Corticosteroides inhalados versus sistémicos para el tratamiento de la displasia broncopulmonar en recién nacidos prematuros ventilados de muy bajo peso al nacer
Resumen
Antecedentes
Esta es una actualización de una revisión publicada en 2012. También se ha actualizado una revisión relacionada, «Corticosteroides inhalados versus corticosteroides sistémicos para la prevención de la displasia broncopulmonar en recién nacidos prematuros de muy bajo peso al nacer sometidos a ventilación". La displasia broncopulmonar (DBP) es un problema grave y frecuente entre los recién nacidos de muy bajo peso al nacer a pesar del uso prenatal de esteroides y del tratamiento posnatal con surfactante para reducir la incidencia y la gravedad del síndrome de dificultad respiratoria. Los corticosteroides se han usado ampliamente para el tratamiento o la prevención de la DBP debido a sus propiedades antiinflamatorias. Sin embargo, el uso de esteroides sistémicos se ha asociado con efectos adversos graves a corto y a largo plazo. La administración tópica de corticosteroides a través de las vías respiratorias podría tener efectos beneficiosos en el sistema pulmonar con menos efectos secundarios sistémicos no deseados.
Objetivos
Comparar la efectividad de los corticosteroides inhalados versus los corticosteroides sistémicos administrados a los recién nacidos prematuros dependientes del respirador con un peso al nacer ≤ 1500 g o una edad gestacional ≤ 32 semanas después de siete días de vida sobre la incidencia de muerte o DBP a las 36 semanas de edad posmenstrual.
Métodos de búsqueda
Se utilizó la estrategia de búsqueda estándar del Grupo Cochrane de Neonatología para buscar en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials, CENTRAL 2017, número 1), MEDLINE vía PubMed (1966 hasta el 23 de febrero 2017), Embase (1980 hasta el 23 de febrero 2017) y CINAHL (1982 hasta el 23 de febrero 2017). También se realizaron búsquedas en registros de ensayos clínicos, resúmenes de congresos y listas de referencias de los artículos recuperados para obtener ensayos controlados aleatorizados y ensayos cuasialeatorizados.
Criterios de selección
Ensayos controlados aleatorizados o cuasialeatorizados que compararan el tratamiento con corticosteroides inhalados versus sistémicos (independientemente de la dosis y la duración) iniciado después de la primera semana de vida en recién nacidos de muy bajo peso al nacer dependientes del respirador.
Obtención y análisis de los datos
Se utilizaron los procedimientos metodológicos estándar previstos por la Colaboración Cochrane.
Resultados principales
Se incluyeron tres ensayos con un total de 431 participantes que compararon los corticosteroides inhalados versus sistémicos para el tratamiento de la DBP. No se incluyeron ensayos nuevos para la actualización de 2017.
Aunque un estudio asignó al azar a los recién nacidos < 72 horas (N = 292), el tratamiento comenzó cuando los recién nacidos tenían > 15 días. En este estudio más grande, las muertes se incluyeron desde el momento de la asignación al azar y antes de comenzar el tratamiento. Dos estudios (N = 139) realizaron la asignación al azar y comenzaron el tratamiento entre los 12 y los 21 días.
Dos ensayos informaron de diferencias no significativas entre los grupos para el resultado primario: incidencia de muerte o DBP a las 36 semanas de edad posmenstrual entre todos los recién nacidos asignados al azar. Las estimaciones para el ensayo más grande fueron el riesgo relativo (RR) 1,04 (intervalo de confianza [IC] del 95%: 0,86 a 1,26), y la diferencia de riesgos (DR) 0,03 (IC del 95%: ‐0,09 a 0,15); (evidencia de calidad moderada). Las estimaciones para el otro ensayo que informó el resultado primario fueron RR 0,94 (IC del 95%: 0,83 a 1,05), DR ‐0,06 (IC del 95%: ‐0,17 a 0,05); (evidencia de baja calidad).
Los resultados secundarios que incluían datos de los tres ensayos no mostraron diferencias significativas en la duración de la ventilación mecánica o el oxígeno suplementario, la duración de la estancia hospitalaria o la incidencia de hiperglucemia, hipertensión, enterocolitis necrotizante, hemorragia gastrointestinal, retinopatía del prematuro o sepsis comprobada con cultivo (evidencia de calidad moderada a baja).
En un subconjunto de 75 recién nacidos supervivientes reclutados en el Reino Unido e Irlanda, no hubo diferencias significativas en los resultados del desarrollo a los siete años de edad entre los grupos (evidencia de calidad moderada). Un estudio recibió apoyo financiero y la industria proporcionó aerocámaras e inhaladores de dosis medidas de budesonida y placebo para el mismo estudio. No se identificó ningún conflicto de intereses.
Conclusiones de los autores
Esta revisión no encontró evidencia de que los corticosteroides inhalados otorgaran ventajas netas sobre los corticosteroides sistémicos en el tratamiento de los recién nacidos prematuros dependientes del respirador. No hubo evidencia de diferencias en la efectividad o en los perfiles de eventos secundarios para los esteroides inhalados versus sistémicos.
Un mejor sistema de administración que garantice una administración selectiva de los esteroides inhalados a los alvéolos quizá pueda tener efectos clínicos beneficiosos sin aumentar los eventos adversos.
Para resolver este problema, se necesitan estudios que identifiquen la proporción riesgo/beneficio de diferentes técnicas de administración y regímenes de dosificación para la administración de estos medicamentos. En los estudios futuros se deben considerar los efectos a largo plazo de los esteroides inhalados, con especial atención a los resultados del neurodesarrollo.
PICOs
Resumen en términos sencillos
Corticosteroides inhalados versus sistémicos para el tratamiento de la displasia broncopulmonar en recién nacidos prematuros ventilados de muy bajo peso al nacer
Pregunta de la revisión
Comparar la efectividad de los corticosteroides inhalados versus corticosteroides sistémicos administrados a los recién nacidos prematuros dependientes del respirador con un peso al nacer ≤ 1500 g o una edad gestacional ≤ 32 semanas después de siete días de vida sobre la incidencia de enfermedades pulmonares crónicas a las 36 semanas de edad posmenstrual corregida.
Antecedentes
Los bebés prematuros (bebés nacidos antes de término, 40 semanas de embarazo) a menudo necesitan apoyo respiratorio (respirador). Los bebés que necesitan apoyo respiratorio mecánico invasivo (colocación de un tubo de respiración en la tráquea) durante un período prolongado suelen desarrollar displasia broncopulmonar (definida como la necesidad de oxígeno suplementario a las 36 semanas de edad posmenstrual). Se piensa que parte de la causa puede residir en la inflamación de los pulmones. Los corticosteroides reducen la inflamación y la hinchazón de los pulmones, pero pueden tener efectos secundarios graves. El uso de corticosteroides se ha asociado con parálisis cerebral (problema motor) y con retraso en el desarrollo. La inhalación de esteroides, para que el fármaco llegue directamente a los pulmones, se ha intentado como una forma de limitar los efectos adversos.
Fecha de la búsqueda
23 de febrero 2017.
Características de los estudios
Los tres ensayos incluidos fueron aleatorizados, pero el cegamiento de la intervención y la medición de los resultados variaron. Se combinaron los datos de dos ensayos (en los que se incluyó a 139 recién nacidos) debido a que reclutaron a recién nacidos de entre 12 y 21 días de edad, aunque los datos de un ensayo (en el que se incluyó a 292 lactantes) se informaron por separado debido a que los investigadores asignaron al azar a recién nacidos de menos de 72 horas de edad. El momento adecuado en que se midieron los resultados varió entre los estudios, por lo que no fue apropiado combinar algunos resultados. En un estudio, todas las muertes que ocurrieron fueron informadas desde el momento en que los bebés fueron asignados al azar y no desde el momento en que se inició el tratamiento, por lo que hubo un mayor número de bebés que murieron en dicho estudio.
Un estudio recibió apoyo financiero y la industria farmacéutica proporcionó aerocámaras e inhaladores de dosis fijas de budesonida y placebo para el mismo estudio. No se identificó ningún conflicto de intereses.
Resultados clave
La evidencia de dos estudios en 370 recién nacidos, que fueron asignados al azar entre los 12 y los 21 días de edad y que contribuyeron con datos al resultado primario de esta revisión, mostró que los esteroides inhalados administrados después de los siete días de edad en comparación con los esteroides sistémicos no disminuyeron la incidencia de muerte o displasia broncopulmonar (DBP) a las 36 semanas de edad posmenstrual. La evidencia del único estudio en el que se asignó al azar a recién nacidos de menos de 72 horas de edad no mostró diferencias en la incidencia de muerte o de DBP.
La evidencia de tres estudios en 431 recién nacidos que contribuyeron a los resultados secundarios mostró que los esteroides inhalados administrados después de los siete días de edad en comparación con los esteroides sistémicos no alteraron significativamente la incidencia de DBP a las 36 semanas de edad posmenstrual, la hiperglucemia, la hipertensión, la duración de la ventilación, la duración de la administración de oxígeno suplementario, la duración de la estancia hospitalaria, la hemorragia intraventricular de grado III‐IV, la leucomalacia periventricular, la enterocolitis necrotizante, la hemorragia gastrointestinal, la retinopatía del prematuro en estadio > 3; la sepsis comprobada con cultivo o la incidencia de efectos adversos.
Los perfiles de eventos adversos no difieren entre los esteroides inhalados y los esteroides sistémicos, aunque no se ha informado de algunas complicaciones potenciales del tratamiento con esteroides. Se necesita más investigación para demostrar si cualquier forma habitual de administración de esteroides da lugar a mejorías en la salud general de los neonatos en riesgo de displasia broncopulmonar.
Calidad de la evidencia
La calidad de la evidencia (según los criterios GRADE) fue de moderada a baja.
Conclusiones de los autores
Summary of findings
| Inhaled steroids compared with systemic steroids for BPD (infants randomised at < 72 hours of age) | ||||||
| Patient or population: Neonates with developing BPD Settings: Neonatal intensive care unit Intervention: Inhaled steroids Comparison: Systemic steroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic steroids | Inhaled steroids | |||||
| Death or BPD at 36 weeks' postmenstrual age | High risk population | RR 1.04 (95% CI 0.86 to 1.26) | 292 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this single study was high. The study was not blinded at all sites. Only 35/150 infants randomised to systemic steroids received full course while 33/142 infants randomised to inhaled steroids received full course. Results were presented in intention to treat analyses including deaths occurring after 72 hours of age. We downgraded the quality of the evidence by one step. Precision: Precison for the point estimate was acceptable Presence of publication bias: N/A. | |
| 580 per 1000 | 606 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Inhaled steroids compared with systemic steroids for BPD (infants randomised between 12 and 21 days of age) | ||||||
| Patient or population: Neonates with developing BPD Settings: Neonatal intensive care unit Intervention: Inhaled steroids Comparison: Systemic steroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic steroids | Inhaled steroids | |||||
| Death or BPD at 36 weeks' postmenstrual age | High risk population | RR 0.94 (95% CI 0.83 to 1.05) | 78 (1) | ⊕⊕⊝⊝ | Bias: The risk of bias for this single study was high. There was no blinding of the intervention or outcome measurements. We downgraded the quality of the evidence by one level. Precision: The precision for the point estimate was low as the sample size was small Presence of publication bias: N/A. | |
| 963 per 1000 | 902 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Inhaled steroids compared with systemic steroids for BPD (infants randomised at < 72 hours or between 12 and 21 days of age) | ||||||
| Patient or population: Neonates with developing BPD Settings: Neonatal intensive care unit Intervention: Inhaled steroids Comparison: Systemic steroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic steroids | Inhaled steroids | |||||
