Abdominal surgical incisions for caesarean section
Abstract
Background
Caesarean section is the commonest major operation performed on women worldwide. Operative techniques, including abdominal incisions, vary. Some of these techniques have been evaluated through randomised trials.
Objectives
To determine the benefits and risks of alternative methods of abdominal surgical incisions for caesarean section.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (28 February 2013).
Selection criteria
Randomised controlled trials of intention to perform caesarean section using different abdominal incisions.
Data collection and analysis
We extracted data from the sources, checked them for accuracy and analysed the data.
Main results
Four studies (666 women) were included in this review.
Two studies (411 women) compared the Joel‐Cohen incision with the Pfannenstiel incision. Overall, there was a 65% reduction in reported postoperative febrile morbidity (risk ratio (RR) 0.35, 95% confidence interval (CI) 0.14 to 0.87) with the Joel‐Cohen incision. One of the trials reported reduced postoperative analgesic requirements (RR 0.55, 95% CI 0.40 to 0.76); operating time (mean difference (MD) ‐11.40, 95% CI ‐16.55 to ‐6.25 minutes); delivery time (MD ‐1.90, 95% CI ‐2.53 to ‐1.27 minutes); total dose of analgesia in the first 24 hours (MD ‐0.89, 95% CI ‐1.19 to ‐0.59); estimated blood loss (MD ‐58.00, 95% CI ‐108.51 to ‐7.49 mL); postoperative hospital stay for the mother (MD ‐1.50, 95% CI ‐2.16 to ‐0.84 days); and increased time to the first dose of analgesia (MD 0.80, 95% CI 0.12 to 1.48 hours) compared with the Pfannenstiel group. No other significant differences were found in either trial.
Two studies compared muscle cutting incisions with Pfannenstiel incision. One study (68 women) comparing Mouchel incision with Pfannenstiel incision did not contribute data to this review. The other study (97 women) comparing the Maylard muscle‐cutting incision with the Pfannenstiel incision, reported no difference in febrile morbidity (RR 1.26, 95% CI 0.08 to 19.50); need for blood transfusion (RR 0.42, 95% CI 0.02 to 9.98); wound infection (RR 1.26, 95% CI 0.27 to 5.91); physical tests on muscle strength at three months postoperative and postoperative hospital stay (MD 0.40 days, 95% CI ‐0.34 to 1.14).
Authors' conclusions
The Joel‐Cohen incision has advantages compared with the Pfannenstiel incision. These are: less fever, pain and analgesic requirements; less blood loss; shorter duration of surgery and hospital stay. These advantages for the mother could be extrapolated to savings for the health system. However, these trials do not provide information on severe or long‐term morbidity and mortality.
PICO
Plain language summary
Abdominal surgical incisions for caesarean section
In a caesarean section operation, there are various types of incisions in the abdominal wall that can be used. These include vertical and transverse incisions, and there are variations in the specific ways the incisions can be undertaken. The review of studies identified four trials involving 666 women. The Joel‐Cohen incision showed better outcomes than the Pfannenstiel incision in terms of less fever for women, less postoperative pain, less blood loss, shorter duration of surgery and shorter hospital stay. However, the trials did not assess possible long‐term problems associated with different surgical techniques.
Authors' conclusions
Background
Caesarean section is the commonest major operation performed on women worldwide. Operative techniques used for caesarean section vary and some of these techniques have been evaluated through randomised trials.
Various abdominal incisions have been used for caesarean delivery. These include vertical (midline and paramedian) incisions and transverse incisions (Pfannenstiel, Maylard, Cherney, Joel‐Cohen). The type of incision used may depend on many factors including the clinical situation and the preferences of the operator.
