Corticosteroides inhalados versus sistémicos para el tratamiento de la displasia broncopulmonar en recién nacidos prematuros ventilados de muy bajo peso al nacer
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Multicentre, randomised open study. 1. Blinding of randomisation: Yes. 2. Blinding of intervention: Not in all centres. 11 centres ‐ Yes; 36 centres ‐ No 3. Blinding of outcome measurement: No 4. Complete follow up: Yes Study period: February 1994 to December 1998. Study location: 47 neonatal intensive care units worldwide (UK, Ireland, Canada, Switzerland, Norway, Greece, Portugal, Sweden, Slovenia, Poland, Israel, Singapore, UAE) | |
| Participants | 570 infants from 47 neonatal intensive care units worldwide (UK, Ireland, Canada, Switzerland, Norway, Greece, Portugal, Sweden, Slovenia, Poland, Israel, Singapore, UAE) were enrolled. Inclusion criteria: Gestational age < 30 weeks, postnatal age < 72 hours and need for mechanical ventilation and inspired FiO₂ > 30%. Delayed selective treatment was started if infants needed mechanical ventilation and > 30% FiO₂ for > 15 days. Infants of 30 ‐ 31 weeks GA could also be included if they needed > 50% FiO₂. Exclusion criteria: congenital lethal anomalies, severe intraventricular haemorrhage (grade 3 or 4) and proven systemic infection before entry. A strong suspicion of infection, uncontrolled hypertension and hyperglycaemia were considered to be indications to postpone trial entry until they resolved, provided that this occurred within 72 hours of birth. The trial had factorial design and a similar number of infants was allocated to each group. Group 1 received early (< 72 hours) dexamethasone (N = 135); group 2 received delayed (> 15 days) dexamethasone (N = 150); group 3 received early budesonide (N = 143); Group 4 received delayed selective budesonide Demographic data: values presented as mean (SD) or as appropriate Group 1: Early (< 72 hours) dexamethasone (N = 135) Data not included in this systematic review Group 2: Delayed (> 15 days) dexamethasone (N = 150) Gestational age: 27.1 weeks (1.9) Group 3: Early budesonide (N = 143) Data not included in this systematic review Group 4: Delayed selective budesonide group N = 142 | |
| Interventions | 1. Dexamethasone was administered IV or orally in initial dose of 0.5 mg/kg/day in 2 divided doses for 3 days, followed by 0.25 mg/kg/day in 2 divided doses for 3 days, then 0.10 mg/kg/day for 3 days, and finally 0.05 mg/kg/day in 2 divided doses for 3 days for a total of 12 days of treatment. 2. Budesonide was administered using a metered dose inhaler (200 µg/puff; Pulmicort, Astra Draco, Lund, Sweden) connected to spacing device (Aerochamber MV 15; Trudell Medical, Canada). The aero chamber was a rigid, clear plastic cylinder, 11 by 4.1 cm with an approximate capacity of 145 mL. After endotracheal suctioning, the metered dose inhaler was shaken and inserted into the spacing chamber. The spacer was then filled with 100% oxygen and the infant's FiO₂ was increased by 20%. The aero chamber was connected into the ventilatory circuit and manual inflations were given through the chamber using an inflatable bag. Budesonide was administered as soon as chest wall movements were established. A 500 to 1000 g infant was given 2 puffs twice daily and 1000 to 1500 g infant was given 3 puffs twice daily. The puffs were given one at a time, activating metered dose inhaler at end expiration and allowing 10 breaths after each activation. After each administration, the chamber was removed from the ventilator circuit and the infant was reconnected to the ventilator at the previous settings. The duration of budesonide treatment was up to 12 days provided the infant remained intubated. If the infant was extubated before 12 days budesonide was discontinued | |
| Outcomes | 1. Primary outcome measure was death or oxygen dependency at 36 weeks' postmenstrual age. 2. Secondary outcome measures included death or major cerebral abnormality on ultrasound nearest to 6 weeks' postnatal age, death or oxygen dependency at 28 days and expected date of delivery, duration > 40% FiO₂, duration of any supplemental oxygen, duration of assisted ventilation by endotracheal tube and duration of hospital stay. 3. Complications such as pneumothorax, other pulmonary air leaks, necrotising enterocolitis, acquired pneumonia, patent ductus arteriosus requiring treatment, pulmonary haemorrhage requiring increased ventilation, seizures treated with anticonvulsants, recurrent apnoea needing treatment, retinopathy of prematurity at 36 weeks' postmenstrual age, gastric haemorrhage and GI perforation were noted. All neonates were monitored daily for blood pressure and blood glucose. Also, withdrawals from the intervention because of hypertension, hyperglycaemia, sepsis, gastric bleeding, or intestinal perforation were noted. An intention‐to‐treat analysis was performed | |