| BPD at 36 weeks' postmenstrual age | High risk population | RR 1.08 (95% CI 0.88 to 1.32) | 429 (3) | ⊕⊕⊝⊝ | Bias: The risk of bias for these three studies was high. There was blinding of randomisation in all three studies. There was no blinding of the intervention or outcome measurements at all sites in the largest study (Halliday 2001). In Rozycki 2003 there was blinding of the intervention but blinding of outcome assessment was unclear. In Suchomski 2002 there was no blinding of the intervention or outcomes measurements. We downgraded the quality of the evidence by two levels. Precision: The precision for the point estimate was high as the sample size was quite large. Presence of publication bias: N/A. We did not create a funnel plot as there were only three trials included in the analysis. | |
| 422 per 1000 | 485 per 1000 | |||||
| Hyperglycaemia | High risk population | RR 0.86 (95% CI 0.61 to 1.22) | 429 (3) | ⊕⊕⊝⊝ | Bias: The risk of bias for these three studies was high. There was blinding of randomisation in all three studies. There was no blinding of the intervention or outcome measurements at all sites in the largest study (Halliday 2001). In Rozycki 2003 there was blinding of the intervention but blinding of outcome assessments was unclear. In Suchomski 2002 there was no blinding of the intervention or outcome measurements. We downgraded the quality of the evidence by two levels. Heterogeneity/consistency: There was no heterogeneity (I² = 8%). Precision: The precision for the point estimate was high as the sample size was quite large. Presence of publication bias: N/A. We did not create a funnel plot as there were only three trials included in the analysis. | |
| 255 per 1000 | 177 per 1000 | |||||
| Hypertension | High risk population | RR (RR 0.86, 95% CI 0.73 to 1.01) | 429 (3) | ⊕⊕⊝⊝ | Bias: The risk of bias for these three studies was high. There was blinding of randomisation in all three studies. There was no blinding of the intervention or outcome measurements at all sites in the largest study (Halliday 2001). In Rozycki 2003 there was blinding of the intervention but blinding of outcome assessments was unclear. In Suchomski 2002 there was no blinding of the intervention or outcome measurements. We downgraded the quality of the evidence by two steps. Heterogeneity/consistency: There was no heterogeneity (I² = 0%). Precision: The precision for the point estimate was high as the sample size was quite large. Presence of publication bias: N/A. We did not create a funnel plot as there were only three trials included in the analysis. | |
| 604 per 1000 | 430 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Inhaled steroids compared with systemic steroids for BPD ‐ long‐term outcomes at 7 years of age (infants randomised at < 72 hours of age) | ||||||
| Patient or population: Neonates with developing BPD Settings: NICU Intervention: Inhaled steroids Comparison: Systemic steroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic steroids | Inhaled steroids | |||||
| General conceptual ability (GCA) score at 7 years The test has a standardisation mean of 100 and SD of 15 | The mean GCA score in the control group was 90.2 | The mean GCA score in the intervention groups was 3.4 units lower | MD ‐3.40 (95% CI ‐12.38 to 5.58) | 74 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the Quality of the evidence by one step. Presence of publication bias: N/A. |
| Moderate/severe disability at 7 years | 135 per 1000 | 189 per 1000 | RR 1.40 (95% CI 0.49 to 4.01) | 74 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the Quality of the evidence by one step. Presence of publication bias: N/A. |
| Death or moderate/severe disability at 7 years | 418 per 1000 | 423 per 1000 | RR 1.01 (95% CI 0.65 to 1.58) | 107 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the Quality of the evidence by one step. Presence of publication bias: N/A. |
| Systolic blood pressure > 95th percentile at 7 years | 353 per 1000 | 194 per 1000 | RR 0.55 (95% CI 0.25 to 1.23) | 70 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the quality of the evidence by one step. Presence of publication bias: N/A. |
| Diastolic blood pressure > 95th percentile at 7 years | 121 per 1000 | 167 per 1000 | RR (1.38, 95% CI 0.43 to 4.45) | 69 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the quality of the evidence by one step. Presence of publication bias: N/A. |
| Ever diagnosed as asthmatic by 7 years | 528 per 1000 | 459 per 1000 | RR 0.87 (95% CI 0.55 to 1.39) | 73 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the quality of the evidence by one step. Presence of publication bias: N/A. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
Antecedentes
Descripción de la afección
La displasia broncopulmonar (DBP) es un problema grave y común entre los recién nacidos de muy bajo peso al nacer, a pesar de que se utilizan esteroides prenatales (Roberts 2017) y el tratamiento posnatal con surfactante (Soll 1998; Bahadue 2012) para disminuir la incidencia y la gravedad del síndrome de dificultad respiratoria. La incidencia de DBP varía entre el 23 y el 26% (Lee 2000; Lemons 2001) en recién nacidos de muy bajo peso al nacer (< 1500 g) y tiene una relación inversa tanto con la edad gestacional como con el peso al nacer (Lee 2000; Sinkin 1990).
Descripción de la intervención
Varios ensayos controlados aleatorizados (Avery 1985; CDTG 1991; Cummings 1989; Harkavy 1989; Kazzi 1990; Ohlsson 1992) y revisiones sistemáticas (Bhuta 1998; Doyle 2014; Doyle 2014a; Halliday 1999; Shah 2001) han demostrado que, entre los recién nacidos con DBP, el tratamiento con corticosteroides sistémicos facilita la extubación y mejora el cumplimiento del sistema respiratorio. Entre los ensayos se ha observado una importante heterogeneidad con respecto a la dosis y la duración de la administración de dexametasona. Sin embargo, los corticosteroides parecen tener poco efecto en la duración de la administración de oxígeno suplementario, la duración de la hospitalización o la mortalidad (Avery 1985; CDTG 1991; Harkavy 1989; Kazzi 1990; Ohlsson 1992). En esta población existen inquietudes con respecto a los efectos secundarios a corto y a largo plazo de los esteroides sistémicos. Las mismas incluyen hiperglucemia, hipertensión, miocardiopatía hipertrófica, hemorragia y perforación gastrointestinal, aumento del catabolismo y deficiencia de crecimiento, nefrocalcinosis, mineralización ósea deficiente y susceptibilidad a las infecciones (Ng 1993; Stark 2001).
Los potenciales efectos en el crecimiento del cerebro y en el desarrollo neurológico son los más alarmantes. Modelos animales (rata y monos rhesus) en un estadio similar de ontogenia que el feto humano han mostrado que los esteroides afectan de manera permanente la división, la diferenciación y la mielinización de las células cerebrales, así como la ontogenia del desarrollo cortical cerebral (Johnson 1979; Weichsel 1977). Estos efectos son duraderos y se asociaron con un menor diámetro cefálico y anormalidades neuromotoras. Varios estudios de seguimiento sobre el tratamiento posnatal sistémico con corticosteroides de recién nacidos prematuros han mostrado una mayor incidencia de alteraciones del neurodesarrollo en neonatos supervivientes tratados con dexametasona (O'Shea 1999; Shinwell 2000; Yeh 1998).
En teoría, el uso de corticosteroides inhalados podría producir efectos beneficiosos en el sistema pulmonar sin concentraciones sistémicas altas concomitantes y con un menor riesgo de efectos adversos. Los resultados de un estudio multicéntrico amplio sobre el uso temprano de esteroides inhalados concluyeron que, entre los recién nacidos extremadamente prematuros, la incidencia de DBP era menor entre los que recibieron budesonida inhalada de forma temprana que entre los que recibieron placebo, pero es posible que la ventaja se haya obtenido a expensas del aumento de la mortalidad (Bassler 2015). Los resultados de este estudio han sido incorporados en una revisión Cochrane (Shah 2017) y un metanálisis (Shinwell 2016). Shinwell 2016 estableció la conclusión de que «Los recién nacidos muy prematuros parecen beneficiarse con la administración de corticosteroides inhalados con un riesgo reducido de DBP y sin efectos sobre la muerte, otras morbilidades ni eventos adversos. Se esperan ansiosamente los datos sobre los resultados respiratorios, del crecimiento y del desarrollo a largo plazo». Los hallazgos de Shah 2017 fueron los siguientes: «Hay cada vez más evidencia proveniente de los ensayos examinados de que la administración temprana de corticosteroides inhalados a los recién nacidos de muy bajo peso al nacer es efectiva para reducir la incidencia de muerte o EPC a las 36 semanas de edad posmenstrual en los recién nacidos asignados al azar o en los supervivientes. Aunque existe significación estadística, la relevancia clínica está abierta al debate ya que el límite superior del IC para el resultado de la muerte o la DBP a las 36 semanas de edad posmenstrual es infinito. Los resultados del seguimiento a largo plazo del estudio Bassler 2015 podrían afectar las conclusiones de esta revisión. Es necesario realizar más estudios para identificar el cociente riesgo:beneficio de las diferentes técnicas de administración y de los regímenes de dosificación para estos fármacos. Los estudios deben considerar los efectos beneficiosos y adversos a corto y a largo plazo de los corticosteroides inhalados, con especial atención a los resultados del neurodesarrollo».
Cabe destacar que una revisión Cochrane por Onland 2017b concluyó: «A pesar de que algunos estudios informaron un efecto modulatorio de los regímenes de tratamiento a favor de los regímenes de dosis alta en la incidencia de la DBP y el deterioro del neurodesarrollo, no pueden efectuarse recomendaciones sobre el tipo, la dosificación o el momento adecuado de la administración de corticosteroides óptimos para la prevención de la DBP en recién nacidos prematuros en función del nivel actual de evidencia. Se necesita de manera urgente un ECA bien diseñado y de tamaño grande para establecer el régimen de dosificación de corticosteroides sistémicos posnatal óptimo».
Aparte de los estudios incluidos en esta revisión, no se conocen otras comparaciones directas del uso temprano de corticosteroides inhalados versus corticosteroides sistémicos.
De qué manera podría funcionar la intervención
Se piensa que parte de la causa de la DBP puede residir en la inflamación de los pulmones (Nelin 2017). Como parte de un ensayo aleatorizado y controlado con placebo del tratamiento temprano con beclometasona inhalada, Gupta 2000 midió las concentraciones de interleucina‐8 (IL‐8) y del antagonista del receptor de interleucina‐1 (IL‐1ra) en los aspirados traqueales como marcadores de la inflamación pulmonar. Los recién nacidos tratados con beclometasona con niveles iniciales de IL‐8 moderadamente elevados recibieron menos tratamiento sistémico posterior con glucocorticosteroides y tuvieron una menor incidencia de DBP que los recién nacidos no tratados. Gupta 2000 y sus coautores establecieron la conclusión de que el tratamiento con beclometasona inhalada a tiempo se asociaba con una reducción de la inflamación pulmonar después de una semana de tratamiento. Los corticosteroides, cuando se administran por vía oral o intravenosa, reducen esta inflamación en los pulmones (Nelin 2017). El uso de corticosteroides se ha asociado con parálisis cerebral y con el retraso del desarrollo (AAP & CPS 2002; Nelin 2017). En un estudio retrospectivo de recién nacidos < 29 semanas de edad posmenstrual y evaluados entre los 18 y los 21 meses de edad corregida, se encontró que la exposición a esteroides inhalados no estaba asociada con el aumento de las probabilidades de muerte o de deterioro del neurodesarrollo (Kelly 2017). Sin embargo, en el mismo estudio el uso de esteroides sistémicos antes de las cuatro semanas de edad se asoció con resultados significativamente peores (Kelly 2017). Es importante reducir al mínimo la exposición a los efectos potencialmente nocivos de los corticosteroides, en particular en el cerebro en desarrollo (Doyle 2017).
Por qué es importante realizar esta revisión
Las revisiones Cochrane han considerado el uso de corticosteroides sistémicos o inhalados en la prevención o el tratamiento de la DBP o de la enfermedad pulmonar crónica. Las mismas incluyen revisiones del uso temprano (< 8 días) de corticosteroides sistémicos posnatales para prevenir la enfermedad pulmonar crónica (Doyle 2014) y el uso tardío (> 7 días) de corticosteroides sistémicos posnatales para la enfermedad pulmonar crónica (Doyle 2014a).
