hb E trait with normal Hb gene (A‐adults) and an abnormal Hb gene (E). This is usually a clinically insignificant condition. | |
| Interventions | Women were divided into two groups according to Hb (AA) and Hb (AE) and studied separately. Women from each group were randomly assigned to one of the following interventions: group 1: placebo, supervised; group 2, 120 mg of elemental iron and 5 mg folic acid daily supervised; group 3, 240 mg of elemental iron daily supervised; group 4: 240 mg of elemental iron daily supervised; group 5: 120 mg elemental iron and 5 mg of folic acid, motivated but unsupervised; and group 6: 240 mg of elemental iron and 5 mg of folic acid daily, motivated but unsupervised. For the Hb (AE) group, women were randomly assigned to one of the following groups: group 7: placebo, supervised; group 8: 240 mg elemental iron and 5 mg of folic acid daily, supervised; group 9: 240 mg of elemental iron daily, supervised; group 10: 120 mg of elemental iron and 5 mg of folic acid daily, motivated but unsupervised, and group 11: 240 mg of elemental iron and 5 mg of folic acid daily, motivated but unsupervised. Elemental iron was given as ferrous sulphate. | |
| Outcomes | Maternal: haemoglobin, serum ferritin after 10 and 15 wks of supplementation, and side effects. | |
| Notes | Groups 1, 2, 3, 4, 7, 8, 9 supervised. Groups 5, 6, 10 and 11 motivated but unsupervised. For purposes of analysis, the groups were merged by iron alone or iron‐folic acid, and included as daily higher doses in both cases. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate, using a set of random tables. |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? | Low risk | Unclear ‐ participant and outcome assessor blinded. Provider blinding unclear. |
| Incomplete outcome data addressed? | Low risk | Adequate. Ranged from 10%‐15%. |
| Methods | Randomisation: adequate ‐ by computerised random numbers. Allocation concealment: adequate by sealed envelopes. Blinding: inadequate ‐ participant, care provider and outcome assessor not blinded. Loss to follow up: inadequate ‐ more than 20% lost to follow up. | |
| Participants | 256 clinically healthy pregnant women from low socio‐economic status attending one antenatal care clinic in Guatemala City, Guatemala and Hb > 80 g/L were recruited. City of Guatemala is at 1500 m above sea level, so values were adjusted by altitude subtracting 5 g/L in Hb. | |
| Interventions | Women were randomly assigned to one of two groups: group 1: daily supervised intake of 60 mg elemental iron and 500 ug folic acid; group 2: weekly supervised intake of 180 mg of elemental iron and 3.5 mg of folic acid in one intake once a week. Iron given as ferrous sulphate. | |
| Outcomes | Maternal: haemoglobin concentration at baseline and at term (38th week of gestation); side effects and total iron intake. | |
| Notes | Supervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate ‐ by computerised random numbers. |
| Allocation concealment? | Low risk | Adequate, by sealed envelopes. |
| Blinding? | High risk | Inadequate ‐participant, care provider and outcome assessor not blinded. |
| Incomplete outcome data addressed? | High risk | Inadequate ‐ more than 20% lost to follow up. |
| Methods | Randomisation: adequate by computerised random numbers. Allocation concealment: adequate by sealed envelopes. Blinding: inadequate. Participant and provider not blinded. Outcome assessor for laboratory blinded to groups. Loss to follow up: inadequate. | |
| Participants | 120 clinically healthy pregnant women attending one antenatal care clinic in Guatemala City, Guatemala with Hb >80 g/L were recruited. Women were from low SES. City of Guatemala is 1500 m above sea level, so values were adjusted by altitude subtracting 5 g/L in Hb. | |
| Interventions | Women from low SES were randomly assigned to one of two groups: group 3: daily unsupervised intake of 60 mg elemental iron as ferrous sulphate and 500 ug folic acid; or group 4: weekly unsupervised intake of 180 mg of elemental iron as ferrous sulphate and 3.5 mg of folic acid in one intake once a week. | |
| Outcomes | Maternal: haemoglobin concentration at baseline and at term (38th week of gestation); side effects and total iron intake. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate, by computerised random numbers. |
| Allocation concealment? | Low risk | Adequate, by sealed envelopes. |
| Blinding? | High risk | Inadequate. Participant and provider not blinded. |
| Incomplete outcome data addressed? | High risk | Inadequate |
| Methods | Randomisation: method unclear. Allocation concealment: adequate. Bottles containing the tablets had been numbered by random selection at source and the code was unknown during trial. Blinding: adequate. participant and provider blinded. Loss to follow up: adequate. No losses to follow up. | |
| Participants | 360 non‐anaemic women attending antenatal clinic at Ridcliffe Infirmary, Oxford, England United Kingdom before 28th week of gestation, who had not taken iron supplements in the preceding 8 wks and with Hb >= 102 g/L or a normal serum iron reading. Exclusion criteria: Hb < 110 g/L and serum iron less than 60 ug/L. | |
| Interventions | Women were randomly assigned to one of various combinations of elemental iron as ferrous gluconate and folic acid, as follows: | |
| Outcomes | Maternal: haemoglobin, haematocrit, serum iron, serum folic acid activity, serum vitamin B12 estimation at 28 wks of gestation and predelivery. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method unclear. |
| Allocation concealment? | Low risk | Adequate. Bottles containing the tablets had been numbered by random selection at source and the code was unknown during trial. |
| Blinding? | Low risk | Adequate. Participant and provider blinded. |
| Incomplete outcome data addressed? | Low risk | Adequate. No losses to follow up. |
| Methods | Randomisation: adequate. Cluster randomisation. Allocation concealment: adequate. Coded. Blinding: adequate. Participant, provider and outcome assessors blinded. Loss to follow up: inadequate. More than 20% losses to follow up. | |
| Participants | 4998 married pregnant women (with positive pregnancy test) living in the south eastern plains district of Sarlahi, Nepal. Widows were excluded. | |
| Interventions | Women were randomly assigned to one of five groups: group 1 received 1000 ug retinol equivalents vitamin A (control) daily; group 2 received 1000 ug retinol equivalents vitamin A and 400 ug folic acid daily; group 3 received 1000 ug retinol equivalents vitamin A, 400 ug folic acid and 60 mg elemental iron as ferrous fumarate daily; group 4 received 1000 ug retinol equivalents vitamin A, 400 ug folic acid, 60 mg of elemental iron as ferrous fumarate and 30 mg of zinc sulphate daily; and group 5 received 1000 ug retinol equivalents vitamin A, 400 ug folic acid, 60 mg elemental iron as ferrous fumarate, 30 mg of zinc, 10 ug vitamin D, 10 mg vitamin E, 1.6 mg thiamine, 1.8 mg riboflavin, 20 mg niacin, 2.2 mg vitamin B6, 2.6 ug vitamin B12, 100 mg vitamin C, 65 ug vitamin K, 2 mg cooper, and 100 mg magnesium daily. Only groups 1 and three are considered in this review. Supplementation started at recruitment and continued until 3 month post‐partum in the case of live births of 5 wks or more after a miscarriage or stillbirth. All participating women were offered deworming treatment (albendazole 400 mg single dose) in the second and third trimester. | |
| Outcomes | Maternal: premature delivery, Hb and iron status at baseline in the third trimester and Hb at 6 wk postpartum, prevalence of anemia in third trimester and at 6 wk postpartum, severe anemia postpartum, moderate anaemia during third trimester, moderate anaemia postpartum, moderate haemoconcentration during third trimester | |
| Notes | Unsupervised but trial personnel visited women twice each week to monitor supplement intake. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate. Cluster randomisation. |
| Allocation concealment? | Low risk | Adequate. Coded. |
| Blinding? | Low risk | Adequate ‐ participant, provider and outcome assessors blinded. |
| Incomplete outcome data addressed? | High risk | Inadequate. More than 20% losses to follow up. |
| Methods | Randomisation: adequate by computerised random numbers. Allocation concealment: adequate. Blinding: adequate ‐ participant and care provider blinded. Outcome assessor unclear. Loss to follow up: inadequate ‐ more than 20% lost to follow up. | |
| Participants | 275 legally competent, non‐imprisoned, non‐anaemic, low‐income pregnant women at < 20 wks of gestation with ferritin levels above 20 ug/L enrolled at the Cuyahoga County, MetroHealth Center, Supplemental Nutrition Program for Women, Infants and Children in Cleveland, Ohio, USA. | |
| Interventions | Women were randomly assigned to one of two groups: group 1 received 1 gelatin capsule containing 30 mg of elemental iron as ferrous sulphate daily; group 2 received 1 placebo soft gelatin capsule daily for 119 days. | |
| Outcomes | Maternal: prevalence of anaemia at 28 and 38 wks, side effects, compliance to treatment, maternal weight gain, iron status (mean cell volume, haemoglobin concentration, serum ferritin, erythrocyte protoporphyrin concentrations at 28 and 38 wks. | |
| Notes | Unsupervised. Women were re‐evaluated at 28 wks of gestation, and according to haemoglobin concentrations at that time were prescribed treatment following the Institute of Medicine guidelines for iron supplementation during pregnancy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate, by computerised random numbers. |
| Allocation concealment? | Low risk | Adequate |
| Blinding? | Low risk | Adequate ‐ participant and care provider blinded. Outcome assessor unclear. |
| Incomplete outcome data addressed? | High risk | Inadequate ‐ more than 20% lost to follow up. |
| Methods | Randomisation: unclear ‐ randomised but method used unclear. Allocation concealment: adequate. Blinding: adequate. Participant and provider blinded. Loss to follow up: adequate ‐ less than 20%. | |
| Participants | 191 non‐anaemic pregnant women with 12‐18 wks of gestation attending antenatal care clinic at the Maternity at Poissy Hospital, Paris, France. Exclusion criteria included women who had taken iron or folate supplements in the prior 6 months and those with language barriers for proper communication. | |
| Interventions | Women were randomly allocated to one of 2 groups: group 1: daily intake of 45 mg of elemental iron as ferrous betainate hydrochloride (15 mg elemental iron per tablet) and group 2: placebo tablets. | |
| Outcomes | Maternal: haemoglobin, MCV, serum iron, total iron binding capacity, transferrin saturation, serum ferritin at baseline, at 5 months, at 7 months, at delivery and 2 months postpartum. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear ‐ randomised but method used unclear. |
| Allocation concealment? | Low risk | Adequate. |
| Blinding? | Low risk | Adequate. Participant and provider blinded. |
| Incomplete outcome data addressed? | Low risk | Adequate, less than 20%. |
| Methods | Randomisation: adequate by cluster. Allocation concealment: inadequate ‐ not used. Blinding: inadequate ‐ neither participant nor provider blinded. Outcome assessor unclear. Loss to follow up: inadequate ‐ more than 20% loss to follow up. | |
| Participants | 209 apparently healthy women attending antenatal care clinics in rural areas of Mymemsingh thana, Bangladesh, with fundal height of 14‐22 cm (18‐24 wks of gestation), who had not used iron supplements prior to the study. Exclusion criteria: women with haemoglobin concentrations < 80 g/L. | |
| Interventions | Each clinic was randomly assigned to one of two interventions: 60 mg of elemental iron as ferrous sulphate and 250 ug folic acid given in one tablet daily, or 120 mg of elemental iron as ferrous sulphate and 500 ug folic acid once a week (given in two tablets one day of the week). Supplementation continued until 6 wks postpartum. | |
| Outcomes | Maternal: haemoglobin concentration at baseline and after 12 wks of supplementation. Compliance, side‐effects, serum ferritin and serum transferrin receptors at 6 wks postpartum. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate by cluster. |
| Allocation concealment? | High risk | Not used. |
| Blinding? | High risk | Inadequate ‐ neither participant nor provider blinded. Outcome assessor unclear. |
| Incomplete outcome data addressed? | High risk | Inadequate ‐ more than 20% loss to follow up. |
| Methods | Randomisation: adequate ‐ computer generated. Allocation concealment: adequate ‐ central allocation at trials office, sequentially numbered. Blinding: adequate ‐ participant and care provider blinded. Loss to follow up: inadequate. 23% and 21% in groups included. | |
| Participants | 90 healthy non‐anaemic pregnant women with singleton pregnancy of less than 13 wks, attending an inner city maternity centre in Bergen, Norway and willing to participate. Exclusion criteria: uncertain gestational age according to menstrual history, haemoglobin concentration < 110 g/L, chronic disease or pregnancy complications (hypertension, diabetes, bleeding), multiple pregnancy, liver enzymes out of normal range and logistic difficulties foreseen at baseline (moving out of area). | |
| Interventions | Women were randomly allocated to one of the following: group 1: three tablets containing 1.2 mg heme iron from porcine blood and 9 mg of elemental iron as ferrous fumarate (Hemofer®) and one placebo tablet (total 27 mg elemental iron a day); group 2: one tablet containing 27 mg elemental iron as iron fumarate with 100 mg vitamin C (Collet®) and three placebo tablets; or group 3: four placebo tablets. | |
| Outcomes | Maternal: haemoglobin, erythrocytes count, haematocrit, mean corpuscular volume, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration, reticulocytes, serum iron, total iron binding capacity, serum transferrin, erythrocyte protoporphyrin at baseline and at 20, 28, 38 wks, 8 wks postpartum, and 6 months postpartum; pregnancy complications: hypertension, pre‐eclampsia, forceps, postpartum haemorrhage, maternal wellbeing and breastfeeding duration. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate, computer generated. |
| Allocation concealment? | Low risk | Adequate |
| Blinding? | Low risk | Adequate ‐ participant and care provider blinded. |
| Incomplete outcome data addressed? | High risk | Inadequate. 23% and 21% in groups included. |
| Methods | Randomisation: inadequate ‐ alternate by day of the week. Allocation concealment: inadequate. Blinding: inadequate. Open. Loss to follow up: adequate. Less than 5% excluded. | |
| Participants | 174 primigravidae or secundigravidae at their first visit at the antenatal Clinic of Queen Elizabeth Hospital in Woodville, Australia with ability to write and speak English. | |
| Interventions | Women were divided into a supplemented group receiving a daily dose of 100 mg of elemental iron as ferrous gluconate or a control group that was unsupplemented. | |
| Outcomes | Maternal: haemoglobin and haematocrit at 20‐30 wk, 30‐40 wk, at 5 days, at 6 wk and at 3 months postpartum. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Inadequate ‐ quasi‐randomised, alternate by day of the week. |
| Allocation concealment? | High risk | Inadequate. |
| Blinding? | High risk | Inadequate. Open. |
| Incomplete outcome data addressed? | Low risk | Adequate. Less than 5% excluded. |
| Methods | Randomisation: adequate. Allocation concealment: adequate ‐ supplied in coded opaque bottles. Blinding: inadequate ‐ participant blinded. Care provider and outcome assessor unblinded. Loss to follow up: adequate. No losses to follow up. | |
| Participants | 13 apparently healthy non‐anemic non‐smokers pregnant women aged 18‐40 years and < 14 wks of gestation with singleton pregnancy recruited through local medical practitioners and the Maternity Department of the Norfolk and Norwich University Hospital, England, United Kingdom. | |
| Interventions | Women were randomly assigned to one of two groups: group 1 received 100 mg elemental iron as ferrous gluconate daily after food and group 2 received a placebo. Supplementation started at 16th week of gestation until delivery. | |
| Outcomes | Maternal: haemoglobin, serum ferritin, transferrin receptor, plasma zinc, exchangeable zinc pool, zinc excretion and zinc absorption at 16, 24 and 34 wks of gestation. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate. |
| Allocation concealment? | Low risk | Adequate, supplied in coded opaque bottles. |
| Blinding? | High risk | Inadequate ‐ participant blinded. Care provider and outcome assessor unblinded. |
| Incomplete outcome data addressed? | Low risk | Adequate ‐ no losses to follow up. |
| Methods | Randomisation: adequate. Random codes created by computer in blocks of ten by maternity centre. Allocation concealment: adequate. Sealed numbered envelopes stored in containers from which midwives were asked to take them in order. Blinding: inadequate ‐ neither participants nor provider blinded. Outcome assessor blinded. Loss to follow up: adequate ‐ less than 5% lost to follow up. | |
| Participants | 2994 pregnant women with less than 16 wks of gestation attending 15 communal maternity centres and 12 centres in five neighbouring communities in Tampere, Finland who consented to participate. Exclusion criteria included: chronic illness, anemia (hematocrit under 0.32 or Hb <110 g/L), late arrival, likelihood of moving away from the area before child birth, or twin pregnancies. | |
| Interventions | Women were randomly assigned to one of two policy groups: group 1 (routine) were recommended to take 100 mg elemental iron alone (iron compounds and dosage varied as per midwife recommendation) daily after the 16th week gestation; or group 2 (selective) who received no iron supplements. Women in the selective group who had a haematocrit of < 0.30 (Hb < 100 g/L) on two consecutive visits were provided 100 mg elemental iron (as ferrous sulphate) to be taken one tablet (50 mg) twice a day for two months or until haematocrit increased to 0.31 (Hb 100 g/L) | |
| Outcomes | Maternal: Haematocrit at 28th and 36th week gestation, weight increase (kg), systolic and systolic blood pressure at 36th wk, proteinuria, haematocrit at 28th and 36th wk gestation, overall estimation of health, symptoms of tiredness, sick days, fever, adverse effects from iron supplements, symptoms related to iron supplements, duration of first stage of labor, C‐section, blood transfusion, fever I hospital, postpartum stay in hospital for more than 7 days, not breastfeeding in postpartum check up, spontaneous abortions, length of gestation in wk, proportion of premature births. Infant: birth weight, low birth weight, death, perinatal mortality, 1 min Apgar score < 7, special care unit, malformations, infections, hiperviscosity as a discharge diagnosis, weight gain, overall health. | |
| Notes | Average iron intake in the routine group was 124 mg elemental iron a day. 32 women were excluded: 20 twin pregnancies, 4 discovered not to be pregnant, and 8 for other (unintentional) reasons. Of the remaining 2912, 218 participants miscarried. Final samples were therefore 2694: 1336 women in the routine iron group and 1358 women in the selective group. The limit to prescribe treatment on the selective group was changed in the middle of the study to haematocrit <0.31 (Hb < 105 g/L) after the 33rd wk of gestation. Compliance assessed daily through self reporting. Women in the routine group who reported not having taken the iron supplements during the preceding two wks was only 8.2% at 28th week gestation and 14% at 36 wks. It is reported that 7.4% of mothers in the selective group (i.e. no iron unless anaemic) reported having taken iron either regularly or "every now and then" in the preceding two wks, at 28th wk gestation, while this proportion increased to 14% in the 36th wk. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate. Random codes created by computer in blocks of ten by maternity centre. |
| Allocation concealment? | Low risk | Adequate ‐ sealed numbered envelopes stored in containers from which midwives were asked to take them in order. |
| Blinding? | High risk | Inadequate ‐ neither participants nor provider blinded. Outcome assessor blinded. |
| Incomplete outcome data addressed? | Low risk | Adequate ‐ less than 5% lost to follow up. |
| Free of selective reporting? | Low risk | |
| Methods | Randomisation: unclear. Allocation concealment: unclear. Blinding: inadequate ‐ neither participants nor provider blinded. Outcome assessor unclear. Loss to follow up: unclear. | |
| Participants | 207 pregnant women with less than 26 wks of gestation and Hb > 100 g/L attending antenatal care clinic in Nebraska, USA. | |
| Interventions | Women were randomly assigned to one of 3 groups: group 1 received 1 g of an iron salt daily; group 2 received 0.8‐1.2 g of ferrous sulphate and 60‐90 mg of cobalt chloride daily, and group 3 received no treatment. | |
| Outcomes | Maternal: haemoglobin, haematocrit, serum iron, erythrocyte protoporphyrin at 3‐6 months and pre‐delivery. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear. |
| Allocation concealment? | Unclear risk | Unclear. |
| Blinding? | High risk | Inadequate ‐ neither participants nor provider blinded. |
| Incomplete outcome data addressed? | Unclear risk | Unclear. |
| Methods | Randomisation: unclear. Allocation concealment: unclear. Blinding: inadequate ‐ neither participant nor provider blinded. Outcome assessor unclear. Loss to follow up: adequate. Less than 20%. | |
| Participants | 75 consecutive apparently healthy pregnant women with 32‐34 wks of gestation attending the maternity clinic at St Anthony's Hospital, Oklahoma City, Oklahoma, USA. | |
| Interventions | Women were randomly divided in three groups: group 1 served as control and received no treatment; group 2 received 220 mg elemental iron as ferrous sulphate daily; and group 3 received 55 mg elemental iron as sustained release ferrous sulphate daily. | |
| Outcomes | Maternal: haemoglobin, haematocrit, incidence and severity of side effects on a weekly basis until delivery. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear. |
| Allocation concealment? | Unclear risk | Unclear. |
| Blinding? | High risk | Inadequate ‐ neither participant nor provider blinded. Outcome assessor unclear. |
| Incomplete outcome data addressed? | Low risk | Adequate. Less than 20% losses to follow up. |
| Methods | Randomisation: adequate by cards shuffle. Allocation concealment: unclear. Blinding: inadequate ‐ participant blinded. Provider blinded to treatments but not to controls. Outcome assessor unclear. Loss to follow up: inadequate. 23% of participants were lost to follow up. | |
| Participants | 430 apparently healthy women with 24‐25 wks of singleton pregnancy and Hb equal or above 104 g/L attending antenatal clinic at Simpson Memorial Maternity Pavillion, Edinburgh, Scotland, United Kingdom. | |
| Interventions | Women were randomly allocated to one of 4 groups: group 1 received 35 mg of elemental iron as ferrous sulphate three times a day; group 2 received 35 mg of elemental iron as ferrous gluconate three times a day; group 3 received 35 mg of elemental iron as ferrous gluconate with 25 mg of ascorbic acid, three times a day; group 4 received placebo. | |
| Outcomes | Maternal: haemoglobin, red cell count, haematocrit at baseline and at 37th week. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate by cards shuffle. |
| Allocation concealment? | Unclear risk | Unclear. |
| Blinding? | High risk | Inadequate ‐ participant blinded. Provider blinded to treatments but not to controls. Outcome assessor unclear. |
| Incomplete outcome data addressed? | High risk | Inadequate. 23% of participants were lost to follow up. |
| Methods | Randomisation: adequate. Allocation concealment: unclear. Blinding: inadequate: subject, provider and outcome assessor unclear. Loss to follow up: adequate: more than 80% of participants were included in the analysis. 16.2% of participants were lost to follow up. | |
| Participants | 154 apparently healthy pregnant women seeking prenatal care in Gwangju, South Korea during first trimester of pregnancy who did not receive other supplements or medications throughout pregnancy and who were willing to participate. | |
| Interventions | Women were randomly allocated to one of 5 groups: group 1 received 30 mg elemental iron and 175 ug folic acid daily from first trimester until delivery; group 2 received 60 mg of elemental iron with 350 ug of folic acid from first trimester until delivery; group 3 received 30 mg elemental iron and 175 ug of folic acid from 20th week of gestation until delivery; group 4 received 60 mg elemental iron and 350 ug of folic acid from 20th week of gestation until delivery; or control group with no supplement. Elemental iron was given as ferrous sulphate. | |
| Outcomes | Maternal: haemoglobin, haematocrit, serum ferritin, serum soluble transferrin receptor concentrations at baseline and during first, second, third trimester of pregnancy and at delivery. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate. |
| Allocation concealment? | Unclear risk | Unclear. |
| Blinding? | High risk | Unclear: subject, provider and outcome assessor blinding unclear. |
| Incomplete outcome data addressed? | Low risk | Adequate ‐ less than 20% lost to follow up. |
| Methods | Randomisation: method unclear. Non‐supplemented group was self‐selected. | |
| Participants | 395 healthy, anaemic and non anaemic, pregnant women attending prenatal care at 2 outpatient clinics at Changji Hospital and Shihezi Maternal and Child Health Station in Xianjiang, China. Women with Hb < 80 g/L were excluded. Maternal age was 25.15 ± 2.28 years. | |
| Interventions | Women were randomly assigned to one of 3 groups: group 1: 60 mg elemental iron as ferrous sulphate and 0.25 mg of folic acid daily; group 2: 120 mg of elemental iron as ferrous sulphate and 0.5 mg of folic acid daily; group 3: 120 mg elemental iron as ferrous sulphate and 0.5 mg of folic acid once weekly. A control group that received no iron was composed of women who did not want to participate in the study and did not receive any iron supplements. | |
| Outcomes | Maternal: haemoglobin concentration at 3, 5, 8 months and at term; serum ferritin concentrations at 3 months and at term in a subgroup; side effects. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Method unclear. Non‐supplemented group was self‐selected. |
| Allocation concealment? | Low risk | Adequate by sealed closed envelopes. |
| Blinding? | High risk | Inadequate. Neither participant nor provider blinded. Outcome assessor blinded. |
| Incomplete outcome data addressed? | Low risk | Adequate ‐ less than 20% lost to follow up. |
| Methods | Randomisation: adequate by means of computer generated with balanced blocks and stratified for parity. Allocation concealment:adequate ‐ opaque bottles marked with sequential numerical code prepared by the Pharmacy Department of Women's & Children's Hospital. Blinding: adequate ‐ participant and care provider blinded. Loss to follow up: adequate. Less than 20% lost to follow up. | |
| Participants | 430 non‐anaemic pregnant women attending antenatal clinics at Women's and Children's Hospital in Adelaide, Australia with singleton or twin pregnancies and informed consent. Exclusion criteria: diagnosis of thalassaemia, history of drug or alcohol abuse and history of vitamin and mineral preparations containing iron prior to enrolment in study. | |
| Interventions | Women were randomly assigned to receive one tablet containing 20 mg of elemental iron daily between meals from week 20 until delivery or a placebo tablet. | |
| Outcomes | Maternal: haemoglobin concentration at 28 wk, at delivery, and at 6 months postpartum; ferritin concentration at delivery and at 6 months postpartum; maternal gastrointestinal side effects at 24 and 36 wk of gestation; serum zinc at delivery and at 6 month postpartum; maternal well being at 36 wk of gestation, at 6 wk and at 6 months postpartum; pregnancy outcomes: type of birth, blood loss at delivery, gestational age. At 4 years postpartum: general health of mothers using the SF‐36, a self administered questionnaire that assesses 8 concepts of health. | |
| Notes | Unsupervised but monthly phone calls to encourage compliance. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate by means of computer generated with balanced blocks and stratified for parity. |
| Allocation concealment? | Low risk | Aadequate, opaque bottles marked with sequential numerical code prepared by the Pharmacy Department of Women's & Children's Hospital. |
| Blinding? | Low risk | Adequate ‐ participant and care provider blinded. |
| Incomplete outcome data addressed? | Low risk | Adequate. Less than 20% lost to follow up. |
| Methods | Randomisation: adequate stratified by age group. Allocation concealment: unclear. Blinding: adequate. Participant and provider blinded. Outcome assessor unclear. Loss to follow up: inadequate. More than 20% lost to follow up. | |
| Participants | 144 non‐iron deficient adolescents 15‐18 years old in their first pregnancy and adult women 19 or older in their first or greater pregnancy attending prenatal care at Marshfield Clinic, Wisconsin, USA. | |
| Interventions | Women were randomly assigned to receive once daily 60 mg of elemental iron as ferrous sulphate or a placebo. All women received 1 mg of folic acid daily. | |
| Outcomes | Maternal: prevalence of iron deficiency anaemia, compliance to treatment, side effects, vomiting, nausea, constipation, diarrhoea, C‐section, serum ferritin and haemoglobin concentrations at 24‐28 wk gestation and at 36‐40 wk gestation. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate stratified by age group. |
| Allocation concealment? | Unclear risk | Unclear. |
| Blinding? | Low risk | Adequate. Participant and provider blinded. Outcome assessor unclear. |
| Incomplete outcome data addressed? | High risk | Inadequate. More than 20% lost to follow up. |
| Methods | Randomisation: unclear ‐ randomised but method unclear. Allocation concealment: inadequate. Blinding: inadequate. Participant and provider not blinded. Outcome assessor blinded. Loss to follow up: inadequate. More than 20% lost to follow up. | |
| Participants | 550 multi gravidae pregnant women with less than 34 wks of gestation attending antenatal care clinics in 18 villages near the town of Farafenni, in North Bank Division, Gambia where malaria is endemic with high transmission during 4‐5 months a year. | |
| Interventions | Women were allocated randomly by compound of residence to receive 60 mg of elemental iron as ferrous sulphate or placebo. All pregnant women received a weekly tablet of 5 mg of folic acid but no antimalarial chemoprophylaxis. | |
| Outcomes | Maternal: haemoglobin concentrations at baseline, 4‐6 wks before delivery and one week postpartum; plasma iron, total iron binding capacity, transferrin saturation, deposition of malaria pigment in placenta. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear ‐ randomised but method unclear. |
| Allocation concealment? | High risk | Inadequate. |
| Blinding? | High risk | Inadequate. Participant and provider not blinded. Outcome assessor blinded. |
| Incomplete outcome data addressed? | High risk | Inadequate. More than 20% lost to follow up. |
| Methods | Randomisation: method unclear. Allocation concealment: unclear. Blinding: adequate. Participant and provider blinded. Outcome assessor unclear. Loss to follow up: adequate. Less than 20% lost to follow up. | |
| Participants | 248 healthy Caucasian Danish women attending Birth Clinic in Copenhagen, Denmark within 9‐18 wks of gestation and normal pregnancy. Exclusion criteria: complicated delivery, excessive smoking (> 9 cigarettes/day). | |
| Interventions | Women were randomly assigned to receive 66 mg of elemental iron as ferrous fumarate daily (n = 121) or placebo (n = 127) until delivery. | |
| Outcomes | Maternal: haemoglobin, haematocrit, erythrocyte indices, iron status, serum ferritin, serum transferrin saturation, serum erythropoietin at baseline and every 4th week until delivery, and 1‐8 wks after delivery in sub sample; pregnancy complications. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method unclear. |
| Allocation concealment? | Unclear risk | Unclear. |
| Blinding? | Low risk | Adequate. Participant and provider blinded. Outcome assessor unclear. |
| Incomplete outcome data addressed? | Low risk | Adequate. Less than 20% lost to follow up. |
| Methods | Randomisation: adequate. computer generated random numbers. Block randomisation (block size = 10). Allocation concealment: inadequate ‐ not used. Blinding: inadequate. Open to participants, care providers and outcome assessor. Loss to follow up: inadequate. More than 20% excluded. | |
| Participants | 111 apparently healthy pregnant women with less than 20 wks and no prior intake of iron supplements during this pregnancy with Hb equal or higher than 100 g/L and singleton pregnancy in New Delhi, India. Women who were taking anti‐epileptics or anti‐thyroid medications, had history of menorrhagia, bleeding disorders, chronic peptic ulcers, bleeding piles, thalassaemia or other haemoglobinopathies, or history of haemorrhage in present or past pregnancies were excluded. | |
| Interventions | Women were randomly assigned to one of two groups: group 1 received two tablets of 100 mg elemental iron and 500 ug folic acid each (total 200 mg iron and 1000 ug folic acid), to be taken only once a week, one tablet before lunch and another tablet before dinner; group 2 received one tablet of 100 mg elemental iron and 500 ug folic acid daily. Women were advised to take the supplements 30 minutes before the meals and not with tea, coffee or milk. Also, women were advised to take calcium supplements after meals (500 mg elemental calcium twice daily). Iron supplementation started between 14 and 20 wks until delivery. Deworming, if required, was carried out with Mebendazole 100 mg twice a day for 3 days in the second trimester. | |
| Outcomes | Maternal: Hb, serum ferritin concentrations at baseline and at 32‐34 wks, prevalence of anaemia, compliance to treatment, presence of intestinal parasites. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate. computer generated random numbers. Block randomisation (block size=10). |
| Allocation concealment? | High risk | Not used. |
| Blinding? | High risk | Inadequate ‐ open to participants, care providers and outcome assessor. |
| Incomplete outcome data addressed? | High risk | Inadequate. More than 20% excluded. |
| Methods | Randomisation: method unclear. Allocation concealment: adequate ‐ sequentially numbered. Blinding: adequate ‐ participant and provider blinded. Loss to follow up: adequate ‐ less than 5%. | |
| Participants | 180 primigravidae women with less than 20 wk gestation and Hb > 100 g/L attending antenatal clinic in Aberdeen Maternity Hospital, Scotland, United Kingdom. | |
| Interventions | Women were randomly assigned to one of three groups: group 1 received 3 tablets containing 4 mg elemental iron each (total 12 mg daily); group 2 received 3 tablets containing 35 mg elemental iron (total 105 mg elemental iron daily) and group 3 received placebo. Intervention was from week 20 to week 36 of gestation. | |
| Outcomes | Maternal: haemoglobin, haematocrit at baseline, and at wks 20, 30, 36 of gestation and 7‐13 days postpartum; plasma volume at 30 wks, total red cell volume, serum iron and total iron binding capacity at 30 wks, subjective health and side effects at 30 wks. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method unclear. |
| Allocation concealment? | Low risk | Adequate, sequentially numbered. |
| Blinding? | Low risk | Adequate ‐ participant and provider blinded. |
| Incomplete outcome data addressed? | Low risk | Adequate ‐ less than 5%. |
| Methods | Randomisation: inadequate ‐ quasi randomised. Allocation concealment: not used. Blinding: inadequate ‐ neither participant nor provider blinded. Outcome assessor blinded. Loss to follow up: inadequate. More than 20% lost to follow up. | |
| Participants | 203 healthy pregnant women with normal blood pressure at first visit, attending antenatal care clinic at Diego Paroissien Hospital in the Province of Buenos Aires, Argentina. | |
| Interventions | Women were assigned to one of three groups: group 1 received 60 mg of elemental iron as ferrous fumarate daily; group 2 received 60 mg elemental iron every three days; and group 3 received no treatment. Supplementation started at 8‐28 wks until 34‐37 wks of gestation. | |
| Outcomes | Maternal: Hb, haematocrit, erythroporphyrin, serum ferritin concentration at baseline and at 34‐37wk gestation, premature delivery. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Inadequate ‐ quasi randomised. |
| Allocation concealment? | High risk | Not used |
| Blinding? | High risk | Inadequate ‐ neither participant nor provider blinded. Outcome assessor blinded. |
| Incomplete outcome data addressed? | High risk | Inadequate. More than 20% lost to follow up. |
| Free of selective reporting? | High risk | |
| Free of other bias? | High risk | |
| Methods | Randomisation: adequate ‐ by random numbers. Allocation concealment: adequate ‐ packages of tablets numbered by manufacturer. Blinding: adequate ‐ participant and provider blinded. Outcome assessor blinded. Loss to follow up: unclear. | |
| Participants | 197 healthy pregnant women 17‐40 years of age, with 28 +/‐ 3 wks of gestation attending antenatal care clinic in a Mother‐Child Health Center in Niamey, Niger. | |
| Interventions | Women were randomly assigned to one of two groups: group 1 received 100 mg of elemental iron as ferrous betainate daily; group 2 received placebo. | |
| Outcomes | Maternal: haemoglobin concentration, mean corpuscular volume, haematocrit, erythrocyte protoporphyrin, serum iron, transferrin, total iron binding capacity, serum ferritin concentrations, at baseline and at the first stage of labor and at 3 and 6 months postpartum, prevalence of iron deficiency and iron deficiency anaemia. | |
| Notes | Supervised by physicians who recorded tablet consumption. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate ‐ by random numbers. |
| Allocation concealment? | Low risk | Adequate, packages of tablets numbered by manufacturer. |
| Blinding? | Low risk | Adequate ‐ participant and provider blinded. Outcome assessor blinded. |
| Incomplete outcome data addressed? | Unclear risk | Unclear. |
| Methods | Randomisation: method unclear. Allocation concealment: unclear. Blinding: inadequate. Neither participant nor provider blinded. Outcome assessor not blinded. Loss to follow up: unclear. | |
| Participants | 172 pregnant women believed to be in the second trimester of pregnancy by date of last menstrual period attending antenatal care clinic in Parland Memorial Hospital, Dallas, Texas, USA. | |
| Interventions | Women were randomly assigned to one of three interventions: group 1 received 1000 mg of iron intramuscularly as iron‐dextran; group 2 received 112 mg of elemental iron as ferrous gluconate daily in 3 tablets; group 3 received placebo tablets. | |
| Outcomes | Maternal: haemoglobin concentration at baseline and at delivery. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method unclear. |
| Allocation concealment? | Unclear risk | Unclear. |
| Blinding? | High risk | Inadequate. Neither participant no provider blinded. Outcome assessor not blinded. |
| Incomplete outcome data addressed? | Unclear risk | Unclear. |
| Free of other bias? | High risk | |
| Methods | Randomisation: method unclear. Allocation concealment: unclear. Blinding: inadequate ‐ open. Loss to follow up: adequate ‐ less than 20% lost to follow up. | |
| Participants | 32 healthy non‐anaemic pregnant women attending antenatal care at maternity centres of Oulu University Central Hospital, Finland with uncomplicated pregnancy of less than 16 wks, and no earlier haematological problems. | |
| Interventions | Women were randomly assigned to one of two groups: group 1 received 200 mg of elemental iron as ferrous sulphate daily; group 2 received no treatment. | |
| Outcomes | Maternal: haemoglobin, haematocrit, red cell count, leucocyte count, reticulocytes, mean corpuscular volume, mean corpuscular haemoglobin concentration, mean corpuscular haemoglobin, serum iron, total iron binding capacity, transferrin, vitamin B12, whole folate, and serum ferritin concentration at baseline, and at wks, 16, 20, 24, 28, 32, 36, 40 and 5 days, 1, 2, and 6 months postpartum. Bone marrow aspirates at 16th and 32nd week and at 2 months postpartum. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method unclear. |
| Allocation concealment? | Unclear risk | Unclear. |
| Blinding? | High risk | Inadequate ‐ open. |
| Incomplete outcome data addressed? | Low risk | Adequate ‐ less than 20% lost to follow up. |
| Methods | Randomisation: adequate ‐ block randomised by randomised numbers table. Allocation concealment: not used. Blinding: inadequate ‐ participant and care provider not blinded. Outcome assessor blinded. Loss to follow up: inadequate. More than 20% lost to follow up. | |
| Participants | 176 pregnant women with 8‐24 wks of gestation attending antenatal care at six health centres in West Java, Indonesia. | |
| Interventions | Health centres were randomised to one of two interventions: weekly regimen, where women received 120 mg of elemental iron as ferrous sulphate with 0.50 mg of folic acid; or daily regimen where women received 60 mg of elemental iron as ferrous sulphate with 0.25 mg of folic acid daily until week 28‐32 of gestation. | |
| Outcomes | Maternal: haemoglobin concentration, serum ferritin, weight at baseline and at 28‐32 wks of gestation; compliance to treatment and prevalence of parasitic infections. | |
| Notes | Unsupervised but frequent contact with participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate ‐ block randomised by randomised numbers table. |
| Allocation concealment? | High risk | Not used. |
| Blinding? | High risk | Inadequate ‐ participant and care provider not blinded. Outcome assessor blinded. |
| Incomplete outcome data addressed? | High risk | Inadequate. More than 20% lost to follow up. |
| Methods | Randomisation: inadequate ‐ by alternating numbers. Allocation concealment: unclear. Blinding: inadequate ‐ participant and provider not blinded. Outcome assessor blinded. Loss to follow up: inadequate ‐ more than 20% lost to follow up. | |
| Participants | 680 pregnant women served by 11 health centres from five sub districts on or near the western end of the island of Seram in the Province of Maluku, Indonesia. | |
| Interventions | Women were assigned to one of two interventions: group 1 received 60 mg of elemental iron as ferrous sulphate with 0.25 mg of folic acid daily by a traditional birth attendant; group 2 received 120 mg of elemental iron as ferrous sulphate with 0.5 mg of folic acid once a week by the traditional home visiting birth attendants. A control group was formed by participants receiving traditional iron supplements (60 mg elemental iron) with folic acid from health centres, self administered without incentive. | |
| Outcomes | Maternal: haemoglobin concentration at baseline and after 12 and 20 wks of supplementation; serum ferritin at baseline and after 12 wks of supplementation; compliance. | |
| Notes | Daily group and control unsupervised. Weekly group supervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Inadequate ‐ quasi‐randomised, by alternating numbers. |
| Allocation concealment? | Unclear risk | Unclear. |
| Blinding? | High risk | Inadequate ‐ participant and provider not blinded. Outcome assessor blinded. |
| Incomplete outcome data addressed? | High risk | Inadequate ‐ more than 20% lost to follow up. |
| Methods | Randomisation: method unclear. Allocation concealment: unclear. Blinding: inadequate ‐ participant blinded. Provider and outcome assessor unclear. Loss to follow up: adequate ‐ less than 20%. | |
| Participants | 52 healthy pregnant women attending outpatient Women's clinic at Haukeland Hospital, Bergen, Norway within first 10 wks of a normal singleton pregnancy with uncomplicated delivery at 37‐42 wks. | |
| Interventions | Women were randomly assigned to one of two groups: group 1 received 200 mg of elemental iron as ferrous sulphate daily; group 2 received placebo. | |
| Outcomes | Maternal: haemoglobin, haematocrit, plasma cell volume, erythrocyte count, leucocyte count, mean corpuscular volume, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration, serum iron, iron binding capacity, erythrocyte protoporphyrin, serum ferritin at baseline and every month during 2nd trimester and every 2 wks until delivery. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method unclear. |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? | High risk | Inadequate ‐ participant blinded. Provider and outcome assessor unclear. |
| Incomplete outcome data addressed? | Low risk | Adequate ‐ less than 20%. |
| Methods | Randomisation: adequate ‐ by using random number generator. Allocation concealment: adequate. Blinding: adequate ‐ participant, provider and outcome assessor blinded to treatment. Loss to follow up: inadequate. More than 20% lost to follow up. | |
| Participants | 429 non anaemic iron replete women with less than 20 wks of gestation attending who had not taken supplements containing iron in the last month, with a singleton pregnancy attending the prenatal clinic at the Wake County Human services in Raleigh, North Carolina, USA. | |
| Interventions | Women were randomly assigned to one of two groups: group 1 received multivitamin/mineral supplements containing 30 mg of iron as ferrous sulphate daily or group 2 received multivitamin/mineral supplements containing 0 mg of iron (no iron) until 29 wks of gestation. Supplementation started on average at 12 wks. The multivitamin/mineral supplement contained the following: vitamin A 4000 IU; vitamin D 400 IU; vitamin C 70 mg; folic acid 0.5 mg; thiamine 1.5 mg; riboflavin 1.6 mg; niacin 17 mg; vitamin B6 2.6 mg; vitamin B12 2.5 ?g; calcium 200 mg; magnesium 100 mg; copper 1.5 mg; zinc 15 mg. Folic acid supplements were prescribed for all women who had received the positive pregnancy test until the first prenatal visit. | |
| Outcomes | Maternal: prevalence of anaemia, iron repletion andiron deficiency anaemia at 26‐29 wks, side effects, compliance to treatment, iron status (haemoglobin concentration, serum ferritin at 26‐29 wks, preterm delivery. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate, by using random number generator. |
| Allocation concealment? | Low risk | Adequate. |
| Blinding? | Low risk | Adequate ‐ participant, provider and outcome assessor blinded to treatment. |
| Incomplete outcome data addressed? | High risk | Inadequate. More than 20% lost to follow up. |
| Methods | Randomisation: unclear. Allocation concealment: unclear. Blinding: adequate. Participants blind, care provider blind and outcome assessor blinded. Loss to follow up: adequate ‐ less than 20% lost to follow up. | |
| Participants | 60 healthy primiparous women attending antenatal care clinic in Goteborg, Sweden with uncomplicated pregnancy and less than 14 wks of gestation and with Hb concentrations above 120 g/L who had not received iron supplements in the previous 6 months or parenteral iron at any previous time. Women whose Hb concentration fell below 100 g/L during the study period were excluded and received immediate therapy. | |
| Interventions | Women were randomly allocated to receive 200 mg of elemental iron as a sustained release preparation of ferrous sulphate daily or placebo from 12 wks of gestation until 9 wks postdelivery. | |
| Outcomes | Maternal: iron absorption measurements; haemoglobin concentration, haematocrit, bone marrow haemosiderin, mean corpuscular haemoglobin concentration, total iron binding capacity, transferrin saturation at baseline, and at wks 16, 20, 24, 28, 32, and 35; and 8‐10 wks after delivery. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method unclear. |
| Allocation concealment? | Unclear risk | Unclear. |
| Blinding? | Low risk | Adequate. Participants blind, care provider blind and outcome assessor blinded. |
| Incomplete outcome data addressed? | Low risk | Adequate ‐ less than 20% lost to follow up. |
| Methods | Randomisation: randomised but method unclear. Allocation concealment: unclear. Blinding: inadequate ‐ open. Loss to follow up: adequate ‐ less than 20% lost to follow up. | |
| Participants | 48 healthy pregnant women with no adverse medical or obstetric history attending antenatal care clinic in Newcastle, England, United Kingdom before 12 wks of gestation. | |
| Interventions | Women were randomly allocated to one of two groups: group 1 receive 325 mg of ferrous sulphate (about 65 mg elemental iron) and 350 ug of folic acid daily from 12 wks until delivery and group 2 received no supplements. | |
| Outcomes | Maternal: haemoglobin concentration, serum ferritin, mean cell volume at 12 wks and every 4 wks until delivery, and at 6 days, 6 wks and 6 months after delivery; plasma volume at 12 and 36 wks of gestation. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method unclear. |
| Allocation concealment? | Unclear risk | Unclear. |
| Blinding? | High risk | Inadequate ‐ open. |
| Incomplete outcome data addressed? | Low risk | Adequate ‐ less than 20% lost to follow up. |
| Methods | Randomisation: adequate by random number lists. Allocation concealment: adequate, sealed envelopes progressively numerated. Blinding: inadequate ‐ open. Loss to follow up: adequate ‐ less than 20%. | |
| Participants | 254 non‐anaemic non‐iron deficient healthy pregnant women from multiple centres in Italy. Exclusion criteria: acquired or congenital anaemia, haemoglobinopathies, thalassaemia, medically or surgically treated cardiopathy, abortion, hypertension, gastric resection, metabolic or endocrine disorder, hepatic or renal disease, epilepsy or another neurological disease, previously treated for cancer, alcohol or substance dependence. | |
| Interventions | Women were randomly assigned to receive 40 mg of elemental iron containing 250 g of ferritin in a microgranulated gastric resistant capsule daily or no treatment from 12‐16 wks of gestation until the end of puerperium. | |
| Outcomes | Maternal: haemoglobin concentration, red cell count, mean corpuscular volume, serum iron, total transferrin, transferrin saturation, serum ferritin at 12‐16 wks, two times during pregnancy, at 38‐42 wks, and at puerperium 48‐52 wks. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate by random number lists |
| Allocation concealment? | Low risk | Adequate, sealed envelopes progressively numerated. |
| Blinding? | High risk | Inadequate ‐ open |
| Incomplete outcome data addressed? | Low risk | Adequate ‐ less than 20% losses to follow up. |
| Methods | Randomisation: method unclear. Allocation concealment: not used. Blinding: inadequate ‐ open. Loss to follow up: adequate ‐ less than 20%. | |
| Participants | 30 pregnant women with uncomplicated pregnancies and deliveries attending antenatal care clinic at the University Hospital Obstetric Unit in Rotterdam, Netherlands. | |
| Interventions | Women received 100 mg of elemental iron as ferrous sulphate daily or no treatment from the third month of gestation until delivery. Follow up was until 12 wks after delivery. | |
| Outcomes | Maternal: haemoglobin concentration, serum iron, serum ferritin, transferrin concentration at baseline and every 3‐4 wks until delivery, and three months after delivery. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method unclear. |
| Allocation concealment? | High risk | Not used. |
| Blinding? | High risk | Inadequate ‐ open. |
| Incomplete outcome data addressed? | Low risk | Adequate ‐ less than 20% losses to follow up. |
| Methods | Randomisation: adequate ‐ by random table numbers. Allocation concealment: adequate by means of sealed envelopes. Blinding: inadequate. Participant nor provider blinded. No placebo used. Loss to follow up: adequate ‐ less than 20%. | |
| Participants | 44 non‐anaemic Caucasian women with singleton pregnancy and no major illnesses attending the University Hospital Obstetrical Clinic of the Erasmus University in Rotterdam who had not received iron supplementation during their first visit. | |
| Interventions | Women were randomly assigned to one of two groups: | |
| Outcomes | Maternal: haemoglobin, serum iron, serum transferrin and serum ferritin concentrations at 16, 28, 36 wks, delivery, 6 and 12 wks postpartum. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate ‐ by random table numbers. |
| Allocation concealment? | Low risk | Adequate, by means of sealed envelopes. |
| Blinding? | High risk | Inadequate. Participant nor provider blinded. No placebo used. |
| Incomplete outcome data addressed? | Low risk | Adequate ‐ less than 20% losses to follow up. |
| Methods | Randomisation: method unclear. Allocation concealment: unclear. Blinding: unclear. Loss to follow up: adequate ‐ less than 20%. | |
| Participants | 3599 pregnant women with Hb above 100 g/L at their antenatal care clinic visit at Queen's Mother's Hospital in Glasgow, Scotland, United Kingdom. Women who reported not taken the tablets regularly ere excluded as well as those diagnosed with anaemia during the study. | |
| Interventions | Women were randomly allocated to one of five interventions: group 1 received no prophylactic supplements; group 2 received 105 mg of elemental iron daily as chelated iron aminoates; group 3 received 105 mg of elemental iron with 100 ug of folic acid; group 4 received 105 mg of elemental iron daily with 300 ug of folic acid; and group 5 received 105 mg elemental iron daily with 450 ug of folic acid. | |
| Outcomes | Maternal: haemoglobin concentration at baseline and in every visit, at early puerperium and during postnatal visit; incidence of obstetric complications. incidence of megaloblastic anaemia. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method unclear. |
| Allocation concealment? | Unclear risk | Unclear. |
| Blinding? | Unclear risk | Unclear. |
| Incomplete outcome data addressed? | Unclear risk | Adequate ‐ less than 20% losses to follow up. However, women were excluded from the trial and the analysis if they were diagnosed as anaemic. |
| Free of selective reporting? | High risk | |
| Methods | Randomisation: inadequate ‐ alternate. Allocation concealment: not used. Blinding: adequate ‐ participant and care provider blinded. Outcome assessor blinded. Loss to follow up: inadequate. More than 20% lost to follow up. | |
| Participants | 500 pregnant women attending antenatal care clinic at the Royal Free Hospital in London, England, United Kingdom during wartime, with ages between 18‐43 years. Women with severe anaemic or rheumatoid arthritis were excluded. | |
| Interventions | Women were alternatively allocated to receive 580 mg of elemental iron as ferrous gluconate daily or placebo from their first visit. | |
| Outcomes | Maternal: haemoglobin concentration using the Haldane method at baseline and every 4 wks until delivery, then 1 day, 2‐4 days, 5‐16 days and 6 wks postpartum; serum protein and pregnancy complications (not reported by group). | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Inadequate ‐ quasi randomised, alternate. |
| Allocation concealment? | High risk | Not used. |
| Blinding? | Low risk | Adequate ‐ participant and care provider blinded. Outcome assessor blinded. |
| Incomplete outcome data addressed? | Unclear risk | Inadequate. More than 20% lost to follow up. |
| Methods | Randomisation: cluster randomisation but method unclear. Allocation concealment: not used. Blinding: inadequate ‐ open. Loss to follow up: inadequate ‐ more than 20% lost to follow up. | |
| Participants | 484 apparently healthy pregnant women with 13‐17 wks of gestation who had not received iron supplements before enrolling in the study, and who had a haemoglobin concentration > 80 g/L attending antenatal care clinics at the district hospital and 7 health centres from 54 villages in the Province of Khon‐Kaen in northeast Thailand. | |
| Interventions | The villages were grouped according to size and then randomised in 4 clusters to one of three interventions: group 1 received a daily regimen providing 60 mg of elemental iron as ferrous sulphate with 0.25 mg of folic acid daily; group 2 received 120 mg of elemental iron with 3.5 mg of folic acid once a week; and group 3 received 180 mg of elemental iron as ferrous sulphate with 3.5 mg of folic acid once a week. | |
| Outcomes | Maternal: haemoglobin concentration, serum ferritin, free erythrocyte protoporphyrin at baseline and at 35 +/‐ 2 wks of gestation, and 4‐6 months postpartum; haematocrit prior to delivery; weight at baseline and at 35 wks of gestation; compliance, haemoglobin type, and hookworm prevalence. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear ‐ cluster randomisation but method unclear. |
| Allocation concealment? | High risk | Not used. |
| Blinding? | High risk | Inadequate ‐ open. |
| Incomplete outcome data addressed? | High risk | Inadequate ‐ more than 20% lost to follow up. |
| Free of other bias? | High risk | |
| Methods | Randomisation: adequate by computer‐generated random number table. Allocation concealment: unclear. Blinding: inadequate ‐ neither participant nor provider blinded. Outcome assessor unclear. Loss to follow up: inadequate. More than 47% lost to follow up. | |
| Participants | 413 healthy non‐severely anaemic pregnant women attending antenatal care at Ekwendeni Hospital or its mobile clinics in northern Malawi with less than 30 wks of gestation at their first visit, stratified by initial haemoglobin concentration before randomisation. | |
| Interventions | Women were randomly assigned within each anaemia grade category to one of two interventions: group 1 received 120 mg of elemental iron as ferrous sulphate with 0.5 mg of folic acid once a week; group 2 received 60 mg of elemental iron as ferrous sulphate with 0.25 mg of folic acid daily. | |