| Notes | The study was performed double blind in 11 centres, and in these centres placebo metered dose inhalers and intravenous saline were used to mask treatment allocation. The study design was open rather than double‐blind because some clinicians wanted to prescribe broad spectrum antibiotics or H₂ blockers such as cimetidine or ranitidine to infants receiving dexamethasone. However, in 11 centres, the trial was conducted double‐blind, and in these centres placebo metered dose inhalers and intravenous saline were used to mask treatment allocation This study was supported by a grant from Action Research, United Kingdom. Trudell Medical, London Ontario, Canada supplied Aerochambers, and Astra, Draco, Lund, Sweden supplied the metered dose inhalers (MDIs) of budesonide and placebo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Multicentre, randomised open study. Random number sequence generation performed by the trial statistician, independent of researchers |
| Allocation concealment (selection bias) | Low risk | Once an infant had full filled entry criteria, the supervising clinician telephoned the randomisation centre in Belfast to enrol an infant and determine the treatment group |
| Blinding (performance bias and detection bias) | High risk | Not in all centres. 11 centres ‐ Yes; 36 centres ‐ No |
| Blinding of participants and personnel (performance bias) | High risk | Not in all centres. 11 centres ‐ Yes; 36 centres ‐ No |
| Blinding of outcome assessment (detection bias) | High risk | Not in all centres. 11 centres ‐ Yes; 36 centres ‐ No |
| Incomplete outcome data (attrition bias) | Low risk | Outcome data reported on all enrolled infants |
| Selective reporting (reporting bias) | Low risk | There was no selective reporting according to the first author (HH) |
| Other bias | Unclear risk | Appears free of other bias |
| Methods | Prospective randomised controlled trial. Study period: January 1992 to March 1995. Study location: Division of Neonatal‐Perinatal Medicine, Medical College of Virginia at Virginia Commonwealth University, Richmond, Virginia, USA | |
| Participants | 61 preterm infants with birth weights between 650 g and 2000 g if at 14 days of age were at significant risk for developing moderate to severe BPD, defined as need for mechanical ventilation and oxygen, along with X‐ray changes beyond 28 days of life were enrolled. Demographic data: values presented as mean (SE) or as appropriate Group A: Dexamethasone group N = 15 Group B: High dose beclomethasone group N = 16 Group C: Medium dose beclomethasone group N = 15 Group D: Low dose beclomethasone group N = 15 | |
| Interventions | Infants were randomised into 4 groups: Dexamethasone group: 42 day course of dexamethasone as described by Avery et al followed by a 7 day course of placebo to ensure that all subjects ended the study at the same time. Subjects in the aerosol steroid groups (B, C, or D) began a similar 42 day systemic dexamethasone course on day 8 if extubation was unsuccessful while on inhaled steroids. Aerosol steroid groups (limited to data from infants 650 to 1000 g) randomised to receive: High dose beclomethasone group (2.4 to 3.69 µg/kg/day) Beclomethasone dipropionate (42 µg/actuation, Vanceril, Schering‐Plough, Kenilworth, NJ) was administered using a metered dose inhaler placed between the bag and the spacer. After disconnecting the ventilator circuit, a 250 mL Laerdal resuscitation bag with oxygen reservoir connected to an oxygen source (> 90% FiO₂) was connected through a spacer to the endotracheal tube. After activating the metered dose inhaler, infants were given three manual breaths. Infants < 1001 g at birth received one dose of beclomethasone every 12 hourly while larger infants received a dose every 8 hourly. | |
| Outcomes | 1. Primary outcome measure was extubation within the first 7 days of the study 2. Secondary outcome measures included changes in ventilator settings and oxygen delivery Long‐term outcomes were not included. Our primary outcome of death or BPD at 36 weeks' postmenstrual age was not reported on. BPD at 36 weeks' postmenstrual age and deaths during hospital stay were reported on. There were 3 deaths in the dexamethasone group (3/15) and 4 deaths in the beclomethasone groups (4/46) | |
