Corticosteroides inhalados versus sistémicos para el tratamiento de la displasia broncopulmonar en recién nacidos prematuros ventilados de muy bajo peso al nacer
Información
- DOI:
- https://doi.org/10.1002/14651858.CD002057.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 16 octubre 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Neonatología
- Copyright:
-
- Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Sachin Shah (SS): performance of literature search, identification of studies, abstraction of the data, analysis of data and writing of the review.
Arne Ohlsson (AO): writing of the protocol, identification of studies (literature search), abstraction of data, analysis of data and editing of the review.
Henry Halliday (HH): writing of protocol, identification of studies, abstraction of data, analysis of data and editing of review.
Vibhuti Shah (VS): writing of the protocol, identification of studies (literature search), abstraction of data, analysis of data and editing of the review.
The searches for the 2011 update were completed by SS, AO, VS. The administrative update was conducted centrally by the Cochrane Neonatal Review Group staff (Yolanda Montagne, Diane Haughton, and Roger Soll).
The literature searches for the 2017 update were conducted by Jennifer Spano, Information Specialist, Cochrane Neonatal.
This 2017 update was reviewed and approved by SS, AO, VS, HH.
Sources of support
Internal sources
-
Mount Sinai Hospital, Toronto, Ontario, Canada.
External sources
-
No sources of support supplied
Declarations of interest
Sachin S Shah, no conflict of interest to declare.
Arne Ohlsson, no conflict of interest to declare.
Henry L Halliday, is the author of an included trial. He did not assess the quality of his own trial.
Vibhuti S Shah, no conflict of interest to declare.
Acknowledgements
We thank Dr HL Halliday and Dr Chris Patterson for providing additional data for the infants included in the OSECT trial.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Oct 16 | Inhaled versus systemic corticosteroids for the treatment of bronchopulmonary dysplasia in ventilated very low birth weight preterm infants | Review | Sachin S Shah, Arne Ohlsson, Henry L Halliday, Vibhuti S Shah | |
| 2012 May 16 | Inhaled versus systemic corticosteroids for the treatment of chronic lung disease in ventilated very low birth weight preterm infants | Review | Sachin S Shah, Arne Ohlsson, Henry L Halliday, Vibhuti S Shah | |
| 2007 Oct 17 | Inhaled versus systemic corticosteroids for the treatment of chronic lung disease in ventilated very low birth weight preterm infants | Review | Sachin S Shah, Arne Ohlsson, Henry L Halliday, Vibhuti S Shah | |
| 2003 Jan 20 | Inhaled versus systemic corticosteroids for the treatment of chronic lung disease in ventilated very low birth weight preterm infants | Review | Sachin S Shah, Arne Ohlsson, Henry L Halliday, Vibhuti Shah | |
Differences between protocol and review
There is no published protocol. In this 2017 update the primary outcome was changed to death or bronchopulmonary dysplasia at 36 weeks' postmenstrual age. The title was changed from "Inhaled versus systemic corticosteroids for the treatment of chronic lung disease in ventilated very low birth weight preterm infants" to "Inhaled versus systemic corticosteroids for the treatment of bronchopulmonary dysplasia in ventilated very low birth weight preterm infants" to reflect this change. These changes were made following recommendations from the Editorial team so that different reviews on the topic of postnatal steroids would use the same primary outcome and the same nomenclature. These changes necessitated changes to the secondary outcomes that were listed in the previous update of the review.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- *Infant, Very Low Birth Weight;
- *Respiration, Artificial;
- Administration, Inhalation;
- Beclomethasone [administration & dosage];
- Bronchopulmonary Dysplasia [*drug therapy, mortality];
- Chronic Disease;
- Dexamethasone [administration & dosage];
- Glucocorticoids [*administration & dosage];
- Infant, Premature;
- Randomized Controlled Trials as Topic;
- Respiratory System Agents [administration & dosage];
Medical Subject Headings Check Words
Humans; Infant, Newborn;
PICO