Otras revisiones Cochrane consideran el uso de corticosteroides inhalados en la prevención o el tratamiento de la enfermedad pulmonar crónica. En Shah 2017 se examinaron los efectos de la administración temprana de corticosteroides inhalados para prevenir la enfermedad pulmonar crónica en los recién nacidos prematuros de muy bajo peso al nacer sometidos a ventilación y en Onland 2017a se examinó el uso tardío (≥ 7 días) de corticosteroides inhalados para reducir la displasia broncopulmonar en los recién nacidos prematuros.
Las revisiones Cochrane también han comparado los corticosteroides sistémicos e inhalados. Shah 2012 comparó el uso de corticosteroides inhalados versus sistémicos para la prevención de enfermedades pulmonares crónicas en recién nacidos prematuros de muy bajo peso al nacer sometidos a ventilación, y el uso de corticosteroides inhalados versus sistémicos para el tratamiento de enfermedades pulmonares crónicas en recién nacidos prematuros de muy bajo peso al nacer sometidos a ventilación (Shah 2012a).
En las revisiones Cochrane se ha evaluado el uso de corticosteroides para otras indicaciones en los recién nacidos, incluida la dexametasona intravenosa para facilitar la extubación (Davis 2001), los corticosteroides para el tratamiento de la hipotensión (Ibrahim 2011) y los corticosteroides para el tratamiento del síndrome de aspiración de meconio (Ward 2003).
En las declaraciones publicadas por la European Association of Perinatal Medicine (Halliday 2001a), la American Academy of Pediatrics (Watterberg 2010) y la Canadian Pediatric Society (Jefferies 2012) no se recomienda el uso habitual de dexametasona sistémica para la prevención o el tratamiento de la DBP. Esta recomendación se basó en las preocupaciones con respecto a las complicaciones a corto y largo plazo, especialmente la parálisis cerebral.
Por lo tanto, es necesario investigar alternativas al tratamiento con corticosteroides sistémicos que quizás presenten menos consecuencias adversas. La administración tópica de corticosteroides a través de las vías respiratorias puede tener efectos beneficiosos en el sistema pulmonar con menos efectos secundarios sistémicos no deseados.
El objetivo de esta revisión fue examinar la efectividad del tratamiento con corticosteroides inhalados versus sistémicos administrados a recién nacidos de muy bajo peso al nacer de 1500 g o menos que dependían del respirador después de la primera semana de vida, para el tratamiento de la progresión de la DBP. Esta es una actualización de la revisión publicada por última vez en 2012 (Shah 2012a).
Objetivos
El objetivo principal fue comparar la efectividad de los corticosteroides inhalados versus corticosteroides sistémicos administrados a los recién nacidos prematuros dependientes del respirador con un peso al nacer ≤ 1500 g o una edad gestacional ≤ 32 semanas después de siete días de vida sobre la incidencia de muerte o displasia broncopulmonar (DBP) a las 36 semanas de edad posmenstrual.
Objetivos secundarios
Comparar la efectividad de los corticosteroides inhalados versus corticosteroides sistémicos en otros indicadores de la DBP, la incidencia de eventos adversos y el resultado del neurodesarrollo a largo plazo.
Métodos
Criterios de inclusión de estudios para esta revisión
Tipos de estudios
Ensayos clínicos aleatorizados o cuasialeatorizados.
Tipos de participantes
Recién nacidos prematuros dependientes del respirador con un peso al nacer ≤ 1500 g o una edad gestacional ≤ 32 semanas y una edad posnatal de más de siete días de edad.
Tipos de intervenciones
Corticosteroides inhalados en comparación con corticosteroides sistémicos, independientemente del tipo, la dosis y la duración del tratamiento, siempre que el tratamiento se iniciara antes de los siete días de edad.
Tipos de medida de resultado
Para las siguientes dos comparaciones:
1. Esteroides inhalados versus esteroides sistémicos en recién nacidos ‐ los resultados incluyen las muertes (recién nacidos asignados al azar a < 72 horas de edad)
2. Esteroides inhalados versus esteroides sistémicos en recién nacidos ‐ los resultados incluyen las muertes (recién nacidos asignados al azar entre los 12 y los 21 días de edad)
Resultados primarios
-
Muerte o displasia broncopulmonar (DBP) a las 36 semanas de edad posmenstrual.
Resultados secundarios
-
Muerte o DBP a los 28 días de edad
-
Muerte a las 36 semanas de edad posmenstrual
-
Muerte a los 28 días de edad
Para la siguiente comparación:
3. Esteroides inhalados versus esteroides sistémicos en los recién nacidos ‐ (recién nacidos asignados al azar a < 72 horas de edad o entre los 12 y los 21 días de edad)
Resultados secundarios
-
DBP a las 36 semanas de edad posmenstrual (requerimiento de oxígeno suplementario a las 36 semanas de edad posmenstrual)
-
DBP a los 28 días de edad (requerimiento de oxígeno suplementario a los 28 días de edad)
-
Necesidad de ventilación entre los supervivientes a las 36 semanas de edad posmenstrual
-
Duración de la ventilación mecánica entre los supervivientes (días)
-
Duración de la administración de oxígeno suplementario entre los supervivientes (días)
-
Duración de la estancia en el hospital entre los supervivientes (días)
-
Hemorragia intraventricular grado III‐IV (definida según Papile 1978)
-
Leucomalacia periventricular (definida como quistes en el área periventricular en el ultrasonido o la TC)
-
Hiperglucemia (definida como un nivel de glucosa en sangre > 10 mmol/L) durante el curso de la intervención
-
Hipertensión (definida como presión arterial sistólica o diastólica > 2 desviaciones estándar [DE] por encima de la media de edad gestacional y posnatal del recién nacido [Zubrow 1995]) durante la intervención
-
Enterocolitis necrotizante (Bell, etapas II y III) (Bell 1978)
-
Hemorragia gastrointestinal (definida como presencia de aspirados nasogástricos u orogástricos sanguinolentos)
-
Retinopatía del prematuro ≥ etapa 3 (ICROP 1984)
-
Sepsis comprobada por cultivo
-
Supresión del eje hipotalámico‐pituitario‐suprarrenal evaluado con la prueba de estimulación de la hormona adrenocorticotropa (ACTH, por sus siglas en inglés) o metirapona
-
Conducto arterioso permeable definido por la presencia de signos o síntomas clínicos o por demostración con ecocardiografía
-
Miocardiopatía hipertrófica definida como engrosamiento del tabique intraventricular o de la pared del ventrículo izquierdo en la ecocardiografía; sepsis definida por la presencia de síntomas y signos clínicos de infección y un cultivo positivo de un sitio normalmente estéril
-
Neumonía basada en signos clínicos y radiológicos y en un cultivo positivo en la muestra de aspiración endotraqueal
-
Crecimiento (peso, largo/altura y perímetro cefálico) a las 36 semanas de edad posmenstrual; cataratas (definidas por la presencia de opacidades en el cristalino)
-
Hipertrofia de la lengua
-
Nefrocalcinosis (definida por la presencia de ecodensidades en la médula renal en la ecografía) (Saarela 1999)
Resultado del neurodesarrollo a largo plazo (en los recién nacidos supervivientes)
-
El deterioro del neurodesarrollo se definió como la presencia de parálisis cerebral o deterioro mental (escalas de Bayley de desarrollo infantil, Mental Developmental Index < 70) o ceguera legal (agudeza visual < 20/200) o sordera (con ayuda o < 60 dB en las pruebas audiométricas) evaluado a los 18 a 24 meses.
Se presentaron los siguientes resultados a los siete años de edad: estos análisis post hoc se basaron en los datos disponibles de una submuestra del estudio Halliday 2001
-
British Ability Scales, Second Edition (proporciona una medida global del funcionamiento cognitivo, la puntuación de la habilidad conceptual general [GCA, por sus siglas en inglés], con una media estandarizada de 100 y DE de 15)
-
Escalas de actividades, sociales y de competencia escolar de la Child Behaviour Checklist (CBCL) para niños de cuatro a 18 años de edad
-
Strengths and Difficulties Questionnaire (SDQ) del cual se derivan las puntuaciones generales de los problemas de comportamiento, emocionales, de conducta, de hiperactividad y con los pares
-
Parálisis cerebral
-
La discapacidad grave se definió como una puntuación de GCA < 55; incapacidad para caminar de forma independiente y para vestirse o alimentarse por sí mismo, necesidad de oxigenoterapia domiciliaria continua, alteración de la conducta que requiere supervisión constante, ausencia de visión útil, o ausencia de audición útil
-
La discapacidad moderada se definió como un puntaje de la GCA de 55 a 69; movilidad restringida, ingreso en una UCI y ventilación en el último año, derivación secundaria para obtener ayuda especializada con el comportamiento, capacidad de ver solo movimientos bruscos o pérdida de audición no corregida con ayuda
-
Muerte o discapacidad moderada/grave
-
Presión arterial sistólica > percentil 95
-
Presión arterial diastólica > percentil 95
-
Diagnosticado en algún momento como asmático alrededor de los siete años de edad
-
La discapacidad grave se definió como una puntuación de GCA < 55; incapacidad para caminar de forma independiente y para vestirse o alimentarse por sí mismo, necesidad de oxigenoterapia domiciliaria continua, alteración de la conducta que requiere supervisión constante, ausencia de visión útil, o ausencia de audición útil
-
La discapacidad moderada se definió como un puntaje de la GCA de 55 a 69; movilidad restringida, ingreso en una UCI y ventilación en el último año, derivación secundaria para obtener ayuda especializada con el comportamiento, capacidad de ver solo movimientos bruscos o pérdida de audición no corregida con ayuda
-
Muerte o discapacidad moderada/grave
-
Presión arterial sistólica > percentil 95
-
Presión arterial diastólica > percentil 95
-
Diagnosticado en algún momento como asmático alrededor de los siete años de edad
Métodos de búsqueda para la identificación de los estudios
Búsquedas electrónicas
We used the criteria and standard methods of Cochrane and Cochrane Neonatal for the 2017 update (see the Cochrane Neonatal search strategy for specialized register).
We conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL 2017, Issue 1) in The Cochrane Library; MEDLINE via PubMed (1 January 2011 to 23 February 2017); Embase (1 January 2011 to 23 February 2017); and CINAHL (1 January 2011 to 23 February 2017) using the following search terms: (bronchopulmonary dysplasia OR lung diseases OR chronic lung disease OR BPD OR CLD) AND ((anti‐inflammatory agents OR steroid* OR dexamethasone OR budesonide OR beclomethasone dipropionate OR flunisolide OR fluticasone propionate OR corticosteroid* OR betamethasone OR hydrocortisone) AND (inhalation OR aerosol OR inhale*)), plus database‐specific limiters for RCTs and neonates. See Appendix 1 for previous search methodologies and Appendix 2 for the full search strategies for each database searched for the 2017 update.
We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization International Trials Registry Platform (WHO ICTRP); and the ISRCTN Registry). We searched Abstracts2View for abstracts from the Pediatric Academic Societies Annual Meetings from 2010 to 2016.
Búsqueda de otros recursos
We searched the reference lists of identified trials.
Obtención y análisis de los datos
We used the methods of the Cochrane Neonatal Review Group for data collection and analysis.
Selección de los estudios
Three review authors (SS, AO, VS) independently screened titles and abstracts for inclusion of all potential studies identified as a result of the search.
We retrieved full‐text study reports and three review authors (SS, AO, VS) independently screened the reports and identified studies for inclusion, and noted and recorded reasons for exclusion of ineligible studies. We resolved disagreement through discussion or, if required, we consulted a fourth review author (HH). We identified and excluded duplicates and collated multiple reports of the same study so that each study rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Figure 1) and Characteristics of excluded studies. We did not impose any language restrictions
Extracción y manejo de los datos
For each trial, information was sought regarding the method of randomisation, blinding and reporting of outcomes of all infants enrolled in the trial. Data from primary investigators were obtained for unpublished trials or when published data were incomplete. Retrieved articles were assessed and data extracted independently by four review authors (SS, VS, AO, HH). This update was performed by two review authors (VS, AO). Discrepancies were resolved by discussion and consensus.