| Notes | Infants were ventilated with time‐cycled, pressure limited, non synchronized ventilators during this study. Ventilator management was prescribed during the first 2 weeks of the study. If the pCO₂ was < 50 mm Hg, the peak pressure was lowered until it was < 15 cm H₂O. Then, if the pCO₂ was < 50 mm Hg, the ventilator rate was lowered, FiO₂ was adjusted to maintain oxygen saturation between 88 to 92%. No subjects received bronchodilators during the study period. The use of caffeine or diuretics was left to the discretion of the attending physician No funding resources were reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random number table was used. |
| Allocation concealment (selection bias) | Low risk | After parental consent, infants were stratified into two birth weight groups (650 to 1000 g and 1001 to 2000 g birth weight) and then into four dosing group using a random table number. Only the pharmacy was aware of the individual group assignment. |
| Blinding (performance bias and detection bias) | Low risk | There was aerosol and systemic placebo in all participants in the control groups |
| Blinding of participants and personnel (performance bias) | Low risk | Yes |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Low risk | Outcome data were provided on all enrolled infants |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us so we cannot judge if there were any deviations from the protocol |
| Other bias | Unclear risk | Appears free of other bias |
| Methods | Prospective randomised controlled trial. 2. Blinding of intervention: No 3. Blinding of outcome measurement: No 4. Complete follow up: Yes Study period: July 1994 to July 1996. Study location: Neonatal intensive care unit (NICU) at Children’s Hospital of Buffalo, State of New York. | |
| Participants | 78 preterm infants ≤ 30 weeks, birth weight ≤ 1500 g and conventional ventilator dependence at 12 to 21 days of age with a rate > 15/min and FiO₂ > 0.30 with persistence of these ventilator settings for a minimum of 72 hours were enrolled in the study. Demographic data: Values presented as mean (SD) or as appropriate Inhaled beclomethasone 800 µg/d group N = 25 Inhaled beclomethasone 400 µg/day group N = 26 Intravenous dexamethasone group N = 27 | |
| Interventions | 1. The inhaled beclomethasone was delivered through a metered dose inhaler with a spacer device (Aerovent) connected in line with the ventilator at 50 µg per puff. Between each puff, the infant was ventilated with 4 or 5 manual breaths delivered at a peak pressure identical to that delivered during mechanical ventilation. 2. The intravenous dexamethasone group (N = 27) received a 42 day tapering course (Avery 1985), starting with 0.5 mg/kg/day, divided every 12 hours. Cross over from either of the inhaled beclomethasone groups to intravenous dexamethasone was allowed if, after 4 to 5 days of inhaled beclomethasone, the infant's ventilator and oxygen support had not decreased and the attending neonatologist felt that the infant might benefit from intravenous dexamethasone. 3. All data were analysed according to original group assignment. | |
| Outcomes | 1. Primary outcome measures: hypertension or hyperglycaemia needing treatment or culture positive sepsis. 2. Other outcome variables: ventilatory settings, specifically rate, mean airway pressure (MAP), peak inspiratory pressure, positive end expiratory pressure, and supplemental oxygen requirement every 6 hours daily on all enrolled patients starting from 5 days before initiation of steroid therapy and daily thereafter, until extubation. After extubation, the supplemental oxygen was recorded daily until patient was discharged or supplemental oxygen was discontinued. 3. The occurrence of the following was also recorded: intraventricular haemorrhage, periventricular leukomalacia, necrotising enterocolitis, retinopathy of prematurity, GI bleed and death. 4. For infants who completed at least a 10 day course of either inhaled or intravenous steroids, an ACTH stimulation test was done 2 weeks after completion of steroid course. The test was completed in 24 neonates and the morning cortisol levels were noted before and one hour after intravenous bolus of 36 µg/kg synthetic ACTH (Cortrosyn, Organon, West Orange, NJ). | |
| Notes | Sample size was determined by assuming a rate of adverse effects (as defined by sepsis or hyperglycaemia or hypertension requiring treatment) of 80% in intravenous steroid‐treated infants. The sample size was calculated to detect a 30% difference in adverse effects at 80% power and P < 0.05. No funding resources were reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Low risk | Random allocation using 3 sets of 27 assembled, opaque envelopes |
| Blinding (performance bias and detection bias) | High risk | No placebo was used |