Traditionally, vertical incisions were used for caesarean delivery (Myerscough 1982). Here the skin is incised in the midline between the umbilicus and the pubic symphysis. The rectus sheath and the peritoneum are incised in the midline. This area is the least vascular. Vertical subumbilical midline incisions have the presumed advantage of speed of abdominal entry and less bleeding. A vertical midline incision may be extended upwards if more space is required for access. Moreover, this incision may be used if a caesarean delivery is planned under local anaesthesia (WHO 2000). The disadvantages of a vertical midline incision include the greater risk of postoperative wound dehiscence and development of incisional hernia. The scar is cosmetically less pleasing. In the paramedian incision, the skin incision is made to one side of the midline (usually right). The anterior rectus sheath is opened under the skin incision. The belly of the underlying rectus abdominus muscle is then retracted laterally and the posterior rectus sheath and peritoneum are opened. Because of a shutter‐like effect, the stress on the scar is presumed to be less. The paramedian incision is reportedly stronger (Kendall 1991) than the midline scar but has no cosmetic advantage.
The lower abdominal transverse incision is adequate for the vast majority of caesarean operations. It has the advantages of cosmetic approval and minimal risk of postoperative disruption. The risks of incisional hernia are less than those following vertical incisions. However, transverse abdominal incisions usually involve more dissection and may require more surgical skills. Blood loss following dissection may be more. Also, this incision may be difficult to make under local anaesthesia, though successful techniques have been described (Sreenivasan 2006). Transverse incisions are difficult to extend if increased access is required.
The traditional lower abdominal incision for caesarean delivery is the incision described in 1900 by Pfannenstiel. Classically, this incision is located two fingers‐breadth above the pubic symphysis. Here the skin may be entered via a low transverse incision that curves gently upward, placed in a natural fold of skin (the 'smile' incision). After the skin is entered, the incision is rapidly carried through subcutaneous tissue to the fascia, which is then nicked on either side of the midline. The subcutaneous tissue is incised sharply with a scalpel. Once the fascia is exposed, it is incised transversely with heavy curved Mayo scissors. In the standard technique, the upper and then the lower fascial edges are next grasped with a heavy toothed clamp, such as a Kocher, and elevated. Under continuous tension, the fascia is then separated from the underlying muscles by blunt and sharp dissection. Once the upper and lower fascia have been dissected free, and any perforating vessel sutured or electrocoagulated, the underlying rectus abdominus muscles are separated with finger dissection. If the muscles are adherent, sharp dissection is necessary to separate them. The peritoneum is then opened sharply in the midline. The initial entry is then widened sharply with fine scissors exposing intraperitoneal contents.
When exposure is limited and additional space is required, the Maylard or Cherney modification may be used. In the Maylard procedure, the rectus abdominus muscles are divided either sharply or by electrocautery to allow greater access to the abdomen. However, this may result in a good deal of tissue damage and the underlying artery may be entered (O'Grady 1995). The Maylard incision length is usually longer than the Pfannenstiel incision. However, difficulty in delivery of the fetus is minimal with Pfannenstiel incisions measuring at least 15 cm in length (Ayers 1987), the length of a standard Allis clamp ‐ the Allis clamp test (Finan 1991). Shorter incisions may lead to difficulty in general exposure or delivery of the baby's head, or both.
In the Cherney procedure, the lower fascia is reflected exposing the tendinous attachment of the rectus abdominus muscle bodies to the fascia of the pubis (O'Grady 1995). The muscle is severed as low as possible and the proximal and distal ends suture ligated. One or both muscle attachments may be divided as required.
The Mouchel incision is similar to the Maylard incision. This transverse incision runs at the upper limit of the pubic hair and is thus lower than the Maylard incision. The muscles are divided above the openings of the inguinal canals (Mouchel 1981).
In the Pelosi technique (Wood 1999) for caesarean delivery, the skin is cut in a low transverse fashion with a knife. The subcutaneous tissues and fascia are incised with electrocautery. The upper aspect of the fascial incision is elevated and the median raphe (line or ridge) is dissected cephalad (towards the head) 2 cm to 3 cm using electrocautery. The rectus muscles are separated bluntly with fingers to identify the underlying peritoneum, which is then entered by inserting the index finger inwards and upwards or sharply as required. The peritoneum and muscles are stretched to the full extent of the skin. In this technique, no bladder flap is created before incision of the uterus (hysterotomy). After delivery of the baby, the obstetrician awaits spontaneous placental expulsion before closing the hysterotomy in one layer. The fascia is closed and the skin edges are approximated with staples. The Pelosi technique was reported to be associated with decreased operative time, decreased blood loss, improved patient outcome and decreased overall cost (Wood 1999).