| Outcomes | Maternal: haemoglobin concentration at baseline and after 8 wks of supplementation; compliance, presence of side effects, and prevalence of anaemia. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate by computer‐generated random number table. |
| Allocation concealment? | Unclear risk | Unclear. |
| Blinding? | High risk | Inadequate ‐ neither participant nor provider blinded. Outcome assessor unclear. |
| Incomplete outcome data addressed? | High risk | Inadequate. More than 47% lost to follow up. |
| Free of selective reporting? | High risk | Compliance estimated by self reporting was 76% and 60% in the weekly and daily groups respectively. |
| Free of other bias? | High risk | |
| Methods | Randomisation: inadequate ‐ quasi randomised. Allocation concealment: inadequate. Blinding: inadequate. Participant and care provider not blinded. Outcome assessor blinded. Loss to follow up: inadequate. More than 54% lost to follow up. | |
| Participants | 51 healthy pregnant women with 18‐22 wks of gestation who had not taken supplements or medication in the previous six months attending public health centre in Ulsan, South Korea. | |
| Interventions | Women were randomly assigned to one of two treatments: group 1 received 160 mg of elemental iron in one intake once a week; group 2 received 80 mg of elemental iron daily. Elemental iron was given in the form of ferrous sulphate. Women with low Hb were assigned by the trialists to daily regimen. | |
| Outcomes | Maternal: haemoglobin concentration, serum ferritin, red blood cell count, haematocrit, mean corpuscular volume, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration, serum iron, total iron binding capacity, transferrin saturation at baseline and after treatment; zinc status before and after treatment, weight gain, nutrient intake before and after treatment. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Inadequate ‐ quasi randomised. |
| Allocation concealment? | High risk | Inadequate |
| Blinding? | High risk | Inadequate. Participant and care provider not blinded. Outcome assessor blinded |
| Incomplete outcome data addressed? | High risk | Inadequate. More than 54% lost to follow up. |
| Free of selective reporting? | High risk | No compliance reported for all the groups. Analysis reported on high compliers only. |
| Methods | Randomisation: adequate with table of random numbers. Allocation concealment: adequate. Coded bottles. Blinding: adequate ‐ participants and care provider and outcome assessor blinded. Loss to follow up: adequate. Less than 5% lost to follow up. | |
| Participants | 750 apparently healthy non‐smoking non‐anaemic (with Hb higher or equal to 132 g/L) pregnant women in early stage of second trimester, BMI 19.8‐26 kg/m2 and age 17‐35 years with singleton pregnancy attending prenatal care in Tehran, Iran. Women with history of threatened abortion in the present pregnancy or diseases related with polycythaemia such as asthma and chronic hypertension were not included. | |
| Interventions | Women were randomly assigned to one of two groups: group 1 received 50 mg of elemental iron as ferrous sulphate with 1 mg folic acid daily and group 2 received placebo and 1 mg of folic acid daily. | |
| Outcomes | Maternal: haemoglobin at 24‐28 wk, 32‐36 wk, premature delivery, weight gain, C‐sections, hypertensive disorders, severe anaemia, haemoconcentration, iron deficiency, iron deficiency anaemia, mean corpuscular volume, mean corpuscular haemoglobin and Hb concentrations at term, severe anaemia and haemoconcentration at any time during 2‐3 trimesters, symptomatic tract infection, puerperal infection, antepartum and postpartum haemorrhage, transfusion provided, side effects (any), diarrhoea, constipation, nausea, heartburn, vomiting, placental abruption, premature rupture of membranes. Infant: birthweight, perinatal mortality rate, low Apgar at 10th minute, small for gestational age. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate with table of random numbers. |
| Allocation concealment? | Low risk | Adequate. Coded bottles. |
| Blinding? | Low risk | Adequate ‐ participants and care provider and outcome assessor blinded. |
| Incomplete outcome data addressed? | Low risk | Adequate. Less than 5% lost to follow up. |
| Methods | Randomisation: adequate with table of random numbers. Allocation concealment: adequate. Coded bottles. Blinding: adequate ‐ participants and care provider blinded. Loss to follow up: adequate. Less than 5% lost to follow up. | |
| Participants | 244 pregnant women 17‐35 years of age attending prenatal care in Tehran, Iran, with BMI between 19.8‐26 kg/m2, and 13‐18 wks of gestation, with singleton pregnancy and non‐anaemic (Hb 132 g/L or higher) and normal serum ferritin (15 ug/L or higher). Women who smoked, had history of diseases such as polycythaemia, asthma, or chronic hypertension, or a history or threatened abortion in the present pregnancy were excluded. | |
| Interventions | Women were randomly assigned to one of two groups: group 1 received 50 mg of elemental iron as ferrous sulphate daily and group 2 received placebo from 20th week of gestation until delivery. All women received 50 mg elemental iron as ferrous sulphate after delivery for 6 wks. | |
| Outcomes | Maternal: haemoglobin, haematocrit, serum ferritin at baseline, at time of delivery, one week post‐partum and six wks post‐partum, postpartum haemorrhage, C‐sections. | |
| Notes | Unsupervised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate with table of random numbers. |
| Allocation concealment? | Low risk | Adequate. Coded bottles. |
| Blinding? | Low risk | Adequate ‐ participants and care provider blinded. |
| Incomplete outcome data addressed? | Low risk | Adequate. Less than 5% lost to follow up. |
Fe: iron
Hb: haemoglobin
ITT: intention to treat
MCV: mean corpuscular volume
PCV: plasma cell volume
SES: socioeconomic status
vs: versus
wk(s): week(s)
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| 67 non‐anaemic pregnant women attending prenatal care clinics in Kingsvinger Hospital, in Kingsvinger, Norway were allocated to a daily regimen of either 100 mg Fe or 15 mg Fe. Both groups received iron at different doses. No comparisons allowed within the scope of this review. | |
| Community based study in Vellore district, India using a pre‐post experimental design measuring the impact of an iron supplementation program, helminthic treatment and education intervention in the prevalence of anaemia in the different trimesters of pregnancy. The same pregnant women were not followed. | |
| 260 pregnant women from Cairo, United Arab Republic were randomly allocated to two forms of iron: a slow release ferrous sulphate preparation and ferrous sulphate in addition to folic acid. Both groups received iron supplementation in different preparations. No comparisons allowed within the scope of this review. | |
| 209 pregnant women between 18 and 45 years of age, attending outpatient obstetric clinics at North York General Hospital and the Hospital for Sick Children in Toronto, Canada were randomly assigned to receive multiple micronutrient supplements containing 60 mg of iron as ferrous fumarate (Materna) or another supplement (PregVit) to be taken twice daily with the morning dose containing 35 mg of iron and the evening dose containing 300 mg calcium, among other vitamins and minerals. Both groups received iron in different doses as well as other vitamins and minerals. No comparisons allowed within the scope of this review. | |
| 744 apparently healthy pregnant (with less than 20 wks) and non‐pregnant women of reproductive age (15‐49 years) from the municipalities of Calasiao, Binmaley and Santa Barbara, Philippines who were pregnant or most likely to become pregnant within the 12‐month duration of the study, and who volunteered to participate in the study were provided two preparations of iron‐folic acid supplements. Women with severe anaemia or history of malaria were excluded. Non‐pregnant women were prescribed four capsules monthly each containing 60 mg of elemental iron and 3.5 mg folic acid to be taken once weekly before bedtime (to be purchased by the women in local drugstores). Pregnant women received free of cost four capsules monthly each containing 120 mg of elemental iron and 3.5 mg of folic acid to be taken once a week before bedtime until delivery and for 3 months thereafter. Pregnant women seen at the health centres with 20 wks or more of gestation were advised to take their usual daily dose of iron‐folic acid tablets containing 60 mg of elemental iron and 0.5 mg of folic acid. Women were followed for 12 months. Haemoglobin, haematocrit, mean corpuscular volume, mean corpuscular haemoglobin concentration, serum ferritin, transferrin receptors, prevalence of iron deficiency and anaemia, compliance were assessed at baseline, 4.5, 9 and 12 months. There was not randomisation and the control group was not appropriate for comparisons. | |
| Fifteen healthy pregnant women 22‐32 years old, in the first trimester of pregnancy from Boston, Massachusetts, USA were randomly assigned to three different multiple micronutrient preparations to assess absorption of iron. | |
| 260 women with singleton pregnancy in Zurich, Germany, were randomised at 21‐24 wks of gestation to receive either intravenous iron group (further divided into two doses of 200 mg iron saccharate or three doses of 200 mg iron) or 80 mg elemental iron as ferrous sulphate daily. Both groups received iron in different routes of administration. No comparisons allowed within the scope of this review. | |
| 864 apparently healthy married pregnant and non‐pregnant nulliparous women of reproductive age planning to have a child soon from 19 rural communes of the Thanh Mien district in Hai Duong province, Vietnam were invited to participate and assigned to one of the following interventions according to their pregnancy status at baseline: women who were pregnant received free of charge UNICEF tablets containing 60 mg of elemental iron and 0.25 mg of folic acid to be taken daily and women who were non‐pregnant were prescribed pink packs of tablets containing 60 mg of elemental iron and 3.5 mg of folic acid that they could buy at their village from the Women's Union, to be taken once weekly. If these women became pregnant, women received red packs of tablets containing 120 mg of elemental iron and 3.5 mg of folic acid free of charge to be taken once weekly. After delivery women were given tablets containing 60 mg of iron and 0.5 mg of folic acid free of charge for 3 months to be taken weekly. Haemoglobin concentration, serum ferritin, and serum ferritin receptors, prevalence of anaemia and iron deficiency and compliance were measured at baseline, at 4.5, 9 and 12 months. This is not a randomised study and no comparisons can be made for the aims of this review. | |
| Planned study registered at the Oxford Database of Perinatal Trials. Author contacted and informed the project was not completed. | |
| 203 pregnant women attending prenatal care clinics during their 6th month visit were randomly allocated to either 105 mg of elemental iron with 500 mg of ascorbic acid or a placebo. Both groups received iron. | |
| 109 pregnant women attending prenatal care clinics in Manchester, England were randomly allocated to one of three groups: group A: one tablet daily given in 'reminder packs', group B: one tablet daily given in loose forms, or group C two tablets daily given in loose form. Tablets contained 50 mg of elemental iron as slow release ferrous sulphate and 400 ug of folic acid. All groups received iron. No comparisons allowed within the scope of this review. | |
| 472 pregnant women attending the booking clinic were alternatively allocated to two forms of iron: a slow release ferrous sulphate preparation and folic acid or combined conventional ferrous sulphate/folic acid. Both groups received iron supplementation in different preparations. No comparisons allowed within the scope of this review. | |
| 18 pregnant women were randomly assigned to receive either a tablet containing 80 mg of elemental iron with a new mucous membrane vaccine (Tardyferon® or a tablet containing 80 mg elemental iron with 0.35 mg folic acid (Tardyferon‐Fol®) for a period of 3 months. All women received iron. No comparisons allowed within the scope of this review. | |
| Two liquid preparations were used in this study: one with D‐sorbitol and the other without. Both preparations contained vitamin B12, vitamin B6, ferric pyrophosphate and folic acid. | |
| 120 singleton pregnant women attending the Instituto Nacional de Perinatologia in Mexico City, Mexico with haemoglobin concentrations higher than 115 g/L at 20 wks of gestation (equivalent to 105 g/L at sea level) were randomly assigned to one of two groups, group 1: one tablet containing 60 mg of elemental iron, 200 ug folic acid and 1 ug vitamin B12 given daily, and group 2: two tablets (total 120 mg of elemental iron, 400 ug folic acid, and 2 ug vitamin B12) to be taken once weekly. Haemoglobin and serum ferritin concentrations were measured every 4 wks from wks 20 until 36, side effects, compliance, birth weight, gestational age at birth, anaemia, iron deficiency. The addition of vitamin B12 to the formulation makes this study ineligible to the inclusion criteria. No comparisons allowed within the scope of this review. | |
| 190 pregnant women attending antenatal clinic in St Mary's Hospital in London, England were randomly assigned to one of three groups: ferrous fumarate, ferrous fumarate and folic acid, or placebo. The outcomes measured include full blood count at 20th, 30th, 35th and 39th week of gestation and 6th day after delivery. The paper does not report standard deviations in the variables measured and no data can be extracted. | |
| 81 pregnant women with 20 +/‐ wks of gestation from Ludhiana City, India were divided to one of three groups: group 1, 60 mg of elemental iron ad 500 ug of folic acid daily; group 2, 60 mg of elemental iron and 2,000,000 IU of vitamin A, or group 3, who did not receive any supplements. Supplementation was for a period of 15 wks. Outcomes measured included haemoglobin, red blood cell count, total iron binding capacity, transferrin saturation, serum iron, serum vitamin A at baseline and at 36 +/‐ 2 wks of gestation. Poor methodological quality. None of the outcomes pre‐specified in our protocol were recorded due to the varied time of final measurements. | |
| 100 pregnant women with 20‐34 wks of gestation attending the antenatal clinic at The Bandra Holy Family Hospital, Bandra, Mumbai India were randomly assigned to receive 30 mg elemental iron + other essential micronutrients daily or 116 mg elemental iron, folic acid, zinc and vitamin C daily. Outcomes included haemoglobin concentration, maternal weight gain, infant birth weight and maternal compliance and side effects Both groups received iron supplementation. No comparisons allowed within the scope of this review. | |
| 200 women were randomly assigned to receive 50 mg iron daily given either as Gastric Delivery System (GDS) or conventional ferrous sulphate. Gastrointestinal side effects were evaluated. The participants were non‐pregnant women. | |
| 42 healthy women with less than 16 wks of pregnancy were randomly assigned to receive either a multiple micronutrient supplement containing 65 mg of elemental iron or one multiple micronutrient supplement with no iron, calcium, zinc and copper and pantothenic acid. Both groups received different multivitamin/multi mineral supplement formulations. No comparisons allowed within the scope of this review. | |
| 170 pregnant women with less than 20 wk gestation from 13 adjacent villages in a rural area in Bogor District, West Java, Indonesia were randomly assigned to receive daily supplementation with B‐carotene (4.5 mg), zinc (30 mg), both, or placebo containing iron (30 mg) and folic acid (0.4 mg). Both groups received iron and folic acid. No comparisons allowed within the scope of this review. | |
| 146 pregnant women with less than 20 wks of gestation were randomly allocated to receive either a multivitamin tablet twice a day or a multivitamin tablet in conjunction with a standard ferrous sulphate tablet twice a day providing a total of 120 mg of elemental iron daily. Both groups received a multivitamin supplement. No data can be extracted from the published article. | |
| 179 pregnant women with Hb levels below 105 g/L and more than 16 wks of gestation volunteered for this study and were divided into four supplementation groups according to the stage of pregnancy: 16th week, 20th week, 24th week, and non‐supplemented controls. 37% of these women were lost to follow up and were excluded from the final analysis. Data are presented without standard deviation. No data can be extracted from the publication for this review. | |
| 176 pregnant women attending Ilula Lutheran Health Center's antenatal service in Iringa region, Tanzania with 21‐26 wks of gestational age and haemoglobin > 80 g/L were randomly assigned to receive 120 mg elemental iron as ferrous sulphate in conventional form or 50 mg elemental iron as gastric delivery system (GDS). Both groups received iron supplementation in different preparations. No comparisons allowed within the scope of this review. | |
| 154 pregnant women with less than 14 wks of gestation, and who had not received or were receiving treatment for a blood disorder were divided into 2 groups according to the day in which they attended the clinic in Cardiff: group 1 received 60 mg of ferrous sulphate and group 2 received placebo. Haemoglobin concentration, mean corpuscular volume (MCV), serum ferritin, serum iron and total iron binding capacity were measured at 10‐14 wk and at term. The data in the paper are presented with no standard deviation values. No data can be extracted from the publication for this review. | |
| 146 consecutive pregnant women attending a public antenatal clinic in Western Australia before the 20th week of gestation who had not received iron supplements and were willing to participate were randomly assigned in sequences of 50 to one of the 5 interventions groups: group 1 received placebo; group 2 received 60 mg of elemental iron as ferrous sulphate; group 3 received 0.5 mg of folic acid; group 4 received 60 mg of elemental iron as ferrous sulphate and 0.5 mg of folic acid; and group 5 received 60 mg of elemental iron as ferrous sulphate and 5 mg of folic acid. Supplementation with iron was from 20th week of gestation until delivery. All women had received 50 mg of ascorbic acid daily from the first visit until week 20th. More than 20% of the women were lost to follow up. No data can be extracted from the publication for this review. | |
| 200 apparently healthy primigravidae Hausa women living in Zaria, Nigeria and planning to deliver in Zaria, with less than 24 wks of gestation, who had not taken any antimalarial treatment or iron supplements in current pregnancy were randomly assigned to one of five groups: group 1: received no active treatment; group 2: received chloroquine 600 mg base once, followed by proguanil 100 mg per day; group 3 received in addition to chloroquine and proguanil, 60 mg elemental iron daily; group 4 received in addition to chloroquine and proguanil, 1 mg of folic acid daily, and group 5: in addition to chloroquine and proguanil received 60 mg of elemental iron and 1 mg of folic acid daily. Eighty‐nine out of 200 women delivered in the hospital and no other complete clear data can be extracted for the outcomes of interest in this review. | |
| 643 pregnant women attending antenatal clinic in London were randomly assigned to one of two groups: group 1 received 200 mg of ferrous sulphate daily; group 2 received 200 mg of ferrous sulphate with 5 mg of folic acid daily. Both groups received iron. No comparisons allowed within the scope of this review. | |
| 568 apparently healthy pregnant women with less than 20 wks of pregnancy and no prior iron supplementation were allocated alternatively to receive 100 mg of elemental iron and 350 ug of folic acid daily or no treatment. Ferritin and haemoglobin concentrations were measured at baseline and at 28 and 36 wks of gestation and 2 days postpartum. Mean corpuscular volume and mean corpuscular haemoglobin were measured at 2 days postpartum. Only means and median are presented. No standard deviation is shown and for ferritin concentrations no ln‐transformed data are presented. No data were extractable from the paper and subsequent communication with author. | |
| 412 non‐black pregnant women with 26 ± 2 wks of gestation, who had not received iron supplements in the previous 6 months, from low SES using the prenatal unit of Quito's public obstetric hospital, Ecuador were randomly assigned to receive two tablets containing 78 mg of elemental iron as ferrous sulphate daily or placebo during a period of 2 months. Overall loss to follow up rate was 41.7%. Haemoglobin, PCV, red cell indices, serum ferritin, total iron binding capacity, serum folate, serum vitamin B12 at baseline and after 2 months. Prevalence of iron deficiency was estimated by response to therapy. No prespecified outcomes from this review are presented in the paper. No further data were available. | |
| 40 apparently healthy women with singleton pregnancy in their second trimester (between 16‐24 wks of gestation), living in urban slums, from low socio‐economic status attending Guru Teg Bahadur Hospital, Delhi, India were randomly assigned to receive one tablet containing 100 mg of elemental iron as ferrous sulphate with 500 ug of folic acid daily or once a week. Weekly intake was supervised. Duration of supplementation was 100 days. Haemoglobin and haematocrit concentrations at baseline, at 4 wks, 8 wks and 14 wks of supplementation, serum ferritin concentration, at baseline, at 14 wks of supplementation and at delivery. | |
| 92 pregnant women from 14‐24 wks of gestation attending the university antenatal clinic, in Galle, Sri Lanka were randomly assigned to one of three regimens: group 1 (n = 26) received a tablet containing 100 mg of elemental iron as ferrous fumarate, with additional micronutrients once a week; group 2 (n = 35) received the same tablet but three times a week; and group 3 (n = 31) received the same supplement in a daily fashion. All groups were receiving multiple micronutrients. No comparisons allowed within the scope of this review. | |
| 900 pregnant women of poor socio‐economic status females attending government antenatal care clinics were grouped in three groups: group 1 (n = 300) received routine antenatal care; group 2 (n = 300) received 100 mg of elemental iron and 500 ug folic acid daily from the 20th week of gestation and group 3 (n = 300) received 100 mg of elemental iron and 500 ug folic acid daily from the 20th week of gestation and additionally 900 mg of alpha linolenic acid from the 22nd week of gestation. Outcomes assessed included birth weight, low birth weigh, premature delivery. The study is not reported as randomised and is excluded in the first screening for eligibility. | |
| 40 pregnant women attending antenatal care clinic were given a tablet containing 47 mg of elemental iron, as ferrous sulphate and 0.5 mg of folic acid daily or a tablet containing 100 mg of elemental iron as ferrous glycine sulphate daily. Both groups received iron. No comparisons allowed within the scope of this review. | |
| 40 pregnant women attending antenatal care at the Adolescent Pregnancy Clinic and Obstetrics Clinics at the John Hopkins and Sinai Hospital in Baltimore, Maryland, USA at or before 16 wks of pregnancy with haematocrit equal or above 31% were randomly assigned to one of two groups: group 1 (n = 16) received 60 mg of elemental iron as ferrous fumarate and prenatal vitamins daily; or group 2 (n = 9) received only the prenatal vitamins with no iron. Two women objected to the randomisation and 13 dropped out of the study. Both groups received multiple micronutrients. Supplementation lasted a month. Psychometric tests (arithmetic, total digit span, digit symbol, vocabulary and others) were performed and hematologic status was measured at baseline and after a month. Hematologic outcomes cannot be extracted from the paper. None of the other outcomes were sought. | |
| 192 pregnant women were consecutively randomised to receive one of two treatments: group 1: received a daily vitamin‐mineral tablet containing 15 mg of elemental iron or group 2: received a daily vitamin‐mineral tablet containing 100 mg of elemental iron. Both groups received iron in different doses. No comparisons allowed within the scope of this review. | |
| 65 untreated and 54 treated pregnant women in West Berlin, Germany were assessed during pregnancy for haemoglobin concentrations, iron an folate levels, total iron binding capacity, and red cell count. No data are presented for outcomes prespecified in the review. Women were of different gestational age. No outcomes can be extracted from the paper. | |
| No report available of the study results. | |
| 120 unselected pregnant women were given 114 mg of elemental iron daily from week 15 until delivery, or not treatment. Only an abstract with insufficient data available. | |
| 202 apparently healthy pregnant women 20‐32 years of age attending health clinics from 12 communes in Dong HungDistrict, Thai Binh Province, Vietnam with 14‐18 wks of gestation who agreed to participate in the study were selected to participate. Women were assigned through block randomly assigned to one of 4 interventions: group 1 (n = 44) received 400 ml fortified milk with iron (ferrous fumarate), vitamin C and folic acid daily; group 2 (n = 41) received 400 ml of milk fortified with vitamin C and folic acid but no iron daily; group 3 (n = 40) received one tablet containing 60 mg of elemental iron (as ferrous sulphate) and 250 ug of folic acid daily and group 4 (n = 43) received one placebo tablet daily. For purposes of this review groups 3 and 4 comparing iron and folic acid supplements to placebo could be included. However, no data on outcomes of interest could be extracted from the published report. | |
| 42 apparently healthy pregnant women attending two antenatal care clinics in London, England were assigned to one of three interventions: group 1 received 200 mg ferrous sulphate with 5 mg of folic acid three times a day; group 2 received 350 mg of ferrous aminoate with 50 ug of folic acid three times a day; and group 3 received 200 mg of ferrous sulphate with 500 ug of folic acid once a day. Intervention period was 3 wks. All groups received iron and folic acid. No comparisons allowed within the scope of this review. | |
| 84 anaemic women seeking antenatal care in the Department of Obstetrics and Gynaecology of the Fukui School of Medicine Hospital, Japan were randomly assigned to receive 100 mg of elemental iron as ferrous sulphate daily after the evening meal, or the same dose + vitamin C for 4 wks. Both groups received iron. No comparisons allowed within the scope of this review. | |
| 800 pregnant women with less than 24 wks of gestation and Hb > 85 g/L in India were assigned by rotation to one of four groups: group 1 received placebo tablets; group 2 received 30 mg of elemental iron as ferrous fumarate in a single tablet daily; group 3 received 30 mg of elemental iron as ferrous fumarate with 500 ug of folic acid in a single tablet; and group 4 received in addition to iron and folic acid, 2 ug of vitamin B12 in a single tablet. Loss to follow up was 65%. None of the pre‐specified outcomes in the protocol was reported and no data were extractable from the paper. | |
| 2100 pregnant women (22 +/‐ 7 wk gestation at entry) attending antenatal clinics in Bissau, Guinea‐Bissau or who were identified by The Bandim Health project were randomly assigned to receive daily multi micronutrient tablet containing one Recommended Dietary Allowance (RDA) of 15 micronutrients, or daily multi micronutrients containing two times the RDA except for iron that was maintained at one RDA or a conventional prenatal daily iron (60 mg) and folic acid (400 ug) supplement. All groups receive iron and folic acid daily. No comparisons allowed within the scope of this review. | |
| 36 healthy non‐anaemic pregnant women in second or third trimesters of gestation were randomly assigned to receive one of four prenatal commercial multivitamin/multi mineral preparations daily: Stuartnatal 1+1; Stuart Prenatal; Materna; and Natalins Rx. All participants received multiple micronutrients. No comparisons allowed within the scope of this review. | |
| 220 pregnant women with a singleton pregnancy and Hb between 80‐110 g/L at 16‐24 wk gestation from New Delhi, India were randomly allocated to receive daily oral iron therapy of 100 mg elemental iron as ferrous sulphate with 500 ug folic acid or 250 mg of iron sorbitol intramuscularly and repeated at an interval of 4‐6 wks. This trial compares the effects of daily oral iron with two injections of high dose parenteral iron. No comparisons allowed within the scope of this review. | |
| 109 apparently healthy pregnant women with 16‐24 wks of gestation who had not received iron supplements were randomly assigned to one of three groups: group 1 received 60 mg of elemental iron + 0.5 mg of folic acid once daily; group 2 received 120 mg of elemental iron + 0.5 mg of folic acid once daily; group 3 received 120 mg of elemental iron twice daily + 0.5 mg of folic acid. Duration of supplementation was 12‐14 wks. All participants received iron and folic acid daily. No comparisons are allowed within the scope of this review. | |
| 1035 pregnant women attending mother and child health clinics near the town of Farafenni, The Gambia were randomised to receive either folic acid (500‐1500 ug/day) together with oral iron (47 mg of ferrous sulphate per tablet) or oral iron alone (60 mg of ferrous sulphate per tablet) daily for 14 days. All women received treatment with three tablets of SP (25 mg of pyrimethamine and 500 mg of sulfadoxine). Both groups received iron daily. No comparisons allowed within the scope of this review. | |
| 102 healthy pregnant women attending antenatal clinics at the Royal Jubilee Maternity Hospital in Belfast, Ireland with a singleton pregnancy and haemoglobin > 104 g/L and known gestational age of less than 20 wks who were non‐compliers with routine prescription of 200 mg of ferrous sulphate daily, were randomly assigned to receive 2 sachets of 24 ml each of Spatone® water containing 10 mg of elemental iron or placebo. Participants were instructed to take the two sachets daily half an hour before breakfast diluting it in orange juice. Primary outcomes were compliance and side effects. Duration of intervention was from week 22 to week 28 of gestation. | |
| 273 healthy pregnant women with 16‐24 wks of gestation and haemoglobin concentrations at or above 105 g/L attending antenatal care clinics were divided in order in which they were registered in three groups: group 1 was given 5 g of ferrous sulphate daily; group 2 received 5 mg of folic acid daily; and group 3 received 5 g of ferrous sulphate and 5 mg of folic acid daily. All participants were given 3 multivitamin tablets daily containing vitamin A, vitamin B, C and D. The study was not randomised. | |
| 427 healthy Danish pregnant women living in the northeastern part of Copenhagen County, Denmark were randomly allocated to receive iron (as ferrous fumarate) in daily doses of 20 mg (n = 105), 40 mg (n = 108), 60 mg (n = 106), and 80 mg (n = 108) from 18 wks of gestation. Hemoglobin (Hb), serum ferritin, and serum soluble transferrin receptor concentrations were measured at 18 wks (inclusion), 32 wks, and 39 wks of gestation and 8 wks postpartum. All women received iron daily. No comparisons allowed within the scope of this review. | |
| 356 pregnant women attending two different antenatal care clinics at the King Edward Memorial Hospital for Women in Subiaco, Australia received according to the clinic they visited, either no treatment or 100 mg of elemental iron as ferrous gluconate daily. No systematic allocation was used in this open trial. | |
| 105 pregnant women attending the University Unit, Mater Misericordiae Mothers' Hospital, South Brisbane, Australia, with normal height, weight and nutrition for the Australian population and with no previous adverse medical, surgical or obstetrical history were allotted by random selection to one of four types of supplements: group 1 received 50 mg of elemental iron as dried ferrous sulphate daily; group 2 received 80 mg elemental iron as dried ferrous sulphate with 300 ug of folic acid daily; group 3 received 105 mg elemental iron as ferrous sulphate and group 4 received 105 mg of elemental iron as ferrous sulphate with 300 ug of folic acid. All groups received iron daily. No comparisons allowed within the scope of this review. | |
| 191 anaemic pregnant women between the ages of 17‐35 years of age, and uneventful obstetric history attending the Maternity wing of the Federal Government Services Hospital in Islamabad and the Maternal & Child Health Clinic at the Christian Mission Hospital in Taxila, Pakistan were randomly assigned to one of two interventions: group 1 received 200 mg of ferrous sulphate (40 mg elemental iron) with 1 mg of folic acid once daily; and group 2 received 200 mg of ferrous sulphate with 1 mg of folic acid on two days of the week and placebo the rest of the days. Subjects and care providers were blinded to the treatments. Outcomes measured included haemoglobin concentration and serum ferritin at baseline and during the three following consecutive visits as well as compliance and weight. Change in haemoglobin Z‐scores after supplementation was the main outcome variable, in women from different gestational ages and duration of intervention, thus not allowing outcomes prespecified in this review. | |
| 167 pregnant women with less than 20 wks of gestation who called either Motherisk General Information line or the Motherisk Nausea and Vomiting of Pregnancy (NVP) Helpline (Hospital for Sick Children, Toronto) and had not started taking or had discontinued any multivitamin due to adverse events were randomly assigned to one of two groups: group 1 were provided, PregVit® (a small‐size, containing 35 mg elemental iron as ferrous fumarate and multivitamins; or group 2 who received Orifer F® (high iron content, small size) containing 60 mg elemental iron as ferrous sulphate and multivitamins. Follow‐up interviews documented pill intake and adverse events. Participants from both groups received iron in different amounts and compounds. | |
| 74 low‐income pregnant adolescents ranging from 13‐18 years of age attending antenatal care at the Evangelina Rosa Maternity Hospital in Teresina, Piaui State, Brazil were distributed into five groups: group 1 received 120 mg elemental iron as ferrous sulphate and 250 ug of folic acid daily; group 2 received 80 mg elemental iron as ferrous sulphate and 250 ug folic acid daily; group 3 received 120 mg of elemental iron, with 5 mg of zinc sulphate and 250 ug of folic acid daily; and group 4 received 80 mg of elemental iron as ferrous sulphate, with 5 mg of zinc sulphate and 250 ug of folic acid daily. All groups received iron and two groups received zinc in addition to iron and folic acid. No comparisons allowed within the scope of this review. | |
| 80 apparently healthy non‐anaemic pregnant women attending University College Hospital and Inalende Maternity Hospital in Ibadan, Nigeria during the first and second trimesters of pregnancy were randomly allocated to one of two groups: group 1 (n = 39) received one tablet Ferrograd Folic 500 Plus® daily, a sustained‐released formulation containing ferrous sulphate and folic acid (composition is not available); or group 2 (n = 41) received a capsule containing 200 mg ferrous sulphate and 5 mg of folic acid. All patients were also provided 25 mg weekly of pyrimethamine throughout pregnancy as an anti‐malarial agent. Patients who became anaemic during pregnancy were excluded of the study and analysis. Outcomes measured included reticulocyte count, haematocrit, anaemia, side effects. Both groups received iron and folic acid supplements, thus making the comparisons not suitable for this review. | |
| 315 apparently healthy pregnant women attending four prenatal care clinics in 4 geographical areas of Nigeria with mild to moderate anaemia (as defined by haematocrit between 26%‐34%) and 18‐28 wks of gestation, single pregnancies, no complications and who consented to participate in the study were randomly allocated to one of two groups: group 1 (n = 159) received one daily capsule of a multiple micronutrient supplement Chemiron® containing 300 mg of ferrous fumarate, 5 mg folic acid, 10 ug vitamin B12, 25 mg of vitamin C, 0.3 mg magnesium sulphate and 0.3 mg of zinc sulphate; group 2 (n = 156) received a capsule containing 200 mg ferrous sulphate and 5 mg of folic acid. All patients were also provided 600 mg of cloroquine to be taken under supervision and 25 mg weekly of pyrimethamine throughout pregnancy. Patients who became anaemic during pregnancy were excluded of the study and analysis. Outcomes measured included blood haemoglobin, anaemia, haematocrit, serum ferritin levels, side effects. A second published study followed these same women and their infants. Both groups received iron and folic acid supplements, thus making the comparisons not suitable for this review. | |
| 41 healthy pregnant women, attending prenatal care clinics at Hospital Diego Paroissien in La Matanza, Province of Buenos Aires, Argentina with serum ferritin below 50 mg/mL. Women were assigned to one of two groups: group 1 received 100 mg of elemental iron daily as ferric maltosate, and group 2 received no treatment. | |
| 1200 healthy pregnant women with a singleton pregnancy and less than 20 wk gestation attending an antenatal clinic at Janakpur zonal hospital in Nepal, were randomly assigned to receive routine daily iron (60 mg) and folic acid (400 ug) supplements or a multiple micronutrient supplement containing 15 vitamins and minerals including iron (30 mg) and folic acid (400 ug). Both groups received iron and folic acid. No comparisons allowed within the scope of this review. | |
| 200 pregnant women attending antenatal clinics in Glasgow, Scotland with less than 20 wk gestation, whose antenatal care was undertaken wholly by the hospital antenatal clinic and who subsequently had a normal delivery, were randomly allocated to receive 200 mg of ferrous sulphate daily or 200 mg of ferrous sulphate with 1.7 mg of folic acid daily throughout pregnancy. Both groups received iron. No comparisons allowed within the scope of this review. | |
| 116 pregnant women of 10‐30 wk of gestational age attended antenatal care clinics in Trujillo, Venezuela were randomly allocated to receive a 120 mg oral dose of iron as ferrous sulphate and 0.5 mg of folic acid weekly (n = 52) or 60 mg iron and 0.25 mg folic acid and a placebo twice weekly (n = 44). Haemoglobin, hematocrit, serum ferritin and transferrin saturation were estimated at baseline and at 36‐39 wk of gestation. All groups received iron and folic acid in two intermittent regimens with no control group. No comparisons allowed within the scope of this review. | |
| In a randomised double‐blind study the new effervescent iron tablet Loesferron® was tested in 57 postpartum women. The participants were not pregnant women. | |
| 107 healthy pregnant women with 6‐20 wks of gestation who had not received iron supplements during the current pregnancy attending 19 health units in the State of Morelos, Mexico were randomly assigned by block pairs to receive either 120 mg of elemental iron as ferrous sulphate in a single dose daily or once weekly. Haemoglobin concentration, prevalence of anaemia and nutrient consumption at baseline and after 10 wks of supplementation were measured. None of the prespecified outcomes of this review were available. Gestational ages were variable among the participants. | |
| 873 pregnant women living near Cuernavaca, Morelos, Mexico with less than 13 wks of gestation who did not use micronutrient supplements were randomly assigned to receive a daily multiple micronutrient supplement or a daily iron‐only supplement. Both supplements contained 60 mg of elemental iron as ferrous sulphate. Supplement intake was supervised by trained workers from registration until delivery by home visits 6 days a week. No comparison allowed within the scope of this review. | |
| 394 healthy non‐anaemic adult pregnant women with 24‐32 wks of gestation and singleton pregnancy from Fuentalabra, Spain were randomly assigned to one of two groups: group 1 received 40 mg of elemental iron as iron mannitol albumin daily; and group 2 received 40 mg elemental iron as iron protein succinylate daily. Both groups received iron daily. No comparisons allowed within the scope of this review. | |
| 110 pregnant women attending the antenatal clinic at Comprehensive Rura Health Services Project Hospital, Ballabgarh, India, with 16‐24 wks of gestation were randomly assigned to one of three groups: group 1 received 60 mg elemental iron and 0.5 mg of folic acid daily; group 2 received 120 mg elemental iron with 0.5 mg of folic acid daily; and group 3 received 240 mg elemental iron and 0.5 mg of folic acid daily. Elemental iron was given as ferrous sulphate. All groups received iron daily. No comparisons allowed within the scope of this review. | |
| 84 non‐anaemic pregnant women at Mazarik University Brno in Czech Republic were treated from 20‐24 wks with one capsule of Actiferrin Compositum®, and from 36 wks to delivery with 2 capsules. The group was compared with 57 non‐anaemic pregnant women who received no supplements. The supplement contained 34.5 mg of elemental iron as ferrous sulphate, 0.5 mg of folic acid, and 0.3 mg of cyanocobalamin. No comparisons allowed within the scope of this review. | |
| 117 pregnant women between 20‐29 wks of gestation were alternatively assigned during three consecutive two wks periods to receive daily tablets containing 200 mg of elemental iron as ferrous sulphate, 200 mg of elemental iron as a sustained released iron or placebo. After each 2‐wks treatment period women were questioned about possible side effects. No side effects are reported by group assigned. No comparisons are allowed within the scope of this review. | |
| 233 pregnant women attending their second antenatal care visit at the University Health Services of Oslo, Norway with serum ferritin concentration < 60 ug/L were randomised to two different iron preparations, group 1 received one tablet containing 60 mg of elemental iron as ferrous sulphate daily; group 2 received three tablets each containing 1.2 mg of heme iron from porcine blood plus 8 mg of elemental iron as ferrous fumarate per tablet (total 3.6 heme iron and 24 mg elemental iron) daily. A third group (n = 93) of pregnant women who had been given advice to take or not the iron supplements according to the centre recommendations were enrolled in the trial at 6 wks postpartum and served as control. The study groups were not randomised to the interventions and no comparisons can be made within the scope of this review. | |
| 221 apparently healthy pregnant women, had not used iron supplements prior to enrolment, who were 12 to 16 wks were recruited from six health centres in Dakar, Senegal during their first prenatal visit, and randomly assigned to receive either a prescription to purchase iron/folic acid tablets to be taken daily, according to official policy, or to receive free tablets. Compliance was assessed 20 wks after enrolment through interviews and pill count. All women received iron. No comparisons allowed within the scope of this review. | |
| 115 healthy pregnant women with 20‐28 wks of gestation attending the antenatal clinic of the National Institute of Nutrition, Government Maternity Hospital, India were randomly assigned to one of 11 different formulations and doses of iron and then undergo iron tolerance tests. They received ferrous sulphate tablets containing 60 mg, 12 mg, and 180 mg of elemental iron; formulations containing 60 mg of elemental iron as pure ferrous sulphate salt, ferrous fumarate tablets, ferrous fumarate syrup, excipients added to pure ferrous sulphate salts; powdered ferrous sulphate tablets, iron tablets distributed by the National Nutritional Anaemia Prophylaxis Programme and pure ferrous salt in gelatin capsules. | |
| 376 pregnant women with ages between 16‐35 y, with mild anaemia (Hb concentrations between 80‐110 g/L) attending eight maternal and child health centres in Kingston, St. Andrews and Spanish Town, Jamaica, with gestational age between 14‐22 wks were randomly assigned to one of three groups: group 1 received one placebo tablet daily; group 2 received 100 mg of elemental iron as ferrous sulphate daily; group 3 received gastric delivery system capsule containing 50 mg of elemental iron daily. All women received 400 mg of folic acid. Outcomes measure included haemoglobin, haematocrit, MCV, white cell count, serum iron, total iron binding capacity, serum ferritin, serum transferrin receptor, at baseline, at 6 wks and at 12 wks after start of supplementation as well as side effects. No prespecified outcomes are presented at the paper as gestational ages differed in the participants. | |
| 300 pregnant women attending the Maternity Welfare Center, in Oulu, Finland before the 5th month of pregnancy were randomly assigned to one of three interventions: group 1 received 100 mg of elemental iron daily as sustained‐release tablets daily; group 2 received 200 mg of elemental iron daily as sustained‐release tablets and group 3 received 200 mg of elemental iron daily as rapidly disintegrating ferrous sulphate tablets. All groups received iron in different doses and formulations. | |
| 151 healthy pregnant women with Hb > 50 g/L who had not received iron supplements during the last 6 months from Delhi and Vellore, India were divided in one of three strata according to Hb concentration (50‐79 g/L; 80‐109 g/L;110 g/L and above) and within each strata were allocated randomly to one of five interventions: group 1 received 120 mg of elemental iron as ferrous sulphate 6 days a week; group 2 received 100 mg of elemental iron as iron dextran complex intramuscular twice per week; group 3 received iron as group 1 + pteroylmonoglutamic acid 5 mg/d 6 days a week + cyanocobalamin 100 ug intramuscular once per 14 d; group 4 received 100 mg of elemental iron intramuscular + pteroylmonoglutamic acid + cyanocobalamin 100 ug intramuscular; and group 5 received iron dextran complex intramuscular in a single total dose infusion + 5 mg/d pteroylmonoglutamic acid + 100 ug intramuscular cyanocobalamin once per 14 days. All groups received iron at different doses and routes. No comparisons allowed within the scope of this review. | |
| Trial abandoned. No data available. | |
| 248 healthy pregnant women attending hospital antenatal clinic in London, England, were allocated randomly to receive a slow‐release dose of 105 mg of elemental iron as ferrous sulphate and 350 ug of folic acid daily or 80 mg of elemental iron as ferrous fumarate and 400 ug of folic acid daily in a standard preparation. Both groups received iron in different doses and preparations. No comparisons allowed within the scope of this review. | |
| 251 pregnant women aged 17‐35 years, parity 0‐4 and haemoglobin concentrations between 80 and 109 g/L were randomly allocated to one of four groups: group 1 received 2.4 mg of retinol and one placebo iron tablet daily; group 2 received 60 mg of elemental iron as ferrous sulphate and a placebo vitamin A tablet daily; group 3 received 2.4 mg of retinol and 60 mg of elemental iron; and group 4 received two placebos for 8 wks. Outcomes measured include: haemoglobin, haematocrit, serum ferritin, serum iron, total iron binding capacity, serum retinol, transferrin saturation, at baseline and after 8 wks of supplementation. None of the pre‐specified outcomes in this review can be extracted from this paper. | |
| 82 pregnant women with haemoglobin concentrations 140 g/L or above attending clinic in Thessaloniki, Greece were randomised to receive 80 mg iron protein succinylate daily or a placebo. Serial haemoglobin, haematocrit and serum erythropoietin were measured from maternal blood and cord blood on delivery; serum ferritin measured in frequent intervals. Abstract only available. Insufficient information to assess characteristics of the trial. | |
| 285 healthy middle‐class pregnant women with haemoglobin concentration above 100 g/L attending antenatal clinic at the University Hospital at Kuala Lumpur, Malaysia were assigned to receive daily iron supplements or no treatment. Abstract only available. No additional information was available, including doses, regimens and other characteristics of the trial. | |
| 128 anaemic and non‐anaemic pregnant females aged 10‐19 years old, with an average gestation of 16 wks, were grouped for three levels of iron supplementation: group 1 (n = 42 non‐anaemic participants) received placebo (no iron); group 2 (n = 41 anaemic and non‐anaemic participants) received 22 mg of elemental iron daily and group 3 (n = 45 anaemic and non‐anaemic participants) received 55 mg elemental iron daily. Women were supplemented from 16 wks until delivery. Outcomes assessed included Hb, haematocrit, red cell count, mean corpuscular volume, serum iron, serum transferring and serum, ferritin measured every four wks. The study is not reported as randomised and is excluded in the first screening for eligibility. | |
| 135 healthy pregnant women between 22‐28 wks of gestation attending antenatal clinic in Burma, were randomly assigned to receive a daily dose of 60 mg, 120 mg or 240 mg of elemental iron as ferrous sulphate. A control group was composed by 47 apparently healthy adults (17 males and 30 single women). Control groups are not appropriate. No comparisons allowed within the scope of this review. | |
| 83 healthy nulliparous non vegetarian, non‐anaemic pregnant women with serum ferritin concentrations above 10 ug/L were randomly assigned to one of three groups: group 1 received 100 mg of elemental iron as ferrous sulphate daily; group 2 received placebo, and group 3 received dietary advice only. Blood haemoglobin, serum ferritin and blood manganese were determined at baseline before 15th week of gestation, between 25‐28 wks, and between 35‐40 wks of gestation. Median and ranges are presented. No outcomes were extractable from this report for this review. | |
| 52 healthy non‐anaemic nulliparous women with normal singleton pregnancy and serum ferritin levels above 15 mg/L at 16th week in Herlev, Denmark were randomly assigned to receive either a daily tablet containing 18 mg or a daily tablet containing 100 mg of elemental iron from 16 wks until delivery. All women received 0.3 mg of folic acid daily. All women received iron in different doses. No comparisons allowed within the scope of this review. | |
| 191 consecutive pregnant when attending antenatal care clinics and at 32 wks of gestation were divided in two groups by alternate allocation by clinic: group 1 received 140 mg of elemental iron daily as ferrous gluconate in four tablets; group 2 received 150 mg elemental iron daily as ferrous glutamate in 3 tablets. All women received iron in different dose and number of tablets. No comparisons allowed within the scope of this review. | |
| 60 iron deficiency anaemic pregnant women with the gestational age of 12‐34 wks were randomly assigned to one of 3 groups: Group A (n = 15) received intravenous 500 mg of iron sucrose for storage; group B (n = 20) received intravenous iron sucrose according to deficit calculated as per formula with 200 mg of iron was given for storage and group C received intra muscular iron Sorbitol in the dose used as practice. All groups received iron intravenous or intramuscular. | |
| 350 consecutive pregnant women attending antenatal care clinic were allocated to one of five groups: group 1 received no hematinic supplements; group 2 received 105 mg of elemental iron daily as iron chelate aminoates; group 3 received 105 mg of elemental iron daily with 100 ug of folic acid; group 4 received 105 mg of elemental iron daily with 300 ug of folic acid; and group 5 received 105 mg of elemental iron daily th 450 ug of folic acid. All women received a multivitamin preparation (Vivatel) free of folic acid. | |
| 68 pregnant women attending antenatal care clinic in Queen Mother's Hospital in Scotland, were randomly allocated to receive 195 mg of elemental iron alone daily or 195 mg of elemental iron in conjunction with 300 ug of folic acid daily. | |
| 369 pregnant women attending antenatal care at Beijing Hospital, China were divided into two groups according to their initial haemoglobin concentrations. Women with Hb 110 g/L or above were randomly assigned to one of two groups: group 1 (n = 96) received one daily tablet of maternal supplement containing 60 mg of elemental iron in addition to other micronutrients including calcium and magnesium ; group 2 (n = 95) served as control and received no supplements. Another group of women with Hb < 110 g/L (treatment group) were randomly assigned to one of three groups: group 1 received 1 tablet of maternal supplement daily; group 2 received 0.9 g of ferrous sulphate daily; and group 3 received one tablet of Ferroids, a sustained released preparation daily. In the preventive group, women entered the study from 20‐24 gestational wks. In the treatment groups, women less than 36 gestational wks were accepted. No comparisons allowed due to the addition of other micronutrients in the treatment. | |
| 180 anaemic women (Hb < 110 g/L) attending antenatal care at the Children, Youth and Women's Health Service, Adelaide, Australia with 24‐32 wks of gestation and a singleton pregnancy. Women were excluded if they were taking iron or vitamin and mineral supplements, had presumptive diagnosis of non iron deficiency related anaemia, history of thalassaemia, drug or alcohol abuse and/or diabetes requiring insulin or a known fetal abnormality. Women were randomly assigned to receive a daily dose of 20, 40 or 80 mg of elemental iron as ferrous sulphate for 8 wks or until birth. The primary outcomes measured were Hb levels, anaemia at the end of the intervention and gastrointestinal side effects during treatment. All women received iron at different doses. No comparisons allowed within the scope of this review. | |
| 203 pregnant women attending antenatal clinic in Paris, France, with 28 +/‐ 2 wks of gestation were studied. Women with Hb below 110 g/L (n = 48) were provided 105 mg of elemental iron and 500 mg of ascorbic acid daily. Women with Hb concentration above 110 g/L were randomly assigned to receive 105 mg of elemental iron and 500 mg of ascorbic acid daily until delivery or placebo. Iron was provided in conjunction with vitamin C. No comparisons allowed within the scope of this review. | |
| 200 apparently pregnant women with 24‐26 wks of gestation, with singleton pregnancy with moderate anaemia (Hb > 80 g/L and < 110 g/L) were randomly assigned to receive injectable iron‐sorbitol‐citrate in three intramuscular doses of 150 mg each at 4 wks intervals or 100 mg of elemental iron daily. Haemoglobin concentrations were measured at baseline, every 4 wks and at delivery. The study compares two routes of iron administration. Both groups receive iron. No comparisons allowed within the scope of this review. |
IU: international units
Hb: Haemoglobin
wk(s): week(s)
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomisation: adequate. Computer‐generated random numbers. Allocation concealment: inadequate ‐ not used. Blinding: inadequate. Open to participants, care providers and outcome assessor. Loss to follow up: adequate. Less than 20% were lost to follow up. |
| Participants | 109 pregnant non‐anaemic women between 14 and 18 wks with no prior intake of iron supplements in the Department of Obstetrics and Gynaecology of the All India Institute of Medical Sciences in New Delhi, India were invited to participate in the study. Exclusion criteria were: haemoglobin < 110 g/L, packed cell volume (PCV) < 30; cigarette smoking; preexisting hypertension or diabetes; history of chronic illness, e.g. liver or renal disease, tuberculosis, heart disease, malaria; history of bleeding disorders, bleeding piles, chronic peptic ulcer; thalassaemia or other haemoglobinopathies; intake of drugs such as antiepileptics, non‐steroidal anti‐inflammatory drugs (NSAIDs), antithyroid medication, vitamins, antioxidants; multiple pregnancy; and prior history of blood transfusion. |
| Interventions | Participants were randomly allocated into one of three different groups: group 1 (n = 37) received the standard Government of India supply of Irofol® tablets containing 100 mg of elemental iron as ferrous sulphate and 500 ug folic acid (Nestor Pharmaceuticals Ltd., Faridabad, Haryana, India) to be taken once weekly; group 2 (n = 36) received the standard Government of India supply of Irofol® tablets containing 100 mg of elemental iron as ferrous sulphate and 500 ug folic acid and were instructed to take two tablets on any one day of the week ? one before lunch and the other before dinner (total 200 mg elemental iron and 1000 ug folic acid a week) with no tablets were taken during the rest of the week; group 3 (n = 36) received Ferium® tablets iron (III)‐hydroxide polymaltose complex tablets daily containing Iron (III) Hydroxide Polymaltose containing 100 mg elemental iron and 350 ug folic acid to be taken one tablet daily (Emcure Pharmaceuticals Ltd., Pune). All groups received health education regarding the importance of diet in pregnancy, iron‐rich foods and appropriate dietary practices and were instructed to take the tablets 30 min before meals and not with tea, coffee or milk. They were also advised to take calcium supplements after meals. |
| Outcomes | Maternal: miscarriage, intrauterine demise, haemoglobin, haematocrit, MCV and MCHC, thiobarbituric acid reactive substances (TBARS) and glutathione levels at baseline (14‐16 wks) and at 30‐34 wks, compliance, side effects, nausea, vomiting, diarrhoea, constipation, metallic taste, epigastric discomfort, premature delivery, hypertension during pregnancy, preeclampsia, C‐section. Infant: birth weight, low birth weight (LBW), placental weight, 1 min Apgar score and incidence of meconium. |