Study flow diagram: review update

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
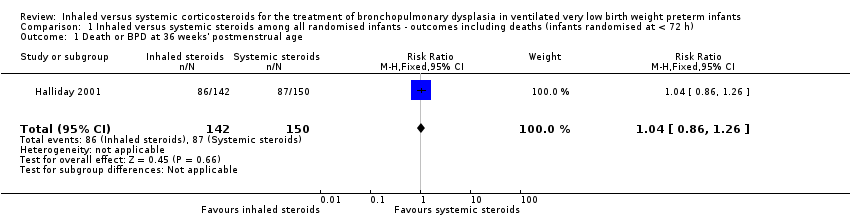
Comparison 1 Inhaled versus systemic steroids among all randomised infants ‐ outcomes including deaths (infants randomised at < 72 h), Outcome 1 Death or BPD at 36 weeks' postmenstrual age.

Comparison 1 Inhaled versus systemic steroids among all randomised infants ‐ outcomes including deaths (infants randomised at < 72 h), Outcome 2 Death or BPD at 28 days of age.

Comparison 1 Inhaled versus systemic steroids among all randomised infants ‐ outcomes including deaths (infants randomised at < 72 h), Outcome 3 Death at 36 weeks' postmenstrual age.

Comparison 1 Inhaled versus systemic steroids among all randomised infants ‐ outcomes including deaths (infants randomised at < 72 h), Outcome 4 Death at 28 days of age.

Comparison 2 Inhaled versus systemic steroids among infants ‐ outcomes including deaths (Infants randomised between 12 and 21 days of age), Outcome 1 Death or BPD at 36 weeks' postmenstrual age.
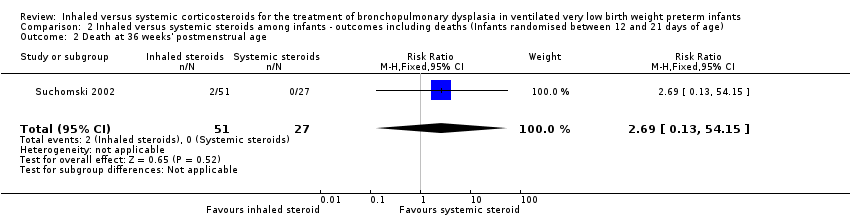
Comparison 2 Inhaled versus systemic steroids among infants ‐ outcomes including deaths (Infants randomised between 12 and 21 days of age), Outcome 2 Death at 36 weeks' postmenstrual age.

Comparison 2 Inhaled versus systemic steroids among infants ‐ outcomes including deaths (Infants randomised between 12 and 21 days of age), Outcome 3 Death at 28 days of age.

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 1 BPD at 36 weeks' postmenstrual age.

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 2 BPD at 28 days of age.

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 3 Need for ventilation among survivors at 36 weeks' postmenstrual age.

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 4 Duration of mechanical ventilation among survivors (days).

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 5 Duration of supplemental oxygen among survivors (days).

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 6 Length of hospital stay among survivors (days).

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 7 Intraventricular haemorrhage grade III‐IV.

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 8 Periventricular leukomalacia.
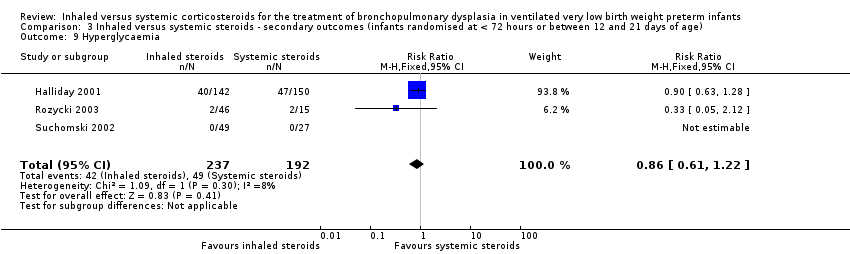
Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 9 Hyperglycaemia.
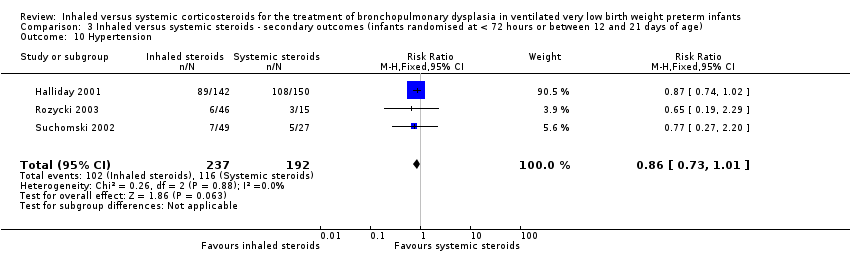
Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 10 Hypertension.