For each study, data were entered into RevMan by one review author and checked for accuracy by a second reviewer author. We resolved discrepancies through discussion.
We attempted to contact authors of original reports to provide further details when information in published reports was unclear.
Evaluación del riesgo de sesgo de los estudios incluidos
Three review authors (VS, SS, AO) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool (Higgins 2011) for the following domains:
-
Sequence generation (selection bias).
-
Allocation concealment (selection bias).
-
Blinding of participants and personnel (performance bias).
-
Blinding of outcome assessment (detection bias).
-
Incomplete outcome data (attrition bias).
-
Selective reporting (reporting bias).
-
Other bias.
Disagreements were resolved by discussion or by consulting a third review author. See Appendix 3 for a more detailed description of risk of bias for each domain.
Medidas del efecto del tratamiento
We performed statistical analyses using Review Manager software (Review Manager 2014). Dichotomous data were analysed using relative risk (RR), risk difference (RD) and the number needed to benefit (NNTB) or number needed to harm (NNTH). The 95% confidence interval (CI) was reported for all estimates.
We analysed continuous data using weighted mean difference (WMD) or the standardised mean difference (SMD) to combine trials that measured the same outcome but used different methods.
Cuestiones relativas a la unidad de análisis
For clinical outcomes, such as episodes of sepsis, we analysed data as the proportion of neonates having one or more episodes.
Manejo de los datos faltantes
Levels of attrition were noted for the included studies. The impact of including studies with high levels of missing data in the overall assessment of treatment effect was explored by conducting sensitivity analyses.
All outcome analyses were on an intention‐to‐treat basis i.e. we included all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Evaluación de la heterogeneidad
We examined heterogeneity among trials by inspecting the forest plots and quantifying the impact of heterogeneity using the I² statistic. If noted, we planned to explore the possible causes of statistical heterogeneity using prespecified subgroup analysis (e.g. differences in study quality, participants, intervention regimens, or outcome assessments). Heterogeneity tests, including the I² statistic, were performed to assess the appropriateness of pooling data. We used the following criteria to describe heterogeneity: < 25% no heterogeneity, ≥ 25% to 49% low heterogeneity, ≥ 50% to 74% moderate heterogeneity and ≥ 75% high heterogeneity.
Evaluación de los sesgos de notificación
We planned to assess possible publication bias and other biases by inspecting the symmetry or asymmetry of funnel plots had there been at least 10 trials included in an analysis.
For included trials that were recently performed (and therefore prospectively registered), we explored possible selective reporting of study outcomes by comparing the primary and secondary outcomes in the reports with the primary and secondary outcomes proposed at trial registration, using the web sites www.clinicaltrials.gov and www.controlled‐trials.com. If such discrepancies were found, we planned to contact the primary investigators to obtain missing outcome data on outcomes pre‐specified at trial registration.
Síntesis de los datos
Meta‐analysis was conducted using Review Manager software (Review Manager 2014). We used the Mantel‐Haenszel method for estimates of typical relative risk and risk difference. We analysed continuous measures using the inverse variance method. We used the fixed‐effect model for all meta‐analyses.
Quality of evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) primary outcomes: death or BPD at 36 weeks' postmenstrual age for infants randomised at < 72 hours of age and for infants randomised between 12 and 21 days of age. For secondary outcomes we included infants randomised at < 72 hours or between 12 and 21 days; BPD at 36 weeks' postmenstrual age; hyperglycaemia; hypertension. For infants randomised at < 72 hours of age we included the following outcomes at 7 years of age: general conceptual ability (GCA); moderate/severe disability; death or moderate/severe disability; systolic blood pressure > 95th percentile; diastolic blood pressure > 95th percentile; and ever diagnosed as asthmatic.
Three authors (VS, SS, AO) independently assessed the quality of the evidence for each of these outcomes. We considered evidence from randomised controlled trials as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias. We used GRADEpro GDT to create ‘Summary of findings’ tables to report the quality of the evidence.
The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades:
-
High: We are very confident that the true effect lies close to that of the estimate of the effect.
-
Moderate: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
-
Low: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
-
Very low: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Análisis de subgrupos e investigación de la heterogeneidad
Groups were analysed based on all randomised and survivors only.
Análisis de sensibilidad
We planned to conduct sensitivity analyses for situations where this might affect the interpretation of significant results (e.g. where there is risk of bias associated with the quality of some of the included trials or missing outcome data). However, it was determined this was unnecessary for this review.
Results
Description of studies
Three trials were identified and included in the previous review. The updated searches of databases and trials registers in 2017 identified a total of 427 records. After removal of duplicates, we assessed 395 records by title and abstract and excluded all 395 records. The study flow from the searches is illustrated in Figure 1. One study received grant support and the industry provided Aerochambers and metered dose inhalers of budesonide and placebo for the same study. No conflict of interest was identified.
Results of the search
Five trials comparing inhaled versus systemic corticosteroids in treatment of BPD were identified. Two trials (Dimitriou 1997; Nicholl 2002) were excluded as both included non ventilator‐dependent patients and the groups of ventilated infants could not be identified separately. No new studies were included for this update.
Included studies
Three trials fulfilled the inclusion criteria and were included in the previous update of the review (Shah 2012a): Halliday 2001; Suchomski 2002 and Rozycki 2003. Although Rozycki 2003 enrolled preterm infants with birth weights between 650 g and 2000 g, on review of the published data, 93% of the enrolled infants had birth weights < 1000 g with postmenstrual age ranging from 23 to 31 weeks. Therefore, data from this trial were included in this review. Details of each trial are given in Characteristics of included studies.
Halliday 2001 enrolled infants born at < 30 weeks gestation, postnatal age < 72 hours, needing mechanical ventilation and fractional inspired oxygen concentration (FiO₂) > 0.30. Infants of 30 and 31 weeks gestation could also be included if they needed FiO₂ > 0.50. Infants with lethal congenital anomalies, severe intraventricular haemorrhage (grade 3 or 4), or proven systemic infection before entry were excluded from the trial. The trial was designed to evaluate the effectiveness of early (< 72 hours) or delayed (> 15 days) administration of systemic dexamethasone or inhaled budesonide. Infants were randomly allocated to one of four treatment groups in a factorial design: early (< 72 hours) dexamethasone, early budesonide, delayed selective (> 15 days) dexamethasone and delayed selective budesonide. Only the delayed budesonide and delayed dexamethasone groups are included in this review. Halliday 2001 randomised 142 babies to delayed selective budesonide and 150 to delayed selective dexamethasone groups. Budesonide was administered by metered dose inhaler and a spacing chamber at 400 µg/kg twice daily for 12 days. Dexamethasone was given intravenously (IV) or orally in a tapering course beginning with 0.5 mg/kg/day in two divided doses for three days reducing by half every three days for a total of 12 days of therapy. Delayed selective treatment was started if infants needed mechanical ventilation and more than 30% oxygen for > 15 days. Of 142 infants randomised to the delayed selective budesonide group, 33 received a full course, 21 received a partial course and 88 babies did not receive budesonide. Of 150 infants randomised to the delayed selective dexamethasone group, 35 received a complete course, 25 received a partial course and 90 infants did not receive dexamethasone. An intention‐to‐treat analysis was performed. The primary outcome was death or oxygen dependency at 36 weeks. Secondary outcome measures included death or major cerebral abnormality, duration of oxygen treatment, and complications of preterm birth. Long‐term outcomes at 7 years of age were assessed in a sample from the UK and Ireland by assessors blinded to the treatment assignments.
Suchomski 2002 compared inhaled beclomethasone, either 400 or 800 µg/d, to intravenous dexamethasone in preterm infants dependent on conventional mechanical ventilation and supplemental oxygen at two weeks of age. The study included 78 preterm infants with birth weight ≤ 1500 g, gestational age ≤ 30 weeks and ventilatory dependence at 12 to 21 days of age with rate > 15/min and FiO₂ > 0.30 with a persistence of these ventilator settings for a minimum of 72 hours. Infants on high frequency ventilation were ineligible for inclusion in the study. Infants were excluded from the study if they had major congenital malformations, culture‐proven sepsis, hypertension or hyperglycaemia needing treatment, or persistent patent ductus arteriosus. Infants were randomly assigned to one of the three treatment groups: inhaled beclomethasone at 400 µg/d or 800 µg/d, or intravenous dexamethasone. Inhaled beclomethasone was continued until extubation. Post‐extubation the same dose was continued for another 48 hours. After that, the dose was halved every other day for six days, after which the steroids were stopped. Based on our inclusion criteria (to include all studies regardless of dosage of inhaled steroids), and because there was no significant difference in the effects of the two different doses, the two inhaled beclomethasone groups in Suchomski 2002 were combined to form one group in the present review. Intravenous dexamethasone was given as a 42 day tapering course starting with 0.5 mg/kg/day in two divided doses (Avery 1985). Cross‐over from either of the inhaled beclomethasone groups to intravenous dexamethasone was allowed if after four to five days of inhaled beclomethasone, the infant's ventilator and oxygen support had not decreased and the attending neonatologist felt that the infant could benefit from intravenous dexamethasone. It was reported that 18 infants from the inhaled steroid group crossed over to systemic dexamethasone. An intention‐to‐treat analysis was performed by the investigators. Outcome measures included adverse effects including sepsis, hypertension and hyperglycaemia; short‐term ventilatory requirements, duration of mechanical ventilation, duration of supplemental oxygen, length of stay in the hospital and need for respiratory support at 28 days or 36 weeks' postmenstrual age. Deaths at 36 weeks' postmenstrual age were not reported. For infants completing a 10 day course of either inhaled or intravenous steroids, an adrenocorticotropic hormone (ACTH) stimulation test was done two weeks after completion of the steroid course.