| Notes | Mean gestation at which supplementation was started was 16.1 1.3 wks and mean duration of iron supplementation before final sampling was 17.9 1.4 wks |
| Methods | Randomisation: (A) adequate. Cluster randomised. Allocation concealment: (A) adequate. A treatment colour code was assigned to each village based on the treatment allocation schedule and opened only once all data had been collected and blinded analysis completed. Blinding: (A) adequate. Participant and care provider blinded. Loss to follow up: (A) adequate. Less than 20% loss to follow up. |
| Participants | 5828 eligible pregnant women with less than 28 wks and residents in two poor rural counties in Shaanxi Province of north west China participated in the study. Their villages were randomly assigned for women to receive one of three groups. |
| Interventions | Their villages were randomly assigned for women to receive one of three groups: group 1, daily antenatal multiple micronutrients containing 30 mg iron, 400 µg folic acid and 15 mg zinc, 2 mg copper, 65 µg selenium, 150 µg iodine, 800 µg vitamin A, 1.4 mg vitamin B‐1 (thiamine), 1.4 mg vitamin B‐2 (riboflavin), 1.9 mg vitamin B‐6, 2.6 µg vitamin B‐12, 5 µg vitamin D, 70 mg vitamin C, 10 mg vitamin E, and 18 mg niacin; group 2 who received a tablet containing 60 mg iron and 400 ug of folic acid; and group 3 received a tablet containing 400 ug of folic acid alone (control). |
| Outcomes | Birth weight within one hour of delivery, low birth weight, birth length, gestational age at birth, preterm delivery, small for gestational age babies, maternal haemoglobin concentration in the third trimester (gestation 28‐32 wks), anaemia in the third trimester, fetal losses during pregnancy, birth outcome, delivery information, neonatal and maternal deaths; neonatal survival at the six wks, perinatal deaths, neonatal deaths, stillbirths. |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Impact of iron/folic acid versus multimicronutrient versus folic acid supplements during pregnancy on mortality, morbidity, and complications during pregnancy, labor, and delivery: a randomised controlled trial in China. |
| Methods | |
| Participants | Pregnant women 20 years or older who live in one of the study counties (Laoting, Mancheng, Fengrun, Xianghe, Yuanshi), who can follow instructions and can swallow pills. |
| Interventions | Daily prenatal supplements that contain 400 ug folic acid alone, or daily supplements that contain 30 mg iron and 400 ug folic acid. |
| Outcomes | Perinatal mortality, i.e., the number of stillbirths (fetal deaths of 28 wks or more of gestation) and the number of deaths within the first 0‐6 days of life per 1000 births (live births and stillbirths); Gastrointestinal side effects at monthly visits. |
| Starting date | May 2006; expected completion: December 2010. |
| Contact information | Mary E Cogswell, DrPH, RN 770‐488‐6053 [email protected] |
| Notes |
| Trial name or title | Routine Iron Prophylaxis During Pregnancy (PROFEG) |
| Methods | A pragmatic randomised controlled trial with non‐blind design. Total intended sample size is 4000 women. Hypothesis: group 2 will have better health outcomes. Study site: Mozambique, Maputo City. |
| Participants | Pregnant women 18 years of age or older attending prenatal care in two health centres, one in Maputo city and one in Maputo province. The women are followed in prenatal visits and until delivery. |
| Interventions | Women will be randomised individually and allocated into two different groups: group 1, women in the routine iron prophylaxis will receive 65 mg ferrous sulphate and 400 ug of folic acid daily; group 2, women will be screened in the antenatal visits with measurements of Hb. If Hb is lower than 90 g/L women will receive a monthly supply of 130 mg of iron to be taken daily and folic acid. If Hb is 90 g/L or higher then women receive 1 tablet containing 1 mg of folic acid. |
| Outcomes | Primary outcomes: preterm delivery, low birth weight, malaria reactivation during pregnancy (mother) [Time Frame: Until birth]. Secondary Outcome Measures: perinatal mortality, complications during pregnancy and birth [Time Frame: pregnancy and neonatal period]. |
| Starting date | The project consists of three interlinked phases: the preparatory phase, pilot study and trial as such. The research project started in April 2005 with the preparatory phase, the pilot study of the second phase tested the data collection methods and procedures in the study protocol. The third phase is currently ongoing. |
| Contact information | Principal Investigator:Elina Hemminki; Study Director: Baltazar Chilundo Elina Hemminki, Research Professor Baltazar Chilundo, MD, PhD Universidade Eduardo Mondlande, Faculty of Medicine, Department of Community Health Phone: +258 84 3158350 E‐mail: [email protected] |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Low birthweight (less than 2500 g) (ALL) Show forest plot | 9 | 6275 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.61, 1.03] |
| Analysis 1.1  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 1 Low birthweight (less than 2500 g) (ALL). | ||||
| 2 Low birthweight (less than 2500 g) (BY SUBGROUPS) Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 2 Low birthweight (less than 2500 g) (BY SUBGROUPS). | ||||
| 2.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 7 | 5771 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.61, 1.08] |
| 2.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 2 | 504 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.22, 1.38] |
| 2.5 Non‐anaemic at start of supplementation | 6 | 4452 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.46, 1.25] |
| 2.6 Unspecified/mixed anaemic status at start of supplementation | 3 | 1823 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.71, 0.94] |
| 2.7 Daily lower dose (60 mg elemental iron or less) | 7 | 3247 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.53, 1.18] |
| 2.8 Daily higher dose (more than 60 mg elemental iron) | 2 | 3028 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.45, 1.26] |
| 3 Birthweight (g) (ALL) Show forest plot | 10 | 5956 | Mean Difference (IV, Random, 95% CI) | 36.05 [‐4.84, 76.95] |
| Analysis 1.3  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 3 Birthweight (g) (ALL). | ||||
| 4 Birthweight (g) (BY SUBGROUPS) Show forest plot | 11 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 4 Birthweight (g) (BY SUBGROUPS). | ||||
| 4.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 9 | 5822 | Mean Difference (IV, Random, 95% CI) | 30.89 [‐13.87, 75.65] |
| 4.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 2 | 251 | Mean Difference (IV, Random, 95% CI) | 39.72 [‐67.69, 147.12] |
| 4.5 Non‐anaemic at start of supplementation | 8 | 4496 | Mean Difference (IV, Random, 95% CI) | 29.32 [‐27.08, 85.71] |
| 4.6 Unspecified/mixed anaemic status at start of supplementation | 3 | 1577 | Mean Difference (IV, Random, 95% CI) | 52.33 [10.16, 94.51] |
| 4.7 Daily low dose (60 mg elemental iron or less) | 6 | 2804 | Mean Difference (IV, Random, 95% CI) | 37.22 [‐17.82, 92.27] |
| 4.8 Daily higher dose (more than 60 mg elemental iron) | 6 | 3382 | Mean Difference (IV, Random, 95% CI) | 17.99 [‐41.28, 77.26] |
| 5 Premature delivery (less than 37 weeks of gestation) (ALL) Show forest plot | 8 | 5730 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.67, 1.09] |
| Analysis 1.5  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 5 Premature delivery (less than 37 weeks of gestation) (ALL). | ||||
| 6 Premature delivery (less 37 weeks of gestation) (BY SUBGROUPS) Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 6 Premature delivery (less 37 weeks of gestation) (BY SUBGROUPS). | ||||
| 6.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 6 | 2989 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.66, 1.20] |
| 6.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.48] |
| 6.5 Non‐anaemic at start of supplementation | 6 | 1775 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.54, 1.12] |
| 6.6 Unspecified/mixed anaemic status at start of supplementation | 1 | 1261 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.85, 1.28] |
| 6.7 Daily lower dose (60 mg elemental iron or less) | 6 | 3023 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.68, 1.18] |
| 6.8 Daily higher dose (more than 60 mg elemental iron) | 2 | 2707 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.48, 1.06] |
| 7 Maternal Hb concentration at term (g/L) (ALL) Show forest plot | 17 | 2463 | Mean Difference (IV, Random, 95% CI) | 8.83 [6.55, 11.11] |
| Analysis 1.7  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 7 Maternal Hb concentration at term (g/L) (ALL). | ||||
| 8 Maternal Hb concentration at term (g/L) (BY SUBGROUPS) Show forest plot | 17 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.8  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 8 Maternal Hb concentration at term (g/L) (BY SUBGROUPS). | ||||
| 8.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 13 | 2306 | Mean Difference (IV, Random, 95% CI) | 8.14 [5.61, 10.67] |
| 8.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 3 | 130 | Mean Difference (IV, Random, 95% CI) | 10.24 [2.45, 18.04] |
| 8.3 Unspecified/mixed gestational age at start of supplementation | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 14.0 [8.07, 19.93] |
| 8.5 Non‐anaemic at start of supplementation | 11 | 2002 | Mean Difference (IV, Random, 95% CI) | 7.77 [4.86, 10.69] |
| 8.6 Unspecified/mixed anaemic status at start of supplementation | 6 | 461 | Mean Difference (IV, Random, 95% CI) | 11.03 [7.51, 14.56] |
| 8.7 Daily low dose (60 mg elemental iron or less) | 8 | 1956 | Mean Difference (IV, Random, 95% CI) | 7.16 [4.14, 10.17] |
| 8.8 Daily higher dose (more than 60 mg elemental iron) | 9 | 507 | Mean Difference (IV, Random, 95% CI) | 10.94 [7.06, 14.82] |
| 9 Anaemia at term (Hb less than 110 g/L) (ALL) Show forest plot | 14 | 4390 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.17, 0.42] |
| Analysis 1.9  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 9 Anaemia at term (Hb less than 110 g/L) (ALL). | ||||
| 10 Haemoconcentration at term (Hb more than 130 g/L) (ALL) Show forest plot | 10 | 4643 | Risk Ratio (M‐H, Random, 95% CI) | 2.62 [1.21, 5.67] |
| Analysis 1.10  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 10 Haemoconcentration at term (Hb more than 130 g/L) (ALL). | ||||
| 11 Haemoconcentration at term (Hb more than 130 g/L) (BY SUBGROUPS) Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.11  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 11 Haemoconcentration at term (Hb more than 130 g/L) (BY SUBGROUPS). | ||||
| 11.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 5 | 4173 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [0.72, 5.45] |
| 11.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 3 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 3.94 [0.31, 50.47] |
| 11.3 Unspecified/mixed gestational age at start of supplementation | 2 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 4.67 [2.53, 8.60] |
| 11.5 Non‐anaemic at start of supplementation | 5 | 4011 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [0.64, 4.66] |
| 11.6 Unspecified/mixed anaemic status at start of supplementation | 5 | 632 | Risk Ratio (M‐H, Random, 95% CI) | 4.03 [1.39, 11.72] |
| 11.7 Daily low dose (60 mg elemental iron or less) | 4 | 1317 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.54, 3.86] |
| 11.8 Daily higher dose (more than 60 mg elemental iron) | 6 | 3326 | Risk Ratio (M‐H, Random, 95% CI) | 3.55 [1.89, 6.66] |
| 12 Haemoconcentration during second or third trimester (ALL) Show forest plot | 10 | 4841 | Risk Ratio (M‐H, Random, 95% CI) | 2.27 [1.40, 3.70] |
| Analysis 1.12  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 12 Haemoconcentration during second or third trimester (ALL). | ||||
| 13 Haemoconcentration during second or third trimester (BY SUBGROUPS) Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.13  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 13 Haemoconcentration during second or third trimester (BY SUBGROUPS). | ||||
| 13.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 7 | 4522 | Risk Ratio (M‐H, Random, 95% CI) | 2.62 [1.49, 4.60] |
| 13.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.72, 2.86] |
| 13.3 Unspecified/mixed gestational age at start of supplementation | 2 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [0.30, 12.29] |
| 13.5 Non‐anaemic at start of supplementation | 6 | 4088 | Risk Ratio (M‐H, Random, 95% CI) | 2.10 [1.13, 3.90] |
| 13.6 Unspecified/mixed anaemic status at start of supplementation | 4 | 753 | Risk Ratio (M‐H, Random, 95% CI) | 2.67 [0.99, 7.17] |
| 13.7 Daily low dose (60 mg elemental iron or less) | 5 | 1655 | Risk Ratio (M‐H, Random, 95% CI) | 2.35 [1.21, 4.57] |
| 13.8 Daily higher dose (more than 60 mg elemental iron) | 5 | 3186 | Risk Ratio (M‐H, Random, 95% CI) | 2.14 [1.05, 4.37] |
| 14 Iron deficiency at term (as defined by two or more indicators) (ALL) Show forest plot | 6 | 1108 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.27, 0.70] |
| Analysis 1.14  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 14 Iron deficiency at term (as defined by two or more indicators) (ALL). | ||||
| 15 Iron deficiency at term (as defined by two or more indicators) (BY SUBGROUPS) Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.15  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 15 Iron deficiency at term (as defined by two or more indicators) (BY SUBGROUPS). | ||||
| 15.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 4 | 867 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.35, 0.90] |
| 15.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 2 | 241 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.17, 0.44] |
| 15.5 Non‐anaemic at start of supplementation | 4 | 944 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.41, 0.90] |
| 15.6 Unspecified/mixed anaemic status at start of supplementation | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.07, 0.29] |
| 15.7 Daily low dose (60 mg elemental iron or less) | 4 | 944 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.41, 0.90] |
| 15.8 Daily higher dose (more than 60 mg elemental iron) | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.07, 0.29] |
| 16 Iron deficiency anaemia at term (ALL) Show forest plot | 6 | 1667 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.16, 0.69] |
| Analysis 1.16  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 16 Iron deficiency anaemia at term (ALL). | ||||
| 17 Iron deficiency anaemia at term (BY SUBGROUPS) Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.17  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 17 Iron deficiency anaemia at term (BY SUBGROUPS). | ||||
| 17.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 5 | 1622 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.18, 0.76] |
| 17.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.13] |
| 17.5 Non‐anaemic at start of supplementation | 5 | 1547 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.20, 0.74] |
| 17.6 Unspecified/mixed anaemic status at start of supplementation | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.04 [0.00, 0.72] |
| 17.7 Daily low dose (60 mg elemental iron or less) | 5 | 1547 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.20, 0.74] |
| 17.8 Daily higher dose (more than 60 mg elemental iron) | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.04 [0.00, 0.72] |
| 18 Side‐effects (Any) (ALL) Show forest plot | 8 | 3667 | Risk Ratio (M‐H, Random, 95% CI) | 3.92 [1.21, 12.64] |
| Analysis 1.18  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 18 Side‐effects (Any) (ALL). | ||||
| 19 Side‐effects (Any) (BY SUBGROUPS) Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.19  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 19 Side‐effects (Any) (BY SUBGROUPS). | ||||
| 19.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 4 | 3034 | Risk Ratio (M‐H, Random, 95% CI) | 3.69 [0.42, 32.71] |
| 19.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 3 | 428 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.99, 3.08] |
| 19.3 Unspecified/mixed gestational age at start of supplementation | 1 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 62.79 [3.89, 1013.31] |
| 19.5 Non‐anaemic at start of supplementation | 4 | 2897 | Risk Ratio (M‐H, Random, 95% CI) | 3.27 [0.39, 27.31] |
| 19.6 Unspecified/mixed anaemic status at start of supplementation | 4 | 770 | Risk Ratio (M‐H, Random, 95% CI) | 3.94 [1.09, 14.28] |
| 19.7 Daily low dose (60 mg elemental iron or less) | 4 | 566 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.99, 1.87] |
| 19.8 Daily higher dose (more than 60 mg elemental iron) | 5 | 3148 | Risk Ratio (M‐H, Random, 95% CI) | 12.12 [1.76, 83.43] |
| 20 Very low birthweight (less than 1500 g) (ALL) Show forest plot | 5 | 2687 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.31, 1.74] |
| Analysis 1.20  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 20 Very low birthweight (less than 1500 g) (ALL). | ||||
| 21 Perinatal death (ALL) Show forest plot | 3 | 5036 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.67, 1.29] |
| Analysis 1.21  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 21 Perinatal death (ALL). | ||||
| 24 Infant Hb concentration at 3 months (g/L) (ALL) Show forest plot | 1 | 197 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐3.21, 3.21] |
| Analysis 1.24  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 24 Infant Hb concentration at 3 months (g/L) (ALL). | ||||
| 25 Infant serum ferritin concentration at 3 months (ug/L) (ALL) Show forest plot | 1 | 197 | Mean Difference (IV, Random, 95% CI) | 19.0 [2.75, 35.25] |
| Analysis 1.25  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 25 Infant serum ferritin concentration at 3 months (ug/L) (ALL). | ||||
| 26 Infant Hb concentration at 6 months (g/L) (ALL) Show forest plot | 2 | 533 | Mean Difference (IV, Random, 95% CI) | ‐1.25 [‐8.10, 5.59] |
| Analysis 1.26  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 26 Infant Hb concentration at 6 months (g/L) (ALL). | ||||
| 27 Infant serum ferritin concentration at 6 months (ug/L) (ALL) Show forest plot | 1 | 197 | Mean Difference (IV, Random, 95% CI) | 11.0 [4.37, 17.63] |
| Analysis 1.27  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 27 Infant serum ferritin concentration at 6 months (ug/L) (ALL). | ||||
| 29 Admission to special care unit (ALL) Show forest plot | 2 | 2805 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.73, 1.23] |
| Analysis 1.29  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 29 Admission to special care unit (ALL). | ||||
| 30 Very premature delivery (less than 34 weeks' gestation) (ALL) Show forest plot | 4 | 1417 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.16, 1.24] |
| Analysis 1.30  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 30 Very premature delivery (less than 34 weeks' gestation) (ALL). | ||||
| 31 Severe anaemia at term (Hb less than 70 g/L) (ALL) Show forest plot | 8 | 1751 | Risk Ratio (M‐H, Random, 95% CI) | 4.83 [0.23, 99.88] |
| Analysis 1.31  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 31 Severe anaemia at term (Hb less than 70 g/L) (ALL). | ||||
| 32 Moderate anaemia at term (Hb more than 70 g/L and less than 90 g/L) (ALL) Show forest plot | 9 | 1868 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.55, 1.62] |
| Analysis 1.32  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 32 Moderate anaemia at term (Hb more than 70 g/L and less than 90 g/L) (ALL). | ||||
| 33 Severe anaemia at any time during second and third trimester (ALL) Show forest plot | 9 | 2089 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.01, 34.52] |
| Analysis 1.33  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 33 Severe anaemia at any time during second and third trimester (ALL). | ||||
| 34 Moderate anaemia at any time during second or third trimester (ALL) Show forest plot | 10 | 2266 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.19, 0.92] |
| Analysis 1.34  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 34 Moderate anaemia at any time during second or third trimester (ALL). | ||||
| 35 Infection during pregnancy (including urinary tract infections) (ALL) Show forest plot | 2 | 3421 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.83, 1.63] |
| Analysis 1.35  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 35 Infection during pregnancy (including urinary tract infections) (ALL). | ||||
| 36 Puerperal infection (ALL) Show forest plot | 2 | 2169 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.25, 2.10] |
| Analysis 1.36  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 36 Puerperal infection (ALL). | ||||
| 37 Antepartum haemorraghe (ALL) Show forest plot | 2 | 1157 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.51, 4.31] |
| Analysis 1.37  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 37 Antepartum haemorraghe (ALL). | ||||
| 38 Postpartum haemorraghe (ALL) Show forest plot | 5 | 1554 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.51, 1.78] |
| Analysis 1.38  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 38 Postpartum haemorraghe (ALL). | ||||
| 39 Transfusion provided (ALL) Show forest plot | 3 | 3453 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.38, 0.96] |
| Analysis 1.39  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 39 Transfusion provided (ALL). | ||||
| 40 Haemoglobin concentration within one month postpartum (ALL) Show forest plot | 6 | 904 | Mean Difference (IV, Random, 95% CI) | 7.08 [4.70, 9.47] |
| Analysis 1.40  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 40 Haemoglobin concentration within one month postpartum (ALL). | ||||
| 41 Severe anaemia at postpartum (Hb less than 80 g/L) (ALL) Show forest plot | 7 | 1094 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.00, 1.05] |
| Analysis 1.41  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 41 Severe anaemia at postpartum (Hb less than 80 g/L) (ALL). | ||||
| 42 Moderate anaemia at postpartum (Hb more than 80 g/L and less than 100 g/L) (ALL) Show forest plot | 4 | 831 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.12, 2.51] |
| Analysis 1.42  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 42 Moderate anaemia at postpartum (Hb more than 80 g/L and less than 100 g/L) (ALL). | ||||
| 43 Diarrhoea (ALL) Show forest plot | 3 | 1088 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.32, 0.93] |
| Analysis 1.43  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 43 Diarrhoea (ALL). | ||||
| 44 Constipation (ALL) Show forest plot | 4 | 1495 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.62, 1.43] |
| Analysis 1.44  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 44 Constipation (ALL). | ||||
| 45 Nausea (ALL) Show forest plot | 4 | 1377 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.72, 2.03] |
| Analysis 1.45  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 45 Nausea (ALL). | ||||
| 46 Heartburn (ALL) Show forest plot | 3 | 1323 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.86, 1.66] |
| Analysis 1.46  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 46 Heartburn (ALL). | ||||
| 47 Vomiting (ALL) Show forest plot | 4 | 1392 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.59, 1.30] |
| Analysis 1.47  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 47 Vomiting (ALL). | ||||
| 48 Maternal death (death while pregnant or within 42 days of termination of pregnancy) (ALL) Show forest plot | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 1.48  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 48 Maternal death (death while pregnant or within 42 days of termination of pregnancy) (ALL). | ||||
| 49 Maternal wellbeing/satisfaction (ALL) Show forest plot | 2 | 2604 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.91, 1.09] |
| Analysis 1.49  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 49 Maternal wellbeing/satisfaction (ALL). | ||||
| 50 Placental abruption (ALL) Show forest plot | 2 | 2169 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.32, 4.06] |
| Analysis 1.50  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 50 Placental abruption (ALL). | ||||
| 51 Premature rupture of membranes (ALL) Show forest plot | 1 | 727 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.74, 1.34] |
| Analysis 1.51  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 51 Premature rupture of membranes (ALL). | ||||
| 52 Pre‐eclampsia (ALL) Show forest plot | 2 | 774 | Risk Ratio (M‐H, Random, 95% CI) | 2.58 [0.81, 8.22] |
| Analysis 1.52  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 52 Pre‐eclampsia (ALL). | ||||
| 91 Small for gestational age (less than 10th percentile weight at birth for gestational age) (not pre‐specified) Show forest plot | 4 | 2511 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.58, 1.30] |
| Analysis 1.91  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 91 Small for gestational age (less than 10th percentile weight at birth for gestational age) (not pre‐specified). | ||||
| 93 Cesarean delivery (not pre‐specified) Show forest plot | 7 | 4283 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.78, 1.13] |
| Analysis 1.93  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 93 Cesarean delivery (not pre‐specified). | ||||
| 94 Birth length in cm (not pre‐specified) Show forest plot | 5 | 2140 | Mean Difference (IV, Random, 95% CI) | 0.38 [0.10, 0.65] |
| Analysis 1.94  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 94 Birth length in cm (not pre‐specified). | ||||
| 95 Forceps or vacuum delivery (not pre‐specified) Show forest plot | 2 | 477 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.94, 2.40] |
| Analysis 1.95  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 95 Forceps or vacuum delivery (not pre‐specified). | ||||
| 96 Breastfeeding at least 4 months (not pre‐specified) Show forest plot | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.89, 1.13] |
| Analysis 1.96  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 96 Breastfeeding at least 4 months (not pre‐specified). | ||||
| 97 Haemoglobin concentration at 4‐8 weeks' postpartum (g/L) (not pre‐specified) Show forest plot | 10 | 1188 | Mean Difference (IV, Random, 95% CI) | 5.13 [0.24, 10.02] |
| Analysis 1.97  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 97 Haemoglobin concentration at 4‐8 weeks' postpartum (g/L) (not pre‐specified). | ||||
| 98 Apgar score < 7 at 5 minutes (not pre‐specified) Show forest plot | 2 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.17, 3.28] |
| Analysis 1.98  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 98 Apgar score < 7 at 5 minutes (not pre‐specified). | ||||
| 99 Apgar Score at 5 min (not pre‐specified) Show forest plot | 2 | 228 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.07, 0.62] |
| Analysis 1.99  Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 99 Apgar Score at 5 min (not pre‐specified). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3 Birthweight (ALL) Show forest plot | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐68.0 [‐398.33, 262.33] |
| Analysis 2.3  Comparison 2 Intermittent iron alone versus daily iron alone, Outcome 3 Birthweight (ALL). | ||||
| 5 Premature delivery (less than 37 weeks of gestation) (ALL) Show forest plot | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.02, 8.96] |
| Analysis 2.5  Comparison 2 Intermittent iron alone versus daily iron alone, Outcome 5 Premature delivery (less than 37 weeks of gestation) (ALL). | ||||
| 12 Haemoconcentration during second or third trimester (Hb more than 130 g/L) (ALL) Show forest plot | 2 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.18, 1.58] |
| Analysis 2.12  Comparison 2 Intermittent iron alone versus daily iron alone, Outcome 12 Haemoconcentration during second or third trimester (Hb more than 130 g/L) (ALL). | ||||
| 33 Severe anaemia at any time during second and third trimester (Hb less than 70 g/L) (ALL) Show forest plot | 2 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 2.33  Comparison 2 Intermittent iron alone versus daily iron alone, Outcome 33 Severe anaemia at any time during second and third trimester (Hb less than 70 g/L) (ALL). | ||||
| 34 Moderate anaemia at any time during second or third trimester (ALL) Show forest plot | 2 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 2.42 [0.16, 35.56] |
| Analysis 2.34  Comparison 2 Intermittent iron alone versus daily iron alone, Outcome 34 Moderate anaemia at any time during second or third trimester (ALL). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Low birthweight (less than 2500 g) (ALL) Show forest plot | 2 | 1368 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.28, 4.02] |
| Analysis 3.1  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 1 Low birthweight (less than 2500 g) (ALL). | ||||
| 2 Low birthweight (less than 2500 g) (BY SUBGROUPS) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.2  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 2 Low birthweight (less than 2500 g) (BY SUBGROUPS). | ||||
| 2.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 2 | 1368 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.28, 4.02] |
| 2.6 Unspecified/mixed anaemic status at start of supplementation | 2 | 1368 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.28, 4.02] |
| 2.7 Daily low dose (60 mg elemental iron or less) | 1 | 1320 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.69, 0.91] |
| 2.8 Daily higher dose (more than 60 mg elemental iron) | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.25, 98.96] |
| 3 Birthweight (ALL) Show forest plot | 2 | 1365 | Mean Difference (IV, Random, 95% CI) | 57.73 [7.66, 107.79] |
| Analysis 3.3  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 3 Birthweight (ALL). | ||||
| 4 Birthweight (BY SUBGROUPS) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.4  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 4 Birthweight (BY SUBGROUPS). | ||||
| 4.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 2 | 1365 | Mean Difference (IV, Random, 95% CI) | 57.73 [7.66, 107.79] |
| 4.6 Unspecified/mixed anaemic status at start of supplementation | 2 | 1365 | Mean Difference (IV, Random, 95% CI) | 57.73 [7.66, 107.79] |
| 4.7 Daily low dose (60 mg elemental iron or less) | 1 | 1320 | Mean Difference (IV, Random, 95% CI) | 65.0 [17.46, 112.54] |
| 4.8 Daily higher dose (more than 60 mg elemental iron) | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐32.0 [‐213.62, 149.62] |
| 5 Premature delivery (less than 37 weeks of gestation) (ALL) Show forest plot | 3 | 1497 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [0.40, 6.00] |
| Analysis 3.5  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 5 Premature delivery (less than 37 weeks of gestation) (ALL). | ||||
| 6 Premature delivery (less than 37 weeks of gestation) (BY SUB GROUPS) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.6  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 6 Premature delivery (less than 37 weeks of gestation) (BY SUB GROUPS). | ||||
| 6.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 2 | 1362 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.92, 1.39] |
| 6.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.6 Unspecified/mixed anaemic status at start of supplementation | 2 | 1449 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.92, 1.39] |
| 6.7 Daily low dose (60 mg elemental iron or less) | 2 | 1449 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.92, 1.39] |
| 7 Haemoglobin concentration at term (ALL) Show forest plot | 4 | 179 | Mean Difference (IV, Random, 95% CI) | 12.00 [2.93, 21.07] |
| Analysis 3.7  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 7 Haemoglobin concentration at term (ALL). | ||||
| 8 Haemoglobin concentration at term (BY SUBGROUPS) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.8  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 8 Haemoglobin concentration at term (BY SUBGROUPS). | ||||
| 8.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 2 | 93 | Mean Difference (IV, Random, 95% CI) | 15.65 [11.84, 19.46] |
| 8.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 2 | 86 | Mean Difference (IV, Random, 95% CI) | 7.92 [‐11.68, 27.52] |
| 8.5 Non‐anaemic at start of supplementation | 1 | 48 | Mean Difference (IV, Random, 95% CI) | 17.10 [8.44, 25.76] |
| 8.6 Unspecified/mixed anaemic status at start of supplementation | 3 | 131 | Mean Difference (IV, Random, 95% CI) | 10.47 [‐1.07, 22.00] |
| 8.7 Daily low dose (60 mg elemental iron or less) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.8 Daily higher dose (more than 60 mg elemental iron) | 4 | 179 | Mean Difference (IV, Random, 95% CI) | 12.00 [2.93, 21.07] |
| 9 Anaemia at term (Hb less than 110 g/L) (ALL) Show forest plot | 3 | 346 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.12, 0.56] |
| Analysis 3.9  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 9 Anaemia at term (Hb less than 110 g/L) (ALL). | ||||
| 10 Haemoconcentration at term (Hb more than 130 g/L) (ALL) Show forest plot | 3 | 353 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [0.34, 8.94] |
| Analysis 3.10  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 10 Haemoconcentration at term (Hb more than 130 g/L) (ALL). | ||||
| 11 Haemoconcentration at term (Hb more than 130 g/L) (BY SUBGROUPS) Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.11  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 11 Haemoconcentration at term (Hb more than 130 g/L) (BY SUBGROUPS). | ||||
| 11.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 3.37 [0.19, 60.03] |
| 11.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 3 | 298 | Risk Ratio (M‐H, Random, 95% CI) | 1.97 [0.32, 12.08] |
| 11.6 Unspecified/mixed anaemic status at start of supplementation | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 4.31 [0.26, 70.41] |
| 11.7 Daily low dose (60 mg elemental iron or less) | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 4.31 [0.26, 70.41] |
| 11.8 Daily higher dose (more than 60 mg elemental iron) | 2 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.24, 6.78] |
| 12 Haemoconcentration during second or third trimester (ALL) Show forest plot | 2 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.63, 5.04] |
| Analysis 3.12  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 12 Haemoconcentration during second or third trimester (ALL). | ||||
| 13 Haemoconcentration during second or third trimester (BY SUBGROUPS) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.13  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 13 Haemoconcentration during second or third trimester (BY SUBGROUPS). | ||||
| 13.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 2 | 390 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.33, 6.32] |
| 13.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.50, 3.56] |
| 13.3 Unspecified/mixed gestational age at start of supplementation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.5 Non‐anaemic at start of supplementation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.6 Unspecified/mixed anaemic status at start of supplementation | 2 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.63, 5.04] |
| 13.7 Daily low dose (60 mg elemental iron or less) | 2 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.63, 5.04] |
| 13.8 Daily higher dose (more than 60 mg elemental iron) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Iron deficiency at term (as defined by two or more indicators) (ALL) Show forest plot | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.06, 0.99] |
| Analysis 3.14  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 14 Iron deficiency at term (as defined by two or more indicators) (ALL). | ||||
| 15 Iron deficiency at term (as defined by two or more indicators) (BY SUBGROUPS) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.15  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 15 Iron deficiency at term (as defined by two or more indicators) (BY SUBGROUPS). | ||||
| 15.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.01, 1.10] |
| 15.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.08, 1.63] |
| 15.5 Non‐anaemic at start of supplementation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.6 Unspecified/mixed anaemic status at start of supplementation | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.06, 0.99] |
| 15.7 Daily low dose (60 mg elemental iron or less) | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.06, 0.99] |
| 15.8 Daily higher dose (more than 60 mg elemental iron) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Iron deficiency anaemia at term (ALL) Show forest plot | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.17, 1.09] |
| Analysis 3.16  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 16 Iron deficiency anaemia at term (ALL). | ||||
| 17 Iron deficiency anaemia at term (BY SUBGROUPS) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.17  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 17 Iron deficiency anaemia at term (BY SUBGROUPS). | ||||
| 17.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.12, 1.12] |
| 17.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.18, 1.40] |
| 17.5 Non‐anaemic at start of supplementation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.6 Unspecified/mixed anaemic status at start of supplementation | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.17, 1.09] |
| 17.7 Daily low dose (60 mg elemental iron or less) | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.17, 1.09] |
| 17.8 Daily higher dose (more than 60 mg elemental iron) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Side effects (Any) (ALL) Show forest plot | 1 | 456 | Risk Ratio (M‐H, Random, 95% CI) | 44.32 [2.77, 709.09] |
| Analysis 3.18  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 18 Side effects (Any) (ALL). | ||||
| 20 Very low birthweight (less than 1500 g) (ALL) Show forest plot | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.25, 98.96] |
| Analysis 3.20  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 20 Very low birthweight (less than 1500 g) (ALL). | ||||
| 21 Perinatal death (ALL) Show forest plot | 3 | 1862 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.58, 1.17] |
| Analysis 3.21  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 21 Perinatal death (ALL). | ||||
| 29 Admission to special care unit (ALL) Show forest plot | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 3.29  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 29 Admission to special care unit (ALL). | ||||
| 30 Very premature delivery (less than 34 weeks' gestation) (ALL) Show forest plot | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.25, 98.96] |
| Analysis 3.30  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 30 Very premature delivery (less than 34 weeks' gestation) (ALL). | ||||
| 31 Severe anaemia at term (Hb less than 70 g/L) (ALL) Show forest plot | 3 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 3.31  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 31 Severe anaemia at term (Hb less than 70 g/L) (ALL). | ||||
| 32 Moderate anaemia at term (Hb more than 70g/L and less than 90 g/L) (ALL) Show forest plot | 3 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 3.32  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 32 Moderate anaemia at term (Hb more than 70g/L and less than 90 g/L) (ALL). | ||||
| 33 Severe anaemia at any time during second and third trimester (Hb less than 70 g/L) (ALL) Show forest plot | 4 | 523 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 0.83] |
| Analysis 3.33  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 33 Severe anaemia at any time during second and third trimester (Hb less than 70 g/L) (ALL). | ||||
| 34 Moderate anaemia at any time during second or third trimester (ALL) Show forest plot | 4 | 523 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.11, 1.04] |
| Analysis 3.34  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 34 Moderate anaemia at any time during second or third trimester (ALL). | ||||
| 35 Infection during pregnancy (including urinary tract infections) (ALL) Show forest plot | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.15, 6.53] |
| Analysis 3.35  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 35 Infection during pregnancy (including urinary tract infections) (ALL). | ||||
| 36 Puerperal infection (ALL) Show forest plot | 1 | 2863 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.13, 2.28] |
| Analysis 3.36  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 36 Puerperal infection (ALL). | ||||
| 37 Antepartum haemorrhage (ALL) Show forest plot | 2 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.22, 7.12] |
| Analysis 3.37  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 37 Antepartum haemorrhage (ALL). | ||||
| 38 Postpartum haemorrhage (ALL) Show forest plot | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.00, 2.71] |
| Analysis 3.38  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 38 Postpartum haemorrhage (ALL). | ||||
| 40 Haemoglobin concentration within one month postpartum (ALL) Show forest plot | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 10.40 [4.03, 16.77] |
| Analysis 3.40  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 40 Haemoglobin concentration within one month postpartum (ALL). | ||||
| 41 Severe anaemia at postpartum (Hb less than 80 g/L) (ALL) Show forest plot | 3 | 525 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.00, 0.76] |
| Analysis 3.41  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 41 Severe anaemia at postpartum (Hb less than 80 g/L) (ALL). | ||||
| 42 Moderate anaemia at postpartum (Hb more than 80 g/L and less than 100 g/L) (ALL) Show forest plot | 3 | 525 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.17, 0.69] |
| Analysis 3.42  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 42 Moderate anaemia at postpartum (Hb more than 80 g/L and less than 100 g/L) (ALL). | ||||
| 48 Maternal death (death while pregnant or within 42 days of termination of pregnancy) (ALL) Show forest plot | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 3.48  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 48 Maternal death (death while pregnant or within 42 days of termination of pregnancy) (ALL). | ||||
| 50 Placental abruption (ALL) Show forest plot | 1 | 2863 | Risk Ratio (M‐H, Random, 95% CI) | 8.19 [0.49, 138.16] |
| Analysis 3.50  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 50 Placental abruption (ALL). | ||||
| 52 Pre‐eclampsia (ALL) Show forest plot | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 70.16] |
| Analysis 3.52  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 52 Pre‐eclampsia (ALL). | ||||
| 91 Small for gestational age (less than 10th percentile weight at birth for gestational age) (not pre‐specified) Show forest plot | 1 | 1318 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.80, 0.97] |
| Analysis 3.91  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 91 Small for gestational age (less than 10th percentile weight at birth for gestational age) (not pre‐specified). | ||||
| 92 Oedema during pregnancy (not pre‐specified) Show forest plot | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 2.82 [0.99, 8.09] |
| Analysis 3.92  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 92 Oedema during pregnancy (not pre‐specified). | ||||
| 93 Caesarean delivery (not pre‐specified) Show forest plot | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.22, 3.13] |
| Analysis 3.93  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 93 Caesarean delivery (not pre‐specified). | ||||
| 94 Birth length in cm (not pre‐specified) Show forest plot | 1 | 1320 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.06, 0.46] |
| Analysis 3.94  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 94 Birth length in cm (not pre‐specified). | ||||
| 97 Haemoglobin concentration at 4‐8 weeks postpartum (not prespecified) Show forest plot | 3 | 526 | Mean Difference (IV, Random, 95% CI) | 4.88 [‐0.85, 10.62] |
| Analysis 3.97  Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 97 Haemoglobin concentration at 4‐8 weeks postpartum (not prespecified). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Low birthweight (less than 2500 g) (ALL) Show forest plot | 4 | 730 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.58, 1.91] |
| Analysis 4.1  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 1 Low birthweight (less than 2500 g) (ALL). | ||||
| 2 Low birthweight (less than 2500 g) (BY SUBGROUPS) Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.2  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 2 Low birthweight (less than 2500 g) (BY SUBGROUPS). | ||||
| 2.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 2 | 455 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.55, 3.01] |
| 2.3 Unspecified/mixed gestational age at start of supplementation | 2 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.36, 1.99] |
| 2.5 Non‐anaemic at start of supplementation | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.36, 4.32] |
| 2.7 Daily low dose (60 mg elemental iron or less) | 3 | 650 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.50, 1.97] |
| 2.8 Daily higher dose (more than 60 mg elemental iron) | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.36, 4.32] |
| 3 Birthweight (ALL) Show forest plot | 4 | 730 | Mean Difference (IV, Random, 95% CI) | ‐7.10 [‐67.20, 53.01] |
| Analysis 4.3  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 3 Birthweight (ALL). | ||||
| 4 Birthweight (BY SUBGROUPS) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 4.4  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 4 Birthweight (BY SUBGROUPS). | ||||
| 4.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 2 | 455 | Mean Difference (IV, Random, 95% CI) | 1.19 [‐70.50, 72.87] |
| 4.3 Unspecified/mixed gestational age at start of supplementation | 2 | 275 | Mean Difference (IV, Random, 95% CI) | ‐26.71 [‐137.00, 83.58] |
| 4.5 Non‐anaemic at start of supplementation | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐154.95, 154.95] |
| 4.7 Daily low dose (60 mg elemental iron or less) | 3 | 650 | Mean Difference (IV, Random, 95% CI) | ‐8.36 [‐73.56, 56.85] |
| 4.8 Daily higher dose (more than 60 mg elemental iron) | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐154.95, 154.95] |
| 5 Premature delivery (less than 37 weeks of gestation) (ALL) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.39, 10.31] |
| Analysis 4.5  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 5 Premature delivery (less than 37 weeks of gestation) (ALL). | ||||
| 7 Haemoglobin concentration at term (ALL) Show forest plot | 3 | 475 | Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐4.74, 3.08] |
| Analysis 4.7  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 7 Haemoglobin concentration at term (ALL). | ||||
| 8 Haemoglobin concentration at term (BY SUBGROUPS) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 4.8  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 8 Haemoglobin concentration at term (BY SUBGROUPS). | ||||
| 8.3 Unspecified/mixed gestational age at start of supplementation | 2 | 301 | Mean Difference (IV, Random, 95% CI) | ‐2.19 [‐6.85, 2.47] |
| 8.7 Daily low dose (60 mg elemental iron or less) | 3 | 422 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐5.15, 4.95] |
| 8.8 Daily higher dose (more than 60 mg elemental iron) | 1 | 109 | Mean Difference (IV, Random, 95% CI) | ‐0.82 [‐4.99, 3.35] |
| 9 Anaemia at term (Hb < 110 g/L) (ALL) Show forest plot | 3 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.78, 1.83] |
| Analysis 4.9  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 9 Anaemia at term (Hb < 110 g/L) (ALL). | ||||
| 10 Haemoconcentration at term (Hb more than 130 g/L) (ALL) Show forest plot | 3 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.47, 1.82] |
| Analysis 4.10  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 10 Haemoconcentration at term (Hb more than 130 g/L) (ALL). | ||||
| 11 Haemoconcentration at term (Hb more than 130 g/L) (BY SUBGROUPS) Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.11  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 11 Haemoconcentration at term (Hb more than 130 g/L) (BY SUBGROUPS). | ||||
| 11.7 Daily low dose (60 mg elemental iron or less) | 3 | 422 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.42, 3.66] |
| 11.8 Daily higher dose (more than 60 mg elemental iron) | 1 | 109 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.19, 2.11] |
| 12 Haemoconcentration during second or third trimester (Hb more than 130 g/L) (ALL) Show forest plot | 6 | 1111 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.24, 0.77] |
| Analysis 4.12  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 12 Haemoconcentration during second or third trimester (Hb more than 130 g/L) (ALL). | ||||
| 13 Haemoconcentration during second or third trimester (Hb more than 130 g/L) (BY SUBGROUPS) Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.13  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 13 Haemoconcentration during second or third trimester (Hb more than 130 g/L) (BY SUBGROUPS). | ||||
| 13.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 2 | 250 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.26, 1.01] |
| 13.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 1 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.10, 0.55] |
| 13.3 Unspecified/mixed gestational age at start of supplementation | 3 | 695 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.13, 1.65] |
| 13.4 Anaemic at start of supplementation (Hb <110 g/L if in first or <105 g/L if in second trimester) | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.5 Non‐anaemic at start of supplementation | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.6 [0.15, 2.34] |
| 13.6 Unspecified/mixed anaemic status at start of supplementation | 4 | 966 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.21, 0.80] |
| 13.7 Daily low dose (60 mg elemental iron or less) | 5 | 953 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.20, 0.82] |
| 13.8 Daily higher dose (more than 60 mg elemental iron) | 2 | 247 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.42, 1.31] |
| 16 Iron deficiency anaemia at term (based on two or more indicators) (ALL) Show forest plot | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.08, 6.63] |
| Analysis 4.16  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 16 Iron deficiency anaemia at term (based on two or more indicators) (ALL). | ||||
| 18 Side effects (any) (ALL) Show forest plot | 7 | 1307 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.45, 1.04] |
| Analysis 4.18  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 18 Side effects (any) (ALL). | ||||
| 19 Side effects (any) (BY SUBGROUPS) Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.19  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 19 Side effects (any) (BY SUBGROUPS). | ||||
| 19.1 Early gestational age (less than 20 weeks or pre‐pregnancy) at start of supplementation | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.08, 0.53] |
| 19.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 1 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.79, 1.27] |
| 19.3 Unspecified/mixed gestational age at start of supplementation | 5 | 1055 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.42, 1.26] |
| 19.4 Non‐anaemic at start of supplementation | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.08, 0.53] |
| 19.7 Daily low dose (60 mg elemental iron or less) | 6 | 1171 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.71, 1.19] |
| 19.8 Daily higher dose (more than 60 mg elemental iron) | 2 | 253 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.07, 0.25] |
| 20 Very low birthweight (less than 1500 g) (ALL) Show forest plot | 4 | 737 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 4.20  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 20 Very low birthweight (less than 1500 g) (ALL). | ||||
| 21 Perinatal death (ALL) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 4.21  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 21 Perinatal death (ALL). | ||||
| 27 Infant ferritin concentration at 6 months (ug/L) (ALL) Show forest plot | 1 | 88 | Mean Difference (IV, Random, 95% CI) | 0.09 [0.05, 0.13] |
| Analysis 4.27  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 27 Infant ferritin concentration at 6 months (ug/L) (ALL). | ||||
| 30 Very premature delivery (less than 34 weeks of gestation) (ALL) Show forest plot | 1 | 111 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.06, 15.31] |
| Analysis 4.30  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 30 Very premature delivery (less than 34 weeks of gestation) (ALL). | ||||
| 31 Severe anaemia at term (Hb less than 70 g/L) (ALL) Show forest plot | 4 | 555 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 4.31  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 31 Severe anaemia at term (Hb less than 70 g/L) (ALL). | ||||
| 32 Moderate anaemia at term (Hb more than 70g/L and less than 90 g/L) (ALL) Show forest plot | 3 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.07, 16.23] |
| Analysis 4.32  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 32 Moderate anaemia at term (Hb more than 70g/L and less than 90 g/L) (ALL). | ||||
| 33 Severe anaemia at any time during second and third trimester (Hb less than 70 g/L) (ALL) Show forest plot | 6 | 1240 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 4.33  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 33 Severe anaemia at any time during second and third trimester (Hb less than 70 g/L) (ALL). | ||||
| 34 Moderate anaemia at any time during second or third trimester (ALL) Show forest plot | 6 | 1111 | Risk Ratio (M‐H, Random, 95% CI) | 2.54 [0.63, 10.17] |
| Analysis 4.34  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 34 Moderate anaemia at any time during second or third trimester (ALL). | ||||
| 37 Antepartum haemorraghe (ALL) Show forest plot | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 15.59] |
| Analysis 4.37  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 37 Antepartum haemorraghe (ALL). | ||||
| 41 Severe anaemia at postpartum (Hb less than 80 g/L) (ALL) Show forest plot | 1 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.04, 4.64] |
| Analysis 4.41  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 41 Severe anaemia at postpartum (Hb less than 80 g/L) (ALL). | ||||
| 42 Moderate anaemia at postpartum (Hb more than 80 g/L and less than 100 g/L) (ALL) Show forest plot | 1 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.26, 4.95] |
| Analysis 4.42  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 42 Moderate anaemia at postpartum (Hb more than 80 g/L and less than 100 g/L) (ALL). | ||||
| 43 Diarrhoea (ALL) Show forest plot | 4 | 553 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.38, 2.34] |
| Analysis 4.43  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 43 Diarrhoea (ALL). | ||||
| 44 Constipation (ALL) Show forest plot | 4 | 553 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.48, 2.06] |
| Analysis 4.44  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 44 Constipation (ALL). | ||||
| 45 Nausea (ALL) Show forest plot | 5 | 854 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.30, 1.16] |
| Analysis 4.45  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 45 Nausea (ALL). | ||||
| 46 Heartburn (ALL) Show forest plot | 3 | 473 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.29, 2.06] |
| Analysis 4.46  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 46 Heartburn (ALL). | ||||
| 47 Vomiting (ALL) Show forest plot | 5 | 854 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.82, 2.53] |
| Analysis 4.47  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 47 Vomiting (ALL). | ||||
| 50 Placental abruption (ALL) Show forest plot | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.01] |
| Analysis 4.50  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 50 Placental abruption (ALL). | ||||
| 51 Premature rupture of membranes (ALL) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.95] |
| Analysis 4.51  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 51 Premature rupture of membranes (ALL). | ||||
| 68 Ln (serum ferritin concentration) 4‐8 wk postpartum (not pre‐specified) Show forest plot | 1 | 160 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.42, 0.16] |
| Analysis 4.68  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 68 Ln (serum ferritin concentration) 4‐8 wk postpartum (not pre‐specified). | ||||
| 70 Low serum ferritin concentration at postpartum (4‐8 wk) (not pre‐specified) Show forest plot | 1 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.40, 3.57] |
| Analysis 4.70  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 70 Low serum ferritin concentration at postpartum (4‐8 wk) (not pre‐specified). | ||||
| 71 High serum transferrin receptors at 6 weeks postpartum (not pre‐specified) Show forest plot | 1 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.36, 1.33] |
| Analysis 4.71  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 71 High serum transferrin receptors at 6 weeks postpartum (not pre‐specified). | ||||
| 93 Caesarean delivery (not pre‐specified) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.38, 2.07] |
| Analysis 4.93  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 93 Caesarean delivery (not pre‐specified). | ||||
| 97 Haemoglobin concentration at 4‐8 weeks postpartum (not pre‐specified) Show forest plot | 1 | 146 | Mean Difference (IV, Random, 95% CI) | 2.0 [‐3.86, 7.86] |
| Analysis 4.97  Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 97 Haemoglobin concentration at 4‐8 weeks postpartum (not pre‐specified). | ||||