| Blinding of participants and personnel (performance bias) | High risk | No placebo was used |
| Blinding of outcome assessment (detection bias) | High risk | No placebo was used |
| Incomplete outcome data (attrition bias) | Low risk | Outcome data were reported on all enrolled infants |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us, so we cannot judge if there were any deviations from the protocol or not |
| Other bias | Unclear risk | Appears free of other bias |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| The authors included non ventilator‐dependent infants and the age of commencement of steroids varied from 5 to 118 days of life | |
| Non ventilator‐dependent infants were included in the study |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
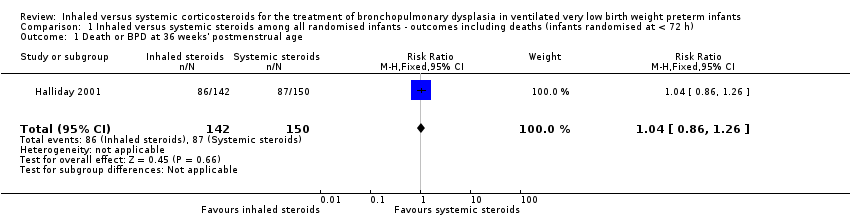
| 1 Death or BPD at 36 weeks' postmenstrual age Show forest plot | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.86, 1.26] |
| Analysis 1.1  Comparison 1 Inhaled versus systemic steroids among all randomised infants ‐ outcomes including deaths (infants randomised at < 72 h), Outcome 1 Death or BPD at 36 weeks' postmenstrual age. | ||||
| 2 Death or BPD at 28 days of age Show forest plot | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.90, 1.12] |
| Analysis 1.2  Comparison 1 Inhaled versus systemic steroids among all randomised infants ‐ outcomes including deaths (infants randomised at < 72 h), Outcome 2 Death or BPD at 28 days of age. | ||||
| 3 Death at 36 weeks' postmenstrual age Show forest plot | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.62, 1.49] |
| Analysis 1.3 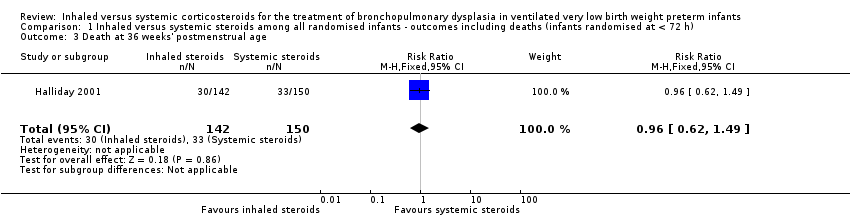 Comparison 1 Inhaled versus systemic steroids among all randomised infants ‐ outcomes including deaths (infants randomised at < 72 h), Outcome 3 Death at 36 weeks' postmenstrual age. | ||||
| 4 Death at 28 days of age Show forest plot | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.52, 1.37] |
| Analysis 1.4  Comparison 1 Inhaled versus systemic steroids among all randomised infants ‐ outcomes including deaths (infants randomised at < 72 h), Outcome 4 Death at 28 days of age. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or BPD at 36 weeks' postmenstrual age Show forest plot | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.83, 1.05] |
| Analysis 2.1  Comparison 2 Inhaled versus systemic steroids among infants ‐ outcomes including deaths (Infants randomised between 12 and 21 days of age), Outcome 1 Death or BPD at 36 weeks' postmenstrual age. | ||||
| 2 Death at 36 weeks' postmenstrual age Show forest plot | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [0.13, 54.15] |
| Analysis 2.2 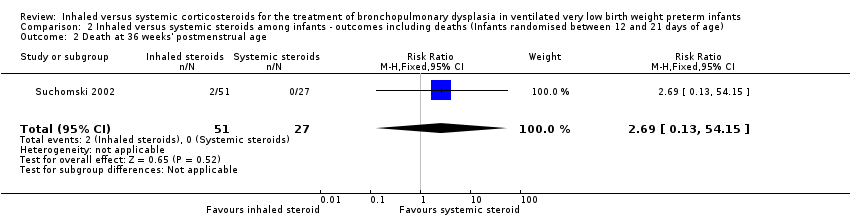 Comparison 2 Inhaled versus systemic steroids among infants ‐ outcomes including deaths (Infants randomised between 12 and 21 days of age), Outcome 2 Death at 36 weeks' postmenstrual age. | ||||
| 3 Death at 28 days of age Show forest plot | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [0.13, 54.15] |
| Analysis 2.3  Comparison 2 Inhaled versus systemic steroids among infants ‐ outcomes including deaths (Infants randomised between 12 and 21 days of age), Outcome 3 Death at 28 days of age. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 BPD at 36 weeks' postmenstrual age Show forest plot | 3 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.88, 1.32] |
| Analysis 3.1  Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 1 BPD at 36 weeks' postmenstrual age. | ||||
| 2 BPD at 28 days of age Show forest plot | 2 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| Analysis 3.2 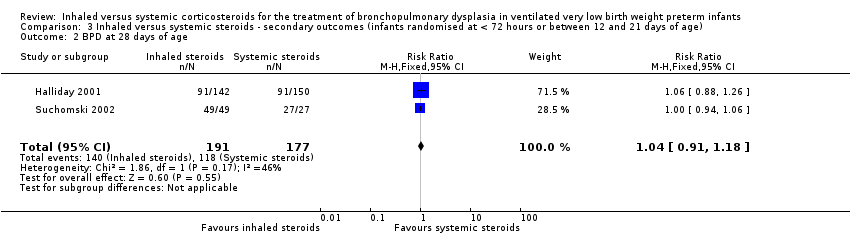 Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 2 BPD at 28 days of age. | ||||
| 3 Need for ventilation among survivors at 36 weeks' postmenstrual age Show forest plot | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.30, 4.06] |
| Analysis 3.3  Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 3 Need for ventilation among survivors at 36 weeks' postmenstrual age. | ||||
| 4 Duration of mechanical ventilation among survivors (days) Show forest plot | 2 | 368 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐5.22, 4.63] |
| Analysis 3.4  Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 4 Duration of mechanical ventilation among survivors (days). | ||||
| 5 Duration of supplemental oxygen among survivors (days) Show forest plot | 2 | 368 | Mean Difference (IV, Fixed, 95% CI) | ‐4.91 [‐20.87, 11.06] |
| Analysis 3.5 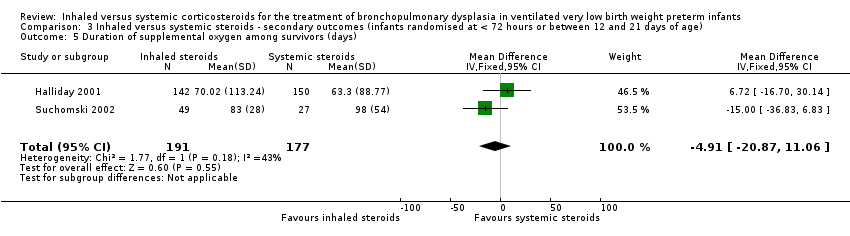 Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 5 Duration of supplemental oxygen among survivors (days). | ||||