Rozycki 2003 enrolled 61 preterm infants with birth weights between 650 g and 2000 g who at 14 days of age were at significant risk of developing moderate to severe BPD (defined as the need for mechanical ventilation and oxygen) with x‐ray changes beyond 28 days of life. Infants with culture‐proven sepsis and who were receiving FiO₂ of ≥ 0.30 were eligible if they had a ventilatory index (10,000/ventilator rate x peak pressure x pCO₂) of < 0.8. Infants without previous sepsis were eligible if the ventilatory index was < 0.51. Infants meeting these criteria had a 75% risk of developing moderate‐severe BPD. Infants with the following were excluded: pre‐existing hyperglycaemia with blood glucose > 200 mg/dL for > 24 hours, hypertension with systolic pressures > 70 to 90 mm Hg, depending on birth weight, surgery within previous seven days, active bacterial infection unless repeat blood, urine or cerebrospinal fluid cultures were sterile after 72 hours of antibiotics, thrombocytopenia with platelet count < 100,000, any gastrointestinal bleeding within the previous seven days, significant weaning from ventilator support in the previous three days and previous exposure to postnatal steroids. Eligibility was determined at 14 days of age but the study could be delayed up to six days while awaiting resolution of infections. Infants were randomised to the following four groups: Group A: aerosol placebo‐systemic dexamethasone; Group B: high beclomethasone‐systemic placebo; Group C: medium beclomethasone‐systemic placebo; and Group D: low beclomethasone‐systemic placebo. Those receiving aerosol steroids who remained ventilator‐dependent after seven days were switched to standard 42‐day tapering doses of systemic dexamethasone. The primary outcome variable was extubation within the first seven days of the study. Secondary outcome measures included: changes in ventilatory settings and oxygen delivery over the first seven days, the incidence of hypertension, hyperglycaemia, infection and growth.
Excluded studies
We excluded two trials (Dimitriou 1997; Nicholl 2002). Both trials included non ventilator‐dependent participants and the groups of ventilated infants could not be identified separately. See Characteristics of excluded studies.
Studies awaiting classification
There are no studies awaiting classification.
Ongoing studies
Our searches did not find any ongoing studies.
Risk of bias in included studies
The risk of bias in the included trials are illustrated in Figure 2 and Figure 3.
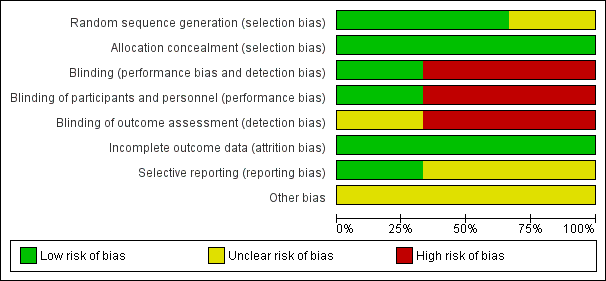
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
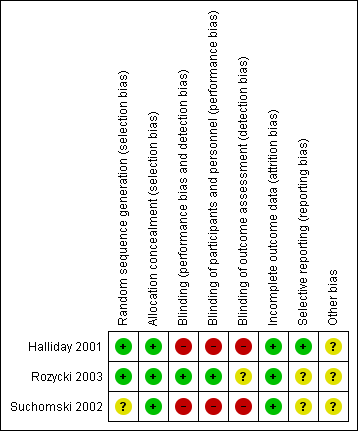
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
The risk of bias is taken into account in the 'Summary of findings' tables for the primary outcome and important secondary outcomes (summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3; summary of findings Table 4). Reasons for downgrading the quality of evidence is explained in the comments columns in 'Summary of findings' tables.
There were elements of risk of bias in the three included studies. For details see the information below.
Allocation
In the study by Halliday 2001 after identifying an eligible infant, the clinician telephoned the randomisation centre to enrol the infant and to determine the treatment group (low risk of bias).
Suchomski 2002 was a prospective randomised controlled trial. Three sets of 27 cards were assembled followed by placement of one card each into one of 81 opaque envelopes. As infants were enrolled, a card was sequentially pulled and the infant assigned to the appropriate study group (low risk of bias).
Rozycki 2003 was a prospective randomised double‐blind controlled trial. The infants were randomised using a random table number and only the pharmacy was aware of the individual group assignment (low risk of bias).
Blinding
Halliday 2001 was a multi‐centre RCT involving 47 centres. The interventions and outcome measures were not blinded in all the centres (high risk of bias). However, in 11 centres the trial was conducted double blind. In these centres, placebo metered dose inhalers and intravenous saline were used to mask treatment allocation. Comparisons were made for the primary outcome variables between the centres observing double blind strategy and the other centres. The long‐term assessments at 7 years of age were performed by assessors blinded to the group assignments (low risk of bias).
In Suchomski 2002 blinding of the intervention was not performed (high risk of bias). Blinding of outcome measurement was not ensured (high risk of bias). Cross‐over from inhaled steroid groups to intravenous dexamethasone was allowed at the discretion of attending neonatologist.
In Rozycki 2003 blinding of intervention was performed (low risk of bias). Blinding of outcome measurement was unclear (unclear risk of bias).
Incomplete outcome data
There was complete follow up of all randomised infants in all three studies (low risk of bias in all three studies).
Selective reporting
There was no selective reporting in the trial by Halliday 2001 (low risk of bias ). The protocols for the other trials were not available, so we can not judge if there were any deviations or not.
Other potential sources of bias
We are not aware of any other sources of bias in the included trials (unclear risk).
Effects of interventions
See: Summary of findings for the main comparison Inhaled steroids compared with systemic steroids for BPD (infants randomised at < 72 hours of age); Summary of findings 2 Inhaled steroids compared with systemic steroids for BPD (infants randomised between 12 and 21 days of age); Summary of findings 3 Inhaled steroids compared with systemic steroids for BPD (infants randomised at < 72 hours or between 12 and 21 days of age); Summary of findings 4 Inhaled steroids compared with systemic steroids for BPD ‐ long‐term outcomes at 7 years of age (infants randomised at < 72 hours of age)
Halliday 2001 randomised infants at < 72 hours of age; Suchomski 2002 randomised infants at 12 to 21 days; and Rozycki 2003 randomised after 14 days of age. All trials reported outcomes from the age of randomisation. Although infants received steroids after the first two weeks of life in all trials, the time period over which outcomes were measured differed among studies. Data from all three trials were combined for meta‐analyses of secondary outcomes (Halliday 2001; Suchomski 2002; Rozycki 2003).
For outcomes that included death we performed separate analyses for Halliday 2001 which reported on deaths from randomisation at < 72 hours and we combined results from Suchomski 2002 and Rozycki 2003.
1. Inhaled versus systemic steroids among infants ‐ outcomes including deaths (infants randomised at < 72 hours of age)
Because only Halliday 2001 was included in these analyses, tests for heterogeneity were not applicable.
Primary outcome
Death or BPD at 36 weeks' postmenstrual age
There was no statistically significant difference between the groups for the combined outcome of death or BPD at 36 weeks' postmenstrual age (RR 1.04, 95% CI 0.86 to 1.26; RD 0.03, 95% CI ‐0.09 to 0.14; 1 study, N = 292; Analysis 1.1; moderate‐quality evidence).
Secondary outcomes
Death or BPD at 28 days of age
There was no statistically significant difference between the groups for the combined outcome of death or BPD at 28 days of age (RR 1.00, 95% CI 0.90 to 1.12; RD 0.00, 95% CI ‐0.09 to 0.09; 1 study, N = 292; Analysis 1.2).
Death at 36 weeks' postmenstrual age
There was no statistically significant effect on death at 36 weeks' postmenstrual age between groups (RR 0.96, 95% CI 0.62 to 1.49; RD ‐0.01, 95% CI ‐0.10 to 0.09; 1study, N = 292; Analysis 1.3).
Death at 28 days of age
No statistically significant effect on mortality by 28 days was noted between the groups (RR 0.85, 95% CI 0.52 to 1.37; RD ‐0.03, 95% CI ‐0.12 to 0.06; 1 study, N = 292; Analysis 1.4).
2. Inhaled versus systemic steroids among infants ‐ outcomes including deaths (infants randomised between 12 and 21 days of age)
Because only Suchomski 2002 was included in these analyses, tests for heterogeneity were not applicable. Rozycki 2003 did not report on deaths at 36 weeks' PMA or at 28 days of age.
Primary outcome
Death or BPD at 36 weeks' postmenstrual age
There was no statistically significant difference between groups for the combined outcome of death or BPD at 36 weeks' postmenstrual age (RR 0.94, 95% CI 0.83 to 1.05; RD ‐0.06, 95% CI ‐0.17 to 0.05; 1 study, N = 78; Analysis 2.1; low‐quality evidence).
Secondary outcomes
Death at 36 weeks' postmenstrual age
There was no statistically significant effect on death at 36 weeks' postmenstrual age between groups (RR 2.69, 95% CI 0.13 to 54.15; RD 0.04, 95% CI ‐0.04 to 0.12; 1 study, N = 78; Analysis 2.2).
Death at 28 days of age
No statistically significant effect on mortality by 28 days was noted between groups (RR 2.69, 95% CI 0.13 to 54.15; RD 0.04, 95% CI ‐0.04 to 0.12; 1 study, N = 78; Analysis 2.3).
3. Inhaled versus systemic steroids among infants ‐ secondary outcomes (infants randomised at < 72 hours of age or between 12 and 21 days of age)
Secondary outcomes
BPD at 36 weeks' postmenstrual age
There was no statistically significant difference in the incidence of BPD at 36 weeks' postmenstrual age in the inhaled steroid compared to systemic steroid group (typical RR 1.08, 95% CI 0.88 to 1.32; typical RD 0.03, 95% CI ‐0.06 to 0.12; 3 studies, N = 429; Analysis 3.1; low‐quality evidence). There was low heterogeneity for both RR (39%) and RD (28%).
BPD at 28 days of age
There was no statistically significant difference in the incidence of BPD at 28 days between groups (typical RR 1.04, 95% CI 0.91 to 1.18; typical RD 0.03, 95% CI ‐0.06 to 0.12; 2 studies, N = 368; Analysis 3.2). There was low heterogeneity for both RR (46%) and RD (0 %).
Need for ventilation amongst survivors at 36 weeks' postmenstrual age
There was no statistically significant difference for this outcome between groups (RR 1.10, 95% CI 0.30 to 4.06; RD 0.01, 95% CI ‐0.14 to 0.16; 1 study, N = 76; Analysis 3.3).
Duration of mechanical ventilation among survivors (days)
The duration of mechanical ventilation was not statistically significantly different between groups (typical WMD ‐ 0.3 days, 95% CI ‐5.2 to 4.6; 2 studies, N = 368; Analysis 3.4). There was no heterogeneity for WMD (0%).
Duration of supplemental oxygen among survivors (days)
The duration of supplemental oxygen was not statistically significantly different between groups (typical WMD ‐4.91 days, 95% CI ‐21 to 11; 2 studies, N = 368; Analysis 3.5).
Length of hospital stay among survivors (days)
There was no statistically significant difference in the length of hospital stay among survivors between groups (MD ‐13, 95% CI ‐33 to 7; 1 study, N = 76; Analysis 3.6). Test for heterogeneity not applicable.
Intraventricular haemorrhage grade III‐IV
There was no statistically significant difference in length of hospital stay among survivors between groups (RR 0.90, 95% CI 0.33 to 2.40; RD ‐0.03, 95% CI ‐0.28 to 0.23; 1 study, N = 61; Analysis 3.7). Test for heterogeneity not applicable.
Periventricular leukomalacia
There was no statistically significant difference in the incidence of periventricular leukomalacia between groups (typical RR 0.85, 95% CI 0.34 to 2.13; typical RD ‐0.02, 95% CI ‐0.15 to 0.10; 2 studies, N = 137; Analysis 3.8). There was no heterogeneity for either RR (0%) or RD (0%).
Hyperglycaemia
There was no statistically significant difference in the incidence of hyperglycaemia between groups (typical RR 0.86, 95% CI 0.61 to 1.22; typical RD ‐0.03, ‐0.11 to 0.05; 3 studies, N = 429; Analysis 3.9; low‐quality evidence). There was no heterogeneity for either RR (8%) or RD (0 %).
Hypertension
There was no statistically significant difference in the incidence of hypertension between groups (typical RR 0.86, 95% CI 0.73 to 1.01; typical RD ‐0.08, 95% Ci ‐0.17 to 0.00; 3 studies, N = 429; Analysis 3.10; low‐quality evidence). There was no heterogeneity for either RR (0%) or RD (0%).
Necrotising enterocolitis
There was no statistically significant difference in the incidence of necrotising enterocolitis between groups (typical RR 0.96, 95% CI 0.50 to 1.85; typical RD ‐0.00, 95% CI ‐0.06 to 0.06; 2 studies, N = 368; Analysis 3.11). There was no heterogeneity for either RR (0%) or RD (0%).
Gastrointestinal bleed
There was no statistically significant difference in the incidence of gastrointestinal bleed between groups (typical RR 0.89, 95% CI 0.41 to 1.93; typical RD ‐0.01, 95% CI ‐0.06 to 0.04; 2 studies, N = 368; Analysis 3.12). As there were no outcomes in either group in one trial, test for heterogeneity for RR was not applicable. There was no heterogeneity for RD (0%).
Retinopathy of prematurity ≥ stage 3
There was no statistically significant difference in the incidence of retinopathy of prematurity between groups (typical RR 1.32, 95% CI 0.77 to 2.25; typical RD 0.04, 95% CI ‐0.03 to 0.11; 3 studies, N = 363; Analysis 3.13). There was no heterogeneity for either RR (0%) or RD (0%).
Culture‐proven sepsis