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 11 Necrotising enterocolitis.

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 12 Gastrointestional bleed.
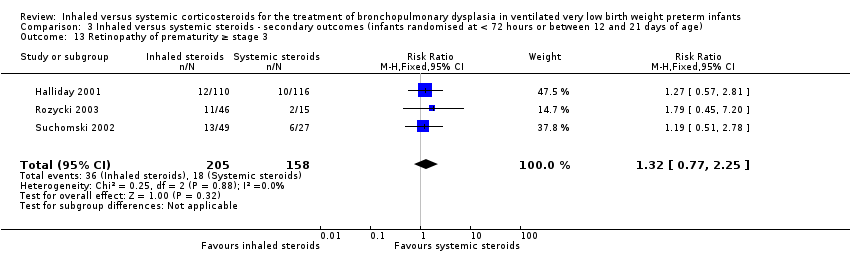
Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 13 Retinopathy of prematurity ≥ stage 3.

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 14 Culture‐proven sepsis.

Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 1 General conceptual ability (GCA) score at 7 years.

Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 2 Child behaviour check list (CBLC) at 7 years.
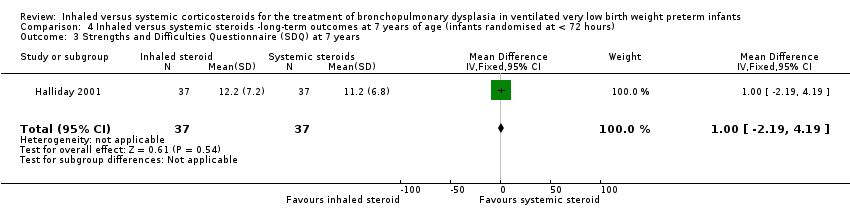
Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 3 Strengths and Difficulties Questionnaire (SDQ) at 7 years.

Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 4 Cerebral palsy at 7 years.

Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 5 Moderate/severe disability at 7 years.

Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 6 Death or moderate/severe disability at 7 years.

Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 7 Systolic blood pressure of > 95th percentile at 7 years.

Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 8 Diastolic blood pressure of > 95th percentile at 7 years.

Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 9 Ever diagnosed as asthmatic by 7 years.
| Inhaled steroids compared with systemic steroids for BPD (infants randomised at < 72 hours of age) | ||||||
| Patient or population: Neonates with developing BPD Settings: Neonatal intensive care unit Intervention: Inhaled steroids Comparison: Systemic steroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic steroids | Inhaled steroids | |||||
| Death or BPD at 36 weeks' postmenstrual age | High risk population | RR 1.04 (95% CI 0.86 to 1.26) | 292 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this single study was high. The study was not blinded at all sites. Only 35/150 infants randomised to systemic steroids received full course while 33/142 infants randomised to inhaled steroids received full course. Results were presented in intention to treat analyses including deaths occurring after 72 hours of age. We downgraded the quality of the evidence by one step. Precision: Precison for the point estimate was acceptable Presence of publication bias: N/A. | |
| 580 per 1000 | 606 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Inhaled steroids compared with systemic steroids for BPD (infants randomised between 12 and 21 days of age) | ||||||
| Patient or population: Neonates with developing BPD Settings: Neonatal intensive care unit Intervention: Inhaled steroids Comparison: Systemic steroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic steroids | Inhaled steroids | |||||
| Death or BPD at 36 weeks' postmenstrual age | High risk population | RR 0.94 (95% CI 0.83 to 1.05) | 78 (1) | ⊕⊕⊝⊝ | Bias: The risk of bias for this single study was high. There was no blinding of the intervention or outcome measurements. We downgraded the quality of the evidence by one level. Precision: The precision for the point estimate was low as the sample size was small Presence of publication bias: N/A. | |
| 963 per 1000 | 902 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Inhaled steroids compared with systemic steroids for BPD (infants randomised at < 72 hours or between 12 and 21 days of age) | ||||||
| Patient or population: Neonates with developing BPD Settings: Neonatal intensive care unit Intervention: Inhaled steroids Comparison: Systemic steroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic steroids | Inhaled steroids | |||||
| BPD at 36 weeks' postmenstrual age | High risk population | RR 1.08 (95% CI 0.88 to 1.32) | 429 (3) | ⊕⊕⊝⊝ | Bias: The risk of bias for these three studies was high. There was blinding of randomisation in all three studies. There was no blinding of the intervention or outcome measurements at all sites in the largest study (Halliday 2001). In Rozycki 2003 there was blinding of the intervention but blinding of outcome assessment was unclear. In Suchomski 2002 there was no blinding of the intervention or outcomes measurements. We downgraded the quality of the evidence by two levels. Precision: The precision for the point estimate was high as the sample size was quite large. Presence of publication bias: N/A. We did not create a funnel plot as there were only three trials included in the analysis. | |
| 422 per 1000 | 485 per 1000 | |||||
| Hyperglycaemia | High risk population | RR 0.86 (95% CI 0.61 to 1.22) | 429 (3) | ⊕⊕⊝⊝ | Bias: The risk of bias for these three studies was high. There was blinding of randomisation in all three studies. There was no blinding of the intervention or outcome measurements at all sites in the largest study (Halliday 2001). In Rozycki 2003 there was blinding of the intervention but blinding of outcome assessments was unclear. In Suchomski 2002 there was no blinding of the intervention or outcome measurements. We downgraded the quality of the evidence by two levels. Heterogeneity/consistency: There was no heterogeneity (I² = 8%). Precision: The precision for the point estimate was high as the sample size was quite large. Presence of publication bias: N/A. We did not create a funnel plot as there were only three trials included in the analysis. | |
| 255 per 1000 | 177 per 1000 | |||||
| Hypertension | High risk population | RR (RR 0.86, 95% CI 0.73 to 1.01) | 429 (3) | ⊕⊕⊝⊝ | Bias: The risk of bias for these three studies was high. There was blinding of randomisation in all three studies. There was no blinding of the intervention or outcome measurements at all sites in the largest study (Halliday 2001). In Rozycki 2003 there was blinding of the intervention but blinding of outcome assessments was unclear. In Suchomski 2002 there was no blinding of the intervention or outcome measurements. We downgraded the quality of the evidence by two steps. Heterogeneity/consistency: There was no heterogeneity (I² = 0%). Precision: The precision for the point estimate was high as the sample size was quite large. Presence of publication bias: N/A. We did not create a funnel plot as there were only three trials included in the analysis. | |
| 604 per 1000 | 430 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Inhaled steroids compared with systemic steroids for BPD ‐ long‐term outcomes at 7 years of age (infants randomised at < 72 hours of age) | ||||||
| Patient or population: Neonates with developing BPD Settings: NICU Intervention: Inhaled steroids Comparison: Systemic steroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic steroids | Inhaled steroids | |||||