| 6 Length of hospital stay among survivors (days) Show forest plot | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐13.0 [‐33.22, 7.22] |
| Analysis 3.6 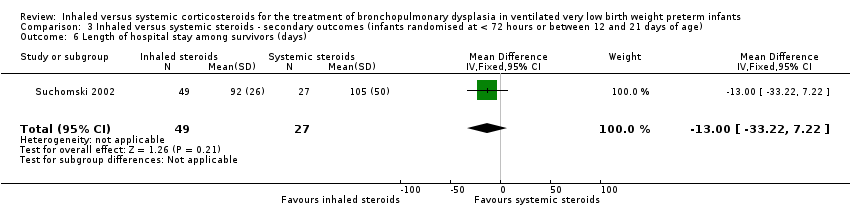 Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 6 Length of hospital stay among survivors (days). | ||||
| 7 Intraventricular haemorrhage grade III‐IV Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.33, 2.40] |
| Analysis 3.7 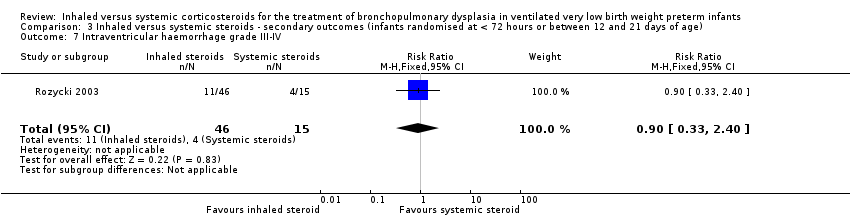 Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 7 Intraventricular haemorrhage grade III‐IV. | ||||
| 8 Periventricular leukomalacia Show forest plot | 2 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.34, 2.13] |
| Analysis 3.8 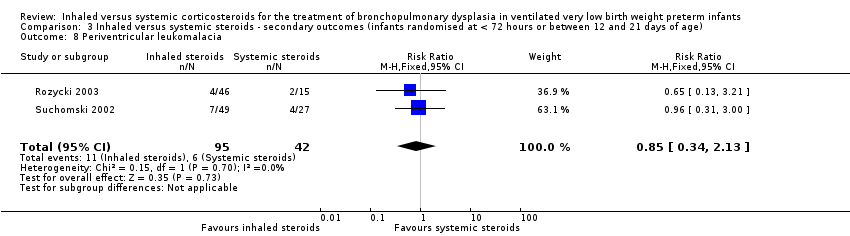 Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 8 Periventricular leukomalacia. | ||||
| 9 Hyperglycaemia Show forest plot | 3 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.61, 1.22] |
| Analysis 3.9  Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 9 Hyperglycaemia. | ||||
| 10 Hypertension Show forest plot | 3 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.73, 1.01] |
| Analysis 3.10  Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 10 Hypertension. | ||||
| 11 Necrotising enterocolitis Show forest plot | 2 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.50, 1.85] |
| Analysis 3.11  Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 11 Necrotising enterocolitis. | ||||
| 12 Gastrointestional bleed Show forest plot | 2 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.41, 1.93] |
| Analysis 3.12  Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 12 Gastrointestional bleed. | ||||
| 13 Retinopathy of prematurity ≥ stage 3 Show forest plot | 3 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.77, 2.25] |
| Analysis 3.13 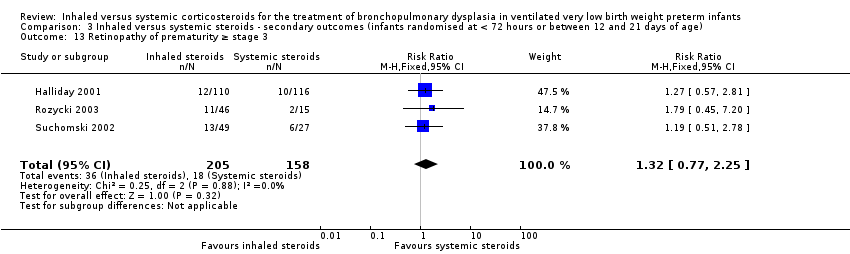 Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 13 Retinopathy of prematurity ≥ stage 3. | ||||
| 14 Culture‐proven sepsis Show forest plot | 2 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.79, 1.45] |
| Analysis 3.14  Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 14 Culture‐proven sepsis. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 General conceptual ability (GCA) score at 7 years Show forest plot | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐3.40 [‐12.38, 5.58] |
| Analysis 4.1 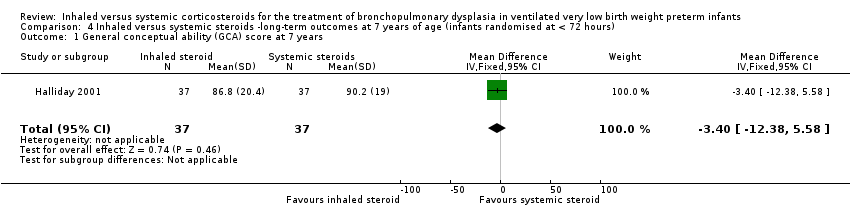 Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 1 General conceptual ability (GCA) score at 7 years. | ||||
| 2 Child behaviour check list (CBLC) at 7 years Show forest plot | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐4.75, 5.15] |
| Analysis 4.2  Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 2 Child behaviour check list (CBLC) at 7 years. | ||||
| 3 Strengths and Difficulties Questionnaire (SDQ) at 7 years Show forest plot | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐2.19, 4.19] |
| Analysis 4.3  Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 3 Strengths and Difficulties Questionnaire (SDQ) at 7 years. | ||||
| 4 Cerebral palsy at 7 years Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.35, 2.72] |
| Analysis 4.4  Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 4 Cerebral palsy at 7 years. | ||||
| 5 Moderate/severe disability at 7 years Show forest plot | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.4 [0.49, 4.01] |
| Analysis 4.5 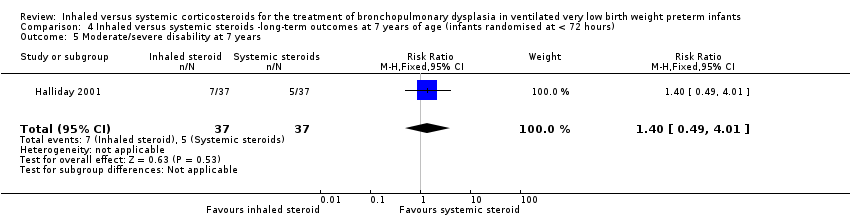 Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 5 Moderate/severe disability at 7 years. | ||||
| 6 Death or moderate/severe disability at 7 years Show forest plot | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.65, 1.58] |
| Analysis 4.6 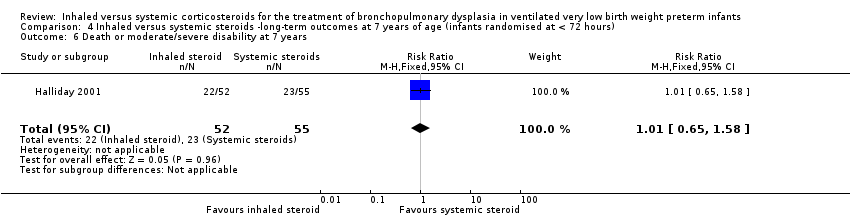 Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 6 Death or moderate/severe disability at 7 years. | ||||
| 7 Systolic blood pressure of > 95th percentile at 7 years Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.25, 1.23] |
| Analysis 4.7  Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 7 Systolic blood pressure of > 95th percentile at 7 years. | ||||
| 8 Diastolic blood pressure of > 95th percentile at 7 years Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.43, 4.45] |
| Analysis 4.8  Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 8 Diastolic blood pressure of > 95th percentile at 7 years. | ||||
| 9 Ever diagnosed as asthmatic by 7 years Show forest plot | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.55, 1.39] |
| Analysis 4.9 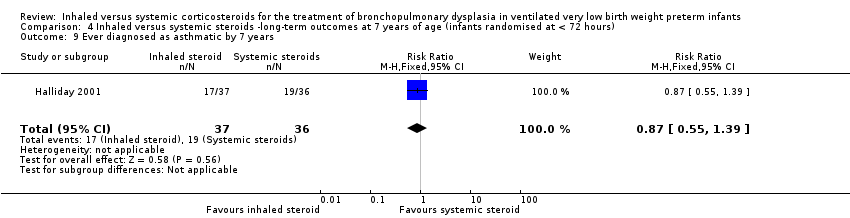 Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 9 Ever diagnosed as asthmatic by 7 years. | ||||