There was no statistically significant difference in the incidence of culture‐proven sepsis between groups (typical RR 1.07, 95% CI 0.79 to 1.45; RD 0.02, 95% CI ‐0.07 to 0.12; 2 studies, N = 368; Analysis 3.14). There was no heterogeneity for either RR (0%) or RD (0%).
Other outcomes
Adrenocorticotropic hormone (ACTH) stimulation test
Suchomski 2002 reported that the ACTH test was completed for 24 infants. The baseline cortisol levels before the ACTH stimulation test for the 800 µg/d inhaled group (3 ± 2.3 µg/dL; N = 7) and the intravenous group (1.6 ± 1.3 µg/dL; N = 10) were statistically significantly lower than for the 400 µg/d inhaled group (7.3 ± 4.2 µg/dL; N = 7). However, the response to ACTH (i.e. relative rise in cortisol level) was similar in all three groups: 12 ± 5.7 µg/dL in the 400 µg/d inhaled group, 15.6 ± 8.5 µg/dL in the 800 µg/d inhaled group and 10.7 ± 4.6 µg/dL in the intravenous group, P = 0.408. Post ACTH stimulation cortisol levels were 18.4 ± 8.0 µg/dL in the 800 µg/d inhaled group, 19.3 ± 5.9 µg/dL in the 400 µg/d inhaled group and 12.3 ± 5.7 µg/dL in the intravenous group, P = 0.048.
No relevant data for the following outcomes were available for analysis: measurement of pulmonary functions, growth at 36 week PMA, nephrocalcinosis, hypertrophy of tongue, cataract, pneumonia or hypertrophic cardiomyopathy.
4. Inhaled versus systemic steroids among children at 7 years of age (infants randomised at < 72 hours of age)
A subset of infants enrolled in the OSECT study (Halliday 2001) were followed to a median age of 7 years. The study followed 127 (84%) of 152 survivors from the United Kingdom and Ireland. Of these, 75 children belonged to the late budesonide and late dexamethasone groups; 38 children belonged to the late budesonide group; and 37 children to the late dexamethasone group.
Tests for heterogeneity were not applicable to any of these analyses because only one study was included in each analysis.
There were no statistically significant differences between the early inhaled and the early systemic corticosteroid groups for the following outcomes in the Halliday 2001 study which reported on 75 children.
General conceptual ability score at 7 years
There was no significant difference between the groups of infants who received inhaled or systemic steroids (MD ‐3.40, 95% CI ‐12.38 to 5.58; 1 study, N = 74; Analysis 4.1; moderate‐quality evidence).
Child Behaviour Checklist at 7 years
There was no significant difference between the groups of infants who received inhaled or systemic steroids (MD 0.20, 95% CI ‐4.75 to 5.15; 1 study, N = 74; Analysis 4.2; moderate‐quality evidence).
Strengths and Difficulties Questionnaire at 7 years
There was no significant difference between the groups of infants who received inhaled or systemic steroids, (MD 1.00, 95% CI ‐2.19 to 4.19; 1 study, N = 74; Analysis 4.3; moderate‐quality evidence).
Cerebral palsy at 7 years
There was no significant difference between the groups of infants who received inhaled or systemic steroids (RR 0.97, 95% CI 0.35 to 2.72; RD ‐0.01, 95% CI ‐0.18 to 0.17; 1 study, N = 69; Analysis 4.4; moderate‐quality evidence).
Moderate/severe disability at 7 years
There was no significant difference between the groups of infants who received inhaled or systemic steroids (RR 1.40, 95% CI 0.49 to 4.01; RD 0.05, 95% CI ‐0.11 to 0.22; 1 study, N = 74; Analysis 4.5; moderate‐quality evidence).
Death or moderate/severe disability at 7 years
There was no significant difference between the group who received inhaled steroids versus the group who received systemic steroids (RR 1.01, 95% CI 0.65 to 1.58; RD 0.00, 95% CI ‐0.18 to 0.19; 1 study, N = 107; Analysis 4.6; moderate‐quality evidence).
Systolic blood pressure > 95th percentile at 7 years
There was no significant difference between the groups of infants who received inhaled or systemic steroids (RR 0.55, 95% CI 0.25 to 1.23; RD ‐0.16, 95% CI ‐0.36 to 0.05; 1 study, N = 70; Analysis 4.7; moderate‐quality evidence).
Diastolic blood pressure > 95th percentile at 7 years
There was no significant difference between the groups of infants who received inhaled or systemic steroids (RR 1.38, 95% CI 0.43 to 4.45; RD 0.05, 95% CI ‐0.12 to 0.21; 1 study, N = 69; Analysis 4.8; moderate‐quality evidence).
Ever diagnosed with asthma by 7 years
There was no significant difference between the groups who received inhaled or systemic steroids (RR 0.87, 95% CI 0.55 to 1.39; RD ‐0.07, 95% CI ‐0.30 to 0.16; 1 study, N = 73; Analysis 4.9; moderate‐quality evidence).
Discusión
Resumen de los resultados principales
Se incluyeron tres estudios con un total de 431 recién nacidos.
La evidencia de dos estudios realizados en 370 recién nacidos que contribuyeron con datos al resultado primario de esta revisión mostró que los esteroides inhalados administrados después de los siete días de edad comparados con los esteroides sistémicos no disminuyeron la incidencia de muerte o displasia broncopulmonar (DBP) a las 36 semanas de edad posmenstrual.
La evidencia de tres estudios en 431 recién nacidos que contribuyeron a los resultados secundarios mostró que los esteroides inhalados administrados después de los siete días de edad en comparación con los esteroides sistémicos no alteraron significativamente la incidencia de DBP a las 36 semanas de edad posmenstrual, la hiperglucemia, la hipertensión, la duración de la ventilación, la duración de la administración de oxígeno suplementario, la duración de la estancia hospitalaria, la hemorragia intraventricular de grado III‐IV, la leucomalacia periventricular, la enterocolitis necrotizante, la hemorragia gastrointestinal, la retinopatía del prematuro en estadio > 3; la sepsis comprobada con cultivo o la incidencia de efectos adversos.
Un estudio recibió apoyo financiero y la industria farmacéutica proporcionó aerocámaras e inhaladores de dosis fijas de budesonida y placebo para el mismo estudio. No se identificó ningún conflicto de intereses.
Compleción y aplicabilidad general de las pruebas
Para el resultado primario de la muerte o la DBP a las 36 semanas de edad posmenstrual, los datos de Halliday 2001 fueron informados por separado ya que aleatorizaron a recién nacidos < 72 horas de edad. Suchomski 2002 informó sobre el resultado primario, pero incluyó a recién nacidos de 12 a 21 días de edad. Rozycki 2003 no informó sobre el resultado primario. Se combinaron los datos de tres ensayos (Suchomski 2002; Rozycki 2003) para los resultados secundarios cuando se inició el tratamiento en los recién nacidos > siete días de edad. El período de medición de los resultados varió entre Halliday 2001 y Suchomski 2002; lo que hace que la combinación de resultados sea inapropiada. Este hecho puede explicar posiblemente las diferencias en la incidencia de eventos adversos como la mortalidad. Halliday 2001 contó las muertes desde < 72 horas en adelante y Suchomski 2002 contó las muertes desde los 12 a los 21 días en adelante. Este hecho significa que en Halliday 2001 todas las muertes < 72 horas se atribuyeron a la política de tratamiento aleatorizado, mientras que en Suchomski 2002, solo se atribuyeron las muertes a los 12 a 21 días. Al considerar la tasa de eventos de control, se observa lo que era de esperar: una tasa de mortalidad mucho más alta en Halliday 2001 que en Suchomski 2002 (la muerte a las 36 semanas fue de 33/150 en Halliday 2001 y 0/27 en Suchomski 2002). Podrían proporcionarse explicaciones similares para los demás resultados de interés. Debido a estas preocupaciones, la agregación de los datos de los dos ensayos no se realizó cuando los resultados incluían las muertes.
Calidad de la evidencia
Según los criterios GRADE, la evaluación de la calidad de la evidencia para el resultado de la muerte o el DBP a las 36 semanas de edad posmenstrual (resultado primario) para los recién nacidos asignados al azar a < 72 horas de edad fue moderada (Resumen de resultados, tabla 1). La calidad de la evidencia según los criterios GRADE para el resultado de la muerte o la DBP a las 36 semanas de edad posmenstrual (resultado primario) para los recién nacidos asignados al azar entre los 12 y los 21 días de edad fue baja (Resumen de resultados, tabla 2).
Para los resultados secundarios se incluyó a los recién nacidos asignados al azar a < 72 horas o entre los 12 y los 21 días: DBP a las 36 semanas de edad posmenstrual, hiperglucemia e hipertensión. La calidad de la evidencia según los criterios GRADE fue baja (Resumen de resultados, tabla 3). Para los recién nacidos asignados al azar a < 72 horas de edad se incluyeron en las evaluaciones según los criterios GRADE los siguientes resultados a los siete años de edad: capacidad conceptual general; discapacidad moderada/grave; muerte o discapacidad moderada/grave; presión sanguínea sistólica > percentil 95; presión sanguínea diastólica > percentil 95 y algún diagnóstico de asma. Según los criterios GRADE, la calidad de la evidencia fue moderada para estos resultados (Resumen de resultados, tabla 4).
Sesgos potenciales en el proceso de revisión
No se conoce ningún sesgo potencial en el proceso de revisión. Un autor (HH) es el primer autor de un ensayo incluido (Halliday 2001). Dicho estudio fue evaluado por los otros tres autores (AO, SS, VS).
Acuerdos y desacuerdos con otros estudios o revisiones
Esta revisión no encontró evidencia de que los esteroides inhalados otorgaran ventajas netas sobre los esteroides sistémicos en el tratamiento de los recién nacidos prematuros dependientes del respirador. Los esteroides sistémicos administrados de forma tardía (después de una semana) sugieren que el tratamiento tardío puede reducir la mortalidad neonatal sin aumentar de forma significativa el riesgo de resultados adversos en el neurodesarrollo a largo plazo (Doyle 2014a; Shah 2001). Sin embargo, los esteroides sistémicos, en especial la dexametasona administrada a temprana edad (< 7 días de edad), se asocian con un aumento de los hallazgos anormales en los exámenes neurológicos y la parálisis cerebral (Doyle 2014). Es necesario realizar estudios adicionales con particular atención a los resultados del neurodesarrollo a largo plazo, antes de que los esteroides tardíos, ya sea inhalados o sistémicos, puedan recomendarse como seguros para el tratamiento de la DBP en recién nacidos prematuros. Los estudios de seguimiento son extremadamente importantes. Se ha hecho un seguimiento de un subconjunto de recién nacidos supervivientes del estudio OSECT (Halliday 2001) hasta los siete años de edad y los resultados se incluyen en esta revisión actualizada.
Otra preocupación importante con los estudios sobre el tratamiento con esteroides inhalados es la falta de certeza con respecto a la administración y la formación de depósitos de esteroides en la orofaringe y en las vías respiratorias periféricas. Numerosos factores afectan el suministro del fármaco y la formación de depósitos, incluido el número de partículas en el rango respirable, la técnica de administración (uso de inhalador de dosis fija con o sin espaciador), los nebulizadores (a chorro o ultrasónico) y la presencia o ausencia de sonda endotraqueal. En los informes anteriores se ha demostrado que la cantidad de administración de aerosol varía del 0,4% al 14% según la técnica utilizada (Arnon 1992; Grigg 1992; O'Callaghan 1992). El retraso en el inicio de la actividad (Dimitriou 1997) y el perfil de riesgo similar de los esteroides inhalados (Shah 2017) sugieren que los efectos pueden ser secundarios a la absorción sistémica.
Se cree que los esteroides inhalados son menos efectivos comparados con los esteroides sistémicos. El tipo, la dosificación y los métodos de administración del fármaco pueden ser inadecuados. El mayor perfeccionamiento en el sistema de administración de fármacos por inhalación que garantice una administración selectiva en los alvéolos y las vías respiratorias más pequeñas podría mejorar la eficacia clínica y reducir el perfil de efectos secundarios de los esteroides inhalados.
En la presente revisión, no hay evidencia de diferencias en la efectividad o en los perfiles de efectos secundarios para los esteroides inhalados versus sistémicos. Un mejor sistema de administración o una dosis más alta de los esteroides inhalados puede dar lugar a una equivalencia en la efectividad en las dos modalidades de administración, pero también puede mostrar evidencia de efectos secundarios. Para resolver este problema, se necesitan estudios que identifiquen la proporción riesgo/beneficio de diferentes técnicas de administración y regímenes de dosificación para la administración de estos medicamentos.