Joel‐Cohen (Joel‐Cohen 1977) described a transverse skin incision, which was subsequently adapted for caesarean sections. This modified incision is placed about 3 cm below the line joining the anterior superior iliac spines. This incision is higher than the traditional Pfannenstiel incision. Sharp dissection is minimised. After the skin is cut, the subcutaneous tissue and the anterior rectus sheath are opened a few centimetres only in the midline. The rectus sheath incision may be extended laterally by blunt finger dissection (Wallin 1999) or by pushing laterally with slightly opened scissor tips, deep to the subcutaneous tissues (Holmgren 1999). The rectus muscles are separated by finger traction. If exceptional speed is required in the transverse entry, the fascia may be incised in the midline and both the fascia and subcutaneous tissue are rapidly divided by blunt finger dissection (Joel‐Cohen 1977). Stark used this incision for caesarean delivery along with single layer closure of the exteriorised uterus and non‐closure of the peritoneum. This package of surgical techniques for caesarean section used at the Misgav‐Ladach hospital, Jerusalem, has been popularised by Stark and others (Holmgren 1999). The reported advantages include shorter operating time (Darj 1999; Franchi 1998; Mathai 2002; Wallin 1999), less use of suture material (Bjorklund 2000), less intraoperative blood loss (Bjorklund 2000; Darj 1999; Wallin 1999), less postoperative pain (Darj 1999; Mathai 2002) and less wound infection (Franchi 1998) in the group undergoing caesarean by these techniques.
There are other Cochrane reviews on surgical techniques used at caesarean section, for example, techniques of repair of the uterine incision (Jokhan‐Jacob 2004), techniques for closure of the abdominal wall (Anderson 2004) and skin (Alderdice 2003) after caesarean section. This review focuses specifically on abdominal surgical incisions for caesarean section.
Objectives
To determine, from the best available evidence, the benefits and risks of alternative methods of abdominal surgical incisions for caesarean section.
Methods
Criteria for considering studies for this review
Types of studies
All comparisons of intention to perform caesarean section using different abdominal incisions. Quasi‐randomised and cross‐over trials were not included.
Types of participants
Pregnant women due for delivery by caesarean section.
Types of interventions
Abdominal incisions for caesarean section performed according to a prespecified technique.
Types of outcome measures
Primary outcomes
-
Postoperative febrile morbidity as defined by trial authors;
-
postoperative analgesia as defined by trial authors;
-
blood loss as defined by the trial authors;
-
blood transfusion.
Secondary outcomes
For the mother
-
Duration of surgery;
-
operative complications;
-
postoperative complications;
-
postoperative haemoglobin level;
-
postoperative anaemia, as defined by trial authors;
-
postoperative pyrexia;
-
postoperative infection requiring additional antibiotic therapy;
-
wound complications (haematoma, infection, breakdown);
-
time to mobilisation;
-
time to oral intake;
-
time to return of bowel function;
-
time to breastfeeding initiation;
-
voiding problems;
-
length of postoperative hospital stay;
-
unsuccessful breastfeeding, as defined by trial authors;
-
mother not satisfied;
-
appearance of scar.
For the baby
-
Time from anaesthesia to delivery;
-
Apgar score;
-
cord blood pH less than 7.20;
-
birth trauma;
-
admission to special care baby unit;
-
encephalopathy.
Other
-
Caregiver not satisfied;
-
cost.
Outcomes were included if these were clinically meaningful; reasonable measures had been taken to minimise observer bias; missing data were insufficient to materially influence conclusions; data were available for analysis according to original allocation, irrespective of protocol violations; data were available in a format suitable for analysis.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register by contacting the Trials Search Co‐ordinator (28 February 2013).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE;
-
weekly searches of EMBASE;
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
Data collection and analysis
For methods used to assess trials included in previous versions of this review, see Appendix 1.
The following methods were used to assess CORONIS 2007 (ongoing); Mahawerawat 2010; Oguz 1998 (excluded) and will be used in future revisions of the review.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, consulted the third author.