| General conceptual ability (GCA) score at 7 years The test has a standardisation mean of 100 and SD of 15 | The mean GCA score in the control group was 90.2 | The mean GCA score in the intervention groups was 3.4 units lower | MD ‐3.40 (95% CI ‐12.38 to 5.58) | 74 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the Quality of the evidence by one step. Presence of publication bias: N/A. |
| Moderate/severe disability at 7 years | 135 per 1000 | 189 per 1000 | RR 1.40 (95% CI 0.49 to 4.01) | 74 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the Quality of the evidence by one step. Presence of publication bias: N/A. |
| Death or moderate/severe disability at 7 years | 418 per 1000 | 423 per 1000 | RR 1.01 (95% CI 0.65 to 1.58) | 107 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the Quality of the evidence by one step. Presence of publication bias: N/A. |
| Systolic blood pressure > 95th percentile at 7 years | 353 per 1000 | 194 per 1000 | RR 0.55 (95% CI 0.25 to 1.23) | 70 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the quality of the evidence by one step. Presence of publication bias: N/A. |
| Diastolic blood pressure > 95th percentile at 7 years | 121 per 1000 | 167 per 1000 | RR (1.38, 95% CI 0.43 to 4.45) | 69 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the quality of the evidence by one step. Presence of publication bias: N/A. |
| Ever diagnosed as asthmatic by 7 years | 528 per 1000 | 459 per 1000 | RR 0.87 (95% CI 0.55 to 1.39) | 73 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the quality of the evidence by one step. Presence of publication bias: N/A. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or BPD at 36 weeks' postmenstrual age Show forest plot | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.86, 1.26] |
| 2 Death or BPD at 28 days of age Show forest plot | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.90, 1.12] |
| 3 Death at 36 weeks' postmenstrual age Show forest plot | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.62, 1.49] |
| 4 Death at 28 days of age Show forest plot | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.52, 1.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or BPD at 36 weeks' postmenstrual age Show forest plot | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.83, 1.05] |
| 2 Death at 36 weeks' postmenstrual age Show forest plot | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [0.13, 54.15] |
| 3 Death at 28 days of age Show forest plot | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [0.13, 54.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 BPD at 36 weeks' postmenstrual age Show forest plot | 3 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.88, 1.32] |
| 2 BPD at 28 days of age Show forest plot | 2 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 3 Need for ventilation among survivors at 36 weeks' postmenstrual age Show forest plot | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.30, 4.06] |
| 4 Duration of mechanical ventilation among survivors (days) Show forest plot | 2 | 368 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐5.22, 4.63] |
| 5 Duration of supplemental oxygen among survivors (days) Show forest plot | 2 | 368 | Mean Difference (IV, Fixed, 95% CI) | ‐4.91 [‐20.87, 11.06] |
| 6 Length of hospital stay among survivors (days) Show forest plot | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐13.0 [‐33.22, 7.22] |
| 7 Intraventricular haemorrhage grade III‐IV Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.33, 2.40] |
| 8 Periventricular leukomalacia Show forest plot | 2 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.34, 2.13] |
| 9 Hyperglycaemia Show forest plot | 3 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.61, 1.22] |
| 10 Hypertension Show forest plot | 3 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.73, 1.01] |
| 11 Necrotising enterocolitis Show forest plot | 2 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.50, 1.85] |
| 12 Gastrointestional bleed Show forest plot | 2 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.41, 1.93] |
| 13 Retinopathy of prematurity ≥ stage 3 Show forest plot | 3 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.77, 2.25] |
| 14 Culture‐proven sepsis Show forest plot | 2 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.79, 1.45] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 General conceptual ability (GCA) score at 7 years Show forest plot | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐3.40 [‐12.38, 5.58] |
| 2 Child behaviour check list (CBLC) at 7 years Show forest plot | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐4.75, 5.15] |
| 3 Strengths and Difficulties Questionnaire (SDQ) at 7 years Show forest plot | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐2.19, 4.19] |
| 4 Cerebral palsy at 7 years Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.35, 2.72] |
| 5 Moderate/severe disability at 7 years Show forest plot | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.4 [0.49, 4.01] |
| 6 Death or moderate/severe disability at 7 years Show forest plot | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.65, 1.58] |
| 7 Systolic blood pressure of > 95th percentile at 7 years Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.25, 1.23] |
| 8 Diastolic blood pressure of > 95th percentile at 7 years Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.43, 4.45] |
| 9 Ever diagnosed as asthmatic by 7 years Show forest plot | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.55, 1.39] |