Study flow diagram: review update

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Inhaled versus systemic steroids among all randomised infants ‐ outcomes including deaths (infants randomised at < 72 h), Outcome 1 Death or BPD at 36 weeks' postmenstrual age.

Comparison 1 Inhaled versus systemic steroids among all randomised infants ‐ outcomes including deaths (infants randomised at < 72 h), Outcome 2 Death or BPD at 28 days of age.
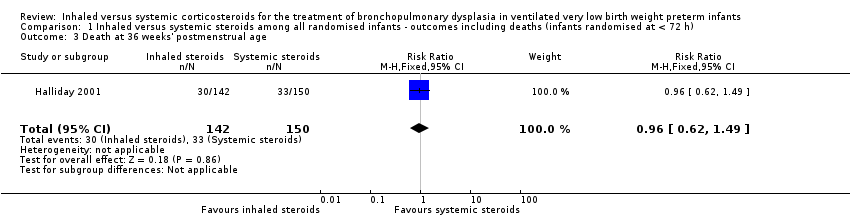
Comparison 1 Inhaled versus systemic steroids among all randomised infants ‐ outcomes including deaths (infants randomised at < 72 h), Outcome 3 Death at 36 weeks' postmenstrual age.

Comparison 1 Inhaled versus systemic steroids among all randomised infants ‐ outcomes including deaths (infants randomised at < 72 h), Outcome 4 Death at 28 days of age.

Comparison 2 Inhaled versus systemic steroids among infants ‐ outcomes including deaths (Infants randomised between 12 and 21 days of age), Outcome 1 Death or BPD at 36 weeks' postmenstrual age.
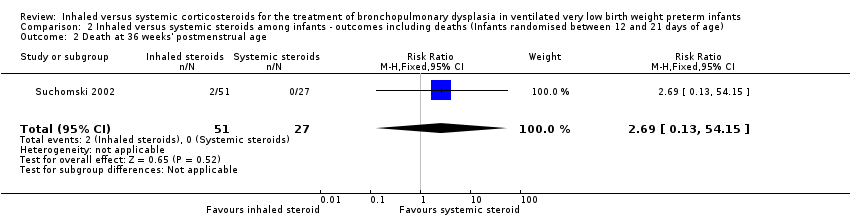
Comparison 2 Inhaled versus systemic steroids among infants ‐ outcomes including deaths (Infants randomised between 12 and 21 days of age), Outcome 2 Death at 36 weeks' postmenstrual age.

Comparison 2 Inhaled versus systemic steroids among infants ‐ outcomes including deaths (Infants randomised between 12 and 21 days of age), Outcome 3 Death at 28 days of age.

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 1 BPD at 36 weeks' postmenstrual age.
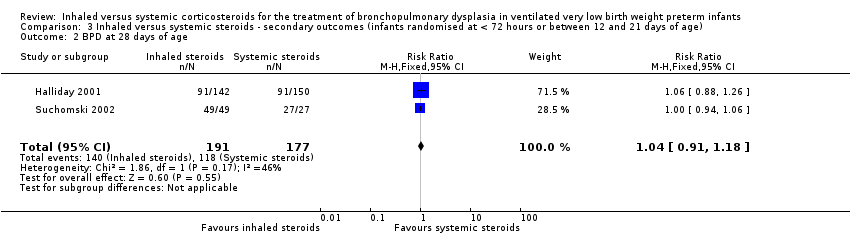
Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 2 BPD at 28 days of age.

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 3 Need for ventilation among survivors at 36 weeks' postmenstrual age.

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 4 Duration of mechanical ventilation among survivors (days).
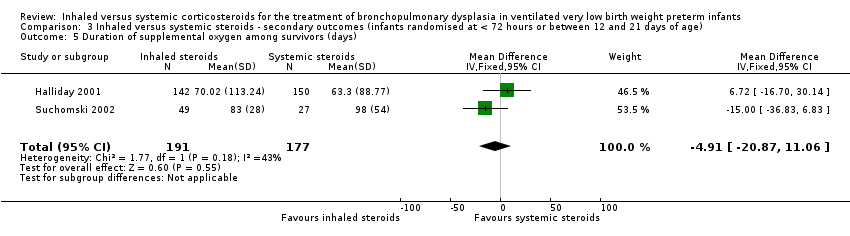
Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 5 Duration of supplemental oxygen among survivors (days).
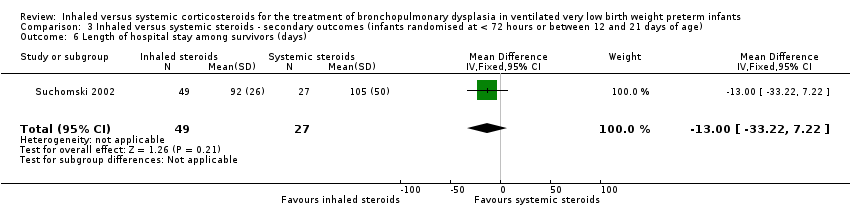
Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 6 Length of hospital stay among survivors (days).
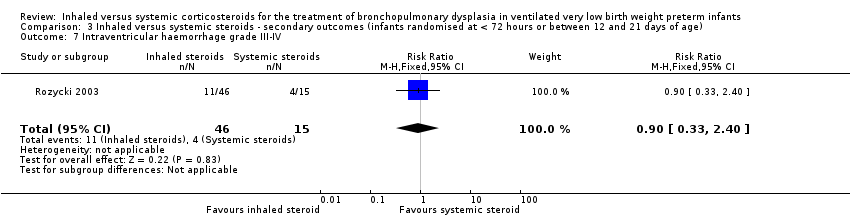
Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 7 Intraventricular haemorrhage grade III‐IV.
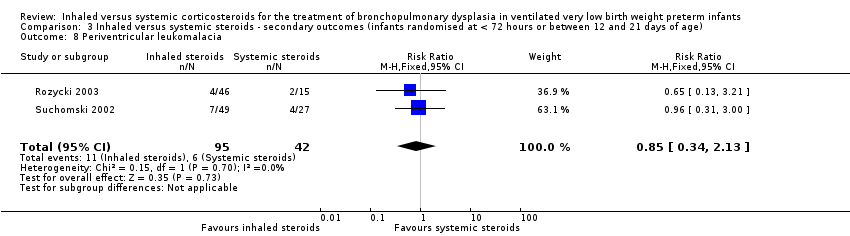
Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 8 Periventricular leukomalacia.

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 9 Hyperglycaemia.

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 10 Hypertension.

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 11 Necrotising enterocolitis.

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 12 Gastrointestional bleed.
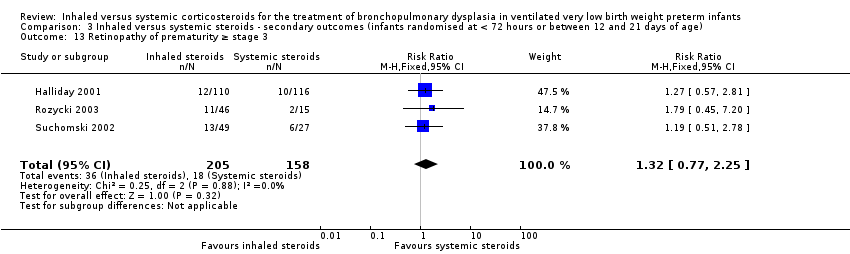
Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 13 Retinopathy of prematurity ≥ stage 3.

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 14 Culture‐proven sepsis.
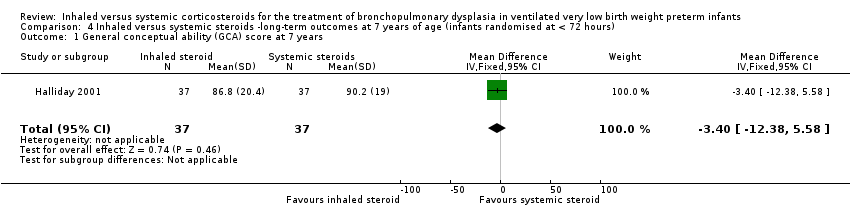
Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 1 General conceptual ability (GCA) score at 7 years.

Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 2 Child behaviour check list (CBLC) at 7 years.

Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 3 Strengths and Difficulties Questionnaire (SDQ) at 7 years.

Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 4 Cerebral palsy at 7 years.
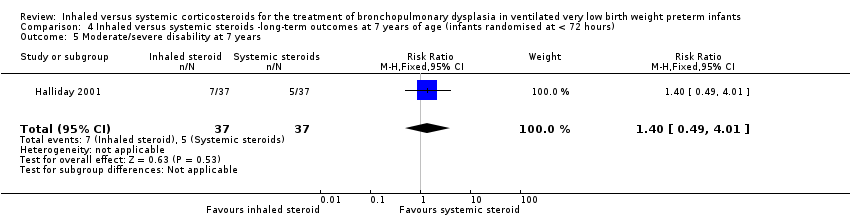
Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 5 Moderate/severe disability at 7 years.
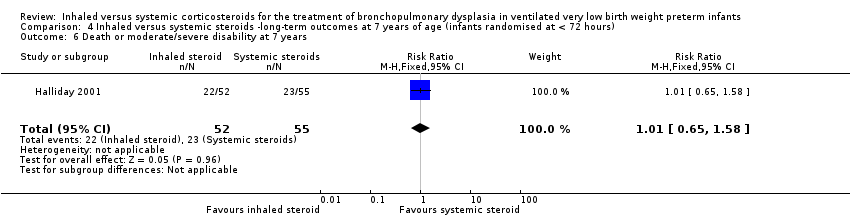
Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 6 Death or moderate/severe disability at 7 years.

Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 7 Systolic blood pressure of > 95th percentile at 7 years.

Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 8 Diastolic blood pressure of > 95th percentile at 7 years.
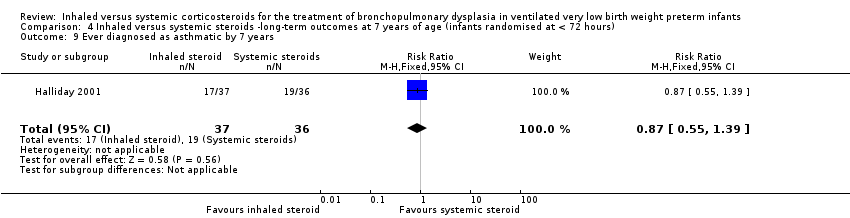
Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 9 Ever diagnosed as asthmatic by 7 years.
| Inhaled steroids compared with systemic steroids for BPD (infants randomised at < 72 hours of age) | ||||||
| Patient or population: Neonates with developing BPD Settings: Neonatal intensive care unit Intervention: Inhaled steroids Comparison: Systemic steroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic steroids | Inhaled steroids | |||||
| Death or BPD at 36 weeks' postmenstrual age | High risk population | RR 1.04 (95% CI 0.86 to 1.26) | 292 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this single study was high. The study was not blinded at all sites. Only 35/150 infants randomised to systemic steroids received full course while 33/142 infants randomised to inhaled steroids received full course. Results were presented in intention to treat analyses including deaths occurring after 72 hours of age. We downgraded the quality of the evidence by one step. Precision: Precison for the point estimate was acceptable Presence of publication bias: N/A. | |
| 580 per 1000 | 606 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Inhaled steroids compared with systemic steroids for BPD (infants randomised between 12 and 21 days of age) | ||||||
| Patient or population: Neonates with developing BPD Settings: Neonatal intensive care unit Intervention: Inhaled steroids Comparison: Systemic steroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic steroids | Inhaled steroids | |||||
| Death or BPD at 36 weeks' postmenstrual age | High risk population | RR 0.94 (95% CI 0.83 to 1.05) | 78 (1) | ⊕⊕⊝⊝ | Bias: The risk of bias for this single study was high. There was no blinding of the intervention or outcome measurements. We downgraded the quality of the evidence by one level. Precision: The precision for the point estimate was low as the sample size was small Presence of publication bias: N/A. | |
| 963 per 1000 | 902 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Inhaled steroids compared with systemic steroids for BPD (infants randomised at < 72 hours or between 12 and 21 days of age) | ||||||
| Patient or population: Neonates with developing BPD Settings: Neonatal intensive care unit Intervention: Inhaled steroids Comparison: Systemic steroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic steroids | Inhaled steroids | |||||
| BPD at 36 weeks' postmenstrual age | High risk population | RR 1.08 (95% CI 0.88 to 1.32) | 429 (3) | ⊕⊕⊝⊝ | Bias: The risk of bias for these three studies was high. There was blinding of randomisation in all three studies. There was no blinding of the intervention or outcome measurements at all sites in the largest study (Halliday 2001). In Rozycki 2003 there was blinding of the intervention but blinding of outcome assessment was unclear. In Suchomski 2002 there was no blinding of the intervention or outcomes measurements. We downgraded the quality of the evidence by two levels. Precision: The precision for the point estimate was high as the sample size was quite large. Presence of publication bias: N/A. We did not create a funnel plot as there were only three trials included in the analysis. | |
| 422 per 1000 | 485 per 1000 | |||||
| Hyperglycaemia | High risk population | RR 0.86 (95% CI 0.61 to 1.22) | 429 (3) | ⊕⊕⊝⊝ | Bias: The risk of bias for these three studies was high. There was blinding of randomisation in all three studies. There was no blinding of the intervention or outcome measurements at all sites in the largest study (Halliday 2001). In Rozycki 2003 there was blinding of the intervention but blinding of outcome assessments was unclear. In Suchomski 2002 there was no blinding of the intervention or outcome measurements. We downgraded the quality of the evidence by two levels. Heterogeneity/consistency: There was no heterogeneity (I² = 8%). Precision: The precision for the point estimate was high as the sample size was quite large. Presence of publication bias: N/A. We did not create a funnel plot as there were only three trials included in the analysis. | |
| 255 per 1000 | 177 per 1000 | |||||
| Hypertension | High risk population | RR (RR 0.86, 95% CI 0.73 to 1.01) | 429 (3) | ⊕⊕⊝⊝ | Bias: The risk of bias for these three studies was high. There was blinding of randomisation in all three studies. There was no blinding of the intervention or outcome measurements at all sites in the largest study (Halliday 2001). In Rozycki 2003 there was blinding of the intervention but blinding of outcome assessments was unclear. In Suchomski 2002 there was no blinding of the intervention or outcome measurements. We downgraded the quality of the evidence by two steps. Heterogeneity/consistency: There was no heterogeneity (I² = 0%). Precision: The precision for the point estimate was high as the sample size was quite large. Presence of publication bias: N/A. We did not create a funnel plot as there were only three trials included in the analysis. | |
| 604 per 1000 | 430 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Inhaled steroids compared with systemic steroids for BPD ‐ long‐term outcomes at 7 years of age (infants randomised at < 72 hours of age) | ||||||
| Patient or population: Neonates with developing BPD Settings: NICU Intervention: Inhaled steroids Comparison: Systemic steroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic steroids | Inhaled steroids | |||||
| General conceptual ability (GCA) score at 7 years The test has a standardisation mean of 100 and SD of 15 | The mean GCA score in the control group was 90.2 | The mean GCA score in the intervention groups was 3.4 units lower | MD ‐3.40 (95% CI ‐12.38 to 5.58) | 74 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the Quality of the evidence by one step. Presence of publication bias: N/A. |
| Moderate/severe disability at 7 years | 135 per 1000 | 189 per 1000 | RR 1.40 (95% CI 0.49 to 4.01) | 74 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the Quality of the evidence by one step. Presence of publication bias: N/A. |
| Death or moderate/severe disability at 7 years | 418 per 1000 | 423 per 1000 | RR 1.01 (95% CI 0.65 to 1.58) | 107 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the Quality of the evidence by one step. Presence of publication bias: N/A. |
| Systolic blood pressure > 95th percentile at 7 years | 353 per 1000 | 194 per 1000 | RR 0.55 (95% CI 0.25 to 1.23) | 70 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the quality of the evidence by one step. Presence of publication bias: N/A. |
| Diastolic blood pressure > 95th percentile at 7 years | 121 per 1000 | 167 per 1000 | RR (1.38, 95% CI 0.43 to 4.45) | 69 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the quality of the evidence by one step. Presence of publication bias: N/A. |
| Ever diagnosed as asthmatic by 7 years | 528 per 1000 | 459 per 1000 | RR 0.87 (95% CI 0.55 to 1.39) | 73 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the quality of the evidence by one step. Presence of publication bias: N/A. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or BPD at 36 weeks' postmenstrual age Show forest plot | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.86, 1.26] |
| 2 Death or BPD at 28 days of age Show forest plot | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.90, 1.12] |
| 3 Death at 36 weeks' postmenstrual age Show forest plot | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.62, 1.49] |
| 4 Death at 28 days of age Show forest plot | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.52, 1.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or BPD at 36 weeks' postmenstrual age Show forest plot | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.83, 1.05] |
| 2 Death at 36 weeks' postmenstrual age Show forest plot | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [0.13, 54.15] |
| 3 Death at 28 days of age Show forest plot | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [0.13, 54.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 BPD at 36 weeks' postmenstrual age Show forest plot | 3 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.88, 1.32] |
| 2 BPD at 28 days of age Show forest plot | 2 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 3 Need for ventilation among survivors at 36 weeks' postmenstrual age Show forest plot | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.30, 4.06] |
| 4 Duration of mechanical ventilation among survivors (days) Show forest plot | 2 | 368 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐5.22, 4.63] |
| 5 Duration of supplemental oxygen among survivors (days) Show forest plot | 2 | 368 | Mean Difference (IV, Fixed, 95% CI) | ‐4.91 [‐20.87, 11.06] |
| 6 Length of hospital stay among survivors (days) Show forest plot | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐13.0 [‐33.22, 7.22] |
| 7 Intraventricular haemorrhage grade III‐IV Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.33, 2.40] |
| 8 Periventricular leukomalacia Show forest plot | 2 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.34, 2.13] |
| 9 Hyperglycaemia Show forest plot | 3 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.61, 1.22] |
| 10 Hypertension Show forest plot | 3 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.73, 1.01] |
| 11 Necrotising enterocolitis Show forest plot | 2 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.50, 1.85] |
| 12 Gastrointestional bleed Show forest plot | 2 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.41, 1.93] |
| 13 Retinopathy of prematurity ≥ stage 3 Show forest plot | 3 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.77, 2.25] |
| 14 Culture‐proven sepsis Show forest plot | 2 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.79, 1.45] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 General conceptual ability (GCA) score at 7 years Show forest plot | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐3.40 [‐12.38, 5.58] |
| 2 Child behaviour check list (CBLC) at 7 years Show forest plot | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐4.75, 5.15] |
| 3 Strengths and Difficulties Questionnaire (SDQ) at 7 years Show forest plot | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐2.19, 4.19] |
| 4 Cerebral palsy at 7 years Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.35, 2.72] |
| 5 Moderate/severe disability at 7 years Show forest plot | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.4 [0.49, 4.01] |
| 6 Death or moderate/severe disability at 7 years Show forest plot | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.65, 1.58] |
| 7 Systolic blood pressure of > 95th percentile at 7 years Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.25, 1.23] |
| 8 Diastolic blood pressure of > 95th percentile at 7 years Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.43, 4.45] |
| 9 Ever diagnosed as asthmatic by 7 years Show forest plot | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.55, 1.39] |