Data extraction and management
For this update of the review we did not identify any additional trials for inclusion. For future updates of this review, we will design a form to extract data. For eligible studies, at least two review authors will extract the data using the agreed form. We will resolve discrepancies through discussion or, if required, we will consult the third author. We will enter data into Review Manager software (RevMan 2011) and check for accuracy.
When information regarding any of the above is unclear, we will attempt to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
For this version of the review, two review authors independently assessed the risk of bias for each previously included study using the revised criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third author.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
-
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel;
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
-
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included the missing data in the analyses which we undertook.
We assessed methods as:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
-
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
-
low risk of bias (where it was clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review had been reported);
-
high risk of bias (where not all the study’s pre‐specified outcomes had been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study fails to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
-
low risk of other bias;
-
high risk of other bias;
-
unclear whether there was risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference as outcomes were measured in the same way between trials. If in future updates we identify trials that measure the same outcome, but use different methods, we will use the standardised mean difference to combine trials.
Unit of analysis issues
Cluster‐randomised trials
If identified, we will include cluster‐randomised trials in the analyses along with individually‐randomised trials. We will adjust their sample sizes using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
This study design is not eligible for inclusion in this review.
Dealing with missing data
For included studies, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T2, I² and Chi² statistics. We regarded heterogeneity as substantial if an I2 was greater than 30% and either the T2 was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates of this review if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2011). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If in future updates of this review, there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary will be treated as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials.
If we use random‐effects analyses, the results will be presented as the average treatment effect with 95% confidence intervals, and the estimates of T2 and I2.
Subgroup analysis and investigation of heterogeneity
We did not carry out any subgroup analyses. If we identify substantial heterogeneity in future versions of this review, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it.
We plan to carry out the following subgroup analyses:
-
primary, repeat and mixed or undefined caesarean sections;
-
general, regional and mixed or undefined anaesthesia;
-
elective, emergency and mixed or undefined caesarean sections.
The following outcomes will be used in subgroup analyses:
-
postoperative febrile morbidity as defined by trial authors;
-
postoperative analgesia as defined by trial authors;
-
blood loss as defined by the trial authors;
-
blood transfusion.
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2011). We will report the results of subgroup analyses quoting the χ2 statistic and P value, and the interaction test I² value.
Sensitivity analysis
In the event of significant heterogeneity, we will perform sensitivity analysis excluding trials with greater risk of bias to determine the effect on the results. Studies with high or unclear risk of bias for selection and/or attrition bias will be considered at high risk of bias and excluded in sensitivity analyses.
Results
Description of studies
Results of the search
Twenty‐nine reports were identified based on the search strategies. Four trials were included (Berthet 1989; Franchi 2002; Giacalone 2002; Mathai 2002) and 24 excluded (Ansaloni 2001; Ayers 1987; Ayres‐de‐Campos 2000; Behrens 1997; Bjorklund 2000; Dani 1998; Darj 1999; Decavalas 1997; Direnzo 2001; Falls 1958; Ferrari 2001; Franchi 1998; Gaucherand 2001; Hagen 1999; Heimann 2000; Hohlagschwandtner; Mahawerawat 2010; Meyer 1997; Meyer 1998; Moreira 2002; Oguz 1998; Redlich 2001; Wallin 1999; Xavier 1999). One study is a trial protocol of an ongoing study (CORONIS 2007).
Included studies
Only two studies (Franchi 2002; Mathai 2002) compared Joel‐Cohen incision with Pfannenstiel incision for laparotomic access. Two studies compared transverse muscle‐cutting incisions ‐ Mouchel (Berthet 1989) and Maylard (Giacalone 2002) ‐ with the Pfannenstiel incision and were included in the review. Thus a total of four studies involving 666 women comparing only different abdominal incisions for caesarean delivery were included in the review. Details of these studies are available in the Characteristics of included studies table.
Excluded studies
Four trials were excluded from the analyses as allocation to intervention groups were not based on randomisation in these trials (Ansaloni 2001; Ayers 1987; Gaucherand 2001; Redlich 2001). Two reports identified in the updated search were also excluded: Mahawerawat 2010 and Oguz 1998 did not compare different abdominal incisions.