Study flow diagram: review update

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Comparison 1 Inhaled versus systemic steroids among all randomised infants ‐ outcomes including deaths (infants randomised at < 72 h), Outcome 1 Death or BPD at 36 weeks' postmenstrual age.

Comparison 1 Inhaled versus systemic steroids among all randomised infants ‐ outcomes including deaths (infants randomised at < 72 h), Outcome 2 Death or BPD at 28 days of age.

Comparison 1 Inhaled versus systemic steroids among all randomised infants ‐ outcomes including deaths (infants randomised at < 72 h), Outcome 3 Death at 36 weeks' postmenstrual age.

Comparison 1 Inhaled versus systemic steroids among all randomised infants ‐ outcomes including deaths (infants randomised at < 72 h), Outcome 4 Death at 28 days of age.

Comparison 2 Inhaled versus systemic steroids among infants ‐ outcomes including deaths (Infants randomised between 12 and 21 days of age), Outcome 1 Death or BPD at 36 weeks' postmenstrual age.
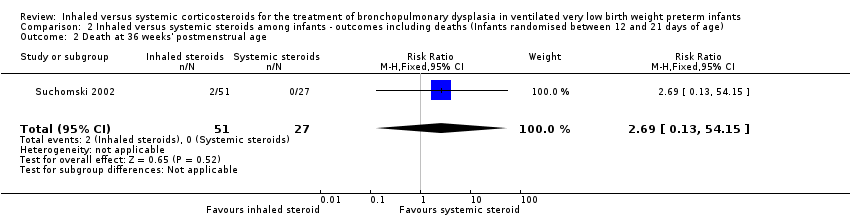
Comparison 2 Inhaled versus systemic steroids among infants ‐ outcomes including deaths (Infants randomised between 12 and 21 days of age), Outcome 2 Death at 36 weeks' postmenstrual age.

Comparison 2 Inhaled versus systemic steroids among infants ‐ outcomes including deaths (Infants randomised between 12 and 21 days of age), Outcome 3 Death at 28 days of age.

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 1 BPD at 36 weeks' postmenstrual age.

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 2 BPD at 28 days of age.

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 3 Need for ventilation among survivors at 36 weeks' postmenstrual age.

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 4 Duration of mechanical ventilation among survivors (days).

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 5 Duration of supplemental oxygen among survivors (days).

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 6 Length of hospital stay among survivors (days).

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 7 Intraventricular haemorrhage grade III‐IV.

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 8 Periventricular leukomalacia.
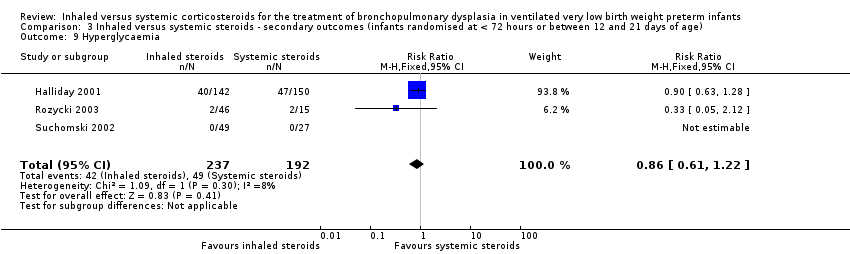
Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 9 Hyperglycaemia.
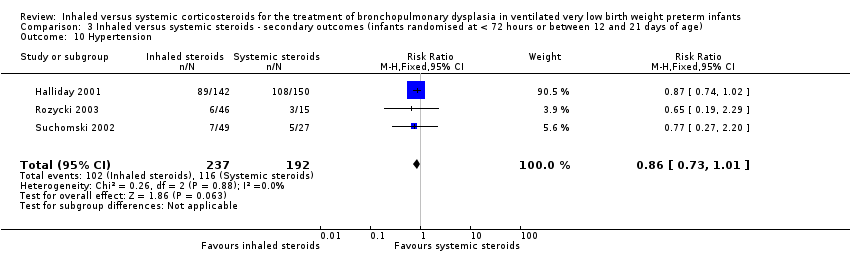
Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 10 Hypertension.

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 11 Necrotising enterocolitis.