Funnel plot of comparison: 1 Daily iron alone versus no intervention/placebo, outcome: 1.7 Maternal Hb concentration at term (g/L) (ALL).
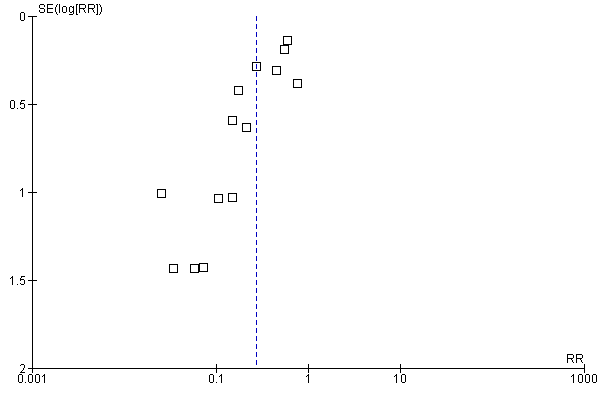
Funnel plot of comparison: 1 Daily iron alone versus no intervention/placebo, outcome: 1.9 Anaemia at term (Hb less than 110 g/L).

Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 1 Low birthweight (less than 2500 g) (ALL).
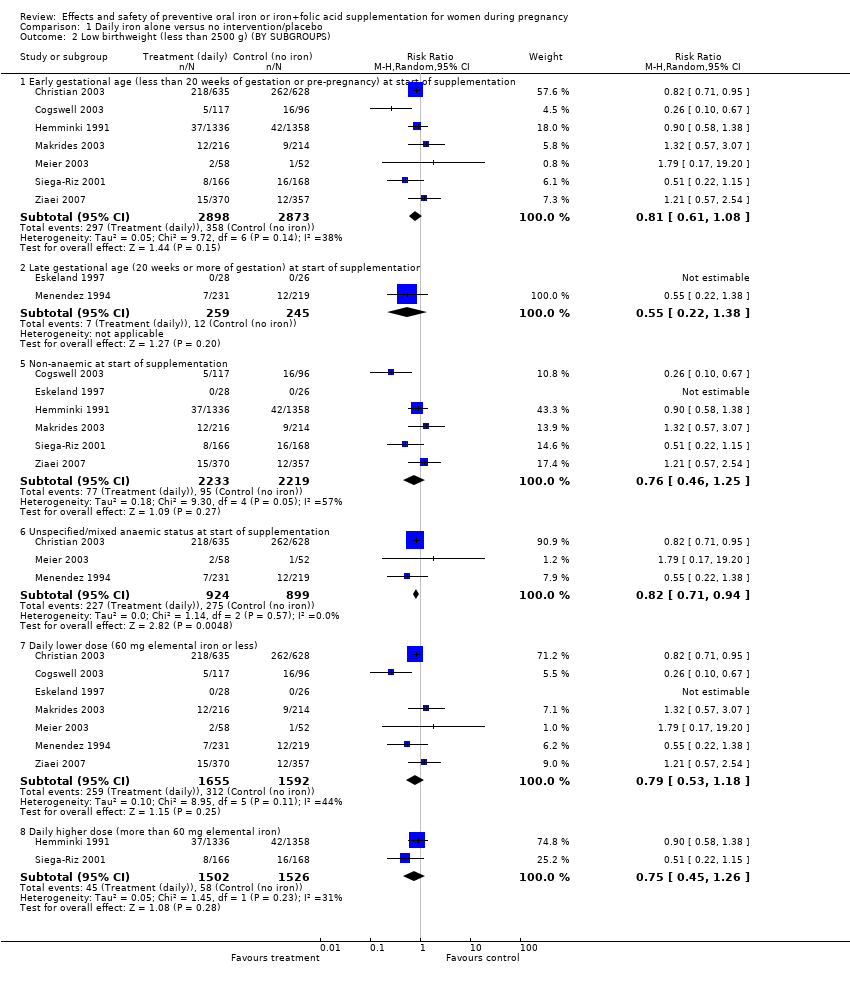
Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 2 Low birthweight (less than 2500 g) (BY SUBGROUPS).

Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 3 Birthweight (g) (ALL).

Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 4 Birthweight (g) (BY SUBGROUPS).
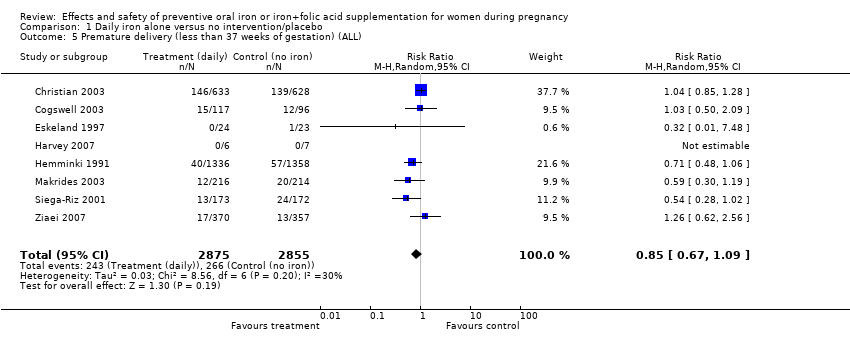
Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 5 Premature delivery (less than 37 weeks of gestation) (ALL).
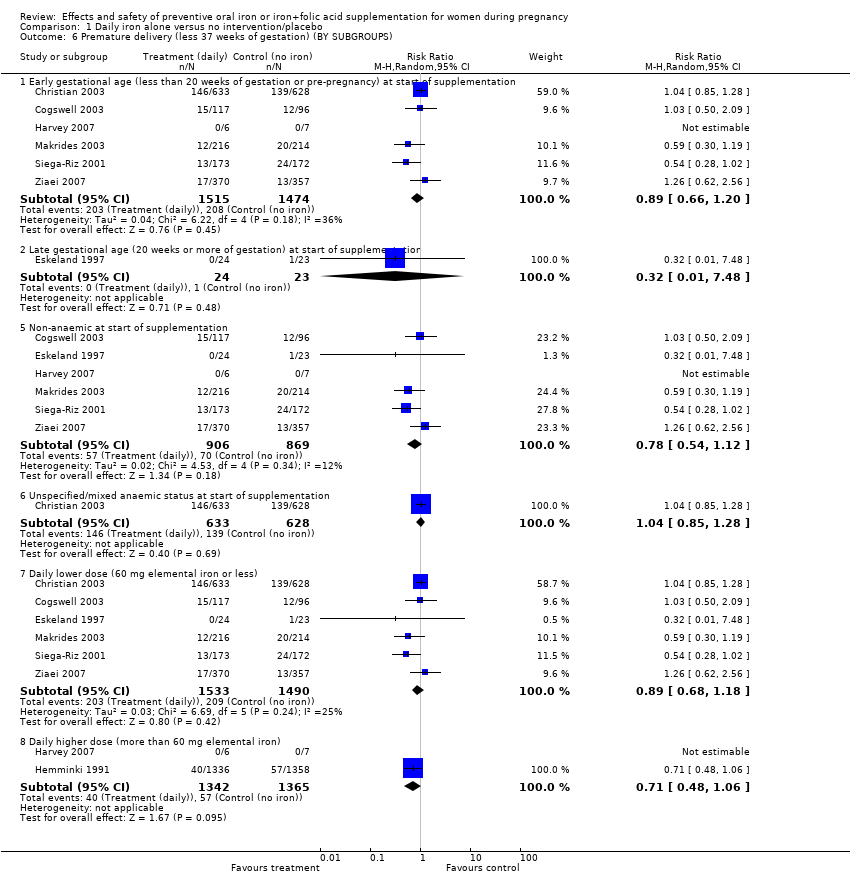
Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 6 Premature delivery (less 37 weeks of gestation) (BY SUBGROUPS).

Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 7 Maternal Hb concentration at term (g/L) (ALL).

Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 8 Maternal Hb concentration at term (g/L) (BY SUBGROUPS).

Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 9 Anaemia at term (Hb less than 110 g/L) (ALL).

Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 10 Haemoconcentration at term (Hb more than 130 g/L) (ALL).
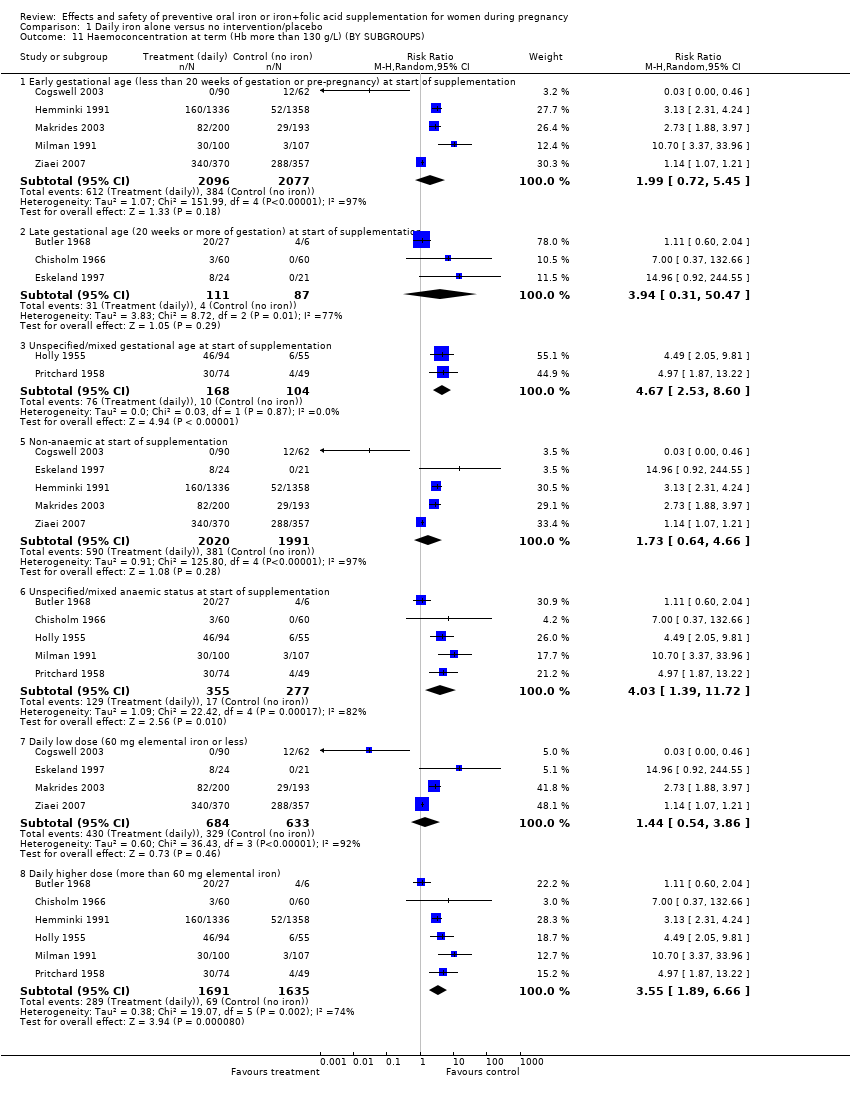
Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 11 Haemoconcentration at term (Hb more than 130 g/L) (BY SUBGROUPS).

Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 12 Haemoconcentration during second or third trimester (ALL).

Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 13 Haemoconcentration during second or third trimester (BY SUBGROUPS).

Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 14 Iron deficiency at term (as defined by two or more indicators) (ALL).

Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 15 Iron deficiency at term (as defined by two or more indicators) (BY SUBGROUPS).

Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 16 Iron deficiency anaemia at term (ALL).
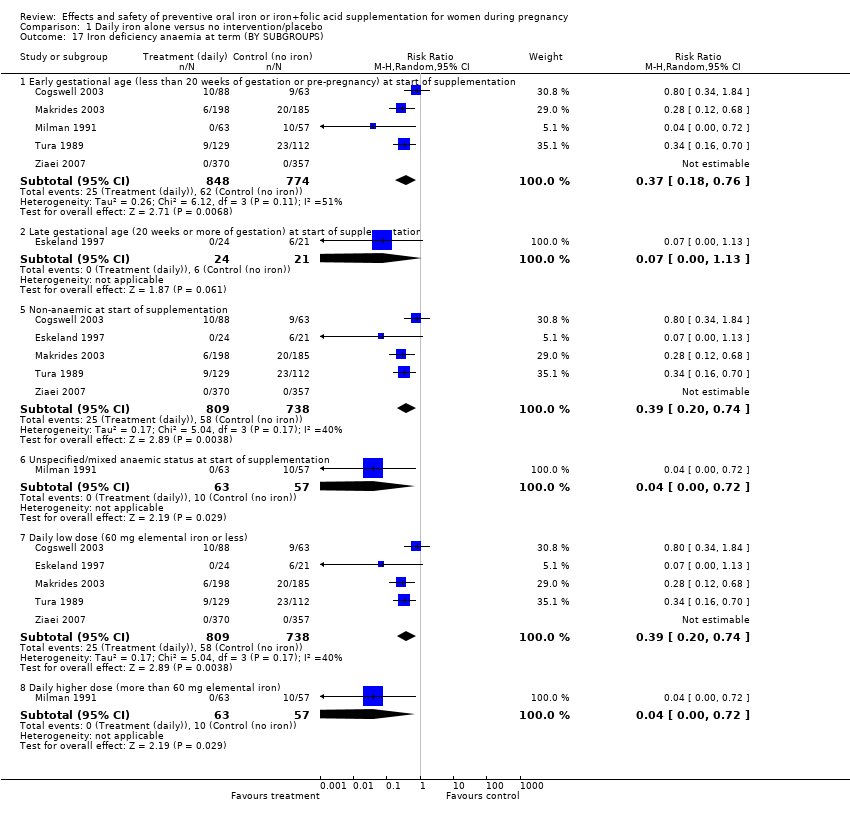
Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 17 Iron deficiency anaemia at term (BY SUBGROUPS).

Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 18 Side‐effects (Any) (ALL).
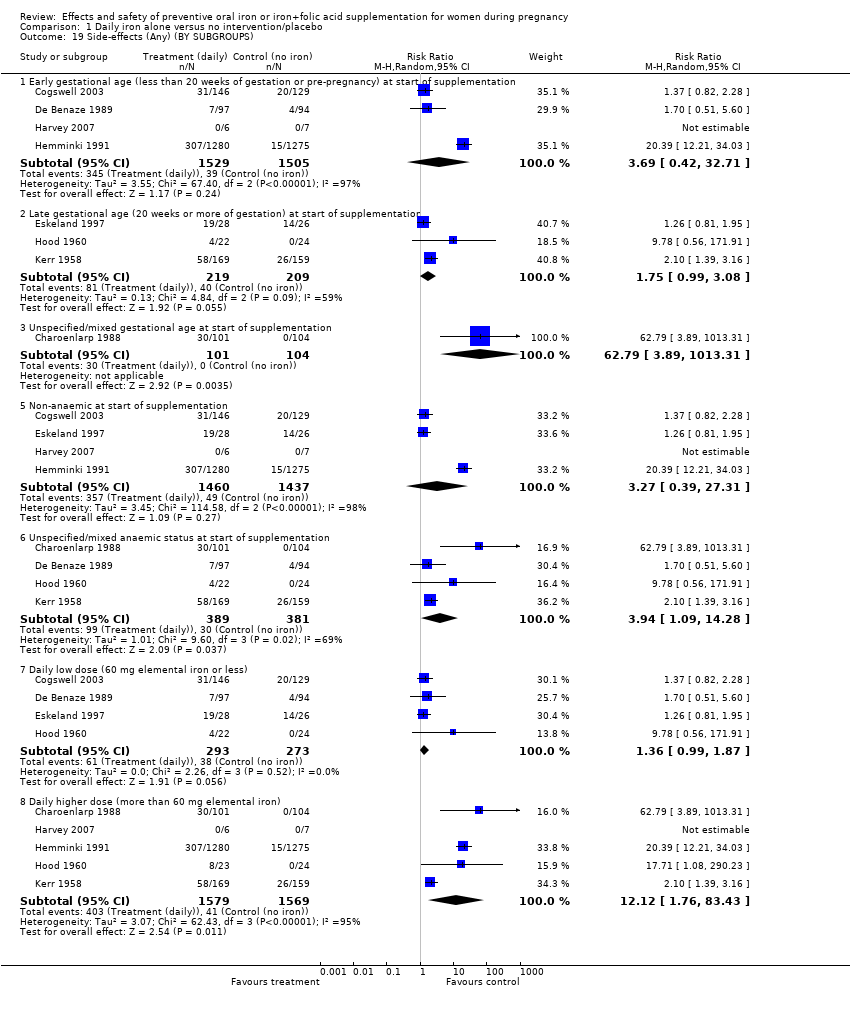
Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 19 Side‐effects (Any) (BY SUBGROUPS).

Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 20 Very low birthweight (less than 1500 g) (ALL).
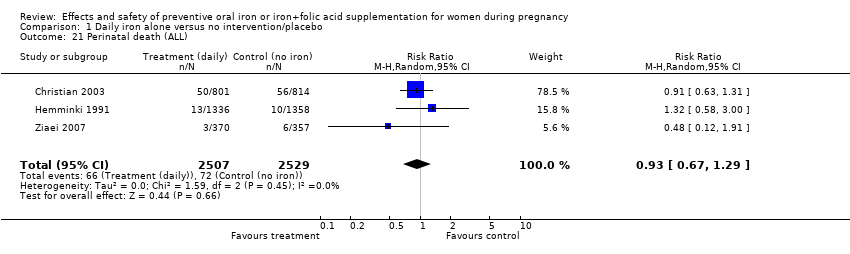
Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 21 Perinatal death (ALL).
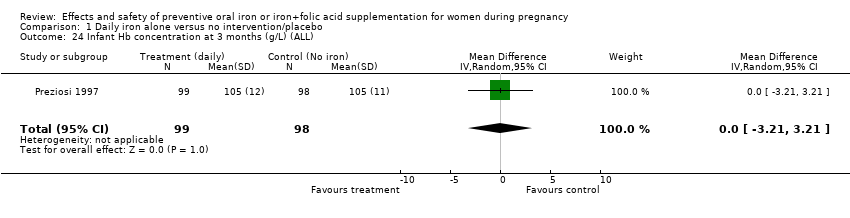
Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 24 Infant Hb concentration at 3 months (g/L) (ALL).

Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 25 Infant serum ferritin concentration at 3 months (ug/L) (ALL).

Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 26 Infant Hb concentration at 6 months (g/L) (ALL).

Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 27 Infant serum ferritin concentration at 6 months (ug/L) (ALL).

Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 29 Admission to special care unit (ALL).
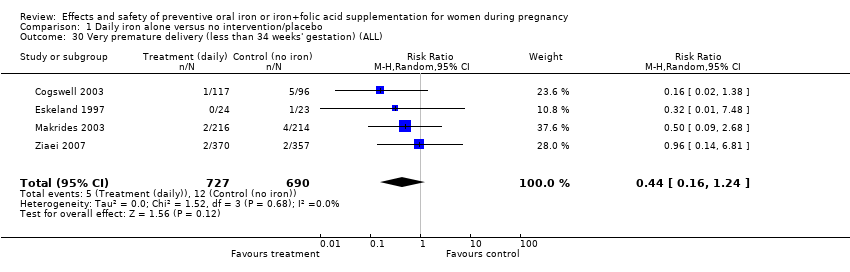
Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 30 Very premature delivery (less than 34 weeks' gestation) (ALL).

Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 31 Severe anaemia at term (Hb less than 70 g/L) (ALL).
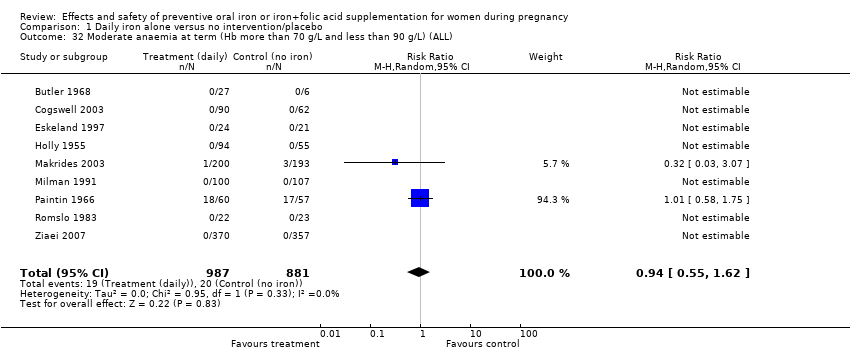
Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 32 Moderate anaemia at term (Hb more than 70 g/L and less than 90 g/L) (ALL).
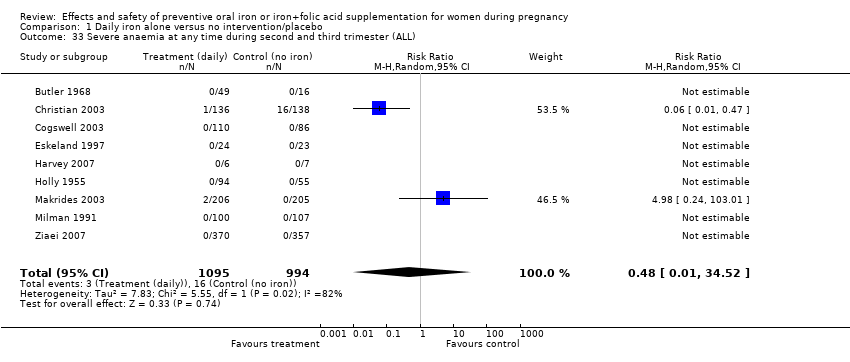
Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 33 Severe anaemia at any time during second and third trimester (ALL).

Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 34 Moderate anaemia at any time during second or third trimester (ALL).
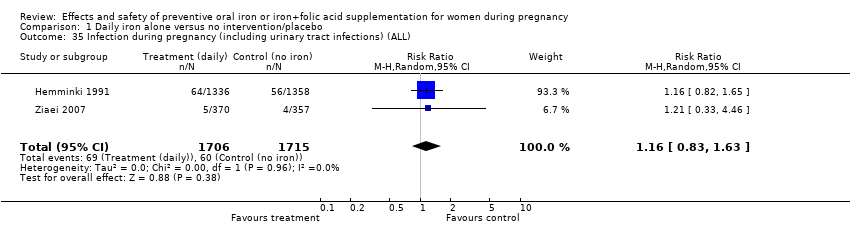
Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 35 Infection during pregnancy (including urinary tract infections) (ALL).
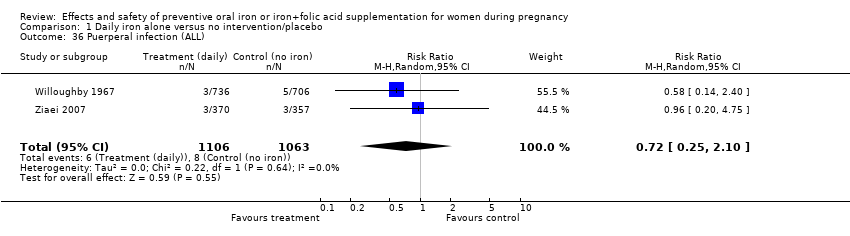
Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 36 Puerperal infection (ALL).
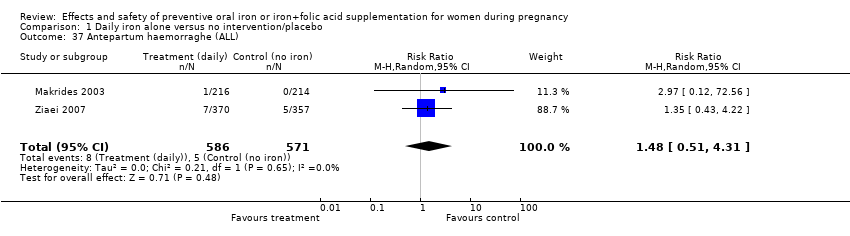
Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 37 Antepartum haemorraghe (ALL).

Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 38 Postpartum haemorraghe (ALL).

Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 39 Transfusion provided (ALL).

Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 40 Haemoglobin concentration within one month postpartum (ALL).

Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 41 Severe anaemia at postpartum (Hb less than 80 g/L) (ALL).

Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 42 Moderate anaemia at postpartum (Hb more than 80 g/L and less than 100 g/L) (ALL).

Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 43 Diarrhoea (ALL).
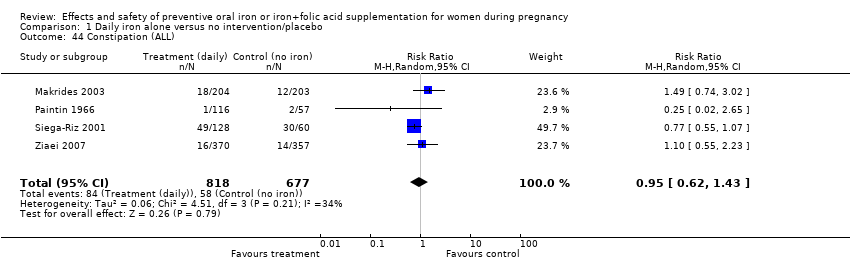
Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 44 Constipation (ALL).
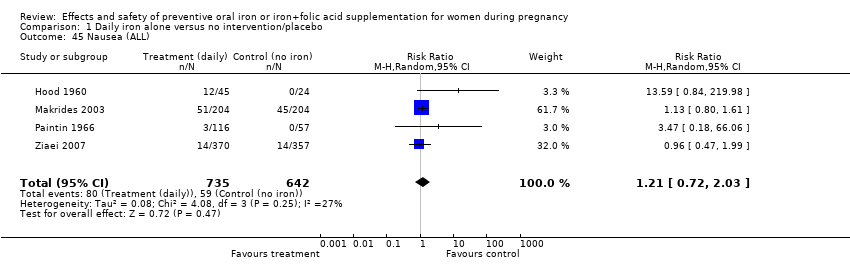
Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 45 Nausea (ALL).
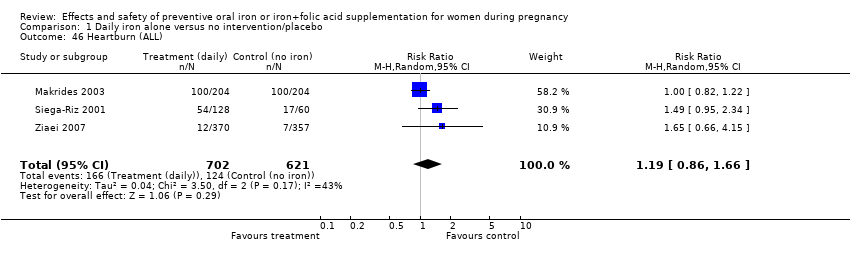
Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 46 Heartburn (ALL).

Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 47 Vomiting (ALL).

Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 48 Maternal death (death while pregnant or within 42 days of termination of pregnancy) (ALL).

Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 49 Maternal wellbeing/satisfaction (ALL).
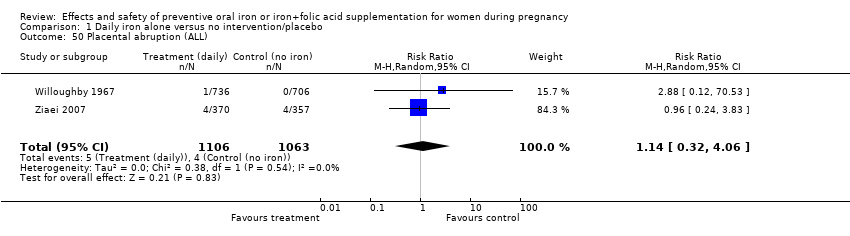
Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 50 Placental abruption (ALL).
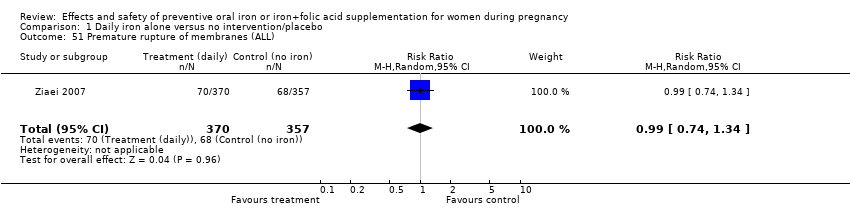
Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 51 Premature rupture of membranes (ALL).
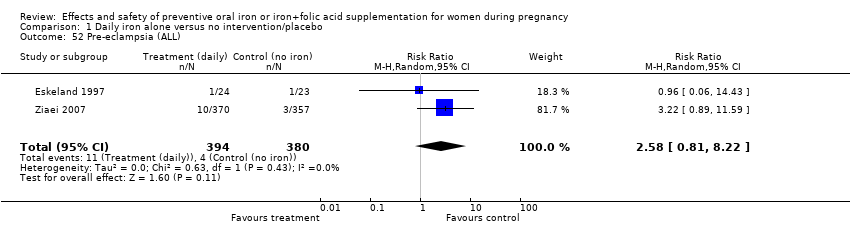
Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 52 Pre‐eclampsia (ALL).

Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 91 Small for gestational age (less than 10th percentile weight at birth for gestational age) (not pre‐specified).

Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 93 Cesarean delivery (not pre‐specified).

Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 94 Birth length in cm (not pre‐specified).

Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 95 Forceps or vacuum delivery (not pre‐specified).
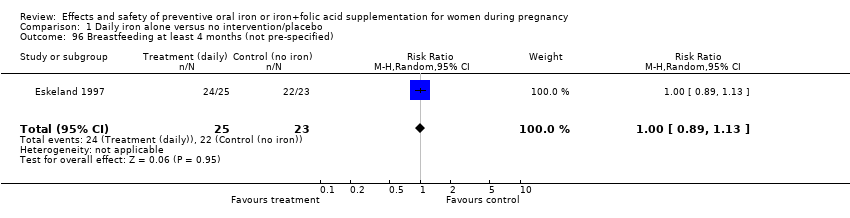
Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 96 Breastfeeding at least 4 months (not pre‐specified).
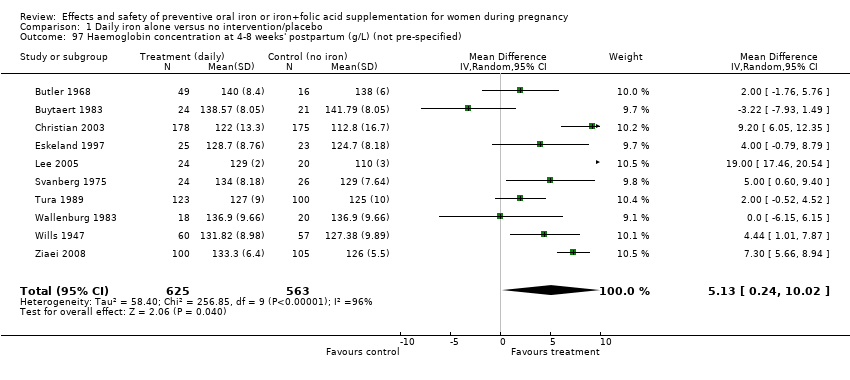
Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 97 Haemoglobin concentration at 4‐8 weeks' postpartum (g/L) (not pre‐specified).
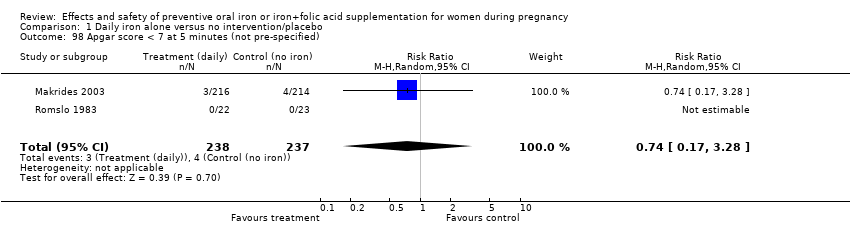
Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 98 Apgar score < 7 at 5 minutes (not pre‐specified).
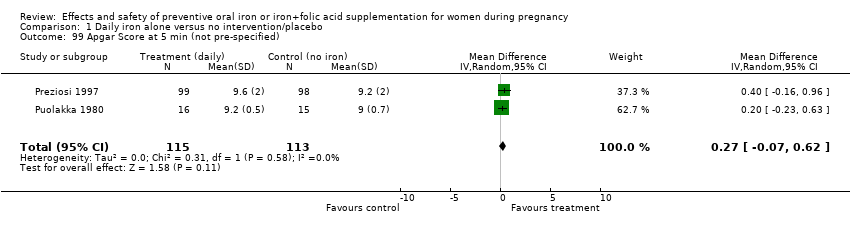
Comparison 1 Daily iron alone versus no intervention/placebo, Outcome 99 Apgar Score at 5 min (not pre‐specified).
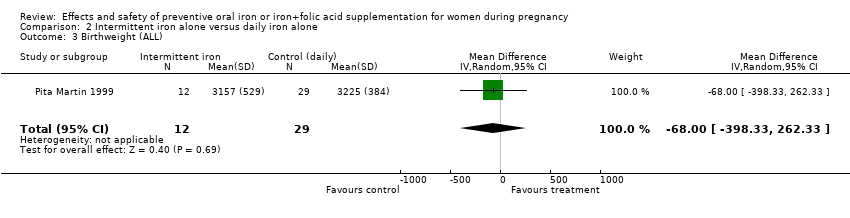
Comparison 2 Intermittent iron alone versus daily iron alone, Outcome 3 Birthweight (ALL).

Comparison 2 Intermittent iron alone versus daily iron alone, Outcome 5 Premature delivery (less than 37 weeks of gestation) (ALL).
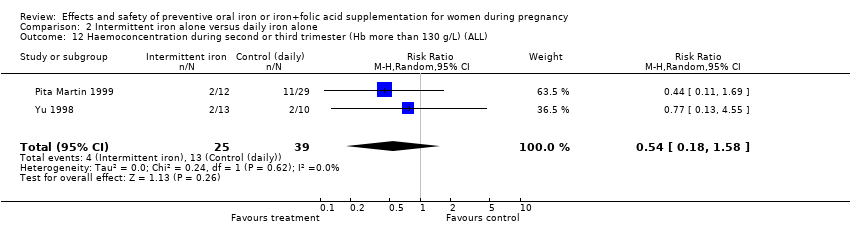
Comparison 2 Intermittent iron alone versus daily iron alone, Outcome 12 Haemoconcentration during second or third trimester (Hb more than 130 g/L) (ALL).
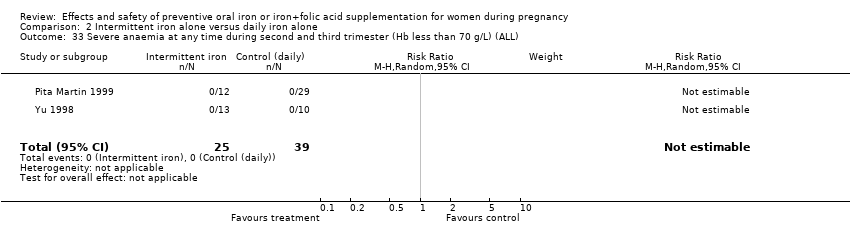
Comparison 2 Intermittent iron alone versus daily iron alone, Outcome 33 Severe anaemia at any time during second and third trimester (Hb less than 70 g/L) (ALL).
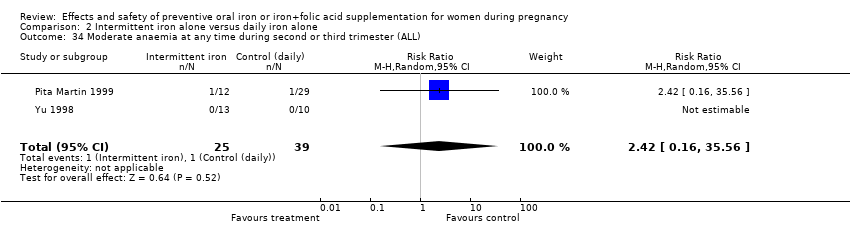
Comparison 2 Intermittent iron alone versus daily iron alone, Outcome 34 Moderate anaemia at any time during second or third trimester (ALL).
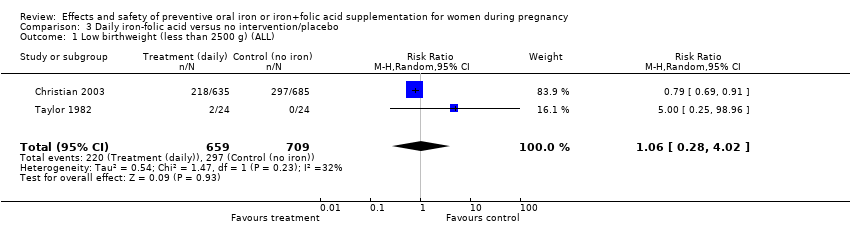
Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 1 Low birthweight (less than 2500 g) (ALL).
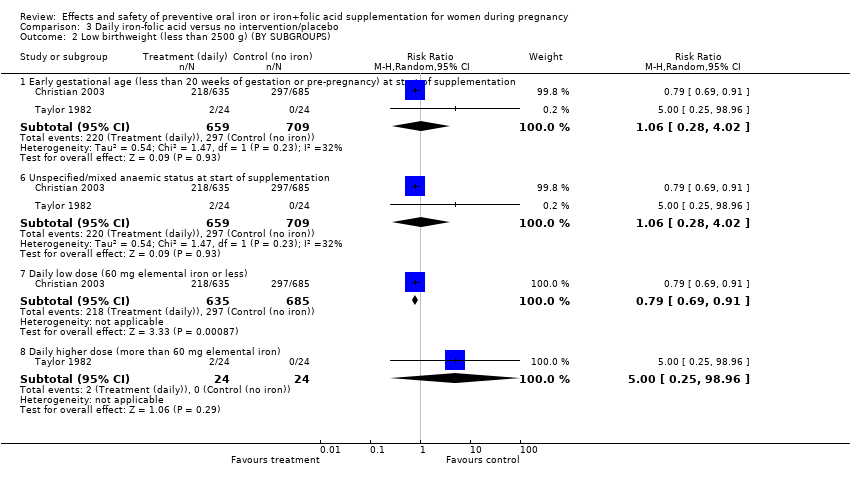
Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 2 Low birthweight (less than 2500 g) (BY SUBGROUPS).
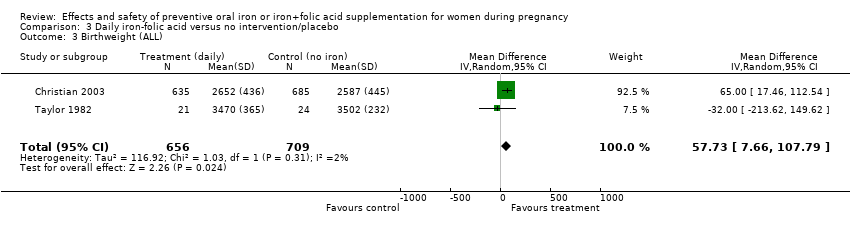
Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 3 Birthweight (ALL).
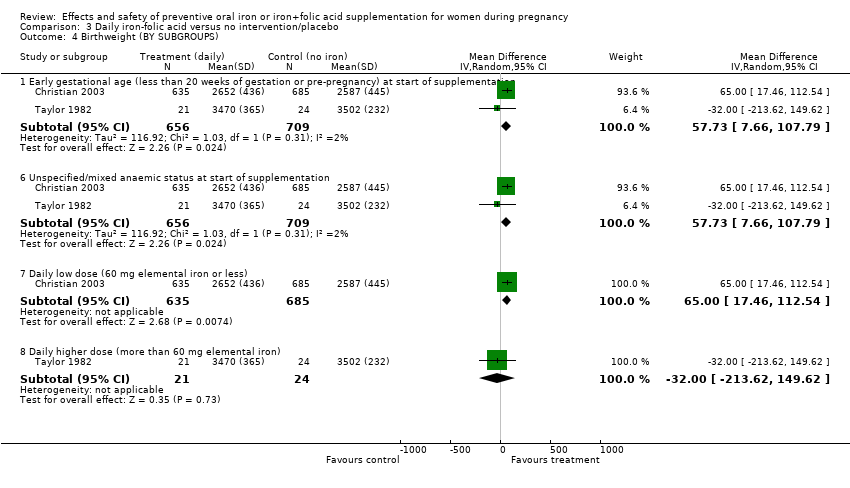
Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 4 Birthweight (BY SUBGROUPS).

Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 5 Premature delivery (less than 37 weeks of gestation) (ALL).
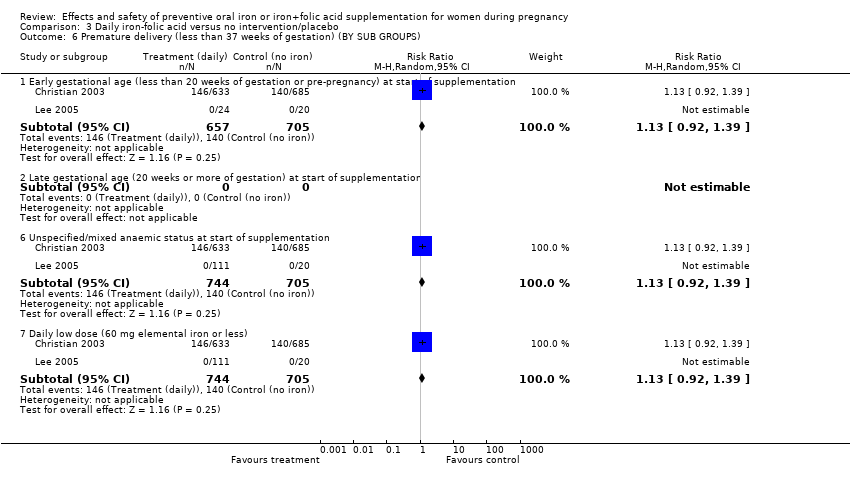
Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 6 Premature delivery (less than 37 weeks of gestation) (BY SUB GROUPS).

Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 7 Haemoglobin concentration at term (ALL).

Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 8 Haemoglobin concentration at term (BY SUBGROUPS).
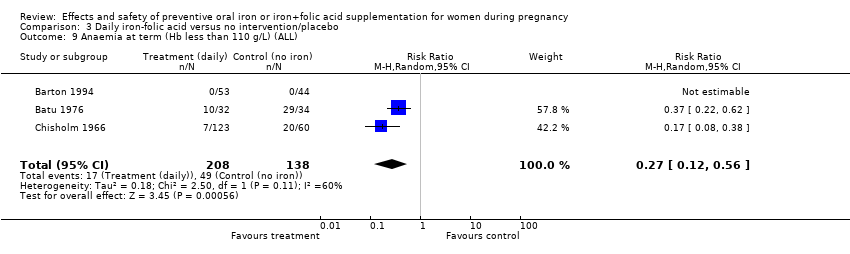
Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 9 Anaemia at term (Hb less than 110 g/L) (ALL).

Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 10 Haemoconcentration at term (Hb more than 130 g/L) (ALL).
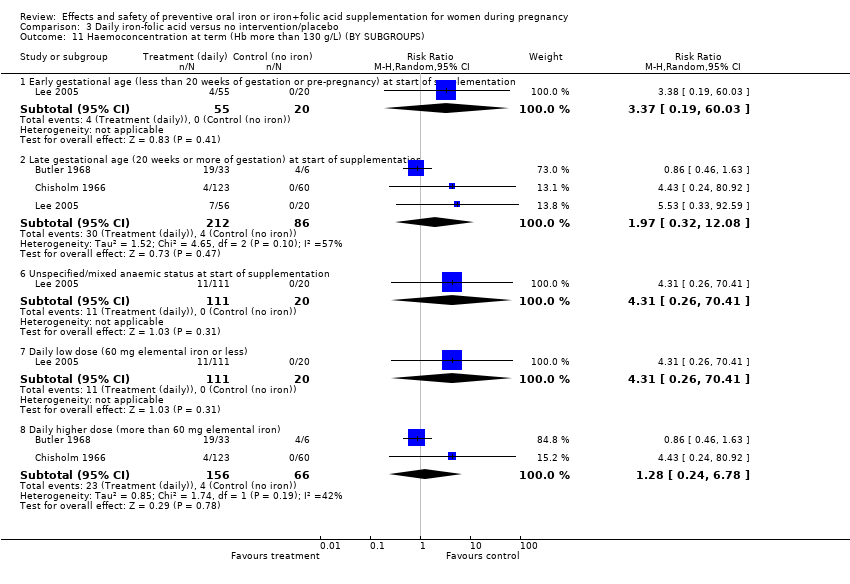
Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 11 Haemoconcentration at term (Hb more than 130 g/L) (BY SUBGROUPS).
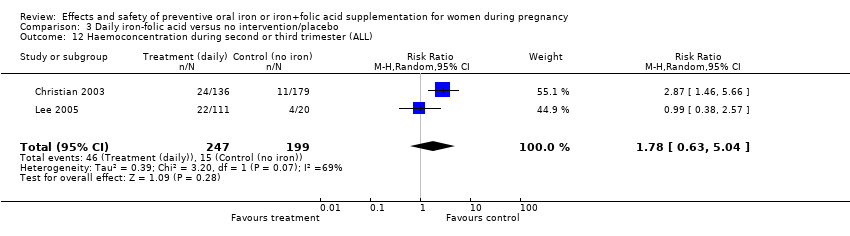
Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 12 Haemoconcentration during second or third trimester (ALL).

Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 13 Haemoconcentration during second or third trimester (BY SUBGROUPS).
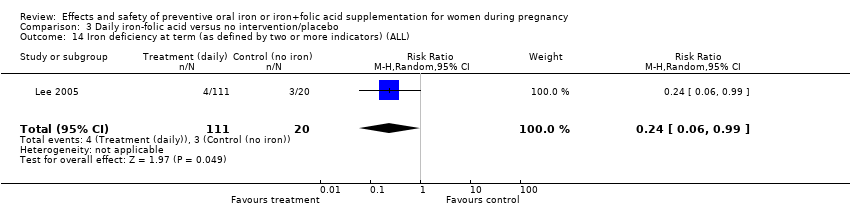
Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 14 Iron deficiency at term (as defined by two or more indicators) (ALL).

Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 15 Iron deficiency at term (as defined by two or more indicators) (BY SUBGROUPS).
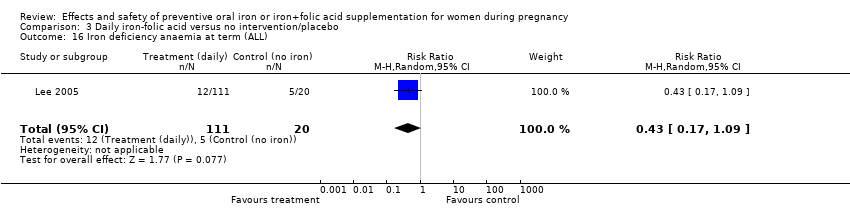
Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 16 Iron deficiency anaemia at term (ALL).

Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 17 Iron deficiency anaemia at term (BY SUBGROUPS).
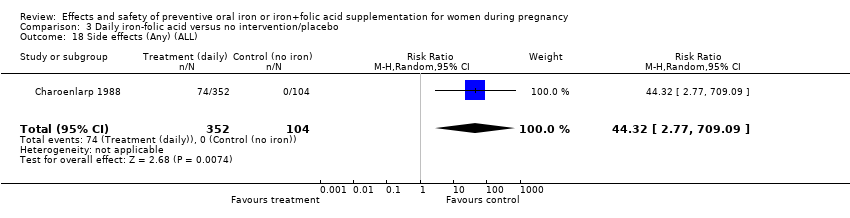
Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 18 Side effects (Any) (ALL).
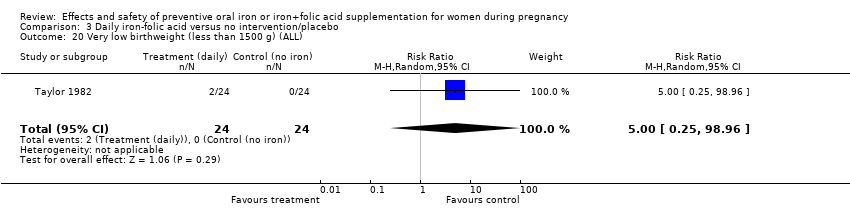
Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 20 Very low birthweight (less than 1500 g) (ALL).

Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 21 Perinatal death (ALL).

Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 29 Admission to special care unit (ALL).
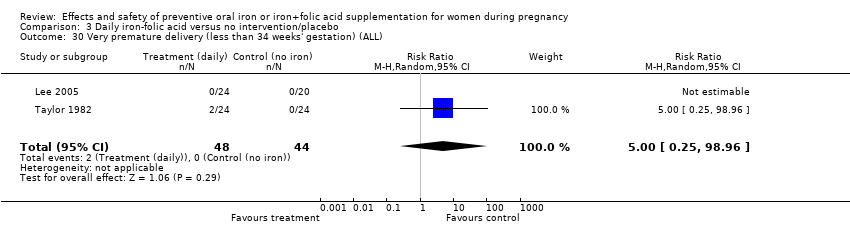
Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 30 Very premature delivery (less than 34 weeks' gestation) (ALL).

Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 31 Severe anaemia at term (Hb less than 70 g/L) (ALL).

Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 32 Moderate anaemia at term (Hb more than 70g/L and less than 90 g/L) (ALL).

Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 33 Severe anaemia at any time during second and third trimester (Hb less than 70 g/L) (ALL).
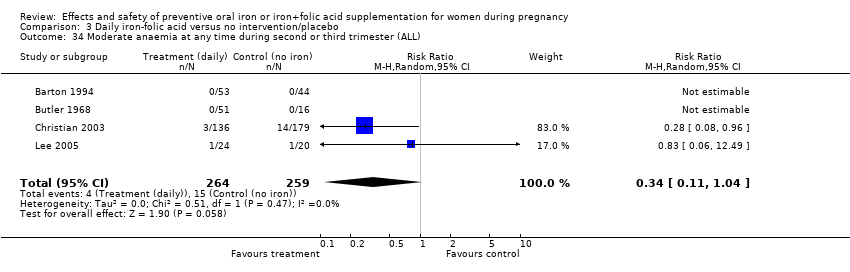
Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 34 Moderate anaemia at any time during second or third trimester (ALL).

Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 35 Infection during pregnancy (including urinary tract infections) (ALL).

Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 36 Puerperal infection (ALL).

Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 37 Antepartum haemorrhage (ALL).

Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 38 Postpartum haemorrhage (ALL).
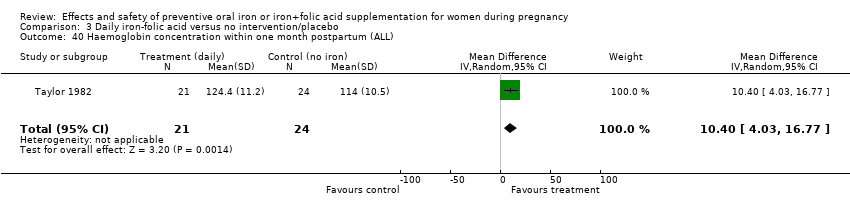
Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 40 Haemoglobin concentration within one month postpartum (ALL).

Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 41 Severe anaemia at postpartum (Hb less than 80 g/L) (ALL).

Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 42 Moderate anaemia at postpartum (Hb more than 80 g/L and less than 100 g/L) (ALL).
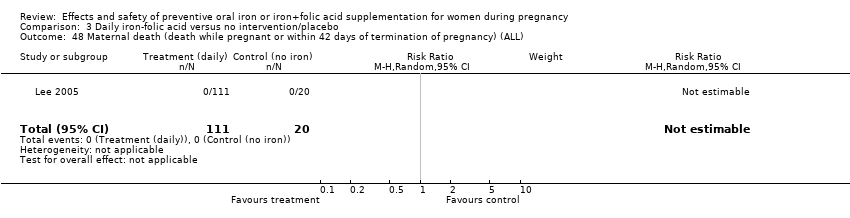
Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 48 Maternal death (death while pregnant or within 42 days of termination of pregnancy) (ALL).
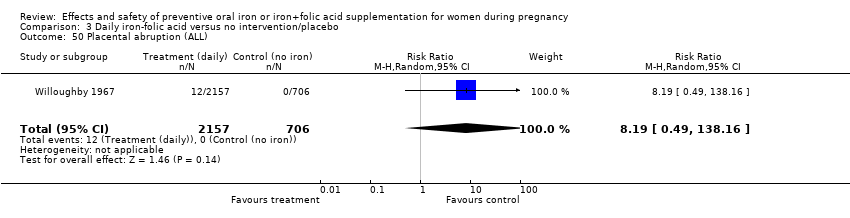
Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 50 Placental abruption (ALL).

Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 52 Pre‐eclampsia (ALL).

Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 91 Small for gestational age (less than 10th percentile weight at birth for gestational age) (not pre‐specified).
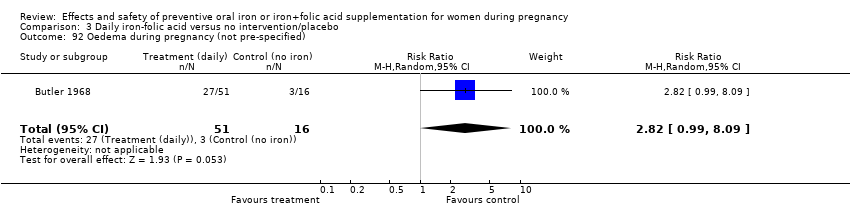
Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 92 Oedema during pregnancy (not pre‐specified).

Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 93 Caesarean delivery (not pre‐specified).

Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 94 Birth length in cm (not pre‐specified).

Comparison 3 Daily iron‐folic acid versus no intervention/placebo, Outcome 97 Haemoglobin concentration at 4‐8 weeks postpartum (not prespecified).
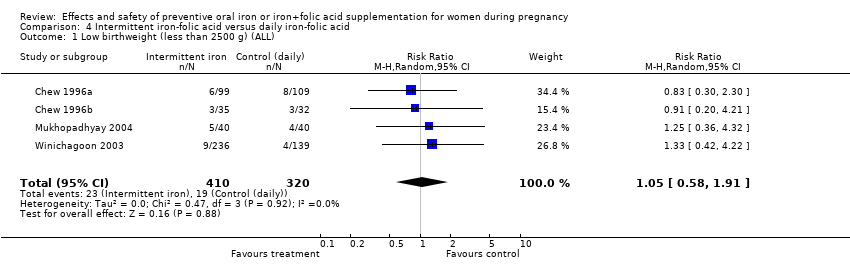
Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 1 Low birthweight (less than 2500 g) (ALL).

Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 2 Low birthweight (less than 2500 g) (BY SUBGROUPS).

Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 3 Birthweight (ALL).

Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 4 Birthweight (BY SUBGROUPS).
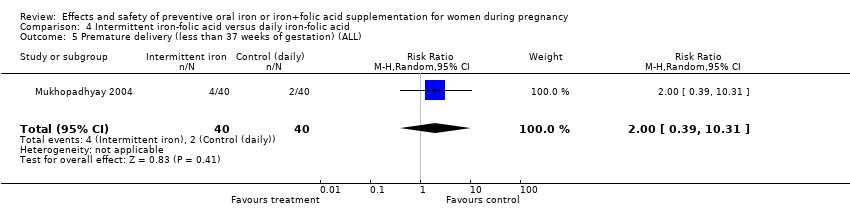
Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 5 Premature delivery (less than 37 weeks of gestation) (ALL).
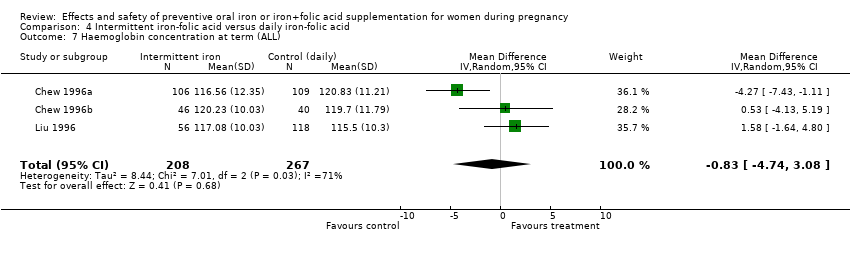
Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 7 Haemoglobin concentration at term (ALL).

Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 8 Haemoglobin concentration at term (BY SUBGROUPS).

Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 9 Anaemia at term (Hb < 110 g/L) (ALL).
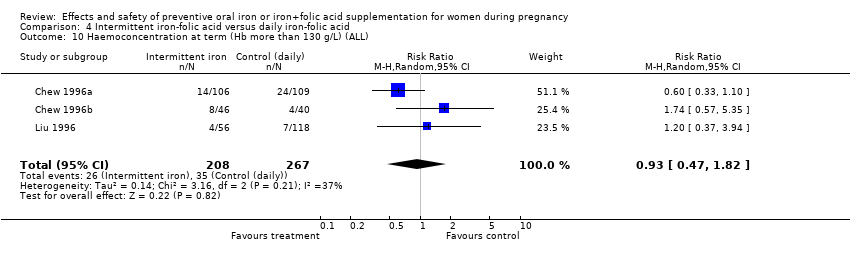
Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 10 Haemoconcentration at term (Hb more than 130 g/L) (ALL).

Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 11 Haemoconcentration at term (Hb more than 130 g/L) (BY SUBGROUPS).

Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 12 Haemoconcentration during second or third trimester (Hb more than 130 g/L) (ALL).

Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 13 Haemoconcentration during second or third trimester (Hb more than 130 g/L) (BY SUBGROUPS).
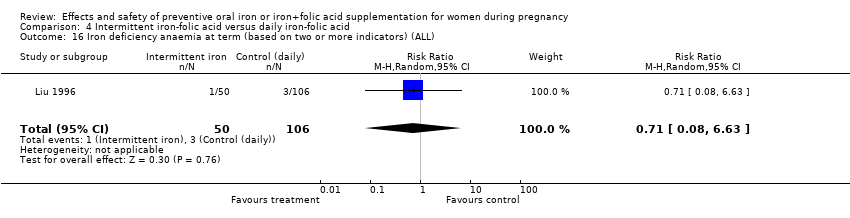
Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 16 Iron deficiency anaemia at term (based on two or more indicators) (ALL).

Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 18 Side effects (any) (ALL).

Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 19 Side effects (any) (BY SUBGROUPS).

Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 20 Very low birthweight (less than 1500 g) (ALL).

Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 21 Perinatal death (ALL).

Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 27 Infant ferritin concentration at 6 months (ug/L) (ALL).
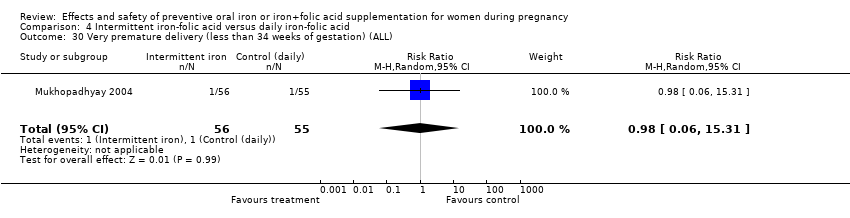
Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 30 Very premature delivery (less than 34 weeks of gestation) (ALL).
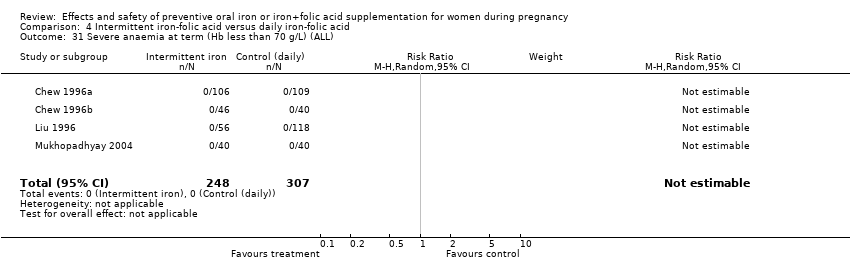
Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 31 Severe anaemia at term (Hb less than 70 g/L) (ALL).

Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 32 Moderate anaemia at term (Hb more than 70g/L and less than 90 g/L) (ALL).
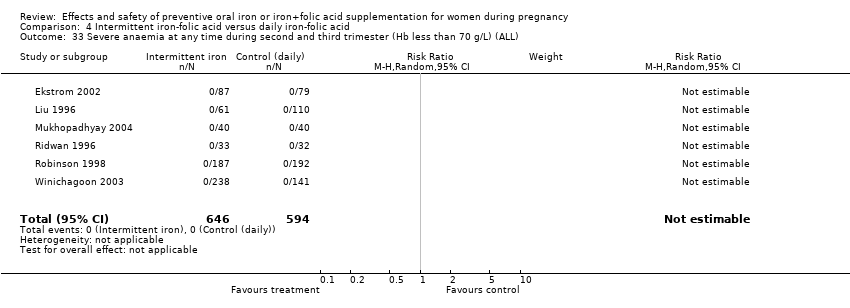
Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 33 Severe anaemia at any time during second and third trimester (Hb less than 70 g/L) (ALL).

Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 34 Moderate anaemia at any time during second or third trimester (ALL).

Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 37 Antepartum haemorraghe (ALL).

Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 41 Severe anaemia at postpartum (Hb less than 80 g/L) (ALL).

Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 42 Moderate anaemia at postpartum (Hb more than 80 g/L and less than 100 g/L) (ALL).

Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 43 Diarrhoea (ALL).
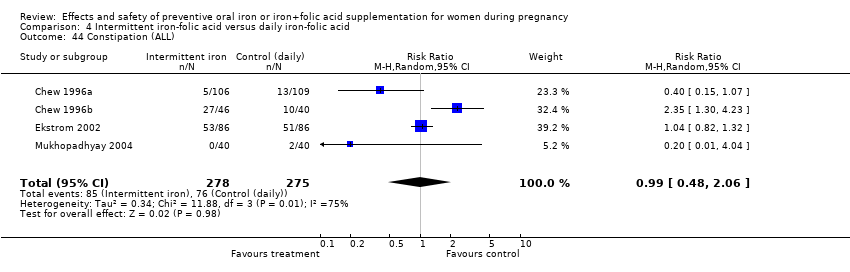
Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 44 Constipation (ALL).

Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 45 Nausea (ALL).

Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 46 Heartburn (ALL).
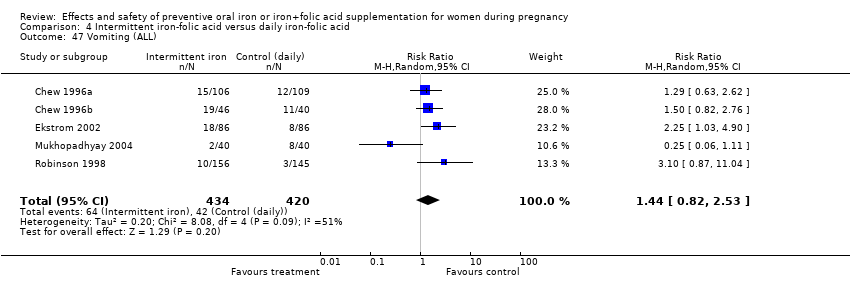
Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 47 Vomiting (ALL).
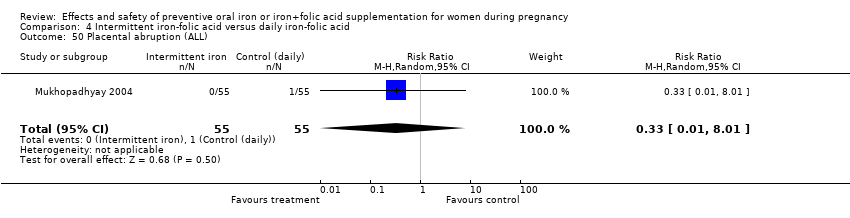
Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 50 Placental abruption (ALL).

Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 51 Premature rupture of membranes (ALL).

Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 68 Ln (serum ferritin concentration) 4‐8 wk postpartum (not pre‐specified).

Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 70 Low serum ferritin concentration at postpartum (4‐8 wk) (not pre‐specified).

Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 71 High serum transferrin receptors at 6 weeks postpartum (not pre‐specified).

Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 93 Caesarean delivery (not pre‐specified).