Twelve studies compared various abdominal incisions either alone or in combinations with other steps carried out during caesarean delivery. Six studies( Dani 1998; Darj 1999; Ferrari 2001; Franchi 1998; Heimann 2000; Wallin 1999), which compared Joel‐Cohen incision as part of the Misgav‐Ladach technique with Pfannenstiel incision had differences in other steps between the two arms, such as closure of the uterotomy and peritoneum. These six studies were therefore excluded from the review, as were two studies (Bjorklund 2000; Moreira 2002) that compared the Misgav‐Ladach technique with the vertical incision.
For details of all excluded studies, see the Characteristics of excluded studies table.
Risk of bias in included studies
The methodological quality of the included studies was variable.
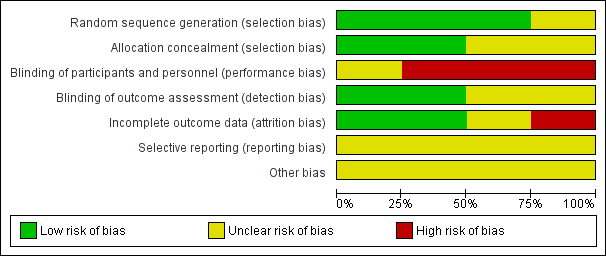
SeeFigure 1 and Figure 2 for a summary of 'Risk of bias' assessments for included studies.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Method of randomisation was unclear in one trial (Berthet 1989) and allocation concealment unclear in two studies (Berthet 1989; Franchi 2002). Other studies were at low risk of selection bias.
Blinding
Given the type of intervention, the surgical team was aware of the allocated intervention. Assessment of some intraoperative variables, for example, time taken for surgery and estimated blood loss, may have been subject to bias. However, outcomes assessed in the immediate postoperative period, for example, febrile morbidity, pain, analgesic requirements, were less subject to bias.
Incomplete outcome data
Data were available for only 81% of women randomised in one trial (Giacalone 2002). The few post‐randomisation exclusions in the other studies were for acceptable clinical reasons.
Selective reporting
There was insufficient information to assess selective reporting.
Other potential sources of bias
There was insufficient information to assess other potential sources of bias.
Effects of interventions
(1) Joel‐Cohen incision versus Pfannenstiel incision
Two studies (Franchi 2002; Mathai 2002) compared the Joel‐Cohen incision with Pfannenstiel incision. All other aspects of surgery in these two trials were similar in the two arms. Both trials (411 women) assessed postoperative febrile morbidity. Overall, there was a 65% reduction in reported postoperative febrile morbidity (risk ratio (RR) 0.35, 95% confidence interval (CI) 0.14 to 0.87) in the Joel‐Cohen group, Analysis 1.1. There was no significant heterogeneity among the trials.
Other outcomes were reported only in Mathai 2002 (101 women). Postoperative analgesic requirements were less in the Joel‐Cohen group (RR 0.55, 95% CI 0.40 to 0.76), Analysis 1.2; operating time was reduced (mean difference (MD) ‐11.40, 95% CI ‐16.55 to ‐6.25 minutes), Analysis 1.17; delivery time was reduced (MD ‐1.90, 95% CI ‐2.53 to ‐1.27 minutes), Analysis 1.21; the time to the first dose of analgesia was increased (MD 0.80, 95% CI 0.12 to 1.48 hours), Analysis 1.3; the total dose of analgesia in the first 24 hours was reduced (MD ‐0.89, 95% CI ‐1.19 to ‐0.59), Analysis 1.4; estimated blood loss was reduced (MD ‐58.00, 95% CI ‐108.51 to ‐ 7.49 mL), Analysis 1.8; and postoperative hospital stay for the mother was reduced (MD ‐1.50; 95% CI ‐2.16 to ‐0.84 days), Analysis 1.25, compared with the Pfannenstiel group. All women in this study had had surgery under spinal analgesia. No other significant differences were found in either trial.