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 12 Gastrointestional bleed.
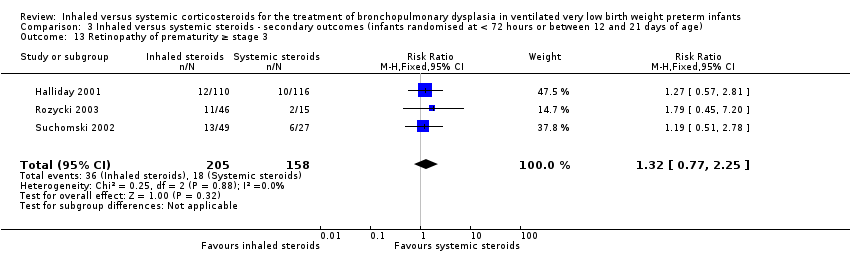
Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 13 Retinopathy of prematurity ≥ stage 3.

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 14 Culture‐proven sepsis.

Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 1 General conceptual ability (GCA) score at 7 years.

Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 2 Child behaviour check list (CBLC) at 7 years.
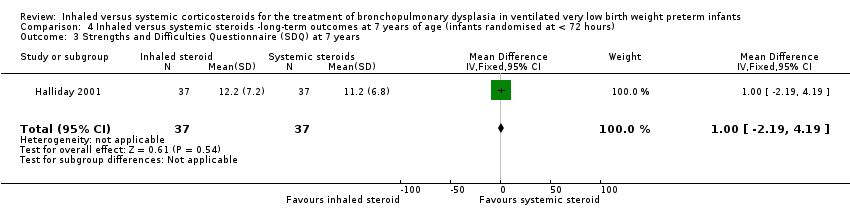
Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 3 Strengths and Difficulties Questionnaire (SDQ) at 7 years.

Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 4 Cerebral palsy at 7 years.

Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 5 Moderate/severe disability at 7 years.

Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 6 Death or moderate/severe disability at 7 years.

Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 7 Systolic blood pressure of > 95th percentile at 7 years.

Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 8 Diastolic blood pressure of > 95th percentile at 7 years.

Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 9 Ever diagnosed as asthmatic by 7 years.
| Inhaled steroids compared with systemic steroids for BPD (infants randomised at < 72 hours of age) | ||||||
| Patient or population: Neonates with developing BPD Settings: Neonatal intensive care unit Intervention: Inhaled steroids Comparison: Systemic steroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic steroids | Inhaled steroids | |||||
| Death or BPD at 36 weeks' postmenstrual age | High risk population | RR 1.04 (95% CI 0.86 to 1.26) | 292 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this single study was high. The study was not blinded at all sites. Only 35/150 infants randomised to systemic steroids received full course while 33/142 infants randomised to inhaled steroids received full course. Results were presented in intention to treat analyses including deaths occurring after 72 hours of age. We downgraded the quality of the evidence by one step. Precision: Precison for the point estimate was acceptable Presence of publication bias: N/A. | |
| 580 per 1000 | 606 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Inhaled steroids compared with systemic steroids for BPD (infants randomised between 12 and 21 days of age) | ||||||
| Patient or population: Neonates with developing BPD Settings: Neonatal intensive care unit Intervention: Inhaled steroids Comparison: Systemic steroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic steroids | Inhaled steroids | |||||
| Death or BPD at 36 weeks' postmenstrual age | High risk population | RR 0.94 (95% CI 0.83 to 1.05) | 78 (1) | ⊕⊕⊝⊝ | Bias: The risk of bias for this single study was high. There was no blinding of the intervention or outcome measurements. We downgraded the quality of the evidence by one level. Precision: The precision for the point estimate was low as the sample size was small Presence of publication bias: N/A. | |
| 963 per 1000 | 902 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Inhaled steroids compared with systemic steroids for BPD (infants randomised at < 72 hours or between 12 and 21 days of age) | ||||||
| Patient or population: Neonates with developing BPD Settings: Neonatal intensive care unit Intervention: Inhaled steroids Comparison: Systemic steroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic steroids | Inhaled steroids | |||||
| BPD at 36 weeks' postmenstrual age | High risk population | RR 1.08 (95% CI 0.88 to 1.32) | 429 (3) | ⊕⊕⊝⊝ | Bias: The risk of bias for these three studies was high. There was blinding of randomisation in all three studies. There was no blinding of the intervention or outcome measurements at all sites in the largest study (Halliday 2001). In Rozycki 2003 there was blinding of the intervention but blinding of outcome assessment was unclear. In Suchomski 2002 there was no blinding of the intervention or outcomes measurements. We downgraded the quality of the evidence by two levels. Precision: The precision for the point estimate was high as the sample size was quite large. Presence of publication bias: N/A. We did not create a funnel plot as there were only three trials included in the analysis. | |
| 422 per 1000 | 485 per 1000 | |||||
| Hyperglycaemia | High risk population | RR 0.86 (95% CI 0.61 to 1.22) | 429 (3) | ⊕⊕⊝⊝ | Bias: The risk of bias for these three studies was high. There was blinding of randomisation in all three studies. There was no blinding of the intervention or outcome measurements at all sites in the largest study (Halliday 2001). In Rozycki 2003 there was blinding of the intervention but blinding of outcome assessments was unclear. In Suchomski 2002 there was no blinding of the intervention or outcome measurements. We downgraded the quality of the evidence by two levels. Heterogeneity/consistency: There was no heterogeneity (I² = 8%). Precision: The precision for the point estimate was high as the sample size was quite large. Presence of publication bias: N/A. We did not create a funnel plot as there were only three trials included in the analysis. | |
| 255 per 1000 | 177 per 1000 | |||||
| Hypertension | High risk population | RR (RR 0.86, 95% CI 0.73 to 1.01) | 429 (3) | ⊕⊕⊝⊝ | Bias: The risk of bias for these three studies was high. There was blinding of randomisation in all three studies. There was no blinding of the intervention or outcome measurements at all sites in the largest study (Halliday 2001). In Rozycki 2003 there was blinding of the intervention but blinding of outcome assessments was unclear. In Suchomski 2002 there was no blinding of the intervention or outcome measurements. We downgraded the quality of the evidence by two steps. Heterogeneity/consistency: There was no heterogeneity (I² = 0%). Precision: The precision for the point estimate was high as the sample size was quite large. Presence of publication bias: N/A. We did not create a funnel plot as there were only three trials included in the analysis. | |
| 604 per 1000 | 430 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Inhaled steroids compared with systemic steroids for BPD ‐ long‐term outcomes at 7 years of age (infants randomised at < 72 hours of age) | ||||||
| Patient or population: Neonates with developing BPD Settings: NICU Intervention: Inhaled steroids Comparison: Systemic steroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic steroids | Inhaled steroids | |||||
| General conceptual ability (GCA) score at 7 years The test has a standardisation mean of 100 and SD of 15 | The mean GCA score in the control group was 90.2 | The mean GCA score in the intervention groups was 3.4 units lower | MD ‐3.40 (95% CI ‐12.38 to 5.58) | 74 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the Quality of the evidence by one step. Presence of publication bias: N/A. |
| Moderate/severe disability at 7 years | 135 per 1000 | 189 per 1000 | RR 1.40 (95% CI 0.49 to 4.01) | 74 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the Quality of the evidence by one step. Presence of publication bias: N/A. |
| Death or moderate/severe disability at 7 years | 418 per 1000 | 423 per 1000 | RR 1.01 (95% CI 0.65 to 1.58) | 107 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the Quality of the evidence by one step. Presence of publication bias: N/A. |
| Systolic blood pressure > 95th percentile at 7 years | 353 per 1000 | 194 per 1000 | RR 0.55 (95% CI 0.25 to 1.23) | 70 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the quality of the evidence by one step. Presence of publication bias: N/A. |
| Diastolic blood pressure > 95th percentile at 7 years | 121 per 1000 | 167 per 1000 | RR (1.38, 95% CI 0.43 to 4.45) | 69 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the quality of the evidence by one step. Presence of publication bias: N/A. |
| Ever diagnosed as asthmatic by 7 years | 528 per 1000 | 459 per 1000 | RR 0.87 (95% CI 0.55 to 1.39) | 73 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the quality of the evidence by one step. Presence of publication bias: N/A. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or BPD at 36 weeks' postmenstrual age Show forest plot | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.86, 1.26] |
| 2 Death or BPD at 28 days of age Show forest plot | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.90, 1.12] |
| 3 Death at 36 weeks' postmenstrual age Show forest plot | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.62, 1.49] |
| 4 Death at 28 days of age Show forest plot | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.52, 1.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or BPD at 36 weeks' postmenstrual age Show forest plot | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.83, 1.05] |
| 2 Death at 36 weeks' postmenstrual age Show forest plot | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [0.13, 54.15] |
| 3 Death at 28 days of age Show forest plot | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [0.13, 54.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 BPD at 36 weeks' postmenstrual age Show forest plot | 3 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.88, 1.32] |
| 2 BPD at 28 days of age Show forest plot | 2 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 3 Need for ventilation among survivors at 36 weeks' postmenstrual age Show forest plot | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.30, 4.06] |
| 4 Duration of mechanical ventilation among survivors (days) Show forest plot | 2 | 368 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐5.22, 4.63] |
| 5 Duration of supplemental oxygen among survivors (days) Show forest plot | 2 | 368 | Mean Difference (IV, Fixed, 95% CI) | ‐4.91 [‐20.87, 11.06] |
| 6 Length of hospital stay among survivors (days) Show forest plot | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐13.0 [‐33.22, 7.22] |
| 7 Intraventricular haemorrhage grade III‐IV Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.33, 2.40] |
| 8 Periventricular leukomalacia Show forest plot | 2 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.34, 2.13] |
| 9 Hyperglycaemia Show forest plot | 3 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.61, 1.22] |
| 10 Hypertension Show forest plot | 3 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.73, 1.01] |
| 11 Necrotising enterocolitis Show forest plot | 2 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.50, 1.85] |
| 12 Gastrointestional bleed Show forest plot | 2 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.41, 1.93] |
| 13 Retinopathy of prematurity ≥ stage 3 Show forest plot | 3 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.77, 2.25] |
| 14 Culture‐proven sepsis Show forest plot | 2 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.79, 1.45] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 General conceptual ability (GCA) score at 7 years Show forest plot | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐3.40 [‐12.38, 5.58] |
| 2 Child behaviour check list (CBLC) at 7 years Show forest plot | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐4.75, 5.15] |
| 3 Strengths and Difficulties Questionnaire (SDQ) at 7 years Show forest plot | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐2.19, 4.19] |
| 4 Cerebral palsy at 7 years Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.35, 2.72] |
| 5 Moderate/severe disability at 7 years Show forest plot | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.4 [0.49, 4.01] |
| 6 Death or moderate/severe disability at 7 years Show forest plot | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.65, 1.58] |
| 7 Systolic blood pressure of > 95th percentile at 7 years Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.25, 1.23] |
| 8 Diastolic blood pressure of > 95th percentile at 7 years Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.43, 4.45] |
| 9 Ever diagnosed as asthmatic by 7 years Show forest plot | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.55, 1.39] |