Comparison 4 Intermittent iron‐folic acid versus daily iron‐folic acid, Outcome 97 Haemoglobin concentration at 4‐8 weeks postpartum (not pre‐specified).
| Trial | Randomisation | Allocation | Blinding | Completeness of data | Quality rating |
| Barton 1994 | Adequate | Adequate | Participants and care provider blinded | Adequate. Less than 5% lost to follow up | HIGH |
| Batu 1976 | Unclear method | Unclear | Unclear. Participants blinded. Care provider and outcome assessor not clear | Inadequate. 28% of women lost to follow up | |
| Butler 1968 | Adequate | Adequate | Inadequate. Participant and provider were blinded for treatment for groups 1 and 2. | Inadequate. More than 20% lost to follow up | |
| Buytaert 1983 | Adequate | Adequate | Inadequate. Open | Unclear | HIGH |
| Cantlie 1971 | Unclear. Method not stated. | Unclear | Unclear | Unclear. Not reported | |
| Chanarin 1971 | Inadequate. Quasi randomised by sequence assignment | Inadequate | Participants and care provider blinded | Adequate. Less than 20% lost to follow up | |
| Charoenlarp 1988 | Adequate | Unclear | Unclear. Participants and outcome assessor blinded. Care provider unclear | Adequate | |
| Chew 1996a | Adequate | Adequate by sealed envelopes | Inadequate. Participants, care provider and outcome assessor not blinded | Adequate. Less than 20% lost to follow up | |
| Chew 1996b | Adequate | Adequate by sealed envelopes | Adequate. Participants and care provider not blinded. Outcome assessor blinded | Inadequate. More than 20% lost to follow up | |
| Chisholm 1966 | Unclear method | Adequate | Participants and care provider blinded | Adequate. Less than 20% lost to follow up | |
| Christian 2003 | Adequate. Cluster randomisation | Adequate. Coded assignment | Adequate. Participants, care providers and outcome assessors blinded | Inadequate. More than 20% lost to follow up | HIGH |
| Cogswell 2003 | Adequate | Adequate | Adequate. Participants, care provider and outcome assessor blinded | Inadequate. More than 20% lost to follow up | HIGH |
| De Benaze 1989 | Unclear method | Adequate | Adequate. Participants and care provider blinded | Adequate. Less than 20% lost to follow up | |
| Ekstrom 2002 | Adequate by cluster | Inadequate. Not used | Inadequate. Participants and care provider not blinded. Outcome assessor unclear | Inadequate. More than 20% lost to follow up | |
| Eskeland 1997 | Adequate | Adequate | Adequate. Participants, care provider and outcome assessor blinded | Inadequate. More than 20% lost to follow up | HIGH |
| Hankin 1963 | Inadequate. Quasi randomised by alternate assignment by day of the week | Inadequate | Inadequate. Open | Adequate. Less than 5% excluded | |
| Harvey 2007 | Adequate | Adequate. Coded opaque bottles | Inadequate. Participants blinded. Care provider and outcome assessor not blinded | Adequate. No losses to follow up. | HIGH |
| Hemminki 1989 | Adequate | Adequate. Sealed numbered envelopes. | Inadequate. Open to participants and care providers. Outcome assessor blind. | Adequate. | HIGH |
| Holly 1955 | Unclear method | Unclear | Inadequate. Participants and care provider not blinded. Outcome assessor unclear | Unclear | |
| Hood 1960 | Unclear method | Unclear | Inadequate. Participants and care provider not blinded. Outcome assessor unclear | Adequate. Less than 20% lost to follow up | |
| Kerr 1958 | Adequate | Unclear | Inadequate. Participants blinded. Care provider not blinded. Outcome assessor unclear | Inadequate. More than 20% lost to follow up | |
| Lee 2005 | Adequate. Method unclear | Unclear | Unclear. | Adequate. Less than 20% lost to follow up | |
| Liu 1996 | Unclear method | Adequate by sealed envelopes | Inadequate. Participants and care provider not blinded. Outcome assessor blinded | Adequate. Less than 20% lost to follow up | |
| Makrides 2003 | Adequate | Adequate | Adequate. Participants and care provider blinded | Adequate. Less than 20% lost to follow up | HIGH |
| Meier 2003 | Adequate. Stratified by age group | Unclear | Adequate. Participants and care provider blinded. Outcome assessor unclear | Inadequate. More than 20% lost to follow up | |
| Menendez 1994 | Unclear method | Inadequate | Inadequate. Participants and care provider not blinded. Outcome assessor blinded | Inadequate. More than 20% lost to follow up | |
| Milman 1991 | Unclear method | Unclear | Adequate. Participants and care provider blinded. Outcome assessor unclear | Adequate. Less than 20% lost to follow up | |
| Mukhopadhyay 2004 | Adequate. Block randomisation | Inadequate. Not used | Inadequate. Open to participants, and care providers. Outcome assessor unclear | Inadequate. More than 20% lost to follow up | |
| Paintin 1966 | Unclear method | Adequate by sequential numbers | Adequate. Participants and care provider blinded | Adequate. Less than 5% lost to follow up | |
| Pita Martin 1999 | Inadequate. Quasi randomised | Inadequate. Not used | Inadequate. Open | Inadequate. More than 20% lost to follow up | |
| Preziosi 1997 | Adequate | Adequate | Adequate. Participant and care provider blinded. Outcome assessor blinded | Unclear | HIGH |
| Pritchard 1958 | Unclear method | Unclear | Inadequate. Open | Unclear | |
| Puolakka 1980 | Unclear method | Unclear | Inadequate. Open | Adequate. Less than 20% lost to follow up | |
| Ridwan 1996 | Adequate | Inadequate. Not used | Inadequate. Participants and care provider not blinded. Outcome assessor blinded | Inadequate. More than 20% lost to follow up | |
| Robinson 1998 | Quasi randomised by alternate numbers | Unclear | Inadequate. Participants and care provider not blinded | Inadequate. More than 20% lost to follow up | |
| Romslo 1983 | Unclear method | Unclear | Inadequate. Participants blinded. Care provider and outcome assessor not blinded | Adequate. Less than 20% lost to follow up | |
| Siega‐Riz 2001 | Adequate. Random number generator | Adequate | Adequate. Participants, care provider and outcome assessor blinded | Inadequate. More than 20% lost to follow up | HIGH |
| Svanberg 1975 | Unclear method | Unclear | Adequate. Participants, care provider, and outcome assessor blinded | Adequate. Less than 20% lost to follow up | |
| Taylor 1982 | Unclear method | Unclear | Inadequate. Open | Adequate. less than 20% lost to follow up | |
| Tura 1989 | Adequate | Adequate | Inadequate. Open | Adequate. Less than 20% lost to follow up | HIGH |
| Van Eijk 1978 | Unclear. Not stated | Inadequate. Not used | Inadequate.Open | Adequate. Less than 20% loss to follow up | |
| Wallenburg 1983 | Adequate | Adequate | Inadequate.Open | Adequate. Less than 20% lost to follow up | HIGH |
| Willoughby 1967 | Unclear method | Unclear | Unclear | A. Adequate. Less than 20% lost to follow up | |
| Wills 1947 | Inadequate. Quasi randomised by alternate allocation | Inadequate. Not used | Adequate. Participants and care provider blinded. Outcome assessor blinded | Inadequate. More than 20% lost to follow up | |
| Winichagoon 2003 | Unclear method of cluster randomisation | Inadequate. Not used | Inadequate.Open | Inadequate. more than 20% lost to follow up | |
| Young 2000 | Adequate | Unclear | Inadequate. Participants and care provider not blinded. Outcome assessor unclear | Inadequate. More than 20% lost to follow up | |
| Yu 1998 | Inadequate. Quasi randomised | Inadequate | Inadequate.Open | Inadequate. More than 20% lost to follow up | |
| Ziaei 2008 | Adequate. Random numbers table | Adequate. Coded assignments | Adequate. Participants and care provider blinded. Outcome assessor unclear | Adequate. Less than 5% lost to follow up | HIGH |
| Ziaei 2007 | Adequate. Table of random numbers | Adequate. Coded bottles | Adequate. participants and care providers blinded. Outcome assessor unclear | Adequate. Less than 5% lost to follow up. | HIGH |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Low birthweight (less than 2500 g) (ALL) Show forest plot | 9 | 6275 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.61, 1.03] |
| 2 Low birthweight (less than 2500 g) (BY SUBGROUPS) Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 7 | 5771 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.61, 1.08] |
| 2.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 2 | 504 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.22, 1.38] |
| 2.5 Non‐anaemic at start of supplementation | 6 | 4452 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.46, 1.25] |
| 2.6 Unspecified/mixed anaemic status at start of supplementation | 3 | 1823 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.71, 0.94] |
| 2.7 Daily lower dose (60 mg elemental iron or less) | 7 | 3247 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.53, 1.18] |
| 2.8 Daily higher dose (more than 60 mg elemental iron) | 2 | 3028 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.45, 1.26] |
| 3 Birthweight (g) (ALL) Show forest plot | 10 | 5956 | Mean Difference (IV, Random, 95% CI) | 36.05 [‐4.84, 76.95] |
| 4 Birthweight (g) (BY SUBGROUPS) Show forest plot | 11 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 9 | 5822 | Mean Difference (IV, Random, 95% CI) | 30.89 [‐13.87, 75.65] |
| 4.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 2 | 251 | Mean Difference (IV, Random, 95% CI) | 39.72 [‐67.69, 147.12] |
| 4.5 Non‐anaemic at start of supplementation | 8 | 4496 | Mean Difference (IV, Random, 95% CI) | 29.32 [‐27.08, 85.71] |
| 4.6 Unspecified/mixed anaemic status at start of supplementation | 3 | 1577 | Mean Difference (IV, Random, 95% CI) | 52.33 [10.16, 94.51] |
| 4.7 Daily low dose (60 mg elemental iron or less) | 6 | 2804 | Mean Difference (IV, Random, 95% CI) | 37.22 [‐17.82, 92.27] |
| 4.8 Daily higher dose (more than 60 mg elemental iron) | 6 | 3382 | Mean Difference (IV, Random, 95% CI) | 17.99 [‐41.28, 77.26] |
| 5 Premature delivery (less than 37 weeks of gestation) (ALL) Show forest plot | 8 | 5730 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.67, 1.09] |
| 6 Premature delivery (less 37 weeks of gestation) (BY SUBGROUPS) Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 6 | 2989 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.66, 1.20] |
| 6.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.48] |
| 6.5 Non‐anaemic at start of supplementation | 6 | 1775 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.54, 1.12] |
| 6.6 Unspecified/mixed anaemic status at start of supplementation | 1 | 1261 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.85, 1.28] |
| 6.7 Daily lower dose (60 mg elemental iron or less) | 6 | 3023 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.68, 1.18] |
| 6.8 Daily higher dose (more than 60 mg elemental iron) | 2 | 2707 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.48, 1.06] |
| 7 Maternal Hb concentration at term (g/L) (ALL) Show forest plot | 17 | 2463 | Mean Difference (IV, Random, 95% CI) | 8.83 [6.55, 11.11] |
| 8 Maternal Hb concentration at term (g/L) (BY SUBGROUPS) Show forest plot | 17 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 13 | 2306 | Mean Difference (IV, Random, 95% CI) | 8.14 [5.61, 10.67] |
| 8.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 3 | 130 | Mean Difference (IV, Random, 95% CI) | 10.24 [2.45, 18.04] |
| 8.3 Unspecified/mixed gestational age at start of supplementation | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 14.0 [8.07, 19.93] |
| 8.5 Non‐anaemic at start of supplementation | 11 | 2002 | Mean Difference (IV, Random, 95% CI) | 7.77 [4.86, 10.69] |
| 8.6 Unspecified/mixed anaemic status at start of supplementation | 6 | 461 | Mean Difference (IV, Random, 95% CI) | 11.03 [7.51, 14.56] |
| 8.7 Daily low dose (60 mg elemental iron or less) | 8 | 1956 | Mean Difference (IV, Random, 95% CI) | 7.16 [4.14, 10.17] |
| 8.8 Daily higher dose (more than 60 mg elemental iron) | 9 | 507 | Mean Difference (IV, Random, 95% CI) | 10.94 [7.06, 14.82] |
| 9 Anaemia at term (Hb less than 110 g/L) (ALL) Show forest plot | 14 | 4390 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.17, 0.42] |
| 10 Haemoconcentration at term (Hb more than 130 g/L) (ALL) Show forest plot | 10 | 4643 | Risk Ratio (M‐H, Random, 95% CI) | 2.62 [1.21, 5.67] |
| 11 Haemoconcentration at term (Hb more than 130 g/L) (BY SUBGROUPS) Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 5 | 4173 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [0.72, 5.45] |
| 11.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 3 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 3.94 [0.31, 50.47] |
| 11.3 Unspecified/mixed gestational age at start of supplementation | 2 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 4.67 [2.53, 8.60] |
| 11.5 Non‐anaemic at start of supplementation | 5 | 4011 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [0.64, 4.66] |
| 11.6 Unspecified/mixed anaemic status at start of supplementation | 5 | 632 | Risk Ratio (M‐H, Random, 95% CI) | 4.03 [1.39, 11.72] |
| 11.7 Daily low dose (60 mg elemental iron or less) | 4 | 1317 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.54, 3.86] |
| 11.8 Daily higher dose (more than 60 mg elemental iron) | 6 | 3326 | Risk Ratio (M‐H, Random, 95% CI) | 3.55 [1.89, 6.66] |
| 12 Haemoconcentration during second or third trimester (ALL) Show forest plot | 10 | 4841 | Risk Ratio (M‐H, Random, 95% CI) | 2.27 [1.40, 3.70] |
| 13 Haemoconcentration during second or third trimester (BY SUBGROUPS) Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 7 | 4522 | Risk Ratio (M‐H, Random, 95% CI) | 2.62 [1.49, 4.60] |
| 13.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.72, 2.86] |
| 13.3 Unspecified/mixed gestational age at start of supplementation | 2 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [0.30, 12.29] |
| 13.5 Non‐anaemic at start of supplementation | 6 | 4088 | Risk Ratio (M‐H, Random, 95% CI) | 2.10 [1.13, 3.90] |
| 13.6 Unspecified/mixed anaemic status at start of supplementation | 4 | 753 | Risk Ratio (M‐H, Random, 95% CI) | 2.67 [0.99, 7.17] |
| 13.7 Daily low dose (60 mg elemental iron or less) | 5 | 1655 | Risk Ratio (M‐H, Random, 95% CI) | 2.35 [1.21, 4.57] |
| 13.8 Daily higher dose (more than 60 mg elemental iron) | 5 | 3186 | Risk Ratio (M‐H, Random, 95% CI) | 2.14 [1.05, 4.37] |
| 14 Iron deficiency at term (as defined by two or more indicators) (ALL) Show forest plot | 6 | 1108 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.27, 0.70] |
| 15 Iron deficiency at term (as defined by two or more indicators) (BY SUBGROUPS) Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 4 | 867 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.35, 0.90] |
| 15.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 2 | 241 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.17, 0.44] |
| 15.5 Non‐anaemic at start of supplementation | 4 | 944 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.41, 0.90] |
| 15.6 Unspecified/mixed anaemic status at start of supplementation | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.07, 0.29] |
| 15.7 Daily low dose (60 mg elemental iron or less) | 4 | 944 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.41, 0.90] |
| 15.8 Daily higher dose (more than 60 mg elemental iron) | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.07, 0.29] |
| 16 Iron deficiency anaemia at term (ALL) Show forest plot | 6 | 1667 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.16, 0.69] |
| 17 Iron deficiency anaemia at term (BY SUBGROUPS) Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 17.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 5 | 1622 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.18, 0.76] |
| 17.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.13] |
| 17.5 Non‐anaemic at start of supplementation | 5 | 1547 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.20, 0.74] |
| 17.6 Unspecified/mixed anaemic status at start of supplementation | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.04 [0.00, 0.72] |
| 17.7 Daily low dose (60 mg elemental iron or less) | 5 | 1547 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.20, 0.74] |
| 17.8 Daily higher dose (more than 60 mg elemental iron) | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.04 [0.00, 0.72] |
| 18 Side‐effects (Any) (ALL) Show forest plot | 8 | 3667 | Risk Ratio (M‐H, Random, 95% CI) | 3.92 [1.21, 12.64] |
| 19 Side‐effects (Any) (BY SUBGROUPS) Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 19.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 4 | 3034 | Risk Ratio (M‐H, Random, 95% CI) | 3.69 [0.42, 32.71] |
| 19.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 3 | 428 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.99, 3.08] |
| 19.3 Unspecified/mixed gestational age at start of supplementation | 1 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 62.79 [3.89, 1013.31] |
| 19.5 Non‐anaemic at start of supplementation | 4 | 2897 | Risk Ratio (M‐H, Random, 95% CI) | 3.27 [0.39, 27.31] |
| 19.6 Unspecified/mixed anaemic status at start of supplementation | 4 | 770 | Risk Ratio (M‐H, Random, 95% CI) | 3.94 [1.09, 14.28] |
| 19.7 Daily low dose (60 mg elemental iron or less) | 4 | 566 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.99, 1.87] |
| 19.8 Daily higher dose (more than 60 mg elemental iron) | 5 | 3148 | Risk Ratio (M‐H, Random, 95% CI) | 12.12 [1.76, 83.43] |
| 20 Very low birthweight (less than 1500 g) (ALL) Show forest plot | 5 | 2687 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.31, 1.74] |
| 21 Perinatal death (ALL) Show forest plot | 3 | 5036 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.67, 1.29] |
| 24 Infant Hb concentration at 3 months (g/L) (ALL) Show forest plot | 1 | 197 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐3.21, 3.21] |
| 25 Infant serum ferritin concentration at 3 months (ug/L) (ALL) Show forest plot | 1 | 197 | Mean Difference (IV, Random, 95% CI) | 19.0 [2.75, 35.25] |
| 26 Infant Hb concentration at 6 months (g/L) (ALL) Show forest plot | 2 | 533 | Mean Difference (IV, Random, 95% CI) | ‐1.25 [‐8.10, 5.59] |
| 27 Infant serum ferritin concentration at 6 months (ug/L) (ALL) Show forest plot | 1 | 197 | Mean Difference (IV, Random, 95% CI) | 11.0 [4.37, 17.63] |
| 29 Admission to special care unit (ALL) Show forest plot | 2 | 2805 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.73, 1.23] |
| 30 Very premature delivery (less than 34 weeks' gestation) (ALL) Show forest plot | 4 | 1417 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.16, 1.24] |
| 31 Severe anaemia at term (Hb less than 70 g/L) (ALL) Show forest plot | 8 | 1751 | Risk Ratio (M‐H, Random, 95% CI) | 4.83 [0.23, 99.88] |
| 32 Moderate anaemia at term (Hb more than 70 g/L and less than 90 g/L) (ALL) Show forest plot | 9 | 1868 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.55, 1.62] |
| 33 Severe anaemia at any time during second and third trimester (ALL) Show forest plot | 9 | 2089 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.01, 34.52] |
| 34 Moderate anaemia at any time during second or third trimester (ALL) Show forest plot | 10 | 2266 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.19, 0.92] |
| 35 Infection during pregnancy (including urinary tract infections) (ALL) Show forest plot | 2 | 3421 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.83, 1.63] |
| 36 Puerperal infection (ALL) Show forest plot | 2 | 2169 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.25, 2.10] |
| 37 Antepartum haemorraghe (ALL) Show forest plot | 2 | 1157 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.51, 4.31] |
| 38 Postpartum haemorraghe (ALL) Show forest plot | 5 | 1554 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.51, 1.78] |
| 39 Transfusion provided (ALL) Show forest plot | 3 | 3453 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.38, 0.96] |
| 40 Haemoglobin concentration within one month postpartum (ALL) Show forest plot | 6 | 904 | Mean Difference (IV, Random, 95% CI) | 7.08 [4.70, 9.47] |
| 41 Severe anaemia at postpartum (Hb less than 80 g/L) (ALL) Show forest plot | 7 | 1094 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.00, 1.05] |
| 42 Moderate anaemia at postpartum (Hb more than 80 g/L and less than 100 g/L) (ALL) Show forest plot | 4 | 831 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.12, 2.51] |
| 43 Diarrhoea (ALL) Show forest plot | 3 | 1088 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.32, 0.93] |
| 44 Constipation (ALL) Show forest plot | 4 | 1495 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.62, 1.43] |
| 45 Nausea (ALL) Show forest plot | 4 | 1377 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.72, 2.03] |
| 46 Heartburn (ALL) Show forest plot | 3 | 1323 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.86, 1.66] |
| 47 Vomiting (ALL) Show forest plot | 4 | 1392 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.59, 1.30] |
| 48 Maternal death (death while pregnant or within 42 days of termination of pregnancy) (ALL) Show forest plot | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 49 Maternal wellbeing/satisfaction (ALL) Show forest plot | 2 | 2604 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.91, 1.09] |
| 50 Placental abruption (ALL) Show forest plot | 2 | 2169 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.32, 4.06] |
| 51 Premature rupture of membranes (ALL) Show forest plot | 1 | 727 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.74, 1.34] |
| 52 Pre‐eclampsia (ALL) Show forest plot | 2 | 774 | Risk Ratio (M‐H, Random, 95% CI) | 2.58 [0.81, 8.22] |
| 91 Small for gestational age (less than 10th percentile weight at birth for gestational age) (not pre‐specified) Show forest plot | 4 | 2511 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.58, 1.30] |
| 93 Cesarean delivery (not pre‐specified) Show forest plot | 7 | 4283 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.78, 1.13] |
| 94 Birth length in cm (not pre‐specified) Show forest plot | 5 | 2140 | Mean Difference (IV, Random, 95% CI) | 0.38 [0.10, 0.65] |
| 95 Forceps or vacuum delivery (not pre‐specified) Show forest plot | 2 | 477 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.94, 2.40] |
| 96 Breastfeeding at least 4 months (not pre‐specified) Show forest plot | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.89, 1.13] |
| 97 Haemoglobin concentration at 4‐8 weeks' postpartum (g/L) (not pre‐specified) Show forest plot | 10 | 1188 | Mean Difference (IV, Random, 95% CI) | 5.13 [0.24, 10.02] |
| 98 Apgar score < 7 at 5 minutes (not pre‐specified) Show forest plot | 2 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.17, 3.28] |
| 99 Apgar Score at 5 min (not pre‐specified) Show forest plot | 2 | 228 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.07, 0.62] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3 Birthweight (ALL) Show forest plot | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐68.0 [‐398.33, 262.33] |
| 5 Premature delivery (less than 37 weeks of gestation) (ALL) Show forest plot | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.02, 8.96] |
| 12 Haemoconcentration during second or third trimester (Hb more than 130 g/L) (ALL) Show forest plot | 2 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.18, 1.58] |
| 33 Severe anaemia at any time during second and third trimester (Hb less than 70 g/L) (ALL) Show forest plot | 2 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34 Moderate anaemia at any time during second or third trimester (ALL) Show forest plot | 2 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 2.42 [0.16, 35.56] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Low birthweight (less than 2500 g) (ALL) Show forest plot | 2 | 1368 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.28, 4.02] |
| 2 Low birthweight (less than 2500 g) (BY SUBGROUPS) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 2 | 1368 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.28, 4.02] |
| 2.6 Unspecified/mixed anaemic status at start of supplementation | 2 | 1368 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.28, 4.02] |
| 2.7 Daily low dose (60 mg elemental iron or less) | 1 | 1320 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.69, 0.91] |
| 2.8 Daily higher dose (more than 60 mg elemental iron) | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.25, 98.96] |
| 3 Birthweight (ALL) Show forest plot | 2 | 1365 | Mean Difference (IV, Random, 95% CI) | 57.73 [7.66, 107.79] |
| 4 Birthweight (BY SUBGROUPS) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 2 | 1365 | Mean Difference (IV, Random, 95% CI) | 57.73 [7.66, 107.79] |
| 4.6 Unspecified/mixed anaemic status at start of supplementation | 2 | 1365 | Mean Difference (IV, Random, 95% CI) | 57.73 [7.66, 107.79] |
| 4.7 Daily low dose (60 mg elemental iron or less) | 1 | 1320 | Mean Difference (IV, Random, 95% CI) | 65.0 [17.46, 112.54] |
| 4.8 Daily higher dose (more than 60 mg elemental iron) | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐32.0 [‐213.62, 149.62] |
| 5 Premature delivery (less than 37 weeks of gestation) (ALL) Show forest plot | 3 | 1497 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [0.40, 6.00] |
| 6 Premature delivery (less than 37 weeks of gestation) (BY SUB GROUPS) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 2 | 1362 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.92, 1.39] |
| 6.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.6 Unspecified/mixed anaemic status at start of supplementation | 2 | 1449 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.92, 1.39] |
| 6.7 Daily low dose (60 mg elemental iron or less) | 2 | 1449 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.92, 1.39] |
| 7 Haemoglobin concentration at term (ALL) Show forest plot | 4 | 179 | Mean Difference (IV, Random, 95% CI) | 12.00 [2.93, 21.07] |
| 8 Haemoglobin concentration at term (BY SUBGROUPS) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 2 | 93 | Mean Difference (IV, Random, 95% CI) | 15.65 [11.84, 19.46] |
| 8.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 2 | 86 | Mean Difference (IV, Random, 95% CI) | 7.92 [‐11.68, 27.52] |
| 8.5 Non‐anaemic at start of supplementation | 1 | 48 | Mean Difference (IV, Random, 95% CI) | 17.10 [8.44, 25.76] |
| 8.6 Unspecified/mixed anaemic status at start of supplementation | 3 | 131 | Mean Difference (IV, Random, 95% CI) | 10.47 [‐1.07, 22.00] |
| 8.7 Daily low dose (60 mg elemental iron or less) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.8 Daily higher dose (more than 60 mg elemental iron) | 4 | 179 | Mean Difference (IV, Random, 95% CI) | 12.00 [2.93, 21.07] |
| 9 Anaemia at term (Hb less than 110 g/L) (ALL) Show forest plot | 3 | 346 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.12, 0.56] |
| 10 Haemoconcentration at term (Hb more than 130 g/L) (ALL) Show forest plot | 3 | 353 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [0.34, 8.94] |
| 11 Haemoconcentration at term (Hb more than 130 g/L) (BY SUBGROUPS) Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 3.37 [0.19, 60.03] |
| 11.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 3 | 298 | Risk Ratio (M‐H, Random, 95% CI) | 1.97 [0.32, 12.08] |
| 11.6 Unspecified/mixed anaemic status at start of supplementation | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 4.31 [0.26, 70.41] |
| 11.7 Daily low dose (60 mg elemental iron or less) | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 4.31 [0.26, 70.41] |
| 11.8 Daily higher dose (more than 60 mg elemental iron) | 2 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.24, 6.78] |
| 12 Haemoconcentration during second or third trimester (ALL) Show forest plot | 2 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.63, 5.04] |
| 13 Haemoconcentration during second or third trimester (BY SUBGROUPS) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 2 | 390 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.33, 6.32] |
| 13.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.50, 3.56] |
| 13.3 Unspecified/mixed gestational age at start of supplementation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.5 Non‐anaemic at start of supplementation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.6 Unspecified/mixed anaemic status at start of supplementation | 2 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.63, 5.04] |
| 13.7 Daily low dose (60 mg elemental iron or less) | 2 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.63, 5.04] |
| 13.8 Daily higher dose (more than 60 mg elemental iron) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Iron deficiency at term (as defined by two or more indicators) (ALL) Show forest plot | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.06, 0.99] |
| 15 Iron deficiency at term (as defined by two or more indicators) (BY SUBGROUPS) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.01, 1.10] |
| 15.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.08, 1.63] |
| 15.5 Non‐anaemic at start of supplementation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.6 Unspecified/mixed anaemic status at start of supplementation | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.06, 0.99] |
| 15.7 Daily low dose (60 mg elemental iron or less) | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.06, 0.99] |
| 15.8 Daily higher dose (more than 60 mg elemental iron) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Iron deficiency anaemia at term (ALL) Show forest plot | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.17, 1.09] |
| 17 Iron deficiency anaemia at term (BY SUBGROUPS) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 17.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.12, 1.12] |
| 17.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.18, 1.40] |
| 17.5 Non‐anaemic at start of supplementation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.6 Unspecified/mixed anaemic status at start of supplementation | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.17, 1.09] |
| 17.7 Daily low dose (60 mg elemental iron or less) | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.17, 1.09] |
| 17.8 Daily higher dose (more than 60 mg elemental iron) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Side effects (Any) (ALL) Show forest plot | 1 | 456 | Risk Ratio (M‐H, Random, 95% CI) | 44.32 [2.77, 709.09] |
| 20 Very low birthweight (less than 1500 g) (ALL) Show forest plot | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.25, 98.96] |
| 21 Perinatal death (ALL) Show forest plot | 3 | 1862 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.58, 1.17] |
| 29 Admission to special care unit (ALL) Show forest plot | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30 Very premature delivery (less than 34 weeks' gestation) (ALL) Show forest plot | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.25, 98.96] |
| 31 Severe anaemia at term (Hb less than 70 g/L) (ALL) Show forest plot | 3 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32 Moderate anaemia at term (Hb more than 70g/L and less than 90 g/L) (ALL) Show forest plot | 3 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33 Severe anaemia at any time during second and third trimester (Hb less than 70 g/L) (ALL) Show forest plot | 4 | 523 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 0.83] |
| 34 Moderate anaemia at any time during second or third trimester (ALL) Show forest plot | 4 | 523 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.11, 1.04] |
| 35 Infection during pregnancy (including urinary tract infections) (ALL) Show forest plot | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.15, 6.53] |
| 36 Puerperal infection (ALL) Show forest plot | 1 | 2863 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.13, 2.28] |
| 37 Antepartum haemorrhage (ALL) Show forest plot | 2 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.22, 7.12] |
| 38 Postpartum haemorrhage (ALL) Show forest plot | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.00, 2.71] |
| 40 Haemoglobin concentration within one month postpartum (ALL) Show forest plot | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 10.40 [4.03, 16.77] |
| 41 Severe anaemia at postpartum (Hb less than 80 g/L) (ALL) Show forest plot | 3 | 525 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.00, 0.76] |
| 42 Moderate anaemia at postpartum (Hb more than 80 g/L and less than 100 g/L) (ALL) Show forest plot | 3 | 525 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.17, 0.69] |
| 48 Maternal death (death while pregnant or within 42 days of termination of pregnancy) (ALL) Show forest plot | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 50 Placental abruption (ALL) Show forest plot | 1 | 2863 | Risk Ratio (M‐H, Random, 95% CI) | 8.19 [0.49, 138.16] |
| 52 Pre‐eclampsia (ALL) Show forest plot | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 70.16] |
| 91 Small for gestational age (less than 10th percentile weight at birth for gestational age) (not pre‐specified) Show forest plot | 1 | 1318 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.80, 0.97] |
| 92 Oedema during pregnancy (not pre‐specified) Show forest plot | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 2.82 [0.99, 8.09] |
| 93 Caesarean delivery (not pre‐specified) Show forest plot | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.22, 3.13] |
| 94 Birth length in cm (not pre‐specified) Show forest plot | 1 | 1320 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.06, 0.46] |
| 97 Haemoglobin concentration at 4‐8 weeks postpartum (not prespecified) Show forest plot | 3 | 526 | Mean Difference (IV, Random, 95% CI) | 4.88 [‐0.85, 10.62] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Low birthweight (less than 2500 g) (ALL) Show forest plot | 4 | 730 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.58, 1.91] |
| 2 Low birthweight (less than 2500 g) (BY SUBGROUPS) Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 2 | 455 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.55, 3.01] |
| 2.3 Unspecified/mixed gestational age at start of supplementation | 2 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.36, 1.99] |
| 2.5 Non‐anaemic at start of supplementation | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.36, 4.32] |
| 2.7 Daily low dose (60 mg elemental iron or less) | 3 | 650 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.50, 1.97] |
| 2.8 Daily higher dose (more than 60 mg elemental iron) | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.36, 4.32] |
| 3 Birthweight (ALL) Show forest plot | 4 | 730 | Mean Difference (IV, Random, 95% CI) | ‐7.10 [‐67.20, 53.01] |
| 4 Birthweight (BY SUBGROUPS) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 2 | 455 | Mean Difference (IV, Random, 95% CI) | 1.19 [‐70.50, 72.87] |
| 4.3 Unspecified/mixed gestational age at start of supplementation | 2 | 275 | Mean Difference (IV, Random, 95% CI) | ‐26.71 [‐137.00, 83.58] |
| 4.5 Non‐anaemic at start of supplementation | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐154.95, 154.95] |
| 4.7 Daily low dose (60 mg elemental iron or less) | 3 | 650 | Mean Difference (IV, Random, 95% CI) | ‐8.36 [‐73.56, 56.85] |
| 4.8 Daily higher dose (more than 60 mg elemental iron) | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐154.95, 154.95] |
| 5 Premature delivery (less than 37 weeks of gestation) (ALL) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.39, 10.31] |
| 7 Haemoglobin concentration at term (ALL) Show forest plot | 3 | 475 | Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐4.74, 3.08] |
| 8 Haemoglobin concentration at term (BY SUBGROUPS) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.3 Unspecified/mixed gestational age at start of supplementation | 2 | 301 | Mean Difference (IV, Random, 95% CI) | ‐2.19 [‐6.85, 2.47] |
| 8.7 Daily low dose (60 mg elemental iron or less) | 3 | 422 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐5.15, 4.95] |
| 8.8 Daily higher dose (more than 60 mg elemental iron) | 1 | 109 | Mean Difference (IV, Random, 95% CI) | ‐0.82 [‐4.99, 3.35] |
| 9 Anaemia at term (Hb < 110 g/L) (ALL) Show forest plot | 3 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.78, 1.83] |
| 10 Haemoconcentration at term (Hb more than 130 g/L) (ALL) Show forest plot | 3 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.47, 1.82] |
| 11 Haemoconcentration at term (Hb more than 130 g/L) (BY SUBGROUPS) Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.7 Daily low dose (60 mg elemental iron or less) | 3 | 422 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.42, 3.66] |
| 11.8 Daily higher dose (more than 60 mg elemental iron) | 1 | 109 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.19, 2.11] |
| 12 Haemoconcentration during second or third trimester (Hb more than 130 g/L) (ALL) Show forest plot | 6 | 1111 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.24, 0.77] |
| 13 Haemoconcentration during second or third trimester (Hb more than 130 g/L) (BY SUBGROUPS) Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 Early gestational age (less than 20 weeks of gestation or pre‐pregnancy) at start of supplementation | 2 | 250 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.26, 1.01] |
| 13.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 1 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.10, 0.55] |
| 13.3 Unspecified/mixed gestational age at start of supplementation | 3 | 695 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.13, 1.65] |
| 13.4 Anaemic at start of supplementation (Hb <110 g/L if in first or <105 g/L if in second trimester) | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.5 Non‐anaemic at start of supplementation | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.6 [0.15, 2.34] |
| 13.6 Unspecified/mixed anaemic status at start of supplementation | 4 | 966 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.21, 0.80] |
| 13.7 Daily low dose (60 mg elemental iron or less) | 5 | 953 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.20, 0.82] |
| 13.8 Daily higher dose (more than 60 mg elemental iron) | 2 | 247 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.42, 1.31] |
| 16 Iron deficiency anaemia at term (based on two or more indicators) (ALL) Show forest plot | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.08, 6.63] |
| 18 Side effects (any) (ALL) Show forest plot | 7 | 1307 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.45, 1.04] |
| 19 Side effects (any) (BY SUBGROUPS) Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 19.1 Early gestational age (less than 20 weeks or pre‐pregnancy) at start of supplementation | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.08, 0.53] |
| 19.2 Late gestational age (20 weeks or more of gestation) at start of supplementation | 1 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.79, 1.27] |
| 19.3 Unspecified/mixed gestational age at start of supplementation | 5 | 1055 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.42, 1.26] |
| 19.4 Non‐anaemic at start of supplementation | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.08, 0.53] |
| 19.7 Daily low dose (60 mg elemental iron or less) | 6 | 1171 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.71, 1.19] |
| 19.8 Daily higher dose (more than 60 mg elemental iron) | 2 | 253 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.07, 0.25] |
| 20 Very low birthweight (less than 1500 g) (ALL) Show forest plot | 4 | 737 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Perinatal death (ALL) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Infant ferritin concentration at 6 months (ug/L) (ALL) Show forest plot | 1 | 88 | Mean Difference (IV, Random, 95% CI) | 0.09 [0.05, 0.13] |
| 30 Very premature delivery (less than 34 weeks of gestation) (ALL) Show forest plot | 1 | 111 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.06, 15.31] |
| 31 Severe anaemia at term (Hb less than 70 g/L) (ALL) Show forest plot | 4 | 555 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32 Moderate anaemia at term (Hb more than 70g/L and less than 90 g/L) (ALL) Show forest plot | 3 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.07, 16.23] |
| 33 Severe anaemia at any time during second and third trimester (Hb less than 70 g/L) (ALL) Show forest plot | 6 | 1240 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34 Moderate anaemia at any time during second or third trimester (ALL) Show forest plot | 6 | 1111 | Risk Ratio (M‐H, Random, 95% CI) | 2.54 [0.63, 10.17] |
| 37 Antepartum haemorraghe (ALL) Show forest plot | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 15.59] |
| 41 Severe anaemia at postpartum (Hb less than 80 g/L) (ALL) Show forest plot | 1 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.04, 4.64] |
| 42 Moderate anaemia at postpartum (Hb more than 80 g/L and less than 100 g/L) (ALL) Show forest plot | 1 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.26, 4.95] |
| 43 Diarrhoea (ALL) Show forest plot | 4 | 553 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.38, 2.34] |
| 44 Constipation (ALL) Show forest plot | 4 | 553 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.48, 2.06] |
| 45 Nausea (ALL) Show forest plot | 5 | 854 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.30, 1.16] |
| 46 Heartburn (ALL) Show forest plot | 3 | 473 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.29, 2.06] |
| 47 Vomiting (ALL) Show forest plot | 5 | 854 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.82, 2.53] |
| 50 Placental abruption (ALL) Show forest plot | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.01] |
| 51 Premature rupture of membranes (ALL) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.95] |
| 68 Ln (serum ferritin concentration) 4‐8 wk postpartum (not pre‐specified) Show forest plot | 1 | 160 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.42, 0.16] |
| 70 Low serum ferritin concentration at postpartum (4‐8 wk) (not pre‐specified) Show forest plot | 1 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.40, 3.57] |
| 71 High serum transferrin receptors at 6 weeks postpartum (not pre‐specified) Show forest plot | 1 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.36, 1.33] |
| 93 Caesarean delivery (not pre‐specified) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.38, 2.07] |
| 97 Haemoglobin concentration at 4‐8 weeks postpartum (not pre‐specified) Show forest plot | 1 | 146 | Mean Difference (IV, Random, 95% CI) | 2.0 [‐3.86, 7.86] |