Women having Joel‐Cohen incisions initiated breastfeeding earlier than those having Pfannenstiel incisions but this difference was not statistically significant (MD ‐5.50, 95% CI ‐13.62 to 2.62 hours), Analysis 1.16. None of the studies reported on postoperative voiding difficulties. There was no difference in the duration of infant's stay in special care baby unit in the one study (101 participants) (Mathai 2002) that reported on this outcome (MD ‐0.46; 95% CI ‐0.95 to 0.03 days), Analysis 1.26.
(2) Joel‐Cohen incision versus vertical incision
No studies directly compared these incisions.
(3) Muscle cutting incision versus Pfannenstiel incision
Two studies compared muscle cutting incisions with Pfannenstiel incision. None of the outcomes of interest for this review were reported by Berthet 1989 comparing Mouchel incision with Pfannenstiel incision. Giacalone 2002 (97 women) compared Maylard incision with Pfannenstiel incision and reported no difference in febrile morbidity (RR 1.26, 95% CI 0.08 to 19.50), Analysis 2.1, need for blood transfusion (RR 0.42, 95% CI 0.02 to 9.98), Analysis 2.2, or wound infection (RR 1.26, 95% CI 0.27 to 5.91), Analysis 2.3, between the two groups. There was no difference in physical tests on muscle strength (Janda's test, Kumar 1995) three months postoperatively between the two incisions (54 women; MD 0.10, 95% CI ‐0.73 to 0.93), Analysis 2.4. No difference was observed in postoperative hospital stay between Maylard muscle‐cutting incision and Pfannenstiel incision (MD 0.40 days, 95% CI ‐0.34 to 1.14), Analysis 2.5.
None of the studies reported on the need for readmission to the hospital for mother or baby. Maternal death, severe disability and thromboembolism were not reported by any of the included trials. There were no reports comparing other long‐term wound problems such as incisional hernia, hypertrophic scar, future fertility problems, complications in later pregnancies and complications at later surgery. No subgroup analysis was done.
Discussion
The limited data comparing muscle‐cutting incisions with Pfannenstiel incisions showed no differences.
The Joel‐Cohen incision was associated with some immediate benefits for women undergoing caesarean delivery in comparison to the Pfannenstiel incision. Postoperative morbidity was less following this incision as indicated by fever, postoperative pain and analgesic requirements. Although measurements were subjective, estimated intraoperative blood loss was reportedly less with Joel‐Cohen incision compared with Pfannenstiel and vertical incisions. The clinical significance of the reported difference (less than 100 mL) in estimated blood loss is probably less important in non‐anaemic women but may be of greater significance in women with anaemia.
Caesarean delivery using the Joel‐Cohen incision took less time than caesarean delivery by Pfannenstiel incision. The time from skin incision to delivery of the baby and the total duration of surgery were both shorter. However, it is unclear if the difference in time for delivery is of clinical significance. However, less time taken for surgery may be significant in situations where there is a shortage of operation theatre facilities and staff availability. Lastly, women having Joel‐Cohen incision had shorter periods of hospitalisation compared with those undergoing the Pfannenstiel incision.
None of the studies reported on significant long‐term outcomes such as long‐term problems associated with surgery and outcomes in subsequent pregnancy.
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Joel‐Cohen versus Pfannenstiel incision, Outcome 1 Postoperative febrile morbidity.

Comparison 1 Joel‐Cohen versus Pfannenstiel incision, Outcome 2 Postoperative analgesia on demand.

Comparison 1 Joel‐Cohen versus Pfannenstiel incision, Outcome 3 Time between surgery and first dose of analgesic (hours).

Comparison 1 Joel‐Cohen versus Pfannenstiel incision, Outcome 4 Total dose of analgesics in 24 hours.

Comparison 1 Joel‐Cohen versus Pfannenstiel incision, Outcome 8 Estimated blood loss (mL).

Comparison 1 Joel‐Cohen versus Pfannenstiel incision, Outcome 10 Blood transfusion.
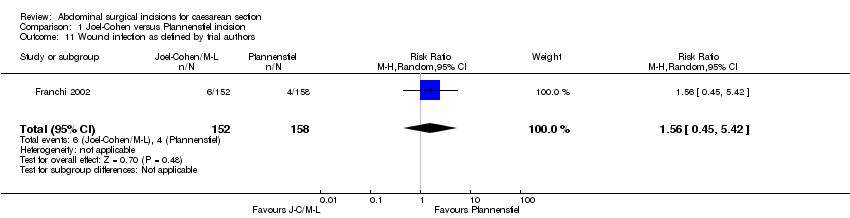
Comparison 1 Joel‐Cohen versus Pfannenstiel incision, Outcome 11 Wound infection as defined by trial authors.

Comparison 1 Joel‐Cohen versus Pfannenstiel incision, Outcome 16 Time (hours) from surgery to start of breastfeeding.
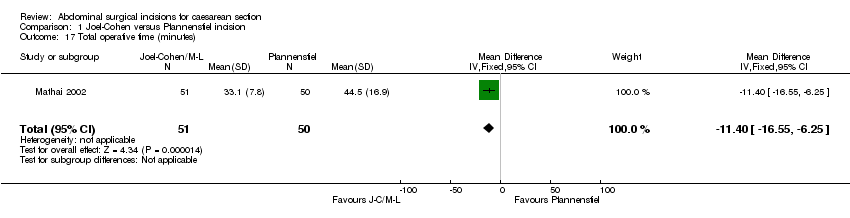
Comparison 1 Joel‐Cohen versus Pfannenstiel incision, Outcome 17 Total operative time (minutes).
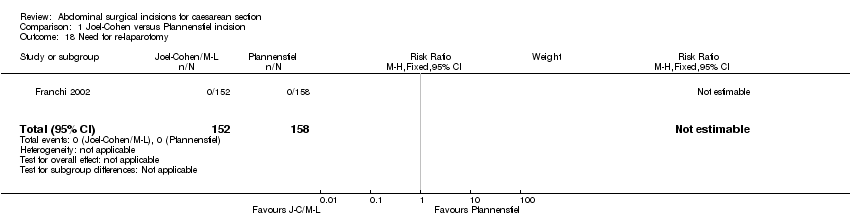
Comparison 1 Joel‐Cohen versus Pfannenstiel incision, Outcome 18 Need for re‐laparotomy.

Comparison 1 Joel‐Cohen versus Pfannenstiel incision, Outcome 21 Delivery time (minutes).

Comparison 1 Joel‐Cohen versus Pfannenstiel incision, Outcome 23 Admissions to special care baby unit ‐ all types.
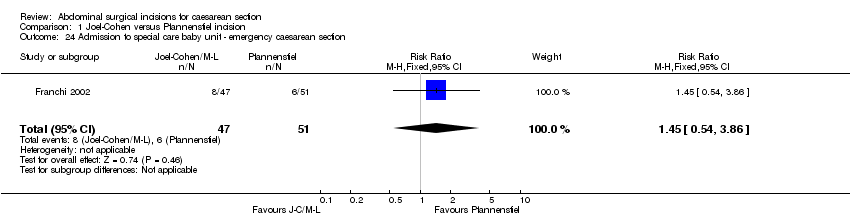
Comparison 1 Joel‐Cohen versus Pfannenstiel incision, Outcome 24 Admission to special care baby unit ‐ emergency caesarean section.

Comparison 1 Joel‐Cohen versus Pfannenstiel incision, Outcome 25 Postoperative hospital stay for mother (days).

Comparison 1 Joel‐Cohen versus Pfannenstiel incision, Outcome 26 Stay in special care nursery (days).

Comparison 2 Muscle‐cutting/Maylard versus Pfannenstiel incision, Outcome 1 Postoperative febrile morbidity.
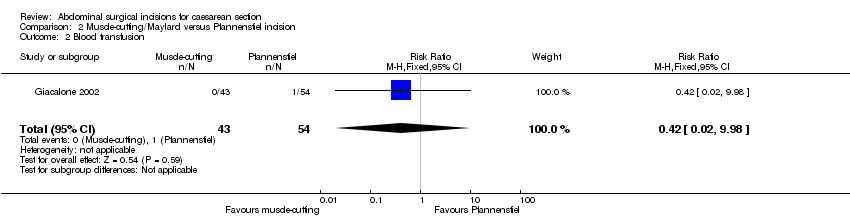
Comparison 2 Muscle‐cutting/Maylard versus Pfannenstiel incision, Outcome 2 Blood transfusion.

Comparison 2 Muscle‐cutting/Maylard versus Pfannenstiel incision, Outcome 3 Wound infection as defined by trial authors.

Comparison 2 Muscle‐cutting/Maylard versus Pfannenstiel incision, Outcome 4 Long‐term complication ‐ physical test at 3 months (Janda's test).
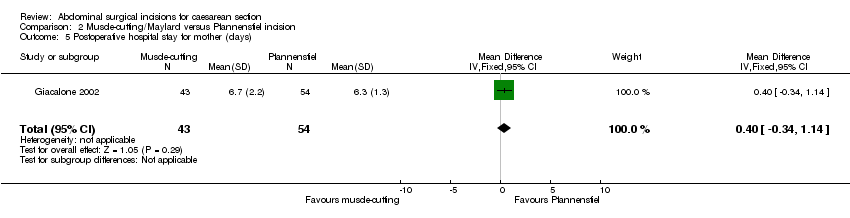
Comparison 2 Muscle‐cutting/Maylard versus Pfannenstiel incision, Outcome 5 Postoperative hospital stay for mother (days).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative febrile morbidity Show forest plot | 2 | 411 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.14, 0.87] |
| 1.1 Joel‐Cohen versus Pfannenstiel incision | 2 | 411 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.14, 0.87] |
| 2 Postoperative analgesia on demand Show forest plot | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.40, 0.76] |
| 3 Time between surgery and first dose of analgesic (hours) Show forest plot | 1 | 101 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [0.12, 1.48] |
| 4 Total dose of analgesics in 24 hours Show forest plot | 1 | 101 | Mean Difference (IV, Fixed, 95% CI) | ‐0.89 [‐1.19, ‐0.59] |
| 5 Number of analgesic injections required | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Duration of analgesics (hours) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Number of analgesic doses required | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Estimated blood loss (mL) Show forest plot | 1 | 101 | Mean Difference (IV, Fixed, 95% CI) | ‐58.0 [‐108.51, ‐7.49] |
| 9 Change in pre‐ and postoperative haemoglobin levels (g) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Blood transfusion Show forest plot | 1 | 310 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Wound infection as defined by trial authors Show forest plot | 1 | 310 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.45, 5.42] |
| 12 Wound haematoma | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Postoperative pain absent on day 1 | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Postoperative pain absent on day 2 | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 "Significant" postoperative pain by visual analogue score | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Time (hours) from surgery to start of breastfeeding Show forest plot | 1 | 101 | Mean Difference (IV, Fixed, 95% CI) | ‐5.5 [‐13.62, 2.62] |
| 17 Total operative time (minutes) Show forest plot | 1 | 101 | Mean Difference (IV, Fixed, 95% CI) | ‐11.40 [‐16.55, ‐6.25] |
| 18 Need for re‐laparotomy Show forest plot | 1 | 310 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Long‐term "significant" wound pain assessed by visual analogue score | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Not satisfied with wound | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Delivery time (minutes) Show forest plot | 1 | 101 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐2.53, ‐1.27] |
| 22 5‐minute Apgar score less than 7 | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Admissions to special care baby unit ‐ all types Show forest plot | 1 | 310 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.44, 3.20] |
| 24 Admission to special care baby unit ‐ emergency caesarean section Show forest plot | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.54, 3.86] |
| 25 Postoperative hospital stay for mother (days) Show forest plot | 1 | 101 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐2.16, ‐0.84] |
| 26 Stay in special care nursery (days) Show forest plot | 1 | 101 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.95, 0.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative febrile morbidity Show forest plot | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.08, 19.50] |
| 2 Blood transfusion Show forest plot | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.02, 9.98] |
| 3 Wound infection as defined by trial authors Show forest plot | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.27, 5.91] |
| 4 Long‐term complication ‐ physical test at 3 months (Janda's test) Show forest plot | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.73, 0.93] |
| 5 Postoperative hospital stay for mother (days) Show forest plot | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.34, 1.14] |

